Abstract
The purpose of this investigation was to consider person characteristics, treatment level variables, and illicit drug use to help explain the HIV antiviral adherence patterns of a community-based, non-drug-treatment-seeking sample of men who have sex with men (MSM). Adherence data were gathered for 300 MSM eight times over the course of 1 year using electronic monitoring. Treatment and person level characteristics were assessed at baseline assessment using computer-administered surveys, and drug usage was established via a diagnostic inventory. These longitudinal data were analyzed via Hierarchical Linear Modeling. The sample was diverse in terms of age and race/ethnicity. Across the span of the year in which the participants were assessed, adherence rates were relatively stable and high (means: 82% to 90%) at each time point and remained relatively stable across the yearlong investigation. Lower adherence rates were evident among those who were drug users, black identified (in terms of race), older, and by pill burden. Individuals on HIV antiretroviral therapy demonstrated consistent although not optimal adherence rates when assessed during the course of a year. The significance of numerous person level factors such as age, race, and drug use suggest that adherence to treatment may in part be impacted by the circumstances that the individual brings to the treatment behavior, and suggests interventions that delve beyond the behavioral to consider and address life social and intrapersonal circumstances that may interfere with adherence behaviors.
INTRODUCTION
A DHERENCE TO ANTIRETROVIRAL therapy (ART; formerly known as HAART) is essential in the treatment of HIV. High levels of adherence (95% or above) are necessary to achieve viral suppression and are associated with greater CD4 lymphocyte count, less chance of developing opportunistic infection, and lower levels of hospitalization.1–3 However, more recent studies have suggested that perhaps for non-nucleoside reverse transcriptase inhibitors, viral suppression might be achieved with lower adherence (i.e., as low as 54%) rates.4 Regardless of these more recent findings, the matter of ART medication adherence remains crucially important because poorer adherence to these regimens has been associated with drug resistance and mutant strains of the virus,5,6 which can be transmitted to uninfected people.7,8 Therefore, researchers have sought to determine antecedents associated with adherence including a variety of person/behavioral and treatment factors, suggesting a biopsychosocial paradigm9 to understanding this behavior.
Research has examined the relations between person-level factors and ART including race/ethnicity, developmental stage, and relationship status. In a recent multicenter clinical trial,10 nonwhite patients indicated lower levels of adherence. Additionally, in several studies, African Americans have been shown to demonstrate lower levels of adherence.11,12 This may be due to lack of self-efficacy, which has been associated with doubting one’s ability to take the medications,13 missing more HIV care appointments,14 or less perceived access to quality health insurance.15 African Americans are more likely to receive HIV care at public clinics as opposed to private clinics15 and are also more likely to have Medicaid or to be uninsured in comparison with their white counterparts.16,17 Lower access to health care is associated with lower levels of adherence in the United States;18 however, quality of health care was found to have no effect on adherence in a recent study conducted in Brazil,19 where all HIV patients have access to generic formulations of ART. Another study found that people of lower socioeconomic status in a universal health care system are less likely to receive ART, which suggests unequal access20 and that people of low financial status, particularly those who are unemployed, demonstrate lower levels of adherence.21 Employment status plays an important role in ART because people who are employed are more likely to have undetectable viral loads.22
Support systems in terms of relationships have provided another lens to examine adherence to ART. A current finding suggests that being in a primary relationship is related to lower levels of adherence regardless of sexual orientation,11 and another recent study found that people in serodiscordant relationships report lower rates of adherence due to concerns of serostatus disclosure.23 Other studies found that these negative associations are not as pronounced in gay men,24,25 and it has also been indicated that adherence is not related to having a primary partner regardless of serostatus in gay men.15 Relationships in which HIV status has been disclosed increase practical and emotional support in gay and bisexual men who are HIV-positive,26,27 yet the impact of primary relationships on ART currently remains ambiguous.
In addition, developmental stage is another area of interest. Older patients, usually over 50 years of age, have demonstrated better adherence to ART than their younger counterparts.19,28–31 This may be because older patients report fewer interruptions to their regimens,32 are more likely to keep their HIV care-related appointments,14 and may be more familiar with medication use and have increased awareness of the detrimental effects of poor adherence.33 Recent findings also suggest that older age is associated with lower levels of virus replication, regardless of quality of adherence,34 so further investigation is needed to determine the impact of age.
In terms of behavioral elements, perhaps the one most examined is recreational drug use. Recreational drug use is consistently associated with lower rates of adherence.35–37 The use of these substances may have a negative effect on adherence due to an increase in cognitive deficits including memory impairment,38,39 but more importantly, drug use can interfere with daily routine (i.e., schedule, meals, sleep), which is a very important component of these oftentimes very complex regimens.40,41 Lower levels of adherence are more pronounced in polydrug users,42 and there is evidence of a possible dose–response relationship between the use of certain drugs and level of adherence, suggesting that heavy or “binge” use might have a more adverse effect on adherence.43 Further investigation is required to determine how patterns or level of drug use are related to adherence.
