Abstract
Male factor infertility accounts for about 50% of the incidence of infertility in couples. In current practice, the men must attend a clinic or hospital facility to provide a semen analysis, which is key to the diagnosis of the male reproductive potential. However, many men are often embarrassed with the process and conventional semen analysis requires complex, labor intensive inspection with a microscope. To mitigate these problems, one of the solutions can be at-home semen analysis. In this review we examine the literature of currently available at home semen analysis test kits, describe their limitations, and compare them to the conventional lab-based methods.
Keywords: Infertility, male; Reproductive technology; Semen analysis; Sperm count
INTRODUCTION
Infertility has been a consistent problem that currently still affects approximately 10% to 20% of reproductive-aged couples worldwide [1,2,3]. Male factor has been found to be responsible for an estimated 40% to 50% of all infertility cases. Male factor infertility is often attributed to poor semen quality with suboptimal sperm motility, limited concentration, or abnormal morphology [1,2,4]. Through evaluation and subsequent treatment, reversible causes of male factor infertility may be determined, which can present a more cost-effective treatment option for couples than immediately employing assisted reproductive technology [4]. Standard semen analysis, in accordance with World Health Organization (WHO) criteria, is commonly done to evaluate the semen parameters in order to assess the male fertility potential. However, standard semen analysis is complex, laborious, time-consuming, and can even be stressful for many patients due to the cost as well as feelings of embarrassment, which may prevent them from seeking medical attention for infertility [5,6,7,8,9,10]. Conversely, at home semen analysis kits can alleviate financial burden and allay concerns with privacy and embarrassment while providing a valuable diagnostic tool for patients who may suffer from male factor infertility that is more convenient than conventional semen analysis.
Regarding the collection of the semen sample, studies have either found no difference between semen parameters in samples collected at home versus in the clinical setting or an improvement in semen quality for samples collected at home, indicating a potential benefit of at home assays for infertility investigation. Moreover, reported satisfaction levels of at home semen collection were higher, and this would be the method relied upon with at home kits [11,12,13]. In a retrospective study of post vasectomy semen analysis compliance, patients reported distance, time commitment, and forgetfulness as primary barriers to completing their semen testing, and 92% of the 503 patients indicated home-based semen analysis would increase their compliance [14]. Furthermore, ease of use for laypeople of home semen analysis kits such as the Trak system has been demonstrated, while other methods of testing incorporate smartphones which can increase accessibility for patients [15,16,17,18]. Convenience, lower cost, and avoidance of potential social stigma and embarrassment make at home semen assays a broadly appealing option for investigating infertility. Herein, we recognize and acknowledge this review is similar to a review performed by Yu et al [5] and Kobori [6], but what differentiates this review is we provided insight into the principle interplaying within each respective kit, whether the kit gained US Food and Drug Administration (FDA) credibility, as well as discuss the limitations according to WHO criteria.
1. Standard semen analysis
The WHO laboratory manual provides standards for semen analysis, including for sample collection, initial macroscopic examination, initial microscopic examination, and for testing sperm motility, concentration, and morphology [19]. In order to meet the standards and guidelines, intensive laboratory training and quality control programs are needed. Unfortunately, methodology lapses lead to mistakes and some laboratories, in order to save time labor and expenses, use methods other than the standard [20,21]. For example, some laboratories utilize different chambers or inadequate dilutions for pipetting, which leads to false results. Furthermore, sperm motility assessment also presents a challenge in the subjective nature of gamete velocity, as well as the standardized time elapsed between sample collection and the result [22,23]. In response to the abovementioned difficulties in the manual seminological assessement, the first computer system for an automatized sperm analysis, or computer assisted semen analysis (CASA), was designed. CASA is able to visualize and estimate sperm concentration, velocity and morphology utilizing a sophisticated electronic imaging system and software algorithm. Repeatability of the measurements and their objectivism constitute a potential advantage of the CASA. However, despite these advantages, the CASA system retails at a costly $30,000 to $40,000, depending on the model purchased.
