Abstract
Purpose
Male infertility is a worldwide problem with limitations in the treatment. Phosphodiesterase-5 inhibitors (PDE5is) is the first choice for the treatment of erectile dysfunction, more and more studies show that it has a certain effect on male infertility in recent years. But there was currently no high quality of systematic review to evaluate the effects of PDE5is on semen quality.
Materials and Methods
We retrieved the electronic databases of MEDLINE, PubMed, Web of Science, EMBASE, etc. Related randomized controlled trials (RCTs) were collected and selected up to May 20, 2020. We have searched literature with terms “male infertility”, “phosphodiesterase-5 inhibitors”, “PDE5i”, “Tadalafil”, “Sildenafil”, “Vardenafil”, “Udenafil”, “Avanafil”, “semen”, and “sperm”. Mean value and its standard deviation were used to perform quantitative analysis. All statistical analyses were conducted by RevMan 5.3 and Stata software.
Results
There were a total of 1,121 participants in the nine included studies. There was a statistically significant improvement treated with PDE5is compared with sham therapy, which including sperm concentration (mean difference [MD]=1.96, 95% confidence interval [CI]=1.70–2.21, p<0.001; MD=3.22, 95% CI=1.96–4.48, p<0.001), straight progressive motility (%) Grade A (MD=3.71, 95% CI=2.21–5.20, p<0.001), sperm motility (MD=8.09, 95% CI=7.83–8.36, p<0.001), morphologically normal spermatozoa (%) (MD=0.67, 95% CI=0.20–1.15, p=0.005; MD=1.27, 95% CI=0.02–2.52, p=0.05), sperm abnormalities (%) (MD=−0.64, 95% CI=−0.81–−0.47, p<0.001), and progressive motile sperm (MD=5.34, 95% CI=3.87–6.81, p<0.001).
Conclusions
In this meta-analysis of nine RCTs, treatment with PDE5is could improve some indicators of male sperm.
Keywords: Infertility, male; Meta-analysis; Phosphodiesterase 5 inhibitors; Spermatozoa; Systematic review
INTRODUCTION
With the process of human industrialization, infertility has become a threat to the health of men all over the world [1]. There are many reasons for male infertility, most of which are unknown [2]. Phosphodiesterase-5 inhibitors (PDE5is) are the first-line drugs to treat erectile dysfunction (ED). PDE5is inhibits the degradation of cyclic guanosine monophosphate (cGMP) derived from nitric oxide (NO) by competitively binding to the catalytic site of PDE5. This can maintain a higher cellular level of cGMP in the cavernous body and blood vessels to continuously activate the NO/cGMP pathway, increasing penile blood flow during sexual stimulation, thereby enhancing penile erection [3]. More and more studies have used this kind of medicine in male infertility patients, but the curative effect is quite different.
In 2015, relevant systematic review [4] have been confirm the improvement effect of PDE5is on sperm quality, but there were many types of studies included, not limited to randomized controlled trials (RCTs). Five years have passed and we have retrieved some new and valuable clinical studies, the increased sample size would help to draw more reliable conclusions. Therefore, this paper makes a systematic review and metaanalysis of this subject again.
This study is registered on PROSPERO. Registration number is CRD42019142980. The protocol of the systematic review also elaborates the details of this study [5].
Given the availability of several RCTs studying the effects of PDE5i in the treatment of male infertility, we performed a meta-analysis to determine whether this novel treatment improves sperm quality in men with male infertility when assessed by the World Health Organization (WHO) sperm criteria compared with men undergoing sham therapy [6,7,8,9,10,11,12,13,14]. At all, we sought to provide evidence-based medical evidence for urologists and andrologists to make clinical decisions.
MATERIALS AND METHODS
1. Search strategy
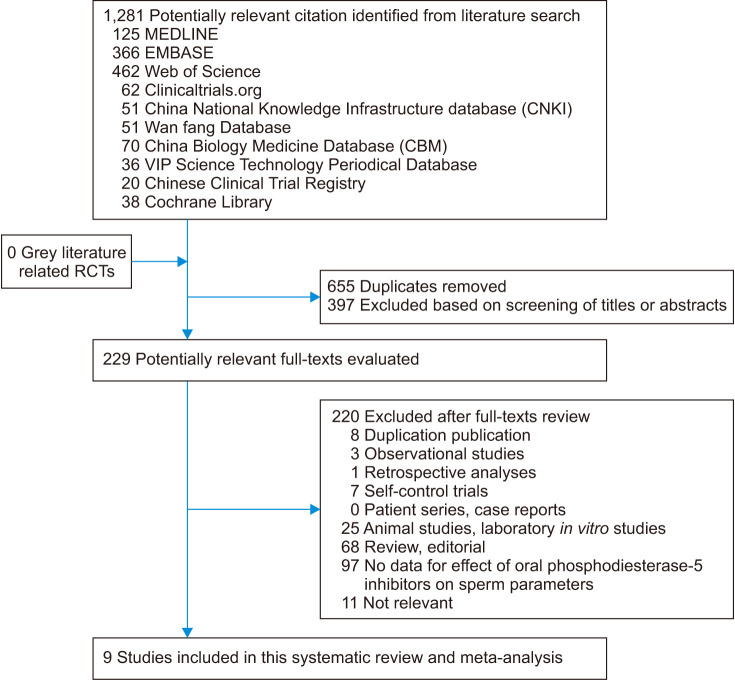
The electronic databases of MEDLINE, PubMed, Web of Science, EMBASE, Clinicaltrials.org., China National Knowledge Infrastructure Database, Wan fang Database, China Biology Medicine Database, VIP Science Technology Periodical Database, Chinese Clinical Trial Registry, and Cochrane Library have been retrieved. Grey literature has been searched in Open Grey. Related RCTs have been collected and selected up to May 20, 2020. As of this month's submission, we have retrieved and updated the data again. Medical Subject Heading or text key words “male infertility” or “semen” or “sperm” AND “phosphodiesterase-5 inhibitors” or “PDE5i” or “Tadalafil” or “Sildenafil” or “Vardenafil” or “Udenafil” or “Avanafil” have been used. The search strategy was adjusted to adapt the different databases. Chinese form of the above terms had been used in Chinese search. This systematic retrospective and meta-analysis have carried out in strict accordance with the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [15]. The data and results used in this paper are almost from published studies, and there are no ethical issues, so the approval of the ethics committee is not required. A flow diagram for study selection is presented in Fig. 1.
Fig. 1. Flow diagram for study selection. RCTs: randomized controlled trials.
2. Inclusion criteria and trial selection
The studies included must be RCTs on the treatment of male infertility with PDE5is, including tadalafil, sildenafil, vardenafil, udenafil and avanafil, and WHO sperm criteria were necessary for the assessment of the sperm quality. In similar patients and studies using the same method, only the largest sample size or recently studies were included. The articles unrelated to the analysis, lack of essential information on patients or intervention measures of the treatment, nonoriginal research, reviews, comments, cohort studies, animal models, and case reports were excluded.
3. Data extraction
Related data in the included articles was extracted independently by three investigators (Liang Dong, Xiaojin Zhang, Xuhong Yan) according to the PRISMA statement, and all discrepancies were resolved through adjudication and discussion by the other reviewer (Yulin Li). The words in abstracts such as “randomized” or “quasi-randomized” were used in all studies, regardless of whether they were blind or not. For each study, the following information were extracted: author, publication time, country, study design, sample size, treatment course, edition of WHO guidelines, diagnostic criteria, outcome indicators, treatment group medicine & dosage and posttreatment data. Study investigators from Jarvi et al [9]'s study were contacted to obtain further information, but the researchers did not reply to our e-mail.
