Abstract
Background/Aims
We evaluated whether anti-Saccharomyces cerevisiae antibody (ASCA) titers are associated with diagnostic findings, disease activity, Paris classification phenotypes, and persistence after infliximab (IFX) treatment in children with Crohn’s disease (CD). We also investigated the role of ASCA as a predictor of mucosal healing (MH) and clinical remission (CR).
Methods
This study included 61 CD patients aged 19 years or younger who were diagnosed and treated between September 2010 and January 2019 and followed for at least 1 year. ASCA was regularly measured at the diagnosis of CD and at least 1 year after IFX therapy.
Results
The average follow-up period was 3.8±3.4 years (range, 1.0 to 7.2 years). Regression analysis showed that the ASCA titer was the only factor associated with Simple Endoscopic Score for Crohn's Disease (SES-CD) or CR among all the parameters. In patients who had achieved MH (SES-CD=0), ASCA immunoglobulin G (IgG) was not associated with MH, but in patients without MH, ASCA IgG was associated with SES-CD (p=0.005) and CR (p<0.001). The cutoff value of ASCA IgG in patients with CR was 21.8 units. However, there was no difference in the relapse rate between the ASCA IgG-positive and -negative groups during the follow-up period.
Conclusions
In patients who have not achieved MH, ASCA IgG is closely related to mucosal damage and CR. Unlike Western studies, ASCA IgG may be more helpful in predicting prognosis than immunoglobulin A in Korean patients, but it is not an appropriate indicator to predict the relapse of CD. (Gut Liver 2021;15-770)
Keywords: Crohn disease, Anti-Saccharomyces cerevisiae, Children, Marker, Mucosal healing
INTRODUCTION
The pathogenesis of Crohn’s disease (CD) is not clearly defined, but the prevalence of pediatric CD worldwide, including Korea, is on the rise.1-4 Despite the availability of many serum biomarkers, there is a lack of reliable tools to predict CD prognosis.5,6 In 1988, Main et al.7 first reported elevated anti-Saccharomyces cerevisiae antibody (ASCA) levels in CD. ASCA is a useful tool to distinguish CD from ulcerative colitis, but its value as a prognostic marker is emerging gradually.8-10
ASCA is generally common in younger age groups, those with ileal involvement, fibrostenosis, and more aggressive and complex disease behavior.11-13 Several studies have investigated whether ASCA titers are altered with disease activity, and successful treatment has shown that its titers are stable or decreased.14-16 However, few studies have investigated the association between ASCA titers and disease prognosis, relapse and biological treatment in children with CD.17-19 The introduction of various biological agents, including monoclonal antibodies that block the inflammatory cytokine tumor necrosis factor-α, has altered the treatment and management options for CD. Serological markers can be used to assess the therapeutic response of these biological agents in pediatric cases of CD.20,21
Our study evaluated whether ASCA titers are associated with diagnostic value, disease activity, Paris classification phenotypes, durability over time after infliximab (IFX) treatment in children with CD. We also investigated the role of ASCA as a predictor of mucosal healing (MH) and clinical remission (CR).
MATERIALS AND METHODS
1. Patients and data collection
A comprehensive medical chart review was performed for all children with CD who were aged below 19 years at the Samsung Medical Center and had quantitative ASCA immunoglobulin A (IgA) and immunoglobulin G (IgG) titers at diagnosis and follow-up. This study was conducted in patients who were treated with IFX for at least 1 year between September 2010 and January 2019.
Paris classification was used to determine the disease phenotype.22 The pediatric Crohn’s disease activity index (PCDAI), serum albumin, hematocrit, erythrocyte sedimentation rate and C-reactive protein values were determined concurrently with the ASCA titers. The PCDAI score ranges from zero to 100, with a score >10 indicating active disease.23 Simple Endoscopic Score for Crohn’s Disease (SES-CD) was also measured by colonoscopy at diagnosis and at least 1 year after IFX treatment, followed by 1 to 2 years intervals. Patients in CR were identified based on PCDAI scores less than 10. Patients with MH were identified based on SES-CD less than 0. Patients were also required to have data on serum IFX trough levels, obtained from blood samples taken immediately before the IFX infusion.
