Abstract
Delivering localized treatment to the paranasal sinuses for diseases such as chronic rhinosinusitis (CRS) is particularly challenging because of the small natural openings leading from the sinuses that can be further obstructed by presence of inflammation. As such, oral steroids, topical nasal sprays or irrigation, and surgery can be utilized to treat persistent sinonasal inflammation, but there exists a need for post-operative options for long-term steroid delivery to prevent disease recurrence. In the present study, a Thermogel, Extended-release Microsphere-based-delivery to the Paranasal Sinuses (TEMPS) is developed with the corticosteroid mometasone furoate. Specifically, the bioactive steroid is released for 4 weeks from poly(lactic-co-glycolic acid) (PLGA) microspheres embedded in a poly(N-isopropylacrylamide) (p-NIPAAm)-based hydrogel. The temperature-responsive system undergoes a reversible sol-gel transition at 34–35 °C such that it can be applied as a liquid at ambient temperature, conforming to the sinonasal epithelium as it gels. In a rabbit model of CRS, TEMPS was maintained in rabbit sinuses and effectively reduced sinonasal inflammation as characterized by micro-computed tomography and histopathology analysis. Ultimately, the combination of controlled release microspheres with a thermoresponsive hydrogel provides flexibility for encapsulating therapeutics in a reversible and conforming system for localized delivery to the sinuses.
Keywords: Thermoresponsive, Microsphere, Hydrogel, Sustained release, Steroid, Sinusitis
1. Introduction
Chronic rhinosinusitis (CRS) is an inflammatory disease of the sinonasal mucosa that affects ~12% of the US population and significantly reduces quality of life [1]. CRS patients present with symptoms of nasal drainage, facial pain and pressure, congestion, and diminished olfaction continuing for over 12 weeks [2,3]. Persistence of these symptoms contributes to a rating of general health status (health utility score) worse than that of patients with chronic obstructive pulmonary disorder or Parkinson’s disease [4]. In over 250,000 cases in the US annually, CRS patients with disease refractory to medical therapies undergo functional endoscopic sinus surgery [2]. In this procedure, inflamed tissue and bone are removed to reestablish sinus outflow tracts and improve access for daily topical maintenance therapy [5]. However, there is need for improved methods of long-term topical administration into the paranasal sinuses following surgery [6].
Currently prescribed intranasal corticosteroids, such as mometasone furoate, are beneficial for CRS symptom relief [2,7], however their local delivery to the sinuses is challenging. Nasal irrigation utilizing pots and squeeze bottles outperforms sprays in the ability to access the sinus cavities, but requires high volumes, practiced technique, and daily dosing [7]. The only local, controlled release devices for the paranasal sinuses are dissolvable stents (Propel® and Sinuva®), which are coated with mometasone furoate and inserted in one of the sinus cavities after surgery. Because the stents are designed to gradually degrade, local epithelial attenuation and cilia loss have been noted in the early response to stent placement [8]. This inflammatory response can lead to clinical adverse events including crusting, granulation, and scarring [9] as well as nasal and ocular irritation [10]. In one severe case, a stent was found to be extending through the skull base into the brain with significant cognitive consequences [11]. In this instance, the patient had undergone extensive surgery and 8 stents were placed throughout the sinus cavities [11]. The varying anatomy of the four sinuses (ethmoid, maxillary, frontal, and sphenoid) necessitates individual stents for each cavity and incorrect placement can lead to complications.
If a system could conform to the sinonasal mucosa, mimicking the native mucus layer, such a device would address the issues with existing devices and be much better suited for long-term application. One such method is a thermoresponsive hydrogel (thermogel) in combination with polymeric microspheres. Previously, we have demonstrated that degradable poly(lactic-co-glycolic acid) (PLGA) microspheres embedded in poly(N-isopropylacrylamide) (pNIPAAm)-based hydrogel safely and effectively deliver long-term glaucoma treatment in rabbits [12]. The sol-gel transition allows for the gel to comfortably conform within the lower fornix of the eye creating a depot for several weeks of sustained drug delivery. Accordingly, we sought to explore the suitability of a combined microsphere-thermogel system for localized drug delivery in complex spaces, such as the sinus cavities, that require a conforming system to maintain long term apposition and retention. Furthermore, this technology, referred to herein as “TEMPS” (Thermogel, Extended-release Microsphere-based-delivery to the Paranasal Sinuses), offers the flexibility of encapsulating different drugs in the polymeric microspheres and broader implications for treatments of various inflammatory or allergic sinonasal conditions.
2. Materials & methods
2.1. Microsphere fabrication and characterization
Drug-loaded polymer microspheres (MSs) were fabricated using a standard single emulsion procedure. 200 mg PLGA (0.32–0.44 dL/g, 50:50 lactic acid:glycolic acid, ester-terminated, RG503, Sigma, St. Louis, MO) and 10 mg mometasone furoate (AcrosOrganics, Thermo Fisher Scientific, Waltham, MA) were mixed with 4 mL dichloromethane. The drug/polymer solution was homogenized (Silverson L4RT-A, East Longmeadow, MA) in 60 mL 2% poly(vinyl alcohol) (PVA, MW ~25 kDa, 98% hydrolyzed, PolySciences, Warrington, PA) at 5000 rpm for 1 min. The emulsion was then mixed with 80 mL 1% PVA and stirred at 600 rpm for 3 h for solvent evaporation. Microspheres were collected, washed 4× with MilliQ water, flash-frozen with liquid nitrogen, and lyophilized (VirTis Benchtop K freeze dryer, Gardiner, NY operated at 100 mTorr) for 48 h before storage at −20 °C. Samples of MSs were visualized by scanning electron microscopy (SEM) (JEOL JSM-6510LV/LGS SEM, Japan) following gold-palladium sputter coating (Denton Sputter Coater, Moorestown, NJ). Size distribution of MSs was determined by volume impedance measurements (Multisizer 3 Coulter Counter, Beckman Coulter, Indianapolis, IN).
Drug encapsulation was evaluated by dissolving 5 mg mometasone-loaded or blank MSs in 2 mL dimethyl sulfoxide (DMSO). The drug concentration was quantified by absorbance measurements at 262 nm using a UV–Vis spectrophotometer (Molecular Devices, Sunnyvale, CA). Encapsulation efficiency was calculated as the ratio of mometasone measured in the dissolved MSs relative to the amount that was initially added during fabrication and normalized based on MS yield.
2.2. Thermogel fabrication and characterization
Thermoresponsive hydrogel was prepared using reagents from Sigma-Aldrich (St. Louis, MO) and methods described by Bellotti et al. [13]. Briefly, 100 μL of 200 Da poly(ethylene glycol) (PEG) was added to 100 mg of N-isopropylacrylamide (NIPAAm). The aqueous free radical polymerization reaction was initiated by the addition of ammonium persulfate and tetramethylethylenediamine. Following overnight polymerization at 4 °C, the gel was washed 10× with warm MilliQ water to remove unreacted monomer and stored at ambient temperature.
The lower critical solution temperature (LCST) of TEMPS was measured as previously described [13]. Additionally, reversibility of the sol-gel transition was demonstrated in vitro, whereby TEMPS and its individual components (gel and MSs alone) were incubated in a clear bottom, 96-well plate in an aqueous salt solution of simulated nasal fluid [14]. At weekly intervals, absorbance was measured at 37 °C and 415 nm. Then the plate was cooled to room temperature and absorbance was measured again. The aqueous solution was replenished as needed and the plate was sealed during incubation to prevent evaporation.
Cytocompatibility of the pNIPAAm gel was evaluated using methods adapted from [13]. A sinonasal epithelial cell line, RPMI 2650 (ATCC® CCL30™) was cultured in Eagle’s Minimum Essential Medium (American Type Culture Collection, Manassas, VA) supplemented with 10% FBS. Cells were seeded at 20,000 cells per well in a 96-well plate. Following incubation for 24 h with the thermogel, 10% PrestoBlue® viability reagent (ThermoFisher Scientific, Waltham, MA) was added. After 3 h incubation, reduction of PrestoBlue reagent was measured by fluorescence readings using 540/580 nm for excitation/emission filters. The mean and standard deviation of fluorescence values (F) were determined (n = 6) and viability was calculated using the following equation:
2.3. In vitro drug release and bioactivity
In vitro release kinetics of mometasone furoate were evaluated using methods adapted from Ammar et al. [15]. To quantify release from MSs only, 10 mg MSs were incubated in 1 mL 2% sodium deoxycholate in water on a roto-shaker at 37 °C (n = 3). At regular time intervals, MS suspensions were centrifuged, the supernatant was collected and diluted 1:3 in methanol, and the absorbance was measured at 248 nm [15]. Absorbance of release samples from blank MSs was subtracted at each timepoint. Drug release from TEMPS was determined using a similar procedure, with the exception that 10 mg MSs were suspended in 100 μL pNIPAAm gel (n = 5).