4. Quality assessment
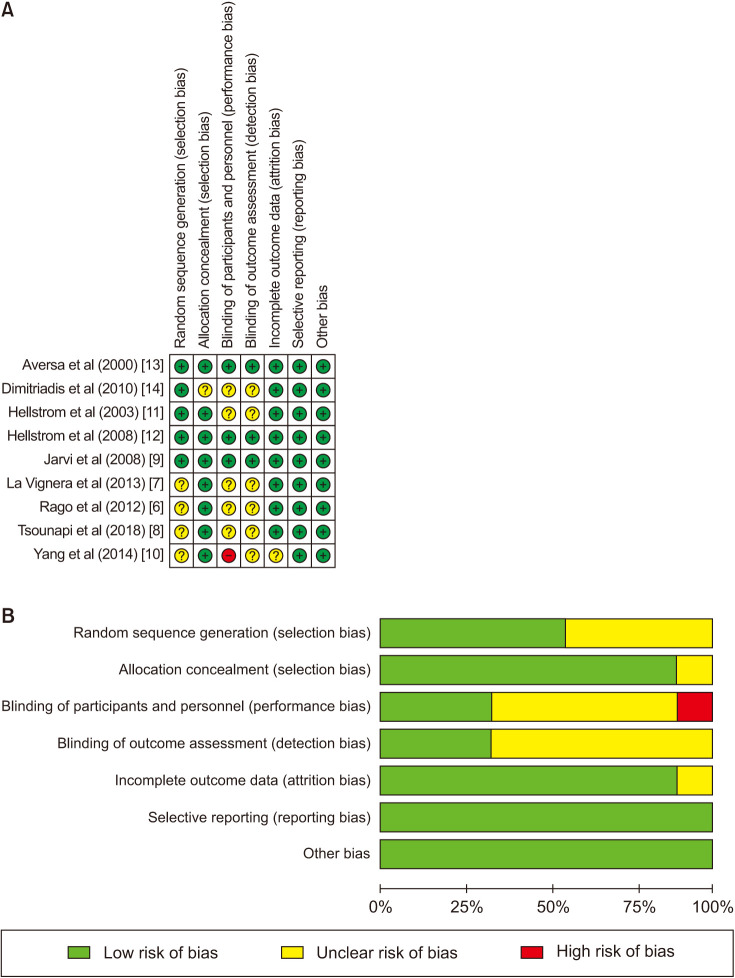
The items of randomization, method to generate the sequence of randomization, randomization concealment, blinding, results data integrity, selective outcome reports and other potential bias sources in the included RCTs were assessed by the Cochrane Risk of Bias Assessment tool [16]. Graph and summary about risk of bias were produced with RevMan 5.3 [17]. All the domains were independently assessed by two trained investigators (Liang Dong, Xiaojin Zhang). All the disputes were resolved by a third professional reviewer (R.R.) through discussion and adjudication.
5. Data synthesis and analysis
The mean values of semen indexes after treatment with PDE5i or sham-therapy in each study were collected, including semen volume, sperm motility, proportion of progressive motile sperm, sperm concentration, normal morphology and so on. Statistics analyses were estimated with RevMan 5.3 and displayed as a forest plot, while a funnel plot has been generated to assess the risk of bias. Statistical tests were two-sided and used p-value less than 0.05 as a significance threshold. The Egger test and Funnel plot were used for investigating publication bias to small study effects [18,19]. The heterogeneity between studies was assessed by standard χ2 test and I2 statistics [20]. Sensitivity analysis was conducted by excluding the effects of individual studies one by one on the overall estimates [21]. Statistical tests were two-sided and the significance threshold p-value used was less than 0.05.
6. Ethics statement
The data and results used in this paper are almost from published studies, and there is no ethical issue, so the approval of the ethics committee is not required.
RESULTS
1. Study characteristics
Nine RCTs involving 1,211 participants were included in this meta-analysis (Table 1). Three studies were conducted in the United States, three studies took place in Italy, two studies from Japan, and one study from China. All nine studies used sham therapy for the control group using probes that looked and tasted similar to the active treatment probe. There are three studies of taking the drug as a one-time use, and one of them took a 15-day course of administration at the same time. The treatment duration of three studies was 3 months, two studies were 6 months, and one study was 9 months. Two studies used the WHO's 1992 version of the semen standard, five studies used the 1999 standard, and only two studies used the 2010 standard. The semen indicators counted in these nine studies include semen volume, sperm count, sperm concentration, grade A sperm ratio, grade B sperm ratio, abnormal sperm morphology ratio, normal sperm morphology ratio, sperm motility, and progressive motile sperm, which were seen in the Table 1. In addition, none of the studies included statistics on sperm DNA fragments. The drugs used in three studies were tadalafil with doses of 5 mg, 10 mg, and 20 mg. One study used only sildenafil at a dose of 100 mg. One study used only avanafil, at a dose of 50 mg. One study used only vardenafil, with a dose of 10 mg. The remaining three studies used multiple groups for controlled experiments, and two types of PDE5i for observation. One study included normal males with normal erectile function and normal fertility. Three studies included normal males or males with mild ED. Four studies included male infertility patients, such as oligoasthenospermia and oligoastheno spermia. Four studies included subjects with different types of male accessory gland infection. Further inclusion diagnostic criteria are listed in Table 1. For most studies, the risk of bias was low. However, the risk of bias was unclear for several domains of published abstracts (Fig. 2).
Table 1. Characteristics of the included study of PDE5is on sperm quality in this systematic review.
| Author | Publishment time | Country | Study design | Sample | Treatment course | Edition of WHO guidelines | Diagnostic criteria | Outcome indicators | Treatment group medicine & dosage | Mean±SD after treatment | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | p-value | |||||||||
| Aversa et al [13] | 2000 | Italy | Double-blind, randomized, placebo-controlled, cross-over | 20 | 20 | Once | 1992 | The inclusion criteria included normal erectile function, proven fertility, a stable relationship, normal electrocardiogram, no prior or concomitant serious illness or consumption of medications during the 3 months period prior to the study. | Sperm number (×106) Straight progressive motility (%) Grade a Straight progressive motility (%) Grade b Sperm abnormalities (%) |
100 mg Sildenafil (Viagra™; Pfizer, Sandwich, UK) | 98.8±14.7 | 94.8±17.2 | - |
| 36.4±2.9 | 31.9±2.4 | ||||||||||||
| 18.6±1.8 | 19.9±2.3 | ||||||||||||
| 52.4±3.1 | 50.4±3.5 | ||||||||||||
| Hellstrom et al [11] | 2003 | USA | Randomized, double-blind, placebo controlled, parallel group | 87 | 88 (10 mg group) | 6 months | 1999 | Healthy men or men with mild ED who were at least 45 years old were eligible. For subjects to be enrolled semen samples including a sperm concentration of 20 million/mL, 50% sperm motility, 50% normal sperm morphology, and 2 and 1.5 mL ejaculate volume in the 10 and 20 mg Tadalafil studies, respectively. | Mean sperm concentration (×106/mL) Mean ejaculate sperm count (×106) Mean % normal sperm morphology Mean % normal sperm motility |
10 and 20 mg Tadalafil | 10 mg study (baseline–change from baseline): 74.9–8.2; 217.5–41.7; 58.9+1.4; 61.5–2.5 | 10 mg study (baseline–change from baseline): 81.5+0.9; 232.6–22.0; 58.9+0.7; 61.8–2.8 | 10 mg study: 0.02; 0.089; 0.388; 0.643 |
| 89 (20 mg group) | 20 mg study (baseline–change from baseline): 76.8–4; 189–10; 62.4–1.1; 59.7–1.7 | 20 mg study (baseline–change from baseline): 78.6–4.2; 210.1–25.8; 63.2–2.3; 61.4–4.4 | 20 mg study: 0.913; 0.231; 0.138; 0.04 | ||||||||||
| Hellstrom et al [12] | 2008 | USA | Multicenter, randomized, double-blind, placebo-controlled | 95 | 96 | 9 months | 1999 | Healthy men or men with mild ED who were at least 45 years of age were eligible to enroll if their semen samples at baseline met the following criteria: sperm concentration of 20 million/mL (geometric mean of two samples), 50% motile sperm, 50% sperm with normal morphology, and semen volume 1.5 mL (arithmetic mean of two samples). | Sperm concentration (106/mL) Sperm count (million) Motile sperm (%) Normal sperm morphology (%) |
