Abstract
Elite performing men continue to record faster record times in running events compared to women. These sex-based differences in running speed and endurance in humans are expected based on sexual dimorphisms that contribute to differences in the determinants of aerobic performance. Comparatively, the sexual dimorphisms contributing to sex-based differences in elite aerobic performance are not ubiquitous across other species that compete in running events. The purpose of this review is to offer a framework and model for ongoing discussions of the physiological determinants and ultimately limits of physical performance. The records for average running speed of champion athletes were delineated by sex for thoroughbred horses, greyhound dogs, and humans. Male and female performances within each of these species are being optimized by training, nutrition, and financial incentives, and are approaching a performance maximum. For horses and greyhounds breeding also plays a role. Analysis of athletic records shows that there is a sex-related difference of ~10% or more in elite athletic performance for humans; however, the upper limit of performance does not appear to be different between sexes for thoroughbred horses and greyhound dogs. In the context of the nil sex differences in running performance in thoroughbreds and greyhounds, we discuss the physiological role of sexual dimorphisms on sex-specific limits to running performance. We highlight that studies on both human and animal performance in athletic events stimulate critical physiological questions and drive novel research.
Keywords: Endurance performance, sex differences, comparative physiology, performance prediction, physiological determinants
1 |. Introduction
Among humans, males have faster running performances than females ubiquitously across all race distances despite faster improvements in record times for females compared to males over the previous century (1–3). Strikingly, however, these sex differences in elite running performance may be blunted or non-existent in other species that engage in competitive running performances, notably the thoroughbred horse and greyhound dog. In this context, elite running performances across species offer a framework to compare the physiological determinants and ultimately limits of physical performance. In this review we utilize between species comparisons to highlight key opportunities for sex-differences research in human exercise physiology.
1.1 |. Athletic Performance as Physiological Model
Elite athletic performance is an ideal ‘experiment of nature’ to determine the role of sexual dimorphisms on sex-specific limits to running performance. Although the determinants of elite athletic performance are multifactorial and complex with many factors that may contribute to success (e.g. enriched environment, genetic predisposition, social support) (4), the world’s best athletes are relatively homogenous within a given athletic event (5). The physiological determinants of elite performances are well-known and have been used to accurately model performance capacities of humans (6, 7) and have been used for selective breeding for speed in racehorses (8) and dogs (9). Additionally, the athletes that comprise top finishers of elite events have commonalities, including, intensive training, standardized and state of the art competition facilities, and access to proper nutrition and medical care. These factors, alongside well-documented, long-term records for elite performances enable comparisons of performances in running speed records across species of humans, horses, and dogs. Because the phenotype and physiology required for elite performances is predictable and different between men and women, elite athletic performances provide an ideal testbed to determine the role of sexual dimorphisms (7). Thus, this review addresses important knowledge gaps using comparative biology to understand sex-related differences in these physiological limits of running capacity.
1.2 |. Sexual dimorphisms in physiological limits to exercise in humans
Distance running performance is determined by a trade-off between three primary physiological factors: maximal oxygen uptake (), ‘lactate threshold’ and running economy. is the maximum amount of oxygen that is able to be consumed; ‘Lactate Threshold’ is the running speed that is associated with a substantial rise in blood lactate levels; and Running Economy is oxygen cost required to run a given pace- an index of efficiency- and is related to biomechanics, muscle fiber type, etc. (10). The physiological determinants of these three factors which determine aerobic performance (, ‘Lactate Threshold’, and Running Economy) are outlined in Figure 1, and physiological determinants with recognized sex-based differences in humans are bolded.
Figure 1. Determinants of distance running performance.