DEVICES FOR HOME BASED SEMEN ANALYSIS
1. SpermCheck Fertility
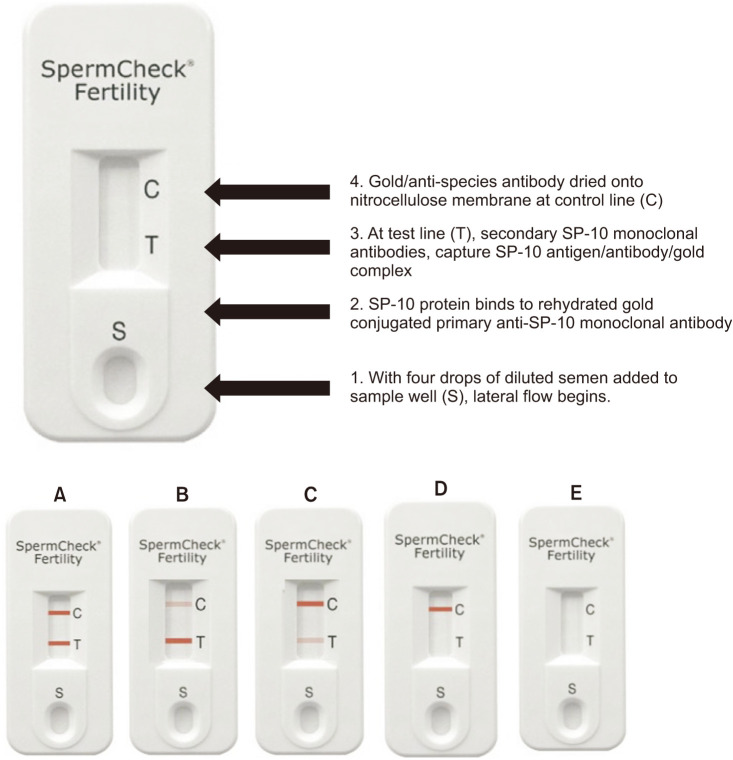
The SpermCheck Fertility test (Princeton BioMeditech Corp., Monmouth Junction, NJ, USA) is a rapid qualitative test that employs two solid-phase chromatographic immunoassays within a single cassette. One test strip is calibrated to give a positive result if the sperm concentration is 2×107 sperm/mL or greater. The device employs a pair of monoclonal antibodies that bind distinct epitopes on the sperm acrosomal antigen SP-10, which is readily released from the sperm head into a detergent containing buffer upon semen addition. SP-10 mRNA and protein has been validated as a selective analyte, since its only expressed in the testis, and not expressed in other organs [24,25,26]. The principle underlying the operation of the device is shown in Fig. 1 and the representative test results with their interpretation are described in Fig. 1.
Fig. 1. SpermCheck Fertility device and results. Device is shown before a sample has been added. Following a 20 minute semen liquefaction period, 100 µL of semen is sampled and mixed with a detergent that solubilizes the sperm's acrosomal membranes, releasing SP-10 protein. Four drops of the solution are applied to the sample well (1). Colloidal gold-conjugated monoclonal SP-10 antibodies present on the absorbent pad bind to the solubilized SP-10 antigen (2). The solution migrates along the nitrocellulose strips by capillary action. At test line (T), a second monoclonal antibody captures the gold-antibody-SP-10 complex, resulting in the appearance of a red line at this position (3). The appearance of lines at the control position indicates the device functioned properly. Diagnostic devices showing a concentration over 20 million (A–C), below 20 million (D), and a type of invalid test result (E). Presence of red control line must appear to ensure result is valid and test fluid has completely flowed over the test line, which must appear within the 7-minute assay period.
The SpermCheck Fertility Test, the first FDA approved at-home screening test for men with normozoospermia or oligozoospermia, has a reported accuracy of 98% and reads out results within 10 minutes (Table 1). In addition, a similar testing device designed for post-vasectomy patients, SpermCheck® Vasectomy Fertility (Princeton BioMeditech Corp.), is calibrated to detect <250,000 sperm, providing the possibility of a useful alternative to standard postvasectomy sperm monitoring. However, Andrusier et al [27] observed that home semen analysis kits failed to significantly improve compliance and suggested that there be partner involvement. Furthermore, its sensitivity is 93% and specificity is 97% (Table 1) [28]. Although the SpermCheck Test is relatively quick and easy to interpret, it doesn't provide information on other parameters, such as motility, volume, and morphology.
Table 1. Summary of available home semen tests.
| Test | Method | Parameters tested | Categories tested | Accuracy (%) | Parameters missing | Cost | No. of tests per kit | Time until result (min) | FDA approval? |
|---|---|---|---|---|---|---|---|---|---|
| SpermCheck Fertility | Immunochromato-graphic assay | Concentration | 20 million | 98 | Motility, semen volume, Morphology, pH | $39.99 | 2 | 30 | Yes; 2010 |
| SpermCheck Vasectomy | Immunochromato-graphic assay | >250,000 | Sensitivity 93 | Motility, semen volume, morphology, pH | $59.99 | 2 | 30 | ||
| Specificity 97 | |||||||||
| Trak | Centrifuge | Concentration & volume | <15 million/mL | 93.3 | Motility, morphology, pH | $89.99 | 2 | 36 | Yes; 2016 |
| 15–55million/mL | 82.4 | $149.99 | 4 | ||||||
| >55 million/mL | 95.5 | $199.99 | 6 | ||||||
| SwimCount | Colorimetric reaction (3-(4,5-di-methyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide,yellow tetrazole dye) | Progressively motile sperm concentration | <5 million | Accuracy 95 | Morphology, pH Total count | $57 | 1 | 60 | Yes; 2019 |
| 5–20 million | Sensitivity 88 | ||||||||
| >20 million | Specificity 74 | ||||||||
