Abstract
Background
This is an updated version of a Cochrane Review previously published in 2019.
Catamenial epilepsy describes worsening seizures in relation to the menstrual cycle and may affect around 40% of women with epilepsy. Vulnerable days of the menstrual cycle for seizures are perimenstrually (C1 pattern), at ovulation (C2 pattern), and during the luteal phase (C3 pattern). A reduction in progesterone levels premenstrually and reduced secretion during the luteal phase is implicated in catamenial C1 and C3 patterns. A reduction in progesterone has been demonstrated to reduce sensitivity to the inhibitory neurotransmitter in preclinical studies, hence increasing risk of seizures. A pre‐ovulatory surge in oestrogen has been implicated in the C2 pattern of seizure exacerbation, although the exact mechanism by which this surge increases risk is uncertain. Current treatment practices include the use of pulsed hormonal (e.g. progesterone) and non‐hormonal treatments (e.g. clobazam or acetazolamide) in women with regular menses, and complete cessation of menstruation using synthetic hormones (e.g. medroxyprogesterone (Depo‐Provera) or gonadotropin‐releasing hormone (GnRH) analogues (triptorelin and goserelin)) in women with irregular menses.
Catamenial epilepsy and seizure exacerbation is common in women with epilepsy. Women may not receive appropriate treatment for their seizures because of uncertainty regarding which treatment works best and when in the menstrual cycle treatment should be taken, as well as the possible impact on fertility, the menstrual cycle, bone health, and cardiovascular health. This review aims to address these issues to inform clinical practice and future research.
Objectives
To evaluate the efficacy and tolerability of hormonal and non‐hormonal treatments for seizures exacerbated by the menstrual cycle in women with regular or irregular menses. We synthesised the evidence from randomised and quasi‐randomised controlled trials of hormonal and non‐hormonal treatments in women with catamenial epilepsy of any pattern.
Search methods
We searched the following databases on 20 July 2021 for the latest update: Cochrane Register of Studies (CRS Web) and MEDLINE Ovid (1946 to 19 July 2021). CRS Web includes randomised controlled trials (RCTs) or quasi‐RCTs from PubMed, Embase, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform, the Cochrane Central Register of Controlled Trials (CENTRAL), and the specialised registers of Cochrane Review Groups including Cochrane Epilepsy. We used no language restrictions. We checked the reference lists of retrieved studies for additional reports of relevant studies.
Selection criteria
We included RCTs and quasi‐RCTs of blinded or open‐label design that randomised participants individually (i.e. cluster‐randomised trials were excluded). We included cross‐over trials if each treatment period was at least 12 weeks in length and the trial had a suitable wash‐out period. We included the following types of interventions: women with any pattern of catamenial epilepsy who received a hormonal or non‐hormonal drug intervention in addition to an existing antiepileptic drug regimen for a minimum treatment duration of 12 weeks.
Data collection and analysis
We extracted data on study design factors and participant demographics for the included studies. The primary outcomes of interest were: proportion seizure‐free, proportion of responders (at least 50% decrease in seizure frequency from baseline), and change in seizure frequency. Secondary outcomes included: number of withdrawals, number of women experiencing adverse events of interest (seizure exacerbation, cardiac events, thromboembolic events, osteoporosis and bone health, mood disorders, sedation, menstrual cycle disorders, and fertility issues), and quality of life outcomes.
Main results
Following title, abstract, and full‐text screening, we included eight full‐text articles reporting on four double‐blind, placebo‐controlled RCTs. We included two cross‐over RCTs of pulsed norethisterone, and two parallel RCTs of pulsed progesterone recruiting a total of 192 women aged between 13 and 45 years with catamenial epilepsy. We found no RCTs for non‐hormonal treatments of catamenial epilepsy or for women with irregular menses.
Meta‐analysis was not possible for the primary outcomes, therefore we undertook a narrative synthesis. For the two RCTs evaluating norethisterone versus placebo (24 participants), there were no reported treatment differences for change in seizure frequency. Outcomes for the proportion seizure‐free and 50% responders were not reported. For the two RCTs evaluating progesterone versus placebo (168 participants), the studies reported conflicting results for the primary outcomes. One progesterone RCT reported no significant difference between progesterone 600 mg/day taken on day 14 to 28 and placebo with respect to 50% responders, seizure freedom rates, and change in seizure frequency for any seizure type. The other progesterone RCT reported a decrease in seizure frequency from baseline in the progesterone group that was significantly higher than the decrease in seizure frequency from baseline in the placebo group.
The results of secondary efficacy outcomes showed no significant difference between groups in the pooled progesterone RCTs in terms of treatment withdrawal for any reason (pooled risk ratio (RR) 1.56, 95% confidence interval (CI) 0.81 to 3.00, P = 0.18, I2 = 0%) or treatment withdrawals due to adverse events (pooled RR 2.91, 95% CI 0.53 to 16.17, P = 0.22, I2 = 0%). No treatment withdrawals were reported from the norethisterone RCTs. The RCTs reported limited information on adverse events, although one progesterone RCT reported no significant difference in the number of women experiencing adverse events (diarrhoea, dyspepsia, nausea, vomiting, fatigue, nasopharyngitis, dizziness, headache, and depression). No studies reported on quality of life.
We judged the evidence for outcomes related to the included progesterone RCTs to be of low to moderate certainty due to risk of bias, and for outcomes related to the included norethisterone RCTs to be of very low certainty due to serious imprecision and risk of bias.
Authors' conclusions
This review provides very low‐certainty evidence of no treatment difference between norethisterone and placebo, and moderate‐ to low‐certainty evidence of no treatment difference between progesterone and placebo for catamenial epilepsy. However, as all the included studies were underpowered, important clinical effects cannot be ruled out.
Our review highlights an overall deficiency in the literature base on the effectiveness of a wide range of other hormonal and non‐hormonal interventions currently being used in practice, particularly for those women who do not have regular menses. Further clinical trials are needed in this area.
Plain language summary
Treatments for seizures in catamenial (menstrual‐related) epilepsy
Background
Catamenial (menstrual) epilepsy describes a worsening of seizures in relation to the menstrual cycle and may affect around 40% of women with epilepsy. There are specific times within the menstrual cycle when women are most at risk: in the days leading up to a menstrual period and during a menstrual period (perimenstrual or catamenial type 1 pattern); at the time of ovulation (catamenial type 2 pattern); and in the second half of their cycle (luteal phase, or catamenial type 3 pattern). The reason for this increased risk may relate to changes in the levels of progesterone (a hormone released by the ovaries) around the time of a menstrual period and oestrogen (a female sex hormone) surge around ovulation. Studies in animals have demonstrated that lower progesterone may affect how the brain reacts to the brain chemical gamma‐Aminobutyric acid (GABA), which is important in preventing seizures. The link between high levels of oestrogen and risk of seizures remains unclear.
Current treatment of catamenial epilepsy depends on whether a woman has regular or irregular menstrual periods. If a woman has regular periods, hormonal (e.g. progesterone supplements) and non‐hormonal treatments (e.g. clobazam or acetazolamide) taken prior to and during a period may be used. In women who do not have regular periods, and who therefore cannot predict their period days, stopping periods using synthetic hormones (e.g. medroxyprogesterone (Depo‐Provera) or gonadotropin‐releasing hormone (GnRH) analogues (triptorelin and goserelin)) are treatment options.
Catamenial epilepsy is common in women with epilepsy, and may have a significant negative impact on quality of life. Women may not receive appropriate treatment for their catamenial seizures. There is uncertainty regarding which treatment works best and when in the menstrual cycle treatments should be taken. There are also concerns about the possible impact on fertility, the menstrual cycle, bone health, and cardiovascular health. This review aimed to address these issues in order to inform clinical practice and future research.
Objectives
The aim of this review was to examine the effectiveness of hormonal and non‐hormonal treatments in stopping seizures in women with catamenial epilepsy.
Methods
We searched electronic databases to find relevant studies in which treatment was continued for at least 12 weeks. Our outcomes of interest were: average change in seizures, percentage of women achieving a reduction in seizures by at least 50%, and percentage of women who became seizure‐free. We also examined the reasons why women dropped out of the studies and any reported side effects.
Results
We included four randomised controlled trials (studies in which participants are randomly assigned to one of two or more treatment groups) of hormonal treatments in the review, two trials evaluating progesterone and two evaluating norethisterone. In all of these studies, the treatment was compared to a placebo (a harmless sugar pill). We did not find any studies testing non‐hormonal treatments or any studies in women with irregular periods. The four included studies involved a total of 192 women aged between 13 and 45 years experiencing catamenial epilepsy. The included studies did not demonstrate any significant differences between groups when comparing progesterone or norethisterone to placebo for seizure outcomes. The included studies reported limited information on side effects, but women taking progesterone were no more likely to withdraw from the study due to side effects than those receiving placebo.
The evidence is current to July 2021.
Certainty of the evidence
We judged the certainty of the evidence to be very low to moderate, as the included studies provided unclear information on methods of blinding, recruited small numbers of participants, and were inconsistent in reporting treatment outcomes.
Conclusions
We found very limited, mostly low‐certainty evidence, of no difference in seizure outcomes for norethisterone and progesterone versus placebo in women with catamenial epilepsy. Our review highlights an overall lack of information on the effectiveness of a wide range of other hormonal and non‐hormonal treatments currently being used. Further studies in women with catamenial epilepsy are needed in this area.
Summary of findings
Background
This is an updated version of a Cochrane review previously published in 2019 (Maguire 2019).
Description of the condition
Studies have shown that in middle to high‐income countries, prevalence rates for active epilepsy are between 4 and 10 per 1000 (Sander 1996). In a systematic review of incidence studies, the median annual incidence of epilepsy was 50.7 per 100,000 for males and 46.2 per 100,000 for females (Kotsopoulos 2002). Globally, 50% of women and girls with epilepsy are in the reproductive age range of 15 to 49 years.
Catamenial epilepsy describes a worsening of seizures in relation to the menstrual cycle, which may affect around 40% of women with epilepsy (Herzog 1997). Studies examining day‐to‐day comparisons of seizures throughout the menstrual cycle have consistently shown a greater likelihood of seizures on day 1 (the start of menstruation), with the lowest risk of seizures on day 20 (the mid‐luteal phase) (Laidlaw 1956; Rosciszewska 1980; Ansell 1986; Tauboll 1991; Herzog 1997). The menstrual cycle is characterised by two phases: the follicular phase (day 1 to day 13), which comprises menstruation (day 1 to 5), followed by ovulation (day 14), and the luteal phase (day 15 to 28). There are two major hormonal changes: a preovulatory surge in oestradiol (day 10 to 15), and a premenstrual drop in progesterone levels (day 25 to 28). In one study of 184 women with focal epilepsy, there was statistically significant evidence for greater seizure occurrences around the time of these two critical hormonal changes, compared with the mid‐follicular and mid‐luteal phases. These time periods were categorised as catamenial type 1 (C1) pattern (day −3 (25) to day 3) and catamenial type 2 (C2) pattern (day 10 to 15). A third pattern ‐ catamenial type 3 (C3) ‐ was noted in women experiencing anovulatory cycles (where no ovulation occurs during the cycle), whereby a lack of progesterone secretion during the luteal phase predisposed to a higher mid‐luteal ratio of oestradiol to progesterone, which placed the woman at risk of seizures throughout the luteal phase (Herzog 1997). The hormonal changes and catamenial seizure patterns during a menstrual cycle are summarised in Figure 1.
