Abstract
Paternal health and behavioral lifestyles affect reproductive and neonatal outcomes and yet the magnitude of these effects remain underestimated. Even though these impacts have been formally recognized as a central aspect of reproductive health, health care services in Europe often neglect the involvement of fathers in their reproductive programs. Following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for systematic reviews, a literature search was carried out to assess the possible impact of paternal health on reproductive outcomes. The comprehensive strategy included cohort studies and meta-analysis available on PubMed, Web of Science, CINAHL, and Google scholar. Cross-referencing of bibliographies of the selected papers ensured wider study capture. Paternal factors were grouped into two categories respectively identified with the terms “Biological Paternal Factors” and “Lifestyle Paternal Factors”. Advanced age may impair male fertility and affect early pregnancy stages. Increased body mass index, smoking, alcohol and recreational drugs, all alter seminal fluid parameters. Hazardous alcohol use correlates with low birthweight in pregnancy and harmful behavioral lifestyles have been linked to congenital heart defects, metabolic and neurodevelopmental disorders in the offspring. Measures targeting paternal health and lifestyle within the first 1,000 days' timeframe need to be implemented in couples undergoing reproductive decisions. Health professionals, as well as future fathers, must be aware of the benefits for the offspring associated with correct paternal behaviors. More research is needed to build guidelines and to implement specific programs aiming at reproductive health promotion.
Keywords: Fertility, Life style, Long term adverse effects, Maternal health, Paternal exposure
INTRODUCTION
Paternal health and behavioral lifestyles affect maternal and neonatal outcomes but remain neglected topics in reproductive health. Even though their importance has been globally acknowledged [1], health care services in Europe have failed so far to attract and increase the involvement of fathers-to-be in reproductive programs [2].
Health campaigns used to focus only on the need to improve maternal health, relegating to a secondary role and only marginally involving fathers in labor and childbirth.
Women are regularly encouraged to take care of their health and monitor lifestyle habits, whereas few specific recommendations concern the male partner [3].
In spite of this prejudice, it has become evident that the male factor is relevant and affects fertility as well as pregnancy outcomes. Medical research has shown that alongside their supportive role, men can improve perinatal outcomes by optimizing their own health and behavioral lifestyles [4,5].
Although the magnitude of male contribution is still undetermined, recent studies provide evidence that justifies the introduction of effective paternal health measures such as genetic factors screening in light of the possible association with recurrent pregnancy loss [4,6,7].
Previous reviews recognize paternal age as a possible risk factor for preterm birth (PTB), genetic abnormalities, cancer development, and other musculoskeletal congenital syndromes in childhood [8]. Considering the existing gap of available studies exploring paternal and maternal health, likely the result of traditional gender stereotypes and a “macho” attitude towards reproductive matters [9], this review aims to draw attention to paternal influence upon the descendants' health.
The broader approach adopted in our paper provides a more comprehensive overview considering every potentially relevant factor related to paternal biological and behavioral lifestyles, as opposed to existing reviews which generally focus on single paternal aspects.
MAIN BODY
1. Materials and methods
1) Data sources and searches
All human studies published up to 2020 reporting on paternal exposure factors and lifestyles associated with reproductive outcomes were identified using PubMed, CINHAL, and Web of Science. Cross-referencing in bibliographies of the appraised papers ensured wider study capture.
Our initial search was not limited to any particular type of study and all potentially eligible studies were reviewed. The electronic search encompassed keywords referring to the periconceptional time period, using the following key concepts and related keywords: “body mass index BMI”, “alcohol consumption”, “smoking habits”, “medical comorbidities and therapeutic treatments”, “occupational hazards”, “environmental hazards”, “recreational or illicit drug use”, “paternal advanced age”, and “reproductive outcomes”. Results were categorized in terms of early periconceptional morbidities (e.g., infertility) and later pregnancy outcomes inclusive of congenital anomalies (CAs), small for gestational age, low birthweight (LBW) babies, and PTBs.
2) Study selection
Studies which assessed associations between paternal health condition/habits and maternal/fetal/neonatal complications, or reproductive outcomes were reviewed.
Inclusion criteria for selected papers were years (from inception of 2008 until March 2020), study type (case-control, cohort, randomized controlled trialss, and meta-analysis), availability of full text, humans as subjects, and English as language of publication. Animal studies, case reports and review articles were excluded.
3) Data extraction and quality assessment
The full text of eligible papers was obtained, and quality was assessed according to the number of subjects involved and the statistical significance of the results presented in each study. Studies providing adjusted odds ratios (aOR), 95% confidence intervals (CI) and p-value were favored. Description of the studied population was also valued as a quality index as well as control for maternal effects in the paternal model. Adjustment habitually accounted for maternal socioeconomic and biological information when performing paternal data analysis [10]. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statements tools, i.e., “Checklist for Systematic reviews and Meta-Analysis items” and “Flow chart template” [11], were used to build the flow chart presented in the Results section and to checklist essential items of the current review.
2. Results
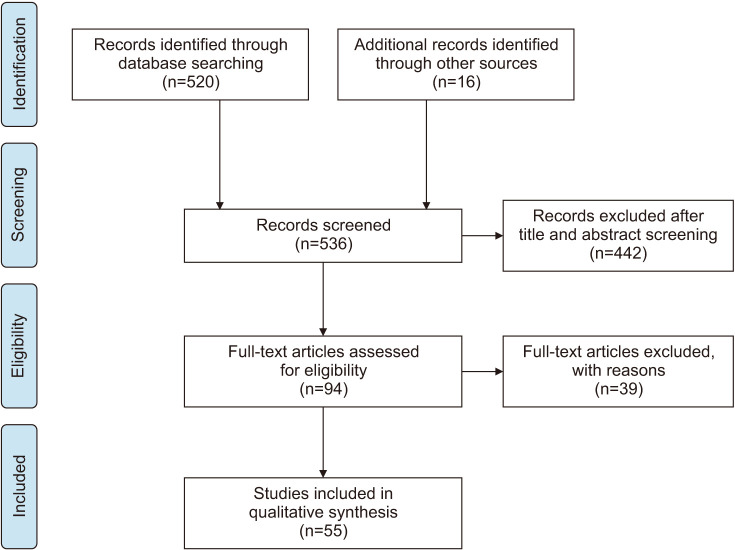
A total of 520 articles were identified. Cross-referencing in bibliographies of the initial selected papers added 16 additional studies. Three of them, despite being published before 2008, were included due to their relevance to this topic. After both title and abstract screening, 94 papers were assessed for eligibility. Thirty-nine studies were excluded, either because they did not meet the criteria for the specific topic searched, or because full text was not available. In the end, 53 articles were included, read and analyzed.
In the following search-flow diagram (Fig. 1) the whole process of identification, screening, eligibility, and inclusion is graphically summarized.
Fig. 1. Flow chart of study-selection process.
Selected paternal indicators identify two distinct categories respectively labelled with the terms “Biological Paternal Factors” and “Lifestyle Paternal Factors”. The former includes variables, such as age, body mass index (BMI), medical comorbidities, and related therapies. The latter comprises paternal exposure to external occupational hazards or acquired health determinants, such as smoking habits, alcohol consumption, and recreational drug use. For each group three kinds of reproductive outcomes were searched: male fertility, early pregnancy complications, fetal and postnatal outcomes.
Results were organized into tables which included the following items: Author's name and year of publication; study design and study period or research timeframe for literature reviews; number of participants/studies; type of exposure factor; specific reproductive outcome; statistical strength of association mainly expressed in OR and CIs; key findings. Additional notes for a better understanding (e.g., abbreviations and comments on results) are at the end of each table.
1) Biological paternal factors
Biological factors related to male partner conditions and their effects upon reproductive outcomes find limited space in the medical literature. Nevertheless, paternal aging, body weight and overall health status (i.e., medical comorbidities and related therapies) are indicators studied by different authors whose findings provide enough information to draw initial conclusions (Table 1, 2).
Table 1. Biological paternal factors and fertility.