With regard to treatment itself, the literature suggests that regimens consisting of a high number of pills are associated with lower levels of adherence.19,22,29,30 The number of pills prescribed per day reflects the number of prescribed medications and the number of required daily dosings, so this serves as a good indicator of complexity of regimen.19 Patients prefer to take fewer pills per day,44 and a recent study found that moving patients from two doses per day to one dosing per day leads to less of a decline of adherence over a 24-week period.45 This suggests that many patients experience pill burden, and regimens containing many pills per day may be too complex and thus lead to lower levels of adherence.
Taken in combination, these factors suggest that adherence to ART can be best understood by examining person/behavioral-level and treatment-level characteristics. The individual on ART must negotiate the realties of his/her life with the demands of the medication regimen.
Many investigations have examined factors related to ART adherence; however, few have utilized longitudinal methodologies. Utilizing the Medication Electronic Monitoring System (MEMS) and a cohort of 108 HIV-positive participants over the course of 48 weeks, Liu et al. found that adherence decreased over time.46 An intervention study of 240 HIV-positive participants over a 6-month period that used MEMS as a measure also found a decrease in adherence over time.47 Investigations with smaller sample sizes have also sought to examine adherence over time and sometimes found conflicting results.28,31,48–50 These results may not be fully accurate because analyses relied on short time spans, which may have provided less accurate estimates of adherence over time. While many of these longitudinal investigations utilized MEMS technology, many assessed participants for less than 12 months and some did not contain large samples. Most studies have engaged participants for periods from only 2 to 28 days in order to assess adherence.51–53 While the majority of investigations measure 28–day periods, measuring adherence by the prior week or 2–3 days prior to assessment may be too short a period of time to gain a correct estimate of adherence. Large samples analyzed over a longer time span are needed to strengthen the understanding of ART adherence over time, as well as the factors that help to explain these adherence trajectories. Moreover, studies investigating adherence to ART tend to be cross-sectional or retrospective, and studies that do utilize prospective longitudinal methodologies tend to use simple pairwise comparisons and repeated-measures analysis of variance, but rarely use more advanced multilevel modeling.46,54
Because of such methodological confounds, we designed and conducted a larger-scale investigation over a more extended period of time in order to gain a better understanding of ART adherence and the predictors, which have been previously studied cross-sectionally. This design, in combination with MEMS technology and more advanced, multilevel statistical methodology, leads to a more powerful understanding of adherence across a time span. Thus, the purpose of our investigation was to conduct a year-long assessment of ART adherence and its correlates in a sample of 300 gay and bisexual men. In what follows we (1) describe the yearlong adherence trajectories of this cohort; and (2) consider the person and treatment level predictors of these trajectories using Hierarchical Linear Modeling.
METHOD
Design
The data presented here are drawn from Project PILLS (Protease Inhibitor Longitudinal Life Study), which sought to characterize the relationship between behavioral and sociodemographic variables in relation to medication adherence of protease inhibitors (PIs) in a cohort of 300 men who have sex with men (MSM) in New York City, funded by the National Institute on Drug Abuse. Since adherence rates tend to be comparable across all medications in an ART regimen,15 this study focused on one class of medications in a regimen: PIs, which are widely prescribed.55 Participants were assessed eight times over the course of 1 year, and the study was concluded in 2004. All aspects of the protocol were approved by the institutional review board of the authors’ university.
Procedure
Participants were recruited using active and passive recruitment strategies. The recruitment efforts were based on a targeted sampling strategy, designed to obtain systematic information because true random sampling was not feasible.56 Although targeted sampling cannot assure diversity of the sample, it can be used to enhance the degree to which the sample includes participants who come from a variety of backgrounds, reside within different social circles and participate in gay, bisexual, and/or HIV/AIDS communities to varying degrees. Participants were actively recruited from AIDS service organizations and mainstream gay venues (e.g., bars, cafes, dance clubs, sex clubs, streets in predominately gay neighborhoods). Participants were also passively recruited using “tear-off flyers,” study cards, advertisements in gay and mainstream publications, and through peer referrals. Some men were enrolled in the study through peer referrals or snowball sampling resulting from participants recommending the study to other HIV-positive men. Substantial efforts were made to ensure that an ethnically diverse sample was obtained by targeting venues serving the needs of HIV-positive gay and bisexual men of color. A targeted strategy was used to ascertain a diverse sample both in terms of race/ethnicity and drug use.