20 mg Tadalafil | Arithmetic mean 70.8; 191.9; 57.2; 58.0 | Arithmetic mean 73.8; 186.3; 59.4; 58.4 | Unadjusted p: 0.10; 0.95; 0.58; 0.63 |
| Jarvi et al [9] | 2008 | USA | Randomized, double-blind, placebo controlled, parallel group | 65 (Sildenafil 100 mg) | 65 | 6 months | 1992 | Subjects were included if they fulfilled the criteria of healthy male volunteers or males with ED, with the ability to produce semen samples without requiring ED therapy, 25 to 64 years old, baseline sperm concentration 20×106/mL or greater, sperm motility 50% or greater (A+B+C), 30% or more sperm with normal morphology and an ejaculate volume of 1 mL or more at each of 3 analyses during screening. | Sperm concentration Percentage normal sperm morphology |
Sildenafil 100 mg and Vardenafil 20 mg | - | - | - |
| 66 (Vardenafil 20 mg) | |||||||||||||
| Dimitriadis et al [14] | 2010 | Japan | Randomized, placebo controlled | 23 (Vardenafil 10 mg/d) | 22 | 3 months | 1999 | Vardenafil (10 mg/d, group A) or Sildenafil (50 mg/d, group B) or L-carnitine (1,000 mg/d, group C; positive controls) were administered to 23 (group A), 25 (group B), and 26 (group C) infertile men with oligoasthenospermia for 12 weeks. Another group of 22 infertile men with oligoasthenospermia (group D) served as a negative control group and receive no pharmacological treatment. | Semen volume (mL) Sperm concentration (×106/mL) Motion spermatozoa (%) Morphologically normal spermatozoa (%) |
Vardenafil 10 mg/d and Sildenafil 50 mg/d | Vardenafil 10 mg/d: 3.1±0.4; 22.6±8.2; 39.2±10.6; 40.6±9.0 | 2.3±0.5; 16.3±7.0; 24.7±10.8; 23.7±8.1 | - |
| 25 (Sildenafil 50 mg/d) | Sildenafil 50 mg/d: 2.8±0.4; 24.3±9.4; 47.1±12.2; 41.3±8.6 | ||||||||||||
| La Vignera et al [7] | 2013 | Italy | Randomized, placebo controlled | 20 (‘fibrosclerotic’ ultrasound forms [FSUF], male accessory gland infection [MAGI]) | 20 (FSUF MAGI) | 3 months | 2010 | A total of 80 infertile patients (31.0±7.0 years [range, 23–38 years]) with MAGI and erectile dysfunction were consecutively selected for this study. From an initial series of patients with MAGI, we selected the patients with ultrasound criteria suggestive of HCUF or FSUF vesiculitis. | Sperm concentration (×106/mL) Sperm count (million) Progressive motility (%) Morphology normal forms (%) Semen volume (mL) |
5 mg Tadalafil | HCUF MAGI: 14±6; 43.4±10.6; 18±8; 12±6; 3.1±1.6 | HCUF MAGI: 14±7; 33.6±5.6; 11±4; 9±5; 2.4±1.1 | - |
| 20 (‘hyper-trophic-congestive’ ultrasound forms [HCUF)] MAGI | 20 (HCUF MAGI) | FSUF MAGI: 9±3; 10.8±1.9; 10±3; 5±3; 1.2±0.6 | FSUF MAGI: 9.5±6.0; 11.4±3.6; 9±3; 4±4; 1.2±0.6 | ||||||||||
| Rago et al [6] | 2012 | Italy | Randomized, open label, parallel group | 73 (a single dose) | 65 | A single dose 15 days | 1999 | Males infertile for at least two years; no endocrine, andrological, organic or genetic disease; normal plasma levels of FSH (range 1–7 mIU/mL), LH (range 2–10 mIU/mL), and testosterone (range 3–9 ng/mL); no azoospermia and criptozoospermia; no antisperm antibodies. Additionally, patients with ED had to present symptoms for at least 6 months and should be treated with PDE5i for the first time. Two hundred and five subjects (mean age 38.0±4.8 year) with idiopathic and primary infertility were enrolled after signing in- formed consent. The patients were randomly assigned into three groups: group A (65 subjects), with non-pharmacological treatment; group B (73 subjects), treated with a single dose of Vardenafil 10 mg; group C (67 subjects), where patients received Vardenafil 10 mg every other day for 15 days. | Semen volume (mL) Sperm concentration (×106/mL) Sperm count (million) Motility spermatozoa (%) Sperm abnormalities (%) |
10 mg vardenafil | A single dose: 2.9±0; 13.1±0.7; 37.9±2.7; 28.3±1; 84.9±0.6 | 2.9±0.1; 13.3±1; 38.9±3.6; 19.7±1.2; 85.6±0.7 | - |
| 67 (15 days) | 15 days: 3.4±0.1; 23.2±2.1; 76.1±7.9; 28.6±1.3; 85.0±0.8 | ||||||||||||
| Yang et al [10] | 2014 | China | Randomized, placebo controlled | Normozoospermic group: 10 (Tadalafil 20 mg), 10 (Sildenafil 100 mg) | Normozoospermic group: 10 (Tadalafil 20 mg), 10 (Sildenafil 100 mg) | A single dose | 1999 | Ten normozoospermic patients and ten asthenozoospermic patients were randomly picked up from our andrological outpatient department respectively. Normozoospermic samples were defined according to the guidelines of WHO 1999). Enrolled patients (age ranged from 26 to 40 years) underwent detailed medical history and physical examination. Patients receiving PDE5i treatment, with chronic prostatitis, leucocytospermia or varicocele were excluded. | Semen volume (mL) Sperm concentration (×106/mL) Straight progressive motility (%) Grade a Motility spermatozoa (%) |
20 mg Tadalafil and 100 mg Sildenafil | Normozoospermic group: (Tadalafil 20 mg) 2.84±1.37; 37.45±10.96; 30.53±7.06; 72.50±6.25 (Sildenafil 100 mg) 2.75±1.32; 37.86±12.44; 30.24±6.95; 69.49±6.38 |
Normozoospermic group: 2.76±1.31; 36.36±13.59; 30.93±7.93; 70.35±7.38 | - |
| Asthenozoospermic group: 10 (Tadalafil 20 mg), 10 (Sildenafil 100 mg) | Asthenozoospermic group: 10 (Tadalafil 20 mg), 10 (Sildenafil 100 mg) | Asthenozoospermic group: (Tadalafil 20 mg) 2.18±0.95; 47.7±15.78; 15.58±11.75; 34.51±10.38 (Sildenafil 100 mg) 2.2±0.86; 45.64±13.35; 14.41±8.09; 33.23±10.94 |
Asthenozoospermic group: 2.38±1.41; 49.14±15.97; 13.93±7.74; 32.76±12.19 | ||||||||||
| Tsounapi et al [8] | 2018 | Japan | Randomized, placebo controlled | 43 | 42 | 3 months | 2010 | This study was initially scheduled to include infertile oligoasthenospermic men (according to the normal values for sperm concentration and sperm motility described in the 5th WHO manual for semen parameter reference values; WHO, 2010) with normal serum testosterone levels. | Semen volume (mL) Sperm concentration (×106/mL) Progressive motility (%) Morphology normal forms (%) |
25 mg Avanafil Bid | 3.2±0.7; 10.4±4.0; 20.0±10.0; 9.0±4.0 | 3.3±0.8; 5.8±2.9; 4.0±2.0; 8.0±4.0 | - |
PDE5is: Phosphodiesterase-5 inhibitors, WHO: World Health Organization, SD: standard deviation, ED: erectile dysfunction, FSH: follicle stimulating hormone, LH: luteinizing hormone, Bid: bis in die (twice a day).
Fig. 2. Nine randomized controlled trials included in our meta-analysis. Quality of studies was assessed with the Cochrane Collaboration's tool. (A) Risk of bias summary. (B) Risk of bias graph.
