Summary:
Objective:
To determine whether a less-invasive approach to surgery for medically-refractory temporal lobe epilepsy is associated with lower health care costs and costs of lost productivity over time, compared to open surgery.
Methods:
We compared direct medical costs and indirect productivity costs associated with treatment with stereotactic radiosurgery (SRS) or anterior temporal lobectomy (ATL) in the Radiosurgery vs. Open Surgery for Epilepsy (ROSE) Trial. Health care use was abstracted from hospital bills, the study database and diaries in which participants recorded health care use and time lost from work while seeking care. Costs of use were calculated using a Medicare-costing approach used in a prior study of the costs of ATL. Power of many analyses was limited by the sample size and data skewing.
Results:
Combined treatment and follow-up costs (in thousands of US dollars) did not differ between SRS (n=20, mean $76.6, 95% CI 50.7 – 115.6) and ATL (n=18, mean $79.0, 95% CI 60.09 – 103.8). Indirect costs also did not differ. More ATL than SRS participants were free of consciousness impairing seizures in each year of follow up (all p < .05). Costs declined following ATL (p = .005). Costs tended to increase over the first 18 months following SRS (p = .17) and declined thereafter (p = .06). This mostly reflected hospitalizations for SRS-related adverse events in the second year of follow-up.
Significance:
Lower initial costs of SRS for medial temporal lobe epilepsy were largely offset by hospitalization costs related to adverse events later in the course of follow-up. Future studies of less-invasive alternatives to ATL will need to assess adverse events and major costs systematically and prospectively in order to understand the economic implications of adopting these technologies.
Keywords: radiosurgery, lobectomy, epilepsy, healthcare, costs
Introduction:
Surgery for epilepsy is highly effective in controlling seizures in carefully selected patients when medications have failed 1–3. The initial costs of evaluation and surgery are high, but successful surgery is associated with improved quality of life and lower health care costs over time 4–6. Therefore, surgery has been considered sufficiently cost-effective to justify broader use on economic grounds 6–9.
Stereotactic radio-surgery (SRS)10, 11 and laser interstitial thermal therapy (LITT)12 have been proposed as less-invasive alternatives to open surgery, since they may result in seizure control similar to that obtained with open surgery and may result in less morbidity. Seizure-free rates following less-invasive approaches have approached rates after open surgery in patients with mesial temporal lobe epilepsy (MTLE) 11, 13. There is preliminary evidence for equivalent or reduced impact on some aspects of cognitive function 11, 14. Less-invasive approaches also may be less costly, since they may not require an extended hospital stay and convalescent period and may be associated with fewer complications. Therefore, less-invasive approaches have the potential to be as cost-effective as open surgery, if not more so.
Health care costs associated with less-invasive alternatives have not been described or compared with costs associated with open surgery. Here, we compare health care costs during the Radiosurgery or Open Surgery for Epilepsy (ROSE) trial, a single-blinded, randomized trial designed to compare SRS and open anterior temporal lobectomy (ATL) in patients with MTLE.
Materials and Methods:
The ROSE Trial:
The participants, treatment protocols, follow-up methods and results with respect to seizure control, adverse events, verbal memory and quality-of-life of the ROSE Trial have been described in detail previously15. Briefly, ROSE was a multicenter, randomized, non-inferiority trial designed to compare the effectiveness of SRS to ATL in treating medically-refractory unilateral MTLE. Persons eligible for ROSE had been determined to be candidates for ATL at one of 15 centers in the USA, UK and India. Institutional review boards at the treatment centers approved the study (https://clinicaltrials.gov/ct2/show/NCT00860145). MTLE diagnosis had been established by video EEG monitoring, and concordant unilateral hippocampal sclerosis on magnetic resonance (MRI) without significant secondary lesions. All participants were at least 18 years old and had had at least three focal onset seizures with impairment of consciousness in the three months prior to enrollment, while on stable anticonvulsant regimens. Patients determined to be appropriate for ATL were randomized to ATL or SRS at the time of enrollment, stratified by center and by hemisphere of speech dominance. Over the next 36 months, participants were scheduled for face-to-face or telephone follow-up every three months with standardized assessment of medical, neuropsychological and psychiatric outcomes. The study protocol directed that all patients remain on stable doses of medications throughout the study period.
Due to lagging recruitment, enrollment in ROSE was halted after treating 58 of 200 planned participants, therefore limiting the power of most planned analyses. By the 36 month end-point, 16/31 (52%) SRS-treated patients had been in remission from consciousness-impairing seizures for the previous year, compared to 21/27 (78%) of ATL-treated patients. Consistent with the delayed effect of SRS, 74% of SRS-treated patients were seizure-free for the final three months of the study, compared to 85% of the ATL-treated group. At 36 months, there were no differences between the groups in verbal memory performance or quality of life.
