Abstract
Loss of function in the von Hippel-Lindau (VHL) tumor suppressor gene occurs in familial and most sporadic renal cell carcinomas (RCCs). VHL has been linked to the regulation of cell cycle cessation (G0) and to control of expression of various mRNAs such as for vascular endothelial growth factor. RCC cells express the Met receptor tyrosine kinase, and Met mediates invasion and branching morphogenesis in many cell types in response to hepatocyte growth factor/scatter factor (HGF/SF). We examined the HGF/SF responsiveness of RCC cells containing endogenous mutated (mut) forms of the VHL protein (VHL-negative RCC) with that of isogenic cells expressing exogenous wild-type (wt) VHL (VHL-positive RCC). We found that VHL-negative 786-0 and UOK-101 RCC cells were highly invasive through growth factor-reduced (GFR) Matrigel-coated filters and exhibited an extensive branching morphogenesis phenotype in response to HGF/SF in the three-dimensional (3D) GFR Matrigel cultures. In contrast, the phenotypes of A498 VHL-negative RCC cells were weaker, and isogenic RCC cells ectopically expressing wt VHL did not respond at all. We found that all VHL-negative RCC cells expressed reduced levels of tissue inhibitor of metalloproteinase 2 (TIMP-2) relative to the wt VHL-positive cells, implicating VHL in the regulation of this molecule. However, consistent with the more invasive phenotype of the 786-0 and UOK-101 VHL-negative RCC cells, the levels of TIMP-1 and TIMP-2 were reduced and levels of the matrix metalloproteinases 2 and 9 were elevated compared to the noninvasive VHL-positive RCC cells. Moreover, recombinant TIMPs completely blocked HGF/SF-mediated branching morphogenesis, while neutralizing antibodies to the TIMPs stimulated HGF/SF-mediated invasion in vitro. Thus, the loss of the VHL tumor suppressor gene is central to changes that control tissue invasiveness, and a more invasive phenotype requires additional genetic changes seen in some but not all RCC lines. These studies also demonstrate a synergy between the loss of VHL function and Met signaling.
von Hippel-Lindau (VHL) disease is an autosomal dominant inheritable cancer syndrome characterized by the development of renal cell carcinomas (RCCs) and vascular tumors of the retinas and the central nervous system (reviewed in references 22 and 25). Moreover, somatic mutation leading to loss of VHL tumor suppressor gene function is common in sporadic RCCs (reviewed in reference 5). RCC cells are known to have the potential for invasion and metastasis, although the clinical course and histopathologic findings vary from case to case (29). Overexpression of growth factors or their receptors has been identified in RCCs, suggesting mechanisms for this invasiveness and collagenolytic activity (35, 36, 39, 47). These factors stimulate in vitro invasiveness and collagenase type IV (gelatinase) expression (35, 36, 39, 47).
Several mechanisms underlying tumorigenesis in VHL-associated human neoplasms have been described. Thus, VHL controls the gene expression of transforming growth factor α (19), GLUT-1 glucose transporter (11), and vascular endothelial growth factor (7, 11, 45). Loss of VHL has been associated with many cellular phenotypes, such as increased vascular endothelial growth factor expression under normoxic conditions (7, 11, 45) and serum-independent growth (38). It was also shown that RCC cells harboring mutant (mut) VHL grow in low serum whereas RCC cells with wild-type (wt) VHL enter G0 and exit the cell cycle (38). Importantly, Iliopoulos et al. (11) showed that the reintroduction of wt VHL into 786-0 cells regulates tumorigenesis in athymic nude mice (10).
Hepatocyte growth factor/scatter factor (HGF/SF) is a multipotential modulator of diverse biological activities in a variety of normal and cancer cells. Acting through the Met tyrosine kinase receptor, HGF/SF functions as a broad-spectrum mitogen. HGF/SF stimulates cell motility and invasion, acts as an in vitro and in vivo angiogenic factor, and participates as a morphogen in mediating lumen formation and tubulogenesis in various epithelial cells (26–28, 42, 53). Met and HGF/SF have been implicated in many human cancers (16) and it has been demonstrated in several rodent and human model systems that Met-HGF/SF signaling induces invasion in vitro and metastatic behavior in vivo (13–16, 42).
It was shown that HGF/SF and Met are expressed in various tissues, including embryonic and adult kidney, in humans as well as other mammals (12, 35, 39, 42, 50, 54, 59). In the early stages of mouse embryogenesis, cells of the metanephric mesenchyme express both HGF/SF and Met whereas only Met is expressed in the ureteric bud epithelia. This suggests a role for Met signaling in renal development (46, 53, 58). HGF/SF stimulates motility and branching morphogenesis in Madin-Darby canine kidney epithelial cells in vitro (20, 30). In addition, HGF/SF plays a role in renal development and regeneration (17, 32) and is a growth-stimulatory factor for rabbit renal tubular cells (9), but HGF/SF and Met null mouse embryos do not show abnormal kidney development (4).
Here we examined the responsiveness of various RCC cell lines to HGF/SF stimulation. RCC cell lines with mut VHL or their isogenic counterparts ectopically expressing wt VHL were tested. RCC cells with mut VHL exhibit branching morphogenesis and invasiveness in vitro in response to HGF/SF, whereas RCC cells with wt VHL do not. We show that VHL regulates the expression of tissue inhibitors of metalloproteinases (TIMPs) and matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9) and that their dysregulation in RCC cells with mut VHL allows HGF/SF-dependent branching morphogenesis and invasion in vitro to occur. These studies also show that the VHL tumor suppressor gene is a negative regulator of tumor cell invasiveness in vitro.
MATERIALS AND METHODS
Cell lines, antibodies, probes, and reagents.
Tissue culture plates and 8-μm-pore-size polycarbonate filters were obtained from Costar (Cambridge, Mass.). The SK-LMS-1 human leiomyosarcoma (15) and HT1080 human fibrosarcoma cell lines were obtained from the American Type Culture Collection (Rockville, Md.) (40). Human embryonic lung fibroblast (MRC-5) cells were kindly provided by C. Medici (University of Parma Medical School, Parma, Italy). Purified recombinant human HGF/SF was a generous gift from R. Schwall, Genetech, Inc. (South San Francisco, Calif.).
The RCC cell line 786-0 contains a single VHL allele with a frameshift mutation at codon 104 (6). Stable transfectants of 786-0 were generated, and clones containing either vector alone (pRC), pRC containing wt VHL (clones WT-7 and WT-8), or vector containing truncated VHL construct (amino acids 1 to 115) (clones ARZ-2 and ARZ-3) (10) were generated. These cells were continuously grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) supplemented with 1 mg of G418 (Gibco/BRL, Life Technologies, Gaithersburg, Md.) per ml. The A498 RCC cell line contains a single VHL allele with a frameshift mutation at codon 142 (6). Transfected subclones (A498-pRC and A498-WT) were generated and propagated as described for the 786-0 cells (24, 38). The RCC cell line UOK-101 was derived from a clear-cell RCC and contains a single VHL allele with a mutation of the splicing recognition site (6). UOK-101 cells were transduced with retroviral vectors expressing either the wt VHL cDNA (101 wt) or a frameshift mutation at codon 187 (101fs; 7). Transduced cells were maintained in 0.8 mg of G418 per ml.
Immunoprecipitation and phosphotyrosine analysis of the Met receptor.
Subconfluent cell cultures grown in DMEM–10% FBS in 100-mm culture dishes were washed and fed with DMEM–0.1% bovine serum albumin (BSA). After an additional 16 h at 37°C, the cells were fed with fresh DMEM–0.1% BSA alone or supplemented with 200 ng of HGF/SF per ml. The cells were then incubated for 10 min, washed with cold TBS (25 mM Tris [pH 7.5], 150 mM NaCl, 1 mM sodium orthovanadate), and incubated for 15 min on ice with lysis buffer (20 mM PIPES [pH 7.4], 150 mM NaCl, 1 mM EGTA, 1% Triton X-100, 1.5 mM MgCl2) containing protease inhibitors (10 μg of aprotinin per ml, 10 μg of leupeptin per ml, 1 mM phenylmethylsulfonyl fluoride) and 1 mM sodium orthovanadate, plus sodium dodecyl sulfate (SDS) at a final concentration of 0.1%. The C-28 anti-Met antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) was then added to the clarified supernatants, which were subsequently incubated with rotation for 18 h at 4°C. At the end of this incubation period, 50 μl of protein G-agarose (Gibco/BRL) was added to the samples, which were incubated with rotation for an additional 1 h at 4°C. The samples were then washed three times with cold lysis buffer and once with cold TBS. After the addition of sample-loading buffer, boiling, and centrifugation, the supernatants were resolved on 4 to 12% polyacrylamide Tris-glycine gels, transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, N.H.), and subjected to Western analysis with antiphosphotyrosine antibody (anti-P-Tyr) (4G10; Upstate Biotechnology, Inc., Lake Placid, N.Y.).
