Abstract
Background/Aims
Data regarding the prognosis of early esophageal cancer are lacking. This study investigated the long-term outcomes and factors affecting the survival of patients with mucosal esophageal squamous cell carcinoma (T1aESCC).
Methods
We analyzed the clinical and tumor-specific parameters of 263 patients who received surgical resection (SR; n=63) or endoscopic resection (ER; n=200) for T1aESCC. Underlying comorbidities were scored using the Charlson comorbidity index (CCI). Overall survival (OS) was the primary outcome, and multivariate regression analysis was performed to predict factors for OS.
Results
Of the study patients (age, 64.5±8.0 years), the CCI was 1.0±1.4 in the ER group and 0.6±0.9 in the SR group (p=0.107). The 5-year OS rate during follow-up (54.4±20.4 months) was 85.7% (ER group, 86.8%; SR group, 82.4%; p=0.631). The cumulative 5-year incidence of esophageal cancer recurrence was 10.5% in the ER group (vs 0% in the SR group). The overall mortality rate was 12.9% (ER group, 12.0%; SR group, 15.9%; p=0.399). The most common cause of mortality was second primary cancers in the ER group (75%) and organ dysfunction or postoperative complications in the SR group (70%). According to multivariate analysis, only CCI was significantly associated with OS (p<0.001). The 5-year OS rate in patients with a CCI >2 and in those with a CCI ≤2 was 60.2% and 88.2%, respectively (p<0.001). The treatment method (ER vs SR) was not a significant affecting factor (p=0.238).
Conclusions
The long-term prognosis of patients with T1aESCC was significantly associated with underlying comorbidities. (Gut Liver 2021;15-712)
Keywords: Esophagus, Squamous cell carcinoma, Endoscopic mucosal resection, Esophagectomy, Prognosis
INTRODUCTION
Recently, the diagnosis of early-stage esophageal cancer is increasing due to screening endoscopy in Korea and Japan.1-3 Resection of mucosal esophageal squamous cell carcinoma (T1aESCC) can be performed, either by surgical or endoscopic method.4,5 Esophagectomy with locoregional lymph node dissection is the standard treatment of T1aESCC.6 In previous studies, patients with ESCC with mucosal invasion showed favorable prognosis and high 5-year survival rates of up to 85%.7,8 As a result of high morbidity and mortality rates with esophagectomy, endoscopic resection (ER) has recently become an alternative to surgical resection (SR) and offers superior safety and acceptable oncologic outcomes, especially confining the tumor depth to mucosa.9 However, there has been no data suggesting factors affecting the long-term prognosis of T1aESCC. Additionally, only some several retrospective studies have compared ER to SR in treating T1aESCC.10,11
In the present study, we investigated the long-term outcomes and factors affecting survival in patients with T1aESCC who were treated with ER versus those treated with SR.
MATERIALS AND METHODS
1. Study design and population
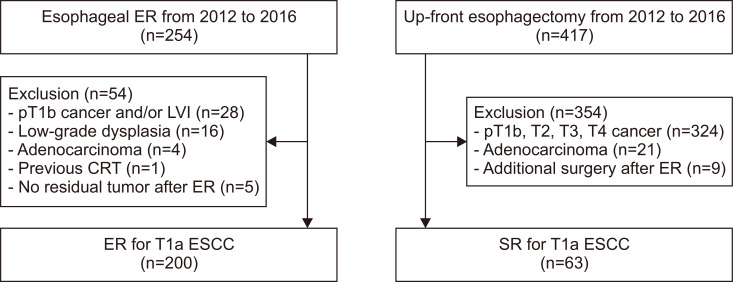
Using a prospectively collected esophageal cancer database between January 2012 and December 2016, patients who underwent ER or up-front esophagectomy for the treatment of esophageal cancer at Asan Medical Center were reviewed. During the study period, 254 patients underwent ER and 417 underwent up-front esophagectomy. ER was indicated when superficial ESCC was less than 3 cm in size without obvious evidence of submucosal invasion on endoscopic ultrasound. The presence of regional or distant lymph node metastasis was detected using chest-abdomen computed tomography (CT) and positron emission tomography-CT scans. For equivocal cases of lymph node metastasis, an endoscopic ultrasound-guided fine needle aspiration/biopsy was performed. Up-front surgery was provided for the cT1-2N0M0 stage. Of 254 patients in the ER group, 54 were excluded due to submucosal and/or lymphovascular tumor invasion (n=28), low-grade dysplasia (n=16), adenocarcinoma (n=4), no residual tumors (n=5), and preoperative chemo-radiotherapy (n=1). In the SR group, 354 patients were excluded due to a deeper invasion depth than the submucosa (n=324), adenocarcinoma (n=21), and additional surgery for non-curative ER (n=9). Finally, a total of 263 T1aESCC cases (200 ER and 63 SR) were included. A flowchart of patient enrollment is shown in Fig. 1. The Institutional Review Board of Asan Medical Center approved the protocols of this (IRB number: 2019-0356). The informed consent was waived because of the retrospective design.