Determining Health Care Use and Costs:
Analysis of health care use was limited to participants at the 12 US-based sites, since patterns of healthcare use and methods for determining costs are different in the UK and India. Costs of treatment and follow-up care were derived from several sources of information using a Medicare costing approach (see Technical Appendix). Inpatient use at the study site hospital (including ATL) was estimated from charges recorded on a Medicare-mandated billing form (UB-04) for all admissions to the hospital where treatment was performed. Medication use was assessed by study coordinators at each visit, including formulation (generic vs. brand), dosage (for AEDs, steroids and psychiatric medications only) and the dates they were started and stopped. All other health care use was assessed via diaries. In the diary that participants used to track seizures for the primary outcome, each participant was also asked to record six different types of health care use and the dates on which they occurred. These were 1) outpatient visits to a health care provider, 2) inpatient admissions, 3) laboratory tests (e.g., AED blood levels), 4) other tests and procedures (e.g., EEG, MRI), 5) dental care and 6) emergency department visits. Diaries were reviewed by study coordinators at each visit. Participants were prompted to use personal calendars and other records to aid recall of any health care use since the prior study visit.
Information about the timing and nature of serious and non-serious adverse events (AE) was extracted from the study database. We included all AEs that were possibly, probably, or definitely treatment-related, because we assumed that the likelihood that participants would seek care for them would be unrelated to the independent safety monitor’s confidence that they were treatment-related. There was insufficient information to determine directly whether hospital admissions were AE-related. For the current analysis, a hospitalization was considered AE-related if the two events occurred within a week of each other or the date of hospitalization fell between the dates of onset and resolution of the AE. For AEs that did not resolve, a hospitalization was considered AE-related if it occurred within a month of AE onset.
Statistical Analyses:
Our primary hypothesis was that SRS would be associated with lower overall direct medical and indirect costs over the course of treatment and follow-up. Secondary hypotheses were that the time course of changes in follow-up costs would differ between the arms (due to expected differences in the timing of seizure remission between the treatments) and that seizure remission would be associated with lower health care use over time. Differences between arms in direct and indirect costs over time were compared using generalized linear mixed models (GLMM) for repeated measures with normal distribution and log link.16 Similar models with Poisson distribution and log link were used for the analysis of the number of other medications used. For analyses of change in follow-up costs over time, seizure remission status during each period was included in these models as a time-varying covariate. The time-trend analysis was based on the ratio of the mean costs during the current 6 months over the mean costs of the preceding 6 months, i.e., the multiplicative effect on mean costs for every 6- month follow-up increment.
Ancillary analyses of rates of health care and medication use over the follow-up period were performed using linear mixed models (LMM) for repeated measures, with treatment arm and year of follow-up treated as categorical variables. An unstructured covariance matrix was used to account for the dependence of the within-subject observations. T-tests, chi-square or Fisher’s exact tests were used to compare participants in the two treatment arms in terms of baseline demographics, clinical characteristics, occurrence of treatment-related AEs and seizure outcome.
Two participants, one in each arm, were lost to follow-up (after the 12-month visit for ATL, after the 24-month visit for SRS). In treatment comparisons of costs and rates of use, values in the missing periods were imputed as the median values for all other participants for those periods. In other analyses examining changes over time, the GLMMs and LMMs used all available data in parameter estimation.
Results:
Participants:
Of the 58 ROSE participants (31 SRS, 27 ATL) included in the analyses of the trial’s primary outcomes, 38 (20 SRS, 18 ATL) were treated at US-based sites and were included in the current analyses. Similar to the main ROSE sample, there were no statistically significant differences between treatment groups in age at surgery, gender distribution, epilepsy duration, number of AEDs, or total number of all medications taken at enrollment (Table 1). SRS participants tended to have a higher monthly seizure frequency at enrollment (p = .06). More participants in the ATL arm were free of consciousness impairing seizures in each of the three years of follow-up (all p < .05) (Table 2).
TABLE 1 –
Demographics at Randomization
| SRS (n=20) | ATL (n=18) | p | |
|---|---|---|---|
| Gender (% female) | 60 | 39 | ns |
| Age at Diagnosis (yrs) | 19.0 (17.3) | 12.1 (12.4) | ns |
| Age at Surgery (yrs) | 44.5 (11.2) | 45.9 (10.7) | ns |
| Epilepsy Duration (yrs) | 25.5 (16.0) | 33.8 (13.8) | ns |
| AEDs (n) | 2.4 (1.2) | 2.5 (2.0) | ns |
| Total Medications (n) | 5.5 (3.0) | 4.3 (3.2) | ns |
| Monthly Seizure Frequency (n) | 6.9 (7.3) | 3.4 (2.4) | .06 |
Numbers in cells are means and standard deviations.