For Western analysis, membranes were blocked with 5% BSA in rinse buffer (0.15 M NaCl, 20 mM Tris, 0.1% Tween 20) for 1 h, washed in rinse buffer for an additional 10 min, and then incubated with the primary anti-P-Tyr antibody (1 μg/ml). The membranes were then washed and incubated with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary antibody (1:1,000 dilution; Boehringer Mannheim, Indianapolis, Ind.) for 1 h at room temperature, washed for 30 min, and treated with the enhanced chemiluminescence detection system from Amersham (Arlington Heights, Ill.). The membranes were then stripped and reprobed with 1 μg of C-28 anti-Met (Santa Cruz Biotechnology) per ml (41). The MRC-5 and SK-LMS-1 cell lines were used as negative and positive controls, respectively (21).
In vitro invasion and migration assay.
Cell invasion assays through extracellular matrix proteins (ECM) were carried out as described previously with minor modifications (21). Transwell filters (8-μm-pore-size polycarbonate filters; Costar) were coated with growth factor-reduced (GFR) Matrigel (20 μg/filter), collagen type IV (6 μg/filter), or laminin (12 μg/filter) (all obtained from Becton Dickinson, Bedford, Mass.) in 100 μl of cold DMEM to form a thin continuous layer. The filters were then left to air dry overnight. The lower compartment of each transwell unit contained 500 μl of DMEM–0.1% BSA. After overnight starvation in serum-free medium, the cells were harvested by trypsinization and counted, and 104 cells were seeded on top of the filter in a final volume of 100 μl of DMEM–0.1% BSA alone or supplemented with HGF/SF (20 ng/ml). Various neutralizing or control antibodies were also added to the upper chambers of the transwell units, including heat-inactivated neutralizing anti-TIMP-1 or anti-TIMP-2 (final concentrations, 0.5 and 5% [vol/vol]) and two different heat-inactivated preimmune rabbit sera. After a 20-h incubation at 37°C, cells in the upper surface of the filters were removed by careful wiping with a cotton swab and the filters were fixed and stained with Diff-Quick (Dade, Aguada, Puerto Rico). Invasion was determined by counting the cells on the lower surface of the filter by phase-contrast microscopy at ×200 magnification. The total filter for each sample was counted. Each sample was assayed in triplicate, and the assays were repeated at least three times.
Branching-morphogenesis assay.
Semiconfluent cell cultures were washed twice with phosphate-buffered saline (PBS) (Ca2+ and Mg2+ free), and 4 ml of Versene was added before the cultures were incubated for 30 min at 37°C. After centrifugation (5 min at 1,000 × g) at 4°C, 5 × 104 cells in 62.5 μl of DMEM–10% FBS were mixed with an equal volume of nondiluted GFR Matrigel on ice, plated at 125 μl per well in a 96-well culture plate, and incubated for 30 min in 10% CO2 at 37°C. After incubation, 125 μl of DMEM–10% FBS, alone or supplemented with 40 ng of HGF/SF (with or without purified recombinant TIMP-1 [6.4 μg/ml], TIMP-2 [10 μg/ml], or PBS), was placed on top of the gel, and the plate was returned to the incubator. After 48 to 72 h, the representative wells were photographed at ×400 magnification. After this incubation period, the viability of the cells harvested from GFR Matrigel was determined to be >95% by dispase treatment and the trypan blue dye exclusion methods.
Gelatin zymography.
Zymogram analyses were performed by the method of Heussen and Dowdle (8), with modifications. Cells were grown in 100-mm culture plates in DMEM–10% FBS to 75% confluence; the medium was then discarded, and the cultures were incubated for two consecutive 4-h periods with 20 ml of DMEM alone (changed after each incubation period) to eliminate serum proteins. The cultures were then incubated at 37°C for 12, 24, 36, or 48 h in serum-free DMEM supplemented with lactalbumin hydrolysate (0.2%, wt/vol) (Sigma Chemical Co., St. Louis, Mo.) with or without HGF/SF (20 ng/ml). In some experiments, cultures were grown in DMEM supplemented with insulin-transferrin-selenium (Gibco/BRL). The conditioned medium was aspirated and immediately frozen at −70°C until use. A 25-μg portion of protein per sample was treated with 20 mM 4-aminophenylmercuric acetate (APMA) (Sigma), and organomercurial activator of MMP proenzymes, for 2 h at 37°C and analyzed by electrophoresis under nonreducing conditions for the presence of gelatin-degrading enzymes (or a nondenaturing 0.1% SDS–10% polyacrylamide gel containing 1 mg of gelatin per ml as a substrate). The gels were prepared by adding powdered gelatin (Sigma) to the water portion of the resolving gel and heating the mixture to 65°C until it was dissolved. The solution was allowed to cool, the remaining ingredients were added, and the gel was cast as described previously (43). After electrophoresis at 4°C, the gels were immersed twice in 2.5% Triton X-100 with gentle shaking at room temperature for 1 h to remove SDS. The gels were then incubated with a developing buffer (10 mM Tris base, 40 mM Tris-HCl, 200 mM NaCl2, 10 mM CaCl, 0.02% Brij 35, 0.02% NaN3) for 18 h at 37°C. The gels were then stained with 0.1% Coomassie brilliant blue R-250 (Bio-Rad, Richmond, Calif.) in water-methanol-acetic acid (5:5:1, vol/vol/vol) for 45 min. Finally, the gels were destained with methanol (45%, vol/vol)–acetic acid (3%, vol/vol) until the bands were visible. Proteolytic activity was demonstrated by the presence of light bands against the dark blue background. The fibrosarcoma cell line HT1080 was used as a positive control cell line for collagenase type IV expression (49), and purified human MMP-2 and MMP-9 (Chemicon) were used as zymography controls. To confirm the specificity of the enzymatic activity of the MMPs, in some experiments 10 mM 1,10-phenanthroline or EDTA (inhibitors of MMP enzymes) was added to the incubation buffer.
Western analyses were used to detect MMP and TIMP expression in the culture supernatants that were prepared for zymography. For TIMP analyses, the culture supernatants were concentrated 10-fold with Centriprep-10 concentrators (Amicon, Beverley, Mass.). Then 25 μg of protein from each sample was resolved under reducing conditions on a 12% polyacrylamide Tris-glycine gel. Anti-TIMP-1, anti-TIMP-2, anti-MMP-2, and anti-MMP-9 at 1 μg/ml each were used as primary antibodies. The remainder of the protocol was then followed as described above. Comparative densetometric analyses were performed with an Alpha Imager 2000 (Alpha Innotech Corp., San Leandro, Calif.).
RNA isolation and Northern analysis.
Total RNA was prepared from subconfluent cell cultures by using 1 ml of RNAzol B (TEL-TEST, Inc., Friendswood, Tex.) per 100-mm culture plate as specified by the manufacturer. For human urokinase (uPA) and human uPA receptor (uPAR) experiments, subconfluent cultures of VHL-negative or VHL-positive RCC cells were incubated for 7 h at 37°C in DMEM–10% FBS supplemented with HGF/SF (200 ng/ml) or unsupplemented. To analyze Fos mRNA expression, cells were starved overnight or washed with DMEM and then incubated with DMEM alone, DMEM–20% FBS, or HGF/SF (200 ng/ml) for 30 min at 37°C. For MMP-2, MMP-9, TIMP-1, and TIMP-2 mRNA expression analysis, subconfluent cultures of cells were incubated for 12, 24, 36, and 48 h in serum-free DMEM supplemented with lactalbumin hydrolysate (0.2%, wt/vol) with or without HGF/SF (20 ng/ml). Northern analysis was performed as described previously (21). A 15-μg portion of total RNA was subjected to gel electrophoresis in 1.2% formaldehyde–agarose gels and capillary transferred to Hybond-N+ nylon membranes (Amersham) by using 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). After transfer, the RNA was UV cross-linked for 2 min and membranes were hybridized at 42°C for 24 h in a buffer consisting of 50% deionized formamide, 5× SSC, 1× Denhardt’s solution, 50 mM NaH2PO4 (pH 6.5), 150 μg of single-stranded DNA per ml, and 0.5% SDS. The blots were probed or reprobed with 32P-labeled probes prepared by random labeling. The probes included a uPA probe isolated as a 1.5-kb fragment (ATCC 57328), a uPAR probe isolated as a 1.1-kb fragment (ATCC 65768), a 1-kb PstI fragment of pFos-1 (2), and the human cDNA probes for TIMP-1 (ATCC 59666), TIMP-2 (ATCC 79069), MMP-2 (ATCC 79067), and MMP-9 (ATCC 1196364). After hybridization, each membrane was washed twice for 5 min in 0.1% SDS–2× SSC at room temperature, twice for 5 min in 0.1% SDS–0.2× SSC at room temperature, and twice for 15 min in 0.1% SDS–0.2× SSC at 68°C. Autoradiography was performed by exposing X-Omat-AR film (Kodak, Rochester, N.Y.) to the hybridized membrane for 6 h at −70°C in the presence of an intensifying screen. The probe was removed by pouring a boiling solution of 0.1% SDS onto each membrane and allowing it to cool to room temperature. The membranes were reprobed with a human glyceraldehyde 3-phosphate dehydrogenase or β-actin probe to assess sample loading (55).