To determine eligibility, men were screened by telephone. To meet inclusion criteria respondents must have been (1) biologically male, (2) predominantly English-speaking, (3) the age of 18 or older, (4) self-identified as HIV-positive, (5) report having had sex with another man in the previous year (MSM), and (6) on an ART regimen that consisted of at least one PI. The list of PIs included at the time of data collection were as follows: Saquinavir (Fortovase), Ritonavir (Norvir), Indinavir (Crixivan), Nelfinavir (Viracept), Amprenavir (Agenerase) and Lopinavir (Kaletra).
Respondents who were eligible and still interested in participating were scheduled for a two-part baseline interview. Baseline assessments consisted of two appointments. At the initial baseline interview, participants gave informed consent. Participants then were provided with an explanation of the project and the use of the MEMS Track Cap, which was used as a primary measure of adherence. Specific information about the PI regimens was assessed because each medication has differential side effects and dosing. ART regimen was confirmed with the participants’ health care providers. Participants then completed a quantitative survey administered via the Audio-CASI (ACASI) system36 to assess demographic and behavioral characteristics, and a structured face-to-face interview was conducted to obtain assessments of treatment characteristics. At the end of the first session, a meeting was scheduled for 2 weeks later to ensure that the MEMS Track Cap was functioning properly and to obtain self-reported adherence data. At this time, participants provided their MEMS Caps to document their adherence behaviors in the previous 2 weeks. Participants were also scheduled to come in for 2-, 4-, 6-, 8-, 10-, and 12-month assessments. Adherence data from the prior 60 days were collected at each follow-up assessment through MEMS data as well as self-report. The assessments each took approximately 90–120 minutes to complete. Participants were provided a monetary incentive at the completion of each data collection visit.
Measures
Sociodemographic and health characteristics.
Using ACASI at baseline assessment, all participants reported age through date of birth, race/ethnicity, relationship status, employment status, and type of health care coverage. Current relationship status was dichotomized into “in a current relationship” or “not in a current relationship.” Participants indicated whether they were employed full time, part time, or whether they were not working. Health care coverage was also dichotomized into either “public” or “private” and age was dichotomized into “<50” and “≥50.” The latter was informed by research on the role of age on adherence.19,28–31
Treatment characteristics.
During the interview at the first baseline assessment, participants indicated via a checklist the PI in their regimen, the year they initiated use of this PI, as well as all of the other types of HIV antiviral medications that were currently part of their ART regimen. In addition, based on their dosing data, we calculated the total number of antiviral pills taken per day. Regimen was confirmed with the participants’ health care provider. We noted any change with regard to the PI each participant was prescribed and created a dichotomous variable to reflect this construct in our analysis.
Drug use.
The Drug Abuse Screening Test (DAST-10),57 which is widely used in drug abuse research, was utilized to assess level of drug use. These data categorized individuals as non-drug users (score = 0), drug users (score = 1–5), and drug abusers (score = 6–10).
Adherence.
At the second portion of the baseline assessment, adherence to the PI in the participants’ regimen was assessed in two manners. First, adherence using an electronic monitoring of pill retrieval with the MEMS Track Cap was assessed. The MEMS Track Cap system consists of a computer chip embedded in a cap that fits all standard size medicine. The chip records the date and time that the bottle is opened, which is defined as an event.58 The chip was then downloaded as a data file, and based on the dosing data and the missed doses indicated, a percentage of adherence for the PI being monitored was calculated. In addition, participants examined the MEMS data output with a research assistant and indicated which doses were correctly accounted for by the MEMS. Furthermore, when participants reported that the MEMS output was incorrect, they corrected the output and listed the time they took a specific medication, and alternatively, the reason they missed a medication dose, if applicable. Similar adjusted models have been utilized in similar studies of ART adherence in the form of composite scores.46 These corrected MEMS data were also used to obtain adherence percentages for the PI being monitored. The corrected MEMS data were implemented because of previous research that has indicated that the system does not necessarily provide a true indicator of adherence and, in fact, may underestimate adherence to HIV medications.59,60 A second assessment of adherence was undertaken to conduct checks on validity via self-report using the ACASI system, and this comparability has been documented elsewhere.35 As has been previously shown, based on the first MEMS assessment, measures using this methodology for collecting adherence data were highly positively correlated with self-report (r = 0.32., p < 0.001), and significantly negatively correlated with baseline viral load (r = −0.21, p = 0.001). In addition, corrected MEMS was significantly related to uncorrected MEMS data (r = 0.37, p < 0.001). None of the participants refused to use the MEMS cap (because use of this system was provided as information to the potential study participants prior to study entry), and we did not detect any significant malfunction in the course of the study of these systems. Thus, for the purposes of our analyses, we utilized the MEMS adjusted data as the measure of adherence.