TABLE 2 –
Estimated Direct Medical and Indirect Costs (In $1000’s) and Seizure Remission during Follow-up (Excludes Treatment Costs)
| Treatment | Follow-up Period (Months) | ||||||
|---|---|---|---|---|---|---|---|
| 1–6 | 7–12 | 13–18 | 19–24 | 25–30 | 31–36 | ||
| Direct Medical Costs | |||||||
| ATL | 23.6 (18.9–28.3) | 12.7 (8.9–18.2) | 9.0 (6.0–13.4) | 9.8 (6.2–15.5) | 7.7 (4.9–12.1) | 7.7 (5.0–11.8) | 7.4 (4.7–11.6) |
| SRS | 11.6 (N/A) | 10.0 (5.8–17.1) | 11.4 (7.5–17.3) | 14.2 (8.3–21.2) | 10.0 (6–16.7) | 10.8 (6.2–18.9) | 10.2 (5.8–17.9) |
| Indirect Costs of Lost Employment and Work Hours | |||||||
| ATL | N/A | 13.7 (9.9–19.1) | 12.1 (8.3–17.9) | 12.1 (8.2–17.8) | 12.9 (9.2–18.2) | 13.0 (9.1–18.6) | 11.9 (8.0–17.7) |
| SRS | N/A | 14.8 (11.4–19.3) | 15.8 (12.3–20.2) | 14.9 (11.4–19.7) | 15.7 (12.3–20.1) | 15.4 (11.8–20.0) | 14.8 (11.1–19.7) |
| Free of Consciousness Impairing Seizures in Past Year (%) | |||||||
| ATL | 0 | 78 | 78 | 78 | |||
| SRS | 0 | 5 | 0 | 35 | |||
Numbers in cells are means and 95% CI’s. The CI for SRS treatment cost is undefined, since the cost of SRS was estimated as the Medicare reimbursement which did not differ across sites and participants (see Technical Appendix).
Total Direct and Indirect Medical Costs:
Combined treatment and follow-up costs did not differ significantly between treatment arms. ATL was associated with an estimated mean direct medical cost per-participant (in thousands of dollars) of 78.98 (95% CI = 50.72 – 115.60), compared to 76.57 (95% CI = 60.09 – 103.82) for SRS (p > .10). Combined direct and indirect costs also did not differ across arms (ATL = 154.9 (95% CI = 122.9 – 195.4) vs. SRS = 167.9 (95% CI = 134.0 – 210.2, p > .10).
Changes in Costs during Follow-up:
In regression analyses, ATL- and SRS-treated groups differed significantly in the ratio of mean direct medical costs over the last six months to those of the first six months of follow-up (ATL = 0.58 (95% CI 0.40–0.85), SRS = 1.02 (95%CI 0.72 – 1.45), p =.03). For the time-trend analyses, in the ATL group, every 6 months of follow-up was associated on average with an approximately 11% decrease in mean direct medical costs (0.89 (95% CI 0.83–0.97), p = .005). In the SRS arm, mean costs tended to increase over the first 18 months of follow-up (1.15 (95% CI 0.94–1.40), p =.17) and then tended to decrease over the remaining 18 months (0.90 (95% CI 0.80–1.01), p = .06). Changes in mean costs over time were not associated with concurrent seizure remission status in either arm (both p > .20). Mean costs for each 6-month period estimated from the regression model are shown in Table 2. Hospitalizations, AEDs and psychiatric medications accounted for > 90% of follow-up costs across all periods (see Table 3). Costs of AEDs did not differ between treatments or across time and were not associated with remission status (all p > .10). Analyses of differences in costs between ATL and SRS for other types of utilization were precluded by the small sample size and skewed nature of cost data.
TABLE 3 –
Mean Annual Cost ($1000s) by Type of Use (excluding study visits)
| ATL | SRS | |||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 1 | Year 2 | Year 3 | |
| Outpatient Visits | 0.4 (0.8) | 0.2 (0.3) | 0.2 (0.2) | 0.3 (0.3) | 0.3 (0.3) | 0.4 (0.4) |
| Hospitalizations | 1.4 (4.2) | 0.0 (0.0) | 0.5 (1.9) | 2.9 (7.7) | 3.7 (6.8) | 1.2 (3.0) |
| Laboratory Tests | 0.03 (0.1) | 0.01 (0.01) | 0.01 (0.02) | 0.01 (0.02) | 0.03 (0.1) | 0.02 (0.04) |
| Other Tests/Procedures | 0.3 (0.4) | 0.2 (0.4) | 0.2 (0.3) | 0.3 (0.4) | 0.3 (0.3) | 0.5 (0.7) |
| Dental Care | 0.1 (0.2) | 0.1 (0.2) | 0.1 (0.2) | 0.01 (0.03) | 0.1 (0.2) | 0.03 (0.1) |
| Emergency Care | 0.02 (0.1) | 0.01 (0.05) | 0.0 (0.0) | 0.1 (0.2) | 0.1 (0.2) | 0.1 (0.2) |
| AEDs | 17.9 (15.2) | 15.9 (15.6) | 12.9 (14.3) | 15.8 (19.4) | 16.4 (23.6) | 15.8 (25.3) |
| Psychiatric Meds | 1.65 (4.5) | 1.2 (2.6) | 1.4 (3.4) | 1.4 (2.5) | 1.2 (2.0) | 0.6 (1.2) |
Numbers in cells are means and standard deviations
Treatment Differences in Rates of Use of Different Types:
The trends noted above were explored by examining rates of use between arms by year of follow-up, since the distribution of rates of use was less skewed than the distribution of costs. The rate of health care use of all types increased over time in the SRS arm and declined in the ATL arm (Treatment x Time interaction p = .04) (Table 4). This was attributable to a decline over time in hospitalizations and laboratory tests following ATL, relative to SRS (p < .05). Rates of use were not associated with seizure remission status, except that participants in remission were less likely to visit the emergency department (p = .03). Change in the number of AEDs used over time tended to differ, with use declining after ATL, relative to SRS (Treatment x Time p = .08), despite the fact that the protocol advised maintaining participants on their pre-treatment AED regimen throughout followup. Participants in the SRS arm used more psychiatric and non-AED medications consistently across all periods (p < 0.03 at each period). Both groups increased their use of other medications over the course of follow-up (both p < .02). Use of other medications was not associated with seizure remission (both p > .20).