RESULTS
VHL inhibits HGF/SF-mediated branching morphogenesis and in vitro invasiveness of RCC cells.
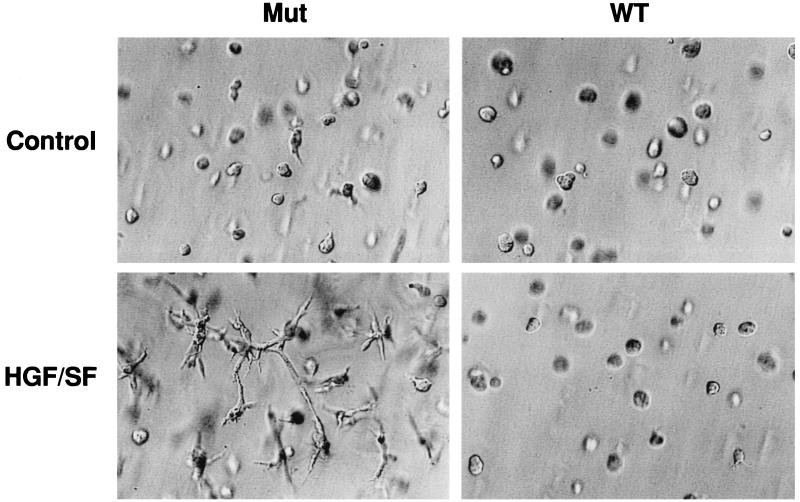
HGF/SF induces a characteristic branching morphogenesis and invasiveness in vitro in many cell types that express the Met receptor (15, 21). We tested multiple independent VHL-negative (mut) RCC cell lines as well as the isogenic VHL-expressing (wt) counterpart of each for branching morphogenesis in three-dimensional (3D) GFR Matrigel plugs. None of the cell lines exhibited branching morphogenesis in the absence of HGF/SF (Table 1; Fig. 1). In contrast, in the presence of HGF/SF, branching was observed in all the VHL-negative cell lines. Two RCC lines, 786-0 and UOK-101, showed particularly marked branching, while the VHL-negative A498 cells showed clear but less dramatic branching. Stable expression of wt VHL abrogated all morphologic response to HGF/SF for all tested cell lines (Table 1; Fig. 1).
TABLE 1.
HGF/SF-dependent branching morphogenesis and invasion
| RCC cell line | VHL status | Branching morphogenesis
|
In vitro invasiona
|
||||
|---|---|---|---|---|---|---|---|
| HGF absent | HGF present | HGF absent | HGF present | HGF presentb
|
|||
| +Neut-T1 | +Neut-T1 and neut-T2 | ||||||
| 786-0 | Mut | − | ++++ | 69 ± 8 | 179 ± 20 | 710 ± 30 | 820 ± 50 |
| WT | − | 11 ± 2 | 16 ± 2.3 | 260 ± 15 | 380 ± 16 | ||
| UOK-101 | Mut | − | ++++ | 116 ± 10 | 305 ± 22 | 1190 ± 40 | 1400 ± 46 |
| WT | − | 25 ± 3 | 39 ± 5 | 450 ± 26 | 570 ± 38 | ||
| A-498 | Mut | − | ++ | 19 ± 2 | 32 ± 4 | 54 ± 7 | 60 ± 7 |
| WT | − | 6 ± 1 | 8 ± 1 | 16 ± 2 | 28 ± 3 | ||
Each value is the mean of three independent experiments ± standard error.
Control rabbit serum had no effect on invasion. Neut-T1, neutralizing TIMP-1 antibody; Neut-T2, neutralizing TIMP-2 antibody.
FIG. 1.
VHL regulation of HGF/SF-mediated RCC cell branching morphogenesis. 786-0 RCC cells were mixed in a GFR Matrigel solution and transferred to tissue culture plates. After a 30-min incubation at 37°C, the cells were fed with DMEM–10% FBS alone (Control) or supplemented with 40 ng of HGF/SF per well. After 3 days, the representative fields were photographed. Magnification, ×348. Each experiment was performed in triplicate, and the assays were repeated three times. WT, VHL-positive 786-0 RCC cells (WT-7); Mut, VHL-negative 786-0 RCC cells (ARZ-2).
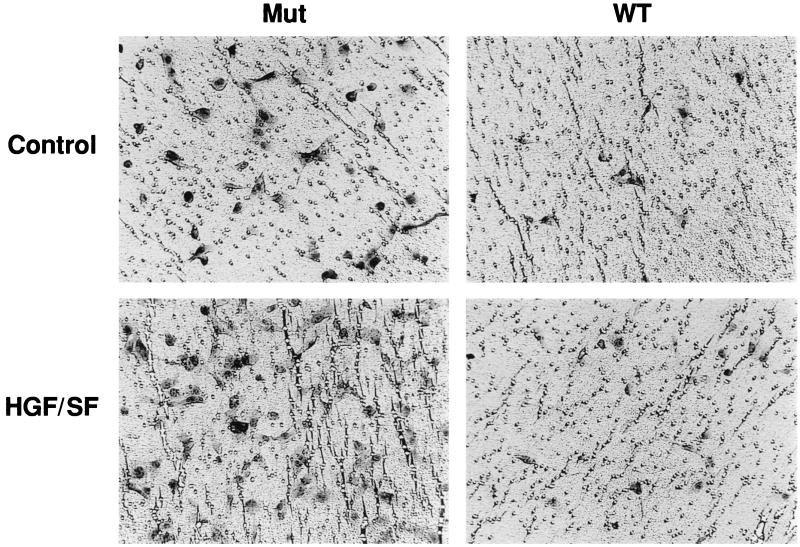
We also tested the same set of RCC cell lines for invasiveness in vitro by using Matrigel-coated filters. The basal level of invasiveness of the VHL-negative 786-0 and UOK-101 cells was relatively high and was markedly stimulated by the addition of HGF/SF (Table 1; Fig. 2). As in the branching-morphogenesis assay, the VHL-negative A498 cells were only weakly invasive in the presence of HGF/SF. The expression of wt VHL dramatically reduced the invasiveness of all of the RCC cell lines (Table 1).
FIG. 2.
VHL regulation of HGF/SF-mediated RCC cell invasion in vitro. After overnight serum starvation, 104 786-0 cells were placed on top of the transwell filters coated with GFR Matrigel (20 μg) in a final volume of 100 μl of DMEM–0.1% BSA alone (Control) or supplemented with HGF/SF (20 ng/ml). The lower compartment of each transwell unit contained 500 μl of DMEM. After a 20-h incubation, the noninvading cells on the upper surface of the filter were removed and the invasive cells attached to the lower surface of the filter were stained. A representative field was photographed. Magnification, ×172. Each sample was assayed in triplicate, and the assays were repeated three times. Mut, VHL-negative 786-0 RCC cells (ARZ-2); WT, VHL-positive 786-0 RCC cells (WT-7).
Met expression in VHL cells.
