Abstract
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has emerged as a pandemic and has caused damage to the lives of the people and economy of countries. However, the therapeutic reagents against SARS-CoV-2 remain unclear. The spike (S) protein of SARS-CoV-2 contains a cleavage motif at the S1/S2 boundary, known to be cleaved by furin. As cleavage is essential for S protein activation and viral entry, furin was selected as the target compound. In this study, we examined the inhibitory effects of two lignans (honokiol and magnolol) on furin-like enzymatic activity using a fluorogenic substrate with whole-cell lysates. Of two compounds tested, honokiol partially inhibited furin-like enzymatic activity. We further examined the anti-SARS-CoV-2 activity of honokiol using VeroE6 cell line, which is stably expressing a transmembrane protease serine 2 (TMPRSS2). It was shown that honokiol exhibited remarkable inhibition of SARS-CoV-2 infection. Therefore, honokiol and crude drugs which contain honokiol such as Magnolia species have a potential therapeutic reagents for SARS-CoV-2.
Keywords: SARS-CoV-2, Furin, COVID-19, Honokiol, Spike protein
Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, transmembrane protease serine 2
Graphical abstract
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been spreading worldwide, causing over 196 million cases of coronavirus disease 2019 (COVID-19) along with more than 4,200,412 deaths (https://covid19.who.int/accessed July 28, 2021).1 The entry of SARS-CoV-2 into host cells is mediated by the transmembrane spike glycoprotein (S protein), which forms homotrimers extending from the virus envelope.2, 3, 4 The S protein is processed and activated by cellular proteases, including a transmembrane protease serine 2 (TMPRSS2), cathepsin, and furin5, 6, 7; it comprises two functional subunits, S1 and S2. The S1 subunit is involved in binding to the host cell receptor, and the S2 subunit participates in the fusion of the virus envelope and host cell membrane.5,8 S protein cleavage occurs at the boundary between the S1 and S2 subunits. The S1 subunit of SARS-CoV-2 initiates virus-receptor binding by interacting with the human host cell receptor angiotensin-converting enzyme 2, and the S2 subunit participates in viral fusion with the target cell.6 The SARS-CoV-2 S protein possesses a multibasic cleavage site for furin at the S1/S2 boundary, which contributes to the activation of the fusion machinery of the virus.7,8
Furin, belonging to the proprotein convertase family, is a calcium-dependent serine endoprotease that cleaves the processing sites of a precursor protein.9, 10 It is ubiquitously expressed and circulates among the trans-Golgi network, plasma membrane, and early endosome and is associated with endocytic and exocytic pathways.11 Furin regulates several physiological pathways, including those of hormones, growth factors, adhesion molecules, and cell surface receptors.12 It also cleaves pathogen-derived proteins, such as viral envelope proteins and bacterial toxins.13 The sequence at the S1/S2 boundary of the S protein contains a furin cleavage site (RRAR↓S). The multibasic cleavage site in the S protein is essential for SARS-CoV-2 entry into lung cells, and furin inhibitors block S protein processing and SARS-CoV-2 infection.5,7,14 Therefore, furin inhibitors are potential antiviral agents for SARS-CoV-2 infection and pathogenesis.15
Honokiol and magnolol, which are lignans isolated from Magnolia species, are active components of the Magnoliae Cortex. The bark of Magnolia species, such as M. officinalis and M. obovata is widely used in traditional Chinese and Japanese herbal medicine for the treatment of gastrointestinal disorders, anxiety, and allergies.16 Other reported actions include neuroprotective, antimicrobial, anti-inflammatory, and anticancer properties.17, 18, 19, 20 Honokiol and magnolol have also been reported to have not only anti-inflammatory, anxiolytic, antidepressant, antioxidant, and anticancer effects, but also antivirus effects (supplemental table).16,21, 22, 23 In this study, we explored honokiol as a potential inhibitor of furin, an enzyme involved in the viral entry to the cell. We further tested if honokiol suppressed viral infection in the whole cell assay of SARS-CoV-2 infection.