The data were collected from all the eligible patients including those with ASCA and simultaneous SES-CD. Approval was obtained from the Institutional Review Board of the Samsung Medical Center Committee on Clinical Investigation (IRB number: 2019-12-152). The requirement for obtaining informed consent from the patients was waived due to the retrospective nature of the study.
2. ASCA IgA and IgG measurements
ASCA IgA and IgG were quantified using commercially available standard calibrated enzyme-linked immunosorbent assay kits, and the tests were performed according to the manufacturer’s protocol (ASCA IgG and ASCA IgA; QUANTA LiteTM, INOVA Diagnostics, San Diego, CA, USA). Results of ASCA IgA and IgG testing were classified as negative (0.0 to 20.0 units), equivocal (20.1 to 24.9 units), or positive (≥25 units).
3. Primary outcome
The primary outcome of this study was the role of ASCA according to disease severity before and after IFX treatment, such as Paris classification phenotypes, laboratory findings, and SES-CD, especially in pediatric CD patients. ASCA titers at diagnosis were compared with those after at least 1 year of IFX treatment, and their correlation with CR and endoscopic MH were evaluated.
4. Statistical analysis
Data were expressed as the mean and standard deviation (continuous variables) and as frequencies and percentages (categorical variables). The chi-square test and Mann-Whitney test were used to compare categorical and quantitative variables. When comparing measurements at baseline and after IFX treatment, the Wilcoxon signed-rank test was used. A receiver operating characteristic analysis was performed to calculate the cutoff value of ASCA IgG for CR. Kaplan-Meier analysis was used to estimate the increase in free survival rate during long-term follow-up.
Simple and multiple linear regression analyses were used to identify the factors associated with MH and CR. A multivariate linear regression analysis was then conducted using a stepwise selection procedure with a 5% significance level for a covariate to enter or remain in the model. Linear correlation was estimated using the Pearson correlation and represented as correlation coefficients (r) on a scatter plot. The results were also expressed as probabilities. All tests were two-sided, and p-values <0.05 are considered statistically significant. All analyses were performed using SPSS, statistical software SPSS version 25 (IBM Corp., Armonk, NY, USA).
RESULTS
1. Baseline characteristics
Serial 167 measurements of ASCA IgA and IgG titers were documented in 61 children with CD. The mean age at diagnosis was 14.6±2.3 years, with age ranging from 8.8 to 18.9 years, and included 41 boys. The patients’ characteristics are summarized in Table 1. Disease phenotype was determined according to the Paris classification.
Table 1.
Clinical Characteristics of Patients with Crohn’s Disease
| Characteristics | Value (n=61) |
|---|---|
| Male sex | 41 (67.2) |
| Age at diagnosis, yr | 14.6±2.3 |
| Paris location | |
| L1 (distal 1/3 of ileum±caecum) | 3 (4.9) |
| L2 (colon) | 2 (3.3) |
| L3 (ileocolonic) | 56 (91.8) |
| L4 (upper GI tract involvement) | 29 (47.5) |
| Paris behavior | |
| B1 (non-stricturing, non-penetrating) | 48 (78.7) |
| B2 (stricturing) | 12 (19.7) |
| B3 (penetrating) | 1 (1.6) |
| Perianal disease | 32 (52.5) |
| Dose intensification during IFX treatment | 7 (11.5) |
| Concomitant AZA treatment | 55 (90.2) |
| Corticosteroid usage | 3 (4.9) |
| Any lifelong CD-related GI surgery | 2 (3.3) |
| Growth impairment | 6 (9.8) |
| Family history of IBD | 5 (8.2) |
Data are presented as number (%) or mean±SD.