| Citation | Study design | Subject | Paternal factor | Reproductive outcome | OR (95% CI), p-value, overall trend | Key finding |
|---|---|---|---|---|---|---|
| Rosiak-Gill et al (2019) [12] | Cohort | 1,124 males | Age≥40 y | DFIa>10% | OR 2.31 (1.36–3.92) | Detrimental effect of advanced paternal age on sperm chromatin integrity. Higher incidence of >10% DFI in subjects aged ≥40 years. |
| p<0.01 | ||||||
| Matorras et al (2011) [13] | Cohort | 454,753 births | Age | Fertility | 21%–23% yearly reduction | Male fertility starts decreasing at 39 years of age. |
| Capelouto et al (2018) [17] | Cohort | 949 female recipients | Age≥51 y | Implantation rate | aRR 0.95 (0.71–1.27) | After adjusting for variables known to affect oocyte donation cycle success, paternal age and BMI were not associated with differences in IVF outcomes. |
| Clinical pregnancy rate | aRR 1.03 (0.82–1.29) | |||||
| Live birth rate | aRR 1.03 (0.75–1.41) | |||||
| BMI≥35 kg/m2 | aRR 0.93 (0.69–1.24) | |||||
| aRR 0.92 (0.72–1.19) | ||||||
| aRR 1.07 (0.81–1.42) | ||||||
| Nguyen et al (2007) [14] | Cohort | 26,303 pregnancies | BMI 25–29.9 kg/m2 | Infertility | OR 1.20 (1.04–1.38) | Direct correlation between BMI and infertility indicators (altered T, E2 levels, and poor semen quality). |
| BMI 30–34.9 kg/m2 | OR 1.36 (1.13–1.63) | |||||
| Tunc et al (2011) [15] | Cohort | 81 males | BMI | Seminal oxidative stress | r=0.23 | Positive correlation between BMI and seminal oxidative stress. An inverse correlation was found between BMI and sperm concentration. Small sample results have limited statistical value. |
| p<0.05 | ||||||
| Sperm concentration | r=-0.33 | |||||
| p<0.01 | ||||||
| Kort et al (2006) [16] | Cohort | 520 males | BMI 18–25 kg/m2 | DFI | 19.9%±1.96% | As paternal BMI increases, chromatin‐intact normal‐motile sperm cells per ejaculate decrease. |
| BMI 25–29.9 kg/m2 | 25.8%±2.23% | |||||
| BMI 30–34.9 kg/m2 | 27.0%±3.16% | |||||
| BMI | p<0.05 | |||||
| Eisenberg et al (2016) [18] | Cohort | 41 males | Diabetes | Fecundity | OR 0.35 (0.13–0.88) | Paternal diabetes diminishes a couple's fecundity expressed by longer TTP. |
| Evans-Hoeker et al (2018) [22] | Cohort | 1,608 males | MD | Fecundity | RR 0.44 (0.20–0.98) | Female partners of currently depressed males (n=34) are less likely to achieve conception. |
| Tanrikut et al (2010) [23] | Cohort | 35 males | Antidepressants | DNA fragmentation | OR 9.33 (2.3–37.9) | The fertility potential of men on paroxetine may be adversely affected by sperm DNA increased fragmentation. |
| Bourke et al (2019) [21] | Cohort | 205 males | Cystic fibrosis | Natural conception | 0.5% | Men with cystic fibrosis are nearly always infertile. Assisted reproduction gives good chances for biological parenthood in males reaching adult age. |
| ART conception | 9% | |||||
| Roessner et al (2012) [19] | Case-control | 27 diabetics males and 18 healthy controls | Healthy cases | DFI | 8.2%±5.4% | ROS and DNA fragmentation affecting sperm quality are increased in both types of diabetes. This effect is more pronounced in men with diabetes type II. |
| Diabetes type I | DFI | 22.5%±8.4% | ||||
| p<0.01 | ||||||
| Diabetes type II | DFI | 28.9%±18.4% | ||||
| p<0.01 | ||||||
| ROS | 19.7%±23.1% | |||||
| p<0.05 | ||||||
| Ananthakrishnan et al (2019) [25] | Cohort | 256 males | IBD | Reduced fecundity | OR 1.99 (1.05–3.78) | IBD likely causes delay in conception. |
| Amino salicylate use | p=0.09 | Amino salicylate used to control IBD has no significant effect on fecundity. | ||||
| Hayashi et al (2008) [24] | Case-control | 73 intervention and 92 control group | H1 receptor antagonists, antiepileptics and antibiotics | Semen quality | 93% vs. 12% | Long-term use of drugs can induce fertility hazard in terms of asthenozoospermia and oligozoospermia. |
| Conception rate | 85% vs. 10% |
OR: odds ratio, CI: confidence interval, DFI: DNA fragmentation index, BMI: body mass index, aRR: adjusted risk ratio, IVF: in-vitro fertilization, T: testosterone, E2: rstradiol, TTP: time to pregnancy, MD: major depression, RR: risk ratio, ART: artificial reproductive techniques, ROS: reactive oxygen species, IBD: inflammatory bowel disease.
aDFI is an expression of sperm quantity and quality.
Table 2. Biological paternal factors and pregnancy outcomes.
| Citation | Study design | Subject | Paternal factor | Reproductive outcome | OR (95% CI), p-value, overall trend | Key finding |
|---|---|---|---|---|---|---|
| Hurley and DeFranco (2017) [27] | Cohort | 1,034,552 births | Age | Preeclampsia | RR 1.00 (0.99–1.00) | Paternal aging is not associated with preeclampsia nor any selected neonatal outcomes after accounting for maternal age. |
| PTB | RR 1.01 (1.01–1.01) | |||||
| Fetal growth restriction | RR 1.01 (1.01–1.02) | |||||
| CAs | RR 1.01 (0.99–1.03) | |||||
| Genetic disorder | RR 1.01 (0.99–1.04) | |||||
| NICU admission | RR 1.01 (1.01–1.01) | |||||
| Tamura et al (2018) [26] | Cohort | 18,059 mother–infant dyads | Age | PTB | RR 1.22 (1.05–1.42) | Compared to newborns with younger fathers (25–34 years), rates of PTB and VLBW among infants with older fathers (≥35 years) are significantly higher. Results adjusted for maternal confounding factors, such as ART, BMI, PTB, SGA, VLBW. |
| p<0.01 | ||||||
| VLBW | RR 2.02 (1.22–3.35) | |||||
| p<0.01 | ||||||
| Magnus et al (2018) [30] | Cohort | 132,331 infants | BMI>30 kg/m2 | Type I diabetes | aHR 1.51 (1.11–2.04) | Childhood-onset type I diabetes possibly influenced by paternal obesity. |
| Noor et al (2019) [28] | Cohort | 429 father-mother-infant triads | BMI≥25 kg/m2 | Increased birthweight | p<0.01 | Higher paternal BMI (≥25 kg/m2) is associated with increased offspring birthweight. |
| Mei et al (2018) [29] | Cohort | 1,178 live births | BMI≥25 kg/m2 | Birthweight | β=0.04 | Paternal overweight/obesity has no effect on BMI_Z score at birth. Significant but mild paternal influence only detected after birth. |
| p>0.05 | ||||||
| Moss and Harris (2015) [32] | Cohort | 372 infants | Diabetes | LBW | p<0.01 | Offspring's lower birthweight associated with paternal diabetes. Δ weight=−783.9 g (range −1,014.2 to −553.6). |
| Ji et al (2018) [33] | Cohort | 15,615 males | Type I diabetes | ADHD | aHR 1.20 (1.03–1.41) | A paternal history of type I diabetes is associated with a 20% increase in risk of being diagnosed with ADHD. |
| Lydholm et al (2019) [89] | Cohort | 1,206,600 infants | Infection/anti-infective agents | Mental disorder in the offspring | HR 1.01 (0.98–1.03) | No paternal association with an increased risk of mental disorders in the child. |
| Buck Louis et al (2018) [35] | Cohort | 4,886 pregnancies | History of mood/anxiety | Ponderal indexa | β 0.05 (0.001–0.09) | Positive association between paternal history of mood/anxiety and ponderal index. Autoimmune disease was associated with larger head circumference. |
| Autoimmune disease | Head circumference | β 0.87 (0.15–1.60) | ||||
| Midtvedt et al (2017) [37] | Cohort | 230 fathers and 350 children | Mycophenolic acid | CAs | 3.9% vs. 2.6% | Mycophenolic acid is the active immunosuppressive substance used after organ transplantation. No significant association with risk of malformations in the offspring. Birthweight is also similar in exposed and unexposed cohorts of children. |
| p=0.49 | ||||||
| Birthweight | 3,381±681 g vs. 3,429±714 g | |||||
| p=0.53 | ||||||
| Lopez-Lopez et al (2018) [38] | Cohort | 33 fathers and 49 children | Mycophenolic acid | CAs | No cases | No increased incidence of malformations in descendants of male kidney transplantation recipients. Small sample. |
| Viktorin et al (2018) [36] | Cohort | 3,983 cases and 164,492 controls | Antidepressants | PTB | aOR 0.91 (0.79–1.04) | Paternal intake of antidepressants around conception is safe with respect to preterm birth, malformations, autism, and intellectual disabilities. |
| CAs | aOR 1.06 (0.90–1.26) | |||||
| Intellectual disabilityb | aOR 0.82 (0.51–1.31) | |||||
| Autism spectrum disorder | aHR 1.13 (0.84–1.53) | |||||
| Yang et al (2018) [34] | Cohort | 781,470 singletons | Antidepressants | ADHD | HR 1.26 (1.06–1.51) | The increased risk of ADHD in offspring associated with paternal SSRI used 12 to 3 months before conception, could be due to the underlying paternal conditions related to drug use. |
| SSRI | ||||||
| Ananthakrishnan et al (2019) [25] | Cohort | 256 males | Biologics | CAs | OR 0.50 (0.05–5.20) | Biologics, thiopurine, corticosteroid use to control IBD has no significant effect on CAs. |
| Thiopurine | OR 0.49 (0.05–4.82) | |||||
| Corticosteroids | OR 1.80 (0.30–10.94) | |||||
| Yang et al (2019) [40] | Cohort | 733,282 singletons | Antiepileptic drugs use | CAs | OR 1.23 (1.10–1.37) | The increased risk of CAs may be attributable to the underlying conditions requiring antiepileptic drugs use. |
| a. Preconceptional | OR 1.29 (1.03–1.61) | |||||
| b. In pregnancy | OR 1.35 (1.12–1.65) | |||||
| Larsen et al (2016) [31] | Cohort | Newborns 372 cases and 399,498 controls | Anti-TNF-α agents' treatment | PTB | OR 1.42 (0.52–3.86) | No association between reproductive outcomes and paternal preconceptional exposure to anti-TNF-α agents in patients with inflammatory bowel, rheumatologic or dermatological diseases. |
| SGA | OR 1.70 (0.74–3.89) | |||||
| Wallenius et al (2015) [39] | Cohort | Newborns 110 cases and 230 controls | DMARDs treatment | CAs | RR 1.22 (0.45–3.31) | Preconceptional paternal exposure to DMARDs, whether synthetic or biological, does not increase Cas. |
| p=0.69 |
OR: odds ratio, CI: confidence interval, PTB: preterm birth, CAs: congenital anomalies, NICU: neonatal intensive care unit, RR: risk ratio, VLBW: very low birthweight, ART: artificial reproductive techniques, BMI: body mass index, SGA: small for gestational age, aHR: adjusted hazard ratio, LBW: low birthweight, ADHD: attention-deficit/hyperactivity disorder, HR: hazard ratio, aOR: adjusted odds ratio, SSRI: selective serotonin reuptake inhibitors, IBD: inflammatory bowel disease, TNF: tumor necrosis factor, DMARDs: disease-modifying antirheumatic drugs.
aPonderal index=[birthweight (g)/length (cm3)]×100. It assesses infant adiposity.
bAs defined by ICD (International Classification of Diseases)-10.