1.

Hormonal changes and catamenial seizures patterns during the menstrual cycle.
Reprinted from Reddy DS. Neurosteroids and their role in sex‐specific epilepsies. Neurobiology of Disease72 (Pt B):198‐209, Copyright (2014), with permission from Elsevier (Reddy 2014).
Approximately 10% of menstrual cycles in healthy women are anovulatory, whereas 35% are anovulatory in women with temporal lobe epilepsy (Herzog 2001). In a study conducted in 1997, around 42% of women with epilepsy demonstrated at least one of the three patterns of catamenial epilepsy. Around 36% had C1 pattern, 29% had C2 pattern, and 42% had C3 pattern (Herzog 1997). Other studies have reported higher prevalence rates (between 63% and 78%); however, they compared seizures in just perimenstrual phases versus other phases of the cycle (Laidlaw 1956; Rosciszewska 1980; Ansell 1986; Tauboll 1991). When a similar comparison was made in the 1997 study, a prevalence rate of 71% was found (Herzog 1997). Reported clinical risk factors for catamenial epilepsy are: younger age, temporal lobe seizures, and a left‐sided epileptogenic foci, which implies that cyclical seizure rhythms are affected by the neuroanatomic substrate of the seizure focus (Quigg 2009).
Description of the intervention
In women with catamenial seizures, non‐hormonal and hormonal treatments may be considered in addition to regular medication. Non‐hormonal treatments include pulsed clobazam and acetazolamide. Hormonal treatments include natural progesterone supplements, synthetic oral or intramuscular progesterones, allopregnanolone, and gonadotropin‐releasing hormone (GnRH) analogues (triptorelin and goserelin).
For women with catamenial epilepsy who have regular menstrual cycles, intermittent treatment approaches are considered. These interventions target vulnerable days of the menstrual cycle perimenstrually (C1 pattern), at ovulation (C2 pattern), and during the luteal phase (C3 pattern). The National Institutes of Health progesterone trial assigned 462 women with drug‐resistant seizures to either oral progesterone or placebo taken during days 14 to 28 (Herzog 2012), and observed changes in seizure frequency (a reduction of more than 50%) between the three‐month baseline and the three‐month treatment period. The study found comparable outcomes for progesterone and placebo overall. However, a secondary analysis identified that the women most likely to respond were those with a C1 pattern seizure type (secondary generalised seizures and focal seizures with altered awareness) and a three‐fold higher perimenstrual seizure frequency. The study demonstrated a favourable short‐term safety profile (Herzog 2012). However, a clear effect in women with C2 or C3 pattern was not shown, which may reflect differences in underlying pathophysiology. Other intermittent cyclic treatments include benzodiazepines, acetazolamide, or increasing the dose of an antiseizure drug already in use.
For women with irregular menstrual cycles, or in those for whom the intermittent cyclic treatments are not effective, the option of pharmacologically stopping the menstrual cycle altogether may be considered, either by using synthetic hormones such as medroxyprogesterone (Depo‐Provera), GnRH analogues (triptorelin and goserelin), or sustained oral contraceptives.
How the intervention might work
Preclinical studies have demonstrated that withdrawal of progesterone or its reduced metabolite allopregnanolone, as occurs premenstrually, can cause insensitivity to the inhibitory neurotransmitter gamma‐Aminobutyric acid (GABA) and also to benzodiazepines that act to enhance GABA transmission (Gangisetty 2010). This is thought to occur by the alteration in the subunit composition of the GABA‐A receptor (Maguire 2005). In animal models, progesterone has been found to reduce neuronal firing and decrease spontaneous and induced epileptiform discharges (Reddy 2004). Progesterone has demonstrated effects on reducing the number of excitatory synapses and the number of oestrogen receptors (McEwen 2001). Other experimental studies support the role of allopregnanolone (a metabolite of progesterone) as conferring seizure protection, with the role of progesterone largely unexplained (Kokate 1999).
The mechanism by which oestradiol causes seizures is uncertain (Osborne 2009). It may regulate the limbic system: there is evidence of oestradiol‐synthesising enzymes present within the hippocampus of the temporal lobe. It has also been hypothesised that oestradiol increases excitation by enhancing glutamate transmission and associated receptors (Woolley 1994; Smejkalova 2010). Several studies of chronic oestrogen administration in females, however, show either anticonvulsant effects or no effect of oestrogen on seizures. Studies have also demonstrated that, in low doses, oestradiol can produce neuroprotective effects (Velísková 2000; Kalkbrenner 2003). Modulation of enzymes involved in glutamate breakdown to GABA have been proposed as neuroprotective mechanisms (Joh 2006; Ledoux 2009).
A detailed understanding of the patterns and pathophysiology is paramount for the development of rational approaches for preventing and treating catamenial epilepsy.
Why it is important to do this review
Catamenial epilepsy and seizure exacerbation is common in women with epilepsy, and may have a significant negative impact on quality of life. Women may not receive appropriate treatment for their catamenial seizures because of uncertainty regarding which treatment works best and when in the menstrual cycle treatment should be taken, as well as the possible impact on fertility, the menstrual cycle, bone health, and cardiovascular health. This review aims to address these issues to inform clinical practice and future research.
Objectives
To evaluate the efficacy and tolerability of hormonal and non‐hormonal treatments for seizures exacerbated by the menstrual cycle in women with regular or irregular menses. We synthesised the evidence from randomised and quasi‐randomised controlled trials of hormonal and non‐hormonal treatments in women with catamenial epilepsy of any pattern.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and quasi‐randomised controlled trials of blinded or open‐label design. We only included studies that randomised participants individually (i.e. cluster‐randomised trials were not included). We included trials with a cross‐over design if each treatment period was at least 12 weeks in length and the trial had a suitable wash‐out period.
Types of participants
We included women of childbearing age who had experienced a catamenial pattern of seizures in at least two baseline cycles, defined as one or more of the following.
C1 pattern: a greater average daily seizure frequency during the perimenstrual phase (days −3 to +3) compared with the mid‐follicular phase (days 4 to 9) and mid‐luteal phase (days −12 to 14) in normal ovulatory cycles.
C2 pattern: a greater average daily seizure frequency during the periovulatory phase (days 10 to −13) compared to the mid‐follicular phase (days 4 to 9) and mid‐luteal phase (days −12 to 14) in normal ovulatory cycles.
C3 pattern: a greater average daily seizure frequency during the luteal phase (days 15 to 28) compared to the follicular phase (days 1 to 14) in anovulatory cycles.
Types of interventions
We included the following intervention and control groups.
Intervention group: women who received a hormonal or non‐hormonal drug intervention in addition to an existing antiepileptic drug regimen for a minimum treatment duration of 12 weeks.
Control group(s): women who received a placebo, comparative drug intervention, or no treatment in addition to an existing antiepileptic drug regimen for a minimum treatment duration of 12 weeks.
Types of outcome measures
Primary outcomes
Seizure freedom, defined as the proportion of women who became seizure‐free over the treatment period.
Responder rate, defined as the proportion of women with a 50% reduction in seizure frequency compared to baseline.
Change in seizure frequency, defined as the absolute and percentage change in seizure frequency compared to baseline.
Secondary outcomes
-
Withdrawals, defined as the number of withdrawals from allocated treatment or from the trial.
Withdrawals for any reason
Withdrawals due to lack of efficacy
Withdrawals due to adverse events
-
Adverse events: of interest (outlined below), including serious adverse events, and other events reported in the trials irrespective of relationship to treatment.
Seizure exacerbation
Cardiac events
Thromboembolic events
Osteoporosis and bone health
Mood disorders
Sedation
Menstrual cycle disorders
Fertility issues
-
Quality of life, according to validated general scales such as the 36‐Item Short Form Health Survey (SF‐36), EuroQol 5‐Dimensions (EQ‐5D), or epilepsy‐specific scales such as the Quality Of Life In Epilepsy‐31 (QOLIE‐31).
Total quality of life score
Domain‐specific scores of quality of life scales
None of the included studies reported on quality of life. If quality of life is reported in future versions of this review, in the first instance, we will report change from baseline in quality of life; if change‐from‐baseline scores are not available, we will report the final scores.
Search methods for identification of studies
Electronic searches
We ran searches for the original review in April 2016, and subsequent searches in January 2018 and January 2019. For the current update, we searched the following databases on 20 July 2021, with no language restrictions. We sought translation of reports published in any languages other than English.
Cochrane Register of Studies (CRS Web), using the search strategy shown in Appendix 1.
MEDLINE (Ovid) (1946 to 19 July 2021), using the search strategy shown in Appendix 2.
CRS Web includes randomised or quasi‐randomised controlled trials from PubMed, Embase, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform, the Cochrane Central Register of Controlled Trials (CENTRAL), and the specialised registers of Cochrane Review Groups including Cochrane Epilepsy.
Searching other resources
We reviewed the reference lists of retrieved trials to check for additional reports of relevant studies.
Data collection and analysis
Selection of studies
The two review authors (MM and SJN) independently assessed trials for inclusion using Cochrane Covidence software (Covidence). We first screened the titles and abstracts of the records, excluding any that were clearly irrelevant. We then screened the full‐text articles for inclusion, recording the excluded studies and the reasons for their exclusion. Any disagreements between review authors regarding eligibility of trials were resolved by discussion. The screening process is displayed in a PRISMA study flow diagram (Figure 2) (Moher 2009).
2.
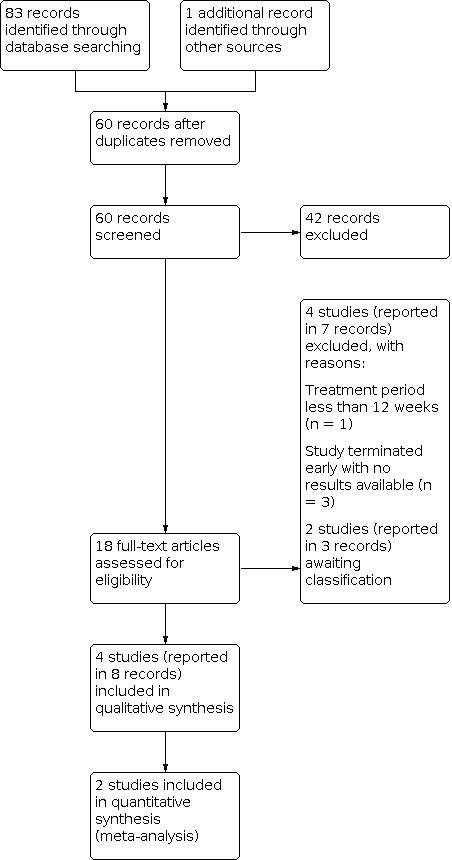
Study flow diagram.
