This systematic review and meta-analysis reviews gut microbiota alterations in general adult psychiatric populations and performs a within- and between-diagnostic comparison.
Key Points
Question
Do psychiatric disorders present with distinct or shared gut microbial alterations?
Findings
This review and meta-analysis of 59 case-control studies found that gut microbiota perturbations were associated with a transdiagnostic pattern with a depletion of certain anti-inflammatory butyrate-producing bacteria and an enrichment of pro-inflammatory bacteria in depression, bipolar disorder, schizophrenia, and anxiety.
Meaning
These findings are in line with genetic and inflammatory marker studies and support the transdiagnostic dimensional model of psychiatric disorders by highlighting the gut microbiota as an additional dimensional component.
Abstract
Importance
Evidence of gut microbiota perturbations has accumulated for multiple psychiatric disorders, with microbiota signatures proposed as potential biomarkers. However, no attempts have been made to evaluate the specificity of these across the range of psychiatric conditions.
Objective
To conduct an umbrella and updated meta-analysis of gut microbiota alterations in general adult psychiatric populations and perform a within- and between-diagnostic comparison.
Data Sources
Cochrane Library, PubMed, PsycINFO, and Embase were searched up to February 2, 2021, for systematic reviews, meta-analyses, and original evidence.
Study Selection
A total of 59 case-control studies evaluating diversity or abundance of gut microbes in adult populations with major depressive disorder, bipolar disorder, psychosis and schizophrenia, anorexia nervosa, anxiety, obsessive compulsive disorder, posttraumatic stress disorder, or attention-deficit/hyperactivity disorder were included.
Data Extraction and Synthesis
Between-group comparisons of relative abundance of gut microbes and beta diversity indices were extracted and summarized qualitatively. Random-effects meta-analyses on standardized mean difference (SMD) were performed for alpha diversity indices.
Main Outcomes and Measures
Alpha and beta diversity and relative abundance of gut microbes.
Results
A total of 34 studies provided data and were included in alpha diversity meta-analyses (n = 1519 patients, n = 1429 control participants). Significant decrease in microbial richness in patients compared with control participants were found (observed species SMD = −0.26; 95% CI, −0.47 to −0.06; Chao1 SMD = −0.5; 95% CI, −0.79 to −0.21); however, this was consistently decreased only in bipolar disorder when individual diagnoses were examined. There was a small decrease in phylogenetic diversity (SMD = −0.24; 95% CI, −0.47 to −0.001) and no significant differences in Shannon and Simpson indices. Differences in beta diversity were consistently observed only for major depressive disorder and psychosis and schizophrenia. Regarding relative abundance, little evidence of disorder specificity was found. Instead, a transdiagnostic pattern of microbiota signatures was found. Depleted levels of Faecalibacterium and Coprococcus and enriched levels of Eggerthella were consistently shared between major depressive disorder, bipolar disorder, psychosis and schizophrenia, and anxiety, suggesting these disorders are characterized by a reduction of anti-inflammatory butyrate-producing bacteria, while pro-inflammatory genera are enriched. The confounding associations of region and medication were also evaluated.
Conclusions and Relevance
This systematic review and meta-analysis found that gut microbiota perturbations were associated with a transdiagnostic pattern with a depletion of certain anti-inflammatory butyrate-producing bacteria and an enrichment of pro-inflammatory bacteria in patients with depression, bipolar disorder, schizophrenia, and anxiety.
Introduction
Despite evidence that probiotic formulations can improve mental health dating back to the early 20th century,1,2 it was only following advances in DNA/RNA sequencing technologies that the involvement of the gut microbiota in the pathophysiology of psychiatric disorders was recognized. Preclinical studies have consistently demonstrated that fecal microbiota transplants from patients with a wide range of psychiatric conditions result in the development of the behavioral and physiological profile of the condition in germ-free mice.3,4,5,6,7 This suggests that psychiatric disorders may be associated with a distinct pattern of microbial perturbations, which may serve as a biomarker.
Attempts to characterize the composition of the microbiota in psychiatric populations have yielded plentiful yet contradictory results. Nevertheless, systematic reviews in individual disorders have been able to identify patterns that may be promising biomarker targets.8,9,10 Indeed, the addition of such biomarkers can improve diagnostic accuracy, guide treatment, and assist the monitoring of treatment response. For the definition of a biomarker to be met, ie, “substance, structure or process that can be measured in the body and influence or predict the incidence of outcome or disease,”11 the specificity and reproducibility of the alteration needs to be demonstrated.12 Therefore, it is crucial to compare microbial perturbations across the wider range of psychiatric conditions.
We performed an umbrella and updated review and meta-analysis of gut microbiota studies in adults with major depressive disorder (MDD), bipolar disorder, psychosis and schizophrenia, anxiety disorders, obsessive compulsive disorder (OCD), eating disorders (anorexia nervosa and bulimia nervosa), autism spectrum disorder, attention-deficit/hyperactivity disorder (ADHD), and posttraumatic stress disorder (PTSD) to evaluate the specificity and reproducibility of gut microbiota alterations and delineate those with potential to become biomarkers.
Methods
The protocol for this review was preregistered with PROSPERO (CRD42021224342). We followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline13 as well as Cochrane guidance for umbrella and updated reviews.14,15
Search Details
We searched Cochrane Library, PubMed, Embase, and PsycINFO on January 27, 2021. The search strings used are available in eAppendix 1 in the Supplement. This search was limited to systematic reviews and meta-analyses in English, including human studies, published since 2005. After reviewing the results, we realized that a large body of recent literature was missed, as numerous studies have become available following the publication of the latest reviews. To ensure thorough coverage, we performed an updated search for each disorder on February 2, 2021, from the search date recorded in the latest available high-quality review for that disorder (eAppendix 1 in the Supplement).
Selection Criteria
Systematic reviews and meta-analyses were considered eligible if they followed established guidelines and included at least 1 eligible original study. Original studies were eligible if they (1) applied an observational case-control design, (2) performed gut microbiota analysis and reported diversity or abundance measures, and (3) sampled a general adult population (age 18-65 years) with a psychiatric diagnosis of interest. Interventional or longitudinal comparisons in the absence of a control group were excluded. Records were screened by 2 authors (V.L.N. and M.R.B.S) and discrepancies resolved via discussion and consultation with a third author (A.H.Y.).
Data Extraction
Information was extracted using a predesigned template by 2 authors (V.L.N. and M.R.B.S) and cross-checked. From systematic reviews and original studies, we extracted publication details, participant demographic and clinical characteristics, and methodological information. As primary outcomes of interest, we extracted community-level measures of gut microbiota composition (alpha and beta diversity) and taxonomic findings at the phylum, family, and genus levels (relative abundance). Alpha diversity provides a summary of the microbial community in individual samples and can be compared across groups to evaluate the role of a particular factor (in this case psychiatric diagnosis) on the richness (number of species) and evenness (how well each species is represented) in the sample.10,16 Beta diversity is a measure of interindividual (between samples) diversity that assesses similarity of communities compared with the other samples analyzed.10 This analysis allows us to see whether patient samples cluster significantly differently (ie, with little or no overlap) compared with control participant samples or whether they overlap, thus suggesting the 2 groups are not distinct. Control samples were defined as individuals without the relevant condition.
Quality Assessment
We performed quality assessment of the systematic reviews using the ROBIS tool17 and of the original studies not covered in any review with the Joanna Briggs Institute Critical Appraisal Checklist for Case-Control Studies.18 No studies were excluded owing to quality concerns. The detailed assessment is available as eAppendix 2 in the Supplement.