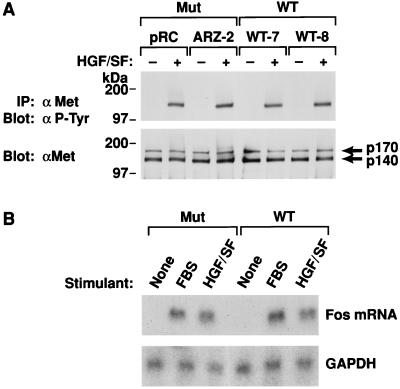
The results presented above could be due to differential Met expression in VHL-positive and VHL-negative RCC cells (these RCC cells do not express endogenous HGF/SF [data not shown]). Met expression was determined by immunoprecipitation from cell lysates with the C-28 anti-Met antibody followed by Western analysis (Fig. 3A). Both the 786-0 VHL-positive (WT-7 and WT-8) and VHL-negative (ARZ-2 and pRC) RCC cells expressed similar levels of p170 and p140 Met (Fig. 3A). In addition, the Met autophosphorylation response after HGF/SF treatment was similar, based on p140 reactivity with anti-P-Tyr antibody (Fig. 3A).
FIG. 3.
Met expression and signaling in 786-0 RCC cells. (A) 786-0 RCC cells, after overnight serum deprivation, were washed and fed with fresh DMEM–0.1% BSA alone or supplemented with 200 ng of HGF/SF per ml for 10 min, and 0.5 mg of cell lysate was immunoprecipitated with anti-Met C-28 and subjected to SDS-polyacrylamide gel electrophoresis and immunoblotting with anti-P-Tyr (top). The membrane was then stripped and reprobed with anti-C-28 antibody (bottom). Mut, VHL-negative RCC cells; WT, VHL-positive 786-0 RCC cells. (B) Fos induction in VHL-negative or -positive 786-0 RCC. Subconfluent cultures of cells were serum starved overnight and incubated for 30 min with DMEM alone or supplemented with either 200 ng of HGF/SF per ml or 20% FBS. Northern analysis was performed as detailed in Materials and Methods. A 1-kb PstI fragment of pFos-1 was used as the probe, and an equal amount of loading per lane was demonstrated by reprobing the membrane with glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Mut, VHL-negative 786-0 RCC cells (ARZ-2); WT, VHL-positive 786-0 RCC cells (WT-7).
We also examined HGF/SF-Met-mediated fos mRNA induction. After a 30-min treatment of quiescent cells with either HGF/SF or serum, fos mRNA was upregulated to similar levels in both VHL-positive and VHL-negative RCC cells (Fig. 3B). In addition, serum-starved VHL-positive and VHL-negative RCC cells treated with HGF/SF for 18 h and then pulsed for 5 h with [3H]thymidine exhibited similar DNA synthesis activity (data not shown). Thus, neither the level of Met expression nor HGF/SF signaling at the levels of fos induction or DNA synthesis could account for differences in VHL-negative and VHL-positive RCC cell invasiveness.
Effect of VHL on extracellular proteases and their regulators.
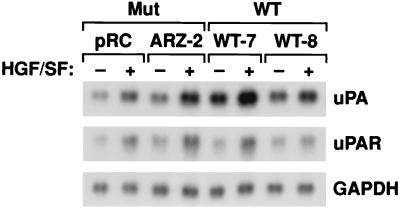
Met-HGF/SF signaling has been shown to induce the expression and activation of the urokinase/plasminogen proteolytic network (15). We used Northern analysis to test whether uPA and uPAR are differentially elevated in VHL-positive and VHL-negative RCC cells in response to HGF/SF (Fig. 4). As in other cell types (15), both uPA and uPAR levels increased in response to HGF/SF, but the increases were similar in both the VHL-positive (WT-7 and WT-8) and VHL-negative (ARZ-2 and pRC) 786-0 RCC cells (Fig. 4). Thus, uPA and uPAR may influence the invasive activity of VHL-negative RCC, but they cannot be responsible for the lack of branching morphogenesis and in vitro invasion activity in the VHL-positive RCC cells.
FIG. 4.
uPA and uPAR induction in 786-0 RCC cells. Subconfluent cultures of cells were incubated for 7 h at 37°C in DMEM–10% FBS supplemented with 200 ng of HGF/SF per ml or unsupplemented. Total RNA extraction and Northern hybridization were performed as described in Materials and Methods. The blots were probed or reprobed with a 32P-labeled human uPA probe, a human uPAR probe, and a human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) probe (52). Mut, VHL-negative 786-0 RCC cells; WT, VHL-positive 786-0 RCC cells.
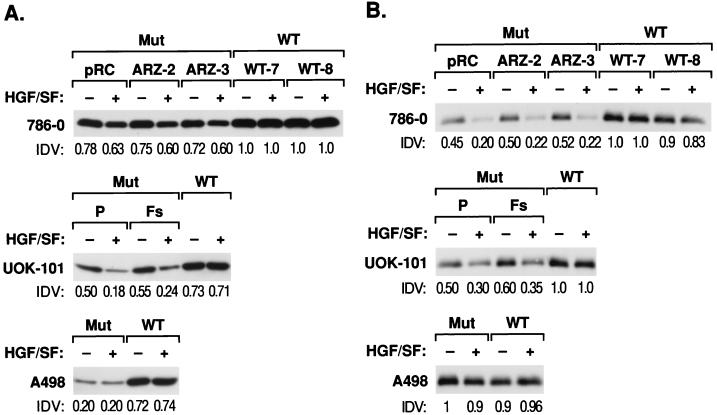
MMPs are responsible for the degradation of ECM and have been associated with cellular invasiveness (48). Their activities, in turn, are regulated by TIMPs. We therefore examined whether VHL expression was able to influence the expression and/or activities of these molecules in the various RCC cell lines. In the cell lines derived from either 786-0 or UOK-101, there were two differences in the expression of TIMP-2 after the stable introduction of wt VHL. First, there was a small but consistent elevation in the baseline levels of TIMP-2 protein with wt VHL (Fig. 5A). Second, and more striking, there was a difference in the response to added HGF/SF. In each of the mut VHL cell lines derived from these two RCC lines, the addition of HGF/SF resulted in a reduction in TIMP-2 protein (20%). Expression of wt VHL abrogated the effect of HGF/SF (Fig. 5A). In the A498 cells, the expression of wt VHL resulted in a significant elevation of TIMP-2 protein levels (50%). In contrast to the two other VHL-negative RCC cell lines, HGF/SF had no effect on TIMP-2 levels in A498 (Fig. 5A).
FIG. 5.
TIMP expression in RCC cells and the influence of HGF/SF. Western analysis of TIMP-2 (A) and TIMP-1 (B) proteins in culture supernatants was performed. Subconfluent cultures in DMEM–0.1% BSA were incubated in the presence or absence of 20 ng of HGF per ml for 12, 24, 36, or 48 h. Only the 36-h sample is presented. Conditioned medium was concentrated and 25 μg of protein was resolved under reducing conditions on an SDS–12% polyacrylamide gel. Western analysis was performed as described in Materials and Methods. Anti-TIMP-2 (A) and TIMP-1 (B) at 1 μg/ml were used as primary antibodies. Mut, VHL-negative RCC; WT, VHL-positive RCC. pRC, ARZ-2, and ARZ-3 are VHL-negative 786-0 RCC cells. P (parental) and Fs are VHL-negative UOK-101 RCC cells; WT is an VHL-positive RCC cell line. IDV relative integrated density value. Comparative densitometric analyses were performed with an Alpha Imager 2000 version 3.2. All values were normalized to 786-0 WT-7.
We also looked at the expression of the related protein, TIMP-1. For the 786-0- and UOK-101-derived cell lines, the results paralleled that seen for TIMP-2, with elevated baseline levels of protein (45%) and abrogation of the HGF/SF-induced diminution of TIMP-1 secretion seen in lines expressing wt VHL (Fig. 5B). In contrast to 786-0 and UOK-101 cells, expression of TIMP-1 in A498 cells was not effected by HGF/SF or wt VHL.
