Abstract
Peroxygenases are an emerging new class of enzymes allowing selective oxyfunctionalisation reactions in a cofactor-independent way different from well-known P450 monooxygenases. Herein, we focused on recent developments from organic synthesis, molecular biotechnology and reaction engineering viewpoints that are devoted to bring these enzymes in industrial applications. This covers natural diversity from different sources, protein engineering strategies for expression, substrate scope, activity and selectivity, stabilisation of enzymes via immobilisation, and the use of peroxygenases in low water media. We believe that peroxygenases have much to offer for selective oxyfunctionalisations and we have much to study to explore the full potential of these versatile biocatalysts in organic synthesis.
Keywords: Oxidation chemistry, Oxyfunctionalisation, Fungal enzyme, Oxygenase, Peroxygenase
1. Introduction
Biocatalysis is steadily gaining importance in pharmaceutical and industrial chemistry. Especially the high stereo- and regioselectivity of many wild type or tailor-made enzymes is mostly valued. Furthermore, the excellent rate accelerations exceeded by enzymes are very beneficial for many processes as they allow for significantly lower reaction temperatures as compared to many heterogeneous and homogeneous catalysts. (Burek et al., 2019) Analysing the use of enzymes in the chemical industry (Sheldon and Woodley, 2018; Truppo, 2017; Woodley, 2019; Woodley, 2020) it becomes clear that enzymes are applied for a very limited range of production processes. Besides the widespread hydrolases, alcohol dehydrogenases, transaminases and imine reductases are receiving increased interest for the chemical and pharmaceutical production. (Huisman and Collier, 2013; Huisman et al., 2010) Selective oxidation chemistry or even selective oxyfunctionalisation of non-activated C–H-, C–C-, or C=C-bonds remains a white spot on the map of industrial biocatalysis with only a few examples from the pharmaceutical industry. This is astonishing insofar as especially in this area the selectivity benefits of enzymes can fully deploy.
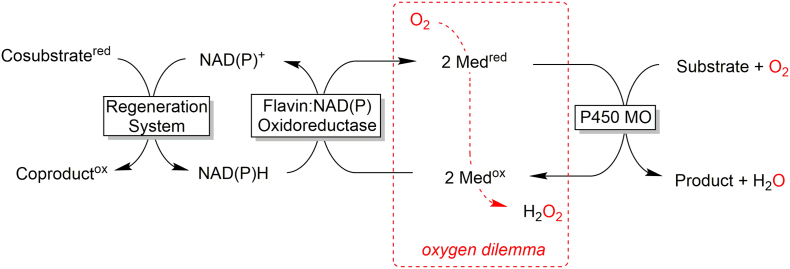
Amongst the oxygenases (E.C. 1.13 and E.C. 1.14), the heme-containing monooxygenases (P450 monooxygenases) are the most popular and best-known catalysts giving access to regio- and stereoselective hydroxylation, epoxidation and heteroatom oxygenation reactions. (Bormann et al., 2015) Given this enormous potential of P450s, the question of why these reactions are not more widespread, especially on industrial scale, suggests itself. Widely discussed reasons are the (i) limited availability of the enzyme, (ii) low substrate loadings, (iii) cofactor dependency of the P450s as well as the so-called (iv) Oxygen Dilemma. (Holtmann and Hollmann, 2016) While the first three reasons can certainly be solved by means of molecular biology and technical systems, the last factor (Oxygen Dilemma) is more difficult to address. Fe-dependent monoxygenases essentially rely on single electron transfer steps. Hence the hydride provided by NAD(P)H cannot directly reduce the monooxygenases’ active sites requiring a relays system to transform the hydride transfer into two successive single electron transfer steps. For this, mediator molecules such as ferredoxins or flavins are used. In their reduced (radical) form, they are however, also prone to direct electron transfer to dissolved O2. Since (in contrast to e.g. the reaction of O2 with NAD(P)H) this reaction is spin-allowed, it also occurs at high rates. As a consequence, a significant portion of the reducing equivalents theoretically provided by NAD(P)H does not reach the monooxygenases’ active sites but is wasted in a futile reaction with O2 (eventually yielding H2O2, Fig. 1).
Fig. 1.
Oxygen Dilemma of P450 monooxygenase reactions. As NAD(P)H cannot directly reduce the (Fe-containing) monooxygenases’ active sites redox mediators are necessary to transform the (NAD(P)H-dependent) hydride transfer into two successive single electron transfer steps. The reduced mediators (radicals) however also react spontaneously with dissolved O2 eventually yielding H2O2. This results in a futile uncoupling of the regeneration reaction from the monooxygenation reaction.
The final consequence thereof is that the reaction systems tend to be inefficient in terms of turnover numbers of the monooxygenase (i.e. moles of product formed per mole of monooxygenase) and hydroxylation (mono-oxygenase) efficiency (i.e. the proportion of reducing equivalents provided by the cosubstrate that are productively used for substrate turnover). An alternative class of enzymes that circumvents the Oxygen Dilemma are the fungal peroxygenases. These enzymes are structurally related to the aforementioned P450 monooxygenases insofar as they also contain the heme prosthetic group coordinated by a cysteine ligand and therefore, in principle, give access to the same enormous range of reactions and products. In contrast to P450 monooxygenases, peroxygenases do not catalyse the reductive activation of molecular oxygen but rather directly use hydrogen peroxide to form the catalytically active oxyferryl. (Wang et al., 2017) In fact, there do exist bacterial P450 peroxygenases (i.e. fed by H2O2) which limit their activity to the α-selective hydroxylation of long fatty acids. (Munro et al., 2018; Onoda et al., 2018; Xu et al., 2017) Thus, the Oxygen Dilemma can be avoided in general. The aim of the current contribution therefore was to review the current state of the art in practical application of fungal peroxygenases. In particular, the catalysed reactions and substrate scope, applied expression systems, protein engineering approaches to optimise the peroxygenases, reaction engineering as well as the immobilisation techniques will be addressed.
2. Natural diversity and protein engineering
The first reported true heme-thiolate peroxygenase isolated from an edible mushroom that produces white rot, the peroxygenase from Agrocybe aegerita (AaeUPO) (Ullrich et al., 2004), represents to date the main model enzyme for all peroxygenase chemistry (Hofrichter et al., 2015). After its initial misclassification as an unusual alkaline lignin peroxidase, later as a haloperoxidase, it was referred to as an aromatic peroxygenase (APO), and finally recognised as unspecific peroxygenase (UPO) constituting the first member of a new sub-subclass of oxidoreductases (EC 1.11.2.1). Four other enzymes are part of this subclass: myeloperoxidase (EC 1.11.2.2; Cl- + H2O2 + H+ ➔ HClO + H2O) (Klebanoff, 2005), plant seed peroxygenase (EC 1.11.2.3; R1H + R2OOH ➔ R1OH + R2OH) (Hanano et al., 2006), fatty acid peroxygenase (EC 1.11.2.4; fatty acid + H2O2 ➔ 3- or 2-hydroxy fatty acid + H2O) (Lee et al., 2003) and 3-methyl-L-tyrosine peroxygenase (EC 1.11.2.5; 3-methyl-L-tyrosine + H2O2 ➔ 3-hydroxy-5-methyl-L-tyrosine+ H2O) (Tang et al., 2012).
UPO is able to insert oxygen into non-activated C-H (both in aliphatic and aromatic compounds), being more enantio- than regioselective and it can be considered a Swiss Army knife for oxyfunctionalisation chemistry whose promiscuity is reflected by its extensive portfolio of transformations (see section 3 on Substrate Scope). Despite its broad substrate specificity (over 400 compounds already described), the peroxygenase sole catalytic requirement is hydrogen peroxide, which acts as both the final electron acceptor and the oxygen donor. The resting state heme binds hydrogen peroxide and through a short-lived compound 0, forms the key intermediate compound I. This strong oxidant can then directly interact with the substrate, forming compound II and subsequently releasing the hydroxylated product. (Hofrichter et al., 2020) While showing a similar chemistry as P450 monooxygenases, UPOs have much less requirements to perform complex oxyfunctionalisation chemistry with high efficiency, in the absence of expensive redox cofactors or auxiliary flavoproteins, being highly active and extracellular secreted enzymes. More significantly, UPOs escape from the O2 uncoupling which for P450s represents a recurrent problem as up to 90% of all the reducing equivalents provided by the sacrificial substrate can be wasted in the futile uncoupling reaction (the Oxygen Dilemma). Given that UPOs can carry out one-electron oxidations (as general peroxidases) and two-electron oxidations (the base for oxyfunctionalisation chemistry), they are considered, from a catalytic point of view the “missing link” between P450s and the classical chloroperoxidase from Caldariomyces fumago (CfuCPO, EC 1.11.1.10) –heme-thiolate containing enzyme with a Cys residue as axial ligand of the heme– as well as with general peroxidases with a His residue as axial ligand. As heme thiolate peroxidases, CfuCPO and UPO share an overall similar reaction mechanism, but CfuCPO cannot perform oxygenations of alicyclic/aromatic rings or n-alkanes like UPO.
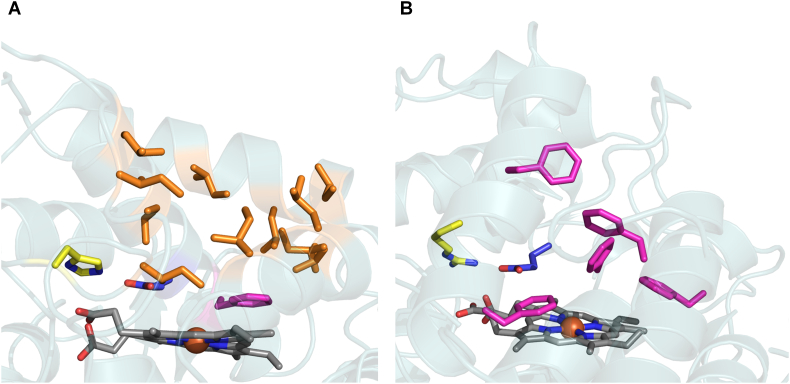
In terms of natural diversity, over 4,000 putative peroxygenase sequences from different fungi have been deposited in the genomic databases, with the characterisation of the following wild type UPOs (i.e. produced from the natural fungus): the original Agrocybe aegerita –AaeUPO– (Ullrich et al., 2004), Agrocybe parasitica -ApaUPO- (Hofrichter et al., 2015), Coprinellus radians –CraUPO–, Coprinopsis verticillata –CveUPO– (Anh, 2008; Anh et al., 2007), Marasmius rotula –MroUPO– (Gröbe et al., 2011), Chaetomium globosum –CglUPO– (Kiebist et al., 2017), Marasmius wettsteinii –MweUPO– (Ullrich et al., 2018) and Psathyrella aberdarensis –PabUPO– (Hofrichter et al., 2020). Given their widespread distribution in fungi, UPOs have been phylogenetically sorted into family I (short peroxygenases) and family II (long peroxygenases). Short UPOs with molecular weights of ~26 kDa (like MroUPO or CglUPO), are broadly found throughout the fungal kingdom. They are dimeric proteins with a histidine residue as a charge stabiliser at the active site and lack intramolecular disulfide bridges, although they do establish an intermolecular disulfide bridge to connect both monomers. By contrast, long UPOs with molecular weights of ~44 kDa (like AaeUPO or PabUPOs) are found in basidiomycetes and ascomycetes, and they are monomeric with an internal disulfide bridge and an arginine residue as a charge stabiliser (Hofrichter et al., 2015). Both families have highly conserved sequences at the active site (i.e. -EHD-S-E- and -EGD-S-R-E for short and long UPOs, respectively). The composition, dimensions and conformation of heme access channel between the short and long clades are reflected in their distinct substrate profiles and functions, Table 1. (Hofrichter et al., 2020) Short UPOs heme access channel is upholstered by flexible aliphatic amino acids and is shorter but wider compared with that of long UPOs, which is translated for the former into a preference for bulky substrates like steroids (Kiebist et al., 2019). Conversely, long UPO’s channel is formed by rigid aromatic amino acids, which determine a substrate preference towards smaller aromatic substrates (Fig. 2). The role of UPO in nature remains uncertain, with several activities proposed, including the synthesis of metabolites, detoxification processes and the lignin degradation by its O-demethylation activity. (Hofrichter et al., 2015)
Table 1.