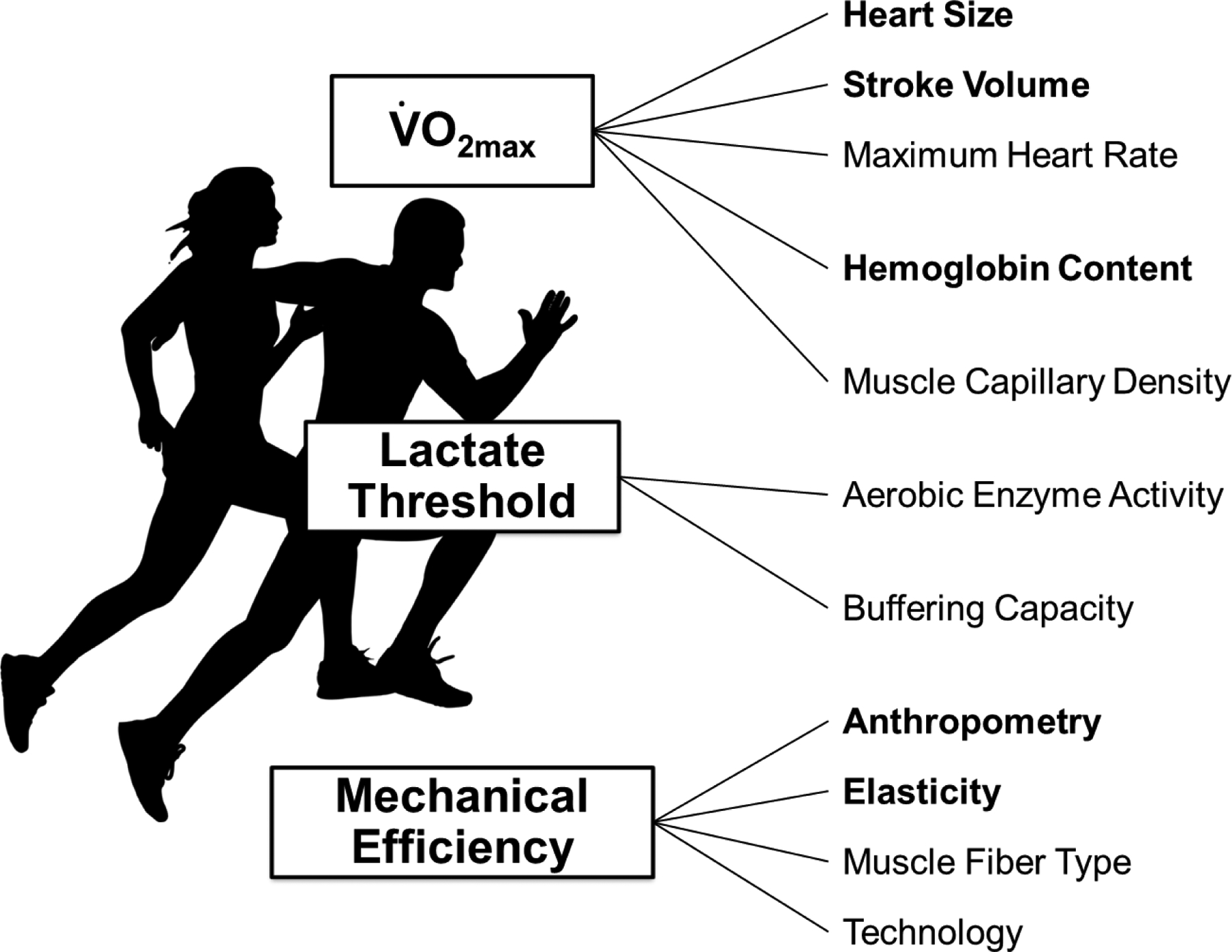
The bolded text highlights physiological factors with recognized sexual dimorphisms and contribute to sex-based differences in distance running performance among humans.
1.2.1 |.
Among the physiological determinants of there are recognized sex-based differences for humans in heart size, stroke volume, and hemoglobin content (1–3). Among humans, heart size is not different between the sexes until the onset of puberty. By adulthood, hearts among males are approximately 30% larger than hearts among females, primarily due to greater myocyte hypertrophy among males (11, 12). The observed sex-based difference in heart size is also the primary factor contributing to larger stroke volume among males compared to females (13–15). However, there does not appear to be a divergence in maximum heart rate by sex (13). The concentration of hemoglobin in blood is higher for males, contributing to a higher oxygen carrying capacity among males compared to females (16). Finally, although males have larger muscle fibers and more capillaries per fiber, capillary density is not different between sexes (17). In combination, the sex-based differences in these factors support the notion and common observation that males have a higher than females, even when is adjusted for total or lean body mass.
1.2.2 |. ‘Lactate Threshold’
The ‘Lactate Threshold’ occurs at a similar percentage of in men and women, although the absolute at which lactate threshold occurs is generally higher among males (10, 18). Aerobic enzyme activity and lactate buffering capacity play an important role in determining the running speed that can be sustained at a given rate of glycogen utilization. As such, the mitochondrial response to training is a key determinant of lactate threshold. There do not appear to be sex differences in the mitochondrial response to training (10, 19). Thus, sex-based differences in the absolute lactate threshold appear to be due to a larger and not the relative percentage of at which lactate threshold occurs. Additionally, the physiological determinates of shorter race distances likely do not include lactate threshold (20).
1.2.3 |. Running Economy
Less is known about running economy compared to the other physiological factors that are determinants of distance running performance. Although it is well understood that running economy is an important predictor of running performance, the biological determinants of running economy are incompletely understood. For example, the running speed that is produced when a runner is running at a given percentage of can vary by 15–20% across well-trained runners (6, 21). There are data that suggest sex-based differences exist in elastic properties of the tendons (22), although Achilles tendon stiffness does not appear to contribute to sex differences in running economy (23). The potential sex-related differences in running economy have been investigated with mixed findings (24–26), likely due to methodological differences in training status of the participants and running speeds evaluated (24). However, among elite, Olympic-level runners, men have greater running economy than women when compared at common running speeds (24).