Throughout the in vitro release assay, release samples were diluted with DMSO and stored at −20 °C for bioactivity measurements. Following the vendor protocol for the Human Glucocorticoid Receptor (NR3C1, GR) Reporter Assay (Indigo Biosciences, State College, PA), release samples from TEMPS between day 25 and 28 of incubation were tested for their ability to bind to the engineered cells and induce luciferase expression, indicative of the presence of bioactive drug. As a control, drug was solvated in DMSO the day of the assay to compare the bioactivity of fresh and released drug (n = 3 per dilution).
2.4. Animal model
This study evaluating TEMPS in male and female New Zealand rabbits (2–4 kg) was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh. When necessary, animals were anesthetized with ketamine (10–50 mg/kg IM) and xylazine (2–5 mg/kg SQ). Lidocaine hydrochloride was applied to the nares to provide topical analgesia for endoscopy. Following all procedures, antisedan (5 mg/kg IV) was dosed for xylazine reversal and rabbits were given SQ saline (10 mL/kg) for higher anesthesia doses. If pain management following procedures was necessary, meloxicam was dosed for 1–3 days (0.2 mg/kg PO or SQ).
2.5. Disease induction and microCT imaging
A reversible obstruction of the left ostiomeatal complex was created to induce disease, simulating CRS in the maxillary sinus. Animals were anesthetized (ketamine, 35 mg/kg IM and xylazine, 5 mg/kg SQ) and the nasal cavity was visualized with a 1.9 mm 0° endoscope (Karl Storz, Tuttlinggen, Germany). A sterile Merocel sponge (aseptically trimmed to approximately 12 × 3 × 1 mm) was inserted through the left naris and packed in the opening of the sinus ostia, lateral to the middle turbinate, as previously described [16]. Two weeks later, the sponge was removed with endoscopic visualization. Over the subsequent 11 weeks, disease phenotype progressed towards a chronic inflammatory pathology, as previously characterized by Cho et al. [16].
Opacification of the sinus cavities was monitored by skull micro-computed tomography (microCT) imaging using a Fidex system (Animage, Pleasanton, CA). For subjects that did not show evidence of opacification after the initial chronic disease induction, disease was re-induced as follows. Three sponges were inserted to ensure full blockage of the left sinus ostia for 2 weeks. Eight weeks after the sponge removal, microCT imaging was performed to assess disease re-induction.
2.6. Treatment application
Following chronic disease induction, animals were divided into 4 groups for bilateral treatment: no treatment, blank vehicle TEMPS (v-TEMPS), mometasone-loaded TEMPS (m-TEMPS), and daily nasal drops. A one-time application of TEMPS was performed by percutaneously injecting the material into the maxillary sinus through the canine fossa using an 18 G needle. Approximately 20 mg of blank MSs or mometasone-loaded MSs were suspended in 0.4 mL of thermogel and injected into the left and right maxillary sinus cavities for the v-TEMPS or m-TEMPS groups, respectively. A no treatment group served as a negative control that also received the one-time percutaneous punctures without material injection. Similarly, a positive control treatment group received the one-time bilateral punctures without injection and then daily nasal drops for 4 weeks of 31.25 μg of steroid in 100 μL (Mometasone Furoate Nasal Spray, Apotex Inc., Toronto, Ontario, diluted with PBS) to the left and right nares via a 0.5-in. 22 G flexible PTFE dispensing needle.
2.7. In vivo measurements
Bilateral intraocular pressure (IOP) was measured at 4 timepoints: baseline, established disease state, 2-weeks and 4-weeks after treatment application. To control for diurnal variations [17], IOP measurements were performed in the morning by the same technician on unrestrained, alert animals using a TonoVet rebound tonometer (Icare, Finland).
Additionally, at these 4 timepoints, bilateral sinus opacification was evaluated using microCT imaging. Using OsiriX Lite (Pixmeo SARL, Switzerland), the left and right maxillary sinuses were identified in a coronal view on three consecutive 155.79 μm thickness scans near the mid-point of the middle turbinate. The image contrast and brightness were adjusted so that only calcified tissue was visible (window level ~ 800, window width ~ 1400) and a region of interest was defined by tracing a polygon along the inner edge of the maxillary sinus wall, excluding calcified bone. The median CT # of this defined region, as calculated by the software, was recorded for analysis by an investigator blinded to the subject ID and group for measurements at 2- and 4-weeks after treatment application.
2.8. Histologic preparation and assessment
At the conclusion of the study, anesthetized animals were euthanized with pentobarbitol (100 mg/kg IV) and tissue was prepared for histological evaluation. The maxillary bones were harvested and fixed by immersion in 10% neutral buffered formalin for 1 week. Following fixation, the specimens were transferred to 10% formic acid for decalcification. After 1 week, the decalcification solution was tested every other day for end-point determination using 5 mL of the used decalcification solution combined with 5 mL ammonium hydroxide and 5 mL ammonium oxalate. If the test solution was cloudy, the tissue was placed in new formic acid until the next test day. Once the test solution yielded a clear solution, sections were cut, placed in cassettes, dehydrated in a graded series of alcohol, and embedded in paraffin blocks. Paraffin-embedded sections were cut to 4 μm using a microtome, mounted on glass slides, and stained with routine hematoxylin and eosin (H&E).
Inflammatory scoring criteria were developed for analysis of maxillary sinus sections. Scoring analysis was performed in a blinded manner by a board-certified veterinary pathologist. Four criteria, including epithelial cell damage, subepithelial edema, inflammation of the submucosal glands, and cilia damage, were scored for their relative involvement in the left or right tissue sections. Involvement was scored from 0 to 3 (none, up to 1/3 of the section, 1/3 to 2/3, or 2/3 to diffuse, respectively). These involvement scores were multiplied by scores for severity ranging from 0 to 3 (none, mild, moderate, or severe) or 0 to 2 (normal cilia, disrupted cilia, or cilia loss). Epithelial hyperplasia was also scored for severity from 0 to 3. Four additional criteria, including involvement of the basement membrane, granulocyte tissue infiltrate, granulocytes in the lumen, and presence of gel-MSs in the lumen, were scored as absent (0) or present (1).
2.9. Statistical analyses
Statistical analyses were performed with GraphPad Prism v7 (San Diego, CA) and SAS JMP® Pro 14 (Cary, NC). Descriptive statistics were used to describe the mean and standard deviations (S.D.) and values are reported as the mean ± S.D., unless specified otherwise. For the in vitro bioactivity assay, EC50 values, 95% confidence intervals (CI), and R2 were determined by nonlinear 3-parameter logistic regression using GraphPad. Using JMP, comparisons among groups with continuous data were performed by one-way ANOVA. After assessing the variances by Levene’s test, Wilcoxon Method (unequal variance) or Tukey (equal variance) post-hoc testing was performed. For nominal data, comparisons among groups were performed by Chi-square testing using Pearson’s p-values. For all analyses, p < 0.050 was considered as significant.
3. Results
3.1. TEMPS provides extended release of bioactive steroid in a thermoresponsive and cytocompatible system in vitro
The combined thermogel and polymer microsphere system, or TEMPS, was engineered to provide extended release of bioactive steroid for 4 weeks. The steroid mometasone furoate was encapsulated in PLGA MSs that had a spherical morphology with mean diameter of 7.8 ± 2.9 μm, which was measured by volume impedance (Fig. 1A) and confirmed by SEM imaging. In Fig. 1A, the second peak near 28 μm was likely aggregates of MSs. Approximately 44 μg of mometasone per mg MS was encapsulated, with a loading efficiency of 88%. The sustained release of mometasone for 4 weeks was demonstrated in vitro from both MSs (Fig. 1B) and TEMPS (Fig. 1C). Additionally, the bioactivity of released mometasone from TEMPS was similar to fresh drug. TEMPS release samples that were collected between day 25 and 28 had an EC50 of 0.33 pM (95% CI [0.17, 0.61] and R2 = 0.92) measured by a glucocorticoid receptor reporter cell line assay, while that of fresh drug was 0.31 pM (95% CI [0.13, 0.65] and R2 = 0.90) (Fig. 1D).
Fig. 1.