GI, gastrointestinal; IFX, infliximab; AZA, azathioprine; CD, Crohn’s disease; IBD, inflammatory bowel disease.
The mean duration between initial diagnosis and the follow-up was 3.8±3.4 years. There were 32 out of 61 (52.5%) ASCA-positive (either IgA or IgG) CD patients at diagnosis and 26 out of 57 (45.6%) patients at follow-up after IFX treatment. Five (8.2%) ASCA IgA-positive and 27 (44.3%) ASCA IgG-positive CD patients were documented at diagnosis. At the time of follow-up, two (3.5%) ASCA IgA-positive and 24 (42.1%) ASCA IgG-positive patients were identified (Fig. 1).
Fig. 1.
ASCA-positive cases at diagnosis and follow-up. ASCA IgA-positive results were found in five cases, and ASCA IgG-positive results were found in 27 cases at diagnosis. During follow-up, two ASCA IgA-positive and 24 ASCA IgG-positive patients were detected.
ASCA, anti-Saccharomyces cerevisiae antibody; IgA, immunoglobulin A; IgG, immunoglobulin G.
2. Durability of ASCA titer
There was no statistically significant difference in the titer of ASCA before and after IFX treatment. The mean duration of follow-up was 3.8±3.4 years, ranging from 1.0 to 7.2 years. The ASCA titer at diagnosis was 14.3±14.4 units and the titer at follow-up was 10.5±10.0 units (p>0.05) (Fig. 2).
Fig. 2.
Durability of the ASCA titer over time. The ASCA titer at diagnosis was 14.3±14.4 units and the titer at follow-up was 10.5±10.0 units. The mean duration of follow-up was 3.8±3.4 and ranged from 1.0 to 7.2 years. There was no significant difference in the titer of ASCA before and after infliximab treatment.
ASCA, anti-Saccharomyces cerevisiae antibody; IgG, immunoglobulin G.
3. Factors associated with MH and CR
The linear regression analysis was used to determine factors associated with MH and CR in 61 CD patients. Factors included sex, age at diagnosis, Paris classification phenotypes, linear growth failure, various laboratory tests, IFX trough levels and ASCA IgA and IgG. In simple and multiple linear regression analyses, ASCA IgA and IgG was the only factor associated with SES-CD (Table 2). Analyses of factors associated with PCDAI also showed similar results (Table 3).
Table 2.
Simple and Multiple Linear Regression Analyses of Factors Associated with SES-CD
| Variable | Simple linear regression analysis | Multiple linear regression analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β±SE | 95% CI | Partial R2 | p-value | β±SE | 95% CI | Partial R2 | p-value | ||
| Sex (male vs female) | –3.500±2.224 | –8.080 to 1.080 | 0.090 | 0.128 | |||||
| Age at diagnosis, yr | 0.107±0.411 | –0.739 to 0.953 | 0.003 | 0.797 | |||||
| Upper GI tract involvement (yes vs no) | 0.767±2.050 | –3.456 to 4.989 | 0.006 | 0.712 | |||||
| B1 disease behavior (yes vs no) | –1.645±2.213 | –6.203 to 2.913 | 0.022 | 0.464 | |||||
| Perianal modifiers (yes vs no) | 2.267±2.005 | –1.864 to 6.397 | 0.049 | 0.269 | |||||
| Linear growth failure (yes vs no) | –0.989±2.869 | –6.898 to 4.920 | 0.005 | 0.733 | |||||
| WBC,/μL | 0.000±0.000 | –0.001 to 0.000 | 0.022 | 0.463 | |||||
| Hct, % | 0.006±0.017 | –0.030 to 0.041 | 0.004 | 0.744 | |||||
| Platelet count,/μL | 0.001±0.010 | –0.020 to 0.022 | 0.000 | 0.920 | |||||
| Albumin, g/dL | –1.601±1.789 | –5.286 to 2.084 | 0.031 | 0.379 | |||||
| ESR, mm/hr | 0.004±0.033 | –0.063 to 0.071 | 0.001 | 0.899 | |||||
| CRP, mg/dL | 0.089±0.295 | –0.519 to 0.697 | 0.004 | 0.766 | |||||
| IFX trough level, μg/mL | 0.130±0.205 | –0.294 to 0.553 | 0.017 | 0.533 | |||||
| ASCA IgA, units | 0.310±0.310 | 0.084 to 0.535 | 0.243 | 0.009* | |||||
| ASCA IgG, units | 0.196±0.064 | 0.064 to 0.328 | 0.273 | 0.005* | 0.219±0.057 | 0.103 to 0.336 | 0.465 | 0.001* | |
SES-CD, Simple Endoscopic Score for Crohn's Disease; SE, standard error; CI, confidence interval; GI, gastrointestinal; WBC, white blood cell; Hct, hematocrit; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; IFX, infliximab; ASCA, anti-Saccharomyces cerevisiae antibody; IgA, immunoglobulin A; IgG, immunoglobulin G.