General comparison between MroUPO (short clade) and AaeUPO (long clade).
| UPO Family |
||
|---|---|---|
| Clade/UPO | Short/MroUPO | Long/AaeUPO |
| Molecular weight | 29 kDa | 44.4 kDa |
| pI | 6.41 | 5.77 |
| Conformation | Dimeric | Monomeric |
| Disulfide bridges | Intermolecular to connect monomers | C-terminal |
| Hydrophobic amino acids lining the channel | Aliphatic amino acids | Aromatic amino acids |
| Charge stabiliser | Histidine 86 | Arginine 189 |
| Other examples | MweUPO, CglUPO | PabUPOs, CraUPO, rCciUPO |
Fig. 2.
Heme access channel of (A) MroUPO (short UPO family) and (B) AaeUPO (long UPO family). Glutamic acid from the acid base pair is depicted in blue (Glu157 in MroUPO and Glu196 in AaeUPO) and charge stabiliser amino acid is colored in yellow (His86 in MroUPO and Arg189 in AaeUPO). Heme access channel lining amino acids are represented in orange (hydrophobic aliphatic in MroUPO) and in pink (hydrophobic aromatic in AaeUPO). The models were visualised with Pymol (http://pymol.org) using the crystal structure of MroUPO at a resolution of 1.83 Å (PDB ID: 5FUJ) and AaeUPO at a resolution of 2.19 Å (PDB ID: 2YOR). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The main hurdle for a new peroxygenase-based chemistry is the production and engineering of UPO in efficient recombinant expression systems. Indeed, the heterologous functional expression of UPOs is the main bottleneck for their industrial application. Missing chaperones along with different post-translational modifications (glycosylation, disulfide bridge, N- and C- terminal processing) among natural and heterologous hosts are high hurdles that must be avoided with only a few successful examples hitherto described in the literature: the evolved AaeUPO mutant (called PaDa-I variant) and the offspring of variants commercialised by EvoEnzyme (Gomez de Santos et al., 2018; Gomez de Santos et al., 2019; Martin-Diaz et al., 2018; Mate et al., 2017; Molina-Espeja et al., 2016; Molina-Espeja et al., 2014; Molina-Espeja et al., 2015; Ramirez-Escudero et al., 2018), as well as the UPOs developed by Novozymes heterologously expressed in Aspergillus oryzae: UPO from Coprinopsis cinerea (rCciUPO) (Babot et al., 2013) and UPO from an undetermined mould (rNOVO) (Peter et al., 2014). Besides, very recently MroUPO (Carro et al., 2019) and Collariella virescens UPO (CvirUPO) (González-Benjumea et al., 2020) have been reported to be expressed in Escherichia coli. Similarly, CfuCPO also suffers the same expression problems as UPO, with only one heterologous expression system developed in the filamentous fungi Aspergillus niger (10 mg/L), as well as some strain engineering in the original fungus to enhance production up to remarkable levels of 1.95 g/L. (Buchhaupt et al., 2011; Conesa et al., 2001)
Within the wish list for UPOs protein engineers we can find: the modification of selectivity, the enhancement of the turnover numbers in a given process, the removal of the unwanted peroxidase activity during oxyfunctionalisation reactions, to stop overoxidation reactivities, to improve oxidative stability as well as its performance in the presence of organic co-solvents and/or at high temperatures. As mentioned above, for all these goals, it is first necessary to heterologously and functionally express the enzyme in a host suited to sculpture its properties by directed evolution (Molina-Espeja et al., 2017). To date, the most successful case story of UPO engineering has been achieved with the AaeUPO, which was first heterologously functional expressed in Saccharomyces cerevisiae and Pichia pastoris (Komagataella phaffii) after several rounds of laboratory directed evolution (Molina-Espeja et al., 2014). With expression levels of 8 mg L–1 in S. cerevisiae and above 200 mg L–1 in P. pastoris in a bioreactor (Molina-Espeja et al., 2015), the highly active and stable AaeUPO secretion variant (PaDa-I) was subjected to new directed evolution campaigns aimed at producing, with high efficiency, from agrochemicals (1-naphthol) (Molina-Espeja et al., 2016) to human drug metabolites (Gomez de Santos et al., 2018; Gomez de Santos et al., 2019). Moreover, the PaDa-I variant was studied by structured-guided evolution and neutral genetic drift in an attempt to make a breakdown of the peroxygenase and peroxidase activities, as well as to expand its substrate promiscuity and stability (Martin-Diaz et al., 2018; Mate et al., 2017). Within this framework, the crystal structure of PaDa-I was solved at a resolution of 1.5 Å. Exhaustive soaking experiments with a panel of peroxidase and peroxygenase substrates combined with the construction of mutant libraries demonstrated an exceptional dynamic trafficking through the heme channel as the main driving force for the substrate promiscuity of PaDa-I (Ramirez-Escudero et al., 2018). Taken together, these findings are opening a new venue for future protein engineering towards more regio- and enantioselective variants by combining computational and directed evolution approaches.
3. Substrate scope
Peroxygenases rely on Compound I as the actual oxidant in their catalytic mechanism. This puts peroxygenases in mechanistic relationship to the well-known P450 monooxygenases. (Fasan, 2012; Hofrichter and Ullrich, 2014; Urlacher and Girhard, 2019) Therefore, it is not very astonishing that peroxygenases have been applied for a broad range of P450-like oxidation and oxyfunctionalisation reactions. The current scope and limitations will be outlined in the following:
3.1. Peroxygenase-catalysed hydroxylation of aliphatic C-H bonds
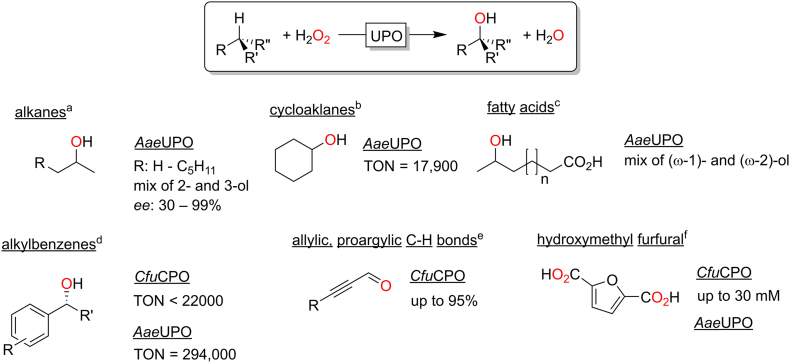
A representative selection of peroxygenase-catalysed hydroxylation of sp3-hybridised C-H bonds is shown in Fig. 3.
Fig. 3.
Selection of hydroxylation products obtained with peroxygenases. a) (Peter et al., 2011); b) (Churakova et al., 2011); c) (Gutierrez et al., 2011); d) (Ni et al., 2016; Park and Clark, 2006; Zaks and Dodds, 1995); e) (Hu and Hager, 1998); f) (Carro et al., 2015; van Deurzen et al., 2006)
In the late 1990s, CfuCPO had been in focus of research (see section 2 on Natural Diversity and Protein Engineering). From the known substrate scope, however, it becomes clear that CfuCPO exhibits significant activity only on activated C-H-bonds such as benzylic, allylic or propargylic C-H bonds. Also the turnover numbers (TONs, molproduct∙molenzyme–1) of CfuCPO observed in these experiments predominantly lie in the several hundreds to few thousands range. The more recent peroxygenases such as the one from Agrocybe aegerita (AaeUPO) exhibit significantly higher activity. Hydroxylation of non-activated (cyclo-)alkanes is possible with these enzymes thereby opening up a range of possible applications in the synthesis of (chiral) fine chemicals and even bulk chemicals.
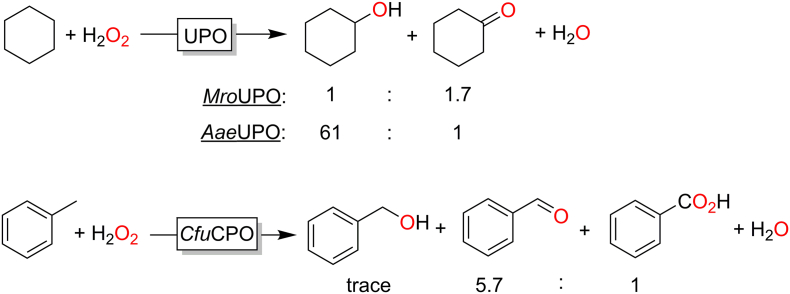
Nevertheless, the current (wild-type) peroxygenases also frequently face issues of poor selectivity: The hydroxylation of CH3- or CH2-groups, for example, is frequently accompanied by overoxidation. In other words, the initially formed (chiral) alcohol is in some cases is further oxidised to the corresponding ketones, aldehydes or carboxylic acids (Fig. 4). In the oxidation of cyclohexane, for example, AaeUPO predominantly forms the cyclohexanol whereas MroUPO forms the ketone in higher concentrations. (Peter et al., 2014)
Fig. 4.
Overoxidation issues observed in peroxygenase-catalysed hydroxylation of C-H bonds. Depending on the UPO used, the oxidation of cyclohexane yields either predominantly cyclohexanol or cyclohexanone. (Peter et al., 2014) Using CfuCPO for the hydroxylation of toluene, Dodds and coworkers observed significant overoxidation of the initial benzyl alcohol to the aldehyde and acid. (Zaks and Dodds, 1995)
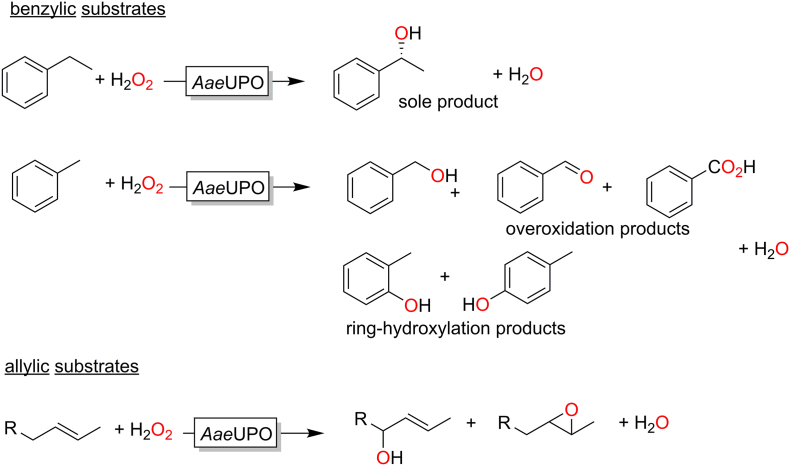
Chemoselectivity issues are frequently observed in the hydroxylation of activated (benzylic or allylic) C-H bonds. The conversion of alkenes, for example, frequently yields mixtures of epoxides and allylic hydroxylation products (Fig. 5). Allylic methyl groups, however, appear to be non-reactive. Similar observations had also been made with CfuCPO. (Zaks and Dodds, 1995)
Fig. 5.