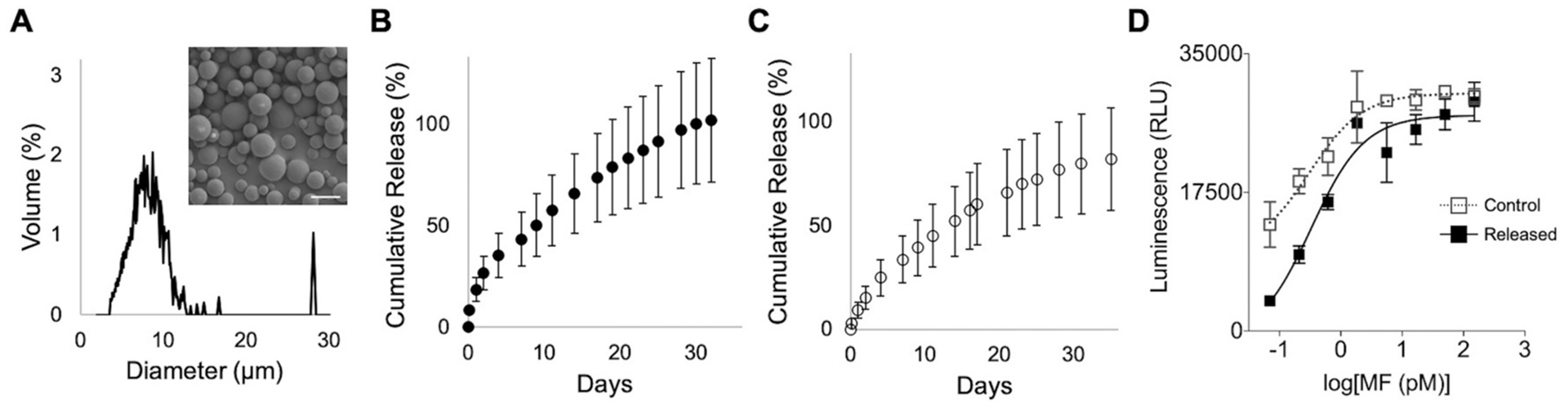
Bioactive mometasone furoate can be released from TEMPS for 4 weeks in vitro. (A) Size distribution and (inset) representative scanning electron micrograph, scale bar = 10 μm, of drug-loaded MSs showing a smooth, spherical morphology with mean diameter of 7.8 μm. (B) Cumulative release of mometasone furoate from MSs (n = 3) and (C) TEMPS, MSs embedded in thermogel (n = 5). Error bars represent mean ± cumulative S.D. (D) Drug released from TEMPS after 28 days of aqueous incubation at 37 °C displays activity (EC50 = 0.33 pM) similar to a control, drug prepared the day of the assay (EC50 = 0.31 pM).
This bioactive steroid is intended for localized delivery to the paranasal sinuses via a biocompatible and reversible thermoresponsive hydrogel. Accordingly, a PEG-pNIPAAm gel formulation with an LCST of 35 °C (characterized in previous work [13]) was selected for the thermoresponsive matrix. The presence of drug-loaded MSs did not alter the LCST, as shown in Fig. 2A, although MSs caused a higher baseline absorbance value in the clear gel. Importantly, the reversible phase change of TEMPS, as compared to MSs alone, was demonstrated by repeat absorbance measurements while the temperature was fluctuated between ambient and body temperature over 28 days (Fig. 2B). Additionally, cytocompatibility of the PEG-pNIPAAm gel with a sinonasal epithelial cell line, RPMI 2650, was tested. After incubating for 24 h with the gel, cellular viability was comparable to that of control cells (Fig. 2C).
Fig. 2.
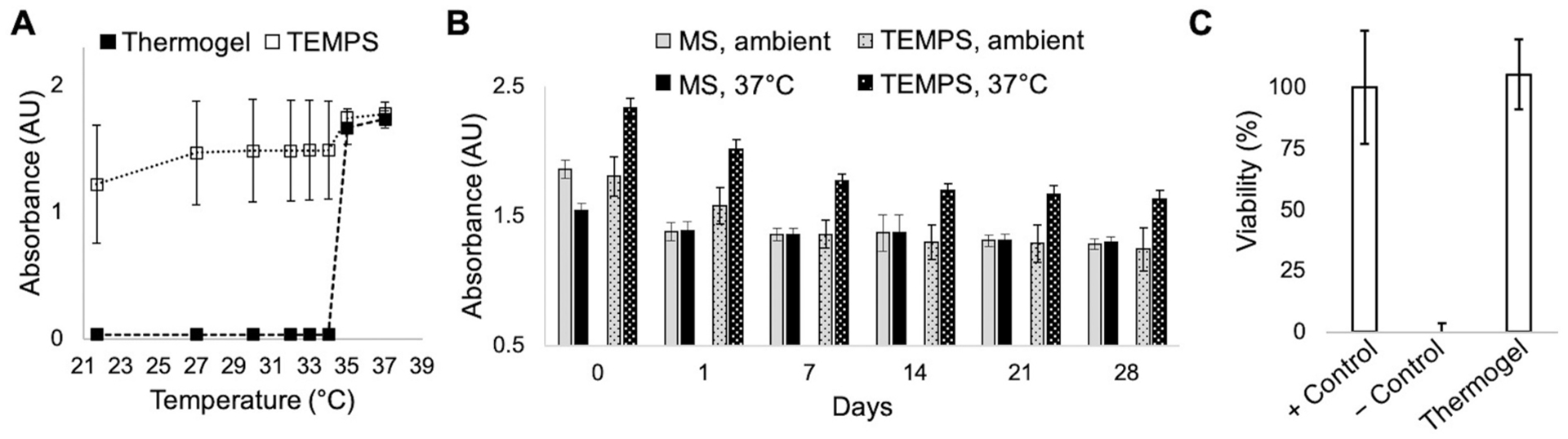
TEMPS is compatible for reversible apposition with the sinonasal epithelium. (A) Absorbance measurements at 415 nm as a function of increasing temperature for samples (n = 4) of thermogel and TEMPS showing that both undergo phase transition at 34–35 °C. (B) The reversible temperature-responsive phase change of TEMPS demonstrated by repeated absorbance measurements at ambient temperature and 37 °C over 28 days. (C) Cytocompatibility of the thermogel with RPMI 2650 cells showing that viability is maintained after 24-h incubation with gel. Cell viability was measured using PrestoBlue reduction and % viability was calculated relative to positive (+) and negative (−) controls.
3.2. Sinonasal inflammation was reduced following treatment with mometasone-loaded TEMPS
Efficacy of extended steroid release from TEMPS was investigated in a CRS rabbit model following the timeline in Fig. 3A and using measurements by microCT imaging as depicted in Fig. 3B. Induction of disease to the left maxillary sinus resulted in a significant increase in opacification between baseline and the disease state bilaterally (p < 0.001, Supplementary Fig. 1A). Due to variability of sinus opacification among subjects at the disease state, induction of disease was repeated in 6 subjects. Furthermore, the change in CT # from the disease state to the 2- or 4-weeks treatment timepoints was compared. On the control (right) side, change in CT # of the v-TEMPS group was significantly increased compared to no treatment (p < 0.001) and m-TEMPS (p < 0.020) at 2-weeks after treatment application. Similarly, at 4-weeks post-treatment, the change in CT # of the v-TEMPS group remained significantly increased compared to the no treatment, m-TEMPS, and daily nasal drop groups (p < 0.001, Fig. 3C). In contrast, the change in CT # at 2- and 4-weeks after treatment on the disease-induced (left) side showed no significant differences between groups (p > 0.050, Supplementary Fig. 1B).
Fig. 3.