*p<0.05.
Table 3.
Simple and Multiple Linear Regression Analyses of Factors Associated with PCDAI
| Variable | Simple linear regression analysis | Multiple linear regression analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β±SE | 95% CI | Partial R2 | p-value | β±SE | 95% CI | Partial R2 | p-value | ||
| Sex (male vs female) | 0.268±3.639 | –7.226 to 7.762 | 0.000 | 0.942 | |||||
| Age at diagnosis, yr | –0.051±0.642 | –1.373 to 1.271 | 0.000 | 0.938 | |||||
| Upper GI tract involvement (yes vs no) | 2.375±3.174 | –4.162 to 8.912 | 0.022 | 0.461 | |||||
| B1 disease behavior (yes vs no) | –1.234±3.484 | –8.408 to 5.941 | 0.005 | 0.726 | |||||
| Perianal modifiers (yes vs no) | 5.750±2.996 | –0.421 to 11.921 | 0.128 | 0.066 | |||||
| Linear growth failure (yes vs no) | –3.016±4.448 | –12.178 to 6.145 | 0.018 | 0.504 | |||||
| WBC,/μL | –0.001±0.001 | –0.002 to 0.000 | 0.055 | 0.240 | |||||
| Hct, % | –0.014±0.027 | –0.069 to 0.042 | 0.010 | 0.617 | |||||
| Platelet count,/μL | –0.020±0.015 | –0.051 to 0.012 | 0.061 | 0.213 | |||||
| Albumin, g/dL | –4.712±2.676 | –10.224 to 0.799 | 0.110 | 0.090 | |||||
| ESR, mm/hr | 0.001±0.051 | –0.104 to 0.106 | 0.000 | 0.985 | |||||
| CRP, mg/dL | 0.212±0.460 | –0.735 to 1.159 | 0.008 | 0.649 | |||||
| IFX trough level, μg/mL | –0.113±0.328 | –0.791 to 0.565 | 0.005 | 0.734 | |||||
| ASCA IgA, units | 0.492±0.170 | 0.142 to 0.842 | 0.252 | 0.008* | |||||
| ASCA IgG, units | 0.377±0.090 | 0.192 to 0.562 | 0.414 | <0.001* | 0.377±0.090 | 0.192 to 0.562 | 0.414 | <0.001* | |
PCDAI, pediatric Crohn’s disease activity index; SE, standard error; CI, confidence interval; GI, gastrointestinal; WBC, white blood cell; Hct, hematocrit; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; IFX, infliximab; ASCA, anti-Saccharomyces cerevisiae antibody; IgA, immunoglobulin A; IgG, immunoglobulin G.
*p<0.05.