Data extraction and management
We extracted the following information for each trial using a data extraction form.
Methodology/trial design
Method of randomisation and concealment
Method of blinding
Trial inclusion and exclusion criteria
Number of people excluded from analyses
Duration of trial periods, e.g. baseline, treatment, and follow‐up periods, and total trial duration
Trial intervention treatment: type of drug and dose
Trial control treatment: type of control (including type of drug and dose if applicable)
Source of funding of the trial and author disclosures
Participant demographics
Total number of women randomised to each group
Age (overall and by treatment group)
Epilepsy/seizure type
Epilepsy duration and aetiology
Existing antiepileptic drug regimen (including dose, overall and by treatment group)
Baseline seizure frequency (overall and by treatment group)
Proportion with C1, C2, and C3 catamenial pattern of seizures
Results
Number of women included in analysis of each outcome by treatment group
Outcome summary data for each intervention (see Types of outcome measures)
The two review authors (MM and SJN) independently extracted the data for each trial and compared extractions. We piloted the content of the data extraction form on an eligible trial, adding to the content where required. Any discrepancies in data extracted between the two review authors were resolved by discussion.
If any of the above information was recorded but not published within the trial reports, or if information was unclear, we contacted the original trial authors for clarification.
Assessment of risk of bias in included studies
The two review authors (MM and SJN) independently assessed the risk of bias for each trial using the Cochrane risk of bias tool, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We rated each of the following six domains as low, high, or unclear risk of bias: method of generating the random sequence, allocation concealment, blinding methods, incomplete outcome data, selective outcome reporting, and other sources of bias.
For included cross‐over studies, we also considered additional criteria for assessing risk of bias in cross‐over studies described in Section 16.4.3 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), and summarised any specific concerns relating to the cross‐over design for the domain 'other sources of bias'.
Any discrepancies in the risk of bias judgements between the two review authors were resolved by discussion.
Measures of treatment effect
We reported dichotomous data (seizure freedom, responder rate, withdrawals, and adverse events) as risk ratios (RRs) with 95% confidence intervals (CIs). Where a large number of different adverse events were reported across the studies (e.g. more than five different adverse events), we reported 99% CIs for this outcome to account for multiplicity of statistical testing.
We reported change in seizure frequency as mean difference (MD) in the change from baseline, with 95% CIs.
None of the included studies reported on quality of life. If quality of life is reported in future versions of the review, we will report it as MD with 95% CIs where the same scales are used across studies. If different quality of life scales are reported across studies, we will consider the similarity of the domains and questions of the scales, and if we deem the different scales to be sufficiently similar, will report pooled quality of life scores as the standardised mean difference (SMD) with 95% CIs. If the different scales are deemed insufficiently similar to combine, we will report each scale in separate analyses (where data allow), or in a narrative review.
Unit of analysis issues
We only included studies that randomised participants individually (i.e. cluster‐randomised trials were not included). We included cross‐over trials if each treatment period was a least 12 weeks in length and the trial had a suitable wash‐out period.
For cross‐over studies, in the first instance, we intended to use methods recommended by Elbourne for pooling cross‐over data, which take account of the correlation between measurements taken from the same group of participants via paired analyses (Elbourne 2002). Alternatively, if suitable data were not available, we may have been able to either use the first‐period data only, or to treat the cross‐over studies as if they were parallel studies, which is a conservative approach and does not take account of within‐participant correlation. However, the two included cross‐over trials reported very limited information about the study design and numerical results, therefore we reported the results of these cross‐over studies narratively (Dana‐Haeri 1983; Cleland 1995).
Had we identified trials with more than two treatment arms (e.g. drug A, drug B, and placebo), we would have constructed separate head‐to‐head comparisons to consider the different pairs of interventions and controls.
Dealing with missing data
We recorded the attrition rates reported in each trial and, if possible and appropriate, contacted the original trial authors if the extent of missing data was unclear. To enable an intention‐to‐treat analysis in the review, we extracted and reported data by randomised treatment groups where possible, irrespective of compliance with allocated treatment, exclusion from analysis, or loss to follow‐up.
In the event of substantial amounts of missing outcome data, we considered the potential bias that may have been introduced when interpreting the results, particularly if the missing data were deemed to not be missing at random.
If appropriate, for the primary outcomes of seizure freedom and responder rate, we planned to consider sensitivity analyses such as best‐case scenario and worst‐case scenario analyses (in the best‐case scenario, individuals in the treatment group are assumed to have a good outcome and those in the control group are assumed to have a bad outcome; in the worst‐case scenario the opposite is assumed).
Assessment of heterogeneity
We assessed clinical heterogeneity by reviewing the differences across trials in design, characteristics of recruited participants, and interventions. Where we were able to perform meta‐analysis, we also estimated heterogeneity statistically using a Chi2 test for heterogeneity (with a conservative judgement of P value less than 0.1 suggestive of heterogeneity) and the I2 statistic. We interpreted the I2 statistic as follows (Deeks 2011):
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
If a sufficient number of trials (10 or more) were included in any comparison, we would investigate publication bias by using a funnel plot and examining any asymmetry. However, fewer than 10 studies were included for the two comparisons of the review, therefore we could not examine funnel plots.
To assess selective reporting bias, we compared the measurements and outcomes planned by the original investigators during the trial with those reported in the published paper by checking the trial protocols (when available) against the information in the final publication. Where published protocols were not available and the trial authors did not provide an unpublished protocol upon request, we compared the methods and the results sections of the published papers. We also used our knowledge of the clinical area to identify where trial investigators had not reported commonly used outcome measures.
Data synthesis
We planned that where trials were deemed sufficiently homogenous in design, participant characteristics, and interventions, we would perform meta‐analysis using Mantel‐Haenszel methodology for dichotomous data and inverse‐variance methodology for continuous data (see Measures of treatment effect). We intended to use a fixed‐effect meta‐analysis model in the first instance. If we found substantial or considerable heterogeneity (i.e. an I2 value of more than 50%), we would repeat the meta‐analysis with a random‐effects model and compare the results of both models.
Where we deemed that the designs, participant characteristics, and interventions were too heterogeneous to combine data, we planned to report the results in a narrative review. Where appropriate, we intended to present outcome data in tables or enter trial‐specific data into forest plots for visual purposes, without the pooling of any outcome data.
For most of the outcomes of the two comparisons in this review, trial‐specific data only were entered into forest plots, or results were reported narratively.
Subgroup analysis and investigation of heterogeneity
We assessed clinical and statistical heterogeneity using the methods outlined in Assessment of heterogeneity.
If appropriate, and if data allowed, we planned to conduct the following subgroup analyses for all outcomes.
Type of epilepsy (focal versus generalised onset, and temporal versus extratemporal onset)
Type of seizure (e.g. focal seizure without altered awareness, focal seizure with altered awareness, secondary generalised seizure, primary generalised seizure, myoclonic seizure, absence seizure)
Catamenial pattern (C1, C2, and C3)
Age groups, as defined by the trials (e.g. puberty, sexual maturity, perimenopausal)
The data reported in the included studies did not permit subgroup analyses of type of epilepsy, catamenial pattern, or age groups. One trial reported seizure outcome results by type of seizure; we have presented these results in Effects of interventions (Herzog 2012).
Sensitivity analysis
As outlined in Dealing with missing data, if substantial outcome data were missing and where appropriate, we would consider sensitivity analyses such as best‐case scenario and worst‐case scenario analyses.
Where appropriate, we would also consider performing a sensitivity analysis excluding studies at high risk of bias across any of the domains outlined in Assessment of risk of bias in included studies.
Summary of findings and assessment of the certainty of the evidence
We generated a summary of findings table for each comparison in the review (Table 1; Table 2), including all outcomes (Schünemann 2011): seizure freedom, responder rate, change in seizure frequency, withdrawals, adverse events, and quality of life.
Summary of findings 1. Norethisterone compared to placebo for seizures in catamenial epilepsy.
| Norethisterone compared to placebo for seizures in catamenial epilepsy | ||||||
|
Patient or population: women with seizures due to catamenial epilepsy Settings: outpatients Intervention: norethisterone Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Norethisterone | |||||
|
Seizure freedom Follow‐up: NA |
Outcome not reported. | NA | ||||
|
Responder rate Follow‐up: NA |
Outcome not reported. | NA | ||||
|
Change in seizure frequency Follow‐up: up to 14 months |
Neither of the studies showed any significant differences between treatments in terms of seizure frequency. | NA | 24 (2 cross‐over studies) | ⊕⊝⊝⊝
very low1 |
1 of the studies also considered tonic‐clonic and complex‐partial catamenial seizures separately. No significant differences were found between treatments by seizure type. | |
|
Number of withdrawals from the study Follow‐up: up to 14 months |
Neither of the studies reported any treatment withdrawals. | NA | 24 (2 cross‐over studies) | ⊕⊝⊝⊝
very low1 |
||
|
Adverse events Follow‐up: up to 14 months |
4 "mild" types of adverse event considered to be related to the trial medication were reported: irregularities in menstrual cycle (5 women), facial rash (1 woman), headaches (2 women), mild swelling of hands and feet (1 woman), and bloated feeling (1 woman). | NA | 15 (1 cross‐over study) | ⊕⊝⊝⊝
very low1 |
It is assumed that these events occurred whilst women were taking norethisterone (and no events were reported in the placebo group), but this is not explicitly stated. | |
|
Quality of life Follow‐up: NA |
Outcome not reported. | NA | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NA: not applicable | ||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded three times due to serious imprecision and risk of bias: the two included studies used a cross‐over design, had a very small sample size, and reported limited information regarding study design and numerical results.