2. Materials and methods
2.1. Materials
Honokiol (>95.0%) and magnolol (>98.0%) were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Cinnamic acid (>99.5%) was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Decanoyl-Arg-Val-Lys-Arg-CMK (Decanoyl-RVKR-CMK) was obtained from Cayman Chemical Company (MI, USA).
2.2. Furin-like assay
Inhibition assays of furin-like activities were performed according to a previous report with minor modifications.24 Briefly, A549 cells (JCRB0076) were cultured and maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cultured cells were scraped and washed with ice-cold Dulbecco's phosphate-buffered saline (D-PBS). Cell counting was performed and lysis buffer (200 mM HEPES-KOH, pH7.4, 0.1% Triton X-100, 10 mM calcium chloride) was added to the cell precipitate. The cell lysates were centrifuged at 13,000 × g for 10 min at 4 °C, and the supernatant was transferred to new tubes and stored at −80 °C until use. The furin-like enzymatic assay was performed as follows: 10 μL honokiol, magnolol, or cinnamic acid and 50 μL cell lysates were added to a 96-well plate containing 30 μL MilliQ water and incubated at 37 °C for 30 min. The reaction mixture and 10 μL 1 mM furin fluorogenic substrate, Pyr-Arg-Thr-Lys-Arg-MCA (PEPTIDE INSTITUTE, Inc., Osaka, Japan) or Pyr-Arg-Arg-Ala-Arg-MCA (Greiner Bio-One, Austria), were mixed and incubated at 37 °C for 30 min. Fluorescence intensity was measured with excitation at 380 nm and emission at 460 nm.
2.3. Honokiol treatment and SARS-CoV-2 infection
Vero E6/TMPRSS2 cells (JCRB1819, Vero E6 cells overexpressing TMPRSS2) were purchased from JCRB Cell Bank (Osaka, Japan) and cultured in DMEM supplemented with 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 mg/mL G418 (Nacalai Tesque, Kyoto, Japan).25 Cell viability in Vero E6/TMPRSS2 cells treated with honokiol was determined using CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Madison, WI, USA). The SARS-CoV-2 infection studies were done in a biosafety level 3 facility at National Institute of Infectious Diseases, Japan. A previously isolated 2019-nCoV/Japan/TY/WK-521/2020 strain (WK-521) of SARS-CoV-2 was propagated in Vero E6/TMPRSS2 cells.25 The viral titer of SARS-CoV 2 was determined through a 50% tissue culture infectious dose (TCID50) assay in Vero E6/TMPRSS2 cells.25 Confluent Vero E6/TMPRSS2 cells cultured in a 96-well plate (CellCarrier-96 Ultra, PerkinElmer, Waltham, MA, USA) were pre-treated with honokiol dissolved in DMSO at the indicated concentrations without agitation for 1 h at 37 °C. The cells were then infected with SARS-CoV-2 at a multiplicity of infection (MOI) of 0.009 in the presence of honokiol for 24 h at 37 °C. The infected cells were then fixed with 4% paraformaldehyde in D-PBS for 30 min and permeabilized with 0.2% Triton X-100 in D-PBS for 15 min. The cells were stained for SARS-CoV-2 S protein using rabbit anti-SARS-CoV-2 Spike RBD monoclonal antibody (1:3,000, clone HL1003, GTX635792; GeneTex, Irvine, CA, USA), followed by goat anti-rabbit IgG Alexa Fluor 488 (1:1,000, Life Technologies, Carlsbad, CA, USA). Cell nuclei were stained with 1 μg/mL 4′,6-diamidino-2-phenylindole (DAPI) solution (Dojindo Laboratories, Kumamoto, Japan). The cells were then imaged using the Operetta CLS High-Content Analysis System (PerkinElmer), and infectivity (percentage of SARS-CoV-2 positive cells) in each well was calculated by counting SARS-CoV-2 S-and DAPI-positive cells using Harmony software (PerkinElmer). Dose response curve was created by nonlinear regression model, the half-maximal inhibitory concentration (IC50) and the half-maximal cytotoxic concentration (CC50) were calculated using GraphPad Prism 9 software.