TABLE 4 –
Mean Annual Number of Use Episodes and Medications Prescribed (excluding study visits)
| ATL | SRS | |||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 1 | Year 2 | Year 3 | |
| Healthcare Use (n) | 9.9 (13.1) | 6.6 (8.0) | 5.0 (3.6) | 6.9 (6.2) | 7.8 (7.2) | 9.8 (7.8) |
| Outpatient Visits | 4.6 (10.0) | 2.4 (3.3) | 2.4 (2.2) | 3.1 (3.9) | 3.0 (3.3) | 4.7 (4.5) |
| Hospitalizations | 0.2 (0.5) | 0.0 (0) | 0.1 (0.2) | 0.3 (0.9) | 0.4 (0.8) | 0.1 (0.4) |
| Laboratory Tests | 0.8 (1.7) | 0.2 (0.4) | 0.1 (0.5) | 0.3 (0.7) | 0.8 (1.6) | 0.5 (1.0) |
| Other Tests/Procedures | 1.1 (1.5) | 0.8 (1.4) | 0.8 (1.1) | 1.1 (1.6) | 0.9 (1.1) | 1.8 (2.5) |
| Dental Care | 0.6 (1.5) | 0.5 (1.7) | 0.5 (1.2) | 0.05 (0.2) | 0.5 (1.6) | 0.2 (0.6) |
| Emergency Care | 0.1 (0.5) | 0.1 (0.2) | 0.0 (0) | 0.6 (0.2) | 0.6 (1.2) | 0.5 (0.9) |
| Medication Use (n) | ||||||
| AEDS | 2.4 (1.1) | 2.4 (1.1) | 2.3 (1.1) | 2.5 (1.1) | 2.6 (1.0) | 2.7 (1.1) |
| Psychiatric | 0.6 (0.8) | 0.5 (0.8) | 0.6 (0.9) | 0.7 (.8) | 0.8 (0.9) | 0.5 (0.7) |
| Other | 1.2 (0.9) | 1.4 (1.2) | 1.6 (1.8) | 2.8 (2.9) | 3.7 (4.3) | 4.2 (5.2) |
Numbers in cells are means and standard deviations
Adverse Events and their Association with Costs:
One ATL participant experienced a serious AE (wound dehiscence) compared to seven SRS participants (p < .05), who experienced eight serious AEs between 7 and 24 months of follow-up (increased headaches (n=3), cerebral edema (n=2), status epilepticus (n=1), increased seizures (n=1), new neurologic deficit (n=1). All serious AEs were associated with hospitalization. Seventeen SRS participants experienced non-serious AEs compared to six ATL participants (p < .01). SRS participants experienced a total of 61 non-serious AEs, most of which were less severe increases in cerebral edema, headaches or seizures, mood or cognitive changes or self-limited neurologic deficits. ATL participants experienced 11 non-serious AEs (decline in memory (n= 5), subdural hematoma (n=2), increase in seizures (n=1), new onset headaches (n=1), double vision (n=1) and hyponatremia (n=1).