Fig. 1.
Flowchart of the study groups.
ER, endoscopic resection; SR, surgical resection; LVI, lymphovascular invasion; CRT, concurrent chemoradiation therapy; ESCC, esophageal squamous cell carcinoma.
2. Procedures of endoscopic and surgical resections
All tumors were evaluated by chromoendoscopy using Lugol solution and/or narrow-band imaging before ER to determine the exact tumor margin. For ER, a single-channel endoscope (GIF-H260 or GIF-HQ290; Olympus, Tokyo, Japan) was used. Briefly, circumferential marking of the lesion was performed; then, normal saline containing a mixture of indigo carmine and epinephrine (0.01 mg/mL) was injected into the submucosal layer, followed by circumferential incision of the lifted mucosa with a hook knife (Olympus) or insulation-tipped knife (Olympus). Then, submucosal dissection was conducted using an insulation-tipped knife (Olympus). The VIO 300D (Erbe Elektromedizin GmbH, Tübingen, Germany) system or the A UES-30system (Olympus) was used as the electrosurgical unit. Hemostatic forceps (FD-410LR; Olympus) were used to coagulate the visible or bleeding vessels on the artificial ulcers.
The surgical method was based on the transthoracic approach (Ivor Lewis operation or McKeown operation), and the transhiatal approach was used for patients in whom the transthoracic approach was difficult. Minimally invasive robot-assisted esophagectomy was considered when the patients and their families agreed to the procedure and the tumor characteristic was indicated for a minimally invasive approach. After resection of esophageal cancer, all patients underwent follow-up endoscopy and chest-abdomen CT to evaluate tumor recurrence every 6 months for the first 2 years and annually thereafter until 5 years.
3. Pathological evaluation and definition
Tumor size, presence of lymphovascular invasion, depth of invasion, and histologic differentiation were evaluated on the resected specimens. The depth of tumor invasion was classified as intraepithelial (m1), invading the lamina propria (m2), muscularis mucosae (m3), or submucosa. Lymphovascular invasion was indicated by the presence of tumor cells within the lymphovascular structures. The degree of differentiation was determined using the World Health Organization classification.12
Metachronous recurrence was defined as esophageal cancers that developed 1-year post-resection at a location different from the primary resection site. Locoregional recurrence was defined as recurrence of the primary tumor or metastasis to regional lymph nodes, as observed on endoscopy, chest-abdomen CT, or positron emission tomography-CT. Second primary cancer (SPC) was defined as tumors clearly designated as malignant on histologic examination and exclude the possibility of esophageal cancer metastasis.
4. Statistical analysis
Overall survival (OS) was the primary outcome and the recurrence-free survival was the secondary outcome. The outcomes were calculated from the first day of procedure until the date of events or the most recent documented follow-up.
Baseline variables are presented as mean±standard deviation or number (%). To compare variables between the study groups, the analysis of variance or the Student t-test was used for continuous variables and the Fisher exact test or the chi-square test was used for categorical variables. The Kaplan-Meier method was used to calculate the OS, which was compared using the log-rank test. In order to identify factors significantly associated with OS, univariate and multivariate analyses with backward elimination using logistic regression analysis were performed. Cox regression analysis was performed to determine the significant factors affecting survival. The results were expressed by estimating the hazard ratios and 95% confidence intervals. All p-values were two-sided and those less than 0.05 were considered significant. IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
RESULTS
1. Patient characteristics
Table 1 lists baseline characteristics of the 263 patients, whose mean age was 64.5±8.0 years and 93.5% of whom were male. Tumor size was 2.1±1.4 cm in the ER group and 3.0±1.5 cm in the SR group (p<0.001). Endoscopic flat-type lesions (88.0% vs 34.9%) and differentiated tumors (99.5% vs 93.6%) were more common in the ER group than in the SR group. Tumors were confined in m1-2 mucosal layers for 85.0% in the ER group and 72.3% in the SR group (p<0.001). We found no significant differences in terms of age, sex, smoking status, alcohol consumption, Charlson comorbidity index (CCI), multiplicity of lesions, and presence of lymphovascular invasion between the groups.