Summary of findings 2. Progesterone compared to placebo for seizures in catamenial epilepsy.
| Progesterone compared to placebo for seizures in catamenial epilepsy | ||||||
|
Patient or population: women with seizures due to catamenial epilepsy Settings: outpatients Intervention: progesterone Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Progesterone | |||||
|
Seizure freedom: all seizures Follow‐up: 12 weeks |
No women in the placebo group achieved freedom from all seizures. | 4% of women in the progesterone group achieved freedom from all seizures. |
RR 4.03 (0.21 to 76.21) |
130 (1 study) | ⊕⊕⊝⊝ low1,2 | There was also no significant difference between treatments in terms of freedom from the most severe seizure type (RR 2.09, 95% CI 0.61 to 7.10). |
|
Responder rate: 50% or greater reduction in all seizures Follow‐up: 12 weeks |
200 per 1000 |
228 per 1000 (112 to 464 per 100) |
RR 1.14 (0.56 to 2.32) | 130 (1 study) | ⊕⊕⊕⊝
moderate1 |
There was also no significant difference between treatments in terms of responder rate for the most severe seizure type (RR 1.24, 95% CI 0.67 to 2.29) or when considering each seizure type individually (complex focal, simple focal, secondary generalised seizures). |
|
Change in seizure frequency Follow‐up: 12 weeks |
1 study (n = 36) reported that the decrease in seizure frequency from baseline in the progesterone group was significantly higher than the decrease in seizure frequency from baseline in the placebo group (P = 0.024). 1 study (n = 130) reported no significant differences between treatments with respect to proportional changes for all seizures combined, most severe seizure type, or each seizure type considered separately (complex focal, simple focal, secondary generalised seizures). |
NA | 166 (2 studies) |
⊕⊕⊝⊝ low1,3 | Due to different methods of data presentation, results could not be combined in meta‐analysis. | |
|
Number of withdrawals from the study: for any reason Follow‐up: 12 weeks |
141 per 1000 |
219 per 1000 (114 to 422 per 1000) |
RR 1.56 (0.81 to 3.00) | 168 (2 studies) |
⊕⊕⊕⊝
moderate1 |
There was also no significant difference between progesterone and placebo in terms of treatment withdrawals due to adverse events (pooled RR 2.91, 95% CI 0.53 to 16.17). |
|
Adverse events: any adverse event Follow‐up: 12 weeks |
511 per 1000 |
434 per 1000 (302 to 634 per 1000) |
RR 0.85 (0.59 to 1.24) | 130 (1 study) | ⊕⊕⊕⊝
moderate1 |
There was no significant difference between progesterone and placebo in the proportion of women experiencing specific adverse events occurring in at least 5% of participants (diarrhoea, dyspepsia, nausea, vomiting, nasopharyngitis, fatigue, dizziness, headache, and depression). In the other study (n = 36), 2 women were excluded from the study due to progesterone side effects (severe headache, nausea and vomiting). No further information on adverse events was provided in this study. |
|
Quality of life Follow‐up: NA |
Outcome not reported. | NA | ||||
| *The basis for the assumed risk is the event rate in the placebo group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NA: not applicable; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded once due to risk of bias: unclear methodological information regarding allocation concealment and attrition in the included trial(s). One of the trials was terminated early due to futility analyses and is therefore statistically underpowered. 2Downgraded once due to imprecision: confidence intervals around the treatment effect are very wide due to the small number of events. 3Downgraded once due to inconsistency: results of the two studies could not be combined in meta‐analysis due to different methods of presenting the outcome. Study‐specific results are not consistent and lead to different conclusions.
For clarity and brevity in the tables, we reported a general statement about the summary of findings for secondary outcomes (withdrawals, adverse events, quality of life), based on different reasons for withdrawal, different adverse events, and different quality of life scales.
We determined the certainty of the evidence using the GRADE approach (Atkins 2004), and downgraded the evidence in the presence of high risk of bias in at least one study due to incompleteness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, and high probability of publication bias. We downgraded the evidence by one level if we considered the limitation to be serious, and by two levels if very serious.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies
Results of the search
The searches outlined above (see Electronic searches) identified 83 records. We found one additional record from other sources. Following removal of duplicates, we screened 60 records and excluded 42 based on title and abstract. We further screened the remaining 18 records, accessing full texts where these were available. We included four studies reported in eight records in the review, excluded four studies reported in seven records, and assessed two studies reported in three records where the published information was insufficient to include these studies at present as awaiting classification (see Characteristics of studies awaiting classification).
A PRISMA study flow diagram is shown in Figure 2.
Included studies
We found four randomised, placebo‐controlled, double‐blind studies of progesterone therapy used for seizures in catamenial epilepsy that reported on the primary efficacy outcome and that met our inclusion criteria. Two of these trials were parallel‐group studies (Herzog 2012; Najafi 2013), and two were cross‐over studies (Dana‐Haeri 1983; Cleland 1995). The included studies randomised a total of 356 women aged between 13 and 45 years. One study also included 164 women with non‐catamenial epilepsy, of which only 130 women with catamenial epilepsy were relevant to this review (Herzog 2012), therefore 192 women from the four studies were included in the review.
Two studies reported on women with focal epilepsy (Dana‐Haeri 1983; Herzog 2012); one study reported on focal and generalised onset epilepsies (Najafi 2013); and one study did not report patient classification of epilepsy (Cleland 1995). Two studies included women with either a C1 or C3 pattern of seizure exacerbation (Dana‐Haeri 1983; Najafi 2013); one study included all catamenial patterns (Herzog 2012); and one study did not report the specific pattern of catamenial seizures (Cleland 1995). Two studies administered progesterone during the second half of the cycle (Herzog 2012; Najafi 2013); one study administered norethisterone during day 5 to 28 (Dana‐Haeri 1983); and one study administered norethisterone but did not specify when during the menstrual cycle it was used (Cleland 1995).
Studies comparing norethisterone and placebo
Cleland 1995 published in abstract form a single‐centre UK, placebo‐controlled, cross‐over study of norethisterone (0.35 mg/day) in 15 women under double‐blind conditions. The only reported inclusion criterion was a documented catamenial exacerbation of epilepsy. Women were randomised to receive six months of either norethisterone (0.35 mg daily) or placebo treatment followed by a two‐month wash‐out period, followed by six months of the other treatment. None of the women withdrew from the study, and all randomised participants were included in the reported analysis.
Dana‐Haeri 1983 reported on a single‐centre UK, placebo‐controlled, cross‐over study of high‐ and low‐dose norethisterone in 9 women aged between 20 and 30 years under double‐blind conditions. Women were included if they demonstrated a catamenial pattern of seizures defined as: an increased seizure frequency or occurrence of generalised seizures before (luteal phase) or during menstruation in at least 5 of 12 menstrual cycles. Women were randomised to either placebo, norethisterone 1.05 mg/day, or norethisterone 15 mg/day and observed through each treatment for four menstrual cycles (day 5 to day 26), then switched to the other treatment arms. At the end of the 12 cycles, each woman was observed for 1 to 2 months without taking any hormonal treatment. None of the women withdrew from the study, and all randomised participants were included in the reported analysis.
Studies comparing progesterone and placebo
Herzog 2012 reported on a multicentre, placebo‐controlled, parallel study of progesterone 600 mg/day in 294 women aged 13 to 45 years under double‐blind conditions. Women were included if they had focal onset epilepsy (as evidenced by electroencephalogram (EEG)) and intractable seizures (persistent seizures despite trials of two or more antiepileptic drugs) and a seizure frequency of two or more per month in a three‐month baseline period. Women were excluded if they had a progressive neurologic or systemic disorder or more than two‐fold elevation in liver enzyme levels. None of the women were taking major tranquillisers or contraceptives during the three months prior to enrolment. Following a three‐month baseline period, women were classified into catamenial (n = 130) or non‐catamenial stratum (n = 164). Catamenial strata included the following types of seizure pattern: C1: perimenstrual, C2: periovulatory, or C3: entire luteal phase. Women were randomised to one of two treatment arms consisting of a placebo or progesterone 600 mg/day taken on days 14 to 28 for three menstrual cycles. Thirty‐three of the 130 catamenial women withdrew from the study. The study reported outcomes for 124 catamenial women, with six women (progesterone arm) excluded from the primary analysis for unknown reasons.
Najafi 2013 reported on a single‐centre, Iranian‐based, placebo‐controlled, parallel study of progesterone (Mejestrol) 80 mg/day in 38 women, mean age 30.5 years, under double‐blind conditions. Women were included if they experienced focal or generalised seizures and a catamenial pattern defined as either a two‐fold increase in seizures during: premenstrual (day −3 to day +2) or whole of luteal phase (day 2 to day 10) together with a low progesterone level (< 5 mg/mL) in the mid‐luteal phase for luteal exacerbations of seizures. Exclusions included pregnancy, lactating, the use of major tranquillisers and antidepressants, abnormal menses, contraceptive use, previous history of thromboemboli, and not willing to consent. The description of an inadequate luteal phase is not typically defined as day 2 to day 10, although the study reports measuring a mid‐luteal progesterone level in the third week of the cycle or 21st day, or both. Following a three‐month baseline period, women were randomly assigned to either placebo or 80 mg/day of progesterone (Mejestrol) taken on day 15 to day 25. Two women in the progesterone group withdrew from the study and were excluded from the primary analysis.
Excluded studies
See: Characteristics of excluded studies
We excluded four studies for the following reasons: one study had a treatment period of less than 12 weeks (Feely 1982), whilst the remaining three studies were terminated early due to poor recruitment (NCT00630630), a change in protocol (NCT00559169), or by the institutional review board (NCT00530413), with no results available.
Risk of bias in included studies
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two trials reported adequate methods of randomisation and were judged to be at low risk of bias: one trial used block randomisation (block size six) conducted separately for women with and without catamenial epilepsy (Herzog 2012), and one trial used random allocation software to randomly divide consecutive patients into two groups (Najafi 2013). The remaining two trials did not report methods used in random sequence generation and were judged to be at unclear risk of bias (Dana‐Haeri 1983; Cleland 1995). None of the trials reported methods of allocation concealment and were therefore all judged to be at unclear risk of bias for this domain.
Blinding
All trials reported adequate methods of blinding of participants and personnel and were judged to be at low risk of bias. All studies were double‐blind and used matched placebo tablets. We assessed the risk of bias for blinding of outcome assessors as unclear in three trials for which no details were reported (Dana‐Haeri 1983; Cleland 1995; Najafi 2013). The fourth trial reported that the statistician, pharmacist, and study safety monitor were all blinded to the outcome, hence this study was judged to be at low risk of bias (Herzog 2012).
Incomplete outcome data
We assessed the risk of bias for incomplete outcome data as unclear in three studies (Dana‐Haeri 1983; Cleland 1995; Herzog 2012), as there were inconsistencies and limited information on the number of women used in calculating outcomes and whether an intention‐to‐treat method was used. We judged the fourth study to be at high risk of bias, as it excluded two participants within the progesterone treatment group due to adverse events, analysing only those who completed the study using an evaluable‐case analysis (Najafi 2013). This form of analysis is prone to inaccurate estimates since it unreasonably assumes that those who discontinue treatment are representative (in terms of responder status) of those who remain.
Selective reporting
The four trials reported on either seizure outcomes, adverse events, number who withdrew, or a combination of these outcomes. None of the studies reported on quality of life outcomes. We assessed two trials as at low risk of reporting bias (Herzog 2012; Najafi 2013). The other two trials did not provide sufficient information to assess selective reporting bias (unclear risk of reporting bias) (Dana‐Haeri 1983; Cleland 1995).