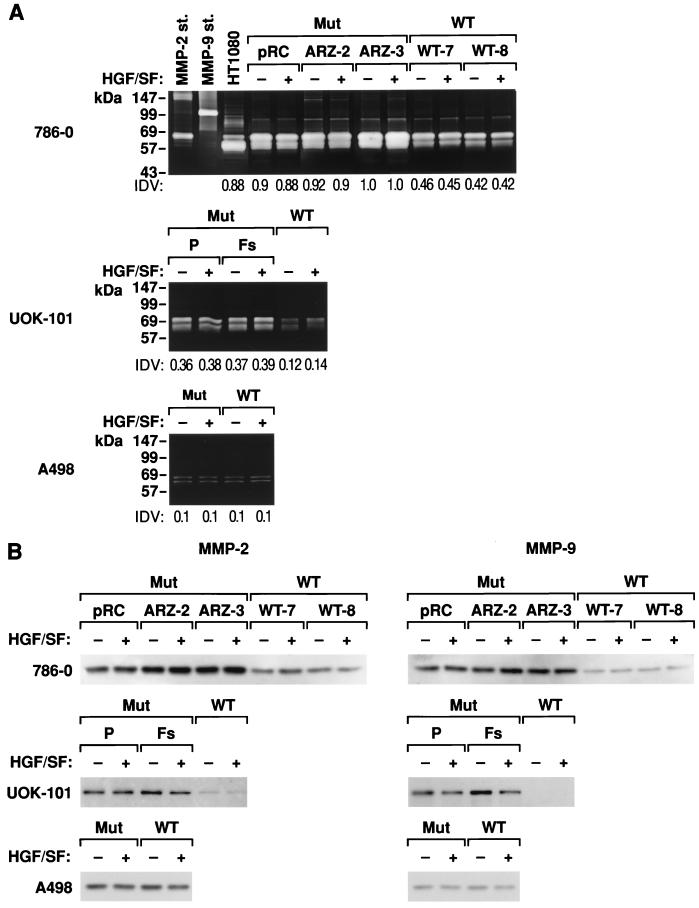
We next examined whether either HGF or VHL effected the expression of MMPs in RCC cells. We first examined MMP activity by substrate zymography of serum-free conditioned media prepared from VHL-negative and VHL-positive RCC cells. Readily detectable levels of gelatinase activity were detected in VHL-negative 786-0 and UOK-101 RCC cells (Fig. 6A). The addition of HGF/SF had no effect on gelatinase activity by these cells. Expression of wt VHL significantly reduced the level of gelatinase activity from both of these cell lines (50%). The less invasive A498 cells showed significantly lower levels of secreted gelatinase activity, and this was not affected after the introduction of wt VHL. MMP expression was further examined by Western blot analysis. Specific antibodies identified the two bands on the zymography gels as MMP-2 or gelatinase A (62 kDa) and MMP-9 or gelatinase B (86 kDa). Once again, the effect of the expression of wt VHL in both the 786-0 and UOK-101 in reducing the expression of both MMP-2 and MMP-9 was apparent (Fig. 6B). These results also confirm the lack of effect of expression of wt VHL in the A498 cells.
FIG. 6.
MMP activity in VHL-positive and VHL-negative RCC. (A) Conditioned medium from HT1080 fibrosarcoma cells was used as a positive control (49). Gelatin zymography of serum-free conditioned medium collected from cells incubated at 37°C for 12, 24, 36, or 48 h in DMEM supplemented with 0.2% (wt/vol) lactalbumin hydrolysate with or without HGF/SF (20 ng/ml) is shown. Only the 36-h sample is shown. Culture supernatants were processed, and 25 μg was subjected to gelatin zymography under nonreducing and nondenaturing conditions. Prestained marker proteins showed approximate molecular masses as indicated. Proteolytic activity, the light bands against the stained background, correspond to MMP-2 and MMP-9. The MMP-2 bands appear as doublets, representing the heavier, inactive form and the fully activated molecule. Linearity of the enzyme-substrate reaction was demonstrated by serial dilution of APMA-activated supernatant. At any dilution, the proteolytic band in the VHL-negative cells was higher than in the VHL-positive cells. In parallel experiments we used either 10 mM 1,10-phenathroline or EDTA to inhibit MMP activity. This completely blocked gelatinase activity (data not shown). The HT1080 fibrosarcoma cell line was used as a positive control for collagenase expression, as was purified human MMP-2 and MMP-9. Comparative densitometric analyses were performed with an Alpha Imager 2000; all values were normalized to VHL-negative ARZ-3 cells. pRC, ARZ-2, and ARZ-3 are VHL-negative 786-0 RCC cell lines; P (parental) and Fs are VHL-negative UOK-101 RCC cell lines; WT is a VHL-positive RCC cell line. (B) Western analysis of MMP-2 and MMP-9 in culture supernatant was performed as in Figure 5 captions. A representative immunoblot at 36 h is shown here. Cell lines are as in panel A.
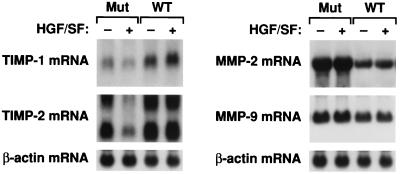
We asked whether the protein expression changes in both TIMPs and MMPs were reflected at the mRNA levels. Shown in Fig. 7 are the results for 786-0 cells. Northern blot analysis qualitatively mirrors the protein expression results for TIMP-1, TIMP-2, MMP-2, and MMP-9. The introduction of wt VHL elevates mRNA levels for the TIMPs and abrogates the HGF/SF effect and lowers the mRNA levels for the two MMPs.
FIG. 7.
Expression of TIMP and MMP mRNA in VHL-positive and VHL-negative RCC cells. Subconfluent cultures of 786-0 RCC cells were incubated for 12, 24, 36, or 48 h in serum-free medium supplemented with HGF/SF (20 ng/ml) or unsupplemented. Only results for the 36-h samples are presented. Total RNA extraction and Northern hybridization were performed as described in Materials and Methods. The blots were probed or reprobed with a 32P-labeled human TIMP-1, TIMP-2, MMP-2, MMP-9, and β-actin probe. Mut, VHL-negative 786-0 RCC cells (ARZ-2); WT, VHL-positive 786-0 RCC cells (WT-7).
Altered TIMP levels may be responsible for the effect of VHL on branching and invasiveness.
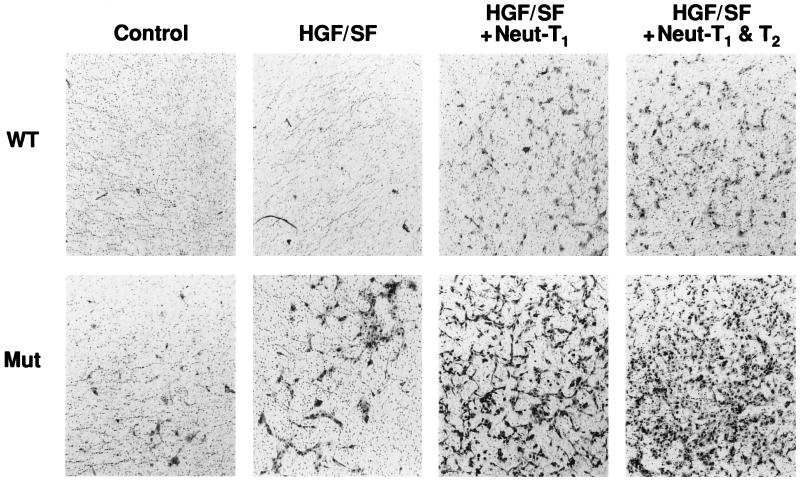
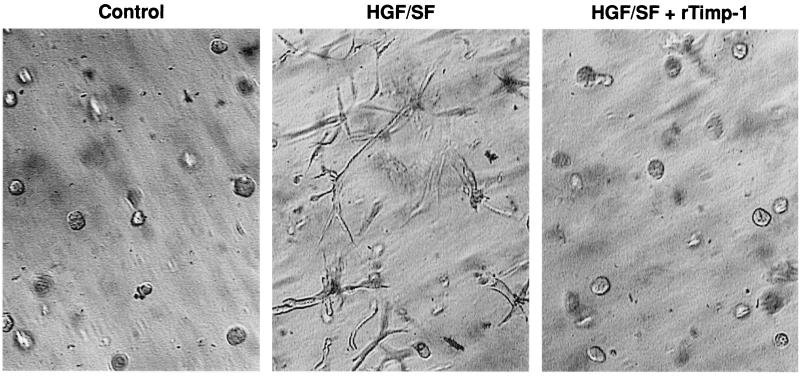
We next asked whether the altered expression of TIMPs could explain, at least in part, the effects of wt VHL on the branching morphogenesis and in vitro invasiveness of the RCC cells. We focused on the TIMPs because VHL altered the expression of TIMP-2 in all three RCC cell lines used. Neutralizing antibodies to either TIMP-1 or TIMP-2 significantly enhanced the invasiveness of both VHL-negative and VHL-positive cells (Fig. 8; Table 1). This was a dose-dependent phenomenon and suggested that increased levels of TIMPs secreted from VHL-positive RCC cells could explain part of the differences in these phenotypes from those of their VHL-negative counterparts. That elevated levels of TIMPs could control invasiveness was tested by the addition of recombinant TIMPs to the 3D GFR Matrigel cultures (Fig. 9). The addition of either TIMP-1 or TIMP-2 to these cultures completely abolished the HGF/SF-stimulated branching morphogenesis of either VHL-negative 786-0 or UOK-101 cells. Taken together, these results strongly suggest that TIMPs and MMPs play a key role in both HGF/SF-stimulated morphogenesis and invasiveness and in the effect of wt VHL expression on these processes.