Selectivity issues observed in AaeUPO-catalysed hydroxylation reactions of activated (benzylic or allylic substrates). (Peter et al., 2013; Ullrich and Hofrichter, 2005)
It is also interesting to note that e.g. in case of AaeUPO-catalysed hydroxylation reactions of alkyl aromatics the chemoselectivity very much depends on the structure of the starting material. The hydroxylation of ethyl benzene for example yields almost exclusively (R)-1-phenyl ethanol (and traces of the overoxidation product) while the conversion of toluene yields a complex mixture of benzyl alcohol and various over oxidation- and ring hydroxylation products (Fig. 5). (Ullrich and Hofrichter, 2005)
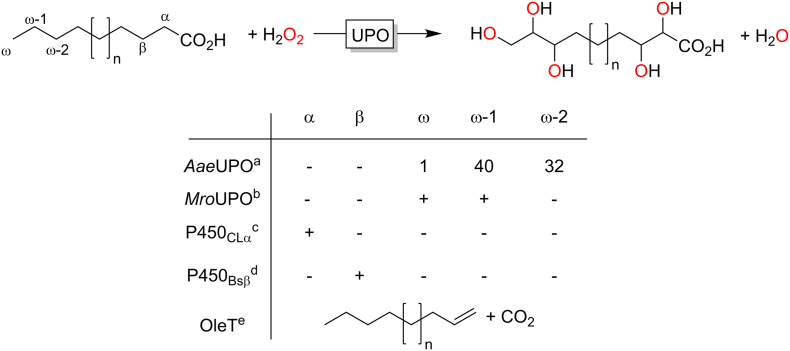
Finally, also the regioselectivity of peroxygenase-catalysed hydroxylation reactions is worth discussing. The peroxygenases available today exhibit a very broad selectivity spectrum for example for the hydroxylation of fatty acids (Fig. 6). While AaeUPO prefers subterminal hydroxylation, the UPO from Marasmius rotula (MroUPO) also exhibits significant activity on the terminal CH3-group as well as α-oxidation followed by decarboxylation (fatty-acid shortening). (Olmedo et al., 2017) Some recently reported P450 peroxygenases prefer C-H-bonds closer to the carboxylate group. Among them, the P450 peroxygenase OleT yields terminal alkenes from fatty acids, presumably via α-hydroxylation followed by decarboxylation. (Zachos et al., 2015)
Fig. 6.
Regioselectivity of some peroxygenases in the conversion of fatty acids. a) (Gutierrez et al., 2011); b) (Olmedo et al., 2016); c) (Girhard et al., 2007); d) (Paul et al., 2014); e) (Zachos et al., 2015).
3.2. Peroxygenase-catalysed hydroxylation of aromatic C-H bonds
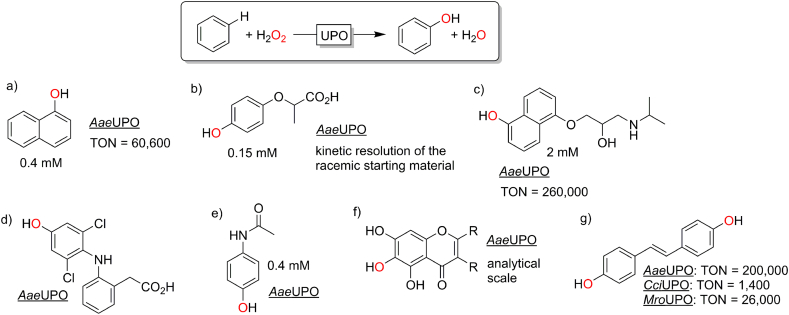
A range of preparatively very relevant aromatic hydroxylation reactions using peroxygenases have been reported in recent years. Apparently, only the new, true peroxygenases such as AaeUPO are efficient in this reaction. Fig. 7 gives an overview over the variety of products obtainable via peroxygenase-catalysis.
Fig. 7.
Selection of peroxygenase-catalysed aromatic hydroxylation products. a) (Molina-Espeja et al., 2016; Ullrich and Hofrichter, 2005); b) (Kinne et al., 2008); c) (Gomez de Santos et al., 2018; Kinne et al., 2009); d) (Kinne et al., 2009); e) (Poraj-Kobielska et al., 2011); f) (Barková et al., 2011); g) (Aranda et al., 2018).
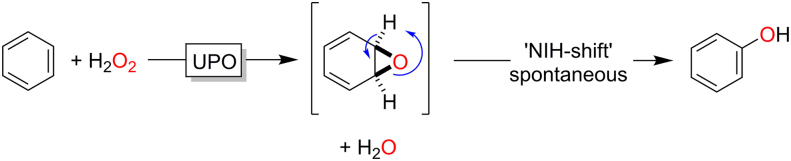
The mechanism of peroxygenase-catalysed aromatic hydroxylation has been investigated intensively by Hofrichter and co-workers (Barková et al., 2011; Hofrichter and Ullrich, 2014; Kluge et al., 2009) and appears to proceed in most cases via an aromatic epoxide intermediate, which then spontaneously rearranges to the corresponding phenol (Fig. 8).
Fig. 8.
Mechanism of the peroxygenase-catalysed hydroxylation proceeding via an intermediate arene oxide, which spontaneously rearranges into the corresponding phenol (NIH-shift).
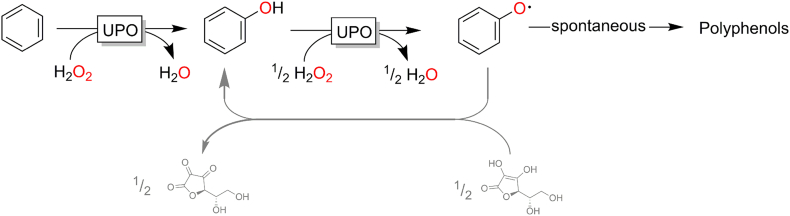
Though already today, a broad range of interesting aromatic hydroxylation reactions are possible, there are nevertheless also a range of challenges that need to be addressed. One of them is the above-mentioned chemoselectivity issue in the hydroxylation of alkyl benzenes (Fig. 5). Another issue is the peroxidase-activity of peroxygenases (Fig. 9). In other words, the phenol (originating from the aromatic hydroxylation reaction) can serve as substrate for a peroxygenase, which through its peroxidase activity (one electron oxidation reaction) undergo an H-atom-abstraction reaction yielding phenoxy radicals. The latter are rather reactive and undergo spontaneous polymerisation reactions, which obviously in most cases are not desired. One possibility to alleviate this polymerisation reaction is to administer radical scavengers to the reaction mixture. Ascorbic acid, for example, is frequently used to suppress the polymerisation reaction.
Fig. 9.
Peroxidase activity of peroxygenases as a challenge for the hydroxylation of aromatic compounds.
More elegantly, Alcalde and co-workers have shown that protein engineering in principle can yield peroxygenase mutants with drastically reduced peroxidase activity. (Molina-Espeja et al., 2016)
3.3. Peroxygenase-catalysed epoxidation reactions
The chemical versatility of epoxides as starting materials for a myriad of synthetic transformations has ever since motivated research on CfuCPO and, more recently, on AaeUPO-catalysed epoxidation reactions. As can be seen from Fig. 10, AaeUPO appears to be the more suitable catalyst for this reaction (as judged by the turnover numbers reported). Even though it appears that epoxidation reactions have not been in focus of the ongoing research efforts for AaeUPO.
Fig. 10.
Selection of peroxygenase-catalysed epoxidation reactions. a) (Kluge et al., 2012; Lakner et al., 1997; Rauch et al., 2019); b) (Lakner et al., 1997); c) (Dexter et al., 1995)
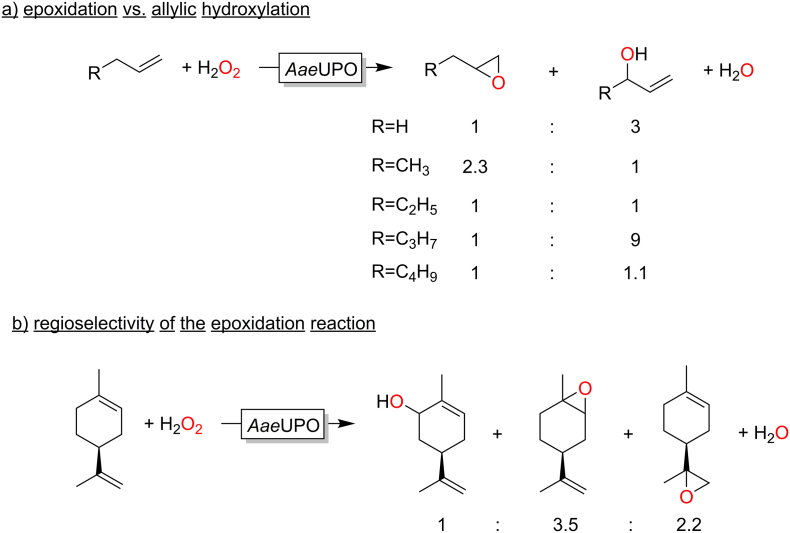
One major limitation of peroxygenases en route to truly applicable epoxidation catalysts is the frequently observed poor chemoselectivity of the reaction also yielding the allylic hydroxylation products. Furthermore, gaining control over the stereo- and (in case of starting materials containing several C=C-double bonds) regioselectivity (Fig. 11). This issue has been studied extensively by Hofrichter and co-workers. (Peter et al., 2013)
Fig. 11.
Selectivity issues observed for the peroxygenase-catalysed epoxidation reaction at the example of AaeUPO. (Peter et al., 2013)
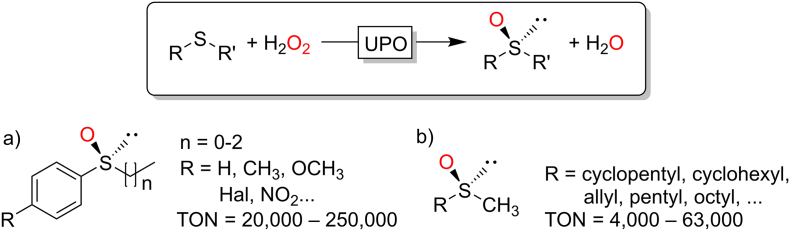
3.4. Peroxygenase-catalysed sulfoxidation reactions
In terms of classical, oxygen transfer reactions, finally, also peroxygenase-catalysed sulfoxidation reactions are worth being mentioned here. In contrast to the aforementioned hydroxylation – and epoxidation reactions, CfuCPO truly excels as catalyst as usually very high turnover numbers are reported for this type of reaction (Fig. 12). It is also worth mentioning that overoxidation to the corresponding sulfones is generally not observed.
Fig. 12.
CfuCPO-catalysed sulfoxidation reactions. a) (Churakova et al., 2013; Kohlmann et al., 2009; Luetz et al., 2004; Perez et al., 2009b; van Deurzen et al., 1997); b) (Colonna et al., 1997; Vargas et al., 1999)
While CfuCPO has been studied extensively, to the best of our knowledge, only one analytical study on AaeUPO-catalysed sulfoxidation (albeit reporting excellent enantioselectivities and conversions) has been published yet. (Bassanini et al., 2017)
3.5. Miscellaneous
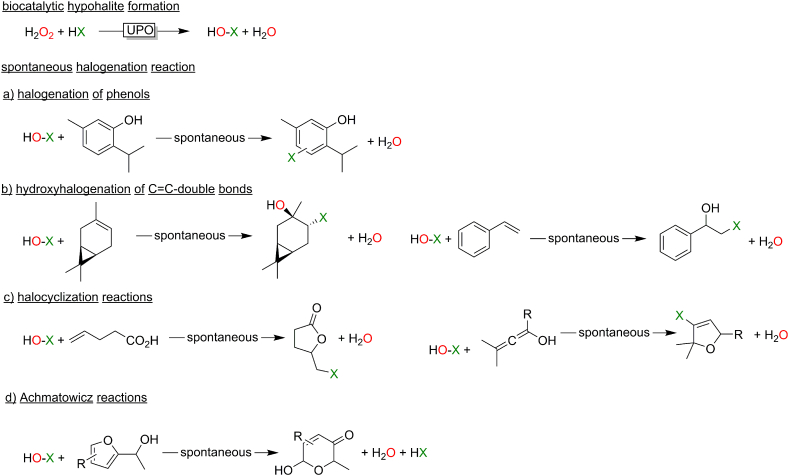
Both, CfuCPO and AaeUPO had first been reported as haloperoxidases catalysing the H2O2-driven oxidation of halides to the corresponding hypohalites. For quite some time, this has been regarded as a synthetically less relevant reaction (because the hypohalite diffuses out of the enzyme active site and undergoes chemical, non-selective transformations). In recent years, the synthetic value, however, is increasingly recognised leading to a range of interesting transformations (Fig. 13). In this respect it is worth mentioning that the classical heme-dependent peroxidases are nowadays increasingly being complemented by the (more H2O2-robust) vanadium-dependent haloperoxidases.