(1) Fertility
Parental aging and declining fertility are generally considered to be correlated. Resembling what happens in women, paternal age could reduce the chance of conceiving in terms of seminal fluid parameters as well as potential CAs. The latest literature confirms that alterations of sperm characteristics include reduced semen volume and teratozoospermia [12]. However evaluation of sperm DNA might have greater clinical utility since seminal DNA fragmentation index (DFI) is found to be higher in men aged 40 or older [12] and this might explain the demographic trend of a steady decrease of men's fertility after the age of 39 [13].
The impact of paternal BMI upon fertility has drawn the attention of researchers: body weight affects hormonal balance and semen quality, the latter undergoing deterioration as body mass increases [14]. Seminal oxidative stress, DFI, abnormal sperm concentration, and motility are often observed in men with higher BMI [15,16]. Given the widespread use of assisted reproductive technologies (ART) to counteract the effects of age on chances of natural conception, recent data are reassuring: in-vitro fertilization (IVF) outcomes in terms of implantation, clinical pregnancy and live birth rates do not seem to be affected by paternal age and BMI [17].
Paternal concurrent diseases and related therapies are a matter of concern when evaluating male fertility. Diabetes, a high prevalence metabolic disease, has been associated with reduced fecundity, longer time to pregnancy (TTP) rates and also with higher reactive oxygen species (ROS) and sperm DNA fragmentation [18], which raises implications for fetal development [19].
Parenting and reproductive health are a concern for individuals affected by chronic conditions. Although a rare disease, estimated to affect approximately 1 in 3,000 individuals, cystic fibrosis (CF) has severe implications on male fertility [20]. The increasing life expectancy of the CF population, with more frequent transition to adulthood, has raised the number of young males wishing to become parents. Documented cases of CF men conceiving naturally are estimated less than 1%, while biological fatherhood may be achieved in 9% to 10% of subjects undergoing ART sperm retrieval [21].
Paternal mental health is relevant when it comes to fecundity: female partners of depressed males are less likely to achieve conception [22]. This finding could depend upon use of antidepressants that have been linked to higher sperm DNA fragmentation and reduction of the couple's fertility [23].
Medical treatments may negatively affect male fertility via different mechanisms. Although evidence suggests that sexual function is reversible after therapy discontinuation, treatment suspension is not always an option for patients with chronic conditions. In a Japanese study, semen of patients treated with antiepileptics and H1 receptor antagonists to cure asthma or chronic bronchitis more often show oligozoospermia and asthenozoospermia [24]. The use of aminosalicylate to control inflammatory bowel disease has been associated with longer TTP [25]. These results suggest that some drugs, used on a long-term basis, cause fertility hazards reversible only when alternative medical treatment is available.
(2) Pregnancy outcomes
Available studies report conflicting results with regard to the implications of age on preterm delivery and birthweight. In a large Japanese cohort study, controlled for maternal age, pregnancies fathered by men of 35 years of age or older end more often prematurely (p<0.01) and with very low birthweight (VLBW) babies (p<0.01) [26]. These findings are not confirmed by a North American study which reports no association between age, increased risk of PTB, fetal growth restriction [27]. Multiple different factors in the two settings, i.e., standard diets, climate, and quality of life, could explain the contrasting conclusions.
Unlike age, paternal excessive BMI seems to correlate with higher birthweight increasing the risks of obesity and type I diabetes in childhood [28,29,30].
Men affected by health conditions are likely to expose their progeny to developmental disorders: paternal diabetes and inflammatory bowel disease increase the risk of reduced fetal weight gain [31,32,33], while history of mood or anxiety disorders, as well as the use of antidepressants at the time of conception, may also lead to sequelae in the offspring ranging from development of attention-deficit/hyperactivity disorders (ADHDs) to increased infant ponderal index [34,35]. Against this background, findings of a Swedish cohort are reassuring since no correlation is observed between paternal antidepressants use and intellectual disabilities, as defined by ICD (International Classification of Diseases)-10 [36].
Generally, no other paternal medical treatments have been correlated to impaired fetal structural development and growth. Specifically, there is no evidence that fetal damage may be caused by antiepileptics, thiopurine and corticosteroid drugs nor disease-modifying antirheumatic drugs or mycophenolic acid [25,31,34,37,38,39,40].
2) Lifestyle paternal factors
A whole set of factors related to environment, occupation and habits is known to exert effects of variable severity upon reproductive outcomes. A prolonged exposure may permanently impair paternal sexual organs' function and have long-term negative implications on the offspring. Table 3, 4 summarize the latest findings on the subject.
Table 3. External paternal factors and fertility.
| Citation | Study design | Subject | Exposure | Reproductive outcome | OR (95% CI), p-value, overall trend | Key finding | |
|---|---|---|---|---|---|---|---|
| Tang et al (2019) [41] | Cohort | 1,631 males | Smoking | Semen quality, semen volume, oligospermia, sperm motility | p<0.001 | Cigarette smoking (≥10 packs/y) is associated with lower semen volume and total sperm count but increased motility. | |
| p<0.05 | |||||||
| p<0.05 | |||||||
| Rehman et al (2019) [42] | Cross-sectional | 165 infertile vs. 211 fertile males | Smoking | T and SHBG levels, semen quality | p<0.05 | Reduced sexual hormone levels which alters sperm total count and morphology but do not impact their motility. | |
| Gaur et al (2010) [43] | Cohort | 100 cases vs. 100 controls | Smoking | Asthenozoospermia | p<0.0001 | Positive correlation between quantity of cigarettes smoked or amount of alcohol intake and altered sperm parameters but alterations are observed even at low degrees of alcohol/tobacco addiction. | |
| Teratozoospermia | p<0.05 | ||||||
| 100 cases vs. 100 controls | Alcohol | Oligozoospermia | p<0.05 | ||||
| Teratozoospermia | p<0.001 | ||||||
| Borges et al (2018) [44] | Cohort | 965 males | Smoking | Semen quality, fertilization and blastocyst formation rate | p<0.05 | Cigarette smoking and alcohol consumption reduce semen quality, in terms of total sperm count, fertilization rates, and blastocyst formation rates. | |
| Alcohol | DNA fragmentation | p<0.01 | |||||
| Sperm concentration, fertilization and blastocyst formation rate | p<0.05 | ||||||
| Kasman et al (2018) [46] | Cohort | 758 males and 1,076 females | Marijuana | TTP | TR 1.08 (0.79–1.47) | No significant impact of marijuana use on TR. | |
| Wise et al (2017) [47] | Cohort | 1,125 couples | Marijuana use | Fecundability | FRs 0.87 (0.66–1.15) | FRs indicate no association between marijuana use and fecundability. | |
| <1 time/wk | FRs 1.24 (0.90–1.70) | ||||||
| ≥1 time/wk | |||||||
| Nassan et al (2019) [48] | Cohort | 662 sub-fertile males | Marijuana use current/past never | Semen quality, concentration and motility | p<0.001 | After adjusting for potential confounders, marijuana users have significantly higher sperm concentration and motility than never users. | |
| Gundersen et al (2015) [100] | Cohort | 1,215 males | Marijuana | Sperm concentration | 1.07 (0.62–1.87) | After adjustment for confounders, regular marijuana smoking was not associated with lower sperm concentration nor total sperm count. | |
| Sperm total count | 1.14 (0.69–1.89) | ||||||
| Pichini et al (2012) [45] | Cohort | 164 couples and 24 individuals | Cannabinoids and cocaine users vs. non-users | Urinary T | p<0.05 | Male drug consumption was associated with significantly lower urinary T concentrations (mean 105.5±21.1 SD vs. 124.5±46.5 mg/24 h). | |
| Tielemans et al (2000) [51] | Cohort | 726 couples | Organic solvents | IVF implantation rate | OR 0.24 (0.06–0.91) | Couples with male partner exposed to organic solvents have reduced IVF implantation rates. Level of exposure matters. | |
| Pesticides | OR 1.57 (0.33–7.44) | ||||||
| Metal dust and fumes | OR 1.44 (0.57–3.61) | No reduction found after exposure to pesticides, metal dust and fumes or welding fumes. | |||||
| Welding fumes | OR 1.20 (0.31–4.68) | ||||||
| Dodge et al (2015) [52] | Cohort | 218 couples | Methyl paraben | Live birth rate after IUI | aOR 0.19 (0.04–0.82) | Paternal professional exposure to methyl paraben at a specific concentration (10.5~29.0 ng/mL) is associated with decreased odds of live birth following IUI. | |
| Campagna et al (2015) [53] | Cohort | 1,223 couples | DDT | Early fecundity | FR 1.22 (0.84–1.77) | Among the spouses of DDT workers (the antimalaria environmental agent), fecundability did not vary during DDT handling nor in the following decade. | |
| Fecundity 10 years after exposure | FR 1.01 (0.67–1.50) | ||||||
| Buck Louis et al (2016) [50] | Cohort | 501 couples | Heavy metals/non-persistent chemicals | Fecundability and specific exposure | OR 0.83 (0.70–0.98) | Specific exposure within persistent metals/chemicals and non-persistent chemicals is associated with a significant reduction in couples' fecundability. | |
| OR 0.69 (0.49–0.97) | |||||||
| Lead benzophenone-2, mBz-phthalate, mM-phthalate | OR 0.80 (0.67–0.97) | ||||||
| OR 0.81 (0.70–0.94) | |||||||
OR: odds ratio, CI: confidence interval, T: testosterone, SHBG: sex hormone binding globulin, TTP: time to pregnancy, TR: TTP ratio, FR: fecundability ratio, SD: standard deviation, IVF: in-vitro fertilization, aOR: adjusted odds ratio, IUI: intra-uterine insemination, DDT: dichloro-diphenyl-trichloroethane.