FIG. 8.
TIMP-1 and TIMP-2 neutralizing antibodies and 786-0 RCC cell in vitro invasiveness. Invasion assays were performed in collagen type IV by the method described in the legend to Fig. 2. Heat-inactivated neutralizing rabbit anti-human TIMP-1 and TIMP-2 antibodies (or two different preimmune rabbit sera) were used at 5% (vol/vol) dilutions in the upper compartment. A greater number of invading cells per filter in the VHL-negative cells than in the VHL-positive cells in the presence of HGF/SF (20 ng/ml) and neutralizing TIMP-1 (Neut-T1) alone or in combination with neutralizing TIMP-2 (Neut-T2) was found (see Table 1). WT, VHL-positive 786-0 RCC cells (WT-7); Mut, VHL-negative 786-0 RCC cells (ARZ-2).
FIG. 9.
TIMP-1 and TIMP-2 and branching morphogenesis in VHL-positive and VHL-negative RCC cells. Branching morphogenesis assays on 786-0 RCC cells were performed as described in the legend to Fig. 1. TIMP-1 (6.4 μg/ml) or TIMP-2 (10 μg/ml) were included in both the gel and the supernatant. Control wells received equal volumes of PBS. The assay was performed for all wt and mut VHL cell lines (data shown for only VHL-negative 786-0 RCC cells). For any cell line, treatment of cells expressing wt VHL with TIMP-1 or TIMP-2 resulted in no response (data not shown). Each experiment was performed in quadruplicate, and the assays were repeated three times.
DISCUSSION
Tumor invasion and metastasis are complex, multistep processes requiring the proteolytic degradation of the basement membrane and tissue matrix, cell motility, and the attachment and detachment of cells to ECM (1, 23). Our data suggest a model whereby tumorigenesis resulting from VHL inactivation may be explained, at least in part, by dysregulation of matrix MMPs and their inhibitors. The invasive potential of a cell is controlled by gene products that regulate cell adhesion, the activation and secretion of proteases, the induction of cell motility, and the stimulation of cell growth. Many of these phenotypes have been associated with Met-HGF/SF signaling, both in vivo and in vitro (16). We found that HGF/SF induced branching morphogenesis in 3D GFR Matrigel in VHL-negative RCC cells but not in RCC cells expressing wt VHL (Fig. 1) and that invasion, both basal level and HGF/SF mediated, was significantly greater in VHL-negative RCC cells (Fig. 2 and 8; Table 1).
Degradation of the ECM is essential for tumor cell invasion and can result from an imbalance between matrix-degrading enzymes and their inhibitors (48). We analyzed the expression of three major families of basement membrane and ECM-degrading enzymes in VHL-positive and VHL-negative RCC cells. There were no apparent differences among the cells tested in the expression of cathepsins B and L (lysosomal cysteine proteinases [data not shown]), and HGF/SF stimulation resulted in increased uPA and uPAR mRNA expression irrespective of the VHL status of the cell (Fig. 4). Therefore, while uPA can contribute to invasion of RCC cells, it cannot account for the differences in invasion and branching morphogenesis exhibited by VHL-positive and VHL-negative RCC cells.
We found that TIMP-2 expression is lower in all three VHL-negative RCC cell lines than in their VHL-positive isogenic counterparts. In addition, the more invasive 786-0 and UOK-101 RCC cells showed lower TIMP-1 levels and elevated levels of MMP-2 and MMP-9, changes that are consistent with the enhanced branching morphogenesis and in vitro invasiveness activity of these RCC cell lines. Similar results for the secreted TIMPs and MMPs were also observed at the mRNA level (Fig. 7). These changes are likely to reflect additional alterations downstream of VHL inactivation, which can contribute to tumor progression. Moreover, treatment of wt and mut VHL 786-0 cells with actinomycin D showed no differences in turnover of mRNA for TIMP-1 and TIMP-2 (data not shown). Thus, the net proteolytic activity increased in VHL-negative cells relative to VHL-positive cells. However, it is interesting that wt VHL not only affects TIMP-2 in all cells tested but also elevates the expression of TIMP-1 and reduces the expression MMPs in 786-0 and UOK-101 RCC cells to levels comparable to those found in A498 RCC cells.
MMP-mediated proteolysis is regulated by naturally occurring TIMPs (57), and decreased levels of TIMP expression have been found in invading tumor cells and tumor metastases (18, 44, 51). Such conditions should favor invasion and branching morphogenesis. Our results supported this by showing that treatment of the VHL-negative RCC cells with biologically active recombinant TIMPs abolished branching morphogenesis; conversely, treatment of either VHL-positive or VHL-negative RCC cells with neutralizing TIMP antibodies enhanced invasion through GFR Matrigel and collagen type IV. MMP activity was already maximal in VHL-negative cells, and HGF/SF stimulation did not change the collagenolytic activity in any of the cells tested. Moreover, Met-HGF/SF signaling in VHL-negative RCC cells could not circumvent TIMP inhibition of in vitro invasion.
Collagen type IV is a major component of the basement membrane and ECM of renal cell tumors of various types and grades of malignancy (3). Of the two major components of GFR Matrigel, collagen type IV and laminin, the former is the critical matrix component through which VHL-negative RCC cells invade (Fig. 8). The production of gelatinases (type IV collagenases) is directly correlated with the invasiveness and metastatic potential of several human and rodent tumor cell lines (31, 33). The expression and enzymatic activities of members of the MMP family, gelatinase A (MMP-2) and gelatinase B (MMP-9), were elevated in the VHL-negative 786-0 and UOK-101 RCC cells relative to the VHL-positive cells tested. Relevant to our in vitro data, an inverse correlation between MMP-2 expression levels and RCC patient survival has been demonstrated (56). Moreover, it has been reported that type IV collagenase activity strongly determines the capacity of RCC cells for in vitro invasiveness and metastatic potential (34, 36, 37).
The consequences of VHL loss characterized in this paper are both puzzling and illuminating. One critical phenotype of cancer is its ability to invade, spread, and metastasize. There is good reason to argue that the dysregulation of MMPs and their inhibitors in RCCs described in this paper is a reflection of this phenotype in vivo. What is surprising is that the phenotype for invasion is linked to the loss of the VHL tumor suppressor gene and can be reversed by the reintroduction of wt VHL. We generally think of tumor cell invasiveness as a late phenotype in the pathway of tumor development. However, these results suggest that the loss of the same tumor suppressor gene that predisposes to the development of RCC and that occurs very early in the process of RCC development is linked to both invasiveness (this paper) and angiogenesis (5, 11, 45). These VHL-associated phenotypes would not be those predicted for a gene whose function should protect cells from the initial steps in the development of cancer.
How might we think about these observations in terms of RCC development? We can consider two models. First, VHL loss primarily affects phenotypes that we traditionally associate with late stages of tumor development and it simply does not matter in which order the collection of genetic changes occur in cancer development. Alternatively, VHL normally is involved in the coordinated regulation of multiple cellular phenotypes whose dysregulation we associate with many different stages of cancer development, from initiation to progression. These include dysregulated growth and survival, altered cell-cell communication, altered responses to local environmental signals such as hypoxia, angiogenesis, invasion, and metastasis. These are cellular processes that must be coordinately regulated to develop, repair, and replace normal tissue architecture. A central role of the VHL tumor suppressor gene as a master coordinator of normal tissue behavior in renal epithelial cells is consistent with the growing list of pathways dysregulated by the loss of VHL (5, 7, 11, 19, 38).