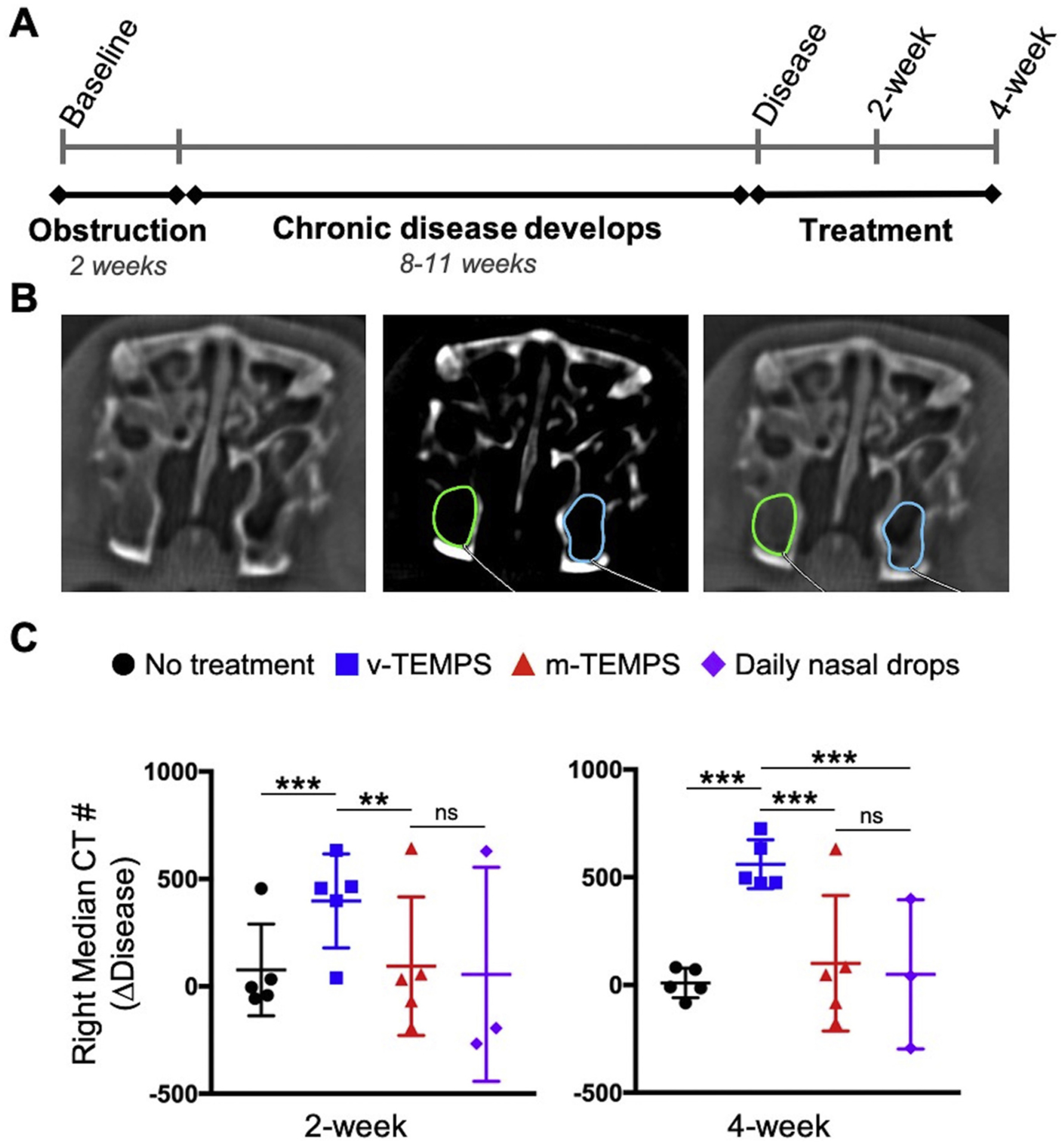
MicroCT imaging showed reduced opacification of the right maxillary sinus following application of m-TEMPS compared to v-TEMPS. (A) Disease was induced by creating a reversible obstruction of the left ostiomeatal complex for 2 weeks. Over the subsequent 11 weeks (disease induction) or 8 weeks (disease re-induction) chronic disease phenotype developed [16]. Subjects were divided into 4 groups for bilateral treatment and monitored for 4 weeks. (B) Representative coronal microCT images of the maxillary sinuses in soft tissue and bone (center) windows. Using the bone window, opacification was measured in a defined region of interest and the median CT # of that region was recorded for 3 consecutive sections. (C) Change in CT # between the disease state and treatment timepoints on the right side. Error bars represent the mean ± S.D. for each group: no treatment (n = 5), v-TEMPS (n = 5), m-TEMPS (n = 5), and daily nasal drops (n = 3). Statistical significance was determined by one-way ANOVA with post hoc Wilcoxon Method testing, ***p < 0.001, **p < 0.020, ns = not significant.
Further evaluation of the tissue response to treatments was performed using histopathology to score inflammation criteria at the study endpoint. On the right side, epithelial cell damage, cilia damage, granulocyte infiltrate, and granulocytes in the lumen were significantly increased in the v-TEMPS group compared to all other groups (p < 0.050). Additionally, inflammation of the submucosal glands was reduced in the m-TEMPS group compared to the v-TEMPS and daily nasal drop groups (p < 0.050, Fig. 4). On the left side, epithelial cell damage and cilia damage were elevated in the m-TEMPS group relative to other groups (p < 0.050), but also exhibited more variability among subjects (Supplementary Fig. 2).
Fig. 4.
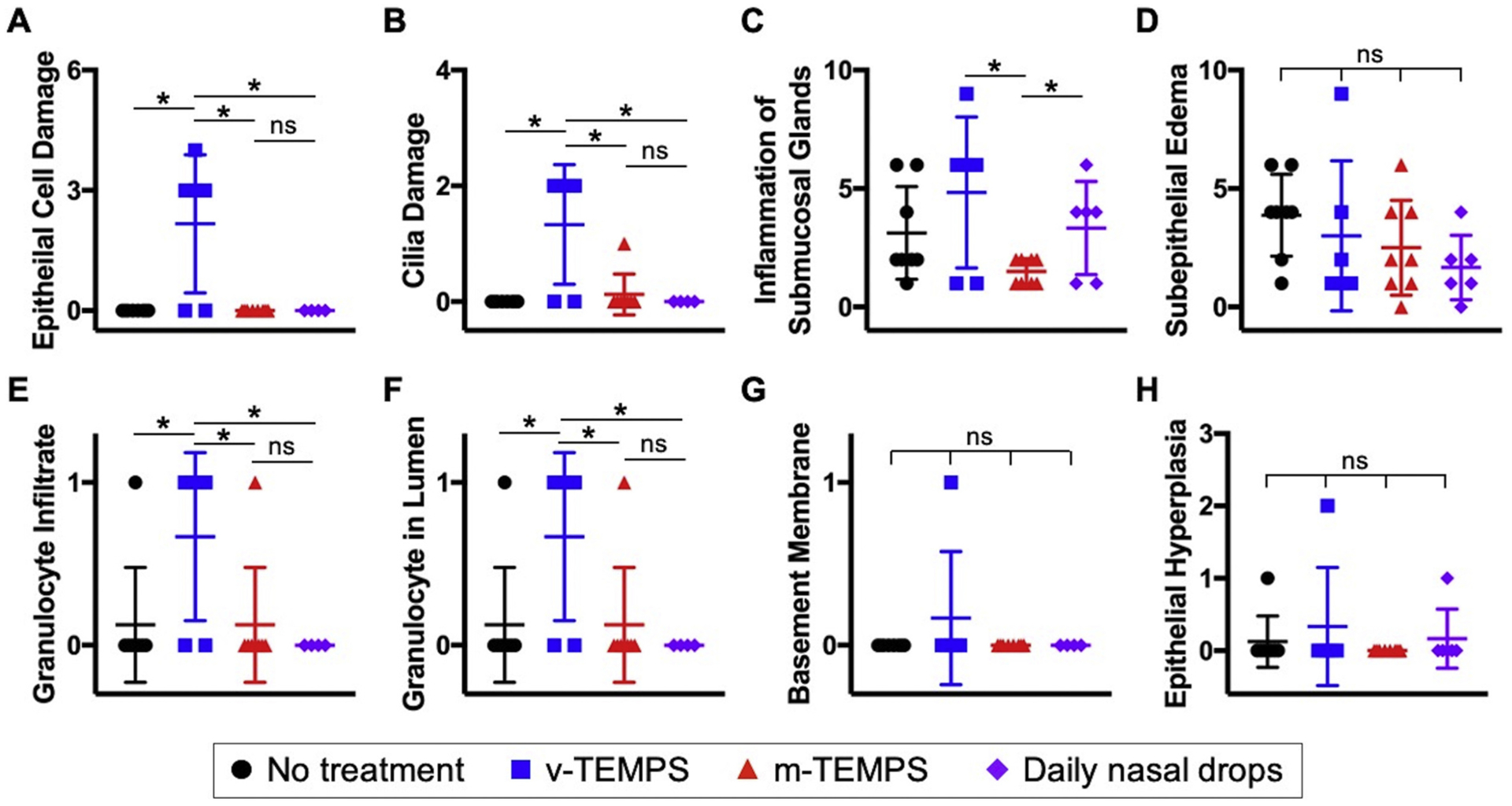
Evidence of iatrogenic trauma due to application of TEMPS was observed in right histopathology sections, but damage was reversed by local steroid delivery. Two sections from each subject were stained with H&E and scored by a blinded veterinary pathologist for (A–D) severity and involvement of (A) epithelial cell damage, (B) cilia damage, (C) inflammation of the submucosal glands, and (D) subepithelial edema, and (E–G) presence (1) or absence (0) of (E) granulocyte infiltrate, (F) granulocytes in the lumen, (G) involvement of the basement membrane, and (H) severity of epithelial hyperplasia. Individual symbols represent the score for each section and error bars represent the mean ± S.D. for treatment groups: no treatment (n = 4), v-TEMPS (n = 3), m-TEMPS (n = 4), and daily nasal drops (n = 3). Statistical significance was determined by Chi-square test using Pearson’s p-value, * p < 0.050, ns = not significant.
3.3. Steroid was safely delivered from TEMPS maintained in the maxillary sinuses for 4 weeks
Throughout the study, bilateral IOP was measured to assess for ocular effects related to localized steroid delivery. Two weeks after treatment application, IOP was not elevated in any group. At 4 weeks, mean IOP of the daily nasal drop group was statistically elevated in comparison with baseline (p < 0.050). Importantly, the m-TEMPS group did not show elevated IOP at any timepoint (Fig. 5A). Additionally, the IOP values reported here are within the range previously reported for healthy New Zealand rabbits, which over a 2 year period was 16.9 mmHg (range: 11.2–25.0, CV: 16.6%, n = 125) [17]. Similarly, the overall mean IOP in this study was 16.7 mmHg (range: 12.0–25.0, CV: 15.3%, n = 72).