Qualitative Synthesis
For the relative abundance of microbial taxa, we performed a qualitative synthesis owing to the large number and limited overlap of findings. Owing to the significant likelihood of false positives noted in previous meta-analyses,19 results reported only by a single study were excluded. Further, results reported only by 1 research group were also excluded because these were considered potentially methodology or population specific. To identify disease-specific and shared alterations, we performed a within- and between-diagnostic comparison. First, we summarized within-disorder findings for each taxon reported in at least 2 studies and labeled those increased, decreased, or not consistent. Not consistent was any finding with less than 75% agreement between studies reporting this taxon. A consistent finding by 2 studies was considered worth noting for future validation, whereas a finding by 3 or more studies (from ≥2 research groups) was considered potentially associated with the disorder. A taxon was considered a candidate for disease-specific response if it was altered (in a consistent direction) in a single disorder only. Alternatively, if a shift was replicated in several disorders with known symptomatic and pathophysiological overlap, this was considered a transdiagnostic alteration. Taxa similarly altered across all/multiple unrelated diagnostic categories were interpreted as general disease response.
Quantitative Synthesis
Meta-analysis was performed on differences in alpha diversity between patients and controls for indices with data reported in 10 or more studies. Detailed methods of data transformation and interpretation thresholds are available in eAppendix 3 in the Supplement. Publication bias was evaluated with funnel plots and Egger test. Preplanned subgroup analyses were disorder, region of study (east/west), and use of psychiatric medication. All analyses were completed in R version 4.17-0 (meta package; R Foundation).20 Two-sided P values were statistically significant at less than .05.
Results
Search Results
We identified 16 systematic reviews (eAppendices 4 and 5 in the Supplement for PRISMA flowcharts and details of the systematic reviews) containing 39 eligible studies. There were no reviews capturing OCD, PTSD, or autism spectrum disorder in adults. In the second search, a further 20 studies were identified, resulting in 59 studies across 8 disorders. The most researched disorder was MDD, followed by psychosis and schizophrenia, bipolar disorder, and anorexia nervosa (Table).
Table. Summary Characteristics of the Identified Reviews and Original Studies by Psychiatric Disorder.
| Disordera | No.b | Region of studiesc | Mean patient age, y | Female, mean % | ||
|---|---|---|---|---|---|---|
| Reviews | Studies | Total patients | ||||
| MDD | 8 | 21 | 930 | East: n = 14; west: n = 7 | 35 | 60 |
| Schizophrenia and psychosis | 5 | 11 | 699 | East: n = 9; west: n = 2 | 36 | 45 |
| Bipolar disorder | 3 | 9 | 465 | East: n = 5; west: n = 4 | 38 | 55 |
| Anorexia nervosa | 3 | 10 | 211 | East: n = 2; west: n = 8 | 26 | 99 |
| Anxiety | 2 | 3 | 84 | East: n = 2; west: n = 1 | 40 | 77 |
| OCD | 0 | 2 | 59 | West: n = 2 | 36 | 54 |
| PTSD | 0 | 1 | 18 | Africa: n = 1 | 42 | 14 |
| ADHDd | 1 | 1 | 19 | West: n = 1 | 20 | 32 |
| MDD + anxiety | NA | 2 | 60 | West: n = 2 | 39 | 82 |
| MDD + bipolar disorder | NA | 2 | 98 | East: n = 1; west: n = 1 | 37 | 69 |
| Total | 16 | 59 | 2643 | East: n = 32; west: n = 24; Africa: n = 1 | NA | NA |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; MDD, major depressive disorder; NA, not applicable; OCD, obsessive compulsive disorder; PTSD, posttraumatic stress disorder.
Studies that examined combined cohorts (MDD + bipolar disorder21,22 or MDD + anxiety23,24) are presented separately.
Some include >1 disorder.
West region includes US, Canada, Europe, Australia, and New Zealand. East region includes China, Japan, and Taiwan. Africa includes South Africa.
Adult populations only.
Characteristics of Included Studies
The 59 studies provided 64 case-control comparisons capturing 2643 patients and 2336 controls (eAppendix 6 in the Supplement provides a detailed summary of study characteristics). Most studies (32 [54.2%]) were conducted in East Asia (China, Japan, and Taiwan), 24 (40.7%) in westernized populations (US, Canada, Europe, Australia, and New Zealand; grouped according to typical diet and lifestyle), and 1 (1.7%) in Africa (South Africa). Most studies had small to moderate sample sizes (median, 62), ranging between 4 and 156 per group (eAppendix 6 in the Supplement). Studies were similar in exclusion criteria; however, few attempted to minimize dietary changes or control dietary intake (12 of 59 [20.3%]) or smoking status (8 of 59 [13.6%]). Use of psychiatric medication also varied substantially, with 11 of 59 studies (18.6%) conducted in medication-free or drug-naive groups, 5 of 59 (8.5%) in groups undergoing treatment and the remainder not controlling this, resulting in anywhere between 20% and 96% of patients taking medication. Methodology of stool processing (eAppendix 7 in the Supplement) and composition analysis (eAppendix 6 in the Supplement) also varied widely, with 16S ribosomal RNA sequencing being most common (44 of 59 studies [74.6%]) followed by 9 studies (15.2%) using quantitative polymerase chain reaction or real-time quantitative polymerase chain reaction and 7 (11.9%) using shotgun metagenomics.
Alpha Diversity
Of 44 studies reporting alpha diversity, 34 provided data and were included in meta-analyses (1519 patients and 1429 controls). Eleven indices were used to assess alpha diversity, including estimates of richness (observed species, Chao1, abundance coverage estimator, and incidence coverage estimator), evenness, richness/evenness (Shannon, Simpson, inverse Simpson, Fisher), biodiversity (Faith phylogenetic diversity), and 1 newly developed index25 (eAppendix 6 in the Supplement). The most widely used were observed species, Chao1, Shannon, Simpson, and phylogenetic diversity. There was no evidence of publication bias in any of the analyses (eAppendix 8 in the Supplement).
Regarding richness, 20 studies provided data on observed species in patients (n = 897) vs controls (n = 789). The pooled estimate showed a significant decrease in patients with a small effect size (standardized mean difference [SMD] = −0.26; 95% CI, −0.47 to −0.06; P = .01) and high heterogeneity (I2 = 75%) (Figure 1A).3,4,22,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42 Within diagnostic categories, there was a significant decrease only in bipolar disorder (SMD = −0.61; 95% CI, −1.19 to −0.03; P = .04; I2 = 80%). Twenty-six studies provided data on Chao1 in patients (n = 956) vs controls (n = 961). The pooled estimate showed a significant decrease in patients with a medium effect size (SMD = −0.5; 95% CI, −0.79 to −0.21; P = .001; I2 = 88%). Regarding individual diagnoses, there was a significant decrease only in bipolar disorder and anorexia nervosa (SMD = −0.53; 95% CI, −1.01 to −0.05; P = .03; I2 = 62% and SMD = −0.86; 95% CI, −1.52 to −0.21; P = .01; I2 = 80%, respectively) (Figure 1B).4,21,25,27,29,31,32,33,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49
Figure 1. Forest Plots of Alpha Diversity Richness Estimators in the Gut Microbiota of Patients With Psychiatric Disorders Compared With Healthy Controls.