Fig. 13.
Chemoenzymatic transformations based on a peroxidase-catalysed hypohalite formation followed by a chemical follow-up reaction. a) (Fernández-Fueyo et al., 2015; Frank et al., 2016; Getrey et al., 2014) b) (Dong et al., 2017; Kaup et al., 2007) c) (Naapuri et al., 2017; Younes et al., 2020); d) (Deska et al., 2018; Thiel et al., 2014).
4. Reaction and medium engineering approaches
The following parts focus on the specific applications of peroxygenases. Firstly, we discuss the essential but also destructive reaction component H2O2 and photobiocatalysis as a possible solution for this challenge. Secondly, enzyme immobilisation approaches followed for peroxygenases are introduced, as it can increase enzyme stability and facilitate reuse of the biocatalyst. At last, a second important aspect of economic feasibility is discussed: Volumetric productivity. This part is on the increase of substrate loading for hydrophobic peroxygenase substrates by using non-conventional media, which may in turn, necessitates the use of immobilised enzymes for increased stability.
4.1. H2O2 challenge and photobiocatalysis
The use of hydrogen peroxide (H2O2) needed at stoichiometric amounts for enzymatic oxidation reactions (e.g. peroxidase-, peroxygenase catalysed) faces the challenge of heme oxidation leading to enzyme inactivation. To alleviate this limitation, several strategies have been developed for in situ supply of H2O2. Those include enzymatic production using glucose oxidase (Fred van Rantwijk, 2000) or formate oxidase (Tieves et al., 2019), electrochemistry (Horst et al., 2016; Krieg et al., 2011), chemical catalysis (Freakley et al., 2019) and photocatalysis.
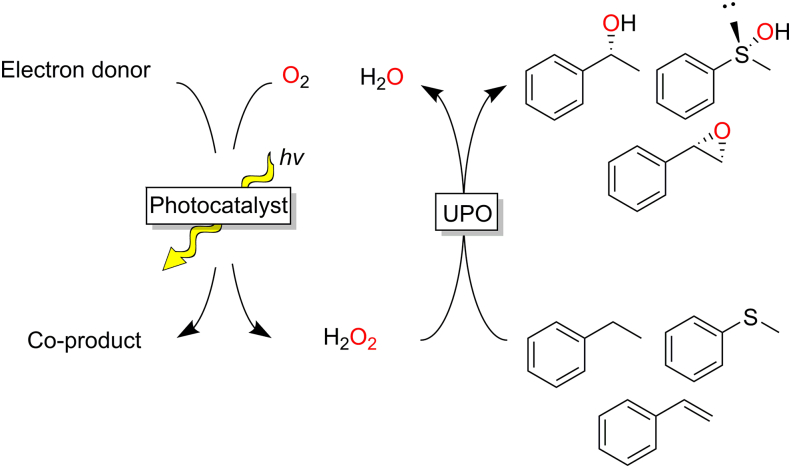
Light-driven methods generally rely on a photocatalyst that, when excited by light, can oxidise an electron donor and can transfer the reducing equivalents to oxygen, generating H2O2 (Fig. 14).
Fig. 14.
Photocatalytic in situ synthesis of H2O2 for peroxygenase catalysis.
One of the first published examples of this light-driven approach was the use of AaeUPO in combination with flavin adenine mononucleotide (FMN) as photocatalyst and ethylenediaminetetraacetic acid (EDTA) as co-substrate. This system was able to hydroxylate a range of alkylic and aromatic substrates and to catalyse the epoxidation reaction of styrene and some of its derivatives. (Churakova et al., 2011)
Later on, an approach with higher atom efficiency was reported by Zhang et al. using gold loaded rutile titanium dioxide (Au-TiO2) as photocatalyst and methanol as sacrificial electron donor, in combination with the engineered AaeUPO PaDa-I. Upon illumination with visible light, full conversion of the model substrate ethylbenzene (15 mM) was reached within 72 hours and (R)-1-phenylethanol was produced with high selectivity (98.2% ee). In line with the previously discussed substrate scope of AaeUPO, they were also able to hydroxylate other alkanes producing only minor amounts of the corresponding ketone as side product. In 1H-NMR analysis, they found methanol was photocatalytically oxidised not only to formaldehyde, but also subsequently to formate. When using formaldehyde and formate as electron donors and comparing it to methanol, the authors reported H2O2 formation rates to be 75% higher and reaction rates to be 32% and 18% higher, respectively. (Zhang et al., 2017) One interesting prospective of using Au-TiO2 is that due to its water oxidation activity, also water as sacrificial electron donor for the in situ generation of H2O2 comes into reach. While the hydroxylation of ethylbenzene achieved lower yields than with co-substrate, a linear correlation between concentration of photocatalyst and concentration of acetophenone, the over-oxidised side product was observed. This prompted the researchers to extend the photocatalytic reaction with a second enzyme. Phenylethanol was oxidised to acetophenone by PaDa-I and rutile Au-TiO2, which was then converted to (R)- and (S)-phenyl ethylamine by addition of ω-transaminases from Aspergillus terreus and Bacillus megaterium, respectively. Similarly, they oxidised toluene to benzaldehyde and then added benzaldehyde lyase from Pseudomonas fluorescence to perform a benzoin condensation, yielding (R)-benzoin. Overall, artificial cascade pathways could be successfully demonstrated initiated with photobiocatalytic oxyfunctionalisation step. Promising results were also achieved with carbon nanodots (CNDs) as non-metal photocatalysts. The combination of PaDa-I, CNDs and FMN yielded a higher initial rate (0.81 mM∙h–1 vs 0.16 mM∙h–1) and higher enzyme TONs (100,000 vs 21,000) when compared with Au-TiO2. Furthermore, Au-BiVO4 and g-C3N4 were investigated for their applicability with PaDa-I. While Au-BiVO4 yielded comparably low product formation, g-C3N4 produced more overoxidation products. (Zhang et al., 2018)
While the system using Au-TiO2 worked well, the reaction proceeded quite slowly, reaching full conversion of 15 mM after 72 h. (Zhang et al., 2018) This was partially due to the fact that the photocatalyst can only be excited by light below 413 nm, which makes up only 0.7% of the light emitted by the lamp used. (Zhang et al., 2017) An effort to accelerate this PaDa-I reaction by expanding the usable spectrum of visible light was made by Willot et al., using a combination of three photosensitizers covering a wide range of wavelengths. They were applied in combination with a formate dehydrogenase (CbFDH from Candida boidinii) reducing NAD+ to NADH and oxidising formate to carbon dioxide. The produced NADH was used by the excited photocatalysts (FMN, phenosafranine and methylene blue) to produce H2O2, which was then utilised by PaDa-I to hydroxylate ethylbenzene (10 mM). After only four hours, full conversion to 10 mM (R)-1-phenylethanol was achieved. While the reaction proceeded remarkably fast, its robustness was lacking. Especially the photocatalyst FMN and CbFDH were rather unstable, exhibiting turnover numbers of only 649 and 135, respectively. (Willot et al., 2019)
Also for CfuCPO photocatalytic H2O2 supply systems were reported. One approach used FMN as a photocatalyst and EDTA or formate as an electron donor for the enantioselective sulfoxidation of thioanisole to the (R)-sulfoxide. While formate would pose a more environmentally benign co-substrate compared to EDTA, it lowered the enantioselectivity (78% ee, >99% ee with using EDTA). (Perez, D.I. et al., 2009) The reason was attributed to binding of the formate to the heme-iron as suggested by crystallographic data, which was shown for CPO-catalysed epoxidation of limonene. (Águila et al., 2008)
Another photobiocatalytic application of AaeUPO added electrochemistry to the system. Choi et al. demonstrated the hydroxylation of ethylbenzene using single walled carbon nanotube (SWNT) electrodes. To reduce the overpotential required to reduce O2 and produce H2O2 the SWNTs were hybridised with lumichrome, a flavin derivative. When exposed to visible light this treatment facilitated the transfer of electrons to O2, reducing the required overpotential substantially, which resulted in a more energy efficient H2O2 synthesis. (Choi et al., 2017)
The choice of the in situ H2O2 generation system will also influence the overall environmental impact of the oxyfunctionalisation system. Sacrificial electron donors such as methanol or formate generate less and less problematic wastes than e.g. EDTA or TRIS. Essentially waste-free systems based on H2 (Al Shameri et al., 2020)), water oxidation or renewable electricity will be preferable. These systems, however, also need to enable robust UPO catalysis at scale as well. Considering that enzyme production is a major contributor to the environmental impact of biocatalytic processes (Delgove et al., 2019; Tieves et al., 2019) those systems enabling the highest biocatalyst utilisation will be the ‘greenest’. Comparative life cycle assessments investigating this issue would be helpful to guide future research and industrial applications.
4.2. Strategies for peroxygenase immobilisation
While photocatalytic provision of H2O2 has been explored using alternative photocatalyst and cosubstrates, it remains as a challenge to develop peroxygenase-catalysed biotransformations with high productivity and low cost contribution of the enzyme. Robust heterogeneous biocatalysts, that are stable at process conditions and that can be recovered and reused, may result in process simplification and reduced processing time combined with a higher product quality and a smaller environmental footprint. Enzyme immobilisation in turn enables the use of non-conventional media for biocatalysis as it restricts conformational mobility and avoids consequent unfolding allowing highly productive biocatalysis in non-aqueous media.
However, the need for enzyme immobilisation has to be assessed carefully, as described below, before making the decision. Based on the TON achievable, a judgement can be made if there is a need for enzyme immobilisation or not. It was proposed that if the TON is too low, immobilisation is not economically viable due to the cost of most of the solid supports, unless they come from a waste stream.(Janssen et al., 2011; Liese and Hilterhaus, 2013) On the other hand, if the TON is very high with a free enzyme or if the product is highly expensive, making a cost contribution of the enzyme <0.05% compared to overall operational costs, then there is no need to immobilise the enzyme. Hence, if the TON value lies in between these borders, then enzyme immobilisation is recommended. The following parts summarizes the studies on immobilisation of UPOs with the aim to have quantitative comparisons mainly on TON values.
To immobilise CfuCPO various carriers have been used so far (Table 2). For instance copolymer hydrogel membranes made from poly(hydroxypropyl methacrylate-co-polyethyleneglycole-methacrylate) (p(HPMA-co-PEG-MA)). While this covalent immobilisation led to an increase in the KM from 24.6 to 47.3 μM in comparison to the free enzyme, it also provided increased stability. The immobilised enzyme exhibited increased robustness against higher temperatures, increased storage stability and excellent operational reusability with no activity loss over 9 cycles and only 27% loss first after 25 cycles. (Bayramoglu et al., 2011) Interesting results were published for the adsorptive immobilisation of CfuCPO on mesoporous silica. (Águila et al., 2011) The immobilisation resulted not only in enhanced stability, like a four- to five fold increase of the TON, it also led to a nine-fold increase of the turnover frequency (kcat). Similar results were reported for adsorptive CfuCPO immobilisation in TiO2 nanotubes. Not only the stability, but also the kcat was increased, even by a factor of 16. The authors attributed this to a confinement effect that reduces enzyme unfolding. (Muñoz-Guerrero et al., 2015) The molecular understanding of this effect needs to be improved. It has been shown that if an enzyme is deposited in a tight pore or shares a larger pore with many other enzymes, its folded structure I favoured over its unfolded form, which increases thermal stability and activity.(Ping et al., 2004; Ping et al., 2003; Ravindra et al., 2004). A carrier-free approach for CfuCPO immobilisation is aggregation and crosslinking. Co-precipitating the enzyme with pentaethylenehexaneamine (PEHA, works as amine donor) yielded a recovered activity of 68% and a biocatalyst more stable towards elevated temperature and inactivation by H2O2 compared to the free enzyme. (Perez, D. et al., 2009)
Table 2.