2. Effect of phosphodiesterase-5 inhibitors on sperm parameters under World Health Organization 1999 criteria
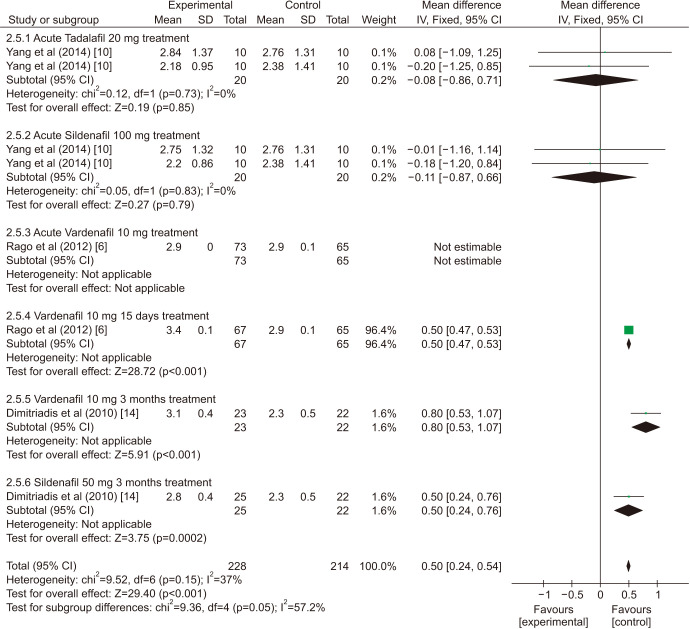
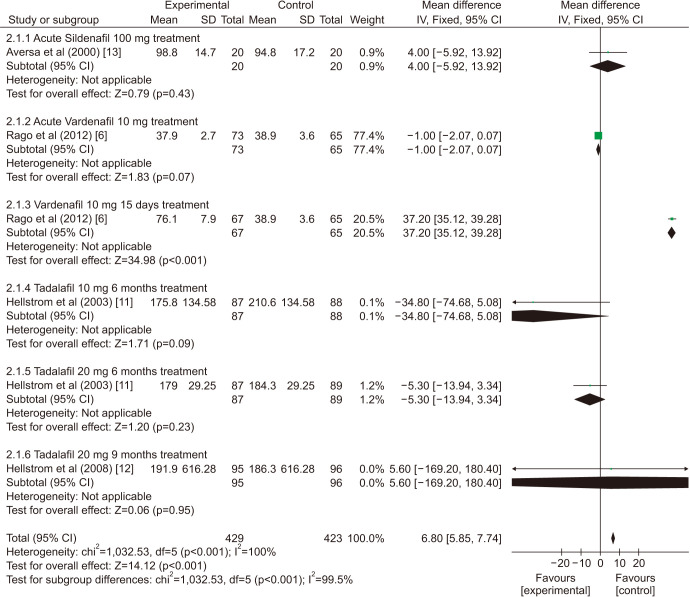
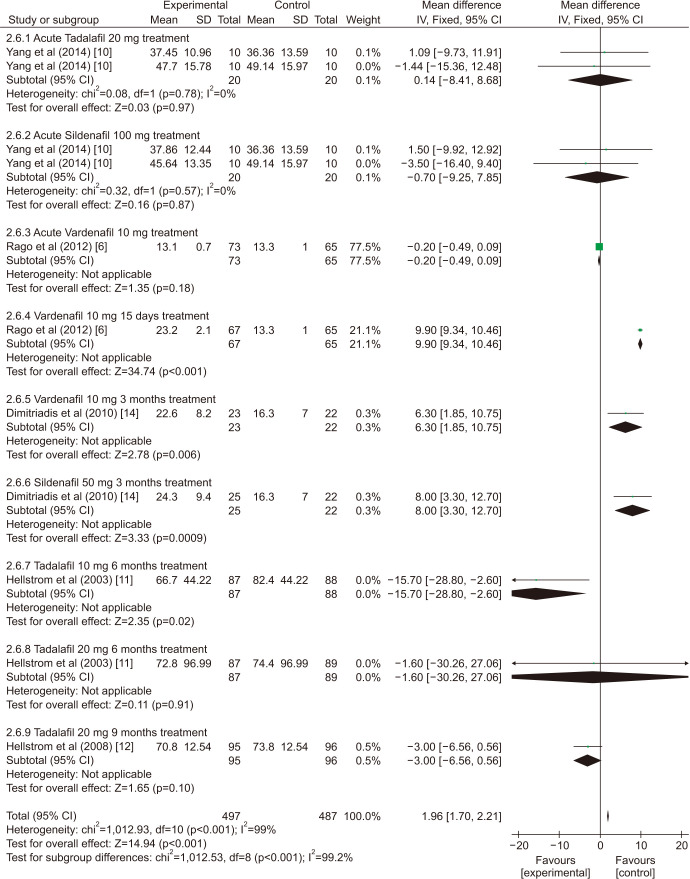
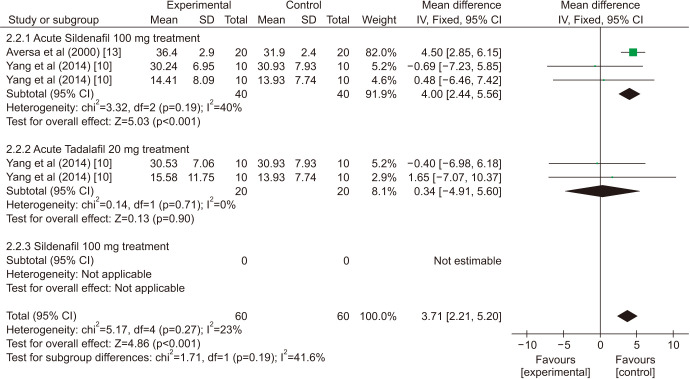
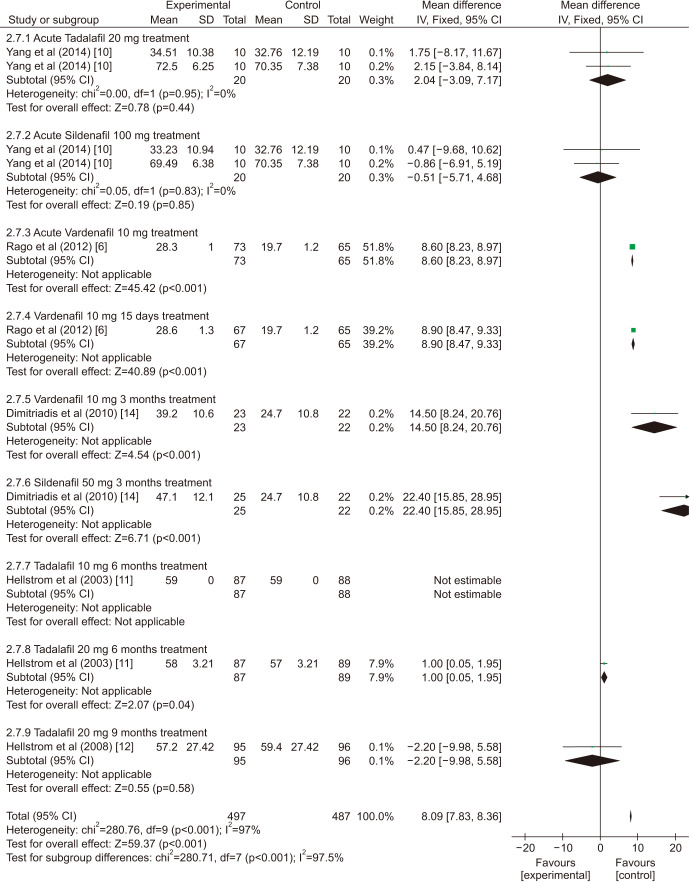
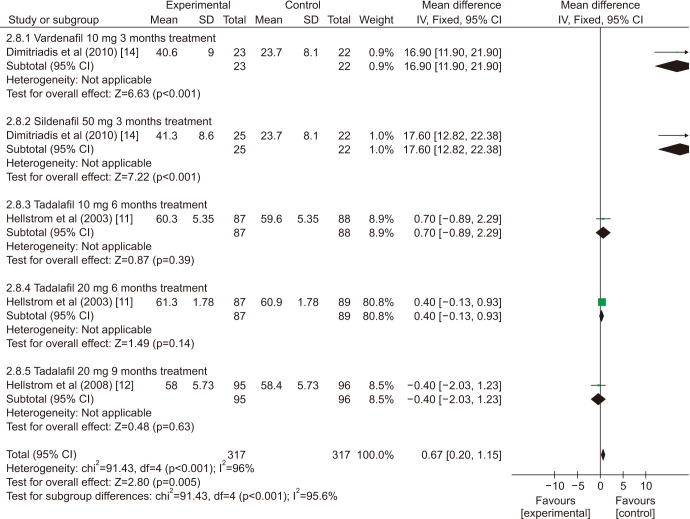
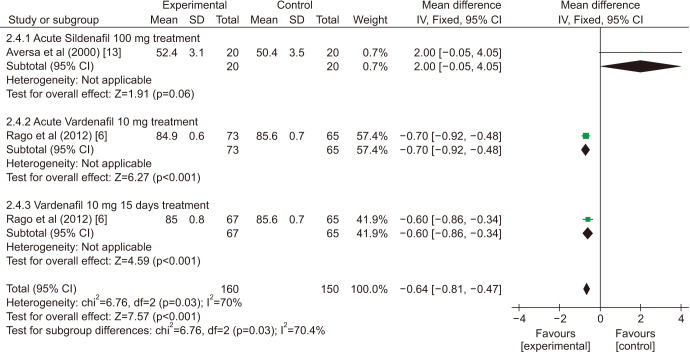
There was a statistically significant improvement treated with PDE5is compared with those receiving sham therapy in sperm parameters, which including semen volume (mean difference [MD]=0.50 points, 95% confidence interval [CI]=0.47–0.54, p<0.001, I2=37%; Fig. 3), sperm number (MD=6.80 points, 95% CI=5.85–7.74, p<0.001, I2=100%; Fig. 4), sperm concentration (MD=1.96 points, 95% CI=1.70–2.21, p<0.001, I2=99%; Fig. 5), straight progressive motility (%) Grade A (MD=3.71 points, 95% CI=2.21–5.20, p<0.001, I2=23%; Fig. 6), sperm motility (MD=8.09 points, 95% CI=7.83–8.36, p<0.001, I2=97%; Fig. 7), morphologically normal spermatozoa (%) (MD=0.67 points, 95% CI=0.20–1.15, p=0.005, I2=96%; Fig. 8), and sperm abnormalities (%) (MD=−0.64 points, 95% CI=−0.81–−0.47, p<0.001, I2=70%; Fig. 9).