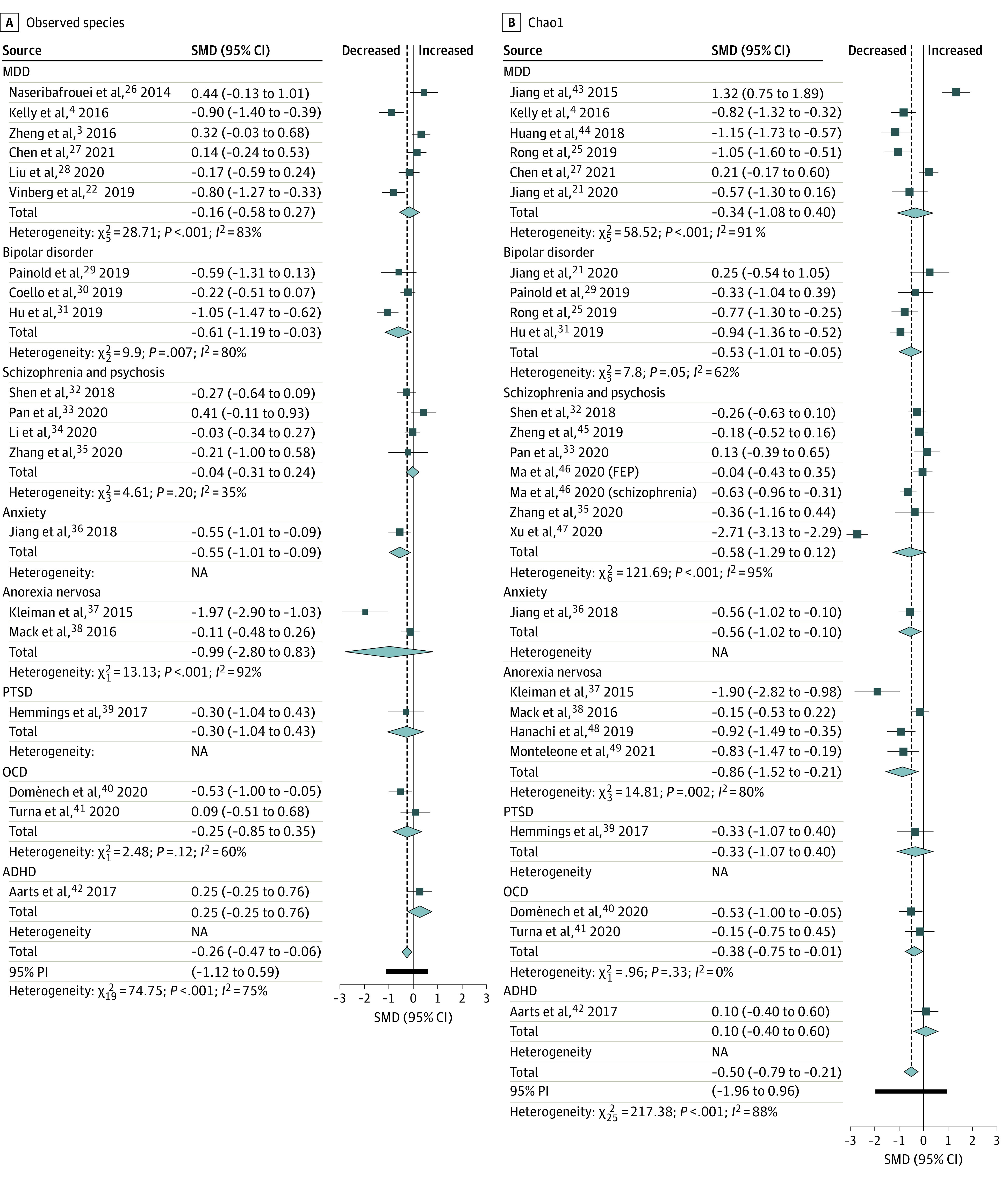
ADHD indicates attention-deficit/hyperactivity disorder; FEP, first episode psychosis; MDD, major depressive disorder; NA, not applicable; OCD, obsessive compulsive disorder; PI, prediction interval; PTSD, posttraumatic stress disorder; SMD, standardized mean difference.
Regarding diversity, 29 studies reported the Shannon index in patients (n = 1176) vs controls (n = 1172). The pooled estimate demonstrated a nonsignificant difference between groups (SMD = −0.12; 95% CI, −0.27 to 0.03; P = .11) (Figure 2A).3,4,21,22,25,27,28,31,32,33,34,35,38,39,40,41,42,43,44,45,46,48,50,51,52,53 Simpson index data were provided by 11 studies (n = 418 patients; n = 377 controls). There was a nonsignificant difference between groups (SMD = 0.04; 95% CI, −0.13 to 0.21; P = .66), with nonsignificant heterogeneity (Figure 2B).21,26,27,31,32,33,40,43,52 Finally, 10 studies provided phylogenetic diversity data in patients (n = 412) vs controls (n = 454). The pooled estimate showed a significant decrease in patients with a small effect size (SMD = −0.24; 95% CI, −0.47 to −0.0012; P = .049; 64%) (Figure 2C).3,4,28,32,33,34,39,40,42,44
Figure 2. Forest Plots of Alpha Diversity in the Gut Microbiota of Patients With Psychiatric Disorders Compared With Healthy Controls.
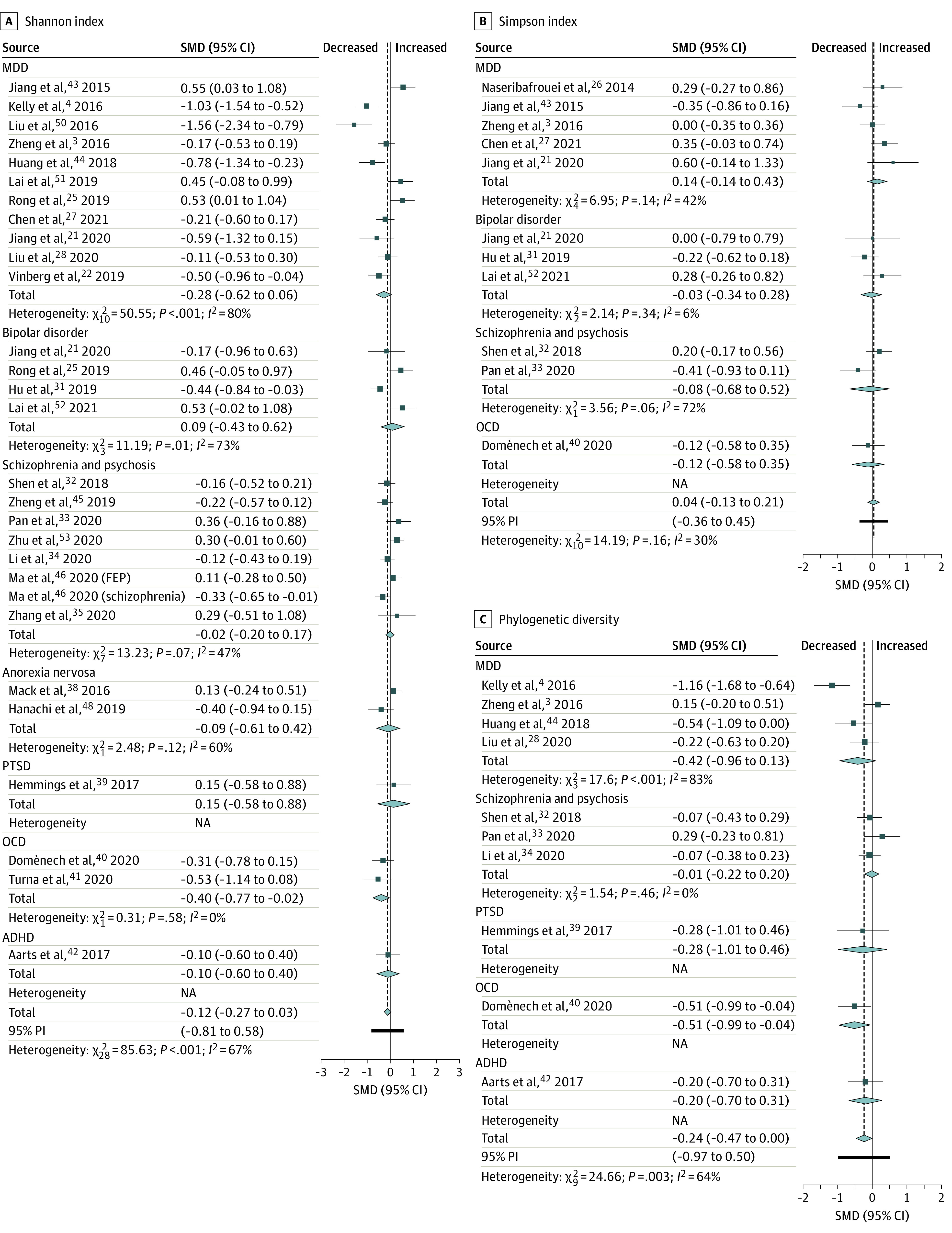
ADHD indicates attention-deficit/hyperactivity disorder; FEP, first episode psychosis; MDD, major depressive disorder; NA, not applicable; OCD, obsessive compulsive disorder; PI, prediction interval; PTSD, posttraumatic stress disorder; SMD, standardized mean difference.