For shorter running performances, the physiological factors dictating performance are less well-known, but likely include an optimal combination of large skeletal muscle and small body size to generate large power outputs. However, there are limits to this relationship, and excessive musculature can be detrimental to athletic performance. For example, there is a genetic mutation in the canine myostatin gene which affects muscle composition and racing speed in whippet racing dogs (27)— which are essentially small greyhounds. Heterozygous whippet racing dogs with one copy of a nonfunctional mutation of the myostatin gene are, on average, more muscular and faster than whippets with two functional copies of the myostatin gene (27). By contrast, homozygous animals with two copies of a nonfunctional myostatin gene have a “bully” phenotype and are over-muscled and significantly slower than the other whippet phenotypes (27), demonstrating the limits to the power outputs associated with large skeletal muscle mass and small body size. Thus, sexual dimorphisms in morphological metrics of size (including body size; body composition; regional distribution of fat and fat-free mass; and skeletal structure and geometry) may contribute to sex-related differences in endurance and running speed, and therefore performance records. However, the precise mechanisms and optimal phenotype contributing to maximized running economy are not well understood.
2 |. Performance Data
The discussion of performances will focus on publicly available records of long-standing athletic competitions with standard race distances and accurate time and record keeping. These procedures did not require ethical review as determined by the Mayo Clinic Institutional Review Board in accordance with the Code of Federal Regulations, 45 CFR 46.102, and the Declaration of Helsinki. For horses, finishing time and sex of the winning horse (n=1) for each competition year of the Kentucky Derby were extracted from a directory on the race website (www.kentuckyderby.com). The Kentucky Derby has been run annually with a distance of 1.25 miles since 1896 and has substantial monetary incentives for top performances. For greyhounds finishing time and sex of the winning dog (n=1) for each competition year of the English Derby were extracted from a robust online directory (www.greyhound-data.com). The English Derby has been run annually since 1927 with a gap during World War II (1941–1944), and the distance was 480m for most years except during the 1980s when the distance was 500m. For humans, there are tactical behaviors demonstrated in championship races, thus, we extracted the performance time of the fastest 800m run within each year for both males and females since 1967. Because annual records were not available before 1967, data prior to 1967 represent world record times. The 800m run was selected because the finishing time (~2 minutes) is similar to the average winning time of the Kentucky Derby (~2 minutes). Notably, however, the average finishing time of the English Derby for greyhounds is ~30 seconds. To account for discrepancies in distance, data for each race are presented as average running velocity. By selecting only elite competitors, each animal model likely has limited genetic variation and represents an ideal model to determine the effect of biological sex per se on running performance.
The physiological regulation of exercise is fundamentally determined by the energy system used to fuel the performance. In that context, there is a predictable hyperbolic relationship between work rate and time to exhaustion of exercise performance in humans (28) and other animals (29), including horses (30). There is a domain of exercise intensity termed ‘severe-intensity’ domain that includes short duration exercise (less than ~10 minutes) using finite anerobic energy sources, primarily muscle phosphocreatine and glucose, which leads to rapid accumulation of metabolic by-products that cause rapid muscle fatigue (28). Thus, we selected events from each species (human, horses and dog) that were in the ‘severe-intensity’ domain for between species comparisons. Although there is a lack of comparisons with similar race duration between the sexes for horses and dogs, for humans, the ~10% sex difference in performance observed in the 800 m is also observed across a wide range of running events from 100 m (~10 seconds) to ~42,000 m (marathon distance; ~2 hours) (31).
3 |. Physiological Limits
In humans, the female world record for the marathon was recently shattered by 84 seconds by Brigid Kosgei (1). However, Kosgei’s record-setting marathon performance does not eclipse the top 5,000 performances of all-time for males, and this observation is generalizable to all race distances. In stark contrast, female horses and dogs have bested elite male horses and dogs on several occasions. Notably, in 1915 a horse named Regret was the first female to win the Kentucky Derby outright. These observations suggest that sex-based physiological limits to running performance are not present in all species. The records of average running speed of champion athletes delineated by sex for the horse, dog, and human are presented in Figure 2. Although the top speed differs, performances within each species appear to be approaching a maximum ceiling of performance, suggesting that performances are nearing the physiological limits. The asymptote in the performance curve is not different between sexes for thoroughbred horses and greyhound dogs; strikingly however, there is a sex-related difference of ~10% or more in human elite athletic performances. These findings are supported by previous analyses of sex differences in running speed by Entin who showed male Thoroughbred and Standardbred horses were only 3.8% and 1.4% faster than females respectively at races longer than one mile (32). Greyhound racing dogs had only a 0.4% difference between sexes in average winning velocities at distances of ~500m. Whereas in humans, there is generally a ~10% sex difference in performance in weight bearing activities (3).
Figure 2.