Table 1.
Baseline Clinicopathologic Characteristics of the Study Patients
| Characteristics | Endoscopic resection (n=200) | Surgical resection (n=63) | p-value |
|---|---|---|---|
| Age, yr | 64.9±8.3 | 63.2±7.1 | 0.194 |
| Male sex | 185 (92.5) | 61 (96.8) | 0.257 |
| Weight, kg | 63.1±10.0 | 65.9±10.4 | 0.064 |
| BMI, kg/m2 | 22.9±3.1 | 23.8±3.1 | 0.054 |
| Charlson comorbidity index | 1.0±1.4 | 0.6±0.9 | 0.107 |
| Smoking | 164 (82.0) | 54 (85.7) | 0.569 |
| Alcohol consumption | 173 (86.6) | 55 (87.3) | 0.536 |
| Tumor location | <0.001 | ||
| Upper third | 19 (9.5) | 4 (6.3) | |
| Middle third | 72 (36.0) | 36 (57.1) | |
| Lower third | 109 (54.5) | 23 (36.5) | |
| Tumor size, cm | 2.1±1.4 | 3.0±1.5 | <0.001 |
| Tumor gross type | <0.001 | ||
| Flat | 176 (88.0) | 22 (34.9) | |
| Non-flat | 24 (12.0) | 41 (65.1) | |
| Differentiation | 0.013 | ||
| Well to moderately | 199 (99.5) | 59 (93.6) | |
| Poorly | 1 (0.5) | 4 (6.4) | |
| Multiplicity of lesion | 10 (5.0) | 5 (7.9) | 0.361 |
| Depth of invasion | <0.001 | ||
| Intraepithelial (m1) | 85 (42.5) | 4 (2.5) | |
| Lamina propria (m2) | 85 (42.5) | 44 (69.8) | |
| Muscularis mucosa (m3) | 30 (15.0) | 15 (23.8) | |
| Lymphovascular invasion | 1 (0.5) | 2 (3.2) | 0.107 |
| Lymph node metastasis | NA | 4 (6.3) | NA |
Data are presented as mean±SD or number (%).
BMI, body mass index; NA, not applicable.
2. Comparison of immediate therapeutic outcomes
In patients undergoing ER, the procedure time was 38.3±24.2 minutes (vs 311.8±45.7 minutes in SR) and the postprocedural hospital stay was 4.0±2.3 days (vs 16.9 ± 9.9 days in SR). The rate of R0 resection was 91% in the ER group and 100% in the SR group (Table 2). Procedure-related immediate adverse events were noted in 10.0% of the ER group and 38.1% of the SR group (p<0.001). Severe undesirable effects including pulmonary, infectious, and hemorrhagic adverse events were 1.5% in the ER and 11.1% in the SR groups (p<0.001). Esophageal stricture was the most common adverse event in patients receiving ER (7.5%; vs 3.2% in SR). Procedure-related immediate mortality was reported at 4.8% in the SR group (0% in the ER group; p=0.013).
Table 2.
Immediate Treatment Outcomes
| Variable | Endoscopic resection (n=200) | Surgical resection (n=63) | p-value |
|---|---|---|---|
| Procedure time, min | 38.3±24.2 | 311.8±45.7 | <0.001 |
| Postprocedural hospital days, day | 4.0±2.3 | 16.9±9.9 | <0.001 |
| R0 resection | 182 (91.0) | 63 (100) | 0.009 |
| Adverse events* | 20 (10.0) | 24 (38.1) | <0.001 |
| Bleeding | 2 (1.0) | 3 (4.8) | |
| Leakage | 0 | 4 (6.3) | |
| Stricture | 15 (7.5) | 2 (3.2) | |
| Micro-perforation | 1 (0.5) | 0 | |
| Fistula | 0 | 1 (1.6) | |
| Subcutaneous emphysema | 1 (0.5) | 0 | |
| Pulmonary events | 1 (0.5) | 3 (4.8) | |
| Wound problem | 0 | 2 (3.2) | |
| Hoarseness | 0 | 6 (9.5) | |
| Chylothorax | 0 | 6 (9.5) | |
| Severe infection | 0 | 1 (1.6) | |
| Procedure related mortality | 0 | 3 (4.8) | 0.013 |
Data are presented as mean±SD or number (%).