Fig. 5.
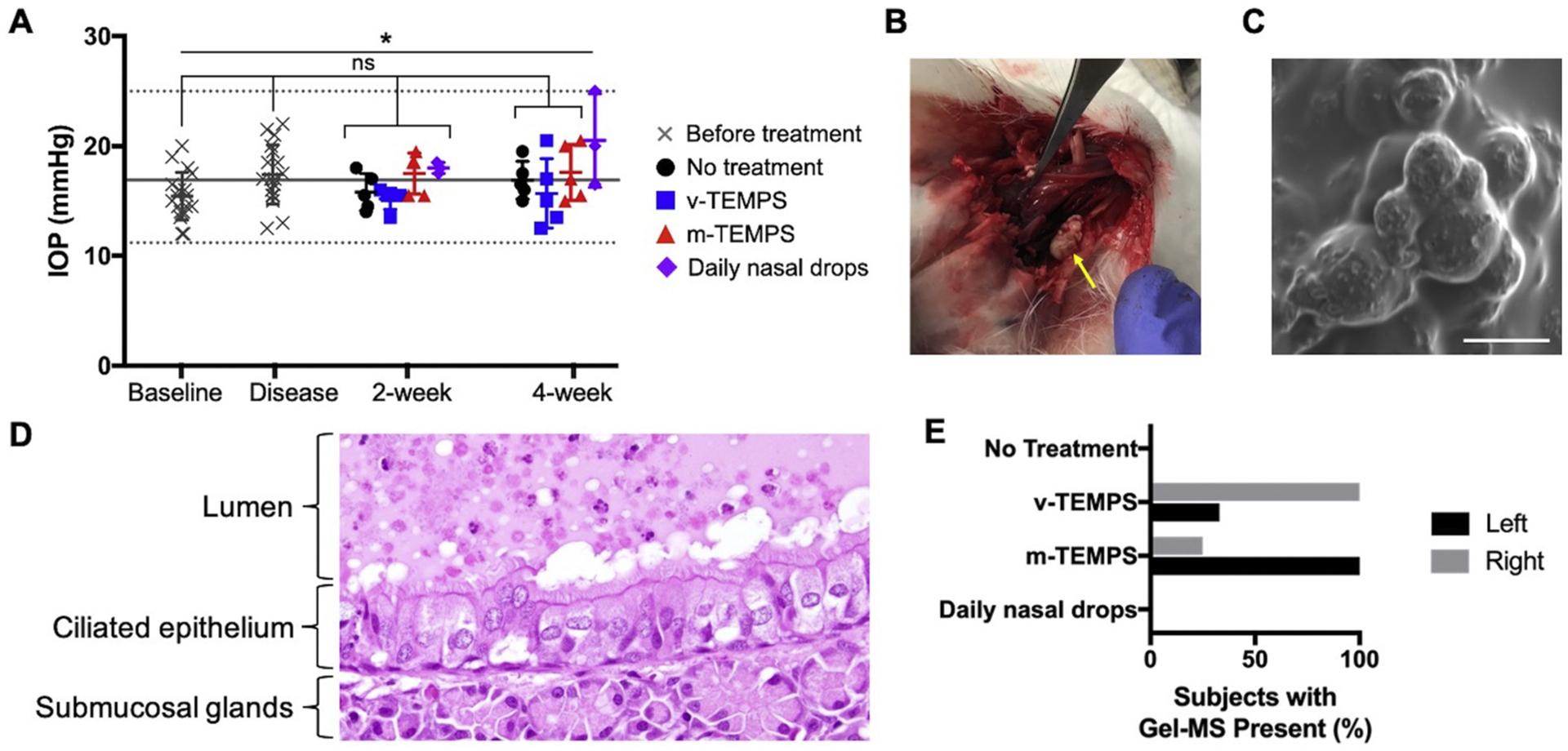
Intraocular pressure was not affected by 4 weeks of local steroid delivery from the retained system. (A) Mean bilateral IOP measurements for each subject are represented as individual symbols and error bars represent the group mean ± S.D. for: before treatment application (n = 18), no treatment (n = 5), v-TEMPS (n = 5), m-TEMPS (n = 5), and daily nasal drops (n = 3). Solid and dotted lines denote the mean and range, respectively, of healthy New Zealand rabbit IOP values [17]. (B) A foreign material (yellow arrow) was recovered during post-mortem analysis from a subject treated with TEMPS. (C) The recovered material was visualized by SEM and spherical MSs were observed, scale bar = 10 μm. (D) Representative H&E section of a subject treated with TEMPS showed a foreign material in the lumen and apposed to the epithelium that contains spherical holes, which are consistent with the expected appearance of TEMPS (40× magnification). (E) Percentage of subjects in each treatment group where TEMPS material was observed in H&E sections. Statistical significance was determined by repeated measures ANOVA with Tukey post-hoc testing, *p < 0.050, ns = not significant.
The ability for TEMPS to be maintained in the maxillary sinuses for 4 weeks was evaluated post-mortem. Within the sinuses of one of the subjects treated with TEMPS, a foreign material was identified (Fig. 5B). This sample was visualized using SEM and microspheres were identified (Fig. 5C). In H&E sections of animals treated with TEMPS, material in the lumen was observed that displayed spherical holes, which were likely the locations of polymer microspheres that dissolved during processing (Fig. 5D). The presence of this material in histological sections was noted on the left and right sides of many subjects in both TEMPS-treated groups (Fig. 5E).
4. Discussion
The sinonasal inflammation that is characteristic of chronic rhinosinusitis (CRS) can be reduced by treatment with steroids [2]. Use of oral steroids, however, can increase risks of adverse effects, such as growth inhibition and decreased bone mineral density [18], and consequently topical methods are preferable if possible. While nasal steroid sprays are convenient, only 30% of the applied dose is deposited in the nasal cavity [18] with very little, if any, reaching the sinus cavities [2,6]. Nasal irrigation is more effective; however, it requires large volumes and daily dosing [7]. CRS patients whose symptoms are not improved by medical therapy can also undergo functional endoscopic sinus surgery to remove inflamed tissue and bone, but challenges of intra-sinus drug delivery still remain in these patients.
In this study, we have presented a potential solution that addresses many existing clinical hurdles with “TEMPS” (Thermogel, Extended-release Microsphere-based-delivery to the Paranasal Sinuses). This system can effectively provide 4 weeks of sustained release of bioactive steroid, mometasone furoate, using a combination of thermogel and polymer microspheres. Importantly, the thermoresponsive nature of the non-biodegradable gel allows its application into the paranasal sinuses as a liquid at ambient temperature, conforming to the sinonasal epithelium as it gels. Within the nasal cavity, inspired air is quickly warmed from 25.3 ± 2.1 °C in the nasal vestibule to 33.9 ± 1.5 °C in the nasopharynx [19] and the paranasal sinuses lie along this temperature transition. Thus, TEMPS can be tuned to reversibly undergo a phase transition at a lower critical solution temperature (LCST) of 34–35 °C [13]. We propose that ambient temperature saline irrigation can return the gel to a liquid state for its removal. While demonstrating removal of the system in vivo was not feasible in the present study, TEMPS that was incubated in vitro at 37 °C for 4 weeks did reversibly undergo a phase change when cooled to ambient temperature at weekly intervals, as expected.
To our knowledge, this is the first hydrogel-based system for the paranasal sinuses that is designed for multiple weeks of sustained, localized steroid release. The advantage of using a gel to adhere topical treatment to the sinonasal mucosa has been previously recognized [20]. In an uncontrolled clinical study, physicians applied a hydrophilic mometasone furoate gel to the sinuses of CRS patients up to 3 times. Drug release kinetics were not specified, and likely not sustained, which is supported by the report of modest, but temporary improvement of the diseased mucosa [20]. Others have designed a thermoresponsive and bioadhesive gel for 4 h of mometasone release [21]. This gel was composed of Pluronic® F-127 and Carbopol® 974P NF and tested for relieving allergic rhinitis in rats and promoting repair of a mucosal injury in rabbits [21–23]. Another mucoadhesive in situ gel designed for the nasal and sinus cavities was synthesized from poloxamer-407, hydroxypropyl methyl cellulose, and chitosan salt and demonstrated the gradual release of steroid, dexamethasone 21-phosphate disodium, for 3 days in vitro [24]. In each of these systems, the shorter duration of drug delivery is dictated by the gel component being the rate limiting step for release. In contrast, release kinetics of TEMPS are intended to be controlled by diffusion and bulk erosion of PLGA microspheres, which can be tuned to release drugs for days, weeks, months, or even years [25]. While in vitro characterization revealed that the pNIPAAm matrix (which surrounds the extended release microspheres) caused a small reduction of the initial burst release from 18.5% (MSs alone) to 11.3% (TEMPS) after 24 h aqueous incubation, the complete 4-week release profile was largely unchanged. It is likely that hydrophobic interactions between mometasone furoate, a lipophilic small molecule [18], and the pNIPAAm chains reduced the rate of initial release but did not limit the ultimate diffusion of drug from the system. Instead, the gel component primarily serves to retain the extended release microspheres and conform within the sinuses.