To explore sources of interstudy heterogeneity, subgroup analyses and meta-regressions were performed for the analyses with sufficient studies (observed species, Chao1, Shannon). Body mass index, age, sex, smoking, region (east/west), psychiatric medication use, subgrouping of psychosis and schizophrenia into first episode, and chronic and sequencing method (including hypervariable region sequenced) did not have a significant association with findings. However, it should be noted that shotgun metagenomics showed increased Shannon diversity in patients (4 studies) in comparison with 16SrRNA V3-V4 sequencing, which showed an overall decrease (12 studies). This could be because the shotgun approach quantifies all genomic DNA (including mycobiome and virome) rather than just specific regions of bacterial DNA. Further studies using shotgun metagenomics or comparing the 2 methodologies on the same population are needed.
Beta Diversity
Beta diversity comparison between patients and controls was reported in 43 studies, with 1 study reporting on 3 separate groups (MDD, anxiety, and MDD + anxiety23), using a variety of measures (eAppendix 9 in the Supplement). Consistent nonsignificant differences were reported by 16 studies, and a further 3 reported conflicting results between the measures used. Patients’ samples clustered differently from controls in 12 of 15 studies in MDD, 7 of 9 in psychosis and schizophrenia, 3 of 6 in bipolar disorder, 3 of 6 in anorexia nervosa, 2 of 3 in anxiety, 0 of 2 in OCD, and 0 of 1 in PTSD (eAppendix 9 in the Supplement). One of 2 combined MDD + bipolar disorder cohort was also significantly different from controls, whereas the MDD + anxiety cohort was not. Although, while Mason et al23 found no differences when looking at diagnostic categories, they found a significant difference when clustering participants according to self-reported symptoms. These findings suggest there is reliable evidence for differences in the shared phylogenetic structure in MDD and psychosis and schizophrenia compared with controls; however, method of measurement and method of patient classification (symptom vs diagnosis based) may affect findings.
Differentially Abundant Microbial Taxa
All studies assessed the relative abundance of gut microbes and 57 of 59 (96.6%) identified significant differences between patients and controls at phylum, family, or genus levels. Overall, in MDD (21 comparisons), 94 taxa were differentially abundant; in psychosis and schizophrenia (11 comparisons), 136; in bipolar disorder (9 comparisons), 60; in anxiety (2 comparisons), 36; in anorexia nervosa (10 comparisons), 32; in OCD (2 comparisons), 15; and in ADHD and PTSD (1 study each), 9 and 3, respectively. After removal of nonreplicated findings, the differences spanned 7 phyla, 28 families, and 67 genera. Study-level findings are presented in eAppendix 10 in the Supplement.
Figure 3 provides the summary of the within- and between-disorder comparison for the disorders with sufficient studies (anorexia nervosa, MDD, bipolar disorder, anxiety, and psychosis and schizophrenia). There was high within-disorder inconsistency and the majority of consistent within-disorder changes were replicated by only 2 studies and thus require further investigation. Considerably fewer were replicated by more than 2 studies from different research groups.
Figure 3. Changes in Relative Abundance of Microbial Taxa Reported by at Least 2 Studies From a Diagnostic Category.
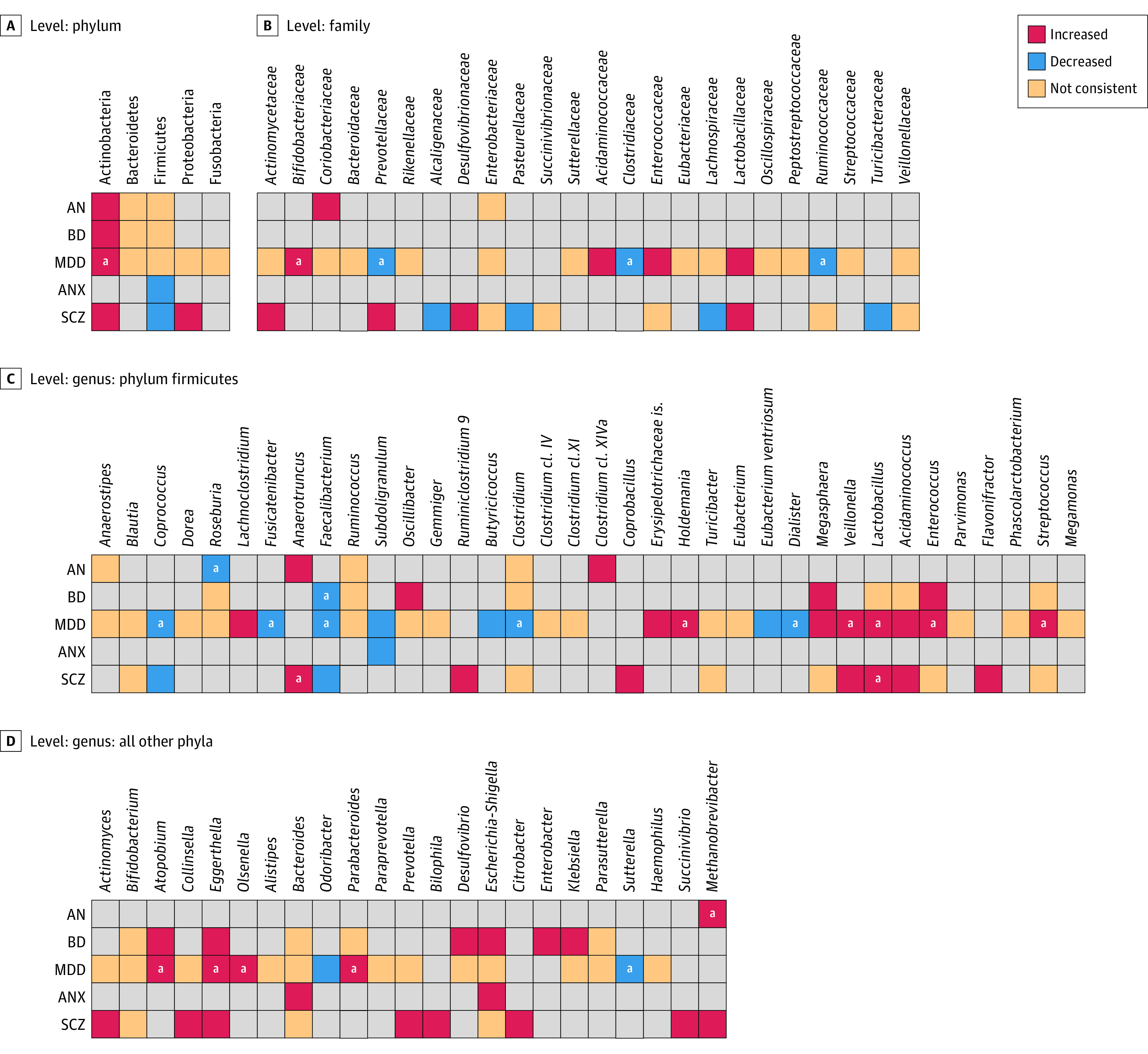
Gray cells indicate not examined, not reported, or not replicated.
aMost replicated findings are indicated here, all of which have been reported by more than 1 research group. Number of studies: anorexia nervosa (AN), 10; bipolar disorder (BD), 9; major depressive disorder (MDD), 21; anxiety (ANX), 2; psychosis and schizophrenia (SCZ), 11.