| FertilitySCORE | Colorimetric reaction (Resazuin dye) | Motile sperm concentration | <20 million/mL | 93 | Morphology Total count | GBP £19.95 ($25) | 2 | 90 | No; available in UK |
| >20 million/mL | |||||||||
| Men's Loupe | Ball Len's microscope with smartphone | Concentration & motility | No categories, manual counting required | Sensitivity 87.5 | pH, morphology | $15 | 4 | 5 | No; product only available in Japan |
| User has to manually count motile & non-motile sperm | Specificity 90.9 | ||||||||
| SEEM | Smartphone based device with optical tracking | Concentration & motility | No categories | Concentration: r=0.382 | Semen volume, morphology, pH | $45 | 2 | 15–30 | No |
| Motility: r=0.594 | |||||||||
| Yo sperm clip | Smartphone based device with optical tracking and internal algorithm | Motile sperm concentration | <6 million/mL | iPhone 7: 98.3 | Volume, concentration, morphology, pH | Mobile: $69.95 | 2 | 10–15 | Yes; 2017 |
| >6 million/mL | Galaxy S2: 97.2 | Mac Window: $69.95 | |||||||
| ExSeed | Smartphone based device with optical tracking | Semen volume | - | - | Morphology | £74.99 ($82) | 2 | 15–20 | No; only available in Denmark & UK |
| Concentration | pH | £149.99 ($199.99) | 5 | ||||||
| Motility | |||||||||
| Total motile sperm count | |||||||||
| Point of care automated-smartphone based system by Kanakasabapathy et al [40] | Microfluidics & optical attachment for smartphone | Concentration | <100 million | Concentration: accuracy 97.1 | Morphology | ~$5 | - | ~1 | No; pre-clinical testing |
| Motility | >100 million | HBA: accuracy 87, sensitivity 100, specificity 69, sperm viability (4.9%)a; 1.2b, DNA fragmentation (5.7%)a; 0.7b | pH | ||||||
| Volume | |||||||||
| Hyaluronic binding assay (HBA) & Sperm viability | |||||||||
| DNA fragmentation |
FDA: US Food and Drug Administration, -: data not provided.
aPercentages reported as standard-deviations. bAbsolute mean bias evaluated by Bland-Altman analysis.
2. Trak
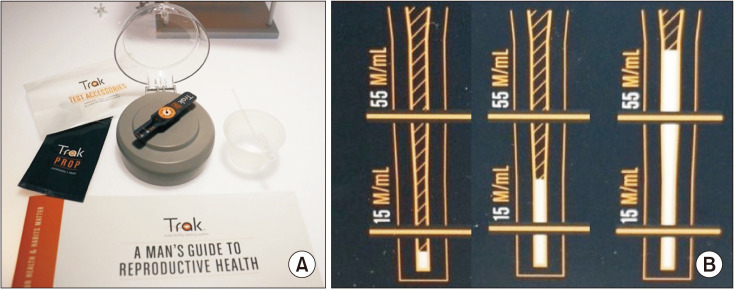
The Trak Male Fertility Testing system (Sandstone Diagnostics, Inc., Livermore CA, USA), shown in Fig. 2, is an FDA 510(k) cleared, small portable device that utilizes centrifugal motion to determine sperm cell count. The system components and operation of the device, as well as example results are shown in Fig. 2, respectively. The device provides a linear sperm concentration with two marks of delineation, 15 million sperm/mL and 55 million sperm/mL, and delivers measurements within three categories, as shown in Fig. 2B: low (<15 million sperm/mL), moderate (15–55 million sperm/mL), and optimal (>55 million/mL). This categorical approach combines the WHO threshold and evidence based reference for a faster time to pregnancy [29]. According to Schaff et al [16], the device achieved an accuracy of 93.3% for results categorized <15 million/mL, 82.4% for results 15–55 million/mL, and 95.5% for results >55 million/mL compared to CASA measurements (Table 1). The device retails for $89.99 for two kits and has a companion mobile application, which provides personalized recommendations on lifestyle changes aimed at improving sperm concentration [16]. Although Trak's linear test results may enable longitudinal measurements, the device only indicates the range of only two semen parameters; volume and concentration, failing to analyze other parameters.
Fig. 2. (A) Trak Male Fertility Testing system components and operation. The system contents include a reusable engine, single-use test cartridges (Props), fluid transfer device, liquefaction cups. Photo shows a prop placed on the engine. To operate the system, the user collects a semen sample in an enzyme-coated collection cup that promotes liquefaction. Then, the user transfers 0.25 mL of semen to the Prop inlet chamber, attaches the Prop to the engine, and closes the lid to initiate the spin sequence, which runs for 6 minutes at 7,000 rpm. When the spin sequence is complete, the sperm cells form a visible, measurable white column that's proportional to the concentration of sperm. (B) Test results of samples with low (<15 million/mL), moderate (15–55 million/mL), and optimal (>55 million/mL) sperm concentrations.