*Adverse events occurred within 60 days of treatment.
3. Long-term outcomes
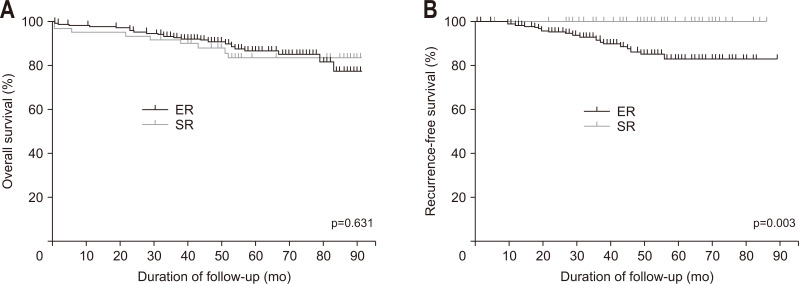
During follow-up 54.4±20.4 months, the 5-year OS rate of the both groups of patients was 85.7%. The 5-year OS rate was not significantly different between the groups (ER group, 86.8%; SR group, 82.4%; p=0.631). The 1- and 3-year survival rates in the ER and SR groups were 98.0% versus 93.7% and 92.9% versus 90.5%, respectively. Excluding three immediate mortality cases, the 5-year OS was 86.8% in ER and 86.5% in SR groups (p=0.662).
The cumulative 5-year incidence of locoregional recurrence of primary esophageal cancer was 1.5% and metachronous esophageal cancer recurrence was 9.0% in the ER group (0% in the SR group) (Fig. 2B). The mean follow-up period for recurrence was 41±18.6 months. During the study period, SPC occurred in 18 patients in the ER groups (9%) and in six in the SR group (9.5%).
Fig. 2.
Kaplan-Meier curves comparing overall survival (A) and recurrence-free survival (B) between the endoscopic resection (ER) and surgical resection (SR) groups.
The overall mortality was 12.9% (ER group, 12.0%; SR group, 15.9%; p=0.399). Esophageal cancer-related death was not reported in either group during the follow-up period. The most common cause of mortality was SPC in the ER group (75%) and organ dysfunction or postoperative complications in the SR group (70%). The long-term outcomes of the study patients are shown in Table 3 and Fig. 2. The three cases of locoregional recurrence after ER was detailed in the Supplementary Table 1.
Table 3.
Long-term Oncologic Outcomes
| Variable | Endoscopic resection (n=200) | Surgical resection (n=63) | p-value |
|---|---|---|---|
| Follow-up duration, mo | 53.2±18.9 | 58.3±24.4 | 0.131 |
| Recurrence-free survival, mo | 40.9±18.7 | 58.3±24.4 | <0.001 |
| Cumulative overall survival rate (%) | 0.631 | ||
| 1 Year | 98.0 | 93.7 | |
| 3 Years | 92.9 | 90.5 | |
| 5 Years | 86.8 | 82.4 | |
| Recurrence of esophageal cancer | 21 (10.5) | 0 | 0.006 |
| Locoregional | 3 (1.5) | 0 | |
| Metachronous | 18 (9.0) | 0 | |
| Cumulative recurrence free survival rate (%) | 0.003 | ||
| 1 Year | 98.5 | 100 | |
| 3 Years | 91.8 | 100 | |
| 5 Years | 83.2 | 100 | |
| Death | 24 (12.0) | 10 (15.9) | 0.399 |
| Disease-specific death | 0 | 0 | 1.000 |
| Cause of death | |||
| Second primary cancers | 18 (75.0) | 2 (20.0) | |
| Organ dysfunction | 2 (8.3) | 4 (40.0) | |
| Postoperative complications | 0 | 3 (30.0) | |
| Other causes | 4 (16.7) | 1 (10.0) |
Data are presented as mean±SD or number (%).