Limited Evidence of Disorder Specificity
Disorder specificity was observed for the enrichment of genera Holdemania and Olsenella and the depletion of genera Fusicatenibacter, Dialister, and Sutterella in MDD (Figure 3C). However, these findings were weakly reproduced (3 to 4 of 21 studies). The archaeon Methanobrevibacter and genus Anaerotruncus may also be candidates for disorder specificity because they were consistently associated with anorexia nervosa and psychosis and schizophrenia, respectively. Interestingly, an alteration in the same direction was also reported in 2 studies from the other disorder, which could not be explained by apparent demographic, clinical, or methodological factors. Nevertheless, specificity in anorexia nervosa cannot be assessed here because no studies in other eating disorders were identified, and conditions such as obesity were beyond the scope. No distinct disorder-specific alterations were observed for the remaining taxa.
Transdiagnostic Alterations
Our findings indicate an overlap between certain disorders: bipolar disorder, psychosis and schizophrenia, and anxiety were associated with MDD. The most consistent changes were depletion of Faecalibacterium (in 15 of 17 studies reporting this genus) and Coprococcus (10 of 10 studies) and the enrichment of Eggerthella (in 10 of 11 studies) (eAppendix 10 in the Supplement). These were followed by enriched Lactobacillus (10 of 13 studies), Enterococcus (8 of 9 studies), and Streptococcus (8 of 10 studies). Further, Atopobium was enriched in bipolar disorder and MDD (5 of 5 studies), while Veillonella was enriched in psychosis and schizophrenia and MDD (5 of 6 studies). There was also evidence for the increase of the pathogen Escherichia-Shigella in bipolar disorder, anxiety, and psychosis and schizophrenia (6 of 7 studies) but not MDD. The Bifidobacterium and Bacteroides genera were reported frequently but inconsistently across these disorders (14 and 16 studies, respectively).
Exploring Confounders: Region and Psychiatric Medication
We explored the association of study region (east/west) with microbial alterations. Owing to the limited overlap in findings and the imbalanced availability of studies by region (eg, MDD and psychosis and schizophrenia were largely investigated in the east, while anorexia nervosa and OCD were investigated in the west), this analysis should be considered preliminary. Clustering according to region identified several taxa that were altered only in studies from Eastern countries: Acidaminococcus (increased), Blautia (not consistent), Megamonas (decreased), Megasphaera (increased), Atopobium (increased), and Bacteroides (not consistent). These differences were driven entirely by studies from China, highlighting the need to distinguish the Chinese microbiome from other East Asian nations as more evidence becomes available.
There is evidence that psychiatric medication can affect microbiota composition.16,54 To investigate this, we compared results from medication-free studies (n = 11) with those in which 80% or more of patients were taking medication (n = 21). We found that increases in the family Lactobacillaceae (although not member genus Lactobacillus) and the genera Clostridium, Klebsiella, and Megasphaera were only reported in medicated groups, while Dialister was decreased in medicated and increased in medication-free groups. Further, 6 of 8 studies in treated patients reported increases in Streptococcus, which was not reported in drug-free studies.
Discussion
To our knowledge, this is the first review to assess gut microbiota perturbations across a spectrum of psychiatric disorders with the aim of evaluating the reproducibility and specificity of potential gut microbial biomarkers. The pattern of alterations observed suggests an increased magnitude and complexity of microbial disorganization for some disorders compared with others. For example, the highest number of differentially abundant taxa was in psychosis and schizophrenia (136 taxa; 11 studies), despite almost twice as many studies in MDD (94 taxa; 21 studies). Conversely, anorexia nervosa was associated with fewer differences (32 taxa; 10 studies), despite the larger number of studies compared with anxiety (36 taxa; 2 studies) and bipolar disorder (60 taxa; 9 studies). This is reminiscent of genome-wide association studies’ findings, in which the highest number of loci have been associated with psychosis and schizophrenia followed by MDD and bipolar disorder, and fewer have been associated with anorexia nervosa, PTSD, and ADHD.55 This increased complexity, also reflected in the microbiota, is consistent with the wider spectrum of clinical presentations associated with the former compared with the latter set of disorders.
Overall, we did not find evidence for disorder specificity: whenever microbial alterations merited specificity, these were weakly reproduced, suggesting they may instead reflect specific population characteristics (eg, depression subtype) and thus need further verification. Instead, our findings indicated that certain disorders share similar patterns of microbial changes. Specifically, we observed an overlap between psychosis and schizophrenia, bipolar disorder, anxiety, and MDD in consistently and inconsistently altered taxa, suggesting these likely harbor transdiagnostic alterations associated with overlapping pathophysiology as has previously been seen in analyses of inflammatory markers, neutrophil-lymphocyte ratios, and genome-wide association studies.12,56,57
Most consistently, the genus Eggerthella was enriched in MDD, bipolar disorder, and psychosis and schizophrenia, while the genera Faecalibacterium and Coprococcus were decreased in all. Eggerthella is associated with gastrointestinal inflammation,10,58 while Faecalibacterium has known anti-inflammatory properties59 and is depleted in immune-mediated inflammatory diseases.58,60 These associations are likely mediated by short-chain fatty acid butyrate, as Faecalibacterium and Coprococcus are involved in its production,61 while Eggerthella has been associated with its depletion.10 Butyrate has a key role in maintaining mucosal integrity and reducing inflammation via macrophage function and decrease in proinflammatory cytokines, while increasing anti-inflammatory mediators.61,62,63 Further, Faecalibacterium was inversely associated with depression severity in 2 MDD studies, 1 bipolar disorder study, and 1 anorexia nervosa study,28,43,64,65 suggesting depletion of this genus may be characteristic of the depressive state, irrespective of diagnosis. Therefore, clinical features and underlying pathophysiology that manifest across diagnoses may be better suited to explain the observed microbial alterations than distinct diagnostic categories. The merits of incorporating the gut microbiota as a dimensional component to the Research Domain Criteria66 have previously been discussed67 and our results reinforce this by demonstrating that while gut microbiota abnormalities were ubiquitously observed, these do not seem to congregate according to distinct diagnoses but instead exhibit a transdiagnostic pattern.
Interestingly, the family Lactobacillaceae and member genus Lactobacillus, strains from which are components of probiotic supplements and linked to positive health outcomes,68 were enriched in MDD, psychosis and schizophrenia, and bipolar disorder. A possible explanation could be that species from this genus have differential effect. For example, 1 study identified the increase in psychosis and schizophrenia to be in subspecies not typically present in the healthy gut.53 Alternatively, increased Lactobacillus has previously been associated with antipsychotic use.69 This finding was somewhat corroborated here, as 4 psychosis and schizophrenia studies reporting increased Lactobacillus were conducted in medicated groups,32,34,46,70 while the one that reported decreased Lactobacillus was in a treatment-naive group.71 In our exploratory analyses, the family Lactobacillaceae was significantly increased only in medicated groups. This suggests that psychotropic medication may be exacerbating the presence of illness-associated Lactobacilli species.