Fig. 3. Clinical outcomes of semen volume under World Health Organization 1999 criteria. SD: standard deviation, CI: confidence interval, df: degree of freedom.
Fig. 4. Clinical outcomes of sperm number under World Health Organization 1999 criteria. SD: standard deviation, CI: confidence interval, df: degree of freedom.
Fig. 5. Clinical outcomes of sperm concentration under World Health Organization 1999 criteria. SD: standard deviation, CI: confidence interval, df: degree of freedom.
Fig. 6. Clinical outcomes of straight progressive motility under World Health Organization 1999 criteria. SD: standard deviation, CI: confidence interval, df: degree of freedom.
Fig. 7. Clinical outcomes of sperm motility under World Health Organization 1999 criteria. SD: standard deviation, CI: confidence interval, df: degree of freedom.
Fig. 8. Clinical outcomes of morphologically normal spermatozoa under World Health Organization 1999 criteria. SD: standard deviation, CI: confidence interval, df: degree of freedom.
Fig. 9. Clinical outcomes of sperm abnormalities under World Health Organization 1999 criteria. SD: standard deviation, CI: confidence interval, df: degree of freedom.
Sensitivity analysis (Table 2) showed that one study [6] was found to affect the overall prevalence estimate by an absolute difference for the indicator of sperm number, one study [13] was found to affect the overall prevalence estimate for the indicator of sperm abnormalities, no individual study affected the overall prevalence estimate for the rest indicators.
Table 2. Sensitivity analysis of some outcomes with World Health Organization 1999 criteria.
| Study | Mean difference | Lower CI | Upper CI | p-value | I2 | |
|---|---|---|---|---|---|---|
| Sperm number | ||||||
| Aversa et al (2000) [13] | 6.82 | 5.87 | 7.77 | <0.001 | 100% | |
| Rago et al (2012) [6] | 33.45 | 31.47 | 35.44 | <0.001 | 97% | |
| Rago et al (2012) [6] | −1.03 | −2.09 | 0.03 | 0.06 | 15% | |
| Hellstrom et al (2003) [11] | 6.82 | 5.88 | 7.76 | 0.0002 | 100% | |
| Hellstrom et al (2003) [11] | 6.94 | 5.99 | 7.89 | <0.001 | 100% | |
| Hellstrom et al (2008) [12] | 6.8 | 5.85 | 7.74 | <0.001 | 100% | |
| Pooled estimate | 6.8 | 5.85 | 7.74 | <0.001 | 100% | |
| Sperm concentration | ||||||
| Yang et al (2014) [10] | 1.96 | 1.70 | 2.21 | <0.001 | 100% | |
| Yang et al (2014) [10] | 1.96 | 1.70 | 2.21 | <0.001 | 100% | |
| Yang et al (2014) [10] | 1.96 | 1.70 | 2.21 | <0.001 | 100% | |
| Yang et al (2014) [10] | 1.96 | 1.70 | 2.21 | <0.001 | 100% | |
| Rago et al (2012) [6] | 9.39 | 8.85 | 9.93 | <0.001 | 88% | |
| Rago et al (2012) [6] | −0.17 | −0.46 | 0.12 | 0.25 | 68% | |
| Dimitriadis et al (2010) [14] | 1.94 | 1.68 | 2.20 | <0.001 | 99% | |
| Dimitriadis et al (2010) [14] | 1.94 | 1.68 | 2.19 | <0.001 | 99% | |
| Hellstrom et al (2003) [11] | 1.96 | 1.71 | 2.22 | <0.001 | 99% | |
| Hellstrom et al (2003) [11] | 1.96 | 1.70 | 2.21 | <0.001 | 99% | |
| Hellstrom et al (2008) [12] | 1.98 | 1.72 | 2.24 | <0.001 | 99% | |
| Pooled estimate | 1.96 | 1.70 | 2.21 | <0.001 | 99% | |
| Sperm motility | ||||||
| Yang et al (2014) [10] | 8.1 | 7.83 | 8.37 | <0.001 | 97% | |
| Yang et al (2014) [10] | 8.11 | 7.84 | 8.37 | <0.001 | 97% | |
| Yang et al (2014) [10] | 8.1 | 7.83 | 8.37 | <0.001 | 97% | |
| Yang et al (2014) [10] | 8.11 | 7.84 | 8.38 | <0.001 | 97% | |
| Rago et al (2012) [6] | 7.55 | 7.16 | 7.93 | <0.001 | 97% | |
| Rago et al (2012) [6] | 7.57 | 7.23 | 7.92 | <0.001 | 97% | |
| Dimitriadis et al (2010) [14] | 8.08 | 7.81 | 8.35 | <0.001 | 97% | |
| Dimitriadis et al (2010) [14] | 8.07 | 7.80 | 8.34 | <0.001 | 97% | |
| Hellstrom et al (2003) [11] | 8.09 | 7.83 | 8.36 | <0.001 | 97% | |
| Hellstrom et al (2003) [11] | 8.71 | 8.43 | 8.98 | <0.001 | 83% | |
| Hellstrom et al (2008) [12] | 8.11 | 7.84 | 8.37 | <0.001 | 97% | |
| Pooled estimate | 8.09 | 7.83 | 8.36 | <0.001 | 97% | |
| Morphologically normal spermatozoa (%) | ||||||
| Dimitriadis et al (2010) [14] | 0.53 | 0.05 | 1.00 | 0.03 | 94% | |
| Dimitriadis et al (2010) [14] | 0.51 | 0.03 | 0.98 | 0.04 | 93% | |
| Hellstrom et al (2003) [11] | 0.67 | 0.18 | 1.17 | 0.008 | 97% | |
| Hellstrom et al (2003) [11] | 1.83 | 0.75 | 2.91 | 0.0009 | 97% | |
| Hellstrom et al (2008) [12] | 0.77 | 0.28 | 1.27 | 0.002 | 97% | |
| Pooled estimate | 0.67 | 0.20 | 1.15 | 0.005 | 96% | |
| Sperm abnormalities (%) | ||||||
| Aversa et al (2000) [13] | −0.66 | −0.82 | −0.49 | <0.001 | 0% | |
| Rago et al (2012) [6] | −0.56 | −0.81 | −0.31 | <0.001 | 84% | |
| Rago et al (2012) [6] | −0.67 | −0.89 | −0.45 | <0.001 | 85% | |
| Pooled estimate | −0.64 | −0.81 | −0.47 | <0.001 | 70% | |
CI: confidence interval.