4. Significant factors affecting overall survival
We calculated multivariate Cox proportional hazard models to identify the factors significantly associated with OS in the overall patients with T1aESCC (Table 4). The following factors were investigated: age, sex, smoking, alcohol consumption, CCI score, tumor size, tumor location, invasion depth, gross shape, differentiation, multiplicity of lesions, lymphovascular invasion, and treatment methods.
Table 4.
Significant Factors of Survival in the Univariate and Multivariate Analyses
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age | 1.04 (0.99–1.09) | 0.093 | 0.083 | ||
| Male sex | 22.40 (0.06–8,171.43) | 0.302 | |||
| Smoking | 3.35 (0.8–13.98) | 0.098 | 3.31 (0.78–14.08) | 0.105 | |
| Alcohol consumption | 2.54 (0.61–10.6) | 0.201 | |||
| Charlson comorbidity index | 1.58 (1.29–1.93) | <0.001 | 1.61 (1.30–2.01) | <0.001 | |
| Tumor size | 1.06 (0.84–1.34) | 0.624 | |||
| Location | |||||
| Upper, mid third | 1 | ||||
| Lower third | 1.1 (0.57–2.18) | 0.762 | |||
| Depth of invasion | |||||
| m1, m2 | 1 | ||||
| m3 | 0.98 (0.38–2.54) | 0.965 | |||
| Tumor shape | |||||
| Flat | 1 | ||||
| Non-flat | 1.76 (0.87–3.56) | 0.115 | |||
| Differentiation | |||||
| Well, moderately- | 1 | 1 | |||
| Poorly- | 3.68 (0.85–15.83) | 0.085 | 2.28 (0.47–11.16) | 0.307 | |
| Multiplicity of lesion | |||||
| Solitary | 1 | ||||
| Multiple | 1.61 (0.49–5.28) | 0.429 | |||
| Lymphovascular invasion | |||||
| Absent | 1 | ||||
| Present | NA | 0.67 | |||
| Resection methods | |||||
| Endoscopic | 1 | 1 | |||
| Surgical | 1.19 (0.57–2.52) | 0.636 | 1.68 (0.71–3.98) | 0.238 | |
HR, hazard ratio; CI, confidence interval; m1, intraepithelial; m2, lamina propria; m3, muscularis mucosae; NA, not applicable.
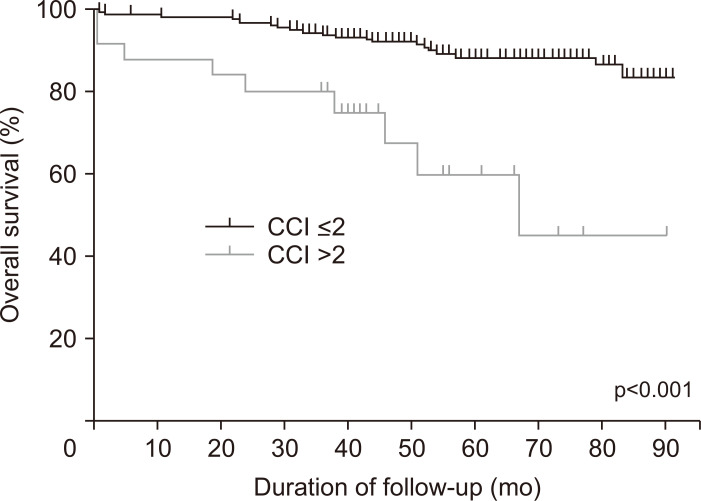
CCI was identified as the only significant factor for OS (hazard ratio, 1.61; 95% confidence interval, 1.30 to 2.01; p<0.001) (Fig. 3). The 1-, 3-, and 5-year survival rates in patients with CCI >2 and those with CCI ≤2 were 88% versus 97.9%, 80% versus 93.6%, and 60.2% versus 88.2%, respectively. The method of treatment (ER vs SR) was not a significant factor for OS (p=0.238).
Fig. 3.
Kaplan-Meier curves comparing overall survival according to the Charlson comorbidity index (CCI) score (≤ 2 and >2).
DISCUSSION
In the present study, the 5-year OS rate of patients with T1aESCC was 85.7% and CCI was the only significant factor affecting survival. There was no difference in the OS between the ER and SR groups.