3. Results and discussion
3.1. Furin-like assay
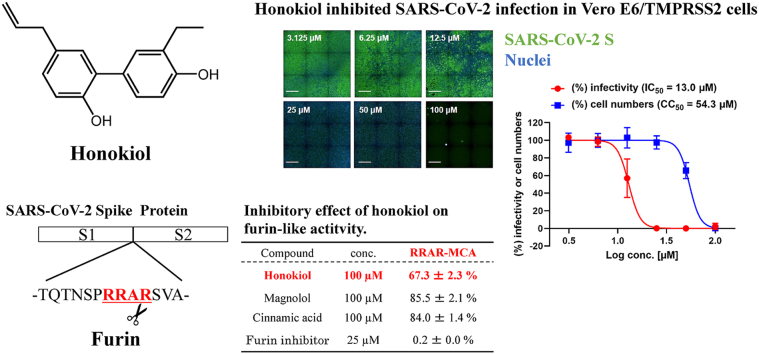
Lignans are polyphenolic substances that feature C18 cores, resulting from the dimerization of phenylpropanoids. We tested two lignans (honokiol and magnolol) and one phenylpropanoid (cinnamic acid) for furin-like enzymatic activities. Decanoyl-RVKR-CMK, a furin inhibitor, was used as a positive control. The furin-like assay was performed using pyr-RTKR-MCA or pyr-RRAR-MCA as cleavage substrates. With pyr-RTKR-MCA as substrate, honokiol suppressed furin-like activities by 71.0 ± 3.0% (% of activity), which is more effective than magnolol and cinnamic acid (89.9 ± 0.8% and 83.8 ± 2.5%, respectively; Table 1). This result is consistent with that using pyr-RRAR-MCA as substrate (honokiol: 67.3 ± 2.3%, magnolol: 85.5 ± 2.1%, and cinnamic acid: 84.0 ± 1.4%). These findings indicate that honokiol has the potential to inhibit multibasic motif cleavage by endogenous proteases.
Table 1.
Inhibitory effect of compounds on furin-like actitvity.
| Compound | conc. | RTKR-MCA | RRAR-MCA |
|---|---|---|---|
| Honokiol | 100 μM | 71.0 ± 3.0% | 67.3 ± 2.3% |
| Magnolol | 100 μM | 89.9 ± 0.8% | 85.5 ± 2.1% |
| Cinnamic acid | 100 μM | 83.8 ± 2.5% | 84.0 ± 1.4% |
| Decanoyl-RVKR-CMK | 25 μM | 0.1 ± 0.0% | 0.2 ± 0.0% |
| Control (no sample) | 100% | 100% | |
| [mean ± standard deviation (SD), n = 3] | |||
3. 2. SARS-CoV-2 infection
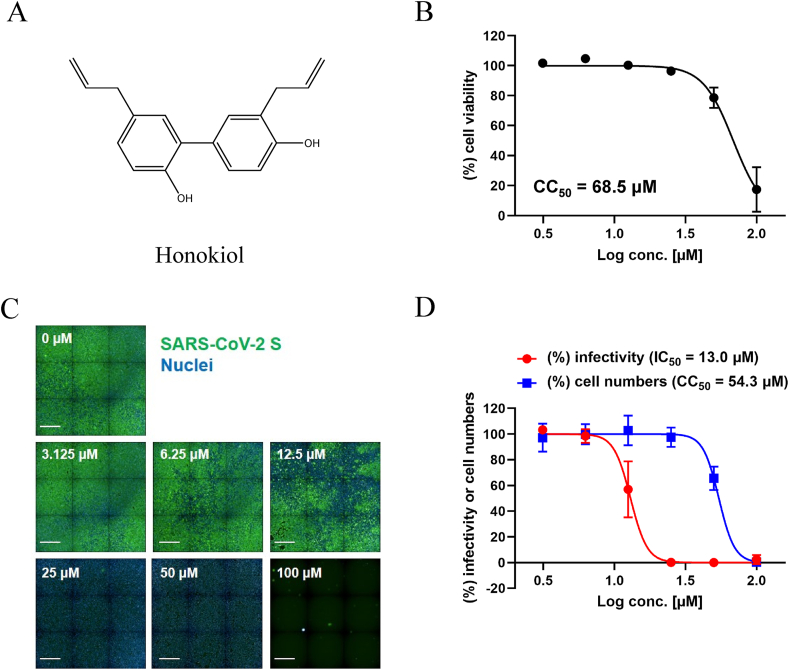
We then evaluated the effect of honokiol on viral infection using Vero E6/TMPRSS2 cells. Treatment of honokiol alone showed no or minor cytotoxicity, except for a highest concentration (100 μM) (Fig. 1A and B). For an infection study, we pretreated Vero E6/TMPRSS2 cells with a dilution series of honokiol (100, 50, 25, 12.5, 6.25, and 3.125 μM) and then infected with SARS-CoV-2 in the presence of honokiol. Cell viability and infectivity were assessed 24 h post-infection (Fig. 1C and D). SARS-CoV-2 infection was suppressed by honokiol in a dose-dependent manner; it completely inhibited viral infection at concentrations of 25 μM (99.83%) and 50 μM (99.96%) with no or minor cytotoxicity (2.49% for 25 μM and 34.5% for 50 μM) (Fig. 1C and D). The IC50 values determined by the infection assay were 13.0 μM for honokiol. The concentration (25 μM) of honokiol used to suppress viral infection in Vero E6/TMPRSS2 cells was lower than that (100 μM) used for the inhibitory assay of furin-like activity. This difference in concentrations might be related to a multitarget