Even if we accept the involvement of VHL in multiple phenotypes associated with both tumor progression and tumor behavior, we still need to account for the consequences of the additional genetic changes that follow VHL loss and that are required to develop cancer. Insight into the nature of some of these additional changes is suggested by the variations in the invasiveness phenotype displayed by the different RCC cell lines. The A498 RCC cells are clearly less “invasive” in these in vitro assays. While they show VHL loss and the associated effect on TIMP-2 expression, these cells do not show the VHL-associated changes in the expression of TIMP-1 and MMP-2 and MMP-9 seen in the 786-0 and UOK-101 RCC cells. It is unlikely that these differences are due to differences in VHL mutations. Thus, UOK-101Fs is transfected with a VHL cDNA harboring a codon 187 frameshift mutation and shows the same dysregulation of TIMPs and MMPs as the parental UOK-101P (Fig. 5 and 6), while the frameshift mutation in A498 resides at codon 142. We can, however, speculate that additional genetic or epigenetic changes in the latter cell lines affecting the expression of TIMP-1 and the MMPs effectively “unmask” the effect of the loss of VHL on those genes. Thus, VHL loss may be necessary and sufficient for the altered expression of TIMP-2, as seen in every VHL-deficient RCC cell line tested, while VHL loss is necessary but not sufficient for the altered expression of TIMP-1, MMP-2, and MMP-9 (Fig. 5B, 6, and 7). Of course, all of these RCC lines have multiple genetic alterations, and the effects for which VHL loss is both necessary and sufficient will have to be established. While the resolution of these speculations requires direct experimental proof, the studies presented here demonstrate that the loss of a specific tumor suppressor gene is critical to the ability of these tumor cells to degrade and move through the ECM. Only after VHL is lost can Met-HGF/SF signaling stimulate branching morphogenesis and invasiveness in vitro. These combined in vitro activities would be expected to enhance tumor growth and invasion in vivo.
ACKNOWLEDGMENTS
We thank Linda Miller and Marianne Oskarsson for technical support. We are also grateful to Richard Frederickson for the artwork and photography and to Ave Cline for typing the manuscript.
This research was sponsored in part by the VHL Family Alliance (J.R.G.), The Murray Foundation (J.R.G.), National Institute of Health grant CA783356 (J.R.G.), and the National Cancer Institute, DHHS, under contract with ABL.
REFERENCES
- 1.Aznavoorian S, Murphy A N, Stetler-Stevenson W G, Liotta L A. Molecular aspects of tumor cell invasion and metastasis. Cancer. 1993;71:1368–1383. doi: 10.1002/1097-0142(19930215)71:4<1368::aid-cncr2820710432>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Curran T, Peters G, Van Beveren C, Teich N M, Verma I M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982;44:674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Droz D, Patey N, Paraf F, Chretien Y, Gogusev J. Composition of extracellular matrix and distribution of cell adhesion molecules in renal cell tumors. Lab Investig. 1994;71:710–718. [PubMed] [Google Scholar]
- 4.Friedhelm B, Riethmacher S, Isenmann S, Aaguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 5.Gnarra J R, Duan D, Weng Y, Humphrey J S, Chen D Y, Lee S, Pause A, Dudley C F, Latif F, Kuzmin I, Schmidt L, Duh F M, Stackhouse T, Chen F, Kishida T, Wei M H, Lerman M I, Zbar B, Klausner R D, Linehan W M. Molecular cloning of the von Hippel-Lindau tumor suppressor gene and its role in renal carcinoma. Biochim Biophys Acta. 1996;1242:201–210. doi: 10.1016/0304-419x(95)00012-5. [DOI] [PubMed] [Google Scholar]
- 6.Gnarra J R, Tory K, Weng Y, Schmidt L, Wei M H, Li H, Latif F, Liu S, Chen F, Duh F-M, Lubensky I, Duan D R, Florence C, Pozzatti R, Walther M M, Bander N H, Grossman H B, Brauch H, Pomer S, Brooks J D, Isaacs W B, Lerman M I, Zbar B, Linehan W M. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 7.Gnarra J R, Zhou S, Merrill M J, Wagner J R, Krumm A, Papavassiliou E, Oldfield E H, Klausner R D, Linehan W M. Post-transcriptional regulation of vascular endothelial growth factor mRNA by the product of the VHL tumor suppressor gene. Proc Natl Acad Sci USA. 1996;93:10589–10594. doi: 10.1073/pnas.93.20.10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heussen C, Dowdle E B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- 9.Igawa T, Kanda S, Kanetake H, Saitoh Y, Ichihara A, Tomita Y, Nakamura T. Hepatocyte growth factor is a potent mitogen for cultured rabbit renal tubular epithelial cells. Biochem Biophys Res Commun. 1991;174:831–838. doi: 10.1016/0006-291x(91)91493-v. [DOI] [PubMed] [Google Scholar]
- 10.Iliopoulos O, Kibel A, Gray S, Kaelin W G J. Tumour suppression by the human von Hippel-Lindau gene product. Nat Med. 1995;1:822–826. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- 11.Iliopoulos O, Levy A P, Jiang C, Kaelin W G, Jr, Goldberg M A. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci USA. 1996;93:10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyer A, Kmiecik T E, Park M, Daar I, Blair D, Dunn K J, Sutrave P, Ihle J N, Bodescot M, Vande Woude G F. Structure, tissue-specific expression, and transforming activity of the mouse met protooncogene. Cell Growth Differ. 1990;1:87–95. [PubMed] [Google Scholar]
- 13.Jeffers M, Fiscella M, Webb C P, Anver M, Koochekpour S, Vande Woude G F. The mutationally activated Met receptor mediates motility and metastasis. Proc Natl Acad Sci USA. 1998;95:14417–14422. doi: 10.1073/pnas.95.24.14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeffers M, Rong S, Oskarsson M, Anver M, Vande Woude G F. Autocrine hepatocyte growth factor/scatter factor-Met signaling induces transformation and the invasive/metastatic phenotype in C127 cells. Oncogene. 1996;13:853–861. [PubMed] [Google Scholar]
- 15.Jeffers M, Rong S, Vande Woude G F. Enhanced tumorigenicity and invasion-metastasis by hepatocyte growth factor/scatter factor-Met signalling in human cells concomitant with induction of the urokinase proteolysis network. Mol Cell Biol. 1996;16:1115–1125. doi: 10.1128/mcb.16.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeffers M, Rong S, Vande Woude G F. Hepatocyte growth factor/scatter factor-Met signalling in tumorigenicity and invasion/metastasis. J Mol Med. 1996;74:505–513. doi: 10.1007/BF00204976. [DOI] [PubMed] [Google Scholar]
- 17.Kawaida K, Matsumoto K, Shimazu H, Nakamura T. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci USA. 1994;91:4357–4361. doi: 10.1073/pnas.91.10.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khokha R, Waterhouse P, Yagel S, Lala P K, Overall C M, Norton G, Denhardt D T. Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science. 1989;243:947–950. doi: 10.1126/science.2465572. [DOI] [PubMed] [Google Scholar]
- 19.Knebelmann B, Ananth S, Cohen H T, Sukhatme V P. Transforming growth factor alpha is a target for the von Hippel-Lindau tumor suppressor. Cancer Res. 1998;58:226–231. [PubMed] [Google Scholar]
- 20.Konishi T, Takehara T, Tsuji T, Ohsato K, Matsumoto K, Nakamura T. Scatter factor from human embryonic lung fibroblasts is probably identical to hepatocyte growth factor. Biochem Biophys Res Commun. 1991;180:765–773. doi: 10.1016/s0006-291x(05)81131-3. [DOI] [PubMed] [Google Scholar]
- 21.Koochekpour S, Jeffers M, Rulong S, Klineberg E, Taylor G, Hudson E A, Resau J H, Vande Woude G F. Met and hepatocyte growth factor/scatter factor expression in human gliomas. Cancer Res. 1997;57:5391–5398. [PubMed] [Google Scholar]
- 22.Linehan W M, Lerman M I, Zbar B. Identification of the von Hippel-Lindau (VHL) gene. Its role in renal cancer. JAMA. 1995;273:564–570. [PubMed] [Google Scholar]
- 23.Liotta L A. Tumor invasion and metastases—role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986;46:1–7. [PubMed] [Google Scholar]