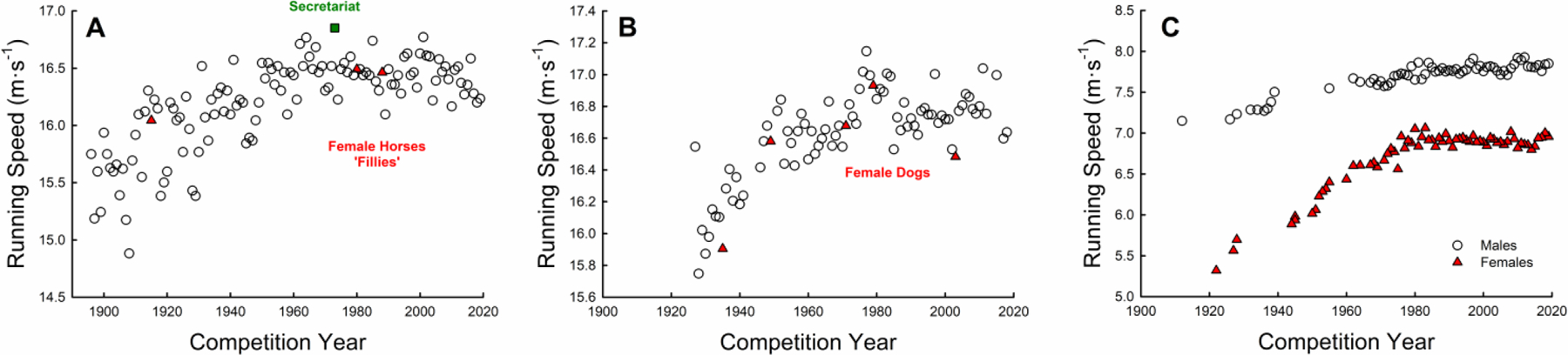
Running speed for the winning performance (n=1) by year for the Kentucky Derby in horses (A) and English Derby in greyhounds (B); running speed for the world record (before 1967) or years’ fastest 800m performance in both male and female humans (C).
3.1 |. Sexual Dimorphisms and Exercise Performance
Considering the highlighted physiological factors contributing to running performance, most sex-related differences in performance among humans are suggested to be sequelae to sexual dimorphisms. The sexual dimorphisms thought to primarily contribute to differences in human performance include smaller anatomical size of the lung, muscle, bones, and heart; lower levels of hemoglobin and hematocrit (oxygen carrying capacity); lower proportions of lean muscle mass; and lower androgen hormone levels in females compared to males (10, 18). These sexual dimorphisms are largely attributed to the divergence in serum testosterone that begins at ~12 years of age, thus, sex-related differences in elite athletic performance are nominal or perhaps non-existent prior to the onset of the puberty-related increase in testosterone for male youths (33). When scaled to body size a 10% difference in (expressed as mL· kg−1 ·min−1) exists between elite male and female athletes (3). Per given body weight, human females have on average about 10% smaller lung sizes, 10% lower hemoglobin content and red cell mass, ~16% smaller spleens, and 14% higher body fat than men (18). While muscle fiber type is not believed to differ between men and women, mechanical cross-bridging within the muscle fibers may differ (34). The ~10% sex-related difference appears to be pervasive among many factors that contribute to running performance, thus, it may not be surprising that there is a nearly ubiquitous sex-related difference of performance in elite sports in adult humans, (e.g. running, cycling, triathlon, rowing) of ~10% (33, 35–37).
Although there is a paucity of comparative biology studies of sex-related differences in elite animals that compete in running events, evidence suggests there are minimal anatomical differences in heart and lung sizes of male and female horses and dog breeds when measurements are adjusted for body mass (38). The sex-related difference in body mass index of horses (39) and dogs also appears to be blunted relative to the ~11% sex-related difference observed in world-class humans (40). Additionally, though substantial evidence supports that testosterone is strongly associated with sex-based differences in sports performance of humans (12, 33, 41), the role of testosterone and androgen hormones may be less definitive among horses and dogs. After the removal of the animal’s reproductive organ through neutering, evidence suggests there are no short or long term decrements in exercise performances for horses or dogs (32). The limited sex-related anatomical differences and blunted impact of androgen hormones on performance among animals likely explain, in part, the lack of sex-related differences observed in exercise performance of elite horses and dogs.
Small observational studies and case studies of elite athletes undergoing hormone therapy, including transgender, cisgender and individuals with disorders of sex development (previously called “intersex”), provide evidence supporting the role of testosterone in potential athletic achievement. For example, gender-affirming testosterone therapy increases muscle mass and strength in transmen by ~15% after 1 year; in contrast, estrogen therapy yields ~5% muscle regression in transwomen (42, 43), lower hemoglobin, and no effects on bone (44). In a longitudinal observational study of the running performance times of eight transgender women runners, athletes ran ~3.5 minutes slower for a five kilometer race, on average, in the female gender than as males (45). Additional studies are underway to investigate the effect of gender transitions on athletic ‘trainability’ and performance by a team of renowned scientists, including Dr. Yannis Pitsiladis who was a leader on the sub2 project and Ms. Joanna Harper who was the first transgender person to advise the International Olympic Committee on matters of gender and sport. In the context of comparative biology, these data provide support for the notion that sexual dimorphisms in endogenous testosterone levels beginning at puberty —which correspond to sex-specific divergence in hematocrit, muscle strength and endurance, cardiac output, and sports performance (33, 41, 46)— are the primary factors associated with sex-based differences in human sports performance. Despite this dispositive evidence supporting a sex-based dichotomy in testosterone and athletic performance among humans, sequelae from sex chromosomes, there is much less known about the impact of sex chromosomes on athletic performance among animals. Hundreds of years of selection by humans have produced breeds of canines with superior speed and athleticism and the primary “performance-enhancing polymorphisms” among dogs encode genes which regulate the cardiovascular and musculoskeletal systems, and there is a limited, if any, impact of the sex chromosomes (47).