effect of honokiol in the whole organism assay, as has been reported for other viral infections.23 It has been reported that honokiol blocks phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) signaling in various cell lines and induces autophagy in neuroblastoma cells by activating the PI3K/Akt/mammalian target of rapamycin (mTOR) and endoplasmic reticular stress/extracellular signal-regulated kinase (ERK)1/2 signaling pathways.26 Since honokiol has been used as an Akt inhibitor, the suppressive effect of viral infection might be attributed to properties other than the inhibition of furin-like activities.
Fig. 1.
Honokiol inhibits SARS-CoV-2 infection in Vero E6/TMPRSS2 cells. (A) The structure of Honokiol. (B) Vero E6/TMPRSS2 cells were treated with honokiol at the indicated concentrations for 24 h. Cell viability was determined using CellTiter-Glo® Luminescent Cell Viability Assay. Results were normalized to those of dimethylsulfoxide (DMSO)-treated cells. (C and D) Vero E6/TMPRSS2 cells were infected with SARS-CoV-2 for 24 h in the presence of the indicated concentration of honokiol, after which the cells were stained with anti-SARS-CoV-2 Spike RBD monoclonal antibody for viral infection and DAPI and analyzed to determine cell number and percent of infection. (C) Representative fluorescence images show SARS-CoV-2 S protein (green) and cell nucleus (blue). Scale bar, 1 mm. (D) The percentages of infected cells and cell numbers were normalized to those of DMSO-treated cells infected with SARS-CoV-2. Values represent the mean ± SD of two independent experiments (n = 6).
Inhibitory effects of virus infection by blocking Akt pathway have been indicated in the previous study on Middle East respiratory syndrome coronavirus (MERS-CoV), which was first identified in 2012 and belongs to the same family and genus as Sars-CoV-2. MERS-CoV infection has been reported to upregulate PI3K/Akt/mTOR, and blocking this pathway significantly inhibited MERS-CoV replication in vitro.27 Reovirus virions activate the PI3K/Akt pathway via clathrin-mediated endocytosis, and it has been shown that SARS-CoV-2 endocytosis occurs through a clathrin-mediated pathway.28,29 A recent study showed that the inhibition of clathrin-dependent endocytosis reduced SARS-CoV-2 infectivity.29 SARS-CoV-2 endocytosis and replication may be associated with the PI3K/Akt/mTOR pathway. Therefore, blocking the PI3K/Akt/mTOR signaling pathway may be important for developing potential therapeutic applications against COVID-19.