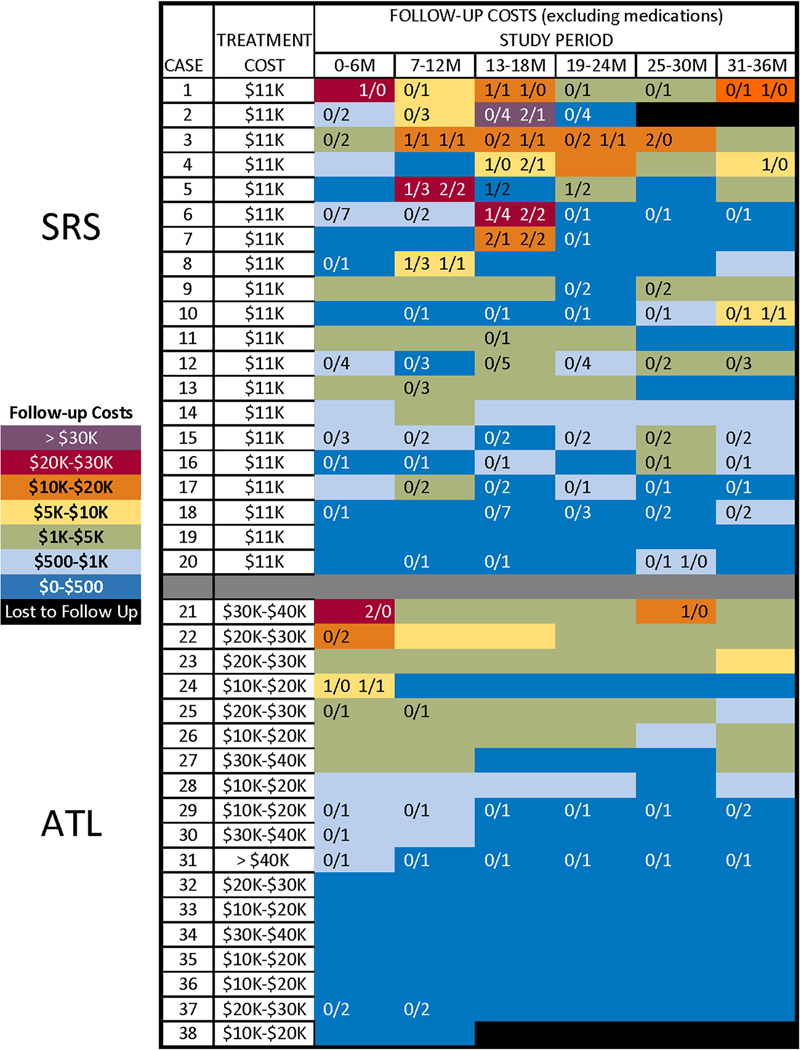
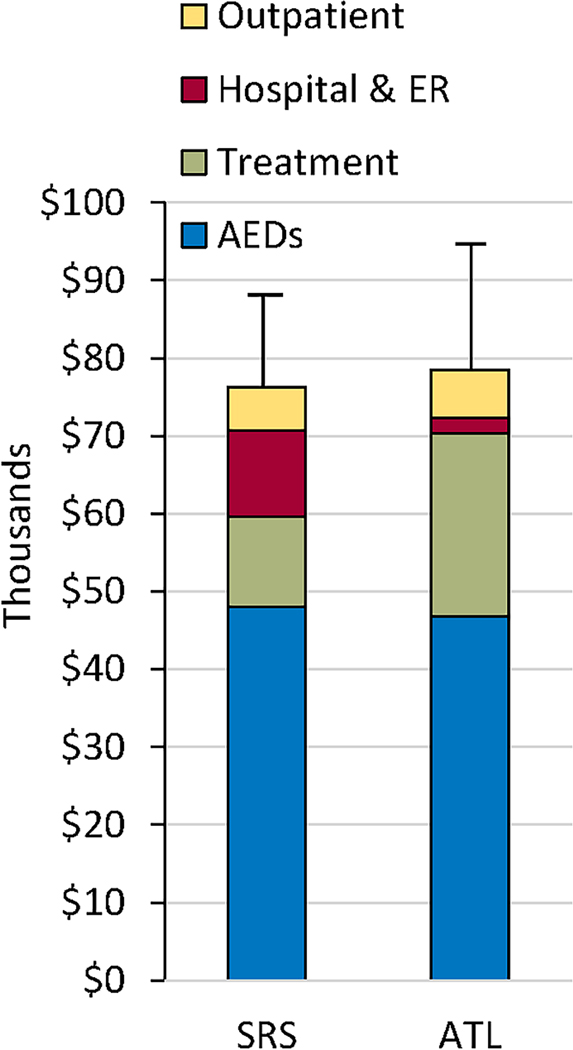
Figure 1 depicts the range of treatment and per-participant direct medical costs (excluding medications) across the follow-up period. Within each arm, participants varied widely in their health care use, as reflected in the large standard deviations (relative to means) in total direct costs and in rates of use. Most periods of high use were associated with hospitalizations and occurrence of AEs, particularly following SRS. Across both treatments, experiencing a serious AE was associated with higher total follow-up costs (excluding AEDs) (r= 0.45, p < .01), but experiencing a non-serious AE was not (r= 0.19, p > .10). Ten SRS participants had a total of 21 hospitalizations, compared to a total of four hospitalizations in two ATL participants (p < .05). Within the SRS arm, all serious AEs and most hospitalizations occurred between the 6 and 18 month visits, corresponding to the period of maximum effect of radiation-induced cerebral edema, with associated increase in headaches or non-consciousness impairing seizures 11. Twelve of the 14 (86%) hospitalizations during this time were AE-related, compared to 2 of the 7 (29%) hospitalizations during other periods (p < .05). Only one of the four hospitalizations after ATL was AE-related (wound dehiscence). The per-participant, mean combined costs of initial treatment, follow-up hospitalizations and emergency treatment were roughly equivalent between the two arms (see Figure 2). This reflects the fact that the lower initial treatment costs of SRS were offset by higher costs associated with more frequent, serious, treatment-related AEs, many of which led to hospitalization.