4. Correlation between ASCA titer and SES-CD score or CR after IFX treatment
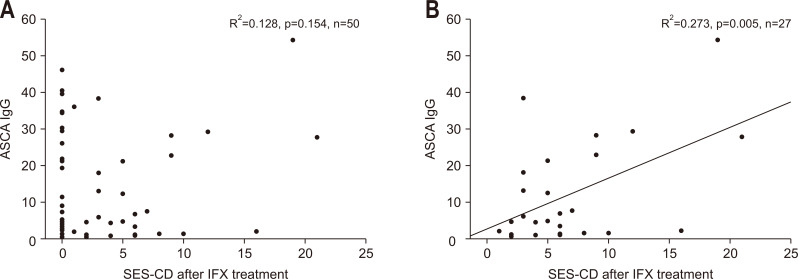
There was no statistically significant difference in correlation between MH based on SES-CD and ASCA titer after IFX treatment (Fig. 3A). However, when ASCA titers of patients with SES-CD score of 0 were removed, a significant correlation was found between the two parameters (p=0.005) (Fig. 3B). The correlation between CR through PCDAI and ASCA IgG after IFX treatment was also observed between the two indicators (p<0.001) (Fig. 4). There was no statistically significant difference in the rate of bowel surgery depending on whether ASCA IgG was positive or not.
Fig. 3.
Correlation between the ASCA titer and SES-CD after IFX treatment. There was no statistically significant difference in the correlation between MH based on SES-CD and the ASCA titer after IFX treatment (A). A significant correlation was observed after eliminating ASCA titers in patients with an SES-CD score of 0 (B).
ASCA, anti-Saccharomyces cerevisiae antibody; SES-CD, Simple Endoscopic Score for Crohn Disease; IFX, infliximab; MH, mucosal healing; IgG, immunoglobulin G.
Fig. 4.
Correlation between the ASCA titer and clinical remission (CR) after infliximab (IFX) treatment. The correlation between CR through PCDAI and the ASCA titer after IFX treatment was observed between the two indicators.
ASCA, anti-Saccharomyces cerevisiae antibody; PCDAI, pediatric Crohn’s disease activity index; IgG, immunoglobulin G.
5. Receiver operating curve for estimation of the diagnostic accuracy of ASCA titer for presumptive CR
The area under the receiver operating characteristic curve for ASCA IgG was associated with CR of CD patients: 0.756 (95% confidence interval, 0.541 to 0.972) (Fig. 5). The cutoff of ASCA IgG associated with CR with the maximum sensitivity and specificity was 21.8 units (sensitivity, 75.0%; specificity, 77.6%).
Fig. 5.
Receiver operating characteristic curve for estimating the diagnostic accuracy of the ASCA titer for the presumptive clinical remission of Crohn’s disease. The cutoff value of ASCA IgG for clinical remission was 21.8 units.
ASCA, anti-Saccharomyces cerevisiae antibody; IgG, immunoglobulin G; AUC, area under the curve; CI, confidence interval.
6. Survival plots for disease relapse during follow-up
After a median follow-up of 3.8 years (range, 1 to 7.2 years), 57.4% (35/61) of patients who were ASCA IgG positive had experienced clinical relapse. According to the Kaplan-Meier survival analysis of ASCA IgG-positive patients, the estimated cumulative relapse rates at 1, 2, 4, and 6 years were 17.3%, 31.2%, 58.8%, and 71.3%, respectively. Among ASCA IgG-negative patients, 45.9% (28/61) had experienced clinical relapse. According to the Kaplan-Meier survival analysis of ASCA IgG-negative patients, the cumulative relapse rates at 1, 2, 4, and 6 years were 16.5%, 29.9%, 27.6%, and 48.5%, respectively. No statistically significant differences were found between ASCA IgG positivity and recurrence rate during the follow-up period (Fig. 6).
Fig. 6.
Survival plots showing disease relapse during follow-up. There was no statistically significant difference between ASCA positivity and the recurrence rate during the follow-up period.
ASCA, anti-Saccharomyces cerevisiae antibody; IgG, immunoglobulin G.