Table 4. External paternal factors and pregnancy outcomes.
| Citation | Study design | Subject | Exposure | Long-term reproductive outcome | OR (95% CI), p-value, overall trend | Key finding | |
|---|---|---|---|---|---|---|---|
| Magnus et al (2019) [55] | Cohort | 1,381 cases and 618,322 controls | Smoking | Diabetes | HR 0.97 (0.82–1.2) | No association between paternal or second-hand smoking during pregnancy with childhood-onset type I diabetes. | |
| Mejia-Lancheros et al (2018) [56] | Cohort | 1,021 newborns | Smoking | Overweight/obesity | aOR 1.76 (1.14–2.71) | After adjustment, positive correlation between paternal smoking and offspring's overweight/obesity at 5- and 9-years follow-up. | |
| p<0.01 | |||||||
| Accordini et al (2018) [54] | Cohort | 1,964 males | Smoking | Asthma | RR 1.43 (1.01–2.01) | Fathers' smoking starting in early adolescence may independently increase asthma risk without allergies in offspring. | |
| Alati et al (2013) [10] | Cohort | 7,062 males | Alcohol | Cognitive development | KS2 score 0.27 (0.07–0.46) | No strong evidence of intrauterine mechanisms linking paternal alcohol use with offspring cognitive development expressed in mean change of KS2 scoresa. | |
| Karalexi et al (2017) [57] | Meta-analysis | 39 studies | Alcohol | Leukemia (any type) | OR 1.05 (0.91–1.22) | No association between paternal preconceptional alcohol consumption and risk of any type of leukemia cancer at 0–14 age. | |
| p=0.931 | |||||||
| Acute lymphoblastic leukemia | OR 1.10 (0.93–1.30) | ||||||
| p=0.361 | |||||||
| Acute myeloid leukemia | OR 1.23 (0.83–1.82) | ||||||
| p=0.489 | |||||||
| Lindblad et al (2011) [59] | Cohort | 7,960 cases and 1,154,564 controls | Addictive drugs disorder | ADHD | Parental addiction to illicit drugs is associated with the highest OR for ADHD medication to treat the offspring. | ||
| Boys | OR 3.7 (3.4–4.2) | ||||||
| Girls | OR 4.6 (3.5–5.9) | ||||||
| Fang et al (2018) [58] | Cohort | 3,210 infants | Opioids | Premature death | aHR 4.79 (1.16–19.79) | The risk of premature death (0–6 years) increases 2.5–5.2 times in relation to the severity of paternal opioid use. | |
| p<0.05 | |||||||
| Sallmén et al (2016) [62] | Cohort | 11,863 cases and 23,720 controls | Lead blood level (μmol/L) | Schizophrenia, spectrum disorder | Paternal occupational exposure to lead does not increase risk for schizophrenia in the offspring no matter the metal blood level. | ||
| <0.5 | aHR 0.97 (0.52–1.83) | ||||||
| 0.5–0.9 | aHR 1.25 (0.85–1.82) | ||||||
| 1.0–1.4 | aHR 0.90 (0.54–1.49) | ||||||
| ≥1.5 μmol/L | aHR 1.38 (0.65–2.92) | ||||||
| Nieuwenhuijsen et al (2013) [60] | Meta-analysis | 3 studies | Solvents | Anencephaly | OR pooled 2.18 (1.52–3.11) | Evidence for association between paternal solvents exposure and child NTDs or anencephaly. No causative role of pesticides for hypospadias. | |
| NTDs | OR pooled 1.86 (1.40–2.46) | ||||||
| Spina bifida | OR pooled 1.59 (0.99–2.56) | ||||||
| Pesticides | Hypospadias | RR 1.19 (1.00–1.41) | |||||
| Jørgensen et al (2014) [63] | Cohort | 600,000 births | Pesticides | Cryptorchidism | aHR 1.04 (0.96–1.12) | No increased risk of cryptorchidism after pesticide exposure. | |
| Messerlian et al (2018) [105] | Cohort | 346 singletons | Benzophenone-3 | Birthweight gain | 137 g (60–214 g) | For each log-unit increase of paternal preconception benzophenone-3 concentration in the urine, an average 137 g gain in birthweight is observed. | |
| Chen et al (2018) [61] | Cohort | 192,492 births | X-ray, CT, PET-CT, or other radionuclide imaging radiation | LBW | p<0.05 | PTB and LBW are both associated with pregestational paternal radiations exposure for medical imaging purposes. | |
| PTB | p<0.01 | ||||||
OR: odds ratio, CI: confidence interval, HR: hazard ratio, aOR: adjusted odds ratio, RR: risk ratio, KS2: Key stage 2, ADHD: attention-deficit/hyperactivity disorder, aHR: adjusted hazard ratio, NTD: neural tube diseases, CT: computed tomography, PET: positron emission tomography, LBW: low birthweight, PTB: preterm birth.
aKey stage 2 scores are considered to be a ‘real world’ measure of academic performance.
(1) Fertility
Very common habits, like smoking or alcohol intake, could influence paternal sexual functions at different levels. Studies suggest a detrimental effect of tobacco smoking mediated by reduced sexual hormones levels and exposure of spermatozoa to oxidative stress [41,42]. Heavy smokers present lower sperm concentration, total count and more pronounced teratozoospermia which entail decreased ART success rates although effects on sperm motility are found unaffected [41,43]. In addition, smoking exposes to hazardous substances like tar, nicotine, carbon monoxide, and heavy metals (e.g., cadmium and lead) with implications for semen DNA integrity leading to aneuploidies and mutations [43]. It is somewhat reassuring that, following smoking cessation, a healing effect on semen quality has been observed [41].
Paternal alcohol consumption can equally cause severe semen damage: two different cohort studies report a positive correlation between alcohol use and sperm abnormalities. Teratozoospermia progressively increases as alcohol consumption goes from moderate to heavy (63% moderate 40–80 g/d and 72% heavy >80 g/d consumers) [43]; sperm count reduction and increased DNA fragmentation are also observed in alcohol users as well as reduced fertilization and blastocyst formation rates [44].
Recreational drugs are largely used by adolescents and young adults during their critical pubertal period, endangering reproductive potential. Additionally, a long suspected detrimental impact on male fertility finds confirmation in the lower urinary testosterone (T) concentrations in adults consuming cannabinoids and cocaine [45]. Other studies analyzing the impact of marijuana on fecundity have raised controversy after excluding adverse effects and a paradoxical increased sperm concentration in sub-fertile men [46,47,48,49].
Occupational and environmental exposures as well are believed to have relevant fertility implications: alarming data already exist linking organic solvents and heavy metals manipulation with fertility reduction. Toxic polluting agents like lead, tetrahydroxybenzophenone, monobenzyl phthalates, monomethyl phthalates are known to affect couples' fecundity by increasing TTP and IVF failure rates [50,51].
Among occupational hazards, the protracted handling of methylparaben can severely cut the odds of live birth rates in ART pregnancies [52]. Studies investigating the impact of paternal exposure to pesticides, metal dust and welding fumes so far exclude effects upon fecundity [51,53].
(2) Pregnancy outcomes
Evidence suggests that paternal smoking has also harmful effects on the overall health of the offspring. Cigarette smoking starting in early adolescence increases the risk of asthma in the progeny [54]. Chronic tobacco use including the time of conception could increase risks of overweight and obesity in children although effects on sugar metabolism seem to be transient and reversible [55,56].
Paternal alcohol consumption raises concern for possible long-term adverse outcomes in the generation-to-be but cognitive sequelae were excluded in a study evaluating neurological development of pupils aged 7 to 11 years old [10]. Likewise, reassuring findings exclude paternal drinking patterns as a risk factor for the development of different forms of leukemia cancers [57].
In addition to the early sequelae on male fertility, recreational drug use has also been associated with long-term adverse outcomes in the offspring which include the positive correlation between the extent of paternal drug addiction and the severity of ADHD [58,59].
Solvents, organic compounds, and medical-related radiations are frequent occupational and environmental factors that endanger the individual and the developing fetus. Neural tube defects (NTDs) and anencephaly have been reported in association with solvents exposure [60]. Repeated findings suggest increased rates of PTBs and LBW in pregnancies fathered after periconceptional exposure to radionuclide imaging radiation [61]. The role of heavy metals (lead), and pesticides on spina bifida, hypospadias, cryptorchidism and children mental disorders (i.e., schizophrenia) is still debated and awaits confirmation [60,62,63].
CONCLUSIONS
1. Discussion
1) Summary of evidence
This review searches for the latest evidence and implications that paternal health and habits exert upon reproductive risks. Indeed, our study reveals that paternal fertility and pregnancy outcomes may be affected by multiple paternal factors related to individual general health as well as to behaviors and occupation.