Figure 1.
Treatment cost ranges and direct medical costs (excluding AEDs) across follow-up for each study participant, by arm. Blue-to-red shading within cells represents increasing cost. Numbers on the left half of each cell are the number of serious AEs (to the left of the “/” mark) and non-serious AEs (to the right of the “/” mark) experienced during the period. Numbers on the right half of each cell are the total number of hospitalizations (to the left of the mark) and AE-related hospitalizations (to the right of the mark) experienced during the period.
Figure 2.
Mean, per-participant total treatment and follow-up direct medical costs (in thousands) between SRS and ATL, by type of cost.
Discussion:
Contrary to our hypothesis, we failed to demonstrate lower direct medical costs associated with SRS compared to ATL over the course of the trial. It is possible that we were unable to detect a small but meaningful relative costs savings of SRS, because of the relatively small sample size and the skewed nature of health care cost data. On the other hand, SRS had two distinct disadvantages in this study that likely contributed to the absence of an expected cost-savings effect.
The first disadvantage was that SRS participants were more likely to experience treatment-related AEs that led to hospitalization. Hospitalization was far and away the most costly type of follow-up care. As shown in Figure 2, the cost of these hospitalizations effectively eliminated the relative economic benefit of the lower initial treatment costs of SRS. This suggests that the ultimate cost-effectiveness of less invasive alternatives to open surgery for epilepsy depends on their achieving similar rates of seizure freedom to ATL, as well as similar or lower rates of post-operative adverse events and hospitalizations.
Adverse events and hospitalizations have not always been assessed and reported consistently in studies of epilepsy surgery. ATL case series have reported relatively low (~5%) post-operative complication rates 17, with higher rates (10–33%) reported in randomized trials 1, 2. In a retrospective medical record review of a subset of 68 patients enrolled in a large prospective, observational study of epilepsy surgery, there were 0.04 hospitalizations per patient-year in patients who were seizure-free after ATL, compared to 0.25 hospitalizations per patient-year among surgical and non-surgical patients with persistent seizures 5. In a recent meta-analysis, the pooled estimate of seizure-free outcome after SRS was 51% (95% CI 38–64%)18. The most common adverse event was headache, which occurred in 9–85% of series in which it was reported (median 43%). The meta-analysis did not address severity and consequences of headaches, but headaches led to hospitalization in 20% of our sample who reported them. In our experience, the presence of transient SRS-associated cerebral edema rather than the severity of headache is typically the driver for inpatient evaluation. Some hospitalizations therefore could have arisen because of center-to-center differences in comfort with the management of this expected result of focal radiation 11. ATL has been used in the treatment of refractory epilepsy for decades and most treatment teams are very comfortable with post-operative and long-term management. This may not be the case for newer procedures, such as SRS. Patients treated with SRS for neoplasms are managed by neurosurgeons and radiation oncologists with considerable experience with these diagnoses and treatments. In the ROSE Trial, neurosurgeons and radiation oncologists performed all of the SRS procedures, but the medical management over time was typically provided by neurologists. It is possible that long-term costs of care would decrease as clinicians become more experienced in outpatient management of post-radiation side effects.
Case series of LITT for mesial temporal lobe epilepsy with MTS have reported seizure-free rates ranging from 36–80% 12. LITT typically requires shorter hospital stays than ATL and patients frequently enter seizure remission immediately after the procedure, so one might predict that overall costs of care would be less than with ATL or SRS. However, rates of adverse events range from 4–28% in series where they have been reported. Several of these (hemorrhage, infarcts, atrial fibrillation, cranial nerve injuries) may have merited hospitalization. Initial LITT failure may lead to additional procedures or to ATL 13, 19, costly interventions that must be included in overall cost estimates of this approach.