DISCUSSION
Several studies have confirmed the association between ASCA antibodies and CD. ASCA antibodies have been known to facilitate the diagnosis and prediction of disease progression in CD patients.12,14,15 However, the association between ASCA titer and MH based on endoscopy is unclear. This study was conducted in patients who were treated with IFX for at least 1 year. The potential of IFX, not only to induce but also to maintain remission, has been well known in adult and pediatric CD patients. It is no longer unfamiliar to set the therapeutic goal of CD based on MH as well as CR. In addition to endoscopy used to identify MH, the development of noninvasive and reliable tools is an ongoing challenge. This study is the first to explore the utility of ASCA as a noninvasive tool in MH screening. Although the association between ASCA IgG and MH was not completely correlated in this study, it was shown that there was an association between ASCA IgG and SES-CD, which was a factor predicting MH. Further, it is not clear whether serum ASCA titers change according to CD activity following treatment and long-term follow-up. Our study showed that the measurement of ASCA titer facilitates the assessment of CR and MH in CD patients. We also demonstrated the persistence of ASCA after treatment.
Ruemmele et al.14 and Canani et al.24 suggest that CD behavior is not associated with ASCA titers in pediatric patients.14,24 However, several studies have reported high ASCA titers among more severe CD patients.25,26 In our study, ASCA is closely related to disease activity and mucosal damage status. Pediatric CD patients who carried high ASCA IgG titers manifested a more clinically severe disease and a higher risk of developing extensive endoscopic luminal disease. Therefore, careful monitoring is required when the titer is high during initial ASCA measurements. The one difference from Western studies is that ASCA IgG, rather than IgA, is a useful predictor of complicated disease behavior in Korea.11,19
Previous studies have suggested a link between ASCA titers and Paris classification phenotypes, that is, positive ASCA titers may be associated with ileocecal disease or correlate with both L3 and L4.19,27 However, we found no significant association between Paris classification phenotypes and positive ASCA status. Considering that most of the previous studies investigating ASCA have focused on Westerners, our results may be attributed to ethnic differences.
Rieder et al.28 found that ASCA titer decreased after corticosteroid therapy. However, Teml et al.15 analyzed ASCA levels in CD patients before and after treatment with either steroids or mesalamine, and reported that the ASCA levels did not change over a period of 2 to 9 months. Other investigators also showed that several antibodies in CD remained stable after 4 months of IFX therapy.29 Our data showed that ASCA titers remained stable even during the follow-up period up to 7 years and it was more reliable than those of other short-term studies. Repeated measurements of ASCA titers after diagnosis are unlikely to be useful.
Chandrakumar et al.19 showed that CD patients who were positive for ASCA IgA and IgG did not show a greater relapse rate than patients with negative titers. These results are similar to our study. There was no significant difference in relapse rate between ASCA IgG-positive and -negative patients, suggesting that ASCA is not an appropriate indicator to predict CD relapse.
The study is limited by a relatively small sample size and retrospective study design. Fecal calprotectin is a useful noninvasive marker of MH and has already been used to monitor therapeutic success in many studies. Unfortunately, in this study, fecal calprotectin was not used as a variable because it was not available during some periods. However, it is significant that ASCA has been proposed as a marker of MH after IFX treatment of children with CD.
In conclusion, the results of this study reveal the importance of ASCA as a noninvasive tool for monitoring disease activity and mucosal damage status in Korean children with CD. In patients who have not achieved MH, ASCA IgG is closely related to mucosal damage status and CR. Therefore, careful monitoring is required when the titer is high during initial ASCA measurements. Unlike Western studies, ASCA IgG may be more helpful in predicting prognosis than IgA in Korean patients, but it is not an appropriate indicator to predict the relapse of CD. Further prospective studies involving a large number of patients are needed to better define the value of ASCA in children with CD.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A2C2007192).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: M.J.K. Data acquisition: Y.L. Data analysis and interpretation: B.K. Drafting of the manuscript; critical revision of the manuscript for important intellectual content: E.S.K. Statistical analysis: E.K. Administrative, technical, or material support; study supervision: Y.H.C. Approval of final manuscript: all authors.