Measures of alpha diversity (within sample) were widely used, following the general assumption that higher diversity is more beneficial to the host72 and thus expected to be decreased in psychiatric patients, as has previously been observed for various diseases.73 However, our meta-analysis demonstrated a nonsignificant association with diversity indices and small to medium decrease in richness, suggesting that while richness is somewhat compromised (although the clinical significance of this decrease is unclear), diversity is overall preserved. The high residual heterogeneity following subgrouping according to disorder type suggests that diagnosis is not a good discriminator of alpha diversity. Regarding beta diversity (between samples), patients with MDD and psychosis and schizophrenia consistently clustered differently from controls. However, it is yet unknown whether psychiatric disorders cluster differently from one another, thus questioning the suitability of diversity measures as biomarkers. From the studies summarized, only 2 studies compared beta diversity cross-diagnostically and neither found a significant difference.21,25
Among the numerous clinical and demographic factors that may have contributed to the widespread inconsistencies between studies, current evidence allowed us to explore 2 key characteristics: geographical region and psychiatric medication. Geographical region and the associated factor of diet can profoundly affect the composition of the microbiota.74,75 Our analysis suggested that some of the observed perturbations may be specific to Chinese populations (eg, increased Acidaminococcus), others may be owing to the effect of psychiatric medication (eg, increased Klebsiella and decreased Dialister), while third may be influenced by a combination of both, such as the genus Megasphaera, which was enriched only in Chinese populations undergoing treatment. Future studies should be encouraged to report findings (even nonsignificant) on all dominant taxa to help delineate the effect of confounders from true disease effects. Additionally, more studies will be needed in currently underrepresented populations from low- to middle-income countries, as mental health problems become an increasing concern.76
For brevity, we have not discussed methodological differences that may have contributed to inconsistent findings such as processing, sequencing, or analysis pipelines because these have been extensively reviewed by others.9,10,74,77,78 Further, some have suggested that the current approaches to microbiome analyses may be unreliable owing to inappropriate handling of inherently compositional data.79 The lack of power calculations is a significant deterrent in the field. To move forward, the reporting of quantitative effect sizes of abundance findings in addition to P values is needed to enable meta-analyses and the evaluation of potentially relevant biological effects.80 Even then, technical and clinical variation between studies may make it difficult to compare effect sizes, which reinforces the need of harmonizing methodologies and encouraging data sharing with sufficient metadata.
Limitations
Although there were insufficient studies to perform in-depth analyses of OCD, PTSD, and anxiety, we believe the inclusion of these disorders provides a comprehensive overview of current evidence. There were no studies in adults with autism spectrum disorder and only 1 study in ADHD, thus precluding us from comparing the association of neurodevelopmental disorders with the microbiota in adulthood. The decision to exclude studies in children and elderly individuals was dictated by an appreciation of the specialist nature of these populations and the substantial age-related differences in the microbiota.81 Next, we acknowledge that the division into Eastern and Western countries is a crude approach to controlling for geographical differences in diet and genetics and does not allow detection of regional variations in the microbiome, which might also explain why we found no alterations specific to Western populations. As more studies become available, more nuanced analyses will be possible. Additionally, most studies had modest sample sizes, suggesting our analyses may still be underpowered and preliminary. Similarly, as most studies included both medicated and unmedicated patients, our analyses of the confounding effects of medication require further verification in larger stratified populations. Our summary may also suffer from the use of different reference databases between studies, as inconsistencies in assigning taxonomy have been described.82 Finally, the aim of this review was to evaluate gut microbial composition, rather than function. Early evidence has suggested that functional potentials associated with psychiatric illness include short-chain fatty acid synthesis, tryptophan metabolism, and neurotransmitter synthesis/degradation.52,53,83,84 Given the noted functional redundancy,85 functional analysis will be key in understanding the role of host-microbiome interactions in neuropsychiatric disorders.
Conclusions
This review suggests a transdiagnostic commonality of microbial disturbances in MDD, bipolar disorder, anxiety, and psychosis and schizophrenia, characterized by depleted anti-inflammatory butyrate-producing bacteria and enriched proinflammatory bacteria. The effect of key confounders such as psychiatric medication and diet should be carefully considered. Researchers should interpret their findings within the larger context of psychiatric disorders to prevent unmerited claims of disorder specificity of gut microbial biomarkers. The evidence summarized here is a good starting point for such comparisons.
eAppendix 1. Systematic search details
eAppendix 2. Quality assessment
eAppendix 3. Detailed methods of the meta-analysis performed
eAppendix 4. PRISMA flowcharts for the umbrella review search and the updated review searches
eAppendix 5. Details of the identified systematic reviews
eAppendix 6. Detailed characteristics of the included studies
eAppendix 7. Stool sample processing methods in the included studies
eAppendix 8. Publication bias assessment for the alpha diversity meta-analyses
eAppendix 9. Beta diversity
eAppendix 10. Figures for study-level findings of relative abundance of microbial taxa
References
- 1.Phillips JGP. The treatment of melancholia by the lactic acid bacillus. J Mental Sci. 1910;56(234):422-430. doi: 10.1192/bjp.56.234.422 [DOI] [Google Scholar]
- 2.Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: part I: autointoxication revisited. Gut Pathog. 2013;5(1):5. doi: 10.1186/1757-4749-5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng P, Zeng B, Zhou C, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21(6):786-796. doi: 10.1038/mp.2016.44 [DOI] [PubMed] [Google Scholar]
- 4.Kelly JR, Borre Y, O’ Brien C, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109-118. doi: 10.1016/j.jpsychires.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 5.Zhu F, Guo R, Wang W, et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol Psychiatry. 2020;25(11):2905-2918. doi: 10.1038/s41380-019-0475-4 [DOI] [PubMed] [Google Scholar]
- 6.Li N, Wang Q, Wang Y, et al. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress. 2019;22(5):592-602. doi: 10.1080/10253890.2019.1617267 [DOI] [PubMed] [Google Scholar]
- 7.Sharon G, Cruz NJ, Kang D-W, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177(6):1600-1618.e17. doi: 10.1016/j.cell.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanada K, Nakajima S, Kurokawa S, et al. Gut microbiota and major depressive disorder: a systematic review and meta-analysis. J Affect Disord. 2020;266:1-13. doi: 10.1016/j.jad.2020.01.102 [DOI] [PubMed] [Google Scholar]
- 9.Di Lodovico L, Mondot S, Doré J, Mack I, Hanachi M, Gorwood P. Anorexia nervosa and gut microbiota: a systematic review and quantitative synthesis of pooled microbiological data. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110114. doi: 10.1016/j.pnpbp.2020.110114 [DOI] [PubMed] [Google Scholar]
- 10.Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CSM. The gut microbiota in anxiety and depression: a systematic review. Clin Psychol Rev. 2021;83:101943. doi: 10.1016/j.cpr.2020.101943 [DOI] [PubMed] [Google Scholar]
- 11.IPCS INCHEM. Environmental health criteria 222: Biomarkers in risk assessment: validity and validation. Accessed February 8, 2021. http://www.inchem.org/documents/ehc/ehc/ehc222.htm
- 12.Yuan N, Chen Y, Xia Y, Dai J, Liu C. Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl Psychiatry. 2019;9(1):233. doi: 10.1038/s41398-019-0570-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 14.Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: overviews of reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions, version 6.1. Cochrane; 2020.