Interestingly, thoroughbreds and greyhounds may have anatomical and physiological advantages for exercise performance relative to humans, which are likely further augmented by selective breeding practices (48). The heart size of the thoroughbred and greyhound represents about 1% of body mass compared to about 0.5% in humans (49, 50). Additionally, both thoroughbreds and greyhounds are able to mobilize large volumes of blood (up to ~20% of blood volume) into circulation using contractions of the spleen (51, 52), which occurs to a much lesser extent among humans, up to ~2% of blood volume (53, 54). These factors likely contribute to the markedly greater observed in thoroughbred horses (up to ~170 ml· kg−1 ·min−1, (55)) and greyhound dogs (up to ~200 mL· kg−1 ·min−1, (56)) compared to humans (up to ~95 mL· kg−1 ·min−1, (57)).
3.3 |. Genotype-phenotype Associations
Although precise genotypes have not been fully elucidated, there are certainly contributing genetic and epigenetic factors that cause a selective bias in the examination of record performances of both elite humans and animals. For example, as mentioned above, the whippet breed originally developed in the late 1800s in canines has a mutation in the myostatin gene contributing to a phenotype of small stature with very fast running speeds. As such, the whippet genetic mutation has been preserved and selectively bred for racing in canines (27). This genotype-phenotype combination is highly-conserved across species and the “whippet” polymorphism is considered the “speed gene” in Thoroughbred horses (58). Thus, in animals, selective breeding likely limits the genetic variability between animals and contributes to the blunted sexual dimorphisms compared to humans; however, the precise “speed genome” is unknown but “gene doping” may represent a final frontier for athletic development in animals.
In humans, the effect of “gene doping” is largely unknown and there are limited observations of selective breeding among humans. Although there is considerable interest in the impact of myostatin mutations and the “whippet” polymorphism on the human genome, particularly for treatment of muscular dystrophies, there is limited scientific evidence beyond a case report of a suspected loss-of-function mutation in the myostatin gene contributing to a heavily muscled phenotype in a child whose mother was reportedly an elite swimmer (59). Previous narrative reviews have suggested that there are very few, if any, apparent relationships between the key physiological determinants of performance (, lactate, etc.) and DNA sequence variation in humans (10, 60). However, anecdotal examples of offspring from world-class athletes or groups of siblings with world-class marks provide a rationale to suggest the presence of “speed genes” in the human genome. Examples that may suggest “speed genes” among humans include parent-child relationships, such as Charles and Chip Jenkins whom both won Olympic gold medals in the 1600m relay, and siblings like the Dibaba family of Olympic medalists (sisters Ejegayehu, Genzebe, and Tirunesh, and cousin Derartu Tulu) and the Brownlee brothers, Alistair and Jonny (both two-time Olympic medalists in triathlon). Notwithstanding the many sociocultural factors that may predispose or contribute to the potential for successful careers in endurance sport, such as early exposure to activity, conducive environmental factors, and social support network, the observations from ‘talented’ families suggest that there may be a favorable genetic profile that, when combined with an optimal training environment, may contribute to elite athletic performance (61).
4 |. Future Directions
Studies on both human and animal performance in athletic events continue to stimulate critical physiological questions and drive novel research experiments of oxygen transport, cardiovascular mechanisms, muscle performance and metabolism (6). Although there have been selective pressures of the environment that have favored endurance running since early in human ancestry (62, 63), there are many physiological questions that persist. Do the dimorphisms in elite performance also represent sex differences in non-elite runners? Is our human sex physiology better suited to one sport versus another? Comparatively, the sex-related performance gap in swimming may be smaller for certain distances and strokes (64) whereas the sex-related performance gap for weight lifting is considerably larger than ~10% (37). What role does pharmacological or genetic alteration of the hormonal balance, such as testosterone, have in narrowing or widening these gaps across the spectrum of sport? Perhaps the human body is better equipped for certain movements such as running versus jumping? Novel advancements of techniques such as the Fosbury flop have had a major impact on sport records. Today much controversy and debate continues as to the role technological developments will have for the future of the sport, such as the line of so-called “neoteric” Nike high tech running shoes (65). Could these inventions be targeted to sex differences thus narrowing or widening the performance gap?
It has been argued that except for reproductive function studies, general human physiology research has traditionally been defined in terms of responses of the ‘typical 70-kg man’ (66). This sentiment applies equally to the study of the physiology of exercise, where many of the classic studies were performed exclusively on male research participants. Physiological studies on sex and gender continue to gain traction thanks to many leaders in history and the 2016 National Institutes of Health policy mandate requiring the study of sex as a biological variable (66). Sex is undoubtedly an important fundamental variable that must be considered when designing and analyzing basic and clinical research. Understanding sex-based differences is of importance to both the physiologist and the clinician because sex differences impact patient populations, exercise guidelines and prescription for disease prevention and ultimately our fundamental understanding of human physiology. Continued integrative physiological research is needed to further the understanding of sex differences in humans and other species. Using ‘experiments in nature’ and cross comparison studies such as our review here of running and performance records can help drive new ideas and questions for future research.