Overview for immobilisation of peroxygenases.
| Enzyme | Carrier (Interaction) | Immobilisation yield | Recovered activity | Reaction catalysed |
Remarks | Ref. |
|---|---|---|---|---|---|---|
| Carrier-bound approaches | ||||||
| CfuCPO | Aminopropyl glass (covalent) |
91% | TMPD oxidation | Improved storage stability | (Kadima and Pickard, 1990) | |
| CfuCPO | Methacrylate polymer (covalent) | n.d. | 83% | Monochloro-dimedon chlorination | Storage and thermal stability increased | (Bayramoglu et al., 2011) |
| CfuCPO | Mesoporous silica (covalent) |
11% | n.d. | Azo dye oxidation | Increased temperature stability and kcat/KM | (Guerrero et al., 2012) |
| CfuCPO | Mesoporous silica (adsorptive) |
80% | n.d. | Styrene oxidation | Increased stability against temperature and acetonitrile | (Águila et al., 2011) |
| CfuCPO | Magnetic beads (covalent) |
n.d. | 58% | Monochloro-dimedon chlorination | Improved thermal and storage stability | (Bayramoğlu et al., 2008) |
| CfuCPO | Chitosan (covalent) |
n.d. | n.d. | Monochloro-dimedon chlorination | Improved thermal and oxidative stability | (Zhang et al., 2009) |
| CfuCPO | Agarose (adsorptive) |
75% | 50% | Monochloro-dimedon chlorination | Increased stability against tert-butyl hydroperoxide | (Pešić et al., 2012) |
| CfuCPO | Agarose (covalent) |
94% | 55% | Monochloro-dimedon chlorination | (Pešić et al., 2012) | |
| CfuCPO | TiO2 nanotubes (adsorptive) |
35% | 26% | Styrene epoxidation | Increased kcat and operational stability | (Muñoz-Guerrero et al., 2015) |
| AaeUPO | Methacrylate polymers (covalent) |
n.d. | 4% | Ethylbenzene hydroxylation | Used in neat substrate | (Fernández-Fueyo et al., 2016) |
| AaeUPO | Methacrylate polymers (covalent) |
n.d. | n.d. | Photo-biocatalytic ethylbenzene hydroxylation | 8x increase of TON compared to free enzyme | (Zhang et al., 2018) |
| AaeUPO | Agarose (covalent) |
n.d. | 15% | Styrene epoxidation | Increased stability against acetonitrile, high temperature, high pH | (Molina-Espeja et al., 2019) |
| AaeUPO | Polyacryl (covalent) |
72.8% | 3% | Styrene epoxidation | Up to 360 mM product | (Rauch et al., 2019) |
| Carrier-free approaches | ||||||
| CfuCPO | Aggregation and cross linking | n.d. | 68% | Thioanisole oxidation | Higher tolerance against H2O2, temperature and pH | (Perez et al., 2009a) |
|
MroUPO/ AaeUPO |
PVA/PEG Gel (entrapment) |
n.d. | 86% | Diclofenac oxidation | 60 fold increase in TON, storage in cyclohexane increased activity | (Poraj-Kobielska et al., 2015) |
| AaeUPO | Calcium alginate entrapment | 81% | - | Hydroxylation of cyclohexane and cyclopentane | Entrapment enabled light driven solvent free alkane hydroxylation | (Hobisch et al., 2020) |
TMPD: N,N,N',N'-tetramethyl-p-phenylenediamine
Looking at Table 2, it is evident that CPOs in general and CfuCPO in particular are well-established biocatalysts as they were used in numerous immobilisation experiments with a wide variety of carrier materials. However, on the immobilisation of UPOs, there are dramatically few publications. Covalent immobilisation of AaeUPO on methacrylate polymer was used to increase enzyme stability in the photobiocatalytic oxidation of ethylbenzene. The removal of the enzyme from the oxidative influence of the used TiO2 photocatalyst proved beneficial for the reaction’s robustness as the enzyme’s TON was increased by a factor of eight. (Zhang et al., 2018) A particularly sophisticated approach, published by Molina et al., was the directed unique-point covalent immobilisation (DUCI) of AaeUPO. For this the previously produced mutant PaDa-I (Ramirez-Escudero, M. et al., 2018) was further modified to carry one Cys residue on the enzyme surface opposite to the entrance of the active site. This Cys was then used for the covalent attachment of the enzyme to two different carriers (epoxy polymethylacrylate and agarose). While for both carriers the recovered activity was around 15%, further experiments with the agarose preparation showed increased robustness against acetonitrile and pH greater than 5 and improved temperature stability. (Molina-Espeja et al., 2019) The only carrier-free immobilisation approach published for UPOs was the encapsulation of MroUPO and AaeUPO in PVA/PEG gel. While the kinetic parameters KM and kcat for the substrates diclofenac, dimethylene-5-nitrobenzene and 1,4-dimethoxybenzene were hardly influenced by the entrapment an interesting effect was reported for the storage of the immobilised enzyme. While the activity towards NBD decreased already after six days of storage in potassium phosphate buffer at pH 7, the storage in non-polar solvents (hexane, cyclohexane) increased the activity by up to 190% after 1 day and still showed 30% higher activity than the fresh preparation after 160 days. A possible explanation for this increase in activity is that the unipolar solvent makes the rather polar immobilisation matrix more accessible for rather non-polar substrates like NBD. Furthermore, the entrapped AaeUPO was used for diclofenac hydroxylation in a stirred tank reactor with a continuous H2O2 feed, where it showed a 60-fold improved TON of 61,041, compared to <1,000 for the free enzyme. (Poraj-Kobielska et al., 2015)
Due to the inconsistent nature of the published data on the various techniques, it is not possible to point out any general recommendations for peroxygenase immobilisation, but the wide variety of the reported methods, can still give valuable input for new immobilisation approaches. There is little research available on immobilisation of AaeUPO; however, we believe that the high biocatalytic potential of AaeUPO will lead to more studies exploring its immobilisation for operational stability in the near future.
4.3. Peroxygenases in non-conventional media
In biocatalysis, many interesting substrates are rather hydrophobic and exhibit low water solubility. Therefore, use of non-conventional media like organic solvents or neat substrate (solvent-free) conditions are a possibility to increase substrate loadings and therefore productivity. One example for such an approach is the use of AaeUPO for ethylbenzene hydroxylation. At first free AaeUPO in combination with an alcohol oxidase from Pseudomonas putida (PpAOx) for continuous H2O2 production from methanol was used in a two-liquid phase system (2LPS), where the aqueous phase holds the enzymes and the organic phase serves as a product sink and substrate reservoir. This approach suffered from poor enzyme stability and ethylbenzene hydroxylation ceased after 24 h. In an effort to increase stability, the enzymes were covalently immobilised on RelizymeTM HA 403/M resin to use them in neat substrate conditions. However, this combination only yielded trace amounts of the hydroxylated product. (Ni et al., 2016) The use of immobilised AaeUPO with a portion wise addition of tert-butyl hydroperoxide in neat ethylbenzene was the next approach. In a reaction volume of 250 mL 40 mM enantiopure (R)-1-phenylethanol was produced in three hours, which corresponded to a TON of 67,500 for AaeUPO. (Fernández-Fueyo et al., 2016) In a recently published article, an immobilised preparation of AaeUPO was employed in the epoxidation of neat styrene derivatives using a continuous feed of tert-butyl hydroperoxide (tertBuOOH). Besides several successful epoxidations performed this way in 1.5 ml vials, the epoxidation of cis-β-methylstyrene was realised on a 10 mL scale using an exponential tertBuOOH feed. The peroxide still caused major enzyme inactivation, but with resupplying fresh enzyme three times, a (1R,2S)-β-methylstyrene oxide concentration of 360 mM was obtained. This reaction was also extended to a chemoenzymatic cascade by adding methylamine to the mix to produce (pseudo)ephedrine. (Rauch et al., 2019) In a recent study, we investigated the hydroxylation of cyclohexane and showed in a solvent screening that the use of neat reaction media, was the simplest and the most efficient approach to follow. (Hobisch et al., 2020) AaeUPO was applied for hydroxylation reactions in neat cyclohexane whereby H2O2 was generated in situ, catalysed by nitrogen-doped carbon nanodots (N-CNDs) under UV-LED illumination. AaeUPO entrapment in alginate beads enabled the product formation in the neat reaction medium, otherwise not possible with the free enzyme. The absorption of hydrophilic N-CNDs into the water-rich alginate beads containing AaeUPO created a 2-in-1 heterogeneous photobiocatalyst. Although the activity was rather low (0.08 mMproduct ∙μMUPO–1 · h–1) the product formation was linear for up to seven days. Herein, the enzyme-to-photocatalyst ratio played an important role for product concentration, which needs further evaluations.
For CfuCPO there was an interesting application reported combining a 2LPS with thioanisole as the organic phase and CfuCPO, FMN and EDTA running the photocatalytic H2O2 production in the aqueous phase. After a monophasic sulfoxidation of thioanisole did not prove very productive, this 2LPS was evaluated. Its effect was an increase in the product formation time from 2 h of the monophasic system to over 24 h. Adding vigorous agitation to increase the interfacial area between the phases, resulted in an eight-fold increase of the initial rate, but also in an end of the sulfoxidation reaction after 2–3 h. To provide sufficient surface area for mass transfer between the phases, sulfosuccinate was used as a surfactant for more stable emulsion at reduced stirring. This resulted in a decrease of the initial rate, but in turn the reaction was sustained for over 20 h and yielded 45 mM (R)-methyl phenyl sulfoxide (>96% ee). (Churakova et al., 2013) While Churakova et al. did not further investigate the cause for CfuCPO inactivation in the 2LPS, a previous publication by Park & Clark elucidates this question. The authors studied the inactivation of CfuCPO in a 2LPS oxidation of toluene and xylene and found that the main cause for activity loss was oxidative destruction of the heme. Optimization of the buffer pH and addition of t-butyl alcohol as radical scavenger improved the total turnover numbers by up to 110%. Neither denaturation nor aggregation of the apo-enzyme seemed to play a major role in enzyme inactivation. (Park and Clark, 2006)
Furthermore, PaDa-I was used in hydroxylation and epoxidation reactions containing natural deep eutectic solvents (NADES). While utilising several different electron bond donors, these NADES were all based on choline chloride as electron bond acceptor. That was essential, since a choline chloride oxidase was used for continuous H2O2 supply. Applying this system, 22.43 mM (R)-1-phenylethanol and 8.68 mM cis-β-methylstyrene oxide were produced equalling 224,300 and 86,800 TONs for UPO respectively. Among others, the researchers found that using 50% (v/v) NADES in the reaction mixture increased process stability. (Ma et al., 2020)
As of yet there are not many published applications of peroxygenases in non-conventional media. The ever-rising interest in reactions catalysed by this enzyme group will certainly be a driving force behind application-oriented research towards alternative reaction media. Since many UPO substrates are rather hydrophobic, especially neat substrate applications are a promising approach that should be further pursued. A neat system could dramatically increase substrate and subsequently product titers.
5. Perspectives
Since their discovery, peroxygenases have attracted a great deal of interest in the biocatalysis community. The simplicity of the reactions owing to independency to external redox cofactors (and hence the regeneration methods) and auxiliary proteins is a big advantage. This easiness and the wide range of H2O2-dependent reactions catalysed by peroxygenases make them promising catalysts for preparative oxyfunctionalisation chemistry. We are convinced that the knowledge and experiences gained during the past two decades will be helpful to explore the potential of peroxygenases further for: (i) Discovery of new peroxygenases, (ii) high-throughput screening strategies for engineered peroxygenase variants, (iii) enhancement of operational stability (via protein engineering and/or immobilisation), (iv) use of non-conventional media for higher substrate loadings and product titers, and (v) heterogenisation of biocatalysts by immobilisation for continuous applications. Herein, collaborative activities between protein engineers, organic chemists and process engineers can bring UPO catalysed reactions to a next level making them attractive for technical applications with more focus on truly interesting and high yield conversions.