3. Effect of phosphodiesterase-5 inhibitors on sperm parameters under World Health Organization 2010 criteria
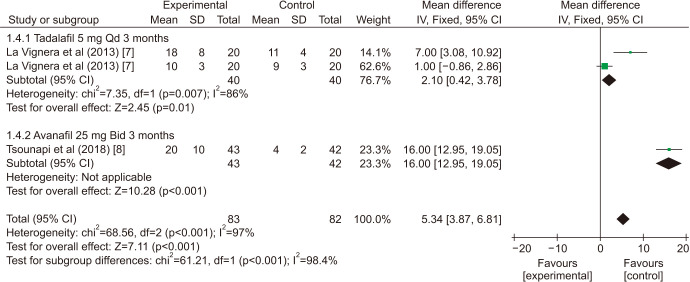
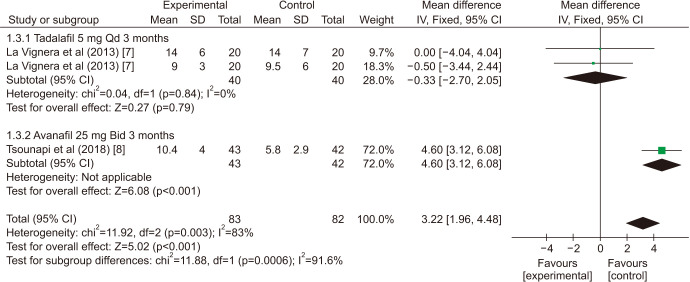
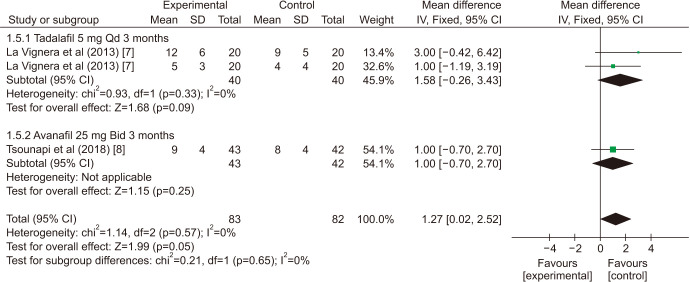
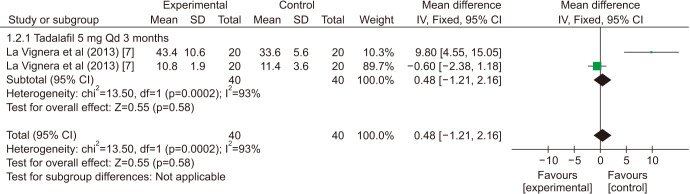
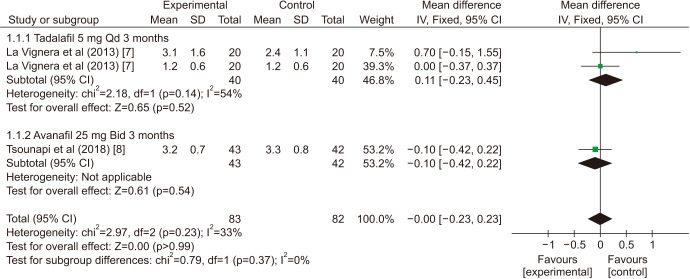
There was a statistically significant improvement treated with PDE5is compared with those receiving sham therapy in progressive motile sperm (MD=5.34 points, 95% CI=3.87–6.81, p<0.001, I2=97%; Fig. 10), sperm concentration (MD=3.22 points, 95% CI=1.96–4.48, p<0.001, I2=83%; Fig. 11), and morphology normal forms (MD=1.27 points, 95% CI=0.02–2.52, p=0.05, I2=0%; Fig. 12). Sperm number (MD=0.48 points, 95% CI=−1.21–2.16, p=0.58, I2=93%; Fig. 13), and semen volume (MD=−0.00 points, 95% CI=−0.23–0.23]; p>0.99, I2=33%, Fig. 14) did not improve significantly after PDE5is treatment.
Fig. 10. Clinical outcomes of the progressive motile sperm under World Health Organization 2010 criteria. SD: standard deviation, CI: confidence interval, Qd: quaque die (every day), df: degree of freedom, Bid: bis in die (twice a day).
Fig. 11. Clinical outcomes of sperm concentration under World Health Organization 2010 criteria. SD: standard deviation, CI: confidence interval, Qd: quaque die (every day), df: degree of freedom, Bid: bis in die (twice a day).
Fig. 12. Clinical outcomes of morphology normal forms under World Health Organization 2010 criteria. SD: standard deviation, CI: confidence interval, Qd: quaque die (every day), df: degree of freedom, Bid: bis in die (twice a day).
Fig. 13. Clinical outcomes of sperm number under World Health Organization 2010 criteria. SD: standard deviation, CI: confidence interval, Qd: quaque die (every day), df: degree of freedom.
Fig. 14. Clinical outcomes of semen volume under World Health Organization 2010 criteria. SD: standard deviation, CI: confidence interval, Qd: quaque die (every day), df: degree of freedom, Bid: bis in die (twice a day).
The sensitivity analysis (Table 3) showed that, one study [8] was found to affect the overall prevalence estimate by an absolute difference for the indicator of sperm concentration, no individual study affected the overall prevalence estimate for the rest indicator.
Table 3. Sensitivity analysis of some outcomes with World Health Organization 2010 criteria.
| Study | Mean difference | Lower CI | Upper CI | p-value | I2 | |
|---|---|---|---|---|---|---|
| Progressive motile sperm | ||||||
| La Vignera et al (2013) [7] | 5.07 | 3.48 | 6.65 | <0.001 | 99% | |
| La Vignera et al (2013) [7] | 12.61 | 10.20 | 15.01 | <0.001 | 92% | |
| Tsounapi et al (2018) [8] | 2.1 | 0.42 | 3.78 | 0.01 | 81% | |
| Pooled estimate | 5.34 | 3.87 | 6.81 | <0.001 | 97% | |
| Sperm concentration | ||||||
| La Vignera et al (2013) [7] | 3.57 | 2.24 | 4.89 | <0.001 | 89% | |
| La Vignera et al (2013) [7] | 4.05 | 2.66 | 5.45 | <0.001 | 77% | |
| Tsounapi et al (2018) [8] | −0.33 | −2.70 | 2.05 | 0.79 | 0% | |
| Pooled estimate | 3.22 | 1.96 | 4.48 | <0.001 | 83% | |
CI: confidence interval.
4. Assessment of publication bias
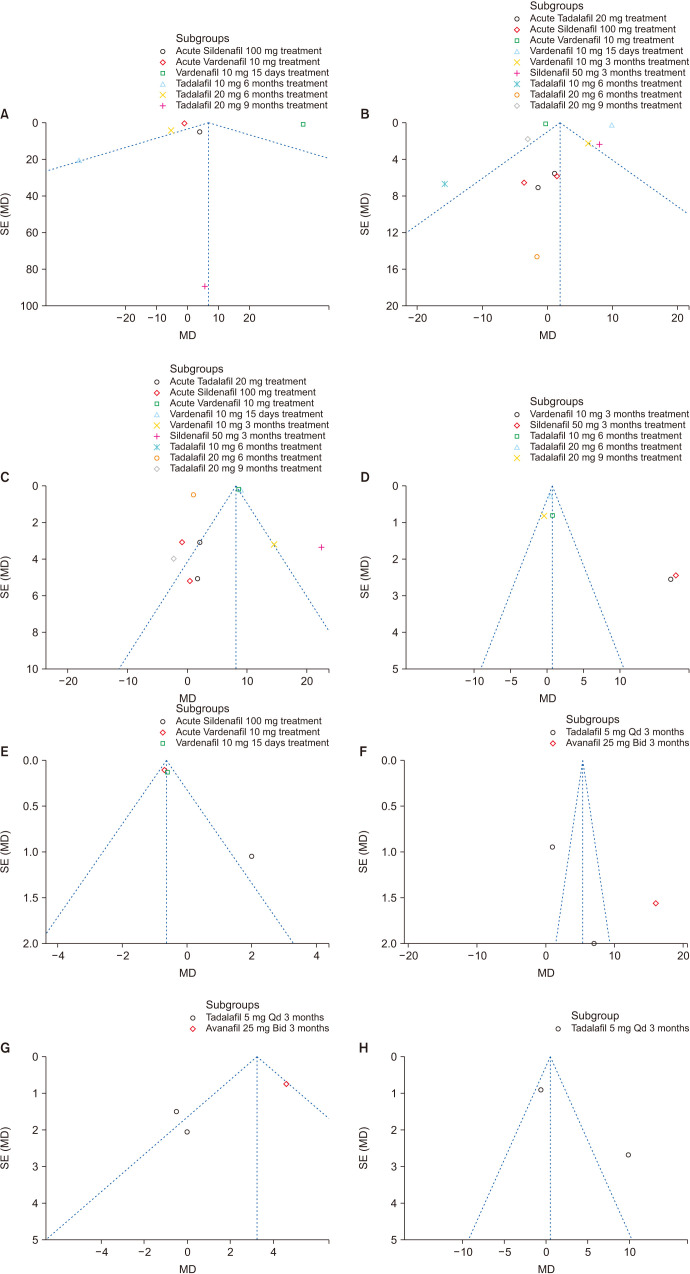
Although only nine studies were included in the meta-analysis, funnel plots were drawn. Egger's and Begg's tests were performed to assess the publication bias with Stata software. The asymmetry were minimal by visual inspection of the funnel plots in the sperm number, sperm concentration, sperm motility, morphologically normal spermatozoa and sperm abnormalities in WHO 1999 criteria and progressive motile sperm, sperm concentration and sperm number in WHO 2010 criteria, which indicates that the pooled estimates were unlikely to be significant biased secondary to small study effects (Fig. 15).