Further research is necessary to elucidate the molecular mechanism for the inhibitory effect of honokiol on SARS-CoV-2 infection. In this study, we performed in vitro assays to evaluate the inhibitory effect of honokiol on furin-like activity. Evaluating the cleavage of S protein is required as well. It is important to understand whether inhibiting Akt pathway causes the inhibition of SARS-CoV-2 infection, and whether inhibiting both furin and Akt pathways have the synergistic effect on inhibition of SARS-CoV-2 infection. As honokiol is contained in the crude drugs from Magnolia plants used in traditional Chinese medicine and Kampo medicine, the inhibitory effect of conventional formulations that contain these crude drugs on SARS-CoV-2 infection should be test in further study.
In summary, we showed that honokiol partially inhibited furin-like activity in vitro tests, which can potentially inhibit the analog site in the S protein of SARS-CoV-2. We also showed that SARS-CoV-2 infection was suppressed in the presence of honokiol at concentrations that are pharmacologically relevant. Since honokiol is known as an inhibitor of PI3K/Akt signaling in various cell lines, honokiol could inhibit SARS-CoV-2 infection not only by inhibiting furin-like activity but also by blocking PI3K/Akt signaling.
Concent for publication
provided by all authors
Highlights of the finding s and novelities
Honokiol inhibits SARS-CoV-2 infection.
Declaration of competing interest
The authors declare no conflict of interest
Acknowledgements
This work was partially supported by JSPS KAKENHI (Grant Number JP20H00259 to T.H. and JP21K15283 to M.K.)
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2021.09.005.
Contributor Information
Takashi Tanikawa, Email: tanikawa@josai.ac.jp.
Masashi Kitamura, Email: kitamura@josai.ac.jp.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kumar A., Singh R., Kaur J., et al. Wuhan to world: the COVID-19 pandemic. Front Cell Infect Microbiol. 2021;11:596201. doi: 10.3389/fcimb.2021.596201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsobaie S. Understanding the molecular biology of SARS-CoV-2 and the COVID-19 pandemic: a review. Infect Drug Resist. 2021;14:2259–2268. doi: 10.2147/IDR.S306441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalathiya U., Padariya M., Mayordomo M., et al. Highly conserved homotrimer cavity formed by the SARS-CoV-2 spike glycoprotein: a novel binding site. J Clin Med. 2020;9(5):1473. doi: 10.3390/jcm9051473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Römer R.A., Römer N.S., Wallis A.K. Flexibility and mobility of SARS-CoV-2-related protein structures. Sci Rep. 2021;11(1):4257. doi: 10.1038/s41598-021-82849-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bestle D., Heindl M.R., Limburg H., et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci Alliance. 2020;3(9) doi: 10.26508/lsa.202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann M., Kleine-Weber H., Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell. 2020;78(4):779–784. doi: 10.1016/j.molcel.2020.04.022. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang J., Wan Y., Luo C., et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molloy S.S., Bresnahan P.A., Leppla S.H., Klimpel K.R., Thomas G. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J Biol Chem. 1992;267(23):16396–16402. [PubMed] [Google Scholar]
- 10.Hatsuzawa K., Nagahama M., Takahashi S., Takada K., Murakami K., Nakayama K. Purification and characterization of furin, a Kex2-like processing endoprotease, produced in Chinese hamster ovary cells. J Biol Chem. 1992;267(23):16094–16099. [PubMed] [Google Scholar]