Analytic plan
To characterize the key variables, descriptive statistics were calculated for the antecedents of adherence utilized in our analysis: person factors (age, race/ethnicity, employment, relationship status, years HIV positive, and drug use), and treatment factors (total ART pills in regimen per day, years on current PI, type of health care). In addition, we computed descriptive statistics for adherence rates based on the adjusted MEMS at each time point. To establish the comparability between the study participants at baseline and those who completed the final assessment, we conducted a comparative analysis of key demographic factors between the baseline and month 12 sample. Thereafter, we conducted our key analysis utilizing Hierarchical Linear Modeling (HLM). HLM is an appropriate statistical method to investigate patterns and predictors of ART adherence over time because its hierarchical structure utilizes a two-level procedure to examine intraindividual growth (level-1) and interindividual differences in growth (level-2) over time.61 A growth curve analysis using HLM confers numerous advantages over more traditional methods of investigating change over time in ART adherence over time among gay and bisexual men, such as repeated measures analysis of variance.61,62 HLM uses empirical Bayes estimation63 to derive the final estimates for each participant, drawing on information at both levels of the analysis. As a result, HLM affords more precise estimates of individual growth over time and greater power to detect predictors of individual differences in change. This process also allows HLM to handle missing data, using any available data points to fit a growth trajectory for each participant.
RESULTS
Sample characteristics
The sample consisted of 300 MSM, the majority of whom were identified as gay (78%, n = 234) or bisexual (17%, n = 50). The mean age of the participants was 42 (SD = 7.72, median = 42) and ranged from 20 to 70. As indicated in Table 1, the sample consisted of an approximate equal number of black and white males: 36.3% and 33.3%, respectively; and Latino: 20.7%. A third (34%) self-reported as being in a relationship.
TABLE 1.
Participant and Treatment Characteristics at Baseline
| n | (%) | |
|---|---|---|
|
| ||
| Race/ethnicity | ||
| Black | 109 | (36.3%) |
| White | 100 | (33.3%) |
| Hispanic/Latino | 62 | (20.7%) |
| Other | 29 | (9.7%) |
| Relationship status | ||
| Single | 198 | (66.0%) |
| Relationship | 102 | (34.0%) |
| DAST-10 | ||
| Non-drug user | 90 | (30.0%) |
| Drug user | 153 | (51.0%) |
| Drug abuser | 55 | (18.3%) |
| Missing | 2 | (0.7%) |
| Health care coverage | ||
| Public | 245 | (81.7%) |
| Medicare/Medicaid | 204 | |
| ADAP+ | 51 | |
| Private | 52 | (17.3%) |
| Employment status | ||
| Employed | 73 | (24.3%) |
| Not employed | 227 | (75.7%) |
| Age | 42.29 | (Md = 42, SD = 7.72) |
| No. of years on current PI | 1.63 | (Md = 1, SD = 1.68) |
| No. of ART pills per day | 14.54 | (Md = 14, SD = 5.22) |
| Years HIV-positive | 9.50 | (Md = 10, SD = 4.34) |
Note: values are n (%) except for age, no. of years on current PI, no. of ART pills per day, and years HIV positive, which are M (Median, SD).
DAST-10, Drug Abuse Screening Test; ADAP, AIDS Drug Assistance Program; PI, protease inhibitor; ART, antiretroviral therapy.
More than half of the men in the sample indicated that they used drugs (51%), and based on the DAST-10, 18.3% were categorized are drug abusers. As indicated in Table 1, the majority of the men in the sample had public health care coverage (81.7%) and the rest had private health care coverage; previously undertaken analyses have established that grouping health care along these dichotomies is appropriate in relation to adherence.35 On average, at the baseline, the men in the sample dosed 14.54 ART pills per day (SD = 5.22; median = 14) and had been on their current PIs for 1.62 years (SD = 1.68; median = 1).