Fig. 15. Funnel plots of this meta-analysis on sperm parameters. (A) Sperm number; World Health Organization (WHO) 1999 criteria. (B) Sperm concentration; WHO 1999 criteria. (C) Sperm motility; WHO 1999 criteria. (D) Morphologically normal spermatozoa; WHO 1999 criteria. (E) Sperm abnormalities; WHO 1999 criteria. (F) Progressive motile sperm; WHO 2010 criteria. (G) Sperm concentration; WHO 2010 criteria. (H) Sperm number; WHO 2010 criteria. SE: standard error, MD: mean difference, Qd: quaque die (every day), Bid: bis in die (twice a day).
DISCUSSION
PDE5is, as a class of epoch-making drugs for the treatment of ED, has been increasingly used in patients with male infertility to improve sperm quality and achieve the purpose of fertility [5,6,8,10]. This systematic review and meta-analysis of nine RCTs involving 1,211 men showed that with PDE5is, sperm concentration, straight progressive motility, morphology normal forms and progressive motile sperm were significantly improved. However, the outcomes of semen volume and sperm number were contradictory [7,8]. The results of this study show that PDE5is could improve the quality of male sperm in clinical practice.
How does PDE5i improve sperm quality? The conclusion of the current study is still unclear. PDE5is inhibits PDE5 in the corpus cavernosum and increases the level of intracellular cGMP, increases the content of NO with vasodilatory effect, thereby promoting the relaxation of smooth muscles and causing penile erection. The role of NO has been confirmed after studies that may play an important role in chromatin concentration, sperm morphology changes and the final release of sperm from the seminiferous epithelium. In addition, NO may also be related to spermatogenesis, steroid production and apoptotic cell death. Therefore, the NO-mediated mechanism in the prostate and testicular parenchyma increased by PDE5i may be the basis for improved sperm quality [22]. Some researchers believe that the positive effect of PDE5i on ejaculation concentration may be due to its effect on the specific pathways of smooth muscle in seminal tract [6]. Different PDE5is increase human testosterone by a direct action on the testis, and significantly upregulate the secretion of INSL3 by human Leydig cells [14], which might lead to the epididymal sperm maturation process and subsequent improvement in spermatozoal potential to obtain optimal sperm motility in oligoasthenospermic men. Sildenafil and Vardenafil could increase the level of serum insulin-like 3-peptide, leading to the enhancement of the secretory function of Leydig cells, which in turn result in an improvement of secretion by Sertoli cells [23]. In addition, vardenafil may directly act on sperm mitochondria or calcium channels to increase intracellular cGMP, thereby having a positive effect on sperm motility [6]. Studies have shown that the psychological stress caused by infertility can affect spermatogenesis [24], also the erectile function. PDE5is could improve erectile function, and reduce this psychological stress, which in turn has a positive impact on sperm parameters [25,26]. In a previous study, different PDE5is such as sildenafil or vardenafil have been shown could enhance the prostatic secretory function [27,28]. Because of the substances secreted by prostate gland play an important role in the maintenance of sperm motility [29]. Avanafil was found to increase the total percentage of motile sperm and the percentage of progressive motile sperm, but did not influence the sperm DNA integrity, which has been proved that this was related to the enhancement in prostatic secretory function [8]. Studies have shown that tadalafil increases erectile function, and increased sexual activity may lead to increased testosterone levels. The effect of androgens on sperm quality is obvious [22].
Hellstrom et al [11,12] only provided the sample size, mean and p-value of the experimental group and the control group. Therefore, we conducted the conversion by professional conversion method [30], and assumed that the standard deviation of the two groups was the same, so we conducted the meta-analysis.
Aversa et al [13] and Jarvi et al [9] adopted the WHO semen quality criteria in 1992. In view of the little change in methodology, only the difference in reference data would not affect the conclusion of this metaanalysis, therefore, the data of these two studies were statistically analyzed together with the WHO criteria of 1999.
This systematic review of multiple pooled data results showed increased heterogeneity. The reasons may be as follows: the WHO semen quality standards used and the indicators observed in each study were not the same, the types of drug, dosages, and treatment courses used in the current nine studies were different, and the types of patients included were also not uniform. After many rounds of discussion, the research team finally decided to group the data according to different versions of WHO semen quality to get the results. Then group according to semen parameters. According to the different types of drugs, dosages, and treatment courses, subgroup analysis was performed.
The existing systematic reviews searched and included studies before April 2016 [4], with the types of RCTs, non-randomized, self-controlled trials, etc., besides only three drugs included (Sildenafil, Vardenafil, and Tadalafil), so the level of evidence-based medicine was slightly lower. Our study differs in that it is the first to publish on a homogenous population of men with all kind of the PDE5is, including tadalafil, sildenafil, vardenafil, and avanafil. And our meta-analysis includes only RTCs and thus can be regarded as level 1a evidence.
Our study has important strengths and limitations. This is the first meta-analysis including only RCTs on the treatment of sperm with PDE5is, demonstrating a significant clinical and statistical improvement. Most of the included studies had small samples, the largest sample size is 264. All included studies are published in public journals in full text. We were unable to obtain valid data for meta-analysis from the original text of one study [9], so we tried to contact the author for the original data via email, but failed to get reply. Therefore, we could not perform statistical analysis on the data included in this study.
Factors related to male infertility, such as age, varicocele, prostate disease, hypertension, diabetes, smoking, drinking, staying up late, sexual discordance, reproductive system infections, chromosomal diseases, genetic diseases, etc., were not elaborated in the original studies. With the stratification of age and comorbidities, more RCTs are needed in the future to analyze the impact of these factors on the efficacy of PDE5is for male infertility patients.
The main goal of male infertility treatment is to aid the fertilization of sperm and egg, reach the final pregnancy and even give birth. Currently, there is no evidence (laboratory/clinical research results or any other data) that improved some indicators of male sperm after PDE5i treatments, thereby improving fertilization, increasing pregnancy or fertility.
Although our findings indicate an improvement in sperm quality in men treated with PDE5is, more RCTs are needed before this treatment is accepted worldwide. After reviewing the literature, we made these recommendations for future research: future research should be random, preferably blinded; the semen analysis method and quality standard recommended by WHO should be used to analyze the semen of subjects to ensure the stability and comparability of the data; other treatment options should be tried to determine the best effect; the control group should receive sham treatment; other drugs should be completely stopped with an appropriate washout period; all studies should be registered in the trial registry; all studies should report all adverse events; add more valuable observation indicators, such as fertilization rate, pregnancy rate, live birth rate, and other assisted reproduction related outcomes.
CONCLUSIONS
In this meta-analysis of RCTs evaluating the effects of PDE5is on sperm quality, some sperm indicators improved in men treated with PDE5is compared to men treated with sham treatment. However, before this treatment is widely accepted, more stringent RCTs must be conducted.