To measure mometasone release kinetics in vitro, a medium that maintained the lipophilic drug in solution was required. Others have measured the release of mometasone from PLGA endotracheal tubes in 1% sodium dodecyl sulfate (SDS) [15]. While an SDS solution was suitable for measuring mometasone release from PLGA MS alone, release from TEMPS could not be performed because SDS is also a detergent and quickly degraded the thermogel. Thus, other surfactants including sodium deoxycholate and tween-80 were evaluated. A solution of 2% sodium deoxycholate demonstrated both compatibility with the thermogel and the ability to maintain mometasone in solution that could be detected by spectrophotometry for in vitro quantification.
A benefit of the lipophilic nature of mometasone is that it can be efficiently encapsulated in polymeric microspheres at concentrations sufficient for clinical use. The PLGA MS formulation described herein encapsulated 44 μg mometasone per mg MS for an overall loading efficiency of 88% and loading content of 4.4%. In contrast, a recent PLGA nanoparticle formulation reported a mometasone loading content of 22.4% [26]. Despite the lower drug content, the MS formulation met the objectives for this study, including sustained release for 4 weeks (in comparison to 7 days from the nanoparticle formulation [26]) and drug loading that was sufficient for a clinical dose. Specifically, ~250 mg MSs would provide a sufficient quantity of drug to locally release ~400 μg mometasone per day for 4 weeks. This dosage is equivalent to that achieved by bilateral, twice daily applications of a mometasone furoate nasal spray. Furthermore, currently available degradable sinus stents have a total loaded dose of 370 μg or 1350 μg mometasone per device and are designed for gradual release over 30 or 90 days, respectively [27,28]. In patients with severe inflammation, increased steroid dosing with sustained delivery may be warranted and provide more effective symptom relief [29]. The PLGA MS component of TEMPS enables sufficient surface area-to-volume loading of mometasone, as well as flexible dosing by adjusting the amount of MSs in the system [30].
The dosing flexibility provided by TEMPS is a product of both the amount of embedded MSs as well as the total volume for application. The system is intended to be applied as a thin coating along the sinus epithelium, either targeted to specific areas of inflammation or distributed throughout the sinuses for widespread treatment. Reported volumes of the maxillary and frontal sinuses of post-operative CRS patients and healthy individuals range from 37 cm3 to 57 cm3 [31]. At the MS concentration tested in this pilot study (50 mg/mL), a patient dose of TEMPS would equate to 5 mL, or just 9–14% of reported sinus volumes. Additionally, this dosing volume is comparable to the clinical study previously mentioned in which 2–10 mL of a mometasone gel was applied in post-operative CRS patients and no adverse effects related to this treatment were reported [20].
The anticipated human dose was scaled for the pilot rabbit study using FDA guidances for Human Equivalent Doses, which specify that a rabbit dose should be 3.1 times a human dose (in mg/kg). Using the assumptions of dosing 400 μg/day to a 60 kg human, the equivalent dose for a 3 kg rabbit is 62 μg/day. To achieve this dosing, 0.4 mL of TEMPS was injected bilaterally. This dosing volume was compared to a model of the rabbit nasal passage, which described the sinuses as a “lateral recess” with a volume of 1029 mm3 on each side [32]. Therefore, the scaled dose was less than half of the rabbit sinus volume.
Another safety consideration for injecting TEMPS is the material’s cytocompatibility, which was evaluated using a human sinonasal cell line derived from a squamous cell carcinoma of the nasal septum (RPMI 2650). While characterization of this cell line has reported several biologic differences from the primary nasal epithelium, it is the only alternative to human nasal epithelial cells (hNECs) [33]. In previous work, RPMI 2650 cells and hNECs were both used for compatibility testing of mucoadhesive and nanostructured microparticles that were developed for experimental treatment of nasal polyps. The delivery vehicle was shown to be compatible with similar trends in viability between the two cell types [34]. Likewise, RPMI 2650 cells that were incubated with PEG-pNIPAAm gel for 24 h showed comparable viability to control cells. Furthermore, these results are consistent with reported cytocompatibility of pNIPAAm gels with human conjunctival epithelial cells [12,13], as well as the numerous biomedical applications that have safely applied PEG-pNIPAAm hydrogels for in vivo testing [12,35].
Herein, the in vivo safety and retention of TEMPS was evaluated in a CRS disease model in rabbits [16]. Rabbits are frequently used for sinusitis studies because their sinus anatomy and immune response are more similar to humans than rodents [36]. However, as is the case in humans, rabbit sinuses are not accessible through the nares without surgically removing bone. For this pilot study, blank vehicle TEMPS (v-TEMPS) and mometasone-loaded TEMPS (m-TEMPS) were percutaneously injected into the left and right maxillary sinuses because the material is flowable at ambient temperatures. Recovery of the gelled material was demonstrated in one subject post-mortem, showing that the system was maintained for at least 4 weeks. Notably, during those 4 weeks, intraocular pressure (IOP) was monitored and the subjects treated with sustained, local steroid from m-TEMPS did not show elevated pressures compared to baseline or the control groups. Additionally, pressures across treatment groups and time remained within the range previously reported for healthy New Zealand rabbits [17]. This is relevant to the safety of high-dose and long-term steroid use, which can pose the risk of elevated IOP and adverse ocular effects, particularly for oral or inhaled corticosteroids [18]. Treatment with the intranasal corticosteroid mometasone furoate, however, has shown no significant differences in IOP following topical delivery via nasal sprays [37,38], stents [9], and as shown in this study, TEMPS.
These topical steroid delivery methods are intended to reduce inflammation, which can be non-invasively monitored by computed tomography (CT) imaging. In clinical settings, CT is the gold standard imaging modality for the diagnosis of CRS and for pre-operative assessment of the extent and location of disease [2,3]. Paranasal sinus inflammation will present on CT imaging as opacification, which is then scored using various staging systems [39]. While scoring systems have been adapted for microCT imaging of disease in rabbit models [16,40,41], we reported opacification as the CT # instead, which is a measurement of the attenuation number of the x-ray beams through the tissue of interest [42,43]. To our knowledge, the presented method for assessing rabbit sinus opacification by reporting the median CT # of a region encompassing the sinuses is new to the field. The advantage of this method is that it allows for objective, blinded measurements that can be performed without prior training on assessing rabbit microCT scans and could be reproducible across investigators and institutions.
In this study, the efficacy of TEMPS was measured by microCT imaging at 4 timepoints and by histopathology analysis at the conclusion of the study. Comparison of the CT # between the TEMPS groups revealed that treatment with m-TEMPS resulted in significantly lower sinus opacification compared to v-TEMPS at 2- and 4-weeks after application. This trend of the presence of inflammation in the v-TEMPS but not the m-TEMPS group was also observed in histopathology analyses, specifically in damage to the ciliated epithelium, submucosal gland inflammation, and presence of granulocytes. Taken together, these results suggest that m-TEMPS was able to provide sustained, local release of mometasone in vivo, particularly because without a sustained release mechanism, the half-life of mometasone furoate is just 5.8 h [44]. Furthermore, evidence that TEMPS could be maintained in the rabbit maxillary sinus was observed 4 weeks after a single application. For comparison, in the clinical study that was previously mentioned, application of a mometasone gel that did not provide sustained release resulted in only temporary improvement of the diseased mucosa [20]. Notably, observations from the present study were made on the control (right) sinus, rather than the disease-induced (left) sinus. The differences between the two sides is likely caused by the variability of disease induction, which is a limitation of this study. While this obstruction-based CRS disease model has been shown to induce persistent unilateral inflammation [16], in this study, changes in CT # from baseline to the disease state were inconsistent. Due to this variability and the small sample size, the effect of m-TEMPS to reduce inflammation caused by sinus obstruction could not be evaluated. In future studies, other disease models or more complete ostium obstruction will be explored, although currently, the development of preclinical models for CRS remains an area of continued investigation [36].
Another limitation of the pilot rabbit study is that the application of TEMPS by percutaneous injection may have caused iatrogenic trauma. Notably, on the control side, this inflammatory trauma was reduced by the sustained release of mometasone from m-TEMPS compared to the blank vehicle, v-TEMPS. However, on the disease-induced side, there may have been an interacting effect of the ostial obstruction and TEMPS injection that limited the anti-inflammatory efficacy of m-TEMPS. In future studies, a different method for TEMPS application will be investigated to confirm safety and efficacy in preclinical studies. Although external approaches for surgically disrupting rabbit sinonasal mucosa or implanting devices exist, these methods likely cause damage that is not representative of sinusitis [36]. Notably, in a clinical setting, TEMPS is intended to be applied transnasally following endoscopic surgery, which we propose would be a more suitable method for application in rabbits as well. Performing this procedure in rabbits will require precise bone removal to surgically open the sinus ostium via the naris in order to access the maxillary sinus while inducing as little added trauma to the surrounding anatomy. Surgical opening of the sinus cavity will also allow visualization of how the material distributes along the sinuses and the maintenance of apposition to the mucosal surfaces, which is critical to its successful drug delivery in vivo.