Esophagectomy is considered the gold standard in the treatment of localized esophageal cancer, particularly superficial tumors.13 However, esophagectomy results in mortality and severe morbidity rates of 2% to 5% and 30% to 40%, respectively, which significantly alter patients’ quality of life.14-16 Compared with SR, ER is a minimally invasive procedure; furthermore, it can perfectly preserve the normal anatomical structures and functions. Patients who underwent ER had shorter postprocedural hospital stays (4.0±2.3 days), shorter procedure times (38.3±24.2 minutes), and lower immediate adverse event rates (10.0%). Thus, ER is now considered a first-line treatment modality for technically feasible early-stage of esophageal cancer.
ER is well-suited for superficial cancers confined to the mucosa, as the likelihood of lymph node metastasis is very low.17,18 The risk of lymph node invasion is closely associated with the tumor invasion depth, lymphovascular invasion, and histological differentiation. Estimates of lymph node metastasis based on these histopathologic features have been established. In previous studies, lymph node metastasis was noted been found in up to 4% of cancers in the epithelium (m1) and lamina propria (m2), 0% to 22% of cancers invading the muscularis mucosa (m3), and 26% to 54% of cancers invading the submucosa.2,19 Therefore, ER is the preferred treatment modality for m1, m2, and m3 cancers and relatively indicated for submucosal cancer invasion of up to 200 μm.4,5,20,21 According to guidelines, esophagectomy and ER are equally recommended for lesions limited to the mucosa for superficial ESCC.20,21 In Japan, ER is considered a radical treatment for lesions confined to mucosal epithelium and lamina propria.4 Lesions extending up to the muscularis mucosae or lightly infiltrating the submucosa (up to 200 μm) are also responsive to mucosal resection.22
The ER group was comparable to the SR group in terms of 5-year OS rate; however, the 5-year recurrence-free survival rate was lower in the ER group than in the SR group, especially, in cases with a higher occurrence of metachronous esophageal cancer. In this study, the 5-year cumulative incidence of locoregional recurrence of primary esophageal cancer was 1.5% and metachronous esophageal cancer recurrence was 9.0% in the ER group. Conversely, in the SR group, locoregional and metachronous recurrence were not reported. Metachronous esophageal cancer is a major concern following ER for T1aESCC. In a previous study, the incidence of metachronous esophageal cancer after endoscopic treatment was reported to be up to 14%.23 Tiny synchronous tumors might be missed at the initial endoscopic examination and might develop into visible recurrence during the 3.4-year surveillance period.23 Therefore, attention should be given to locoregional and metachronous recurrence after ER.
In previous studies, a 5-year survival rate of 90% to 94% was reported in patients with T1aESCC who underwent esophagectomy.24,25 In cases of T1 ESCC with ER, the 5-year OS rates were reported to be 81.6%–99.0%, and in subgroup analysis, 5-year survival rates of patients with m1-2 and m3-sm1 were reported to be 100% and 85%–89.0%, respectively.26,27 In our study, there were no esophageal cancer-related deaths in either group during follow-up.
Data were limited regarding long-term prognostic factors for patients with T1aESCC. The CCI is used for numerical conversion of comorbidities; as such, the CCI is widely established as a predictor for prognosis after multiple surgical procedures.28 Similar to the results of previous studies, we also found the CCI was the strongest prognostic factor (hazard ratio, 1.61; 95% confidence interval, 1.3 to 2.01; p<0.001).29,30 Our findings corroborate the study results that showed the influence of CCI on treatment outcomes in patients with esophageal cancer who underwent esophagectomy,29 in this study, the authors reported that the prognosis of patients with esophageal cancer who after curative esophagectomy was significantly correlated with the CCI.
Alcohol consumption and smoking are major risk factors for squamous cancers,31 and around 4.3% to 10.4% of patients with squamous esophageal cancer develop synchronous SPC.32 SPC associated with esophageal cancer commonly develop in the aerodigestive tract organs, such as the oral cavity, pharynx, larynx, and lung.32,33 In our study, 14 patients (9.1%) were identified with subsequent SPC in both groups. The 5-year survival rates were significantly lower in patients with SPC compared with those without (46.4% and 90.5%, respectively, p=0.003). Furthermore, SPC was the most important cause of death in the ER group.