Adherence across time
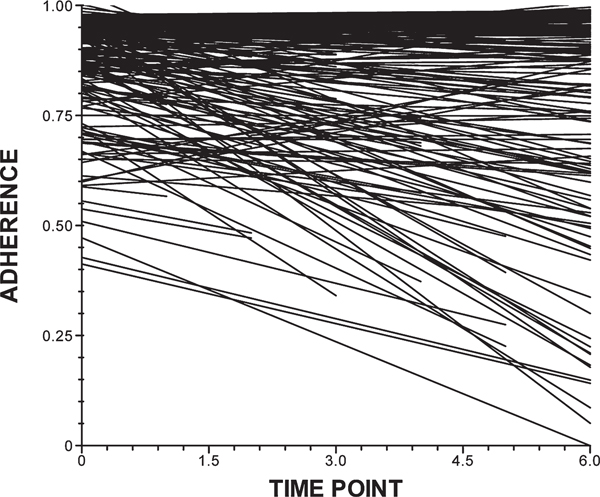
Of the 300 men who completed the first half of the baseline assessment, 276 returned in 2 weeks for the first of our MEMS data collection. Among these, the adherence rate was 0.90 (median = 0.97, SD = 0.18). At the 2-month follow-up assessment, 261 participants reported an average adherence rate of 0.87 (median = 0.98, SD = 20). As reported in Table 2, the average adherence rates were similar at 4, 6, 8, 10, and 12 months post baseline: 0.83 (median = 0.94, SD = 23), 0.84 (median = 0.95, SD = 0.24), 0.86 (median = 0.97, SD = 0.23), 0.83 (median = 0.96, SD = 28), and 0.82 (median = 0.98, SD = 29), respectively. Trajectories of adherence rates over time are illustrated in Figure 1.
TABLE 2.
Sociodemographic and Treatment Predictors of Growth Parameters of Treatment Adherence in Gay and Bisexual Men (n = 249)
| Coefficient | SE | p value | |
|---|---|---|---|
|
| |||
| Fixed effects | |||
| for intercept | |||
| Intercept | 0.89** | 0.06 | <0.001 |
| Person factors | |||
| Age | 0.05 | 0.03 | 0.056 |
| Blacka | −0.11*** | 0.03 | <0.001 |
| Latinoa | −0.03 | 0.03 | 0.404 |
| Othera | −0.02 | 0.04 | 0.591 |
| Employment | 0.03 | 0.05 | 0.571 |
| In a relationship | −0.03 | 0.02 | 0.133 |
| Years HIV-positive | −0.003 | 0.002 | 0.210 |
| Drug useb | −0.05* | 0.02 | 0.048 |
| Drug dependenceb | −0.03 | 0.03 | 0.333 |
| Treatment factors | |||
| Total ART pills | 0.005* | 0.002 | 0.017 |
| Years on current PI | 0.01 | 0.01 | 0.309 |
| Health care | 0.03 | 0.03 | 0.324 |
| For linear slope | |||
| Intercept | −0.01 | 0.02 | 0.486 |
| Person factors | |||
| Age | −0.002 | 0.01 | 0.829 |
| Black | 0.01 | 0.01 | 0.470 |
| Latino | −0.002 | 0.01 | 0.843 |
| Other | 0.004 | 0.01 | 0.774 |
| Employed | −0.01 | 0.02 | 0.421 |
| In a relationship | 0.01 | 0.01 | 0.090 |
| Years HIV positive | −0.000 | 0.001 | 0.955 |
| Drug use | −0.005 | 0.01 | 0.557 |
| Treatment factors | |||
| Total ART pills | −0.0001 | 0.001 | 0.353 |
| Years on current PI | 0.001 | 0.003 | 0.578 |
| Health care coverage | −0.002 | 0.01 | 0.824 |
| Random effects | |||
| Intercept | 0.017*** | ||
| Linear slope | 0.002*** | ||
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001.
White set as reference group.
Non-drug use set as reference group.
ART, antiretroviral therapy; PI, protease inhibitor.
FIG. 1.
Adherence trajectories. MEMS, Medications electronic Monitoring System.
At 12 months, we retained 65% (N = 195) of those who completed baseline assessment. In order to assess the potential confounds of missing data, we compared those who completed all seven follow-up assessments (N = 148, 49%) to those who did not (N = 152, 51%). There were no significant differences in terms of age, race/ethnicity, DAST-10 score, relationship status, employment status, or type of health care. We also considered the key sociodemographic characteristics of the participants who completed their month 12 assessment to those who did not. Those who did not complete their final assessments were not different from the baseline sample with regard to age, race/ethnicity, years HIV-positive, and years on current PI, employment status, drug use, or type of health care coverage.
Longitudinal analysis
First, using HLM as our analytic approach, an unconditional model was examined to illustrate how the treatment adherence changed over time in the sample. The trajectories of adherence are shown in Figure 1. There was significant variability in the slope (χ2 (153) = 291.05, p < 0.001) and intercept (χ2 (153) = 296.11, p < 0.001) terms of the unconditional model, thus suggesting the development of a conditional model. A univariate conditional model was constructed to explore whether the trajectories of treatment adherence over the course of the year could be explained as a function of person factors (age, race/ethnicity, employment status, relationship status, years HIV-positive, and drug use), and treatment factors (total ART pills in regimen per day, years on current PI, type of health care coverage). These variables were selected for inclusion as dictated by the previous literature. The conditional model, which was calculated on the 249 participants who had sufficient data for computation, represents an improvement in fit over the unconditional model as indicated by the Δχ2 statistics:Δχ2 (15) = 98.99 p < 0.01 for the intercept; Δχ2 (15) = 32.34 p = 0.01 for the slope. The fit statistics for each of the variables are shown Table 2.