Another consideration for the efficacy of in vivo drug delivery is the pharmacokinetics (PK), however, in the case of local delivery of mometasone furoate, a PK study for TEMPS would be particularly non-trivial given the tissue microenvironment and the drug stability. Traditionally, drug concentration can be measured non-invasively by collecting blood samples. However, plasma concentrations of mometasone are expected to be near or below limits of quantification because mometasone has <0.1% systemic bioavailability [18] due to its degradation at pH > 0.4 [45], which remains a notable benefit of the safety of this intranasal corticosteroid. As TEMPS is intended to locally deliver mometasone to the sinuses, a PK study would require serial sacrifice and tissue harvest in order to measure drug concentration in the sinus tissue, or recovering the material from rabbit sinuses to measure the drug content that is remaining as a function of time. Although several studies suggest that release into surrounding tissue from implanted or injected PLGA microspheres can be considerably faster than what is measured in vitro (likely due to accelerated hydrolysis caused by a dramatic increase in water content in vivo compared to in vitro [46]), this is not expected for TEMPS. With TEMPS, rather, the MSs are surrounded by the non-biodegradable pNIPAAm gel, which above the LCST, is in a collapsed, hydrophobic configuration both when applied to the sinus epithelium in vivo and when the material is incubated in vitro. Accordingly, the rate of hydrolysis (and therefore drug release kinetics) is expected to be similar in vivo as to what has been demonstrated in vitro because the surrounding pNIPAAm gel is controlling the water content accessible to the PLGA MSs.
5. Conclusions
In summary, data suggest that TEMPS could be a promising system to provide sustained, bioactive steroid delivery for reduction of sinonasal inflammation. TEMPS is cyto-compatible in vitro and is well-tolerated in rabbits in vivo. Due to its temperature-sensitive nature, TEMPS can provide unique apposition to tissue architecture, like the paranasal sinuses. Moreover, while the thermogel matrix is non-biodegradable, the system is temporary and can be readily removed and reapplied as needed. Future studies will focus on improving the robustness of disease induction, increasing sample sizes, and applying TEMPS to the sinus epithelia in rabbits transnasally in order to more accurately represent the potential clinical efficacy for treating pathologies of the sinonasal mucosa.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR001858. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Dr. Do-Yeon Cho and Dr. Brad Woodworth at the University of Alabama for their guidance with the rabbit model, and the University of Pittsburgh Division of Laboratory Animal Resources for their animal care during this study. Graphical abstract was created with Biorender.com.
Footnotes
Declaration of Competing Interest
None.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jconrel.2020.10.062.
References
- [1].DeConde AS, Soler ZM, Chronic rhinosinusitis: epidemiology and burden of disease, Am. J. Rhinol. Allergy 30 (2016) 134–139, 10.2500/ajra.2016.30.4297. [DOI] [PubMed] [Google Scholar]
- [2].Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, Brook I, Ashok Kumar K, Kramper M, Orlandi RR, Palmer JN, Patel ZM, Peters A, Walsh SA, Corrigan MD, Clinical practice guideline (update): adult sinusitis, Otolaryngol. - Head Neck Surg. (United States) 152 (2015) S1–S39, 10.1177/0194599815572097. [DOI] [PubMed] [Google Scholar]
- [3].Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, Baroody FM, Batra PS, Bernal-Sprekelsen M, Bhattacharyya N, Chandra RK, Chiu A, Citardi MJ, Cohen NA, Delgaudio J, Desrosiers M, Dhong HJ, Douglas R, Ferguson B, Fokkens WJ, Georgalas C, Goldberg A, Gosepath J, Hamilos DL, Han JK, Harvey R, Hellings P, Hopkins C, Jankowski R, Javer AR, Kern R, Kountakis S, Kowalski ML, Lane A, Lanza DC, Lebowitz R, Lee HM, Lin SY, Lund V, Luong A, Mann W, Marple BF, Mcmains KC, Metson R, Naclerio R, Nayak JV, Otori N, Palmer JN, Parikh SR, Passali D, Peters A, Piccirillo J, Poetker DM, Psaltis AJ, Ramadan HH, Ramakrishnan VR, Riechelmann H, Roh HJ, Rudmik L, Sacks R, Schlosser RJ, Senior BA, Sindwani R, Stankiewicz JA, Stewart M, Tan BK, Toskala E, Voegels R, Wang DY, Weitzel EK, Wise S, Woodworth BA, Wormald PJ, Wright ED, Zhou B, Kennedy DW, International consensus statement on allergy and rhinology: rhinosinusitis, Int. Forum Allergy Rhinol 6 (2016) S22–S209, 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- [4].Soler ZM, Wittenberg E, Schlosser RJ, Mace JC, Smith TL, Health state utility values in patients undergoing endoscopic sinus surgery, Laryngoscope 121 (2011) 2672–2678, 10.1002/lary.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Piromchai P, Kasemsiri P, Laohasiriwng S, Thanaviratananich S, Chronic rhinosinusitis and emerging treatment options, Int. J. Gen. Med 6 (2013) 453–464, 10.2147/IJGM.S29977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Möller W, Schuschnig U, Celik G, Münzing W, Bartenstein P, Häussinger K, Kreyling WG, Knoch M, Canis M, Becker S, Topical drug delivery in chronic rhinosinusitis patients before and after sinus surgery using pulsating aerosols, PLoS One 8 (2013), e74991, 10.1371/journal.pone.0074991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Snidvongs K, Thanaviratananich S, Update on intranasal medications in rhinosinusitis, Curr Allergy Asthma Rep 17 (2017), 10.1007/s11882-017-0720-3. [DOI] [PubMed] [Google Scholar]
- [8].Li PF, Downie D, Hwang PH, Controlled steroid delivery via bioabsorbable stent: safety and performance in a rabbit model, Am. J. Rhinol. Allergy 23 (2009) 591–596, 10.2500/ajra.2009.23.3391. [DOI] [PubMed] [Google Scholar]
- [9].Marple BF, Smith TL, Han JK, Gould AR, Jampel HD, Stambaugh JW, Mugglin AS, Advance II: a prospective, randomized study assessing safety and efficacy of bioabsorbable steroid-releasing sinus implants, Otolaryngol. Neck Surg 146 (2012) 1004–1011, 10.1177/0194599811435968. [DOI] [PubMed] [Google Scholar]
- [10].Lavigne F, Miller SK, Gould AR, Lanier BJ, Romett JL, Steroid-eluting sinus implant for in-office treatment of recurrent nasal polyposis: a prospective, multicenter study, Int. Forum Allergy Rhinol 4 (2014) 381–389, 10.1002/alr.21309. [DOI] [PubMed] [Google Scholar]
- [11].Tang DM, Roxbury CR, Sindwani R, Kshettry VR, Recinos P, Woodard TD, Multiple bioabsorbable corticosteroid-eluting stent placement with associated skull base injury, Laryngoscope 129 (2019) 1494–1496, 10.1002/lary.27659. [DOI] [PubMed] [Google Scholar]
- [12].Fedorchak MV, Conner IP, Schuman JS, Cugini A, Little SR, Long term glaucoma drug delivery using a topically retained gel/microsphere eye drop, Sci. Rep 7 (2017) 8639, 10.1038/s41598-017-09379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bellotti E, Fedorchak MV, Velankar S, Little SR, Tuning of thermoresponsive pNIPAAm hydrogels for the topical retention of controlled release ocular therapeutics, J. Mater. Chem B 7 (2019) 1276–1283, 10.1039/C8TB02976H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Galgatte UC, Kumbhar AB, Chaudhari PD, Development of in situ gel for nasal delivery: design, optimization, in vitro and in vivo evaluation, Drug Deliv. 21 (2014) 62–73, 10.3109/10717544.2013.849778. [DOI] [PubMed] [Google Scholar]
- [15].Ammar AA, Gruber M, Martin P, Stern O, Jahshan F, Ertracht O, Sela E, Srouji S, Zussman E, Local delivery of mometasone furoate from an eluting endotracheal tube, J. Control. Release 272 (2018) 54–61, 10.1016/j.jconrel.2018.01.005. [DOI] [PubMed] [Google Scholar]