Other potential sources of bias
In two trials there was insufficient information available to assess for other sources of bias (Dana‐Haeri 1983; Cleland 1995). We judged one trial reporting balanced baseline characteristics across treatment groups to be at low risk, with no other sources of bias detected (Najafi 2013). We assessed the fourth trial as at high risk of other sources of bias (Herzog 2012). The trial was significantly underpowered, with only 130 catamenial women recruited out of a target sample size of 640 (to include a sample size of 192 women within the catamenial stratum) to demonstrate a significant difference between progesterone and placebo (power = 0.80; α = 0.05). The trial was stopped early due to futility analyses showing that the blinded conditional power of the comparison for the primary outcome for that stratum had dropped below 50%. The authors identified a biological problem with the original design, reporting that failure of the trial to prove the principal hypothesis may relate to the design, which attempted to treat all three patterns of catamenial epilepsy, which are likely to differ in pathophysiology with a single treatment regimen.
Effects of interventions
Norethisterone versus placebo
Two included studies recruiting 24 women compared norethisterone to placebo (Dana‐Haeri 1983; Cleland 1995). Both were cross‐over studies and provided limited numerical results for the outcomes relevant to this review, therefore results of these studies are described narratively, and the certainty of the evidence for all reported outcomes for this comparison is graded as very low (Table 1).
Primary outcomes
Seizure freedom
Neither study reported on seizure freedom (Dana‐Haeri 1983; Cleland 1995).
Responder rate
Neither study reported on responder rate (Dana‐Haeri 1983; Cleland 1995).
Change in seizure frequency
One study reported that the number of seizures outside key days was higher with norethisterone, whereas the exacerbation of seizure frequency during key days was lower with norethisterone. The authors note that "no statistically significant differences between the treatment groups was shown", but no numerical results were presented to support this (Cleland 1995).
One study reported mean seizure frequencies per menstrual cycle over four menstrual cycles. None of the nine participants in this study showed a significant decrease in seizure frequency whilst taking the high and low doses of norethisterone compared with the placebo. The authors also reported that there was no significant decrease in seizure frequency when tonic‐clonic and complex‐partial catamenial seizures were considered separately (Dana‐Haeri 1983).
Secondary outcomes
Withdrawals
No withdrawals were reported in either of the studies (Dana‐Haeri 1983; Cleland 1995).
Adverse events
One study did not report on adverse events (Dana‐Haeri 1983).
The other study reported limited information on adverse events (Cleland 1995). The study reported four "mild" types of adverse event that were considered to be related to the trial medication in eight out of 14 randomised women: irregularities in menstrual cycle (five women), facial rash (one woman), headaches (two women), mild swelling of hands and feet (one woman), and bloated feeling (one woman). We assume that participants could have reported more than one adverse event, and that these events occurred whilst participants were taking norethisterone, but this information was not explicitly stated.
Quality of life outcomes
Neither study reported on quality of life outcomes (Dana‐Haeri 1983; Cleland 1995).
Progesterone versus placebo
Two included studies recruiting 168 women with catamenial epilepsy compared progesterone to placebo (Herzog 2012; Najafi 2013). One of the studies recruited women with catamenial epilepsy and women with non‐catamenial epilepsy (Herzog 2012); only results for the stratum with catamenial epilepsy are reported in this review. We assessed the certainty of the evidence for this comparison as moderate to low (Table 2).
Primary outcomes
Seizure freedom
One study did not report on seizure freedom (Najafi 2013).
Data from one study contributed to this outcome (Herzog 2012). The difference in the proportion of women achieving seizure freedom of all seizure types was not statistically significant (3/79, 3.8% progesterone versus 0/45, 0% placebo) (risk ratio (RR) 4.03, 95% confidence interval (CI) 0.21 to 76.21, P = 0.35, low‐certainty evidence, Analysis 1.1). However, the CIs around the RR were very wide due to the low number of women achieving seizure freedom, therefore we cannot rule out an advantage to progesterone over placebo, or vice versa, or no difference between treatments.
1.1. Analysis.

Comparison 1: Progesterone versus placebo, Outcome 1: Seizure freedom
The difference in the proportion of women achieving seizure freedom of the most severe seizure type was also not statistically significant (11/79, 13.9% progesterone versus 3/45, 6.7% placebo) (RR 2.09, 95% CI 0.61 to 7.10, P = 0.18, low‐certainty evidence, Analysis 1.1). Again, the CIs around the RR were very wide due to the low number of women achieving seizure freedom, therefore it is difficult to draw any conclusions.
Responder rate
One study did not report on responder rate (Najafi 2013).
Data from one study contributed to this outcome (Herzog 2012). The difference in the proportion of responders for all seizure types was not statistically significant (18/79, 22.8% progesterone versus 9/45, 20% placebo) (RR 1.14, 95% CI 0.56 to 2.32, P = 0.71, moderate‐certainty evidence, Analysis 1.2).
1.2. Analysis.

Comparison 1: Progesterone versus placebo, Outcome 2: Responder rate (50% reduction in seizure frequency)
The difference in the proportion of responders for the most severe seizure type was also not statistically significant (11/79, 13.9% progesterone versus 3/45, 6.7% placebo) (RR 1.24, 95% CI 0.67 to 2.29, P = 0.47, moderate‐certainty evidence, Analysis 1.2). The proportions of responders for each seizure type considered individually (complex focal, simple focal, secondary generalised seizures) did not differ significantly between progesterone and placebo (see Analysis 1.2).
Change in seizure frequency
Both trials contributed to this outcome (Herzog 2012; Najafi 2013), but due to the way the results were presented in the studies, data could not be combined in meta‐analysis. We assessed the certainty of the evidence for this outcome as low.
One study reported the mean seizure frequency in the three months before and the three months after the study (Najafi 2013). There was no difference between treatment groups in terms of seizure frequency in the three months before baseline (mean difference (MD) −1.40, 95% CI −4.39 to 1.59, P = 0.36, Analysis 1.3), but there was a statistically significant advantage for progesterone over placebo in terms of seizure frequency in the three months after baseline (MD −4.50, 95% CI −6.55 to −2.45, P < 0.001, Analysis 1.3). The original study also reports that a repeated‐measures analysis of variance (ANOVA) was conducted to test the difference between treatment groups over time: the authors concluded that the decrease in seizure frequency from baseline in the progesterone group is significantly higher than the decrease in seizure frequency from baseline in the placebo group (P = 0.024).
1.3. Analysis.

Comparison 1: Progesterone versus placebo, Outcome 3: Seizure frequency
One study reported the median and interquartile range of the per cent change in seizure frequency with progesterone treatment compared to placebo (Herzog 2012). The results are summarised in Table 3; the median reductions in seizure frequency were 19.9% and 12.0% in the progesterone and placebo groups respectively, but this difference was not statistically significant. The median reductions for the most severe seizure type or each seizure type considered separately (complex focal, simple focal, secondary generalised seizures) ranged from 15.4% to 38.1% in the progesterone group and 0% to 25.7% in the placebo group; again, none of the differences between the progesterone and placebo groups were statistically significant.
1. Percentage change in seizure frequency in Herzog 2012.
| Seizure type | Progesterone | Placebo | P value | ||
| n | Median (IQR) (%) | n | Median (IQR) (%) | ||
| All seizures | 79 | −19.9 (−49.4 to 7.4) | 45 | −12 (−43.0 to 8.5) | 0.393 |
| Most severe seizure type | 79 | −22.7 (−67.1 to 13.6) | 45 | −12 (−51.6 to 11.4) | 0.483 |
| Secondarily generalised motor seizures | 33 | −38.1 (−95.1 to 15.3) | 17 | −23.7 (−79.4 to 11.9) | 0.797 |
| Complex partial seizures | 76 | −15.4 (−46.2 to 0.0) | 43 | 0 (−45.7 to 21.3) | 0.147 |
| Simple partial seizures | 34 | −25.2 (−84.9 to 26.0) | 17 | −25.7 (−93.5 to 3.16) | 0.527 |
IQR: interquartile range
Secondary outcomes
Withdrawals
Both trials contributed to this outcome (Herzog 2012; Najafi 2013). A total of 26 out of 104 participants (25%) withdrew from progesterone for the following reasons: adverse events (n = 6), withdrew after treatment (n = 6), change in antiepileptic drug (n = 3), inappropriate menstrual cycle length (n = 3), compliance < 80% (n = 3), lost to follow‐up (n = 1), and other, unspecified reason (n = 4). Nine out of 64 participants (14%) withdrew from placebo for the following reasons: adverse events (n = 1), withdrew after treatment (n = 5), change in antiepileptic drug (n = 1), and other, unspecified reason (n = 2). No treatment withdrawals due to lack of efficacy were reported in either study.
There was no significant difference between progesterone and placebo in the two studies in terms of treatment withdrawals for any reason (pooled RR 1.56, 95% CI 0.81 to 3.00, P = 0.18, I2 = 0%, moderate‐certainty evidence, Analysis 1.4) or treatment withdrawals due to adverse events (pooled RR 2.91, 95% CI 0.53 to 16.17, P = 0.22, I2 = 0%, moderate‐certainty evidence, Analysis 1.4).
1.4. Analysis.

Comparison 1: Progesterone versus placebo, Outcome 4: Number of withdrawals from the study
Adverse events
Both trials contributed to this outcome (Herzog 2012; Najafi 2013).
In Najafi 2013, two women were excluded from the study due to progesterone side effects (severe headache, nausea and vomiting). No further information on adverse events was provided in this study.
In Herzog 2012, at least one adverse event was reported in 37 out of 85 women (43.5%) randomised to progesterone and 23 out of 45 women (51%) randomised to placebo. There was no significant difference in the proportion of women experiencing adverse events on progesterone versus placebo (RR 0.85, 95% CI 0.59 to 1.24, P = 0.41, moderate‐certainty evidence, Analysis 1.5).
1.5. Analysis.

Comparison 1: Progesterone versus placebo, Outcome 5: Any adverse events reported
Adverse events reported in at least 5% of participants in Herzog 2012 (diarrhoea, dyspepsia, nausea, vomiting, fatigue, nasopharyngitis, dizziness, headache, and depression) are summarised in Analysis 1.6. There was no significant difference in the proportion of women experiencing any of these adverse events between progesterone and placebo (99% CIs presented to allow for multiple statistical testing).
1.6. Analysis.

Comparison 1: Progesterone versus placebo, Outcome 6: Adverse events reported in > 5% of participants
Nine serious adverse events (SAE) were reported during treatment in Herzog 2012; however, these events were not separated into catamenial epilepsy and non‐catamenial epilepsy subgroups. The most common SAE was hospitalisation for seizures (two women on progesterone and three on placebo). Three additional SAEs were reported on progesterone treatment, but they were considered unlikely to be related to progesterone (stomach flu, thyroid carcinoma, blurred vision). One death occurred on progesterone, which was attributed to sudden unexplained death in epilepsy and was considered unlikely to be related to the progesterone treatment.
Quality of life outcomes
Neither study reported on quality of life outcomes (Herzog 2012; Najafi 2013)
Discussion
Summary of main results
All four randomised controlled trials (RCTs) included in this review trialled hormonal treatments in women with catamenial epilepsy.