3. SwimCount Sperm Quality test and FertilitySCORE
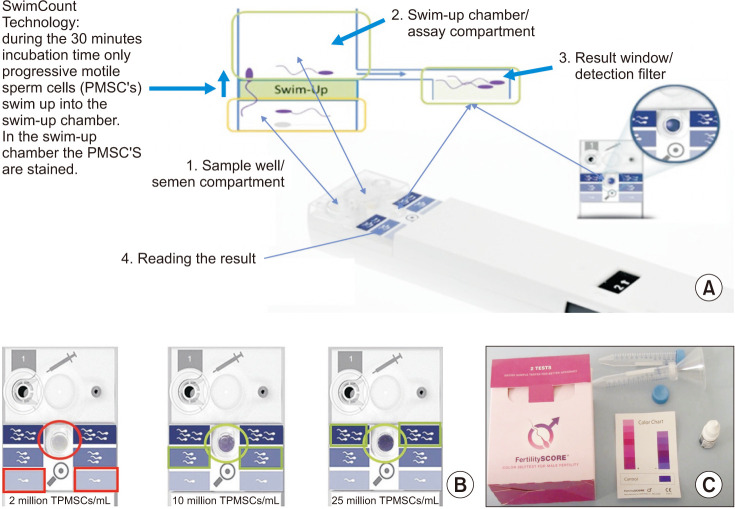
SwimCount Sperm Quality test is a kit that tests the concentration of progressively motile sperm cells within a range when the sperm concentration exceeds a threshold. The principle underlying the operation of the device is shown in Fig. 3A. The “swim-up” technology employed sorts progressively motile spermatozoa as well as spermatozoa with low DNA fragmentation [30]. The SwimCount Sperm Quality is calibrated to give three categorized results, displayed by a light to dark purple color range indicating increased sperm density, respectively. Furthermore, if the motile sperm concentration is below 5 million motile sperm/mL the categorized result will be indicated with the lightest color. Additionally, a sample that contains between 5 million motile sperm/mL and 20 million/mL will result in the middle color (Fig. 3B). If the sample is 20 million sperm/mL or higher, the categorized result will be the darkest of the three colors. Compared with manual microscope methods, and the SwimCount Sperm Quality test has an accuracy of 95% (Table 1) [31]. Comparing 5th generation WHO subnormal semen parameters, defined as below the threshold count of 5 million/mL total progressively motile sperm concentration, the SwimCount's sensitivity and specificity were 88% and 74%, respectively (Table 1) [32]. This kit retails for EUR €49.99 ($57) and has an advantage for men who want to investigate their potential fertility status by giving more detailed information on sperm motility parameters than the Trak and SpermCheck home kits.
Fig. 3. Principles SwimCount and FertilitySCORE device and results of SwimCount shown in results window. (A) Total progressively motile sperm concentration (PMSC). The device is composed of two macro-chambers, which are separated by a filter with a pore of 10 µm. Only progressively motile spermatozoa, with normal morphology pass through this filter once the device is activated. A total of 30 minutes is required for complete dyeing of spermatozoa, utilizing a solution of consisting of phosphate-buffered saline and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide on top of the filter. (B) Example test results that show less than 5 million/mL, between 5–20 million/mL, and more than 20 million/mL total PMSC. (C) FertilitySCORE is based on the principle of metabotically active spermatozoa chemically change the structure of a Resazuin color dye from dark blue to pink and diagnosis whether the motile sperm concentration is 20 million sperm/mL or above.
Similar to the SwimCount Fertility test, the FertilitySCORE test kit measures motile sperm concentration utilizing a colorimetric dye (Fig. 3C). The kit is based on the principle that metabotically active spermatozoa produce reactive oxygen species and y-glutamyltransferase, which chemically changes the structure of a Resazuin color dye from dark blue to pink, and diagnoses whether the motile sperm concentration is 20 million sperm/mL or above [33]. The kit provides two samples and retails for GBP (British Pound) £19.95 ($25). Compared with CASA, the FertilitySCORE has an accuracy of 93% (Table 1) [33]. However, similar to other products, these tests are unable to provide information on parameters other than the concentration of motile sperm, and thus further testing is required.
4. Smartphone based at home semen analysis: Men's Loupe, SEEM, and YO clip
Smartphones have great potential to support medical devices because they are portable, ubiquitous, and can be attached to miniature microscope components, such as a ball lens [34]. Smartphone usage has been expanding rapidly over the past few years, and with the ability to capture more than 5 megapixels of distortion-free imaging over a broad range of magnifications, smartphones are understandably becoming an integral part of the healthcare system [35,36].
The Men's Loupe (Tenga Health Care, Torrance, CA, USA) consists of a 0.8 mm diameter ball lens microscope that is inserted into a plastic jacket, paired with a smartphone camera, and is quite economical, retailing for $15 (Fig. 4A). The ball lens provides an approximate magnification of 555 times, and does not require a dedicated light source, since it utilizes ambient illumination. The apparatus records images and requires manual analysis of the images for both sperm number and motility. The user then inputs the resulting number of motile sperm as well as his smartphone model on the product's website. Kobori et al [37] demonstrated that results by the Men's Loupe showed a strong correlation with CASA results, with a sensitivity of 87.5% and specificity of 90.9% (Table 1). An important limitation to the Men's Loupe is that the periphery of the images are not in focus and consequently do not match the high quality images offered by traditional CASA.