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 764920. MA and PGS thank the FPI fellowship BES-2017-080040, the Spanish Government Project PID2019-106166RB-I00-Oxywave, and the Comunidad de Madrid Synergy CAM project Y2018/BIO-4738-EVOCHIMERA-CM. FH gratefully acknowledges support by The Netherlands Organisation for Scientific Research through a VICI grant (no. 724.014.003) and the European Research Commission (ERC consolidator grant No 648026).
References
- Águila S., Vazquez-Duhalt R., Tinoco R., Rivera M., Pecchi G., Alderete J.B. Stereoselective oxidation of R-(+)-limonene by chloroperoxidase from Caldariomyces fumago. Green Chem. 2008;10(6) 10.1039/b719992a. [Google Scholar]
- Águila S., Vazquez-Duhalt R., Covarrubias C., Pecchi G., Alderete J.B. Enhancing oxidation activity and stability of iso-1-cytochrome c and chloroperoxidase by immobilization in nanostructured supports. J. Mol. Catal. B Enzym. 2011;70(3-4):81–87. 10.1016/j.molcatb.2011.02.008. [Google Scholar]
- Al Shameri A., Willot S.J.-P., Paul C.E., Hollmann F., Lauterbach L. 2020. Chemical Communications. 10.1039/D0CC03229H. [Google Scholar]
- Anh D.H. International Graduate School of Zittau; Zittau: 2008. Novel extracellular haloperoxidase-peroxygenases from the coprophilous fungi Coprinus radians and Coprinus verticillatus: production, purification and biochemical characterization. [Google Scholar]
- Anh D.H., Ullrich R., Benndorf D., Svatoś A., Muck A., Hofrichter M. The Coprophilous Mushroom Coprinus radians Secretes a Haloperoxidase That Catalyzes Aromatic Peroxygenation. Appl. Environ. Microbiol. 2007;73(17):5477–5485. doi: 10.1128/AEM.00026-07. 10.1128/aem.00026-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda C., Ullrich R., Kiebist J., Scheibner K., del Río J.C., Hofrichter M., Martínez A.T., Gutiérrez A. Selective synthesis of the resveratrol analogue 4,4′-dihydroxy-trans-stilbene and stilbenoids modification by fungal peroxygenases. Catal. Sci. Technol. 2018;8(9):2394–2401. [Google Scholar]
- Babot E.D., del Río J.C., Kalum L., Martínez A.T., Gutiérrez A. Oxyfunctionalization of aliphatic compounds by a recombinant peroxygenase from Coprinopsis cinerea. Biotechnol. Bioeng. 2013;110(9):2323–2332. doi: 10.1002/bit.24904. [DOI] [PubMed] [Google Scholar]
- Barková K., Kinne M., Ullrich R., Hennig L., Fuchs A., Hofrichter M. Regioselective hydroxylation of diverse flavonoids by an aromatic peroxygenase. Tetrahedron. 2011;67(26):4874–4878. [Google Scholar]
- Bassanini I., Ferrandi E.E., Vanoni M., Ottolina G., Riva S., Crotti M., Brenna E., Monti D. Peroxygenase-catalyzed enantioselective sulfoxidations. Eur. J. Org. Chem. 2017;2017(47):7186–7189. [Google Scholar]
- Bayramoğlu G., Kiralp S., Yilmaz M., Toppare L., Arıca M.Y. Covalent immobilization of chloroperoxidase onto magnetic beads: Catalytic properties and stability. Biochem. Eng. J. 2008;38(2):180–188. [Google Scholar]
- Bayramoglu G., Altintas B., Yilmaz M., Arica M.Y. Immobilization of chloroperoxidase onto highly hydrophilic polyethylene chains via bio-conjugation: catalytic properties and stabilities. Bioresour. Technol. 2011;102(2):475–482. doi: 10.1016/j.biortech.2010.08.056. [DOI] [PubMed] [Google Scholar]
- Bormann S., Gomez Baraibar A., Ni Y., Holtmann D., Hollmann F. Specific oxyfunctionalisations catalysed by peroxygenases: opportunities, challenges and solutions. Catal. Sci. Technol. 2015;5(4):2038–2052. [Google Scholar]
- Buchhaupt M., Ehrich K., Huttmann S., Guder J., Schrader J. Over-expression of chloroperoxidase in Caldariomyces fumago. Biotechnol. Lett. 2011;33(11):2225–2231. doi: 10.1007/s10529-011-0683-8. [DOI] [PubMed] [Google Scholar]
- Burek B.O., Bormann S., Hollmann F., Bloh J.Z., Holtmann D. Hydrogen peroxide driven biocatalysis. Green Chem. 2019;21(12):3232–3249. [Google Scholar]
- Carro J., Ferreira P., Rodriguez L., Prieto A., Serrano A., Balcells B., Arda A., Jimenez-Barbero J., Gutierrez A., Ullrich R., Hofrichter M., Martinez A.T. 5-hydroxymethylfurfural conversion by fungal aryl-alcohol oxidase and unspecific peroxygenase. FEBS J. 2015;282(16):3218–3229. doi: 10.1111/febs.13177. [DOI] [PubMed] [Google Scholar]
- Carro J., González-Benjumea A., Fernández-Fueyo E., Aranda C., Guallar V., Gutiérrez A., Martínez A.T. Modulating fatty acid epoxidation vs hydroxylation in a fungal peroxygenase. ACS Catal. 2019;9(7):6234–6242. [Google Scholar]
- Choi D.S., Ni Y., Fernandez-Fueyo E., Lee M., Hollmann F., Park C.B. Photoelectroenzymatic oxyfunctionalization on flavin-hybridized carbon nanotube electrode platform. ACS Catal. 2017;7(3):1563–1567. [Google Scholar]
- Churakova E., Kluge M., Ullrich R., Arends I., Hofrichter M., Hollmann F. Specific photobiocatalytic oxyfunctionalization reactions. Angew. Chem. Int. Ed. 2011;50(45):10716–10719. doi: 10.1002/anie.201105308. [DOI] [PubMed] [Google Scholar]
- Churakova E., Arends I.W.C.E., Hollmann F. Increasing the productivity of peroxidase-catalyzed oxyfunctionalization: A case study on the potential of two-liquid-phase systems. ChemCatChem. 2013;5(2):565–568. [Google Scholar]
- Colonna S., Gaggero N., Carrera G., Pasta P. A new enzymatic enantioselective synthesis of dialkyl sulfoxides catalysed by monooxygenases. Chem. Commun. 1997;5:439–440. [Google Scholar]
- Conesa A., van De Velde F., van Rantwijk F., Sheldon R.A., van Den Hondel C.A., Punt P.J. Expression of the Caldariomyces fumago chloroperoxidase in Aspergillus niger and characterization of the recombinant enzyme. J. Biol. Chem. 2001;276(21):17635–17640. doi: 10.1074/jbc.M010571200. [DOI] [PubMed] [Google Scholar]
- Delgove, M.A.F., Laurent, A.B., Woodley, J.M., De Wildeman, S.M.A., Bernaerts, K.V., van der Meer, Y., 2019. A prospective life cycle assessment (LCA) of monomer synthesis: Comparison of biocatalytic and oxidative chemistry. ChemSusChem 12(7), 1349-1360. [DOI] [PMC free article] [PubMed]
- Deska J., Blume F., Sprengart P. Lipase-induced oxidative Furan rearrangements. Synlett. 2018;29(10):1293–1296. [Google Scholar]
- van Deurzen M.P.J., Remkes I.J., van Rantwijk F., Sheldon R.A. Chloroperoxidase catalyzed oxidations in t-butyl alcohol/water mixtures. J. Mol. Catal. A Chem. 1997;117(1-3):329–337. [Google Scholar]
- van Deurzen M.P.J., van Rantwijk F., Sheldon R.A. Chloroperoxidase-catalyzed oxidation of 5-hydroxymethylfurfural. J. Carbohydr. Chem. 2006;16(3):299–309. [Google Scholar]
- Dexter A.F., Lakner F.J., Campbell R.A., Hager L.P. Highly Enantioselective Epoxidation of 1,1-Disubstituted Alkenes Catalyzed by Chloroperoxidase. J. Am. Chem. Soc. 1995;117(23):6412–6413. [Google Scholar]
- Dong J.J., Fernandez-Fueyo E., Li J., Guo Z., Renirie R., Wever R., Hollmann F. Halofunctionalization of alkenes by vanadium chloroperoxidase from Curvularia inaequalis. Chem. Commun. (Camb.) 2017;53(46):6207–6210. doi: 10.1039/c7cc03368k. [DOI] [PubMed] [Google Scholar]
- Fasan R. Tuning P450 Enzymes as Oxidation Catalysts. ACS Catal. 2012;2(4):647–666. [Google Scholar]
- Fernández-Fueyo E., van Wingerden M., Renirie R., Wever R., Ni Y., Holtmann D., Hollmann F. Chemoenzymatic Halogenation of Phenols by using the Haloperoxidase fromCurvularia inaequalis. ChemCatChem. 2015;7(24):4035–4038. [Google Scholar]
- Fernández-Fueyo E., Ni Y., Gomez Baraibar A., Alcalde M., van Langen L.M., Hollmann F. Towards preparative peroxygenase-catalyzed oxyfunctionalization reactions in organic media. J. Mol. Catal. B Enzym. 2016;134:347–352. [Google Scholar]
- Frank A., Seel C.J., Groll M., Gulder T. Characterization of a Cyanobacterial Haloperoxidase and Evaluation of its Biocatalytic Halogenation Potential. Chembiochem. 2016;17(21):2028–2032. doi: 10.1002/cbic.201600417. [DOI] [PubMed] [Google Scholar]
- Freakley, S.J., Kochius, S., van Marwijk, J., Fenner, C., Lewis, R.J., Baldenius, K., Marais, S.S., Opperman, D.J., Harrison, S.T.L., Alcalde, M., Smit, M.S., Hutchings, G.J., 2019. A chemo-enzymatic oxidation cascade to activate C-H bonds with in situ generated H2O2. Nat. Commun. 10(1), 4178. [DOI] [PMC free article] [PubMed]
- Fred van Rantwijk R.A.S. Selective oxygen transfer catalysed by heme peroxidases: synthetic and mechanistic aspects. Curr. Opin. Biotechnol. 2000;11:554–564. doi: 10.1016/s0958-1669(00)00143-9. [DOI] [PubMed] [Google Scholar]
- Getrey L., Krieg T., Hollmann F., Schrader J., Holtmann D. Enzymatic halogenation of the phenolic monoterpenes thymol and carvacrol with chloroperoxidase. Green Chem. 2014;16(3):1104–1108. [Google Scholar]
- Girhard M., Schuster S., Dietrich M., Durre P., Urlacher V.B. Cytochrome P450 monooxygenase from Clostridium acetobutylicum: a new alpha-fatty acid hydroxylase. Biochem. Biophys. Res. Commun. 2007;362(1):114–119. doi: 10.1016/j.bbrc.2007.07.155. [DOI] [PubMed] [Google Scholar]
- Gomez de Santos P., Cañellas M., Tieves F., Younes S.H.H., Molina-Espeja P., Hofrichter M., Hollmann F., Guallar V., Alcalde M. Selective Synthesis of the Human Drug Metabolite 5′-Hydroxypropranolol by an Evolved Self-Sufficient Peroxygenase. ACS Catal. 2018;8(6):4789–4799. [Google Scholar]
- Gomez de Santos P., Cervantes F.V., Tieves F., Plou F.J., Hollmann F., Alcalde M. Benchmarking of laboratory evolved unspecific peroxygenases for the synthesis of human drug metabolites. Tetrahedron. 2019;75(13):1827–1831. [Google Scholar]
- González-Benjumea A., Carro J., Renau-Mínguez C., Linde D., Fernández-Fueyo E., Gutiérrez A., Martínez A.T. Fatty acid epoxidation by Collariella virescens peroxygenase and heme-channel variants. Catal. Sci. Technol. 2020;10:717–725. 10.1039/c9cy02332a. [Google Scholar]
- Gröbe G., Ullrich R., Pecyna M.J., Kapturska D., Friedrich S., Hofrichter M., Scheibner K. High-yield production of aromatic peroxygenase by the agaric fungus Marasmius rotula. AMB Express. 2011;1(1):31. doi: 10.1186/2191-0855-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero E., Aburto P., Terrés E., Villegas O., González E., Zayas T., Hernández F., Torres E. Improvement of catalytic efficiency of chloroperoxidase by its covalent immobilization on SBA-15 for azo dye oxidation. J. Porous. Mater. 2012;20(2):387–396. [Google Scholar]
- Gutierrez A., Babot E.D., Ullrich R., Hofrichter M., Martinez A.T., del Rio J.C. Regioselective oxygenation of fatty acids, fatty alcohols and other aliphatic compounds by a basidiomycete heme-thiolate peroxidase. Arch. Biochem. Biophys. 2011;514(1-2):33–43. doi: 10.1016/j.abb.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Hanano A., Burcklen M., Flenet M., Ivancich A., Louwagie M., Garin J., Blée E. Plant Seed Peroxygenase Is an Original Heme-oxygenase with an EF-hand Calcium Binding Motif. J. Biol. Chem. 2006;281(44):33140–33151. doi: 10.1074/jbc.M605395200. [DOI] [PubMed] [Google Scholar]
- Hobisch, M., van Schie, M.M.C.H., Kim, J., Andersen, K.R., Alcalde, M., Kourist, R., Park, C.B., Hollmann, F., Kara, S., 2020. Solvent-Free Photobiocatalytic Hydroxylation of Cyclohexane. ChemCatChem.