It is important to note that the nonzero intercept (γ0 = 0.90, p < 0.0001) indicates that treatment adherence among these men at baseline is significantly different from 0, at approximately 90%. However, the nonsignificant negative coefficient for the linear slope (γ0 = −0.01, p = 0.486) indicates that, on average, the treatment adherence among these men remains relatively stable across the assessment period. In addition, in this model there are no significant microlevel indicators for the slope. For illustrative purposes, the mean adherence rates at each timepoint are also shown in Table 3.
TABLE 3.
Adherence Rates Across 12 Months
| Time point | N | Mean | SD | Median |
|---|---|---|---|---|
|
| ||||
| 2 Week | 276 | 0.90 | 0.18 | 0.97 |
| 2 Month | 261 | 0.87 | 0.20 | 0.98 |
| 4 Month | 218 | 0.83 | 0.23 | 0.94 |
| 6 Month | 225 | 0.84 | 0.24 | 0.95 |
| 8 Month | 208 | 0.86 | 0.23 | 0.97 |
| 10 Month | 204 | 0.83 | 0.28 | 0.96 |
| 12 Month | 195 | 0.82 | 0.29 | 0.98 |
For the intercept, adherence at baseline is explained by total pills consumed (γ = 0.005, p = 0.017), drug use (γ = −0.05, p = 0.048), being black (γ = −0.11, p < 0.0001), and marginally by age (γ = 0.05, p = 0.056). The results suggest that at baseline, higher levels of treatment adherence are associated with higher number of pills consumed, being a nondrug user, by white men as compared to black men, and by men over 50 years of age.
DISCUSSION
Adherence to ART is an essential component for people who are HIV-positive to maintain good health. Adherence to this type of medication regimen is very complex because ART is associated with a variety of person, behavioral, and treatment factors. The present investigation sought to disentangle the factors associated with adherence using longitudinal data and multilevel modeling.
Unlike most other longitudinal investigations, this study found that adherence did not decrease significantly over the course of a year. There was a trend of decreased adherence, but adherence remained relatively stable over time. Similar to previous findings, men who are black reported poorer levels of adherence at baseline assessment. Black men might miss more doses due to psychological factors such as lack of self-efficacy13 or embarrassment in refilling their ART medications at the pharmacy.64 Black men are also more likely to report perceived barriers and are more likely than their white counterparts to skip doses when they do not feel well.64 Some perceived barriers such as less access to quality heath insurance possibly affects their adherence,15 but this investigation found that type of insurance provider does not affect adherence. Qualitative studies suggest that patients who are HIV-positive prefer health care providers who adequately communicate and involve them in treatment decision making,41 and these perceived qualities may vary according to type of health insurance. Participants in these studies have complained about the quality of government-supplied insurance such as AIDS Drug Assistance Program, but aside from quality, this investigation found that type of health care provider has no significant effect on adherence.
Many studies have found that age is highly related to adherence, with older patients demonstrating better adherence than their younger counterparts.19,28–31 This is related to experience through familiarity with ART and more knowledge of the detrimental effects of missed doses.32 Older patients (particularly over 50 years of age) may have more stable routines than younger people, which allow less interference in the strict regimen required in ART. In this investigation, age approached significance (p = 0.056), which suggests that older patients do display marginally higher levels of adherence to ART. Age in relation to adherence requires further investigation because age is merely an indicator of developmental stage, which includes but is not limited to experience and maturity. Future studies need to incorporate scales to measure what specifically related to age affects adherence.
Recreational drug use is consistently related to poor adherence,35–43 usually due to interference of the required strict routine of ART. Many ART regimens require a strict schedule of multiple doses of multiple medications per day, with or without food. Choosing to ingest substances that temporarily compromise cognitive ability (depending on the substance) can lead to accidental missed doses. Moreover, many users intentionally skip doses of ART when using certain illicit substances in order to prevent negative side effects that arise from combining the two concomitantly,40 and many patients are aware that combining certain illicit drugs with ART medications can strain the liver and lead to possible overdose or other adverse effects.65–67
“Drug use” is perhaps one of the more complex variables related to adherence because there are numerous drugs of different purities that can affect people differently at different times (i.e., depending on food ingestion, sleep, tolerance). Our findings indicate that drug use is related to lower levels of adherence, based on our classification system. This suggests that non-drug-using individuals demonstrate higher levels of adherence. However, people cannot be easily classified as “non-users,” “users,” and “abusers,” and thus our variable is limited in scope. For example, some people who are HIV-positive and suffer from wasting syndrome use marijuana or similar drugs to induce appetite.68 Since certain drugs are used for medicinal purposes, regardless of current fluctuating legal status in the United States, it might be improper to classify these people as users or abusers. Recent evidence suggests that smoking marijuana is also efficacious for people who are HIV-positive and suffer from neuropathy,69 so classifying all drugs into a simple category of “illicit drugs” may be unreasonable. Being a “drug user” also tends to be a stereotype that often erroneously leads practitioners and researchers to assume a certain type of lifestyle, which may not be true.70 Injecting heroin every day is much different from ingesting an Ecstasy pill once a month, yet both behaviors are often dichotomized into “drug use.”