Fig. 4. Men's Loupe (Tenga Health Care) device and smartphone-based SEEM kit. (A) Men's Loupe 0.8 mm diameter ball lens microscope attached to smartphone. (B) Technique for loading semen sample into plastic jacket of ball lens microscope. (C) Magnifying lens semen analysis device with QR code sheet to download the application for operating. (D) Instructions of kit for use. (E) Screenshot of sample test results with concentration and motility.
Like the Men's Loupe (Tenga Health Care), the SEEM® (Recruit Lifestyle Co., Ltd., Tokyo, Japan) sperm self-check service measures the concentration and motility. The kit includes a magnifying lens substrate, paper scoop, collection cup, and a quick response ticket to be read in the smartphone's application, which can be downloaded for free only on the Apple iPhone's iOS store (Apple, Cupertino, CA, USA) (Fig. 4C). The application along with the smartphone records a video of the semen and links to the respective minimum reference numbers set by the WHO, 15 million/mL and 40% motility. Cheon et al [38], demonstrated that sperm concentration and motility measured with SEEM, positively correlated with laboratory-based CASA results, r=0.382 and r=0.594, respectively. The application gives users access to SEEM-lab, which is a portal site that provides informative articles regarding fertility. However, unlike the Men's Loupe, users aren't required to manually count the motile and non-motile sperm.
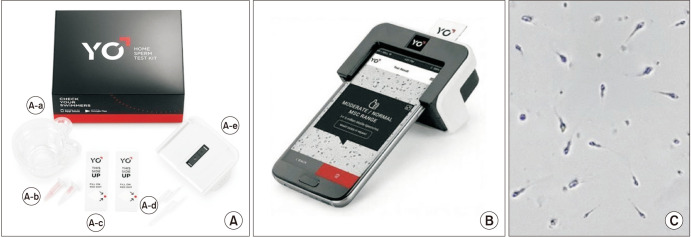
The YO Home Sperm test (Medical Electronics Systems, Los Angeles, CA, USA) entered consumer markets as the first FDA-cleared (K161493), video-based smartphone platform for Home Sperm testing (Fig. 5). The YO clip, a miniature microscope, uses the smartphone's camera and light source to analyze the light and pixel fluctuations caused by the sperm's motility. Utilizing the manufacturer's proprietary algorithms, the apparatus translates these movements into a composite motile sperm concentration and dichotomizes the results into “low” or “moderate/normal” based on the established 6 million/mL cutoff from the 2010 WHO guidelines (Table 1). Furthermore, users can download the YO application, which provides step-by-step instructions and allows users to track their “YO score” with repeat testing (Table 2) [17]. The accuracy of the YO Home Sperm test is 98.3% utilizing the iPhone 7 and 97.2% with the Galaxy S2 (Samsung, Suwon, Korea) [39]. The YO Home Sperm test is also available for Mac and Windows PC Desktops.
Fig. 5. The YO Home Sperm test device and components. (A) YO kit contents, including, (A-a) collection cup, (A-b) liquefaction powder, (A-c) fixed coverslip slide (d) fixed volume transfer pipettes, and (A-e) YO testing clip. (B) The assembled YO clip with inserted testing slide. (C) Sample as seen on the phone screen.
Table 2. YO score based on motile sperm concentration.
| MSC range (million/mL) | YO score | Grouping |
|---|---|---|
| 0–<6 | 0 | - |
| 6–14 | 10 | Low normal |
| 14–22 | 20 | - |
| 22–32 | 30 | - |
| 32–39 | 40 | Average normal |
| 39–51 | 50 | - |
| 51–63 | 60 | - |
| 63–83 | 70 | High normal |
MSC: motile sperm concentration, -: not applicable.
The ExSeed Home Sperm test is a video-based smartphone platform for Home Sperm testing and first entered consumer markets in Denmark and Norway in June 2019. The kit retails for £74.99 for two tests, and £149.99 for five tests. The kit includes five glass test slides, collection cup, and a quick response ticket to be read in the smartphone's application, which can be downloaded for free only on the Apple iPhone's iOS store (Fig. 6). The application gives users access to an informative lifestyle program that focuses on nutrition, exercise, and supplements. The kit measures total motile sperm count and gives a fertility score for interpretation. Unlike the Men's Loupe and SEEM, the ExSeed kit does not provide a magnifying lens substrate and participants must utilize the smartphone's camera and light source. Given its relative novelty, there are still no published studies validating its accuracy compared to the CASA system.
Fig. 6. The ExSeed Home Sperm test device and components. (A) ExSeed kit contents, including, (A-a) collection cup & transfer pipette, (A-b) ExSeed testing clip, and (A-c) fixed coverslip slide.