The second disadvantage of SRS was that fewer SRS-treated participants achieved one-year seizure remission than was expected, based on results of the pilot study 11. Seizure-freedom has been reliably associated with lower costs in most studies of epilepsy samples, including following ATL 5, 6, 20, 21. Consistent with this observation, the majority of ATL-treated participants entered remission early and follow-up costs declined over time. Seizure remission was not statistically associated with follow-up costs in the per-period analyses. Our ability to detect this association within groups and over time may have been limited by the large difference between groups in rates of seizure-freedom and the outsized impact on costs of hospitalizations in the SRS arm for adverse events unrelated to seizure control. Reductions in health care use after ATL also lag achievement of seizure freedom by up to a year 5, 6, presumably because patients and providers are waiting to see whether initial results are reliable. Finally, protocol-averted costs may have led us to underestimate the difference in outpatient follow-up costs associated with remission. Were it not for the prescribed study visits, participants with ongoing seizures likely would have sought care for their seizures elsewhere, whereas patients in remission may have done so less often.
There are several other limitations to these results. We only followed participants for 3 years. Differences in long-term costs might persist or attenuate to the degree that rates of seizure-freedom and adverse events following SRS may converge toward, or remain distinct from those after ATL. Longer-term observation is required. Use of a health care diary may have introduced some error into the assessment of utilization during follow up, but it is unlikely to have biased treatment comparisons substantially. Health utilization diaries provide a generally valid assessment of utilization ( Leggett, L. E., Khadaroo, R. G., Holroyd-Leduc, J., Lorenzetti, D. L., Hanson, H., Wagg, A., … Clement, F. (2016). Measuring Resource Utilization: A Systematic Review of Validated Self-Reported Questionnaires. Medicine, 95(10), e2759.), particularly for hospitalizations, which accounted for the major differences in follow-up costs between treatments. Excluding inpatient utilization, costs estimated from diaries were < 10% of total costs associated with either treatment. Larger treatment and medication costs were estimated from medical record and administrative data. There is no reason to believe that randomized participants would have provided biased estimates of utilization in their diaries. Finally, validity of the diary in this study is supported by the observations that a) the timing of hospitalizations recorded in the diaries by SRS participants corresponded to the peak incidence of adverse events and radiation-induced edema and b) changes in utilization associated with seizure freedom following ATL followed a similar time course reported in prior studies. The indirect costs that we assessed were incomplete. They were selected based on considerations of feasibility, likely effect size, and likelihood of their being affected by treatment outcomes. Seizures can reduce productivity (e.g., decreased efficiency if recovering from seizures, reduced career trajectory), but effects can also be mitigated (e.g., by improved work output to cover for lost time). Accounting for these effects would require assessment methods (e.g., employment records, time and motion studies, long-term employment outcomes) that are not feasible to obtain in the context of a multisite randomized controlled trial where costs were not the primary outcome. We focused on participants’ employment status and caregivers’ lost work hours, since lost work productivity is arguably the largest category of costs in this population, given the high rates of unemployment in this population and the high unit cost. Costs associated with under- and unemployment have also been the primary focus of estimates of the indirect cost of epilepsy in prior studies (Begley et al. 2000).
In conclusion, combined treatment and follow-up costs associated with ATL and SRS appear to be comparable. As in prior studies, costs declined after ATL which rendered most patients free of seizures, but at a higher initial treatment cost. Costs following SRS remained largely stable, with a tendency to increase in the second year and then decline, associated with maximal radiation effect, serious adverse events and associated hospitalizations. The costs associated with these hospitalizations and other care effectively eliminated the cost-savings associated with the less-expensive SRS treatment. In order to fully evaluate the economic implications of adopting less-invasive alternatives to ATL, future studies will need to assess adverse events and major costs systematically and prospectively over the time periods in which they can be expected to occur.
Supplementary Material
Key Points:
Less-invasive approaches to epilepsy surgery may control seizures as well as open surgery, but with potentially lower morbidity and cost.
In a randomized trial comparing radiosurgery to temporal lobectomy, total costs were comparable over the course of the three-year trial.
The lower initial cost of radiosurgery was offset by higher follow-up costs associated with adverse events and hospitalizations.
Future studies of less-invasive approaches should track costs carefully to fully understand the economic implications of their use.
Acknowledgments:
The following individuals and institutions contributed to participant recruitment, treatment and study management: Prof Sarat Chandra, MD; Prof Shashank K. Kale, MD; Ashima N. Washawan, PhD (All India Institute of Medical Science, New Delhi, India); Adam Hebb, MD (Colorado Neurological Institute, Denver, CO); Hyunmi Choi, MD; Catherine Schevon, MD; Gail Iodice RN (Columbia University, New York, NY); Robert Goodman, MD (Icahn School of Medicine at Mount Sinai, New York, NY) Cyndi Herrera (Indiana University, Indianapolis, IN); Robert Beach, MD (State University of New York, Upstate, Syracuse, NY);
Lawrence ver Hoef, MD(University of Alabama at Birmingham, Birmingham, AL); William P. Dillon, MD; Kathleen Lamborn, PhD; Lijun Ma, PhD (University of California San Francisco, San Francisco, CA); Aviva Abosch, MD (University of Colorado, Aurora, CO); Thomas Pittman, MD (University of Kentucky, Lexington, KY); Douglas Kondziolka, MD(NYU Langone Medical Center, NY, NY); Rick Hendrickson, PhD (University of Pittsburgh, Pittsburgh, PA; Beate Diehl, MD (University of Sheffield, Sheffield, United Kingdom); Charles Y. Liu, MD; Laura Kalayjian, MD (University of Southern California, Los Angeles, CA);
Stacy Thompson, RN, Prof W. Jeffery Elias, MD, Prof Jason Sheehan, MD)(University of Virginia, Charlottesville, VA); Jeffrey Ojemann, MD (University of Washington, Seattle, WA).