- 15.Cumpston M, Changler J. Chapter IV: updating a review. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions, version 6.1. Cochrane; 2020. [Google Scholar]
- 16.Ait Chait Y, Mottawea W, Tompkins TA, Hammami R. Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci Rep. 2020;10(1):17878. doi: 10.1038/s41598-020-74934-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whiting P, Savović J, Higgins JPT, et al. ; ROBIS group . ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225-234. doi: 10.1016/j.jclinepi.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moola S, Munn Z, Tufanaru C, et al. Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. Joanna Briggs Institute Reviewer’s Manual. The Joanna Briggs Institute; 2017. [Google Scholar]
- 19.Duvallet C, Gibbons SM, Gurry T, Irizarry RA, Alm EJ. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat Commun. 2017;8(1):1784. doi: 10.1038/s41467-017-01973-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153-160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang HY, Pan LY, Zhang X, Zhang Z, Zhou YY, Ruan B. Altered gut bacterial-fungal interkingdom networks in patients with current depressive episode. Brain Behav. 2020;10(8):e01677. doi: 10.1002/brb3.1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinberg M, Ottesen NM, Meluken I, et al. Remitted affective disorders and high familial risk of affective disorders associate with aberrant intestinal microbiota. Acta Psychiatr Scand. 2019;139(2):174-184. doi: 10.1111/acps.12976 [DOI] [PubMed] [Google Scholar]
- 23.Mason BL, Li Q, Minhajuddin A, et al. Reduced anti-inflammatory gut microbiota are associated with depression and anhedonia. J Affect Disord. 2020;266:394-401. doi: 10.1016/j.jad.2020.01.137 [DOI] [PubMed] [Google Scholar]
- 24.Stevens BR, Goel R, Seungbum K, et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut. 2018;67(8):1555-1557. doi: 10.1136/gutjnl-2017-314759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rong H, Xie XH, Zhao J, et al. Similarly in depression, nuances of gut microbiota: evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. J Psychiatr Res. 2019;113:90-99. doi: 10.1016/j.jpsychires.2019.03.017 [DOI] [PubMed] [Google Scholar]
- 26.Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26(8):1155-1162. doi: 10.1111/nmo.12378 [DOI] [PubMed] [Google Scholar]
- 27.Chen YH, Xue F, Yu SF, et al. Gut microbiota dysbiosis in depressed women: the association of symptom severity and microbiota function. J Affect Disord. 2021;282:391-400. doi: 10.1016/j.jad.2020.12.143 [DOI] [PubMed] [Google Scholar]
- 28.Liu RT, Rowan-Nash AD, Sheehan AE, et al. Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain Behav Immun. 2020;88:308-324. doi: 10.1016/j.bbi.2020.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Painold A, Mörkl S, Kashofer K, et al. A step ahead: exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019;21(1):40-49. doi: 10.1111/bdi.12682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coello K, Hansen TH, Sørensen N, et al. Gut microbiota composition in patients with newly diagnosed bipolar disorder and their unaffected first-degree relatives. Brain Behav Immun. 2019;75:112-118. doi: 10.1016/j.bbi.2018.09.026 [DOI] [PubMed] [Google Scholar]
- 31.Hu S, Li A, Huang T, et al. Gut microbiota changes in patients with bipolar depression. Adv Sci (Weinh). 2019;6(14):1900752. doi: 10.1002/advs.201900752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen Y, Xu J, Li Z, et al. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: a cross-sectional study. Schizophr Res. 2018;197:470-477. doi: 10.1016/j.schres.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 33.Pan R, Zhang X, Gao J, Yi W, Wei Q, Su H. Analysis of the diversity of intestinal microbiome and its potential value as a biomarker in patients with schizophrenia: a cohort study. Psychiatry Res. 2020;291:113260. doi: 10.1016/j.psychres.2020.113260 [DOI] [PubMed] [Google Scholar]
- 34.Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi: 10.7717/peerj.9574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Pan L-Y, Zhang Z, Zhou Y-Y, Jiang H-Y, Ruan B. Analysis of gut mycobiota in first-episode, drug-naïve Chinese patients with schizophrenia: a pilot study. Behav Brain Res. 2020;379:112374. doi: 10.1016/j.bbr.2019.112374 [DOI] [PubMed] [Google Scholar]
- 36.Jiang H-Y, Zhang X, Yu Z-H, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136. doi: 10.1016/j.jpsychires.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 37.Kleiman SC, Watson HJ, Bulik-Sullivan EC, et al. The intestinal microbiota in acute anorexia nervosa and during renourishment: relationship to depression, anxiety, and eating disorder psychopathology. Psychosom Med. 2015;77(9):969-981. doi: 10.1097/PSY.0000000000000247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mack I, Cuntz U, Grämer C, et al. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci Rep. 2016;6:26752. doi: 10.1038/srep26752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hemmings SMJ, Malan-Müller S, van den Heuvel LL, et al. The microbiome in posttraumatic stress disorder and trauma-exposed controls: an exploratory study. Psychosom Med. 2017;79(8):936-946. doi: 10.1097/PSY.0000000000000512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domènech L, Willis J, Alemany M, et al. Changes in the stool and oropharyngeal microbiome in obsessive-compulsive disorder. medRxiv. Preprint posted online May 28, 2020. doi: 10.1101/2020.05.26.20113779 [DOI] [PMC free article] [PubMed]
- 41.Turna J, Grosman Kaplan K, Anglin R, et al. The gut microbiome and inflammation in obsessive-compulsive disorder patients compared to age- and sex-matched controls: a pilot study. Acta Psychiatr Scand. 2020;142(4):337-347. doi: 10.1111/acps.13175 [DOI] [PubMed] [Google Scholar]
- 42.Aarts E, Ederveen THA, Naaijen J, et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS One. 2017;12(9):e0183509. doi: 10.1371/journal.pone.0183509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186-194. doi: 10.1016/j.bbi.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 44.Huang Y, Shi X, Li Z, et al. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr Dis Treat. 2018;14:3329-3337. doi: 10.2147/NDT.S188340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi: 10.1126/sciadv.aau8317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma X, Asif H, Dai L, et al. Alteration of the gut microbiome in first-episode drug-naïve and chronic medicated schizophrenia correlate with regional brain volumes. J Psychiatr Res. 2020;123:136-144. doi: 10.1016/j.jpsychires.2020.02.005 [DOI] [PubMed] [Google Scholar]
- 47.Xu R, Wu B, Liang J, et al. Altered gut microbiota and mucosal immunity in patients with schizophrenia. Brain Behav Immun. 2020;85:120-127. doi: 10.1016/j.bbi.2019.06.039 [DOI] [PubMed] [Google Scholar]
- 48.Hanachi M, Manichanh C, Schoenenberger A, et al. Altered host-gut microbes symbiosis in severely malnourished anorexia nervosa (AN) patients undergoing enteral nutrition: an explicative factor of functional intestinal disorders? Clin Nutr. 2019;38(5):2304-2310. doi: 10.1016/j.clnu.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 49.Monteleone AM, Troisi J, Fasano A, et al. Multi-omics data integration in anorexia nervosa patients before and after weight regain: A microbiome-metabolomics investigation. Clin Nutr. 2021;40(3):1137-1146. doi: 10.1016/j.clnu.2020.07.021 [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Zhang L, Wang X, et al. Similar fecal microbiota signatures in patients with diarrhea-predominant irritable bowel syndrome and patients with depression. Clin Gastroenterol Hepatol. 2016;14(11):1602-1611.e5. doi: 10.1016/j.cgh.2016.05.033 [DOI] [PubMed] [Google Scholar]