The point of care device developed by Kanakasabapathy et al [40], integrates microfluidics, optical sensing modality, and an automated smartphone device, which can provide a powerful platform for easily accessible point of care fertility diagnostic assays. Kanakasabapathy et al [40] demonstrated the ability of the smartphone-based semen analyzer to accurately (97.1%) classify semen samples with sperm counts below and above the 100,000 sperm/mL threshold set for postvasectomy monitoring. The total material cost to fabricate the smartphone accessory and the disposable microfluidic device was $4.45, including $3.59 for the optical attachment and $0.86 for the microfluidic device, making the device particularly affordable. Dimitriadis et al [18] demonstrated that such a smartphone system can also be adapted to accurately measure not only basic semen analysis, but also sperm function quantitatively by estimating the hyaluronic binding ssay (HBA) score, sperm viability, and sperm DNA fragmentation. Dimitriadis et al [18], measured HBA score through microfluidics, the eosin-nigrosin staining-based approach for sperm viability testing, and lastly the Halosperm kit, which is a sperm chromatin dispersion test for assessment of sperm DNA fragmentation. Furthermore, an adaptive thresholding algorithm was applied that made use of sharp gradients to separate the background and foreground for the sperm functionality tests. Compared to CASA results, the smartphone device demonstrated a sensitivity of 100%, specificity of 69.23%, and 87.1% accuracy with HBA (Table 1) [18]. Although conventional HBA assessments require technicians to measure the ratio of bound to unbound cells within an optical microscope, these preliminary results demonstrate an ideal point-of-care portable semen analyzer. When the smartphone device measured sperm viability, results demonstrated a mean bias of 1.2% with a standard deviation of 5.03% [18]. Further advances in software refinements and investigation with microfluidics can aid making these assays suitable.
LIMITATIONS
In their current iterations, home-based semen analysis devices cannot be regarded as a complete replacement of the standard semen analysis conducted in a laboratory setting, and certainly not a substitute for consultation with a fertility specialist. While the at-home semen assay kits we reviewed displayed a high level of accuracy with an affordable price, they only tested sperm concentration or motility. Sperm morphology and other important parameters impacting potential fertility are not tested. Thus, at home assays should not be recommended as the sole test for male factor infertility in couples seeking pregnancy. However, these kits can still be effectively used to detect possible seminal defects early on instead of spending extensive time waiting for natural pregnancy to occur [32]. In this regard, home-based semen analysis kits can serve the role of an indicator to pursue additional evaluation or fertility assistance for patients who do present with suboptimal sperm concentration or motility according to the home test. It should also be emphasized to consumers of these products the potential of false-negative results and that additional testing in clinic may still be necessary. Home-based semen analysis may have the greatest benefit for post-vasectomy patients where sperm count is of the most importance, through the option to test at home increasing compliance because of its convenience [14].
CONCLUSIONS
At-home semen analysis kits provide a rapid, cost-effective tool for evaluating fertility potential in couples seeking pregnancy that may be affected by male factor infertility. While the social stigma of seeking fertility treatment may not be as prevalent as it once was, home-based semen analysis still provides the privacy, convenience, and lower cost that are greatly appealing to men who may be unwilling to otherwise seek clinical evaluation. Technological advancements have allowed at-home semen analysis kits to be accurate and relatively easy to use for the typical patient. While a significant shortcoming of the at-home kits is their inability to assess all semen parameters that may contribute to infertility, they are still valuable in their capacity to test sperm concentration and motility, encourage further testing, and provide a convenient first step for men reluctant to evaluate their fertility clinically.
ACKNOWLEDGEMENTS
The authors thank Manuel Molina, Miller School of Medicine, University of Miami for his technical assistance for this study.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: RR, DG.
- Data curation: DG, MN, JCB, JO.
- Formal analysis: DG, MN, JCB, RR, JO.
- Methodology: DG, MN, JCB, RR, JO.
- Supervision: RR.
- Validation: RR, DG.
- Visualization: DG, RR.
- Writing — original draft: DG, MN, RR.
- Writing — review & editing: DG, MN, JCB, RR, JO.