Finally, certain treatment factors are highly related to adherence. Type of health insurance was not a significant predictor of adherence in this analysis, but number of pills prescribed per day was found to be a predictor. Many studies have found that more prescribed pills per day, usually over 12 or 15, are associated with poor adherence.19,22,29,30 One study also found that being prescribed more than 15 pills per day is less associated with having an undetectable viral load, likely due to poor adherence.22 More complex regimens may be reflected in therapies that require a higher number of pills per day; thus, the problem might be the overall complexities of the therapy and not the actual number of pills to be taken.
This investigation is one of the first to utilize HLM to measure factors related to ART adherence. Race/ethnicity, drug use and number of ART pills prescribed per day, and (tangentially) age were found to be related to adherence at baseline assessment. Unlike many other studies, adherence over a 12-month period remained relatively stable and did not display a significant decrease; however, adherence of many participants in the sample did in fact decrease.
Limitations
Numerous steps were taken to assure the validity of the data generated in this investigation, including but not limited to the “triangulation” of adherence measurement through electronic monitoring, self-reports, and proxy indicators using HIV viral load; the utilization of ACASI technologies for self-reported information; and the use of active recruitment to obtain a more diverse sample of MSM. However, as is the case in any study conducted within the contexts of real lives, the study design does have limitations. First and foremost, the sample was self-selected in that the 300 MSM who chose to participate were not randomly chosen. It is possible that this condition introduced a bias in terms of the main behavior of interest, adherence, in that such participants may have been more motivated with regard to this behavior than those who did not participate. Because we did not obtain adherence measures on those who screened in but chose not to participate in the study, we cannot ultimately make such comparisons. Secondly, despite the diversity of the sample along key demographic states including race, age, and educational attainment, the cohort was rather homogeneous in terms of their adherence trajectories. In addition, our inclusion of variables that have been previously examined may be viewed as a limitation. However, the application of such constructs to longitudinal data represents an innovation in the understanding of adherence over and above the cross-sectional studies in the literature, and further supports the importance of the significant constructs in understanding adherence behaviors. Despite the fact that relationship status was a nonsignificant variable, we note the possibility that our assessment (a simple categorical response) was insufficient to capture the complexities that HIV-positive individuals face in their relationships, especially with regard to serostatus disclosure, which may impact in adherence. The use of MEMS caps may have had intervention-like effects, and thus these adherence data may be more optimal than behaviors exhibited outside of a study condition. Finally, as the advent of new HIV antiviral medications proceeds at a rapid pace, the data presented here are bound by a historical context. For example, one-pill-aday combined formulations were not available at the time that we undertook this study.
CONCLUSIONS
The key finding of our analytic work suggests that many MSM maintain high yet not optimal rates of HIV medication adherence. Moreover, this behavior remains relatively constant over time, as indicated by the longitudinal analysis. Variability of adherence rates across individuals suggests that person-level factors such as race, age, and drug use may be related to poorer adherence. Moreover, more nuanced qualitative investigations could seek to develop typologies of adherence patterns and to consider factors that explain these typologies. However, these findings should not be interpreted to suggest that decisions regarding the initiation and maintenance of HIV treatments be made solely based on these elements. Intervention strategies that seek to optimize HIV treatment adherence should move beyond the simply behavioral and seek to explore intrapersonal and intrapsychic states that individuals bring to their treatment behaviors. In this perspective, motivational-based strategies71 that examine these states and help the individual develop strategies based on his/her own understandings of the obstacles to adherence are highly recommended.
ACKNOWLEDGMENT
This research was funded by a grant from the National Institute on Drug Abuse (R01DA12816).