- 51.Lai W-T, Deng W-F, Xu S-X, et al. Shotgun metagenomics reveals both taxonomic and tryptophan pathway differences of gut microbiota in major depressive disorder patients. Psychol Med. 2021:51(1):90-101. doi: 10.1017/S0033291719003027 [DOI] [PubMed] [Google Scholar]
- 52.Lai WT, Zhao J, Xu SX, et al. Shotgun metagenomics reveals both taxonomic and tryptophan pathway differences of gut microbiota in bipolar disorder with current major depressive episode patients. J Affect Disord. 2021;278:311-319. doi: 10.1016/j.jad.2020.09.010 [DOI] [PubMed] [Google Scholar]
- 53.Zhu F, Ju Y, Wang W, et al. Metagenome-wide association of gut microbiome features for schizophrenia. Nat Commun. 2020;11(1):1612. doi: 10.1038/s41467-020-15457-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Macedo D, Filho AJMC, Soares de Sousa CN, et al. Antidepressants, antimicrobials or both? gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J Affect Disord. 2017;208:22-32. doi: 10.1016/j.jad.2016.09.012 [DOI] [PubMed] [Google Scholar]
- 55.Ikeda M, Saito T, Kanazawa T, Iwata N. Polygenic risk score as clinical utility in psychiatry: a clinical viewpoint. J Hum Genet. 2021;66(1):53-60. doi: 10.1038/s10038-020-0814-y [DOI] [PubMed] [Google Scholar]
- 56.Lee SH, Ripke S, Neale BM, et al. ; Cross-Disorder Group of the Psychiatric Genomics Consortium; International Inflammatory Bowel Disease Genetics Consortium (IIBDGC) . Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984-994. doi: 10.1038/ng.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brinn A, Stone J. Neutrophil-lymphocyte ratio across psychiatric diagnoses: a cross-sectional study using electronic health records. BMJ Open. 2020;10(7):e036859. doi: 10.1136/bmjopen-2020-036859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forbes JD, Chen C-Y, Knox NC, et al. A comparative study of the gut microbiota in immune-mediated inflammatory diseases-does a common dysbiosis exist? Microbiome. 2018;6(1):221. doi: 10.1186/s40168-018-0603-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu X, Zhang M, Yang X, Hong N, Yu C. Faecalibacterium prausnitzii upregulates regulatory T cells and anti-inflammatory cytokines in treating TNBS-induced colitis. J Crohns Colitis. 2013;7(11):e558-e568. doi: 10.1016/j.crohns.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 60.Sokol H, Seksik P, Furet JP, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15(8):1183-1189. doi: 10.1002/ibd.20903 [DOI] [PubMed] [Google Scholar]
- 61.Miquel S, Martín R, Bridonneau C, et al. Ecology and metabolism of the beneficial intestinal commensal bacterium Faecalibacterium prausnitzii. Gut Microbes. 2014;5(2):146-151. doi: 10.4161/gmic.27651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111(6):2247-2252. doi: 10.1073/pnas.1322269111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sublette ME, Cheung S, Lieberman E, et al. Bipolar disorder and the gut microbiome: a systematic review. Bipolar Disord. Published online January 29, 2021. doi: 10.1111/bdi.13049 [DOI] [PubMed] [Google Scholar]
- 64.Evans SJ, Bassis CM, Hein R, et al. The gut microbiome composition associates with bipolar disorder and illness severity. J Psychiatr Res. 2017;87:23-29. doi: 10.1016/j.jpsychires.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y-H, Bai J, Wu D, et al. Association between fecal microbiota and generalized anxiety disorder: severity and early treatment response. J Affect Disord. 2019;259:56-66. doi: 10.1016/j.jad.2019.08.014 [DOI] [PubMed] [Google Scholar]
- 66.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751. doi: 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- 67.Kelly JR, Clarke G, Cryan JF, Dinan TG. Dimensional thinking in psychiatry in the era of the Research Domain Criteria (RDoC). Ir J Psychol Med. 2018;35(2):89-94. doi: 10.1017/ipm.2017.7 [DOI] [PubMed] [Google Scholar]
- 68.Yong SJ, Tong T, Chew J, Lim WL. Antidepressive mechanisms of probiotics and their therapeutic potential. Front Neurosci. 2020;13:1361. doi: 10.3389/fnins.2019.01361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bahr SM, Tyler BC, Wooldridge N, et al. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl Psychiatry. 2015;5(10):e652-e652. doi: 10.1038/tp.2015.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398-403. doi: 10.1016/j.schres.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 71.Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299-306. doi: 10.1016/j.schres.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 72.Shade A. Diversity is the question, not the answer. ISME J. 2017;11(1):1-6. doi: 10.1038/ismej.2016.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gerritsen J, Smidt H, Rijkers GT, de Vos WM. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6(3):209-240. doi: 10.1007/s12263-011-0229-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheung SG, Goldenthal AR, Uhlemann A-C, Mann JJ, Miller JM, Sublette ME. Systematic review of gut microbiota and major depression. Front Psychiatry. 2019;10:34. doi: 10.3389/fpsyt.2019.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simpson HL, Campbell BJ. Review article: dietary fibre-microbiota interactions. Aliment Pharmacol Ther. 2015;42(2):158-179. doi: 10.1111/apt.13248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sankoh O, Sevalie S, Weston M. Mental health in Africa. Lancet Glob Health. 2018;6(9):e954-e955. doi: 10.1016/S2214-109X(18)30303-6 [DOI] [PubMed] [Google Scholar]
- 77.Vindegaard N, Speyer H, Nordentoft M, Rasmussen S, Benros ME. Gut microbial changes of patients with psychotic and affective disorders: a systematic review. Schizophr Res. 2020;S0920-9964(19)30584-5. doi: 10.1016/j.schres.2019.12.014 [DOI] [PubMed] [Google Scholar]
- 78.Bastiaanssen TFS, Cowan CSM, Claesson MJ, Dinan TG, Cryan JF. Making sense of…the microbiome in psychiatry. Int J Neuropsychopharmacol. 2019;22(1):37-52. doi: 10.1093/ijnp/pyy067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gloor GB, Macklaim JM, Pawlowsky-Glahn V, Egozcue JJ. Microbiome datasets are compositional: and this is not optional. Front Microbiol. 2017;8:2224. doi: 10.3389/fmicb.2017.02224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Debelius J, Song SJ, Vazquez-Baeza Y, Xu ZZ, Gonzalez A, Knight R. Tiny microbes, enormous impacts: what matters in gut microbiome studies? Genome Biol. 2016;17(1):217. doi: 10.1186/s13059-016-1086-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Odamaki T, Kato K, Sugahara H, et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16(1):90. doi: 10.1186/s12866-016-0708-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balvočiūtė M, Huson DH. SILVA, RDP, Greengenes, NCBI and OTT: how do these taxonomies compare? BMC Genomics. 2017;18(2)(suppl 2):114. doi: 10.1186/s12864-017-3501-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valles-Colomer M, Falony G, Darzi Y, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 2019;4(4):623-632. doi: 10.1038/s41564-018-0337-x [DOI] [PubMed] [Google Scholar]
- 84.Yang J, Zheng P, Li Y, et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci Adv. 2020;6(49):eaba8555. doi: 10.1126/sciadv.aba8555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heintz-Buschart A, Wilmes P. Human gut microbiome: function matters. Trends Microbiol. 2018;26(7):563-574. doi: 10.1016/j.tim.2017.11.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Systematic search details
eAppendix 2. Quality assessment
eAppendix 3. Detailed methods of the meta-analysis performed
eAppendix 4. PRISMA flowcharts for the umbrella review search and the updated review searches
eAppendix 5. Details of the identified systematic reviews
eAppendix 6. Detailed characteristics of the included studies
eAppendix 7. Stool sample processing methods in the included studies
eAppendix 8. Publication bias assessment for the alpha diversity meta-analyses
eAppendix 9. Beta diversity
eAppendix 10. Figures for study-level findings of relative abundance of microbial taxa