References
- 1.Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci. 2015;8:191–196. doi: 10.4103/0974-1208.170370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dada R, Kumar R, Shamsi MB, Tanwar M, Pathak D, Venkatesh S, et al. Genetic screening in couples experiencing recurrent assisted procreation failure. Indian J Biochem Biophys. 2008;45:116–120. [PubMed] [Google Scholar]
- 3.Gill K, Jakubik J, Rosiak-Gill A, Kups M, Lukaszuk M, Kurpisz M, et al. Utility and predictive value of human standard semen parameters and sperm DNA dispersion for fertility potential. Int J Environ Res Public Health. 2019;16:2004. doi: 10.3390/ijerph16112004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glazer CH, Anderson-Bialis J, Anderson-Bialis D, Eisenberg ML. Evaluation, treatment, and insurance coverage for couples with male factor infertility in the US: a cross-sectional analysis of survey data. Urology. 2020;139:97–103. doi: 10.1016/j.urology.2019.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Yu S, Rubin M, Geevarughese S, Pino JS, Rodriguez HF, Asghar W. Emerging technologies for home-based semen analysis. Andrology. 2018;6:10–19. doi: 10.1111/andr.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobori Y. Home testing for male factor infertility: a review of current options. Fertil Steril. 2019;111:864–870. doi: 10.1016/j.fertnstert.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Raj V, Vijayan AN, Joseph K. Naked eye detection of infertility using fructose blue--a novel gold nanoparticle based fructose sensor. Biosens Bioelectron. 2014;54:171–174. doi: 10.1016/j.bios.2013.10.073. [DOI] [PubMed] [Google Scholar]
- 8.Björndahl L, Kirkman-Brown J, Hart G, Rattle S, Barratt CL. Development of a novel home sperm test. Hum Reprod. 2006;21:145–149. doi: 10.1093/humrep/dei330. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Swerdloff RS. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril. 2014;102:1502–1507. doi: 10.1016/j.fertnstert.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segerink LI, Sprenkels AJ, Oosterhuis GJ, Vermes I, van den Berg A. Microfluidic chips for semen analysis. EJIFCC. 2012;23:66–69. [PMC free article] [PubMed] [Google Scholar]
- 11.Wang JH, Muller CH, Lin K. Optimizing fertility preservation for pre- and postpubertal males with cancer. Semin Reprod Med. 2013;31:274–285. doi: 10.1055/s-0033-1345275. [DOI] [PubMed] [Google Scholar]
- 12.Licht RS, Handel L, Sigman M. Site of semen collection and its effect on semen analysis parameters. Fertil Steril. 2008;89:395–397. doi: 10.1016/j.fertnstert.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 13.Gao J, Duan YG, Yi X, Yeung WSB, Ng EHY. A randomised trial comparing conventional semen parameters, sperm DNA fragmentation levels and satisfaction levels between semen collection at home and at the clinic. Andrologia. 2020;52:e13628. doi: 10.1111/and.13628. [DOI] [PubMed] [Google Scholar]
- 14.Bradshaw A, Ballon-Landa E, Owusu R, Hsieh TC. Poor compliance with postvasectomy semen testing: analysis of factors and barriers. Urology. 2020;136:146–151. doi: 10.1016/j.urology.2019.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Sommer GJ, Wang TR, Epperson JG, Hatch EE, Wesselink AK, Rothman KJ, et al. At-home sperm testing for epidemiologic studies: evaluation of the Trak male fertility testing system in an internet-based preconception cohort. Paediatr Perinat Epidemiol. 2020;34:504–512. doi: 10.1111/ppe.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaff UY, Fredriksen LL, Epperson JG, Quebral TR, Naab S, Sarno MJ, et al. Novel centrifugal technology for measuring sperm concentration in the home. Fertil Steril. 2017;107:358–364. doi: 10.1016/j.fertnstert.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Vij SC, Panner Selvam MK, Agarwal A. Smartphone-based home screening tests for male infertility. Panminerva Med. 2019;61:104–107. doi: 10.23736/S0031-0808.18.03533-4. [DOI] [PubMed] [Google Scholar]
- 18.Dimitriadis I, Bormann CL, Kanakasabapathy MK, Thirumalaraju P, Kandula H, Yogesh V, et al. Automated smartphone-based system for measuring sperm viability, DNA fragmentation, and hyaluronic binding assay score. PLoS One. 2019;14:e0212562. doi: 10.1371/journal.pone.0212562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO) WHO laboratory manual for the examination and processing of human semen, 5th ed [Internet] Geneva: WHO; c2010. [cited 2020 Sep 19]. Available from: http://www.who.int/iris/handle/10665/44261. [Google Scholar]
- 20.Bailey E, Fenning N, Chamberlain S, Devlin L, Hopkisson J, Tomlinson M. Validation of sperm counting methods using limits of agreement. J Androl. 2007;28:364–373. doi: 10.2164/jandrol.106.002188. [DOI] [PubMed] [Google Scholar]