One study reported on the 50% responder rate for progesterone 600 mg/day taken on days 14 to 28 versus placebo in catamenial epilepsy of any pattern (Herzog 2012). There were no statistically significant differences for the proportion of responders for all seizure types between those women randomised to progesterone and those to placebo (RR 1.14, 95% CI 0.56 to 2.32, P = 0.71). Proportions of responders for each seizure type considered individually (complex focal, simple focal, secondary generalised seizures) did not differ significantly between progesterone and placebo. The same study (and the only study to report on the proportion seizure‐free) did not detect any significant differences between treatment groups for all seizures (RR 4.03, 95% CI 0.21 to 76.21, P = 0.35), or when seizures types were considered individually (Herzog 2012).
All studies reported on changes in mean seizure frequency (Dana‐Haeri 1983; Cleland 1995; Herzog 2012; Najafi 2013); however, due to the way that results were presented in the studies, the data could not be combined in meta‐analysis for either progesterone or norethisterone. The norethisterone RCTs did not report any significant change in mean seizure frequency between groups, although the sample sizes were very small, and detail on data outcomes is very limited (Dana‐Haeri 1983; Cleland 1995). The progesterone RCTs reported conflicting results (Herzog 2012; Najafi 2013). One small RCT demonstrated a statistically significant reduction in mean seizure frequency (MD −4.50, 95% CI −6.55 to −2.45, P < 0.001) with progesterone 80 mg/day taken on day 15 to day 25 when compared to placebo in the three months after baseline (Najafi 2013). The other, larger RCT did not demonstrate a significant difference between progesterone 600 mg/day taken on day 14 to 28 and placebo with respect to proportional changes for all seizures combined, most severe seizure type, or each seizure type considered separately (complex focal, simple focal, secondary generalised seizures) (Herzog 2012).
Results for the outcome treatment withdrawal were reported in the two progesterone RCTs but in neither of the norethisterone RCTs. For the two progesterone RCTs (Herzog 2012; Najafi 2013), there was no significant difference between progesterone and placebo in terms of treatment withdrawals for any reason (pooled RR 1.56, 95% CI 0.81 to 3.00, P = 0.18, I2 = 0%) or treatment withdrawals due to adverse events (pooled RR 2.91, 95% CI 0.53 to 16.17, P = 0.22, I2 = 0%).
Limited information was reported for adverse events with norethisterone (Dana‐Haeri 1983; Cleland 1995). One study reported menstrual irregularities and headaches as the most frequently occurring adverse events, although it is unclear whether these outcomes occurred in the norethisterone group (Cleland 1995). For progesterone, one study showed no significant difference between progesterone and placebo in the proportion of women experiencing any adverse event (RR 0.85, 95% CI 0.59 to 1.24, P = 0.41) or any specific adverse event that occurred in at least 5% of participants (diarrhoea, dyspepsia, nausea, vomiting, fatigue, nasopharyngitis, dizziness, headache, and depression) (Herzog 2012). The other study reported limited information on adverse events, although two women were excluded from the study due to severe headache, nausea and vomiting (Najafi 2013).
None of the RCTs reported quality of life outcomes, therefore the effect of norethisterone and progesterone on this outcome is unclear.
Overall completeness and applicability of evidence
This review highlights a significant deficiency within the evidence base for clinical studies of treatments used in catamenial epilepsy. The included RCTs provided very limited data on the effectiveness of norethisterone and progesterone in catamenial epilepsy with regular menses. These trials had small sample sizes, short treatment durations, and differed in their inclusion of different patterns of catamenial seizures. According to the available data, the majority of women included in these RCTs had focal epilepsy. Given that the RCTs were all significantly underpowered, the outcomes lack precision, and therefore a treatment effect for norethisterone and progesterone cannot be ruled out.
We found no RCTs for non‐hormonal treatments of catamenial epilepsy or for women with irregular menses.
Different catamenial patterns of seizures were treated in the same way despite proposed differences in pathophysiological mechanisms. Methodological differences, small sample sizes, differences in definitions of catamenial strata, and incomplete baseline and demographic details make applicability of this evidence very limited.
A post hoc analysis reported by Herzog 2012 ascertained that women with a three‐fold increase in seizure frequency (C1 pattern, 21.4% of the women recruited into the trial) had a statistically significant response to progesterone treatment when compared to the combined placebo group (responder rate 37.8% versus 11.1%, P = 0.037). However, high‐quality clinical trials are required to examine this outcome further.
Quality of the evidence
The included RCTs used appropriate methods of participant and personnel blinding, but other risk of bias domains were judged to be at unclear risk of bias for most studies. None of the RCTs reported an explicit analysis by intention‐to‐treat, and one RCT excluded two women in the final analyses due to adverse events. Three of the four RCTs recruited very small sample sizes, and the largest RCT was terminated early due to under‐recruitment.
We judged the evidence from the included norethisterone RCTs to be of very low certainty due to serious imprecision and risk of bias: the two studies used a cross‐over design, had very small sample sizes, and reported limited information regarding study design and numerical results. We judged the evidence from the included progesterone RCTs to be of low to moderate certainty due to risk of bias (unclear methodological information regarding allocation concealment and attrition); imprecision around treatment effects due to small numbers of events; and inconsistencies between studies in reported methodologies, results, and conclusions.
Potential biases in the review process
There is a possible risk of publication bias in this review given that there are a number of studies awaiting classification. It is also possible that despite the exhaustive searches carried out in this review other sources of data have not been identified.
Agreements and disagreements with other studies or reviews
We are not aware of any other systematic reviews on treatments for perimenstrual seizures in catamenial epilepsy.
Authors' conclusions
Implications for practice.
Overall, this review provides very low‐certainty evidence of comparable effectiveness of norethisterone, and moderate‐ to low‐certainty evidence of comparable effectiveness of progesterone, both versus placebo for catamenial epilepsy. The review provides moderate‐certainty evidence for comparable tolerability of progesterone compared to placebo for adverse events (diarrhoea, dyspepsia, nausea, vomiting, fatigue, nasopharyngitis, dizziness, headache, and depression) and retention. The review provides no information on the comparative tolerability of norethisterone when compared to placebo.
Our review sadly highlights an overall deficiency in the literature base on the effectiveness of a wide range of other hormonal and non‐hormonal interventions currently being used in practice, particularly for those women who do not have regular menses.
Implications for research.
Despite the clinical importance of seizures in catamenial epilepsy and the high frequency of this pattern experienced in women with epilepsy, the literature base for high‐quality randomised controlled trials is lacking. Current trials largely in focal epilepsy attempted to treat various patterns of catamenial epilepsy using a single treatment regimen, which may represent a design fault in view of the likely differences in pathophysiology. For example, progesterone may have greater efficacy where progesterone withdrawal is the key pathophysiological change (C1 or C3 pattern), but may have limited or no effect of pre‐ovulatory seizures, where the proposed mechanism relates to oestrogen surge.
Any further research studies into this area must address the various pathophysiological mechanisms within the design concept. This may necessitate large sample sizes and multicentre collaboration. A future randomised controlled trial examining treatments for those with very high seizure exacerbations as part of a particular catamenial pattern may also be useful.
Areas of trial research might include considering progesterone earlier in the cycle for C2 pattern of seizures, or using alternate strategies, for example depot medroxyprogesterone acetate or gonadotropin‐releasing hormone (GnRH) analogues for this pattern (Haider 1991; Bauer 1992). Similarly, trials are needed to examine the effects of hormonal and non‐hormonal strategies in individuals with primary generalised forms of epilepsy (e.g. juvenile myoclonic epilepsy) where onset of seizures occur in puberty.
What's new
| Date | Event | Description |
|---|---|---|
| 20 July 2021 | New search has been performed | Searches updated 20 July 2021; no new studies identified. |
| 20 July 2021 | New citation required but conclusions have not changed | Conclusions are unchanged. |
History
Protocol first published: Issue 12, 2018 Review first published: Issue 10, 2019
Acknowledgements
We are grateful to Graham Chan for providing an example search strategy, and to the Cochrane Epilepsy Group for editorial support.
We, and the Cochrane Epilepsy Group, are grateful to the following peer reviewers for their time and comments: Marty Chaplin, Robin Grant, Catrin Tudur Smith, and also to our consumer representative who wishes to remain anonymous.
This review was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health and Social Care.
Appendices
Appendix 1. CRS Web search strategy
1 (catamenial NEAR4 epilep*) OR (catamenial NEAR4 seizure*) OR (perimenstrual NEAR4 epilep*) OR (perimenstrual NEAR4 seizure*) AND CENTRAL:TARGET
2 (menstrua* NEAR4 epilep*) OR (menstrua* NEAR4 seizure*) AND CENTRAL:TARGET
3 (#1 OR #2) AND CENTRAL:TARGET
Appendix 2. MEDLINE (Ovid) search strategy
This strategy includes a modification of the Cochrane Highly Sensitive Search Strategy for identifying randomized trials (Lefebvre 2021).
1. (catamenial adj4 epilep$).tw.
2. (catamenial adj4 seizure$).tw.
3. (perimenstrual adj4 seizure$).tw.
4. (perimenstrual adj4 epilep$).tw.
5. (menstrua$ adj4 epilep$).tw.
6. (menstrua$ adj4 seizure$).tw.
7. 1 or 2 or 3 or 4 or 5 or 6
8. exp controlled clinical trial/ or (randomi?ed or placebo or randomly).ab.
9. clinical trials as topic.sh.
10. trial.ti.
11. 8 or 9 or 10
12. exp animals/ not humans.sh.