A proven association occurs between paternal aging and lower sperm quality, often resulting in reduced fertilization, implantation, or increased miscarriage rates; live birth rates have also been found to be reduced [64,65]. As a matter of fact, a precise time when age begins to affect male reproductive potential remains undefined. Some authors report the starting point at age 40, others prefer to use the higher limit of 50 years when there is a sound statistical association [66,67]. In later stages of pregnancy other critical events have been linked to paternal aging: studies in Denmark and the United States have found that paternal age is an independent factor for very PTB and LBW, respectively [8]. Our search provides limited and discordant new information concerning these aspects: the results of a Japanese group suggesting that higher paternal age and assisted reproduction are the main risk factors for VLBW and PTB [26] conflict with those from a United States retrospective cohort study which concludes that older paternal age does not pose an independent risk of adverse perinatal outcomes in pregnancies achieved either with or without ART [27]. A matter of concern is the association of paternal aging with neurodevelopmental disorders in children, such as autism or schizophrenia and CAs [65,67,68]. Previous reviews recognize paternal age as a risk factor for genetic abnormalities, child cancer development and other musculoskeletal congenital syndromes [64,65,69].
Reduced fertility in male adults may also be caused by an increased BMI due to seminal oxidative stress, sexual hormones imbalance, increased DFI as well as reduced sperm concentration and motility [14,15,43,70]. Excessive fat mass alters sex hormone-binding globulin levels as a result of reduced hepatic globulin synthesis in a milieu of high insulin levels [71,72]. Since over-weight and obesity are predominantly determined by the quality of nutrition, the association between ponderal excess and fertility may be explored by taking into account possible mechanisms linked to paternal dietary patterns. “Unhealthy” diets - plentiful in red and processed meat, sweet and sweetened beverages, refined grains and snacks - affect fertility by impairing spermatogenesis, increasing sperm DNA damage and reducing both sperm motility and concentration [73,74,75,76]. Higher intake of food rich in saturated and trans fatty acids enhances their accumulation in the testicular environment leading to poor semen quality, lower testicular volume and low T synthesis [77,78,79,80]. Conversely, Mediterranean and “Prudent” diets, overall considered healthy dietary patterns, rich in seafood, lean meat, whole grains, fruits and vegetables, have been consistently associated with better semen parameters. Their abundance in micronutrients, which include folate and zinc, antioxidant compounds and omega-3 fatty acids, improve spermatogenesis by increasing sperm motility [75,81,82,83]. Effects of paternal weight excess upon their progeny also include fetal growth retardation and child metabolic dysregulation resulting in obesity and type I diabetes via neuronatrin hypomethylation [84,85].
A crucial issue when it comes to paternal comorbidities and related treatments is their impact on reproductive outcomes as well. Cancer has negative implications upon fertility since it often requires antineoplastic treatments, namely chemotherapy, radiotherapy and surgery, which all contribute to sperm quality reduction [86]. Sub-fertility depends on altered sperm chromatin integrity, testicular function and spermatogenesis [87,88]. In all these instances fertility can be preserved with sperm cryo-conservation, neurostimulatory methods of ejaculation or surgical sperm retrieval procedures. ART provides an invaluable back-up also in young men affected by CF, which severely impairs semen quality starting from early adulthood [21]. The high prevalence of diabetes makes it worth mentioning: DFI is significantly higher in high-density sperm fractions and TTP is increased in couples with a diabetic male partner [18,19]. Several other paternal morbidities have been studied in large reviews: varicocele appears to damage sperm DNA while genital tract infections deteriorate the sperm genetic component [88,89]. Finally, altered sperm quality has been associated with neurologic conditions such as multiple sclerosis whereas men with spinal cord injuries have frequent chromatin abnormalities, seminal ROS and DNA fragmentation [90]. The effects of paternal mental conditions on reproduction have been given attention in a large body of the literature. Depression has been diagnosed in up to 49% of male partners affected by infertility and it has been shown that antidepressants use increases DNA sperm fragmentation [22,23,91]. There is also concern for other antidepressants-related effects in the offspring, the most clearly documented one being a higher risk of ADHD [34].
Several different medications, when used on a long term basis can impair sperm function. Treatments for epilepsy, allergies, ulcerative colitis and Crohn's disease (e.g., H1 receptor antagonists and amino salicylate) pose this risk and yet there is reassuring evidence that sperm damage is reversible and no longer present after switching to a safer alternative treatment around conception [24,25].
The chemical content of cigarettes causes mutagenesis, apoptosis and death in rapidly dividing cells with a corresponding decrease in spermatogenesis. Paternal smoking impairs fertility also via sexual hormones reduction, oxidative stress and ROS in sperm [92]. Surprisingly, there is contrasting evidence on smoke-induced sexual hormones imbalance [93]. Recent findings exploring the intensity and duration of smoking and seminal damage, show more frequently permanent abnormal sperm chromatin condensation in heavy (≥20 cigarettes/d) and long-term smokers [94]. Nevertheless, increasing evidence suggests that restoration of semen quality may be accomplished through either progressive reduction of smoking frequency or complete cessation [94]. An association between CAs and smoking has also been observed: clefts, anorectal malformations, septal and left ventricular anomalies, transposition of great arteries and conotruncal heart abnormalities [95,96]. On a long-term perspective, there is alarming evidence that tobacco smoke may cause genetic changes leading to metabolic and chronic conditions in the offspring ranging from asthma to cancer [54,56,97].
Much like smoking, excessive alcohol intake deteriorates sperm quality, oligozoospermia and teratozoospermia being proven effects [43]. While no direct correlation of alcohol use with fetal-neonatal adverse outcomes has been observed, paternal drinking habits are often shared by the female partner, and paternal alcohol consumption may thus be considered an indirect contributing factor to fetal damage. The most frequently reported associations comprise higher rates of miscarriage, LBW, and some delayed effects like mental retardation and cancer in childhood [10,48,98].
The idea that seminal parameters deteriorate whenever male lifestyles include the use of recreational drugs is long rooted in the scientific community and in the general public opinion [99]. It is relevant to consider that psychoactive substances are often consumed during the peripubertal years, when testicular development occurs. The fact that marijuana reduces sperm concentration and total count in regular consumers is therefore a matter of concern [70,100]. Against this background, following the new popularity of marijuana and its alternative therapeutic applications in chronic pain management, new diverging scientific data have reopened the debate questioning the real reproductive hazards correlated with its use. Recent studies carried out in the United States and Canada do not unequivocally endorse the association with male fertility reduction and speculate that sperm count may be unaffected or even increased in regular marijuana users [46,47,48].
Other implications related to illicit drug consumption are worth mentioning: cocaine and opioid addiction disorders have implications on the well-being of the progeny which include LBW, premature deaths before the age of 6 and severe forms of ADHD [31,39,58,59]. Paternal drug addiction is suspected to favor maternal involvement with psychoactive substances during pregnancy just as it happens with alcohol consumption, indirectly amplifying the above-mentioned adverse effects on the child [101].
Some occupations expose workers to the risks of polluted environments and the manipulation of toxic substances. Assuming that this situation exists in both high and low-resource countries, national and international institutions have increased strategies to protect workers' health [102]. The scientific community is also working to limit human damage provoked by the environment by assessing the underlying mechanisms of potential hazards.
Male reproductive germ cells are very vulnerable to external factors and even a moderately raised testicular temperature may be detrimental to spermatogenesis [103]. Reproductive outcomes involving environmental exposure to solvents and heavy metals include increased TTP and IVF failures, while occupational handling of compounds derived from hydroxybenzoic acid (i.e., parabens) has been associated with lower rates of live births after ART [51,52]. Seminal damage deriving from paternal exposure to toxics has serious implications in the progeny: it has been observed that painters and carpenters, who have regular contacts with solvents, carry a higher risk of conceiving newborns suffering from anencephaly, heart or NTDs; moreover, sustained exposure to ionizing radiations increases PTB and LBW rates [60,61,104,105].
2. Strength and limitations
The main strength of this review is the attention given to the neglected role of biological and acquired paternal factors upon reproductive matters: the impact of the male partner's health and habits on conception, pregnancy and newborn health is generally underestimated and left in the background in comparison with maternal factors. Proof of this prevailing attitude is the scarcity of studies addressing the issue. In spite of the paucity of the available literature, we managed to analyze several male determinants, although not necessarily interrelated.
By including a wide selection of different factors and their interaction with reproductive outcomes, we forcedly limited the in depth investigation related to the biological mechanisms behind the effects and this may be considered a major constraint. Another limitation concerns the statistical significance associated with the findings of some included studies. Although providing aORs, residual confounders might exist, potentially interfering with the association between paternal health condition and pregnancy outcomes.
3. Conclusions
The findings of this review allow us to conclude that reproductive outcomes are significantly influenced by paternal factors: biological traits and acquired everyday habits, profoundly affect seminal fluid quality impacting pregnancy, birth and long-term outcomes in the progeny.
The scientific soundness of the selected studies justifies primary prevention campaigns and good clinical practice indications through a preconception strategy focusing on both partners' lifestyles, health and occupation.
ACKNOWLEDGEMENTS
The project was realized with the financial support of the Italian Ministry of Health-CCM.
Footnotes
Conflict of Interest: The authors have nothing to disclose. Renata Bortolus is part of the Funding Board and has a role of supervision.
- Conceptualization: CM, GZ.
- Data curation: CM.
- Formal analysis: CM.
- Funding acquisition: SR, RB.