This study has the following limitations. First, this study has a retrospective design based on consecutive observational data from a single tertiary referral center, which entails possible selection bias. Second, we did not analyze cost-effectiveness and the quality of life. Third, this study included a relatively small number of patients in the SR group and a small number of events, which hindered a detailed subgroup analysis and the detection of small differences with sufficient statistical power.
In conclusion, the long-term prognosis of patients with T1aESCC was significantly affected by underlying comorbidity. Although SR is a gold standard, ER is an excellent alternative therapy when strict endoscopic treatment of early esophageal cancer is indicated. Physicians should be aware of the possibility of metachronous esophageal cancer recurrence in patients undergoing ER and operation-related adverse events in those undergoing SR.
SUPPLEMENTAL MATERIALS
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: H.J.S., Y.H.K., G.H.K. Data acquisition: H.K.N., J.Y.A., J.H.L., K.W.J., D.H.K., H.R.K., K.D.C., G.H.L., H.Y.J., S.I.P. Data analysis and interpretation: H.J.S., Y.H.K., G.H.K. Drafting of the manuscript: H.J.S., Y.H.K., G.H.K. Critical revision of the manuscript for important intellectual content: H.J.S., Y.H.K., G.H.K. Statistical analysis: G.H.K. Approval of final manuscript: all authors.
REFERENCES
- 1.Takubo K, Aida J, Sawabe M, et al. Early squamous cell carcinoma of the oesophagus: the Japanese viewpoint. Histopathology. 2007;51:733–742. doi: 10.1111/j.1365-2559.2007.02766.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim DU, Lee JH, Min BH, et al. Risk factors of lymph node metastasis in T1 esophageal squamous cell carcinoma. J Gastroenterol Hepatol. 2008;23:619–625. doi: 10.1111/j.1440-1746.2007.05259.x. [DOI] [PubMed] [Google Scholar]
- 3.Fujiyoshi T, Tajika M, Tanaka T, et al. Comparative evaluation of new and conventional classifications of magnifying endoscopy with narrow band imaging for invasion depth of superficial esophageal squamous cell carcinoma. Dis Esophagus. 2017;30:1–8. doi: 10.1093/dote/dox037. [DOI] [PubMed] [Google Scholar]
- 4.Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus. 2019;16:25–43. doi: 10.1007/s10388-018-0642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. 2019;16:1–24. doi: 10.1007/s10388-018-0641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishihara R, Arima M, Iizuka T, et al. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig Endosc. 2020;32:452–493. doi: 10.1111/den.13654. [DOI] [PubMed] [Google Scholar]
- 7.Plum PS, Hölscher AH, Pacheco Godoy K, et al. Prognosis of patients with superficial T1 esophageal cancer who underwent endoscopic resection before esophagectomy: a propensity score-matched comparison. Surg Endosc. 2018;32:3972–3980. doi: 10.1007/s00464-018-6139-7. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Huang Y, Xie J, et al. Does delayed esophagectomy after endoscopic resection affect outcomes in patients with stage T1 esophageal cancer? A propensity score-based analysis. Surg Endosc. 2018;32:1441–1448. doi: 10.1007/s00464-017-5830-4. [DOI] [PubMed] [Google Scholar]
- 9.Draganov PV, Wang AY, Othman MO, Fukami N. AGA institute clinical practice update: endoscopic submucosal dissection in the United States. Clin Gastroenterol Hepatol. 2019;17:16–25. doi: 10.1016/j.cgh.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Ding H, Chen T, et al. Outcomes of endoscopic submucosal dissection vs esophagectomy for T1 esophageal squamous cell carcinoma in a real-world cohort. Clin Gastroenterol Hepatol. 2019;17:73–81. doi: 10.1016/j.cgh.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 11.Min YW, Lee H, Song BG, et al. Comparison of endoscopic submucosal dissection and surgery for superficial esophageal squamous cell carcinoma: a propensity score-matched analysis. Gastrointest Endosc. 2018;88:624–633. doi: 10.1016/j.gie.2018.04.2360. [DOI] [PubMed] [Google Scholar]