REFERENCES
- 1.Hong SJ, Cho SM, Choe BH, et al. Characteristics and incidence trends for pediatric inflammatory bowel disease in Daegu-Kyungpook province in Korea: a multi-center study. J Korean Med Sci. 2018;33:e132. doi: 10.3346/jkms.2018.33.e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benchimol EI, Bernstein CN, Bitton A, et al. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: distributed network analysis of multiple population-based provincial health administrative databases. Am J Gastroenterol. 2017;112:1120–1134. doi: 10.1038/ajg.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Matary W, Moroz SP, Bernstein CN. Inflammatory bowel disease in children of Manitoba: 30 years' experience of a tertiary center. J Pediatr Gastroenterol Nutr. 2014;59:763–766. doi: 10.1097/MPG.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 4.Lee KM, Lee JM. Crohn's disease in Korea: past, present, and future. Korean J Intern Med. 2014;29:558–570. doi: 10.3904/kjim.2014.29.5.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou G, Song Y, Yang W, et al. ASCA, ANCA, ALCA and many more: are they useful in the diagnosis of inflammatory bowel disease? Dig Dis. 2016;34:90–97. doi: 10.1159/000442934. [DOI] [PubMed] [Google Scholar]
- 6.Levine A. Pediatric inflammatory bowel disease: is it different? Dig Dis. 2009;27:212–214. doi: 10.1159/000228552. [DOI] [PubMed] [Google Scholar]
- 7.Main J, McKenzie H, Yeaman GR, et al. Antibody to Saccharomyces cerevisiae (bakers' yeast) in Crohn's disease. BMJ. 1988;297:1105–1106. doi: 10.1136/bmj.297.6656.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011;140:1817–1826. doi: 10.1053/j.gastro.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Matary W, Dupuis K, Sokoro A. Anti-Saccharomyces cerevisiae antibody titres correlate well with disease activity in children with Crohn's disease. Acta Paediatr. 2015;104:827–830. doi: 10.1111/apa.13026. [DOI] [PubMed] [Google Scholar]
- 10.Kim BC, Park S, Han J, Kim JH, Kim TI, Kim WH. Clinical significance of anti-Saccharomyces cerevisiae antibody (ASCA) in Korean patients with Crohn's disease and its relationship to the disease clinical course. Dig Liver Dis. 2007;39:610–616. doi: 10.1016/j.dld.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Peeters M, Joossens S, Vermeire S, Vlietinck R, Bossuyt X, Rutgeerts P. Diagnostic value of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease. Am J Gastroenterol. 2001;96:730–734. doi: 10.1111/j.1572-0241.2001.03613.x. [DOI] [PubMed] [Google Scholar]
- 12.Forcione DG, Rosen MJ, Kisiel JB, Sands BE. Anti-Saccharomyces cerevisiae antibody (ASCA) positivity is associated with increased risk for early surgery in Crohn's disease. Gut. 2004;53:1117–1122. doi: 10.1136/gut.2003.030734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Li C, Zhao X, et al. Anti-Saccharomyces cerevisiae antibodies associate with phenotypes and higher risk for surgery in Crohn's disease: a meta-analysis. Dig Dis Sci. 2012;57:2944–2954. doi: 10.1007/s10620-012-2244-y. [DOI] [PubMed] [Google Scholar]
- 14.Ruemmele FM, Targan SR, Levy G, Dubinsky M, Braun J, Seidman EG. Diagnostic accuracy of serological assays in pediatric inflammatory bowel disease. Gastroenterology. 1998;115:822–829. doi: 10.1016/S0016-5085(98)70252-5. [DOI] [PubMed] [Google Scholar]
- 15.Teml A, Kratzer V, Schneider B, et al. Anti-Saccharomyces cerevisiae antibodies: a stable marker for Crohn's disease during steroid and 5-aminosalicylic acid treatment. Am J Gastroenterol. 2003;98:2226–2231. doi: 10.1111/j.1572-0241.2003.07673.x. [DOI] [PubMed] [Google Scholar]