13. 11 not 12
14. 7 and 13
15. remove duplicates from 14
Data and analyses
Comparison 1. Progesterone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Seizure freedom | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 All seizures | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.02 [0.21, 76.21] |
| 1.1.2 Most severe seizure type | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.61, 7.10] |
| 1.2 Responder rate (50% reduction in seizure frequency) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 All seizures | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.56, 2.32] |
| 1.2.2 Most severe seizure type | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.67, 2.29] |
| 1.2.3 Secondarily generalised motor seizures | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.57, 3.13] |
| 1.2.4 Complex partial seizures | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.60, 2.68] |
| 1.2.5 Simple partial seizures | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.50, 2.01] |
| 1.3 Seizure frequency | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 In the 3 months before the study | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐4.39, 1.59] |
| 1.3.2 In the 3 months after the study | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐4.50 [‐6.55, ‐2.45] |
| 1.4 Number of withdrawals from the study | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 Withdrawals for any reason | 2 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.81, 3.00] |
| 1.4.2 Withdrawals due to adverse events | 2 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.53, 16.17] |
| 1.5 Any adverse events reported | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.6 Adverse events reported in > 5% of participants | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 1.6.1 Diarrhoea | 1 | 130 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.53 [0.09, 3.07] |
| 1.6.2 Dyspepsia | 1 | 130 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.53 [0.04, 6.66] |
| 1.6.3 Nausea | 1 | 130 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.08 [0.00, 3.65] |
| 1.6.4 Vomiting | 1 | 130 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.53 [0.04, 6.66] |
| 1.6.5 Fatigue | 1 | 130 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.48 [0.42, 5.20] |
| 1.6.6 Nasopharyngitis | 1 | 130 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.18 [0.00, 11.65] |
| 1.6.7 Dizziness | 1 | 130 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.26 [0.05, 1.54] |
| 1.6.8 Headache | 1 | 130 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.79 [0.08, 7.94] |
| 1.6.9 Depression | 1 | 130 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.12 [0.12, 36.26] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cleland 1995.
| Study characteristics | ||
| Methods | Double‐blind randomised controlled trial, cross‐over design. | |
| Participants | 15 female participants with documented catamenial exacerbation of epilepsy. Age, epilepsy duration, and seizure frequency at baseline of participants not reported. |
|
| Interventions | 6 months of either norethisterone (0.35 mg daily) or placebo treatment, followed by 2‐month wash‐out followed by 6 months of the other treatment; usual medication was continued. | |
| Outcomes | Side effects. Mean menstrual cycle length. Number of seizures outside of "key days". Exacerbation of seizure frequency outside of "key days". |
|
| Funding | Not stated. | |
| Conflict of Interest | Not stated. | |
| Notes | Study reported as an abstract only. Very limited information regarding design reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study described as double‐blind, and a placebo was used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Inconsistent information throughout the abstract: 5 women studied, 8 out of 14 women had adverse events, but no women withdrew from the study. Unclear how many women were studied. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information reported in the abstract to permit a judgement. |
| Other bias | Unclear risk | Insufficient information reported in the abstract to permit a judgement. |
Dana‐Haeri 1983.
| Study characteristics | ||
| Methods | Double‐blind randomised controlled trial, cross‐over design. | |
| Participants | 9 female participants aged 20 to 30 years with catamenial exacerbation occurring in at least 5 of 12 menstrual cycles were included. Participants were either residents at the Chalfont Centre for Epilepsy or outpatients at the National Hospital, London. Epilepsy duration and seizure frequency at baseline not stated. |
|
| Interventions | 3 treatment periods, each of 4 menstrual cycles, followed by observation for 1 to 2 months:
8 women had been taking either single or combination antiepileptic drug therapy for a long time. 1 woman had discontinued taking carbamazepine and was not taking any antiepileptic drugs. |
|
| Outcomes | Seizure frequency during 4 menstrual cycles with each treatment. Results presented separately for women with tonic‐clonic seizures and those with complex partial and simple partial seizures. |
|
| Funding | Not stated. | |
| Conflict of Interest | Not stated. | |
| Notes | Unclear if there was a wash‐out period between treatment periods (but very limited information provided on study design, therefore study included despite unclear information about wash‐out period). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study described as double‐blind, size‐ and colour‐matched placebos used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No specific withdrawals reported, but information insufficient to permit a judgement. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, outcomes and statistical methods reported in brief. Limited data reported relating to seizures, and adverse events not reported; unclear if any further information was measured but not recorded. |
| Other bias | Unclear risk | Very limited information provided on study design and participant characteristics. Unclear if there was a wash‐out period between treatment periods. Unclear if any other bias may be present. |
Herzog 2012.
| Study characteristics | ||
| Methods | Double‐blind, phase III, parallel‐group, randomised (2:1 ratio) controlled trial conducted at 15 hospitals in the USA. | |
| Participants | Female participants, aged 13 to 45 years old, with intractable seizures despite trials of > 2 antiepileptic drugs at therapeutic levels, and monthly menses with intervals of 23 to 35 days. 294 participants with catamenial or non‐catamenial epilepsy were recruited (randomisation stratified by catamenial or non‐catamenial epilepsy); only 130 participants with catamenial epilepsy were relevant to this review. Mean age +/− SD (range): progesterone: 31.4 +/− 8.68 (11 to 45); placebo: 32.31 +/− 8.50 (14 to 45). Duration of epilepsy (years): mean +/− SD (range): progesterone: 18.11 +/− 10.24 (1 to 39); placebo: 18.60 +/− 10.76 (1 to 37). |
|
| Interventions | Treatment consisted of identical progesterone 200 mg or placebo lozenges, taken 3 times daily on days 14 to 28 of treatment cycles. 130 randomised (124 analysed): progesterone: 85 randomised/79 analysed; placebo: 45 randomised/45 analysed. 3 baseline menstrual cycles; 3 treatment menstrual cycles were analysed. |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Funding | This research was supported by a National Institutes of Health (NIH) research grant: NIH NINDS R01 39466. | |
| Conflict of Interest | Full disclosures are available in the journal article and on the online version of the journal article. | |
| Notes | Body mass index, seizure type, age at onset of epilepsy, epilepsy focus, laterality and basis of localisation at baseline were also reported. No significant differences between groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation (block size of 6), conducted separately for women with and without catamenial epilepsy, was conducted by an unblinded statistician. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was double‐blinded, and placebo lozenges used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Unblinded throughout the study (2000‐2010) were the unblinded statistician, research pharmacist and study safety monitor.” ClinicalTrials.gov entry also confirms that outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rates and reasons reported; the paper states that an intention‐to‐treat approach was used, but it is unclear why only 79 women (out of 85 randomised to progesterone) were included in the responder analyses. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods section and on ClinicalTrials.gov are well reported in the results of the paper. All expected outcomes reported. |
| Other bias | High risk | Underpowered ‐ expected recruitment compared to actual recruitment. The trial was stopped early due to futility analyses showing that the blinded conditional power of the comparison for the primary outcome for that stratum dropped below 50%. The trial is underpowered for the original hypothesis, but was stopped for the benefit of the participants, and the authors have identified a biological problem with the original design. “Failure of the trial to prove the principal hypothesis may relate to the design that attempted to treat 3 patterns of catamenial epilepsy which likely differ in pathophysiology with a single treatment regimen.” |
Najafi 2013.
| Study characteristics | ||
| Methods | Double‐blind, parallel‐group randomised controlled trial conducted at the Isfahan University of Medical Sciences between June 2011 and March 2012. | |
| Participants | Female participants with either complex partial seizure, secondary generalised seizure, or primary generalised seizure and received full‑dose antiepileptic drugs. Seizure patterns had to be in the catamenial form, and seizure had to become exacerbated during the premenstrual period (between the 25th day of the previous cycle and the second day of the next cycle) or the whole period of the luteal phase of the cycle (2nd to 10th days of the cycle). Mean age 30.5 ± 8.5 years (overall). Progesterone group (n = 17): mean (SD) 29.2 (8.7); median (IQR) 27 (21.5 to 35). Placebo group (n = 19): mean (SD) 32.1 (8.3); median (IQR) 33 (26 to 36). Epilepsy duration (years, overall): mean 16.3 ± 9.3 years. Progesterone group (n = 17): mean (SD) 15.1 (9.7); median (IQR) 13 (9 to 18). Placebo group (n = 19): mean (SD) 17.5 (8.8); median (IQR) 15 (13 to 20). Seizures in the 3 months before the study (overall): mean 7.8 ± 7.2 years. Progesterone group (n = 17): mean (SD) 6.2 (3.4); median (IQR) 5 (4 to 8). Placebo group (n = 19): mean (SD) 7.6 (5.6); median (IQR) 8 (3 to 15). |
|
| Interventions | Two 40 mg progesterone tablets daily (twice a day) in the 2nd half of the cycle from 15th to 25th day. 2 placebo tablets daily in the same manner. 38 randomised (36 analysed): progesterone: 19 randomised/17 analysed; placebo: 19 randomised/19 analysed. All participants took concomitant antiepileptic drugs. Analysis after 3 months of follow‐up (monthly visits and number of seizures recorded). |
|
| Outcomes | Comparison of number of seizures during 3 months before and after the study. | |
| Funding | No funding provided for the study. | |
| Conflict of Interest | None declared. | |
| Notes | No statistically significant difference in characteristics between groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Consecutive patients meeting the inclusion criteria were randomly divided into 2 groups using Random Allocation Software (Saghaei 2004). |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo tablets were manufactured that were formally the same. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 participants in the progesterone group were excluded from the study due to adverse events. Only those who completed the study were analysed. Small participant numbers and no intention‐to‐treat approach may have affected the results. |
| Selective reporting (reporting bias) | Low risk | Outcome reported in the registry entry (seizure frequency at 3 months) was reported in the publication. Adverse events also reported. |
| Other bias | Low risk | Baseline characteristics across groups are balanced, no other sources of bias detected. |
IQR: interquartile range SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Feely 1982 | Treatment period was less than 12 weeks. |
| NCT00530413 | Study terminated by Institutional Review Board, no results available. |
| NCT00559169 | Study terminated due to a change in protocol, no results available. |
| NCT00630630 | Study terminated prematurely after recruiting only 3 participants, primary and secondary outcomes not analysed. |
Characteristics of studies awaiting classification [ordered by study ID]
NCT01299870.
| Methods | Phase II, randomised, double‐blind, cross‐over trial. |
| Participants | Female participants between the ages of 21 and 45 years with a positive diagnosis of catamenial epilepsy. |
| Interventions | Keishibukuryogan versus placebo (added to usual antiepileptic drug treatment) for 12 weeks. Follow‐up: up to 36 weeks. |
| Outcomes | Safety (up to 36 weeks). Change in seizure frequency (with a focus on an increase in seizure frequency, up to 36 weeks). Change in progesterone levels (up to 36 weeks). |
| Notes | Results "submitted" to ClinicalTrials.gov, but not published online or within any journal article known to us. Awaiting assessment if results can be identified. |
Sperling 2017.
| Methods | Phase II, 18‐week, double‐blind, placebo‐controlled, randomised clinical trial of ganaxolone administered as add‐on therapy in adults with uncontrolled focal onset seizures. |
| Participants | Men or women aged 18 to 69 years inclusive were eligible if they had a diagnosis of epilepsy with focal onset seizures with or without secondarily generalised seizures. 100 out of 147 recruited participants were female, and "the female predominance was likely due to the perceived benefit for women who have catamenial epilepsy based on the ganaxolone’s mechanism of action". |
| Interventions | Ganaxolone (titrated up to 1500 mg/day) or placebo was added to existing antiepileptic drug therapy of up to 3 antiepileptic drugs, which were maintained at a stable dose for at least 30 days prior to enrolment. |
| Outcomes | Primary outcome: Change in mean weekly seizure frequency for all seizure types including complex focal onset seizures, simple focal onset seizures with motor manifestations, and secondarily generalised seizures (but excluding non‐motor simple partial seizures) during the titration plus maintenance periods (weeks 1 to 10). Secondary outcomes:
Exploratory endpoints: the Seizure Severity Questionnaire and Quality Of Life In Epilepsy‐31 Inventory (QOLIE‐31) |
| Notes | Results were not presented separately for participants with catamenial epilepsy. We have contacted the original authors to request results for the subgroup of participants with catamenial epilepsy. |
Differences between protocol and review
There are no differences between the protocol and the review.