- Investigation: CM.
- Methodology: CM.
- Project administration: SR, AET, RB, CB.
- Supervision: GZ, SR, AET.
- Validation: CM, GZ, GC, AET, SR.
- Visualization: CM.
- Writing — original draft: CM, GZ.
- Writing — review & editing: CM, GZ, SR, AET, GC.
References
- 1.United Nations Population Fund (UNFPA) Programme of Action [Internet] New York (NY): United Nations Population Fund (UNFPA); c2014. [cited 2020 Apr 27]. Available from: https://www.unfpa.org/sites/default/files/pub-pdf/programme_of_action_Web%20ENGLISH.pdf. [Google Scholar]
- 2.World Health Organization Regional Office for Europe. Fatherhood and health outcomes in Europe [Internet] Copenhagen: World Health Organization Regional Office for Europe; c2007. [cited 2020 Apr 27]. Available from: http://www.euro.who.int/__data/assets/pdf_file/0017/69011/E91129.pdf. [Google Scholar]
- 3.Soubry A. POHaD: Why we should study future fathers. Environ Epigenet. 2018;4:dvy007. doi: 10.1093/eep/dvy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tokhi M, Comrie-Thomson L, Davis J, Portela A, Chersich M, Luchters S. Involving men to improve maternal and newborn health: a systematic review of the effectiveness of interventions. PLoS One. 2018;13:e0191620. doi: 10.1371/journal.pone.0191620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alio AP, Salihu HM, Kornosky JL, Richman AM, Marty PJ. Feto-infant health and survival: Does paternal involvement matter? Matern Child Health J. 2010;14:931–937. doi: 10.1007/s10995-009-0531-9. [DOI] [PubMed] [Google Scholar]
- 6.Shah PS. Parity and low birth weight and preterm birth: a systematic review and meta-analyses. Acta Obstet Gynecol Scand. 2010;89:862–875. doi: 10.3109/00016349.2010.486827. [DOI] [PubMed] [Google Scholar]
- 7.Puscheck EE, Jeyendran RS. The impact of male factor on recurrent pregnancy loss. Curr Opin Obstet Gynecol. 2007;19:222–228. doi: 10.1097/GCO.0b013e32813e3ff0. [DOI] [PubMed] [Google Scholar]
- 8.Zhu JL, Madsen KM, Vestergaard M, Basso O, Olsen J. Paternal age and preterm birth. Epidemiology. 2005;16:259–262. doi: 10.1097/01.ede.0000152526.63279.da. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Programming for male involvement in reproductive health [Internet] Geneva: World Health Organization; c2002. [cited 2020 May 6]. Available from: https://apps.who.int/iris/bitstream/handle/10665/67409/WHO_FCH_RHR_02.3.pdf?sequence=1. [Google Scholar]
- 10.Alati R, Davey Smith G, Lewis SJ, Sayal K, Draper ES, Golding J, et al. Effect of prenatal alcohol exposure on childhood academic outcomes: contrasting maternal and paternal associations in the ALSPAC study. PLoS One. 2013;8:e74844. doi: 10.1371/journal.pone.0074844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosiak-Gill A, Gill K, Jakubik J, Fraczek M, Patorski L, Gaczarzewicz D, et al. Age-related changes in human sperm DNA integrity. Aging (Albany NY) 2019;11:5399–5411. doi: 10.18632/aging.102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matorras R, Matorras F, Expósito A, Martinez L, Crisol L. Decline in human fertility rates with male age: a consequence of a decrease in male fecundity with aging? Gynecol Obstet Invest. 2011;71:229–235. doi: 10.1159/000319236. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen RH, Wilcox AJ, Skjaerven R, Baird DD. Men's body mass index and infertility. Hum Reprod. 2007;22:2488–2493. doi: 10.1093/humrep/dem139. [DOI] [PubMed] [Google Scholar]
- 15.Tunc O, Bakos HW, Tremellen K. Impact of body mass index on seminal oxidative stress. Andrologia. 2011;43:121–128. doi: 10.1111/j.1439-0272.2009.01032.x. [DOI] [PubMed] [Google Scholar]
- 16.Kort HI, Massey JB, Elsner CW, Mitchell-Leef D, Shapiro DB, Witt MA, et al. Impact of body mass index values on sperm quantity and quality. J Androl. 2006;27:450–452. doi: 10.2164/jandrol.05124. [DOI] [PubMed] [Google Scholar]
- 17.Capelouto SM, Nagy ZP, Shapiro DB, Archer SR, Ellis DP, Smith AK, et al. Impact of male partner characteristics and semen parameters on in vitro fertilization and obstetric outcomes in a frozen oocyte donor model. Fertil Steril. 2018;110:859–869. doi: 10.1016/j.fertnstert.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Eisenberg ML, Sundaram R, Maisog J, Buck Louis GM. Diabetes, medical comorbidities and couple fecundity. Hum Reprod. 2016;31:2369–2376. doi: 10.1093/humrep/dew200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roessner C, Paasch U, Kratzsch J, Glander HJ, Grunewald S. Sperm apoptosis signalling in diabetic men. Reprod Biomed Online. 2012;25:292–299. doi: 10.1016/j.rbmo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Genes and human diseases [Internet] Geneva: World Health Organization; c2020. [cited 2020 May 6]. Available from: http://www.who.int/genomics/public/geneticdiseases/en/ [Google Scholar]
- 21.Bourke SJ, Anderson A, Briggs J, Doe S, Echevarria C, Choudhary M, et al. Current status of fertility and family formation in men with cystic fibrosis. Hum Fertil (Camb) 2019 doi: 10.1080/14647273.2019.1656824. [Epub] [DOI] [PubMed] [Google Scholar]
- 22.Evans-Hoeker EA, Eisenberg E, Diamond MP, Legro RS, Alvero R, Coutifaris C, et al. Major depression, antidepressant use, and male and female fertility. Fertil Steril. 2018;109:879–887. doi: 10.1016/j.fertnstert.2018.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanrikut C, Feldman AS, Altemus M, Paduch DA, Schlegel PN. Adverse effect of paroxetine on sperm. Fertil Steril. 2010;94:1021–1026. doi: 10.1016/j.fertnstert.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T, Miyata A, Yamada T. The impact of commonly prescribed drugs on male fertility. Hum Fertil (Camb) 2008;11:191–196. doi: 10.1080/14647270701739566. [DOI] [PubMed] [Google Scholar]
- 25.Ananthakrishnan AN, Martin C, Kane S, Sandler RS, Long MD. Paternal disease activity is associated with difficulty in conception among men with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17:203–204. doi: 10.1016/j.cgh.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamura N, Hanaoka T, Ito K, Araki A, Miyashita C, Ito S, et al. Different risk factors for very low birth weight, term-small-for-gestational-age, or preterm birth in Japan. Int J Environ Res Public Health. 2018;15:369. doi: 10.3390/ijerph15020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurley EG, DeFranco EA. Influence of paternal age on perinatal outcomes. Am J Obstet Gynecol. 2017;217:566.e1–566.e6. doi: 10.1016/j.ajog.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 28.Noor N, Cardenas A, Rifas-Shiman SL, Pan H, Dreyfuss JM, Oken E, et al. Association of periconception paternal body mass index with persistent changes in DNA methylation of offspring in childhood. JAMA Netw Open. 2019;2:e1916777. doi: 10.1001/jamanetworkopen.2019.16777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mei H, Guo S, Lu H, Pan Y, Mei W, Zhang B, et al. Impact of parental weight status on children's body mass index in early life: evidence from a Chinese cohort. BMJ Open. 2018;8:e018755. doi: 10.1136/bmjopen-2017-018755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnus MC, Olsen SF, Granstrom C, Lund-Blix NA, Svensson J, Johannesen J, et al. Paternal and maternal obesity but not gestational weight gain is associated with type 1 diabetes. Int J Epidemiol. 2018;47:417–426. doi: 10.1093/ije/dyx266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen MD, Friedman S, Magnussen B, Nørgård BM. Birth outcomes in children fathered by men treated with anti-TNF-α agents before conception. Am J Gastroenterol. 2016;111:1608–1613. doi: 10.1038/ajg.2016.405. [DOI] [PubMed] [Google Scholar]
- 32.Moss JL, Harris KM. Impact of maternal and paternal preconception health on birth outcomes using prospective couples' data in Add Health. Arch Gynecol Obstet. 2015;291:287–298. doi: 10.1007/s00404-014-3521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji J, Chen T, Sundquist J, Sundquist K. Type 1 diabetes in parents and risk of attention deficit/hyperactivity disorder in offspring: a population-based study in Sweden. Diabetes Care. 2018;41:770–774. doi: 10.2337/dc17-0592. [DOI] [PubMed] [Google Scholar]
- 34.Yang F, Liang H, Chen J, Miao M, Yuan W, Nørgaard M, et al. Prenatal paternal selective serotonin reuptake inhibitors use and risk of ADHD in offspring. Pediatrics. 2018;141:e20171081. doi: 10.1542/peds.2017-1081. [DOI] [PubMed] [Google Scholar]