- 11.Voorhees P., Deignan E., van Donselaar E., et al. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 1995;14(20):4961–4975. doi: 10.1002/j.1460-2075.1995.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakayama K. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J. 1997;327(Pt 3):625–635. doi: 10.1042/bj3270625. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun E., Sauter D. Furin-mediated protein processing in infectious diseases and cancer. Clin Transl Immunology. 2019;8(8) doi: 10.1002/cti2.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Y.W., Chao T.L., Li C.L., et al. Furin inhibitors block SARS-CoV-2 spike protein cleavage to suppress virus production and cytopathic effects. Cell Rep. 2020;33(2):108254. doi: 10.1016/j.celrep.2020.108254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C., Zheng M., Yang Y., et al. Furin: a potential therapeutic target for COVID-19. iScience. 2020;23(10):101642. doi: 10.1016/j.isci.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo H., Wu H., Yu X., et al. A review of the phytochemistry and pharmacological activities of Magnoliae officinalis cortex. J Ethnopharmacol. 2019;236:412–442. doi: 10.1016/j.jep.2019.02.041. [DOI] [PubMed] [Google Scholar]
- 17.Yang E.J., Lee J.Y., Park S.H., Lee T., Song K.S. Neuroprotective effects of neolignans isolated from Magnoliae Cortex against glutamate-induced apoptotic stimuli in HT22 cells. Food Chem Toxicol. 2013;56:304–312. doi: 10.1016/j.fct.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y., Qiao J., Zhang X., Ge C. Antimicrobial effect of Magnolia officinalis extract against Staphylococcus aureus. J Sci Food Agric. 2011;91(6):1050–1056. doi: 10.1002/jsfa.4280. [DOI] [PubMed] [Google Scholar]
- 19.Walker J.M., Maitra A., Walker J., Ehrnhoefer-Ressler M.M., Inui T., Somoza V. Identification of Magnolia officinalis L. bark extract as the most potent anti-inflammatory of four plant extracts. Am J Chin Med. 2013;41(3):531–544. doi: 10.1142/S0192415X13500389. [DOI] [PubMed] [Google Scholar]
- 20.Ha K.T., Kim J.K., Lee Y.C., Kim C.H. Inhibitory effect of Daesungki-Tang on the invasiveness potential of hepatocellular carcinoma through inhibition of matrix metalloproteinase-2 and -9 activities. Toxicol Appl Pharmacol. 2004;200(1):1–6. doi: 10.1016/j.taap.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Chen X., Hu Y., Shan L., Yu X., Hao K., Wang G.X. Magnolol and honokiol from Magnolia officinalis enhanced antiviral immune responses against grass carp reovirus in Ctenopharyngodon idella kidney cells. Fish Shellfish Immunol. 2017;63:245–254. doi: 10.1016/j.fsi.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Fang C.Y., Chen S.J., Wu H.N., et al. Honokiol, a lignan biphenol derived from the Magnolia tree, inhibits dengue virus type 2 infection. Viruses. 2015;7(9):4894–4910. doi: 10.3390/v7092852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S., Li L., Tan L., Liang X. Inhibition of herpes simplex virus-1 replication by natural compound honokiol. Virol Sin. 2019;34(3):315–323. doi: 10.1007/s12250-019-00104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiba Y., Oyama R., Misawa S., Tanikawa T., Kitamura M., Suzuki R. Screening for inhibitory effects of crude drugs on furin-like enzymatic activities. J Nat Med. 2021:1–6. doi: 10.1007/s11418-021-01519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuyama S., Nao N., Shirato K., et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A. 2020;117(13):7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh P.S., Wang W., Chang Y.A., Lin C.J., Wang J.J., Chen R.M. Honokiol induces autophagy of neuroblastoma cells through activating the PI3K/Akt/mTOR and endoplasmic reticular stress/ERK1/2 signaling pathways and suppressing cell migration. Canc Lett. 2016;370(1):66–77. doi: 10.1016/j.canlet.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Kindrachuk J., Ork B., Hart B.J., et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob Agents Chemother. 2015;59(2):1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian J., Zhang X., Wu H., et al. Blocking the PI3K/AKT pathway enhances mammalian reovirus replication by repressing IFN-stimulated genes. Front Microbiol. 2015;6:886. doi: 10.3389/fmicb.2015.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayati A., Kumar R., Francis V., McPherson P.S. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J Biol Chem. 2021;296:100306. doi: 10.1016/j.jbc.2021.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.