- 16.Oshitani N, Hato F, Matsumoto T, et al. Decreased anti-Saccharomyces cerevisiae antibody titer by mesalazine in patients with Crohn's disease. J Gastroenterol Hepatol. 2000;15:1400–1403. doi: 10.1046/j.1440-1746.2000.02357.x. [DOI] [PubMed] [Google Scholar]
- 17.Esters N, Vermeire S, Joossens S, et al. Serological markers for prediction of response to anti-tumor necrosis factor treatment in Crohn's disease. Am J Gastroenterol. 2002;97:1458–1462. doi: 10.1111/j.1572-0241.2002.05689.x. [DOI] [PubMed] [Google Scholar]
- 18.Dubinsky MC, Mei L, Friedman M, et al. Genome wide association (GWA) predictors of anti-TNFalpha therapeutic responsiveness in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1357–1366. doi: 10.1002/ibd.21174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandrakumar A, Georgy M, Agarwal P, 't Jong GW, El-Matary W. Anti-Saccharomyces cerevisiae antibodies as a prognostic biomarker in children with Crohn disease. J Pediatr Gastroenterol Nutr. 2019;69:82–87. doi: 10.1097/MPG.0000000000002311. [DOI] [PubMed] [Google Scholar]
- 20.Miheller P, Kiss LS, Juhasz M, Mandel M, Lakatos PL. Recommendations for identifying Crohn's disease patients with poor prognosis. Expert Rev Clin Immunol. 2013;9:65–75. doi: 10.1586/eci.12.86. [DOI] [PubMed] [Google Scholar]
- 21.Rieder F, Hahn P, Finsterhoelzl L, et al. Clinical utility of anti-glycan antibodies in pediatric Crohn's disease in comparison with an adult cohort. Inflamm Bowel Dis. 2012;18:1221–1231. doi: 10.1002/ibd.21854. [DOI] [PubMed] [Google Scholar]
- 22.Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–1321. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 23.Hyams J, Markowitz J, Otley A, et al. Evaluation of the pediatric Crohn disease activity index: a prospective multicenter experience. J Pediatr Gastroenterol Nutr. 2005;41:416–421. doi: 10.1097/01.mpg.0000183350.46795.42. [DOI] [PubMed] [Google Scholar]
- 24.Canani RB, Romano MT, Greco L, et al. Effects of disease activity on anti-Saccharomyces cerevisiae antibodies: implications for diagnosis and follow-up of children with Crohn's disease. Inflamm Bowel Dis. 2004;10:234–239. doi: 10.1097/00054725-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Vasiliauskas EA, Kam LY, Karp LC, Gaiennie J, Yang H, Targan SR. Marker antibody expression stratifies Crohn's disease into immunologically homogeneous subgroups with distinct clinical characteristics. Gut. 2000;47:487–496. doi: 10.1136/gut.47.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halfvarson J, Standaert-Vitse A, Jarnerot G, et al. Anti-Saccharomyces cerevisiae antibodies in twins with inflammatory bowel disease. Gut. 2005;54:1237–1243. doi: 10.1136/gut.2005.066860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinton JF, Sendid B, Reumaux D, et al. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–791. doi: 10.1136/gut.42.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rieder F, Lopez R, Franke A, et al. Characterization of changes in serum anti-glycan antibodies in Crohn's disease: a longitudinal analysis. PLoS One. 2011;6:e18172. doi: 10.1371/journal.pone.0018172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Targan SR, Landers CJ, Yang H, et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn's disease. Gastroenterology. 2005;128:2020–2028. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]