- Hofrichter M., Ullrich R. Oxidations catalyzed by fungal peroxygenases. Curr. Opin. Chem. Biol. 2014;19:116–125. doi: 10.1016/j.cbpa.2014.01.015. [DOI] [PubMed] [Google Scholar]
- Hofrichter M., Kellner H., Pecyna M.J., Ullrich R. In: Monooxygenase, Peroxidase and Peroxygenase Properties and Mechanisms of Cytochrome P450. Hrycay E.G., Bandiera S.M., editors. Springer International Publishing; Cham: 2015. Fungal Unspecific Peroxygenases: Heme-Thiolate Proteins That Combine Peroxidase and Cytochrome P450 Properties; pp. 341–368. [DOI] [PubMed] [Google Scholar]
- Hofrichter M., Kellner H., Herzog R., Karich A., Liers C., Scheibner K., Kimani V.W., Ullrich R. Grand Challenges in Fungal Biotechnology. 2020. Fungal Peroxygenases: A Phylogenetically Old Superfamily of Heme Enzymes with Promiscuity for Oxygen Transfer Reactions; pp. 369–403. [Google Scholar]
- Holtmann D., Hollmann F. The Oxygen Dilemma: A Severe Challenge for the Application of Monooxygenases? ChemBioChem. 2016;17(15):1391–1398. doi: 10.1002/cbic.201600176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst A.E.W., Bormann S., Meyer J., Steinhagen M., Ludwig R., Drews A., Ansorge-Schumacher M., Holtmann D. Electro-enzymatic hydroxylation of ethylbenzene by the evolved unspecific peroxygenase of Agrocybe aegerita. J. Mol. Catal. B Enzym. 2016;133:S137–S142. [Google Scholar]
- Hu S., Hager L.P. Unusual propargylic oxidations catalyzed by chloroperoxidase. Biochem. Biophys. Res. Commun. 1998;253(2):544–546. doi: 10.1006/bbrc.1998.9721. [DOI] [PubMed] [Google Scholar]
- Huisman G.W., Collier S.J. On the development of new biocatalytic processes for practical pharmaceutical synthesis. Curr. Opin. Chem. Biol. 2013;17(2):284–292. doi: 10.1016/j.cbpa.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Huisman G.W., Liang J., Krebber A. Practical chiral alcohol manufacture using ketoreductases. Curr. Opin. Chem. Biol. 2010;14(2):122–129. doi: 10.1016/j.cbpa.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Janssen M., Müller C., Vogt D. Recent advances in the recycling of homogeneous catalysts using membrane separation. Green Chem. 2011;13(9) [Google Scholar]
- Kadima T.A., Pickard M.A. Immobilization of Chloroperoxidase on Aminopropyl-Glass. Appl. Environ. Microbiol. 1990;56(11):5. doi: 10.1128/aem.56.11.3473-3477.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup B.A., Piantini U., Wust M., Schrader J. Monoterpenes as novel substrates for oxidation and halo-hydroxylation with chloroperoxidase from Caldariomyces fumago. Appl. Microbiol. Biotechnol. 2007;73(5):1087–1096. doi: 10.1007/s00253-006-0559-3. [DOI] [PubMed] [Google Scholar]
- Kiebist J., Schmidtke K.U., Zimmermann J., Kellner H., Jehmlich N., Ullrich R., Zänder D., Hofrichter M., Scheibner K. A Peroxygenase from Chaetomium globosum Catalyzes the Selective Oxygenation of Testosterone. ChemBioChem. 2017;18(6):563–569. doi: 10.1002/cbic.201600677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebist J., Hofrichter M., Zuhse R., Scheibner K. In: Pan Standford Series on Biocatalysis. Grundwal P., editor. Pan Stanford Publishing; 2019. Oxyfunctionalization of pharmaceuticals by fungal peroxygenases. [Google Scholar]
- Kinne M., Ullrich R., Hammel K.E., Scheibner K., Hofrichter M. Regioselective preparation of (R)-2-(4-hydroxyphenoxy)propionic acid with a fungal peroxygenase. Tetrahedron Lett. 2008;49(41):5950–5953. [Google Scholar]
- Kinne M., Poraj-Kobielska M., Aranda E., Ullrich R., Hammel K.E., Scheibner K., Hofrichter M. Regioselective preparation of 5-hydroxypropranolol and 4'-hydroxydiclofenac with a fungal peroxygenase. Bioorg. Med. Chem. Lett. 2009;19(11):3085–3087. doi: 10.1016/j.bmcl.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Klebanoff S.J. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 2005;77(5):598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- Kluge M., Ullrich R., Dolge C., Scheibner K., Hofrichter M. Hydroxylation of naphthalene by aromatic peroxygenase from Agrocybe aegerita proceeds via oxygen transfer from H2O2 and intermediary epoxidation. Appl. Microbiol. Biotechnol. 2009;81(6):1071–1076. doi: 10.1007/s00253-008-1704-y. [DOI] [PubMed] [Google Scholar]
- Kluge M., Ullrich R., Scheibner K., Hofrichter M. Stereoselective benzylic hydroxylation of alkylbenzenes and epoxidation of styrene derivatives catalyzed by the peroxygenase of Agrocybe aegerita. Green Chem. 2012;14(2):440–446. [Google Scholar]
- Kohlmann C., Greiner L., Leitner W., Wandrey C., Lutz S. Ionic liquids as performance additives for electroenzymatic syntheses. Chemistry. 2009;15(43):11692–11700. doi: 10.1002/chem.200901046. [DOI] [PubMed] [Google Scholar]
- Krieg T., Hüttmann S., Mangold K.-M., Schrader J., Holtmann D. Gas diffusion electrode as novel reaction system for an electro-enzymatic process with chloroperoxidase. Green Chem. 2011;13(10) [Google Scholar]
- Lakner F.J., Cain K.P., Hager L.P. Enantioselective Epoxidation of ω-Bromo-2-methyl-1-alkenes Catalyzed by Chloroperoxidase. Effect of Chain Length on Selectivity and Efficiency. J. Am. Chem. Soc. 1997;119(2):443–444. [Google Scholar]
- Lee D.-S., Yamada A., Sugimoto H., Matsunaga I., Ogura H., Ichihara K., Adachi S.-i., Park S.-Y., Shiro Y. Substrate Recognition and Molecular Mechanism of Fatty Acid Hydroxylation by Cytochrome P450 from Bacillus subtilis : crystallographic, spectroscopic, and mutational studies. J. Biol. Chem. 2003;278(11):9761–9767. doi: 10.1074/jbc.M211575200. [DOI] [PubMed] [Google Scholar]
- Liese A., Hilterhaus L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013;42(15):6236–6249. doi: 10.1039/c3cs35511j. [DOI] [PubMed] [Google Scholar]
- Luetz S., Steckhan E., Liese A. First asymmetric electroenzymatic oxidation catalyzed by a peroxidase. Electrochem. Commun. 2004;6:583–587. [Google Scholar]
- Ma Y., Li Y., Ali S., Li P., Zhang W., Rauch M.C.R., Willot S.J.P., Ribitsch D., Choi Y.H., Alcalde M., Hollmann F., Wang Y. Natural Deep Eutectic Solvents as Performance Additives for Peroxygenase Catalysis. ChemCatChem. 2020;12:989–994. [Google Scholar]
- Martin-Diaz J., Paret C., García-Ruiz E., Molina-Espeja P., Alcalde M. Shuffling the Neutral Drift of Unspecific Peroxygenase in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2018;84(15) doi: 10.1128/AEM.00808-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mate D.M., Palomino M.A., Molina-Espeja P., Martin-Diaz J., Alcalde M. Modification of the peroxygenative:peroxidative activity ratio in the unspecific peroxygenase from Agrocybe aegerita by structure-guided evolution. Protein Eng. Des. Sel. 2017;30(3):191–198. doi: 10.1093/protein/gzw073. [DOI] [PubMed] [Google Scholar]
- Molina-Espeja P., Garcia-Ruiz E., Gonzalez-Perez D., Ullrich R., Hofrichter M., Alcalde M. Directed Evolution of Unspecific Peroxygenase from Agrocybe aegerita. Appl. Environ. Microbiol. 2014;80(11):3496–3507. doi: 10.1128/AEM.00490-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Espeja P., Ma S., Mate D.M., Ludwig R., Alcalde M. Tandem-yeast expression system for engineering and producing unspecific peroxygenase. Enzym. Microb. Technol. 2015;73-74:29–33. doi: 10.1016/j.enzmictec.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Molina-Espeja P., Cañellas M., Plou F.J., Hofrichter M., Lucas F., Guallar V., Alcalde M. Synthesis of 1-Naphthol by a Natural Peroxygenase Engineered by Directed Evolution. ChemBioChem. 2016;17(4):341–349. doi: 10.1002/cbic.201500493. [DOI] [PubMed] [Google Scholar]
- Molina-Espeja P., Gomez de Santos P., Alcalde M. In: Directed Enzyme Evolution: Advances and Applications. Alcalde M., editor. Springer International Publishing; Cham: 2017. Directed Evolution of Unspecific Peroxygenase; pp. 127–143. [Google Scholar]
- Molina-Espeja P., Santos-Moriano P., Garcia-Ruiz E., Ballesteros A., Plou F.J., Alcalde M. Structure-Guided Immobilization of an Evolved Unspecific Peroxygenase. Int. J. Mol. Sci. 2019;20(7) doi: 10.3390/ijms20071627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Guerrero F.A., Águila S., Vazquez-Duhalt R., Torres C.C., Campos C.H., Alderete J.B. Biocatalytic Performance of Chloroperoxidase from Caldariomyces fumago Immobilized onto TiO2 Based Supports. Top. Catal. 2015;59(2-4):387–393. [Google Scholar]
- Munro A.W., McLean K.J., Grant J.L., Makris T.M. Structure and function of the cytochrome P450 peroxygenase enzymes. Biochem. Soc. Trans. 2018;46(1):183–196. doi: 10.1042/BST20170218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naapuri J., Rolfes J.D., Keil J., Manzuna Sapu C., Deska J. Enzymatic halocyclization of allenic alcohols and carboxylates: a biocatalytic entry to functionalized O-heterocycles. Green Chem. 2017;19(2):447–452. [Google Scholar]
- Ni Y., Fernandez-Fueyo E., Gomez Baraibar A., Ullrich R., Hofrichter M., Yanase H., Alcalde M., van Berkel W.J., Hollmann F. Peroxygenase-Catalyzed Oxyfunctionalization Reactions Promoted by the Complete Oxidation of Methanol. Angew. Chem. Int. Ed. Eng. 2016;55(2):798–801. doi: 10.1002/anie.201507881. [DOI] [PubMed] [Google Scholar]
- Olmedo A., Aranda C., Del Rio J.C., Kiebist J., Scheibner K., Martinez A.T., Gutierrez A. From Alkanes to Carboxylic Acids: Terminal Oxygenation by a Fungal Peroxygenase. Angew. Chem. Int. Ed. Eng. 2016;55(40):12248–12251. doi: 10.1002/anie.201605430. [DOI] [PubMed] [Google Scholar]