Footnotes
Ethical Guidelines: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosures: This project was funded by NIH-NINDS R01 NS 058634–01A2; Elekta AB, Stockholm, Sweden. The authors have no other conflicts to disclose.
REFERENCES
- 1.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. New England Journal of Medicine 2001;345:311–318. [DOI] [PubMed] [Google Scholar]
- 2.Engel J, McDermott MP, Wiebe S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. Jama 2012;307:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Téllez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain 2005;128:1188–1198. [DOI] [PubMed] [Google Scholar]
- 4.Spencer SS, Berg AT, Vickrey BG, et al. Health‐related quality of life over time since resective epilepsy surgery. Annals of neurology 2007;62:327–334. [DOI] [PubMed] [Google Scholar]
- 5.Langfitt J, Holloway R, McDermott M, et al. Health care costs decline after successful epilepsy surgery. Neurology 2007;68:1290–1298. [DOI] [PubMed] [Google Scholar]
- 6.Picot MC, Jaussent A, Neveu D, et al. Cost‐effectiveness analysis of epilepsy surgery in a controlled cohort of adult patients with intractable partial epilepsy: A 5‐year follow‐up study. Epilepsia 2016;57:1669–1679. [DOI] [PubMed] [Google Scholar]
- 7.Wiebe S, Gafni A, Blume WT, Girvin JP. An economic evaluation of surgery for temporal lobe epilepsy. Journal of Epilepsy 1995;8:227–235. [Google Scholar]
- 8.King JT, Sperling MR, Justice AC, O’Connor MJ. A cost-effectiveness analysis of anterior temporal lobectomy for intractable temporal lobe epilepsy. Journal of neurosurgery 1997;87:20–28. [DOI] [PubMed] [Google Scholar]
- 9.Langfitt JT. Cost‐effectiveness of anterotemporal lobectomy in medically intractable complex partial epilepsy. Epilepsia 1997;38:154–163. [DOI] [PubMed] [Google Scholar]
- 10.Régis J, Bartolomei F, Rey M, et al. Gamma knife surgery for mesial temporal lobe epilepsy. Epilepsia 1999;40:1551–1556. [DOI] [PubMed] [Google Scholar]
- 11.Barbaro NM, Quigg M, Broshek DK, et al. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 2009;65:167–175. [DOI] [PubMed] [Google Scholar]
- 12.Prince E, Hakimian S, Ko AL, Ojemann JG, Kim MS, Miller JW. Laser interstitial thermal therapy for epilepsy. Current neurology and neuroscience reports 2017;17:63. [DOI] [PubMed] [Google Scholar]
- 13.Gross RE, Stern MA, Willie JT, et al. Stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Annals of neurology 2018;83:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drane DL, Loring DW, Voets NL, et al. Better object recognition and naming outcome with MRI‐guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia 2015;56:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbaro NM, Quigg M, Ward MM, et al. Radiosurgery versus open surgery for mesial temporal lobe epilepsy: The randomized, controlled ROSE trial. Epilepsia 2018;59:1198–1207. [DOI] [PubMed] [Google Scholar]
- 16.McCullagh P NJ. Generalized linear models, 2nd ed.London: Chapman & Hall/CRC, 1989. [Google Scholar]
- 17.Pilcher WH, Rusyniak WG. Complications of epilepsy surgery. Neurosurgery Clinics of North America 1993;4:311–325. [PubMed] [Google Scholar]
- 18.Feng ES, Sui CB, Wang TX, Sun GL. Stereotactic radiosurgery for the treatment of mesial temporal lobe epilepsy. Acta Neurologica Scandinavica 2016;134:442–451. [DOI] [PubMed] [Google Scholar]
- 19.Kang JY, Wu C, Tracy J, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia 2016;57:325–334. [DOI] [PubMed] [Google Scholar]
- 20.Begley CE, Famulari M, Annegers JF, et al. The cost of epilepsy in the United States: an estimate from population‐based clinical and survey data. Epilepsia 2000;41:342–351. [DOI] [PubMed] [Google Scholar]
- 21.Cockerell OC, Hart YM, Sander JW, Shorvon SD. The cost of epilepsy in the United Kingdom: an estimation based on the results of two population-based studies. Epilepsy research 1994;18:249–260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.