- 35.Buck Louis GM, Bell E, Xie Y, Sundaram R, Yeung E. Parental health status and infant outcomes: upstate KIDS study. Fertil Steril. 2018;109:315–323. doi: 10.1016/j.fertnstert.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viktorin A, Levine SZ, Altemus M, Reichenberg A, Sandin S. Paternal use of antidepressants and offspring outcomes in Sweden: nationwide prospective cohort study. BMJ. 2018;361:k2233. doi: 10.1136/bmj.k2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Midtvedt K, Bergan S, Reisæter AV, Vikse BE, Åsberg A. Exposure to mycophenolate and fatherhood. Transplantation. 2017;101:e214–e217. doi: 10.1097/TP.0000000000001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez-Lopez I, Rodelo-Haad C, Agüera ML, Cabello-Jabalquinto R, Esquivias-Motta E, Navarro MD, et al. Administration of mycophenolic acid is not associated with malformations in descendants from kidney transplanted males. PLoS One. 2018;13:e0202589. doi: 10.1371/journal.pone.0202589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallenius M, Lie E, Daltveit AK, Salvesen KÅ, Skomsvoll JF, Kalstad S, et al. No excess risks in offspring with paternal preconception exposure to disease-modifying antirheumatic drugs. Arthritis Rheumatol. 2015;67:296–301. doi: 10.1002/art.38919. [DOI] [PubMed] [Google Scholar]
- 40.Yang F, Yuan W, Liang H, Song X, Yu Y, Gelaye B, et al. Preconceptional paternal antiepileptic drugs use and risk of congenital anomalies in offspring: a nationwide cohort study. Eur J Epidemiol. 2019;34:651–660. doi: 10.1007/s10654-019-00509-2. [DOI] [PubMed] [Google Scholar]
- 41.Tang Q, Pan F, Wu X, Nichols CE, Wang X, Xia Y, et al. Semen quality and cigarette smoking in a cohort of healthy fertile men. Environ Epidemiol. 2019;3:e055. doi: 10.1097/EE9.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rehman R, Zahid N, Amjad S, Baig M, Gazzaz ZJ. Relationship between smoking habit and sperm parameters among patients attending an infertility clinic. Front Physiol. 2019;10:1356. doi: 10.3389/fphys.2019.01356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaur DS, Talekar MS, Pathak VP. Alcohol intake and cigarette smoking: impact of two major lifestyle factors on male fertility. Indian J Pathol Microbiol. 2010;53:35–40. doi: 10.4103/0377-4929.59180. [DOI] [PubMed] [Google Scholar]
- 44.Borges E, Jr, Braga DPAF, Provenza RR, Figueira RCS, Iaconelli A, Jr, Setti AS. Paternal lifestyle factors in relation to semen quality and in vitro reproductive outcomes. Andrologia. 2018;50:e13090. doi: 10.1111/and.13090. [DOI] [PubMed] [Google Scholar]
- 45.Pichini S, De Luca R, Pellegrini M, Marchei E, Rotolo MC, Spoletini R, et al. Hair and urine testing to assess drugs of abuse consumption in couples undergoing assisted reproductive technology (ART) Forensic Sci Int. 2012;218:57–61. doi: 10.1016/j.forsciint.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 46.Kasman AM, Thoma ME, McLain AC, Eisenberg ML. Association between use of marijuana and time to pregnancy in men and women: findings from the National Survey of Family Growth. Fertil Steril. 2018;109:866–871. doi: 10.1016/j.fertnstert.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Wise LA, Wesselink AK, Mikkelsen EM, Cueto H, Hahn KA, Rothman KJ, et al. Dairy intake and fecundability in 2 preconception cohort studies. Am J Clin Nutr. 2017;105:100–110. doi: 10.3945/ajcn.116.138404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nassan FL, Arvizu M, Mínguez-Alarcón L, Gaskins AJ, Williams PL, Petrozza JC, et al. Marijuana smoking and outcomes of infertility treatment with assisted reproductive technologies. Hum Reprod. 2019;34:1818–1829. doi: 10.1093/humrep/dez098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nassan FL, Arvizu M, Mínguez-Alarcón L, Williams PL, Attaman J, Petrozza J, et al. Marijuana smoking and markers of testicular function among men from a fertility centre. Hum Reprod. 2019;34:715–723. doi: 10.1093/humrep/dez002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buck Louis GM, Barr DB, Kannan K, Chen Z, Kim S, Sundaram R. Paternal exposures to environmental chemicals and time-to-pregnancy: overview of results from the LIFE study. Andrology. 2016;4:639–647. doi: 10.1111/andr.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tielemans E, van Kooij R, Looman C, Burdorf A, te Velde E, Heederik D. Paternal occupational exposures and embryoimplantation rates after IVF. Fertil Steril. 2000;74:690–695. doi: 10.1016/s0015-0282(00)00720-2. [DOI] [PubMed] [Google Scholar]
- 52.Dodge LE, Williams PL, Williams MA, Missmer SA, Toth TL, Calafat AM, et al. Paternal urinary concentrations of parabens and other phenols in relation to reproductive outcomes among couples from a fertility clinic. Environ Health Perspect. 2015;123:665–671. doi: 10.1289/ehp.1408605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campagna M, Satta G, Fadda D, Pili S, Cocco P. Male fertility following occupational exposure to dichlorodiphenyltrichloroethane (DDT) Environ Int. 2015;77:42–47. doi: 10.1016/j.envint.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 54.Accordini S, Calciano L, Johannessen A, Portas L, Benediktsdóttir B, Bertelsen RJ, et al. A three-generation study on the association of tobacco smoking with asthma. Int J Epidemiol. 2018;47:1106–1117. doi: 10.1093/ije/dyy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magnus MC, Tapia G, Olsen SF, Granstrom C, Mårild K, Ueland PM, et al. Lifeways Cross-Generation Cohort Study Group. Parental smoking and risk of childhood-onset type 1 diabetes. Epidemiology. 2018;29:848–856. doi: 10.1097/EDE.0000000000000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mejia-Lancheros C, Mehegan J, Murrin CM, Kelleher CC Lifeways Cross-Generation Cohort Study Group. Smoking habit from the paternal line and grand-child's overweight or obesity status in early childhood: prospective findings from the lifeways cross-generation cohort study. Int J Obes (Lond) 2018;42:1853–1870. doi: 10.1038/s41366-018-0039-8. [DOI] [PubMed] [Google Scholar]
- 57.Karalexi MA, Dessypris N, Thomopoulos TP, Ntouvelis E, Kantzanou M, Diamantaras AA, et al. Parental alcohol consumption and risk of leukemia in the offspring: a systematic review and meta-analysis. Eur J Cancer Prev. 2017;26:433–441. doi: 10.1097/CEJ.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 58.Fang SY, Huang N, Tsay JH, Chang SH, Chen CY. Excess mortality in children born to opioid-addicted parents: a national register study in Taiwan. Drug Alcohol Depend. 2018;183:118–126. doi: 10.1016/j.drugalcdep.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 59.Lindblad F, Ringbäck Weitoft G, Hjern A. Maternal and paternal psychopathology increases risk of offspring ADHD equally. Epidemiol Psychiatr Sci. 2011;20:367–372. doi: 10.1017/s2045796011000564. [DOI] [PubMed] [Google Scholar]
- 60.Nieuwenhuijsen MJ, Dadvand P, Grellier J, Martinez D, Vrijheid M. Environmental risk factors of pregnancy outcomes: a summary of recent meta-analyses of epidemiological studies. Environ Health. 2013;12:6. doi: 10.1186/1476-069X-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen S, Yang Y, Qv Y, Zou Y, Zhu H, Gong F, et al. Paternal exposure to medical-related radiation associated with low birthweight infants: a large population-based, retrospective cohort study in rural China. Medicine (Baltimore) 2018;97:e9565. doi: 10.1097/MD.0000000000009565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sallmén M, Suvisaari J, Lindbohm ML, Malaspina D, Opler MG. Paternal occupational lead exposure and offspring risks for schizophrenia. Schizophr Res. 2016;176:560–565. doi: 10.1016/j.schres.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 63.Jørgensen KT, Jensen MS, Toft GV, Larsen AD, Bonde JP, Hougaard KS. Risk of cryptorchidism among sons of horticultural workers and farmers in Denmark. Scand J Work Environ Health. 2014;40:323–330. doi: 10.5271/sjweh.3399. [DOI] [PubMed] [Google Scholar]
- 64.Brandt JS, Cruz Ithier MA, Rosen T, Ashkinadze E. Advanced paternal age, infertility, and reproductive risks: a review of the literature. Prenat Diagn. 2019;39:81–87. doi: 10.1002/pd.5402. [DOI] [PubMed] [Google Scholar]
- 65.Gunes S, Hekim GN, Arslan MA, Asci R. Effects of aging on the male reproductive system. J Assist Reprod Genet. 2016;33:441–454. doi: 10.1007/s10815-016-0663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toriello HV, Meck JM. Statement on guidance for genetic counseling in advanced paternal age. Genet Med. 2008;10:457–460. doi: 10.1097/GIM.0b013e318176fabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Q, Wen SW, Leader A, Chen XK, Lipson J, Walker M. Paternal age and birth defects: How strong is the association? Hum Reprod. 2007;22:696–701. doi: 10.1093/humrep/del453. [DOI] [PubMed] [Google Scholar]
- 68.Anderson RE, Hanson HA, Thai D, Zhang C, Presson AP, Aston KI, et al. Do paternal semen parameters influence the birth weight or BMI of the offspring? A study from the Utah Population Database. J Assist Reprod Genet. 2018;35:793–799. doi: 10.1007/s10815-018-1154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oldereid NB, Wennerholm UB, Pinborg A, Loft A, Laivuori H, Petzold M, et al. The effect of paternal factors on perinatal and paediatric outcomes: a systematic review and meta-analysis. Hum Reprod Update. 2018;24:320–389. doi: 10.1093/humupd/dmy005. [DOI] [PubMed] [Google Scholar]