Contributions of authors
MM and SJN wrote the protocol and completed the review. Both review authors approved the final version of the protocol and the review.
Sources of support
Internal sources
No sources of support provided
External sources
National Institute for Health Research (NIHR), UK
Declarations of interest
MM has no conflicts of interest.
SJN has no conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cleland 1995 {published data only}
- Cleland PG. Controlled double-blind cross-over study of norethisterone (0.35 mg/day) in the treatment of catamenial exacerbation of epilepsy. Epilepsia 1995;36(Suppl 3):S101. [Google Scholar]
Dana‐Haeri 1983 {published data only}
- Dana-Haeri J, Richens A. Effect of norethisterone on seizures associated with menstruation. Epilepsia 1983;24(3):377-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Herzog 2012 {published data only}
- Herzog AG, Fowler KM, Massaro JM, Pennell PB, Sperling MR, Liporace JD, et al. Progesterone therapy for women with epilepsy: results of the phase 3 NIH progesterone trial. Epilepsy Currents 2012;12(Suppl 1):350, Abstract no: 3.191. [Google Scholar]
- Herzog AG, Fowler KM, Smithson SD, Kalayjian LA, Heck CN, Sperling MR, et al. Progesterone vs placebo therapy for women with epilepsy: a randomized clinical trial. Neurology 2012;78(24):1959-66. [DOI: 10.1212/WNL.0b013e318259e1f9] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Frye CA, Progesterone Trial Study Group. Allopregnanolone levels and seizure frequency in progesterone-treated women with epilepsy. Neurology 2014;83(4):345-8. [DOI: 10.1212/WNL.0000000000000623] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Najafi 2013 {published data only}
- Najafi M, Sadeghi MM, Mehvari J, Zare M, Akbari M. Progesterone therapy in women with intractable catamenial epilepsy. Advanced Biomedial Research 2013;2:8. [DOI: 10.4103/2277-9175.107974] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Feely 1982 {published data only}
- Feely M, Calvert R, Gibson J. Catamenial epilepsy as a model for testing a new anticonvulsant (clobazam) [abstract]. Irish Journal of Medical Science 1982;151(11):358-9. [Google Scholar]
- Feely M, Calvert R, Gibson J. Clobazam in catamenial epilepsy. A model for evaluating anticonvulsants. Lancet 1982;2(8289):71-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Feely M, Calvert R, Gibson J. Clobazam in the treatment of catamenial epilepsy [abstract]. British Journal of Clinical Pharmacology 1982;13(2):273P-4P. [Google Scholar]
- Feely M, Gibson J. Intermittent clobazam for catamenial epilepsy: tolerance avoided. Journal of Neurology, Neurosurgery & Psychiatry 1984;47(12):1279-82. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00530413 {published data only}
- NCT00530413. Study of phenobarbital inhibition of catamenial epilepsy. clinicaltrials.gov/show/NCT00530413 (first received 17 September 2007).
NCT00559169 {published data only}
- NCT00559169. Verapamil and catamenial epilepsy. clinicaltrials.gov/show/NCT00559169 (first received 16 November 2007).
NCT00630630 {published data only}
- NCT00630630. Study on safety and efficacy of levetiracetam in the adjunctive treatment of female subjects with C1 catamenial epilepsy. clinicaltrials.gov/show/NCT00630630 (first received 7 March 2008).
References to studies awaiting assessment
NCT01299870 {published data only}
- NCT01299870. Catamenial epilepsy treatment. clinicaltrials.gov/show/NCT01299870 (first received 18 February 2011).
Sperling 2017 {published data only}
- Sperling MR, Klein P, Tsai J. Randomized, double‐blind, placebo‐controlled phase 2 study of ganaxolone as add‐on therapy in adults with uncontrolled partial‐onset seizures. Epilepsia 2017;58(4):558-64. [DOI: 10.1111/epi.13705] [PMID: ] [DOI] [PubMed] [Google Scholar]
Additional references
Ansell 1986
- Ansell B, Clarke E. Ovarian hormones, anticonvulsant drugs and seizures during the menstrual cycle in women with epilepsy. Journal of Neurology, Neurosurgery & Psychiatry 1986;49(1):47-51. [DOI: 10.1136/jnnp.49.1.47] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ (Clinical Research Ed.) 2004;328(7454):1490. [DOI: 10.1136/bmj.328.7454.1490] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bauer 1992
- Bauer J, Wild L, Flügel D, Stefan H. The effect of a synthetic GnRH analogue on catamenial epilepsy: a study in ten patients. Journal of Neurology 1992;239(5):284-6. [DOI: 10.1007/bf00810354] [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Melbourne, Australia: Veritas Health Innovation, Date accessed: 20th July 2021. Available at covidence.org.
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, editor(s), Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI: 10.1093/ije/31.1.140] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gangisetty 2010
- Gangisetty O, Reddy DS. Neurosteroid withdrawal regulates GABA-A receptor α4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience 2010;170(3):865-80. [DOI: 10.1016/j.neuroscience.2010.07.037] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haider 1991
- Haider Y, Barnett DB. Catamenial epilepsy and goserelin (letter). Lancet 1991;338(8781):1530. [PMID: ] [DOI] [PubMed] [Google Scholar]
Herzog 1997
- Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia 1997;38(10):1082-8. [DOI: 10.1111/j.1528-1157.1997.tb01197.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Herzog 2001
- Herzog AG, Friedman MN. Menstrual cycle interval and ovulation in women with localization-related epilepsy. Neurology 2001;57(11):2133–5. [DOI: 10.1212/wnl.57.11.2133] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Joh 2006
- Joh HD, Searles RV, Selmanoff M, Alkayed NJ, Koehler RC, Hurn PD, et al. Estradiol alters only GAD67 mRNA levels in ischemic rat brain with no consequent effects on GABA. Journal of Cerebral Blood Flow & Metabolism 2006;26(4):518–26. [DOI: 10.1038/sj.jcbfm.9600211] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalkbrenner 2003
- Kalkbrenner KA, Standley CA. Estrogen modulation of NMDA-induced seizures in ovariectomized and non-ovariectomized rats. Brain Research 2003;964(2):244-9. [DOI: 10.1016/s0006-8993(02)04065-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kokate 1999
- Kokate TG, Juhng KN, Kirkby RD, Llamas J, Yamaguchi S, Rogawski MA. Convulsant actions of the neurosteroid pregnenolone sulfate in mice. Brain Research 1999;831(1-2):119-24. [DOI: 10.1016/s0006-8993(99)01287-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kotsopoulos 2002
- Kotsopoulos IA, Merode T, Kessels FG, Krom MC, Knottnerus JA. Systematic review and meta-analysis of incidence studies of epilepsy and unprovoked seizures. Epilepsia 2002;43(11):1402-9. [DOI: 10.1046/j.1528-1157.2002.t01-1-26901.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Laidlaw 1956
- Laidlaw J. Catamenial epilepsy. Lancet 1956;271(6955):1235-7. [DOI: 10.1016/s0140-6736(56)90003-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ledoux 2009
- Ledoux VA, Smejkalova T, May RM, Cooke BM, Woolley CS. Estradiol facilitates the release of neuropeptide Y to suppress hippocampus-dependent seizures. Journal of Neuroscience 2009;29(5):1457–68. [DOI: 10.1523/JNEUROSCI.4688-08.2009] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Maguire 2005
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nature Neuroscience 2005;8(6):797-804. [DOI: 10.1038/nn1469] [PMID: ] [DOI] [PubMed] [Google Scholar]
McEwen 2001
- McEwen BS. Invited review: estrogens effects on the brain: multiple sites and molecular mechanisms. Journal of Applied Physiology 2001;91(6):2785–801. [DOI: 10.1152/jappl.2001.91.6.2785] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical Research Ed.) 2009;339:b2535. [DOI: 10.1136/bmj.b2535] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osborne 2009
- Osborne DM, Frye CA. Estrogen increases latencies to seizures and levels of 5alpha-pregnan-3alpha-ol-20-one in hippocampus of wild-type, but not 5alpha-reductase knockout, mice. Epilepsy & Behavior 2009;16(3):411-4. [DOI: 10.1016/j.yebeh.2009.08.016] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quigg 2009
- Quigg M, Smithson SD, Fowler KM, Sursal T, Herzog AG, for NIH Progesterone Trial Study Group. Laterality and location influence catamenial seizure expression in women with partial epilepsy. Neurology 2009;73(3):223-7. [DOI: 10.1212/WNL.0b013e3181ae7adf] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reddy 2004
- Reddy DS, Castaneda DC, O’Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. Journal of Pharmacology and Experimental Therapeutics 2004;310(1):230-9. [DOI: 10.1124/jpet.104.065268] [PMID: ] [DOI] [PubMed] [Google Scholar]
Reddy 2014
- Reddy DS. Neurosteroids and their role in sex-specific epilepsies. Neurobiology of Disease 2014;72(Pt B):198-209. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosciszewska 1980
- Rosciszewska D. Analysis of seizure dispersion during menstrual cycle in women with epilepsy. Monographs in Neural Sciences 1980;5:280-4. [PMID: ] [PubMed] [Google Scholar]
Saghaei 2004
- Saghaei M. Random allocation software for parallel group randomized trials. BMC Medical Research Methodology 2004;4:26. [DOI: 10.1186/1471-2288-4-26] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sander 1996
- Sander JW, Shorvon SD. Epidemiology of the epilepsies. Journal of Neurology, Neurosurgery & Psychiatry 1996;61(5):433-43. [DOI: 10.1136/jnnp.61.5.433] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Smejkalova 2010
- Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. Journal of Neuroscience 2010;30(48):16137-48. [DOI: 10.1523/JNEUROSCI.4161-10.2010] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tauboll 1991
- Taubøll E, Lundervold A, Gjerstad L. Temporal distribution of seizures in epilepsy. Epilepsy Research 1991;8(2):153-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Velísková 2000
- Velísková J, Velísek L, Galanopoulou AS, Sperber EF. Neuroprotective effects of estrogens on hippocampal cells in adult female rats after status epilepticus. Epilepsia 2000;41(Suppl 6):S30-5. [DOI: 10.1111/j.1528-1157.2000.tb01553.x] [DOI] [PubMed] [Google Scholar]
Woolley 1994
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. Journal of Neuroscience 1994;14(12):7680–7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Maguire 2018
- Maguire MJ, Nevitt SJ. Treatments for peri-menstrual seizures in catamenial epilepsy. Cochrane Database of Systematic Reviews 2018, Issue 12. Art. No: CD013225. [DOI: 10.1002/14651858.CD013225] [DOI] [Google Scholar]
Maguire 2019
- Maguire MJ, Nevitt SJ. Treatments for seizures in catamenial (menstrual-related) epilepsy. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: CD013225. [DOI: 10.1002/14651858.CD013225.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