- 12.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 13.Pennathur A, Farkas A, Krasinskas AM, et al. Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg. 2009;87:1048–1054. doi: 10.1016/j.athoracsur.2008.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang AC, Ji H, Birkmeyer NJ, Orringer MB, Birkmeyer JD. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg. 2008;85:424–429. doi: 10.1016/j.athoracsur.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Connors RC, Reuben BC, Neumayer LA, Bull DA. Comparing outcomes after transthoracic and transhiatal esophagectomy: a 5-year prospective cohort of 17,395 patients. J Am Coll Surg. 2007;205:735–740. doi: 10.1016/j.jamcollsurg.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Das A, Singh V, Fleischer DE, Sharma VK. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol. 2008;103:1340–1345. doi: 10.1111/j.1572-0241.2008.01889.x. [DOI] [PubMed] [Google Scholar]
- 17.Choi JY, Park YS, Jung HY, et al. Feasibility of endoscopic resection in superficial esophageal squamous carcinoma. Gastrointest Endosc. 2011;73:881–889. doi: 10.1016/j.gie.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Stein HJ, Feith M, Bruecher BL, Naehrig J, Sarbia M, Siewert JR. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242:566–573. doi: 10.1097/01.sla.0000184211.75970.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery. 1998;123:432–439. doi: 10.1016/S0039-6060(98)70165-5. [DOI] [PubMed] [Google Scholar]
- 20.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829–854. doi: 10.1055/s-0034-1392882. [DOI] [PubMed] [Google Scholar]
- 21.Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:855–883. doi: 10.6004/jnccn.2019.0033. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu Y, Tsukagoshi H, Fujita M, Hosokawa M, Kato M, Asaka M. Long-term outcome after endoscopic mucosal resection in patients with esophageal squamous cell carcinoma invading the muscularis mucosae or deeper. Gastrointest Endosc. 2002;56:387–390. doi: 10.1016/S0016-5107(02)70043-6. [DOI] [PubMed] [Google Scholar]
- 23.Sgourakis G, Gockel I, Lang H. Endoscopic and surgical resection of T1a/T1b esophageal neoplasms: a systematic review. World J Gastroenterol. 2013;19:1424–1437. doi: 10.3748/wjg.v19.i9.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wijnhoven BP, Tran KT, Esterman A, Watson DI, Tilanus HW. An evaluation of prognostic factors and tumor staging of resected carcinoma of the esophagus. Ann Surg. 2007;245:717–725. doi: 10.1097/01.sla.0000251703.35919.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hölscher AH, Bollschweiler E, Schröder W, Metzger R, Gutschow C, Drebber U. Prognostic impact of upper, middle, and lower third mucosal or submucosal infiltration in early esophageal cancer. Ann Surg. 2011;254:802–807. doi: 10.1097/SLA.0b013e3182369128. [DOI] [PubMed] [Google Scholar]
- 26.Nagami Y, Ominami M, Shiba M, et al. The five-year survival rate after endoscopic submucosal dissection for superficial esophageal squamous cell neoplasia. Dig Liver Dis. 2017;49:427–433. doi: 10.1016/j.dld.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009;70:860–866. doi: 10.1016/j.gie.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita K, Watanabe M, Mine S, et al. The impact of the Charlson comorbidity index on the prognosis of esophageal cancer patients who underwent esophagectomy with curative intent. Surg Today. 2018;48:632–639. doi: 10.1007/s00595-018-1630-2. [DOI] [PubMed] [Google Scholar]
- 30.Bernardi D, Asti E, Aiolfi A, Bonitta G, Luporini A, Bonavina L. Outcome of trimodal therapy in elderly patients with esophageal cancer: prognostic value of the Charlson comorbidity index. Anticancer Res. 2018;38:1815–1820. doi: 10.21873/anticanres.12420. [DOI] [PubMed] [Google Scholar]
- 31.Layke JC, Lopez PP. Esophageal cancer: a review and update. Am Fam Physician. 2006;73:2187–2194. [PubMed] [Google Scholar]
- 32.Nagasawa S, Onda M, Sasajima K, Takubo K, Miyashita M. Multiple primary malignant neoplasms in patients with esophageal cancer. Dis Esophagus. 2000;13:226–230. doi: 10.1046/j.1442-2050.2000.00116.x. [DOI] [PubMed] [Google Scholar]
- 33.Matsubara T, Yamada K, Nakagawa A. Risk of second primary malignancy after esophagectomy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21:4336–4341. doi: 10.1200/JCO.2003.12.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.