Additionally, this information may have broad implications for the participation of intersex and transgender athletes in women’s competitions. While striving for fair and inclusive policies for all athletes, the profound influence of testosterone on sports performance in humans should likely be recognized with appropriate consideration. There are specific testosterone levels that could be used to dichotomize competitive cohorts based on relevant biology (46).
4.1 |. Conclusions
Among humans, much of the differences in the current world record performances between males and females are likely due to sexual dimorphisms. Our data supports that the large sex-related difference of ~10% or more in human elite athletic performance are not observed between the sexes in horses and greyhounds. Continued studies on both human and animal performance in athletic events are needed to drive advancement of physiological questions and sex differences.
Acknowledgements
We thank Dr. Chad Wiggins, Dr. Stephen Klassen, Dr. Martin Husen, and Mr. Kevin Webb for intellectual discussions and feedback on earlier versions of the manuscript. This work was supported by the National Heart, Lung, and Blood Institute (NHLBI) grant 5R35HL139854 (to MJJ) and grant 1F32HL154320 (to JWS), and National Institute on Aging grant U54 AG044170 (to SEB).
Nonstandard Abbreviations
maximal oxygen uptake
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- 1.Joyner MJ, Hunter SK, Lucia A, and Jones AM (2020) Physiology and Fast Marathons. J Appl Physiol (1985) [DOI] [PubMed] [Google Scholar]
- 2.Hunter SK, Joyner MJ, and Jones AM (2015) The two-hour marathon: What’s the equivalent for women? J Appl Physiol (1985) 118, 1321–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joyner MJ (2017) Physiological limits to endurance exercise performance: influence of sex. J Physiol 595, 2949–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guilherme J, Tritto A, North K, Lancha A Jr, and Artioli G (2014) Genetics and sport performance: current challenges and directions to the future. Revista Brasileira de Educação Física e Esporte 28, 177–193 [Google Scholar]
- 5.Costa AM, Breitenfeld L, Silva AJ, Pereira A, Izquierdo M, and Marques MC (2012) Genetic inheritance effects on endurance and muscle strength: an update. Sports Med 42, 449–458 [DOI] [PubMed] [Google Scholar]
- 6.Joyner MJ (1991) Modeling: optimal marathon performance on the basis of physiological factors. J Appl Physiol (1985) 70, 683–687 [DOI] [PubMed] [Google Scholar]
- 7.Joyner MJ, Ruiz JR, and Lucia A (2011) The two-hour marathon: who and when? J Appl Physiol (1985) 110, 275–277 [DOI] [PubMed] [Google Scholar]
- 8.Bower MA, McGivney BA, Campana MG, Gu J, Andersson LS, Barrett E, Davis CR, Mikko S, Stock F, Voronkova V, Bradley DG, Fahey AG, Lindgren G, MacHugh DE, Sulimova G, and Hill EW (2012) The genetic origin and history of speed in the Thoroughbred racehorse. Nat Commun 3, 643. [DOI] [PubMed] [Google Scholar]
- 9.Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, and Ostrander EA (2007) A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet 3, e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joyner MJ, and Coyle EF (2008) Endurance exercise performance: the physiology of champions. J Physiol 586, 35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Simone G, Devereux RB, Daniels SR, and Meyer RA (1995) Gender differences in left ventricular growth. Hypertension 26, 979–983 [DOI] [PubMed] [Google Scholar]
- 12.Howden EJ, Perhonen M, Peshock RM, Zhang R, Arbab-Zadeh A, Adams-Huet B, and Levine BD (2015) Females have a blunted cardiovascular response to one year of intensive supervised endurance training. J Appl Physiol (1985) 119, 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa T, Spina RJ, Martin WH 3rd, Kohrt WM, Schechtman KB, Holloszy JO, and Ehsani AA (1992) Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 86, 494–503 [DOI] [PubMed] [Google Scholar]
- 14.Astrand PO, Cuddy TE, Saltin B, and Stenberg J (1964) Cardiac Output during Submaximal and Maximal Work. J Appl Physiol 19, 268–274 [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz DC, Buyukakilli B, Gurgul S, and Rencuzogullari I (2013) Adaptation of heart to training: a comparative study using echocardiography & impedance cardiography in male & female athletes. Indian J Med Res 137, 1111–1120 [PMC free article] [PubMed] [Google Scholar]
- 16.Vahlquist B (1950) The Cause of the Sexual Differences in Erythrocyte, Hemoglobin and Serum Iron Levels in Human Adults. Blood 5, 874–875 [PubMed] [Google Scholar]
- 17.Porter MM, Stuart S, Boij M, and Lexell J (2002) Capillary supply of the tibialis anterior muscle in young, healthy, and moderately active men and women. Journal of applied physiology (Bethesda, Md. : 1985) 92, 1451–1457 [DOI] [PubMed] [Google Scholar]