- 24.Lonergan K M, Iliopoulos O, Ohh M, Kamura T, Conaway R C, Conaway J W, Kaelin W G., Jr Regulation of hypoxia-inducible mRNAs by the von Hippel-Lindau tumor suppressor protein requires binding to complexes containing elongins B/C and Cul2. Mol Cell Biol. 1998;18:732–741. doi: 10.1128/mcb.18.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maher E R, Kaelin W G., Jr von Hippel-Lindau disease. Medicine (Baltimore) 1997;76:381–391. doi: 10.1097/00005792-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto K, Matsumoto K, Nakamura T, Kramer R H. Hepatocyte growth factor/scatter factor induces tyrosine phosphorylation of focal adhesion kinase (p125FAK) and promotes migration and invasion by oral squamous cell carcinoma cells. J Biol Chem. 1994;269:31807–31813. [PubMed] [Google Scholar]
- 27.Matsumoto K, Nakamura T. Hepatocyte growth factor: molecular structure, roles in liver regeneration, and other biological functions. Crit Rev Oncog. 1992;3:27–54. [PubMed] [Google Scholar]
- 28.Matsumoto K, Nakamura T. Roles of HGF as a pleiotropic factor in organ regeneration. In: Goldberg I D, Rosen E M, editors. Hepatocyte growth factor-scatter factor and the Met Receptor. Vol. 65. Basel, Switzerland: Birkhauser-Verlag; 1993. pp. 225–250. [PubMed] [Google Scholar]
- 29.Millan J C. Tumors of the kidney. In: Hill G S, editor. Uropathology. Vol. 2. New York, N.Y: Churchill Livingstone; 1989. pp. 623–701. [Google Scholar]
- 30.Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- 31.Morikawa K, Walker S M, Nakajima M, Pathak S, Jessup J M, Fidler I J. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 1988;48:6863–6871. [PubMed] [Google Scholar]
- 32.Nagaike M, Hirao S, Tajima H, Noji S, Taniguchi S, Matsumoto K, Nakamura T. Renotropic functions of hepatocyte growth factor in renal regeneration after unilateral nephrectomy. J Biol Chem. 1991;266:22781–22784. [PubMed] [Google Scholar]
- 33.Nakajima M, Welch D R, Belloni P N, Nicolson G L. Degradation of basement membrane type IV collagen and lung subendothelial matrix by rat mammary adenocarcinoma cell clones of differing metastatic potentials. Cancer Res. 1987;47:4869–4876. [PubMed] [Google Scholar]
- 34.Nakayama Y, Naito S, Ryuto M, Hata Y, Ono M, Sueishi K, Komiyama S, Itoh H, Kuwano M. An in vitro invasion model for human renal cell carcinoma cell lines mimicking their metastatic abilities. Clin Exp Metastasis. 1996;14:466–474. doi: 10.1007/BF00128963. [DOI] [PubMed] [Google Scholar]
- 35.Natali P G, Prat M, Nicotra M R, Bigotti A, Olivero M, Comoglio P M, Di Renzo M F. Overexpression of the met/HGF receptor in renal cell carcinomas. Int J Cancer. 1996;69:212–217. doi: 10.1002/(SICI)1097-0215(19960621)69:3<212::AID-IJC11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 36.Otani N, Tsukamoto T, Masumori N, Saiki I, Yoneda J, Kumamoto Y. Influence of growth factors on in vitro invasiveness and type IV collagenolysis of human renal cell carcinoma cells. J Urol. 1994;151:223–226. doi: 10.1016/s0022-5347(17)34921-2. [DOI] [PubMed] [Google Scholar]
- 37.Otani N, Tsukamoto T, Saiki I, Yoneda J, Mitaka T, Kumamoto Y. In vitro invasive potential and type IV collagenolytic activity of human renal cell carcinoma cells derived from primary and metastatic lesions. J Urol. 1993;149:1182–1185. doi: 10.1016/s0022-5347(17)36343-7. [DOI] [PubMed] [Google Scholar]
- 38.Pause A, Lee S, Lonergan K M, Klausner R D. The von Hippel-Lindau tumor suppressor gene is required for cell cycle exit upon serum withdrawal. Proc Natl Acad Sci USA. 1998;95:993–998. doi: 10.1073/pnas.95.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prat M, Narsimhan R P, Crepaldi T, Nicotra M R, Natali P G, Comoglio P M. The receptor encoded by the human c-MET oncogene is expressed in hepatocytes, epithelial cells and solid tumors. Int J Cancer. 1991;49:323–328. doi: 10.1002/ijc.2910490302. [DOI] [PubMed] [Google Scholar]
- 40.Rong S, Jeffers M, Resau J H, Tsarfaty I, Oskarsson M, Vande Woude G F. Met expression and sarcoma tumorigenicity. Cancer Res. 1993;53:5355–5360. [PubMed] [Google Scholar]
- 41.Rong S, Oskarsson M, Faletto D, Tsarfaty I, Resau J H, Nakamura T, Rosen E, Hopkins R F, Vande Woude G F. Tumorigenesis induced by co-expression of human hepatocyte growth factor and the human met protooncogene leads to high levels of expression of the ligand and receptor. Cell Growth Differ. 1993;4:563–569. [PubMed] [Google Scholar]
- 42.Rong S, Segal S, Anver M, Resau J H, Vande Woude G F. Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci USA. 1994;91:4731–4735. doi: 10.1073/pnas.91.11.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 44.Schultz R M, Silberman S, Persky B, Bajkowski A S, Carmichael D F. Inhibition by human recombinant tissue inhibitor of metalloproteinases of human amnion invasion and lung colonization by murine B16-F10 melanoma cells. Cancer Res. 1988;48:5539–5545. [PubMed] [Google Scholar]
- 45.Siemeister G, Weindel K, Mohrs K, Barleon B, Martiny-Baron G, Marme D. Reversion of deregulated expression of vascular endothelial growth factor in human renal carcinoma cells by von Hippel-Lindau tumor suppressor protein. Cancer Res. 1996;56:2299–2301. [PubMed] [Google Scholar]
- 46.Sonnenberg E, Meyer D, Weidner K M, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol. 1993;123:223–235. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steiner M S. Review of peptide growth factors in benign prostatic hyperplasia and urological malignancy. J Urol. 1995;153:1085–1096. [PubMed] [Google Scholar]
- 48.Stetler-Stevenson W G, Liotta L A, Kleiner D E J. Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993;7:1434–1441. doi: 10.1096/fasebj.7.15.8262328. [DOI] [PubMed] [Google Scholar]
- 49.Sugiura Y, Shimada H, Seeger R C, Laug W E, DeClerck Y A. Matrix metalloproteinases-2 and -9 are expressed in human neuroblastoma: contribution of stromal cells to their production and correlation with metastasis. Cancer Res. 1998;58:2209–2216. [PubMed] [Google Scholar]
- 50.Tashiro K, Hagiya M, Nishizawa T, Seki T, Shimonishi M, Shimizu S, Nakamura T. Deduced primary structure of rat hepatocyte growth factor and expression of the mRNA in rat tissues. Proc Natl Acad Sci USA. 1990;87:3200–3204. doi: 10.1073/pnas.87.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thorgeirsson U P, Liotta L A, Kalebic T, Margulies I M, Thomas K, Rios-Candelore M, Russo R G. Effect of natural protease inhibitors and a chemoattractant on tumor cell invasion in vitro. J Natl Cancer Inst. 1982;69:1049–1054. [PubMed] [Google Scholar]
- 52.Tsao M S, Zhu H, Giaid A, Viallet J, Nakamura T, Park M. Hepatocyte growth factor/scatter factor is an autocrine factor for human normal bronchial epithelial and lung carcinoma cells. Cell Growth Differ. 1993;4:571–579. [PubMed] [Google Scholar]
- 53.Tsarfaty I, Resau J H, Rulong S, Keydar I, Faletto D L, Vande Woude G F. The met proto-oncogene receptor and lumen formation. Science. 1992;257:1258–1261. doi: 10.1126/science.1387731. [DOI] [PubMed] [Google Scholar]
- 54.Tsarfaty I, Rong S, Resau J H, Rulong S, Pinto da Silva P, Vande Woude G F. Met mediated signaling in mesenchymal to epithelial cell conversion. Science. 1994;263:98–101. doi: 10.1126/science.7505952. [DOI] [PubMed] [Google Scholar]
- 55.Tso J Y, Sun X H, Kao T-H, Reece K S, Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985;13:2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walther M M, Kleiner D E, Lubensky I A, Pozzatti R, Nyguen T, Gnarra J R, Hurley K, Venzon D, Linehan W M, Stetler-Stevenson W G. Progelatinase A mRNA expression in cell lines derived from tumors in patients with metastatic renal cell carcinoma correlates inversely with survival. Urology. 1997;50:295–301. doi: 10.1016/s0090-4295(97)00220-3. [DOI] [PubMed] [Google Scholar]
- 57.Woessner J F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- 58.Woolf A S, Kolatsi-Joannou M, Hardman P, Andermarcher E, Moorby C, Fine L G, Jat P S, Noble M D, Gherardi E. Roles of hepatocyte growth factor/scatter factor and the met receptor in the early development of the metanephros. J Cell Biol. 1995;128:171–184. doi: 10.1083/jcb.128.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zarnegar R, DeFrances M C. Expression of HGF-SF in normal and malignant human tissues. EXS. 1993;65:181–199. [PubMed] [Google Scholar]