ACKNOWLEDGEMENTS
This research was supported by the National Natural Science Foundation of China (Grant Number: 81973647), the Funds of Science Development of Chengdu University of Traditional Chinese Medicine (Item Number: 2017-EL-22), the Scientific research project of Sichuan Provincial Department of Education (Item Number: 18ZA0183), and the project of the Science and Technology Department in Sichuan province (Item Number: 2021JDRC0168).
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: LD, XY.
- Data curation: LD, XZ, XY, YS, YL.
- Formal analysis: LD, XZ, XY, YL.
- Funding acquisition: XY.
- Methodology: LD, XY, YL.
- Project administration: XY.
- Supervision: LD, XY.
- Writing — original draft: LD, XZ, XY, YS, XY.
- Writing — review & editing: LD, YL, XY.
References
- 1.Burnett AL, Nehra A, Breau RH, Culkin DJ, Faraday MM, Hakim LS, et al. Erectile dysfunction: AUA guideline. J Urol. 2018;200:633–641. doi: 10.1016/j.juro.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Dong L, Chang D, Li J, Yang F, Tan K, Yu Y, et al. Efficacy of Shengjing capsules for treatment of male infertility in China: a systematic review and meta-analysis. Int J Clin Exp Med. 2019;12:6594–6603. [Google Scholar]
- 3.Montorsi F, Verheyden B, Meuleman E, Jünemann KP, Moncada I, Valiquette L, et al. Long-term safety and tolerability of tadalafil in the treatment of erectile dysfunction. Eur Urol. 2004;45:339–344. doi: 10.1016/j.eururo.2003.11.010. discussion 344-5. [DOI] [PubMed] [Google Scholar]
- 4.Tan P, Liu L, Wei S, Tang Z, Yang L, Wei Q. The effect of oral phosphodiesterase-5 inhibitors on sperm parameters: a meta-analysis and systematic review. Urology. 2017;105:54–61. doi: 10.1016/j.urology.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 5.Dong L, Zhang X, Yan X, Shen Y, Yu X, Li Y. Effect of phosphodiesterase-5 inhibitors (PDE5is) on the treatment of male infertility: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e18317. doi: 10.1097/MD.0000000000018317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rago R, Salacone P, Caponecchia L, Marcucci I, Fiori C, Sebastianelli A. Effect of vardenafil on semen parameters in infertile men: a pilot study evaluating short-term treatment. J Endocrinol Invest. 2012;35:897–900. doi: 10.3275/8368. [DOI] [PubMed] [Google Scholar]
- 7.La Vignera S. Seminal vesicles of infertile patients with male accessory gland infection: ultrasound evaluation after prolonged treatment with tadalafil, a selective phosphodiesterase-5 inhibitor. Andrologia. 2013;45:386–391. doi: 10.1111/and.12027. [DOI] [PubMed] [Google Scholar]
- 8.Tsounapi P, Honda M, Dimitriadis F, Koukos S, Hikita K, Zachariou A, et al. Effects of a micronutrient supplementation combined with a phosphodiesterase type 5 inhibitor on sperm quantitative and qualitative parameters, percentage of mature spermatozoa and sperm capacity to undergo hyperactivation: a randomised controlled trial. Andrologia. 2018;50:e13071. doi: 10.1111/and.13071. [DOI] [PubMed] [Google Scholar]
- 9.Jarvi K, Dula E, Drehobl M, Pryor J, Shapiro J, Seger M. Daily vardenafil for 6 months has no detrimental effects on semen characteristics or reproductive hormones in men with normal baseline levels. J Urol. 2008;179:1060–1065. doi: 10.1016/j.juro.2007.10.077. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Ma Y, Yang H, Jin Y, Hu K, Wang HX, et al. Effect of acute tadalafil on sperm motility and acrosome reaction: in vitro and in vivo studies. Andrologia. 2014;46:417–422. doi: 10.1111/and.12097. [DOI] [PubMed] [Google Scholar]
- 11.Hellstrom WJ, Overstreet JW, Yu A, Saikali K, Shen W, Beasley CM, Jr, et al. Tadalafil has no detrimental effect on human spermatogenesis or reproductive hormones. J Urol. 2003;170:887–891. doi: 10.1097/01.ju.0000081053.97792.da. [DOI] [PubMed] [Google Scholar]
- 12.Hellstrom WJ, Gittelman M, Jarow J, Steidle C, McMurray J, Talley D, et al. An evaluation of semen characteristics in men 45 years of age or older after daily dosing with tadalafil 20mg: results of a multicenter, randomized, double-blind, placebo-controlled, 9-month study. Eur Urol. 2008;53:1058–1065. doi: 10.1016/j.eururo.2007.09.046. [DOI] [PubMed] [Google Scholar]
- 13.Aversa A, Mazzilli F, Rossi T, Delfino M, Isidori AM, Fabbri A. Effects of sildenafil (Viagra) administration on seminal parameters and post-ejaculatory refractory time in normal males. Hum Reprod. 2000;15:131–134. doi: 10.1093/humrep/15.1.131. [DOI] [PubMed] [Google Scholar]
- 14.Dimitriadis F, Tsambalas S, Tsounapi P, Kawamura H, Vlachopoulou E, Haliasos N, et al. Effects of phosphodiesterase-5 inhibitors on Leydig cell secretory function in oligoasthenospermic infertile men: a randomized trial. BJU Int. 2010;106:1181–1185. doi: 10.1111/j.1464-410X.2010.09243.x. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011] London: The Cochrane Collaboration; 2011. [Google Scholar]
- 17.Review manager (RevMan) 5.3 ed. Copenhagen: Nordic Cochrane Centre, Cochrane Collaboration; 2014. [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 22.Corvasce A, Albino G, Leonetti T, Buonomo AF, Marucco EC. Once-a-day Tadalafil administration improves the spermogram parameters in fertile patients. Arch Ital Urol Androl. 2015;87:210–213. doi: 10.4081/aiua.2015.3.210. [DOI] [PubMed] [Google Scholar]
- 23.Sofikitis N, Giotitsas N, Tsounapi P, Baltogiannis D, Giannakis D, Pardalidis N. Hormonal regulation of spermatogenesis and spermiogenesis. J Steroid Biochem Mol Biol. 2008;109:323–330. doi: 10.1016/j.jsbmb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 24.McGrady AV. Effects of psychological stress on male reproduction: a review. Arch Androl. 1984;13:1–7. doi: 10.3109/01485018408987495. [DOI] [PubMed] [Google Scholar]
- 25.Lenzi A, Lombardo F, Salacone P, Gandini L, Jannini EA. Stress, sexual dysfunctions, and male infertility. J Endocrinol Invest. 2003;26(3 Suppl):72–76. [PubMed] [Google Scholar]
- 26.Jannini EA, Lombardo F, Salacone P, Gandini L, Lenzi A. Treatment of sexual dysfunctions secondary to male infertility with sildenafil citrate. Fertil Steril. 2004;81:705–707. doi: 10.1016/j.fertnstert.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Dimitriadis F, Tsampalas S, Tsounapi P, Giannakis D, Chaliasos N, Baltogiannis D, et al. Effects of phosphodiesterase-5 inhibitor vardenafil on testicular androgen-binding protein secretion, the maintenance of foci of advanced spermatogenesis and the sperm fertilising capacity in azoospermic men. Andrologia. 2012;44 Suppl 1:144–153. doi: 10.1111/j.1439-0272.2010.01153.x. [DOI] [PubMed] [Google Scholar]
- 28.Dimitriadis F, Giannakis D, Pardalidis N, Zikopoulos K, Paraskevaidis E, Giotitsas N, et al. Effects of phosphodiesterase-5 inhibitors on sperm parameters and fertilizing capacity. Asian J Androl. 2008;10:115–133. doi: 10.1111/j.1745-7262.2008.00373.x. [DOI] [PubMed] [Google Scholar]
- 29.La Vignera S, Vicari E, Condorelli RA, D'Agata R, Calogero AE. Male accessory gland infection and sperm parameters (review) Int J Androl. 2011;34(5 Pt 2):e330–e347. doi: 10.1111/j.1365-2605.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- 30.Li YP. Practice of evidence-based medicine. Beijing: People's Medical Publishing House; 2018. [Google Scholar]