- 18.Pate RR, and O’Neill JR (2007) American women in the marathon. Sports medicine (Auckland, N.Z.) 37, 294–298 [DOI] [PubMed] [Google Scholar]
- 19.Holloszy JO, and Coyle EF (1984) Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol 56, 831–838 [DOI] [PubMed] [Google Scholar]
- 20.Ingham SA, Whyte GP, Pedlar C, Bailey DM, Dunman N, and Nevill AM (2008) Determinants of 800-m and 1500-m running performance using allometric models. Med Sci Sports Exerc 40, 345–350 [DOI] [PubMed] [Google Scholar]
- 21.Conley DL, and Krahenbuhl GS (1980) Running economy and distance running performance of highly trained athletes. Med Sci Sports Exerc 12, 357–360 [PubMed] [Google Scholar]
- 22.Kubo K, Kanehisa H, and Fukunaga T (2003) Gender differences in the viscoelastic properties of tendon structures. Eur J Appl Physiol 88, 520–526 [DOI] [PubMed] [Google Scholar]
- 23.Fletcher JR, Pfister TR, and Macintosh BR (2013) Energy cost of running and Achilles tendon stiffness in man and woman trained runners. Physiol Rep 1, e00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniels J, and Daniels N (1992) Running economy of elite male and elite female runners. Med Sci Sports Exerc 24, 483–489 [PubMed] [Google Scholar]
- 25.Helgerud J, Ingjer F, and Stromme SB (1990) Sex differences in performance-matched marathon runners. Eur J Appl Physiol Occup Physiol 61, 433–439 [DOI] [PubMed] [Google Scholar]
- 26.Helgerud J (1994) Maximal oxygen uptake, anaerobic threshold and running economy in women and men with similar performances level in marathons. Eur J Appl Physiol Occup Physiol 68, 155–161 [DOI] [PubMed] [Google Scholar]
- 27.Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, and Ostrander EA (2007) A Mutation in the Myostatin Gene Increases Muscle Mass and Enhances Racing Performance in Heterozygote Dogs. PLOS Genetics 3, e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones AM, Wilkerson DP, DiMenna F, Fulford J, and Poole DC (2008) Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. Am J Physiol Regul Integr Comp Physiol 294, R585–593 [DOI] [PubMed] [Google Scholar]
- 29.Jones AM, Vanhatalo A, Burnley M, Morton RH, and Poole DC (2010) Critical power: implications for determination of V O2max and exercise tolerance. Med Sci Sports Exerc 42, 1876–1890 [DOI] [PubMed] [Google Scholar]
- 30.Lauderdale MA, and Hinchcliff KW (1999) Hyperbolic relationship between time-to-fatigue and workload. Equine Vet J Suppl, 586–590 [DOI] [PubMed] [Google Scholar]
- 31.Cheuvront SN, Carter R, Deruisseau KC, and Moffatt RJ (2005) Running performance differences between men and women:an update. Sports Med 35, 1017–1024 [DOI] [PubMed] [Google Scholar]
- 32.Entin P (2007) Do racehorses and Greyhound dogs exhibit a gender difference in running speed? Equine and Comparative Exercise Physiology 4, 135–140 [Google Scholar]
- 33.Senefeld JW, Clayburn AJ, Baker SE, Carter RE, Johnson PW, and Joyner MJ (2019) Sex differences in youth elite swimming. PLoS One 14, e0225724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeon Y, Choi J, Kim HJ, Lee H, Lim JY, and Choi SJ (2019) Sex- and fiber-type-related contractile properties in human single muscle fiber. J Exerc Rehabil 15, 537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keenan KG, Senefeld JW, and Hunter SK (2018) Girls in the boat: Sex differences in rowing performance and participation. PLoS One 13, e0191504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whipp BJ, and Ward SA (1992) Will women soon outrun men? Nature 355, 25. [DOI] [PubMed] [Google Scholar]
- 37.Thibault V, Guillaume M, Berthelot G, Helou NE, Schaal K, Quinquis L, Nassif H, Tafflet M, Escolano S, Hermine O, and Toussaint JF (2010) Women and Men in Sport Performance: The Gender Gap has not Evolved since 1983. J Sports Sci Med 9, 214–223 [PMC free article] [PubMed] [Google Scholar]
- 38.Trachsel DS, Giraudet A, Maso D, Herve G, Hauri DD, Barrey E, and Robert C (2016) Relationships between body dimensions, body weight, age, gender, breed and echocardiographic dimensions in young endurance horses. BMC Vet Res 12, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armstrong RB, Essen-Gustavsson B, Hoppeler H, Jones JH, Kayar SR, Laughlin MH, Lindholm A, Longworth KE, Taylor CR, and Weibel ER (1992) O2 delivery at VO2max and oxidative capacity in muscles of standardbred horses. J Appl Physiol (1985) 73, 2274–2282 [DOI] [PubMed] [Google Scholar]
- 40.Gagnon CM, Steiper ME, and Pontzer H (2018) Elite swimmers do not exhibit a body mass index trade-off across a wide range of event distances. Proc Biol Sci 285, 20180684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Handelsman DJ, Hirschberg AL, and Bermon S (2018) Circulating Testosterone as the Hormonal Basis of Sex Differences in Athletic Performance. Endocr Rev 39, 803–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiik A, Lundberg TR, Rullman E, Andersson DP, Holmberg M, Mandic M, Brismar TB, Dahlqvist Leinhard O, Chanpen S, Flanagan JN, Arver S, and Gustafsson T (2020) Muscle Strength, Size, and Composition Following 12 Months of Gender-affirming Treatment in Transgender Individuals. J Clin Endocrinol Metab 105 [DOI] [PubMed] [Google Scholar]