- 21.Mortimer ST, van der Horst G, Mortimer D. The future of computer-aided sperm analysis. Asian J Androl. 2015;17:545–553. doi: 10.4103/1008-682X.154312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walczak-Jedrzejowska R, Marchlewska K, Oszukowska E, Filipiak E, Bergier L, Slowikowska-Hilczer J. Semen analysis standardization: Is there any problem in Polish laboratories? Asian J Androl. 2013;15:616–621. doi: 10.1038/aja.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jedrzejczak P, Talarczyk J, Taszarek-Hauke G, Berger A, Hauke J, Pawelczyk L. [External quality assessment of semen analysis in Poland] Ginekol Pol. 2012;83:835–840. Polish. [PubMed] [Google Scholar]
- 24.Kurth BE, Klotz K, Flickinger CJ, Herr JC. Localization of sperm antigen SP-10 during the six stages of the cycle of the seminiferous epithelium in man. Biol Reprod. 1991;44:814–821. doi: 10.1095/biolreprod44.5.814. [DOI] [PubMed] [Google Scholar]
- 25.Kurth BE, Wright RM, Flickinger CJ, Herr JC. Stage-specific detection of mRNA for the sperm antigen SP-10 in human testes. Anat Rec. 1993;236:619–625. doi: 10.1002/ar.1092360405. [DOI] [PubMed] [Google Scholar]
- 26.Freemerman AJ, Wright RM, Flickinger CJ, Herr JC. Tissue specificity of the acrosomal protein SP-10: a contraceptive vaccine candidate molecule. Biol Reprod. 1994;50:615–621. doi: 10.1095/biolreprod50.3.615. [DOI] [PubMed] [Google Scholar]
- 27.Andrusier M, Punjani N, Hayden RP, Dudley V, Matos-Vargas E, Goldstein M. PD25-11: Home testing does not improve post-vasectomy semen analysis compliance. J Urol. 2020;203 Suppl 4:e542–e543. doi: 10.1097/UPJ.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 28.Klotz KL, Coppola MA, Labrecque M, Brugh VM, 3rd, Ramsey K, Kim KA, et al. Clinical and consumer trial performance of a sensitive immunodiagnostic home test that qualitatively detects low concentrations of sperm following vasectomy. J Urol. 2008;180:2569–2576. doi: 10.1016/j.juro.2008.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slama R, Eustache F, Ducot B, Jensen TK, Jørgensen N, Horte A, et al. Time to pregnancy and semen parameters: a cross-sectional study among fertile couples from four European cities. Hum Reprod. 2002;17:503–515. doi: 10.1093/humrep/17.2.503. [DOI] [PubMed] [Google Scholar]
- 30.Quinn MM, Jalalian L, Ribeiro S, Ona K, Demirci U, Cedars MI, et al. Microfluidic sorting selects sperm for clinical use with reduced DNA damage compared to density gradient centrifugation with swim-up in split semen samples. Hum Reprod. 2018;33:1388–1393. doi: 10.1093/humrep/dey239. [DOI] [PubMed] [Google Scholar]
- 31.Castello D, Garcia-Laez V, Buyru F, Bakiricioglu E, Ebbesen T, Gabrielsen A, et al. Comparison of the SwimCount home diagnostic test with conventional sperm analysis. Adv Androl Gynecol. 2018 doi: 10.29011/AAG-101.000001. [DOI] [Google Scholar]
- 32.Yoon YE, Kim TY, Shin TE, Lee E, Choi KH, Lee SR, et al. Validation of SwimCount™, a novel home-based device that detects progressively motile spermatozoa: correlation with World Health Organization 5th semen analysis. World J Mens Health. 2020;38:191–197. doi: 10.5534/wjmh.180095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zalata A, Hafez T, Mahmoud A, Comhaire F. Relationship between resazurin reduction test, reactive oxygen species generation, and gamma-glutamyltransferase. Hum Reprod. 1995;10:1136–1140. doi: 10.1093/oxfordjournals.humrep.a136106. [DOI] [PubMed] [Google Scholar]
- 34.Roy S, Pantanowitz L, Amin M, Seethala RR, Ishtiaque A, Yousem SA, et al. Smartphone adapters for digital photomicrography. J Pathol Inform. 2014;5:24. doi: 10.4103/2153-3539.137728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroemer S, Frühauf J, Campbell TM, Massone C, Schwantzer G, Soyer HP, et al. Mobile teledermatology for skin tumour screening: diagnostic accuracy of clinical and dermoscopic image tele-evaluation using cellular phones. Br J Dermatol. 2011;164:973–979. doi: 10.1111/j.1365-2133.2011.10208.x. [DOI] [PubMed] [Google Scholar]
- 36.Dendere R, Myburg N, Douglas TS. A review of cellphone microscopy for disease detection. J Microsc. 2015;260:248–259. doi: 10.1111/jmi.12307. [DOI] [PubMed] [Google Scholar]
- 37.Kobori Y, Pfanner P, Prins GS, Niederberger C. Novel device for male infertility screening with single-ball lens microscope and smartphone. Fertil Steril. 2016;106:574–578. doi: 10.1016/j.fertnstert.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 38.Cheon WH, Park HJ, Park MJ, Lim MY, Park JH, Kang BJ, et al. Validation of a smartphone-based, computer-assisted sperm analysis system compared with laboratory-based manual microscopic semen analysis and computer-assisted semen analysis. Investig Clin Urol. 2019;60:380–387. doi: 10.4111/icu.2019.60.5.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agarwal A, Panner Selvam MK, Sharma R, Master K, Sharma A, Gupta S, et al. Home sperm testing device versus laboratory sperm quality analyzer: comparison of motile sperm concentration. Fertil Steril. 2018;110:1277–1284. doi: 10.1016/j.fertnstert.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 40.Kanakasabapathy MK, Sadasivam M, Singh A, Preston C, Thirumalaraju P, Venkataraman M, et al. An automated smartphone-based diagnostic assay for point-of-care semen analysis. Sci Transl Med. 2017;9:eaai7863. doi: 10.1126/scitranslmed.aai7863. [DOI] [PMC free article] [PubMed] [Google Scholar]