- [16].Cho D-Y, Mackey C, Van Der Pol WJ, Skinner D, Morrow CD, Schoeb TR, Rowe SM, Swords WE, Tearney GJ, Woodworth BA, Sinus microanatomy and microbiota in a rabbit model of rhinosinusitis, Front. Cell. Infect. Microbiol 7 (2018) 540, 10.3389/fcimb.2017.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bar-Ilan A, The pattern of distribution of intraocular pressure in the albino rabbit, Graefes Arch. Clin. Exp. Ophthalmol 224 (1986) 469–472, 10.1007/BF02173366. [DOI] [PubMed] [Google Scholar]
- [18].Sastre J, Mosges R, Local and systemic safety of intranasal corticosteroids, J Investig Allergol Clin Immunol 22 (2012) 1–12. [PubMed] [Google Scholar]
- [19].Keck T, Leiacker R, Riechelmann H, Rettinger G, Temperature profile in the nasal cavity, Laryngoscope 110 (2000) 651–654, 10.1097/00005537-200004000-00021. [DOI] [PubMed] [Google Scholar]
- [20].Alava I, Isaacs S, Luong A, Citardi MJ, Fakhri S, Mometasone furoate gel: a novel in-office treatment of recalcitrant postoperative chronic rhinosinusitis, J. Otolaryngol. Head Neck Surg 41 (2012) 183–188, 10.2310/7070.2012.00023. [DOI] [PubMed] [Google Scholar]
- [21].Altuntas E, Yener G, Formulation and evaluation of thermoreversible in situ nasal gels containing mometasone furoate for allergic rhinitis, AAPS PharmSciTech (2017), 10.1208/s12249-017-0747-8. [DOI] [PubMed] [Google Scholar]
- [22].Altuntaş E, Yener G, Doğan R, Aksoy F, Şerif Aydın M, Karataş E, Effects of a thermosensitive in situ gel containing mometasone furoate on a rat allergic rhinitis model, Am. J. Rhinol. Allergy 32 (2018) 132–138, 10.1177/1945892418764951. [DOI] [PubMed] [Google Scholar]
- [23].Aksoy F, Dogan R, Ozturan O, Altuntas E, Yener FG, Topcu G, Guler B, Effect of a combination of mometasone furoate, levofloxacin, and retinyl palmitate with an in situ gel-forming nasal delivery system on nasal mucosa damage repair in an experimental rabbit model, Biomed. Pharmacother 96 (2017) 603–611, 10.1016/j.biopha.2017.10.023. [DOI] [PubMed] [Google Scholar]
- [24].Pandey P, Cabot PJ, Wallwork B, Panizza BJ, Parekh HS, Formulation, functional evaluation and ex vivo performance of thermoresponsive soluble gels - a platform for therapeutic delivery to mucosal sinus tissue, Eur. J. Pharm. Sci 96 (2017) 499–507, 10.1016/j.ejps.2016.10.017. [DOI] [PubMed] [Google Scholar]
- [25].Rothstein SN, Kay JE, Schopfer FJ, Freeman BA, Little SR, A retrospective mathematical analysis of controlled release design and experimentation, Mol. Pharm 9 (2012) 3003–3011, 10.1021/mp300388w. [DOI] [PubMed] [Google Scholar]
- [26].Far J, Abdel-Haq M, Gruber M, Abu Ammar A, Developing biodegradable nanoparticles loaded with mometasone furoate for potential nasal drug delivery, ACS Omega. 5 (2020) 7432–7439, 10.1021/acsomega.0c00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Han JK, Marple BF, Smith TL, Murr AH, Lanier BJ, Stambaugh JW, Mugglin AS, Effect of steroid-releasing sinus implants on postoperative medical and surgical interventions: an efficacy meta-analysis, Int. Forum Allergy Rhinol 2 (2012) 271–279, 10.1002/alr.21044. [DOI] [PubMed] [Google Scholar]
- [28].Kern RC, Stolovitzky JP, Silvers SL, Singh A, Lee JT, Yen DM, Iloreta AMC Jr., Langford FPJ, Karanfilov B, Matheny KE, Stambaugh JW, Gawlicka AK, for the RESOLVE II study investigators, A phase 3 trial of mometasone furoate sinus implants for chronic sinusitis with recurrent nasal polyps, Int. Forum Allergy Rhinol 8 (2018) 471–481, 10.1002/alr.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dkhar LK, Bartley J, White D, Seyfoddin A, Intranasal drug delivery devices and interventions associated with post-operative endoscopic sinus surgery, Pharm. Dev. Technol 23 (2018) 282–294, 10.1080/10837450.2017.1389956. [DOI] [PubMed] [Google Scholar]
- [30].Rothstein SN, Little SR, A “tool box” for rational design of degradable controlled release formulations, J. Mater. Chem 21 (2011) 29–39, 10.1039/c0jm01668c. [DOI] [Google Scholar]
- [31].Kumar H, Jain R, Douglas RG, Tawhai MH, Airflow in the human nasal passage and sinuses of chronic rhinosinusitis subjects, PLoS One 11 (2016) 1–14, 10.1371/journal.pone.0156379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xi J, Si XA, Kim J, Zhang Y, Jacob RE, Kabilan S, Corley RA, Anatomical details of the rabbit nasal passages and their implications in breathing, air conditioning, and olfaction, Anat. Rec 299 (2016) 853–868, 10.1002/ar.23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ball SL, Suwara MI, Borthwick LA, Wilson JA, Mann DA, Fisher AJ, How reliable are sino-nasal cell lines for studying the pathophysiology of chronic rhinosinusitis? Ann. Otol. Rhinol. Laryngol 124 (2015) 437–442, 10.1177/0003489414565003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee M, Park CG, Huh BK, Kim S-N, Lee SH, Khalmuratova R, Park J-W, Shin H-W, Bin Choy Y, Sinonasal delivery of resveratrol via Mucoadhesive nanostructured microparticles in a nasal polyp mouse model, Sci. Rep 7 (2017) 40249, 10.1038/srep40249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Alexander A, Khan J, Saraf S, Saraf S, Polyethylene glycol (PEG)–Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications, Eur. J. Pharm. Biopharm 88 (2014) 575–585, 10.1016/j.ejpb.2014.07.005. [DOI] [PubMed] [Google Scholar]
- [36].Al-Sayed AA, Agu RU, Massoud E, Models for the study of nasal and sinus physiology in health and disease: a review of the literature, Laryngoscope Investig. Otolaryngol 2 (2017) 398–409, 10.1002/lio2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Şimşek A, Bayraktar C, Doğan S, Karataş M, Sarıkaya Y, The effect of long-term use of intranasal steroids on intraocular pressure, Clin. Ophthalmol 10 (2016) 1079–1082, 10.2147/OPTH.S106392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bross-Soriano D, Hanenberg-Milver C, Schimelmitz-Idi J, Arrieta-Gomez JR, Astorga Del Toro R, Bravo-Escobar G, Effects of three nasal topical steroids in the intraocular pressure compartment, Otolaryngol. Head Neck Surg 130 (2004) 187–191, 10.1016/j.otohns.2003.09.020. [DOI] [PubMed] [Google Scholar]
- [39].Zinreich SJ, Imaging for staging of rhinosinusitis, Ann. Otol. Rhinol. Laryngol 113 (2004) 19–23, 10.1177/00034894041130s506. [DOI] [PubMed] [Google Scholar]
- [40].Ozcan KM, Ozcan I, Selcuk A, Akdogan O, Gurgen SG, Deren T, Koparal S, Ozogul C, Dere H, Comparison of histopathological and CT findings in experimental rabbit sinusitis, Indian J. Otolaryngol. Head Neck Surg 63 (2011) 56–59, 10.1007/s12070-011-0120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kerschner JE, Cruz MJ, Beste DJ, Donahue KM, Kehl KS, Computed tomography vs. magnetic resonance imaging of acute bacterial sinusitis: a rabbit model, Am. J. Otolaryngol 21 (2000) 298–305, 10.1053/ajot.2000.9874. [DOI] [PubMed] [Google Scholar]
- [42].Hounsfield GN, Computed medical imaging, Science (80) 210 (1980) 22–28. [DOI] [PubMed] [Google Scholar]
- [43].Cho SH, Min HJ, Han HX, Paik SS, Kim KR, CT analysis and histopathology of bone remodeling in patients with chronic rhinosinusitis, Otolaryngol. Head Neck Surg 135 (2006) 404–408, 10.1016/j.otohns.2006.04.005. [DOI] [PubMed] [Google Scholar]
- [44].Szefler SJ, Pharmacokinetics of intranasal corticosteroids, J. Allergy Clin. Immunol 108 (2001) S26–S31, 10.1067/mai.2001.115563. [DOI] [PubMed] [Google Scholar]
- [45].Teng XW, Cutler DC, Davies NM, Degradation kinetics of mometasone furoate in aqueous systems, Int. J. Pharm 259 (2003) 129–141, 10.1016/S0378-5173(03)00226-6. [DOI] [PubMed] [Google Scholar]
- [46].Doty AC, Weinstein DG, Hirota K, Olsen KF, Ackermann R, Wang Y, Choi S, Schwendeman SP, Mechanisms of in vivo release of triamcinolone acetonide from PLGA microspheres, J. Control. Release 256 (2017) 19–25, 10.1016/j.jconrel.2017.03.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