- Olmedo A., Rio J.C.D., Kiebist J., Ullrich R., Hofrichter M., Scheibner K., Martinez A.T., Gutierrez A. Fatty Acid Chain Shortening by a Fungal Peroxygenase. Chem. Eur. J. 2017;23(67):16985–16989. doi: 10.1002/chem.201704773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda H., Shoji O., Suzuki K., Sugimoto H., Shiro Y., Watanabe Y. α-Oxidative decarboxylation of fatty acids catalysed by cytochrome P450 peroxygenases yielding shorter-alkyl-chain fatty acids. Catal. Sci. Technol. 2018;8(2):434–442. [Google Scholar]
- Park J.B., Clark D.S. Deactivation mechanisms of chloroperoxidase during biotransformations. Biotechnol. Bioeng. 2006;93(6):1190–1195. doi: 10.1002/bit.20825. [DOI] [PubMed] [Google Scholar]
- Paul C.E., Churakova E., Maurits E., Girhard M., Urlacher V.B., Hollmann F. In situ formation of H2O2 for P450 peroxygenases. Bioorg. Med. Chem. 2014;22(20):5692–5696. doi: 10.1016/j.bmc.2014.05.074. [DOI] [PubMed] [Google Scholar]
- Perez D.I., Grau M.M., Arends I.W., Hollmann F. Visible light-driven and chloroperoxidase-catalyzed oxygenation reactions. Chem Commun (Camb) 2009;44:6848–6850. doi: 10.1039/b915078a. [DOI] [PubMed] [Google Scholar]
- Perez D., van Rantwijk F., Sheldon R.A. Cross-Linked Enzyme Aggregates of Chloroperoxidase: Synthesis, Optimization and Characterization. Adv. Synthes. Catal. 2009;351(13):2133–2139. [Google Scholar]
- Pešić M., López C., Álvaro G., López-Santín J. A novel immobilized chloroperoxidase biocatalyst with improved stability for the oxidation of amino alcohols to amino aldehydes. J. Mol. Catal. B Enzym. 2012;84:144–151. [Google Scholar]
- Peter S., Kinne M., Wang X., Ullrich R., Kayser G., Groves J.T., Hofrichter M. Selective hydroxylation of alkanes by an extracellular fungal peroxygenase. FEBS J. 2011;278(19):3667–3675. doi: 10.1111/j.1742-4658.2011.08285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter S., Kinne M., Ullrich R., Kayser G., Hofrichter M. Epoxidation of linear, branched and cyclic alkenes catalyzed by unspecific peroxygenase. Enzym. Microb. Technol. 2013;52(6-7):370–376. doi: 10.1016/j.enzmictec.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Peter S., Karich A., Ullrich R., Gröbe G., Scheibner K., Hofrichter M. Enzymatic one-pot conversion of cyclohexane into cyclohexanone: Comparison of four fungal peroxygenases. J. Mol. Catal. B Enzym. 2014;103:47–51. [Google Scholar]
- Ping G., Yuan J.M., Vallieres M., Dong H., Sun Z., Wei Y., Li F.Y., Lin S.H. Effects of confinement on protein folding and protein stability. J. Chem. Phys. 2003;118(17):8042–8048. [Google Scholar]
- Ping G., Yuan J.M., Sun Z., Wei Y. Studies of effects of macromolecular crowding and confinement on protein folding and protein stability. J. Mol. Recognit. 2004;17(5):433–440. doi: 10.1002/jmr.710. [DOI] [PubMed] [Google Scholar]
- Poraj-Kobielska M., Kinne M., Ullrich R., Scheibner K., Kayser G., Hammel K.E., Hofrichter M. Preparation of human drug metabolites using fungal peroxygenases. Biochem. Pharmacol. 2011;82(7):789–796. doi: 10.1016/j.bcp.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Poraj-Kobielska M., Peter S., Leonhardt S., Ullrich R., Scheibner K., Hofrichter M. Immobilization of unspecific peroxygenases (EC 1.11.2.1) in PVA/PEG gel and hollow fiber modules. Biochem. Eng. J. 2015;98:144–150. [Google Scholar]
- Ramirez-Escudero M., Molina-Espeja P., Gomez de Santos P., Hofrichter M., Sanz-Aparicio J., Alcalde M. Structural Insights into the Substrate Promiscuity of a Laboratory-Evolved Peroxygenase. ACS Chem. Biol. 2018;13(12):3259–3268. doi: 10.1021/acschembio.8b00500. [DOI] [PubMed] [Google Scholar]
- Rauch M.C.R., Tieves F., Paul C.E., Arends I.W.C.E., Alcalde M., Hollmann F. Peroxygenase-Catalysed Epoxidation of Styrene Derivatives in Neat Reaction Media. ChemCatChem. 2019;11(18):4519–4523. doi: 10.1002/cctc.201901142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindra R., Zhao S., Gies H., Winter R. Protein encapsulation in mesoporous silicate: the effects of confinement on protein stability, hydration, and volumetric properties. J. Am. Chem. Soc. 2004;126(39):12224–12225. doi: 10.1021/ja046900n. [DOI] [PubMed] [Google Scholar]
- Sheldon R.A., Woodley J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018;118(2):801–838. doi: 10.1021/acs.chemrev.7b00203. [DOI] [PubMed] [Google Scholar]
- Tang M.-C., Fu C.-Y., Tang G.-L. Characterization of SfmD as a Heme Peroxidase That Catalyzes the Regioselective Hydroxylation of 3-Methyltyrosine to 3-Hydroxy-5-methyltyrosine in Saframycin A Biosynthesis. J. Biol. Chem. 2012;287(7):5112–5121. doi: 10.1074/jbc.M111.306316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel D., Doknic D., Deska J. Enzymatic aerobic ring rearrangement of optically active furylcarbinols. Nat. Commun. 2014;5:5278. doi: 10.1038/ncomms6278. [DOI] [PubMed] [Google Scholar]
- Tieves F., Tonin F., Fernández-Fueyo E., Robbins J.M., Bommarius B., Bommarius A.S., Alcalde M., Hollmann F. Energising the E-factor: The E+-factor. Tetrahedron. 2019;75(10):1311–1314. [Google Scholar]
- Truppo M.D. Biocatalysis in the Pharmaceutical Industry: The Need for Speed. ACS Med. Chem. Lett. 2017;8(5):476–480. doi: 10.1021/acsmedchemlett.7b00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich R., Hofrichter M. The haloperoxidase of the agaric fungus Agrocybe aegerita hydroxylates toluene and naphthalene. FEBS Lett. 2005;579(27):6247–6250. doi: 10.1016/j.febslet.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Ullrich R., Nüske J., Scheibner K., Spantzel J., Hofrichter M. Novel Haloperoxidase from the Agaric Basidiomycete Agrocybe aegerita Oxidizes Aryl Alcohols and Aldehydes. Appl. Environ. Microbiol. 2004;70(8):4575–4581. doi: 10.1128/AEM.70.8.4575-4581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich R., Poraj-Kobielska M., Scholze S., Halbout C., Sandvoss M., Pecyna M.J., Scheibner K., Hofrichter M. Side chain removal from corticosteroids by unspecific peroxygenase. J. Inorg. Biochem. 2018;183:84–93. doi: 10.1016/j.jinorgbio.2018.03.011. [DOI] [PubMed] [Google Scholar]
- Urlacher V.B., Girhard M. Cytochrome P450 Monooxygenases in Biotechnology and Synthetic Biology. Trends Biotechnol. 2019;37(8):882–897. doi: 10.1016/j.tibtech.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Vargas R.R., Bechara E.J.H., Marzorati L., Wladislaw B. Asymmetric sulfoxidation of a beta-carbonyl sulfide series by chloroperoxidase. Tetrahedron: Asymmetry(10) 1999:3219–3227. [Google Scholar]
- Wang Y., Lan D., Durrani R., Hollmann F. Peroxygenases en route to becoming dream catalysts. What are the opportunities and challenges? Curr. Opin. Chem. Biol. 2017;37:1–9. doi: 10.1016/j.cbpa.2016.10.007. [DOI] [PubMed] [Google Scholar]
- Willot S.J., Fernandez-Fueyo E., Tieves F., Pesic M., Alcalde M., Arends I., Park C.B., Hollmann F. Expanding the Spectrum of Light-Driven Peroxygenase Reactions. ACS Catal. 2019;9(2):890–894. doi: 10.1021/acscatal.8b03752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley J.M. Accelerating the implementation of biocatalysis in industry. Appl. Microbiol. Biotechnol. 2019;103(12):4733–4739. doi: 10.1007/s00253-019-09796-x. [DOI] [PubMed] [Google Scholar]
- Woodley J.M. New frontiers in biocatalysis for sustainable synthesis. Current Opinion in Green and Sustainable Chemistry. 2020;21:22–26. [Google Scholar]
- Xu H., Ning L., Yang W., Fang B., Wang C., Wang Y., Xu J., Collin S., Laeuffer F., Fourage L., Li S. In vitro oxidative decarboxylation of free fatty acids to terminal alkenes by two new P450 peroxygenases. Biotechnol Biofuels. 2017;10:208. doi: 10.1186/s13068-017-0894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes S.H.H., Tieves F., Lan D., Wang Y., Suss P., Brundiek H., Wever R., Hollmann F. Chemoenzymatic Halocyclization of gamma,delta-Unsaturated Carboxylic Acids and Alcohols. ChemSusChem. 2020;13:97–101. doi: 10.1002/cssc.201902240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos I., Gassmeyer S.K., Bauer D., Sieber V., Hollmann F., Kourist R. Photobiocatalytic decarboxylation for olefin synthesis. Chem. Commun. (Camb.) 2015;51(10):1918–1921. doi: 10.1039/c4cc07276f. [DOI] [PubMed] [Google Scholar]
- Zaks A., Dodds D.R. Chloroperoxidase-catalyzed asymmetric oxidations: substrate specificity and mechanistic study. J. Am. Chem. Soc. 1995;117(42):10419–10424. [Google Scholar]
- Zhang L.H., Bai C.H., Wang Y.S., Jiang Y.C., Hu M.C., Li S.N., Zhai Q.G. Improvement of chloroperoxidase stability by covalent immobilization on chitosan membranes. Biotechnol. Lett. 2009;31(8):1269–1272. doi: 10.1007/s10529-009-0009-2. [DOI] [PubMed] [Google Scholar]
- Zhang W., Burek B.O., Fernandez-Fueyo E., Alcalde M., Bloh J.Z., Hollmann F. Selective Activation of C-H Bonds in a Cascade Process Combining Photochemistry and Biocatalysis. Angew. Chem. Int. Ed. Eng. 2017;56(48):15451–15455. doi: 10.1002/anie.201708668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Fernandez-Fueyo E., Ni Y., van Schie M., Gacs J., Renirie R., Wever R., Mutti F.G., Rother D., Alcalde M., Hollmann F. Selective aerobic oxidation reactions using a combination of photocatalytic water oxidation and enzymatic oxyfunctionalisations. Nat. Catal. 2018;1(1):55–62. doi: 10.1038/s41929-017-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]