- 43.Roberts TA, Smalley J, and Ahrendt D (2020) Effect of gender affirming hormones on athletic performance in transwomen and transmen: implications for sporting organisations and legislators. Br J Sports Med [DOI] [PubMed] [Google Scholar]
- 44.Hilton EN, and Lundberg TR (2020) Transgender Women in the Female Category of Sport: Perspectives on Testosterone Suppression and Performance Advantage. Sports Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harper J (2015) Race Times for Transgender Athletes. Journal of Sporting Cultures and Identities 6, 1–9 [Google Scholar]
- 46.Senefeld JW, Lambelet Coleman D, Johnson PW, Carter RE, Clayburn AJ, and Joyner MJ (2020) Divergence in Timing and Magnitude of Testosterone Levels Between Male and Female Youths. JAMA 324, 99–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J, Williams FJ, Dreger DL, Plassais J, Davis BW, Parker HG, and Ostrander EA (2018) Genetic selection of athletic success in sport-hunting dogs. Proc Natl Acad Sci U S A 115, E7212–E7221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaldivar-Lopez S, Marin LM, Iazbik MC, Westendorf-Stingle N, Hensley S, and Couto CG (2011) Clinical pathology of Greyhounds and other sighthounds. Vet Clin Pathol 40, 414–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young LE (2003) Equine athletes, the equine athlete’s heart and racing success. Exp Physiol 88, 659–663 [DOI] [PubMed] [Google Scholar]
- 50.Shave R, Howatson G, Dickson D, and Young L (2017) Exercise-Induced Cardiac Remodeling: Lessons from Humans, Horses, and Dogs. Vet Sci 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKeever KH, Agans JM, Geiser S, Lorimer PJ, and Maylin GA (2006) Low dose exogenous erythropoietin elicits an ergogenic effect in standardbred horses. Equine Vet J Suppl, 233–238 [DOI] [PubMed] [Google Scholar]
- 52.Longhurst JC, Musch TI, and Ordway GA (1986) O2 consumption during exercise in dogs--roles of splenic contraction and alpha-adrenergic vasoconstriction. Am J Physiol 251, H502–509 [DOI] [PubMed] [Google Scholar]
- 53.Schagatay E, Lunde A, Nilsson S, Palm O, and Lodin-Sundstrom A (2020) Spleen contraction elevates hemoglobin concentration at high altitude during rest and exercise. Eur J Appl Physiol 120, 2693–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bakovic D, Eterovic D, Saratlija-Novakovic Z, Palada I, Valic Z, Bilopavlovic N, and Dujic Z (2005) Effect of human splenic contraction on variation in circulating blood cell counts. Clin Exp Pharmacol Physiol 32, 944–951 [DOI] [PubMed] [Google Scholar]
- 55.Lund RJ, and Guthrie AJ (1995) Measurement of maximal oxygen consumption of thoroughbred horses at an altitude of 1250m using open-circuit flow-through calorimetry. J S Afr Vet Assoc 66, 239–243 [PubMed] [Google Scholar]
- 56.Staaden R (1984) The exercise physiology of the racing greyhound. PhD Thesis, Murdoch University, Murdoch, Western Australia [Google Scholar]
- 57.Haugen T, Paulsen G, Seiler S, and Sandbakk O (2018) New Records in Human Power. Int J Sports Physiol Perform 13, 678–686 [DOI] [PubMed] [Google Scholar]
- 58.Rooney MF, Hill EW, Kelly VP, and Porter RK (2018) The “speed gene” effect of myostatin arises in Thoroughbred horses due to a promoter proximal SINE insertion. PloS one 13, e0205664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, and Lee SJ (2004) Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350, 2682–2688 [DOI] [PubMed] [Google Scholar]
- 60.Joyner MJ (2019) Genetic Approaches for Sports Performance: How Far Away Are We? Sports medicine (Auckland, N.Z.) 49, 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guth LM, and Roth SM (2013) Genetic influence on athletic performance. Curr Opin Pediatr 25, 653–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liebenberg L (2008) The relevance of persistence hunting to human evolution. J Hum Evol 55, 1156–1159 [DOI] [PubMed] [Google Scholar]
- 63.Bramble DM, and Lieberman DE (2004) Endurance running and the evolution of Homo. Nature 432, 345–352 [DOI] [PubMed] [Google Scholar]
- 64.Senefeld J, Joyner MJ, Stevens A, and Hunter SK (2016) Sex differences in elite swimming with advanced age are less than marathon running. Scand J Med Sci Sports 26, 17–28 [DOI] [PubMed] [Google Scholar]
- 65.Kipp S, Kram R, and Hoogkamer W (2019) Extrapolating Metabolic Savings in Running: Implications for Performance Predictions. Front Physiol 10, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller VM, Rocca WA, and Faubion SS (2015) Sex Differences Research, Precision Medicine, and the Future of Women’s Health. J Womens Health (Larchmt) 24, 969–971 [DOI] [PMC free article] [PubMed] [Google Scholar]