- 70.Durairajanayagam D. Lifestyle causes of male infertility. Arab J Urol. 2018;16:10–20. doi: 10.1016/j.aju.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16:293–311. doi: 10.1093/humupd/dmp047. [DOI] [PubMed] [Google Scholar]
- 72.Vanbillemont G, Lapauw B, De Naeyer H, Roef G, Kaufman JM, Taes YE. Sex hormone-binding globulin at the crossroad of body composition, somatotropic axis and insulin/glucose homeostasis in young healthy men. Clin Endocrinol (Oxf) 2012;76:111–118. doi: 10.1111/j.1365-2265.2011.04155.x. [DOI] [PubMed] [Google Scholar]
- 73.Polito M, Conti A, Tiroli M, Capece M, Muzzonigro G. Diet and male infertility. J Androl Sci. 2011;18:60–63. [Google Scholar]
- 74.Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Hekmatdoost A. Intake of food groups and idiopathic asthenozoospermia: a case-control study. Hum Reprod. 2012;27:3328–3336. doi: 10.1093/humrep/des311. [DOI] [PubMed] [Google Scholar]
- 75.Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27:2899–2907. doi: 10.1093/humrep/des298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rato L, Alves MG, Cavaco JE, Oliveira PF. High-energy diets: a threat for male fertility? Obes Rev. 2014;15:996–1007. doi: 10.1111/obr.12226. [DOI] [PubMed] [Google Scholar]
- 77.Chavarro JE, Furtado J, Toth TL, Ford J, Keller M, Campos H, et al. Trans-fatty acid levels in sperm are associated with sperm concentration among men from an infertility clinic. Fertil Steril. 2011;95:1794–1797. doi: 10.1016/j.fertnstert.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jensen TK, Heitmann BL, Blomberg Jensen M, Halldorsson TI, Andersson AM, Skakkebæk NE, et al. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am J Clin Nutr. 2013;97:411–418. doi: 10.3945/ajcn.112.042432. [DOI] [PubMed] [Google Scholar]
- 79.Chavarro JE, Mínguez-Alarcón L, Mendiola J, Cutillas-Tolín A, López-Espín JJ, Torres-Cantero AM. Trans fatty acid intake is inversely related to total sperm count in young healthy men. Hum Reprod. 2014;29:429–440. doi: 10.1093/humrep/det464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.MInguez-Alarcón L, Chavarro JE, Mendiola J, Roca M, Tanrikut C, Vioque J, et al. Fatty acid intake in relation to reproductive hormones and testicular volume among young healthy men. Asian J Androl. 2017;19:184–190. doi: 10.4103/1008-682X.190323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Afeiche MC, Gaskins AJ, Williams PL, Toth TL, Wright DL, Tanrikut C, et al. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. J Nutr. 2014;144:1091–1098. doi: 10.3945/jn.113.190173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu CY, Chou YC, Chao JC, Hsu CY, Cha TL, Tsao CW. The association between dietary patterns and semen quality in a general Asian population of 7282 males. PLoS One. 2015;10:e0134224. doi: 10.1371/journal.pone.0134224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salas-Huetos A, Bulló M, Salas-Salvadó J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update. 2017;23:371–389. doi: 10.1093/humupd/dmx006. [DOI] [PubMed] [Google Scholar]
- 84.Dunford AR, Sangster JM. Maternal and paternal periconceptional nutrition as an indicator of offspring metabolic syndrome risk in later life through epigenetic imprinting: a systematic review. Diabetes Metab Syndr. 2017;11 Suppl 2:S655–S662. doi: 10.1016/j.dsx.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 85.Soubry A, Murphy SK, Wang F, Huang Z, Vidal AC, Fuemmeler BF, et al. Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int J Obes (Lond) 2015;39:650–657. doi: 10.1038/ijo.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coward RM, Kovac JR, Smith RP, Lipshultz LI. Fertility preservation in young men treated for malignancies: options for precancer treatment. Sex Med Rev. 2013;1:123–134. doi: 10.1002/smrj.13. [DOI] [PubMed] [Google Scholar]
- 87.Paoli D, Gallo M, Rizzo F, Spanò M, Leter G, Lombardo F, et al. Testicular cancer and sperm DNA damage: short- and long-term effects of antineoplastic treatment. Andrology. 2015;3:122–128. doi: 10.1111/j.2047-2927.2014.00250.x. [DOI] [PubMed] [Google Scholar]
- 88.Pourmasumi S, Sabeti P, Rahiminia T, Mangoli E, Tabibnejad N, Talebi AR. The etiologies of DNA abnormalities in male infertility: an assessment and review. Int J Reprod Biomed. 2017;15:331–344. [PMC free article] [PubMed] [Google Scholar]
- 89.Lydholm CN, Köhler-Forsberg O, Nordentoft M, Yolken RH, Mortensen PB, Petersen L, et al. Parental infections before, during, and after pregnancy as risk factors for mental disorders in childhood and adolescence: a nationwide Danish study. Biol Psychiatry. 2019;85:317–325. doi: 10.1016/j.biopsych.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 90.Safarinejad MR. Evaluation of endocrine profile, hypothalamic-pituitary-testis axis and semen quality in multiple sclerosis. J Neuroendocrinol. 2008;20:1368–1375. doi: 10.1111/j.1365-2826.2008.01791.x. [DOI] [PubMed] [Google Scholar]
- 91.Chen D, Zhang JP, Jiang L, Liu H, Shu L, Zhang Q, et al. Factors that influence in vitro fertilization treatment outcomes of Chinese men: a cross-sectional study. Appl Nurs Res. 2016;32:222–226. doi: 10.1016/j.apnr.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 92.Harlev A, Agarwal A, Gunes SO, Shetty A, du Plessis SS. Smoking and male infertility: an evidence-based review. World J Mens Health. 2015;33:143–160. doi: 10.5534/wjmh.2015.33.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bundhun PK, Janoo G, Bhurtu A, Teeluck AR, Soogund MZS, Pursun M, et al. Tobacco smoking and semen quality in infertile males: a systematic review and meta-analysis. BMC Public Health. 2019;19:36. doi: 10.1186/s12889-018-6319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mostafa RM, Nasrallah YS, Hassan MM, Farrag AF, Majzoub A, Agarwal A. The effect of cigarette smoking on human seminal parameters, sperm chromatin structure and condensation. Andrologia. 2018;50:e12910. doi: 10.1111/and.12910. [DOI] [PubMed] [Google Scholar]
- 95.Ou Y, Mai J, Zhuang J, Liu X, Wu Y, Gao X, et al. Risk factors of different congenital heart defects in Guangdong, China. Pediatr Res. 2016;79:549–558. doi: 10.1038/pr.2015.264. [DOI] [PubMed] [Google Scholar]
- 96.Baldacci S, Gorini F, Santoro M, Pierini A, Minichilli F, Bianchi F. Environmental and individual exposure and the risk of congenital anomalies: a review of recent epidemiological evidence. Epidemiol Prev. 2018;42(3-4 Suppl 1):1–34. doi: 10.19191/EP18.3-4.S1.P001.057. [DOI] [PubMed] [Google Scholar]
- 97.Beal MA, Yauk CL, Marchetti F. From sperm to offspring: assessing the heritable genetic consequences of paternal smoking and potential public health impacts. Mutat Res. 2017;773:26–50. doi: 10.1016/j.mrrev.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 98.McBride N, Johnson S. Fathers' role in alcohol-exposed pregnancies: systematic review of human studies. Am J Prev Med. 2016;51:240–248. doi: 10.1016/j.amepre.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 99.Fronczak CM, Kim ED, Barqawi AB. The insults of illicit drug use on male fertility. J Androl. 2012;33:515–528. doi: 10.2164/jandrol.110.011874. [DOI] [PubMed] [Google Scholar]
- 100.Gundersen TD, Jørgensen N, Andersson AM, Bang AK, Nordkap L, Skakkebæk NE, et al. Association between use of marijuana and male reproductive hormones and semen quality: a study among 1,215 healthy young men. Am J Epidemiol. 2015;182:473–481. doi: 10.1093/aje/kwv135. [DOI] [PubMed] [Google Scholar]
- 101.Pereira CM, Pacagnella RC, Parpinelli MA, Andreucci CB, Zanardi DM, Souza R, et al. Drug use during pregnancy and its consequences: a nested case control study on severe maternal morbidity. Rev Bras Ginecol Obstet. 2018;40:518–526. doi: 10.1055/s-0038-1667291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.International Labour Organization. Recommendation R197 - promotional framework for occupational safety and health recommendation (No. 197) [Internet] Geneva: International Labour Organization; c2006. [cited 2020 Apr 23]. Available from: https://www.ilo.org/dyn/normlex/en/f?p=NORMLEXPUB:12100:0::NO::P12100_INSTRUMENT_ID:312534. [Google Scholar]
- 103.Durairajanayagam D, Agarwal A, Ong C. Causes, effects and molecular mechanisms of testicular heat stress. Reprod Biomed Online. 2015;30:14–27. doi: 10.1016/j.rbmo.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 104.Hooiveld M, Haveman W, Roskes K, Bretveld R, Burstyn I, Roeleveld N. Adverse reproductive outcomes among male painters with occupational exposure to organic solvents. Occup Environ Med. 2006;63:538–544. doi: 10.1136/oem.2005.026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Messerlian C, Mustieles V, Minguez-Alarcon L, Ford JB, Calafat AM, Souter I, et al. Preconception and prenatal urinary concentrations of phenols and birth size of singleton infants born to mothers and fathers from the Environment and Reproductive Health (EARTH) study. Environ Int. 2018;114:60–68. doi: 10.1016/j.envint.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]