REFERENCES
- 1.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133:21–30. [DOI] [PubMed] [Google Scholar]
- 2.Haubrich RH, Little S, Currier JS, et al. The value of patient-reported adherence to antiretroviral therapy in predicting antivirologic and immunologic response. AIDS 1999;13:1099–1107. [DOI] [PubMed] [Google Scholar]
- 3.Wood E, Hogg RS, Yip B, et al. The impact of adherence on CD4 cell count responses among HIV-infected patients. JAIDS 2004;35:261–268. [DOI] [PubMed] [Google Scholar]
- 4.Bangsberg DR, Acosta EP, Gupta R, et al. Adherenceresistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS 2006;20:223–231. [DOI] [PubMed] [Google Scholar]
- 5.Pillay D. The emergence and epidemiology of resistance in the nucleoside-experienced HIV-infected population. Antiviral Ther 2001;6:15–24. [PubMed] [Google Scholar]
- 6.Condra JH. Resistance to HIV protease inhibitors. Haemophilia 1998;4:610–615. [DOI] [PubMed] [Google Scholar]
- 7.Kalichman SC, Rompa D. HIV treatment adherence and unprotected sex practices in people receiving antiretroviral therapy. Sex Transm Infect 2003;79:59–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozal MJ, Amico KR, Chiarella J, et al. HIV drug resistance and HIV transmission risk behaviors among active injection drug users. JAIDS 2005;40:106–109. [DOI] [PubMed] [Google Scholar]
- 9.Borrell-Carrio F, Suchman AL, Epstein RM. The biopsychosocial model 25 years later: Principles, practice, and scientific inquiry. Ann Fam Med 2004;2: 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis 2002;34:1115–1121. [DOI] [PubMed] [Google Scholar]
- 11.Johnson MO, Catz SL, Remien RH, et al. Theoryguided, empirically supported avenues for intervention on HIV medication nonadherence: Findings from the Healthy Living Project. AIDS Patient Care STDs 2003;17:645–656. [DOI] [PubMed] [Google Scholar]
- 12.Kleeberger CA, Phair JP, Strathdee SA, et al. Determinants of heterogeneous adherence to HIV-antiretroviral therapies in the Multicenter AIDS Cohort Trial. JAIDS 2001;26:82–92. [DOI] [PubMed] [Google Scholar]
- 13.Siegel K, Karus D, Schrimshaw EW. Racial differences in attitudes toward protease inhibitors among older HIV-infected men. AIDS Care 2000;12:423–434. [DOI] [PubMed] [Google Scholar]
- 14.Israelski D, Gore-Felton C, Power R, Wood MJ, Koopman C. Sociodemographic characteristics associated with medical appointment adherence among HIV-seropositive patients seeking treatment in a county outpatient facility. Prev Med 2001;33:470–475. [DOI] [PubMed] [Google Scholar]
- 15.Halkitis PN, Parsons JT, Wolitski RJ, Remien RH. Characteristics of HIV antiretroviral treatments, access and adherence in an ethnically diverse sample of men who have sex with men. AIDS Care 2003;15: 89–102. [DOI] [PubMed] [Google Scholar]
- 16.Fiscella K, Franks P, Doescher MP, Saver BG. Disparities in health care by race, ethnicity, and language among the insured. Med Care 2002;40:52–59. [DOI] [PubMed] [Google Scholar]
- 17.Blendon RJ, Aiken LH, Freeman HE, Corey CR Access to medical care for black and white Americans: A matter of continuing concern. JAMA 1989;261: 278–281. [PubMed] [Google Scholar]
- 18.van Servellen GV, Change B, Garcia L, Lombardi E. Individual and system level factors associated with treatment nonadherence in human immunodeficiency virus-infected men and women. AIDS Patient Care STDs 2002;16:269–281. [DOI] [PubMed] [Google Scholar]
- 19.Nemes MIB, Carvalho HB, Souza MFM. Antiretroviral therapy adherence in Brazil. AIDS 2004;18:S15–S20. [DOI] [PubMed] [Google Scholar]
- 20.Wood E, Montaner JS, Chan K, et al. Socioeconomic status, access to triple therapy, and survival from HIV-disease since 1996. AIDS 2002;16:2065–2072. [DOI] [PubMed] [Google Scholar]
- 21.Carballo E, Cadarso-Suarez C, Carrera I, et al. Assessing relationships between health-related quality of life and adherence to antiretroviral therapy. Qual Life Res 2004;13:587–599. [DOI] [PubMed] [Google Scholar]
- 22.Deloria-Knoll M, Chmiel JS, Moorman AC, et al. Factors related to and consequences of adherence to antiretroviral therapy in an ambulatory HIV-infected patient cohort. AIDS Patient Care 2004;18:721–727. [DOI] [PubMed] [Google Scholar]
- 23.Stirratt MJ, Remien RH, Smith A, et al. The role of HIV serostatus disclosure in antiretroviral medication adherence. AIDS Behav 2006;10:483–493. [DOI] [PubMed] [Google Scholar]
- 24.Peretti-Watel P, Spire B, Schiltz MA, et al. Vulnerability, unsafe sex and non-adherence to HAART: Evidence from a large sample of French HIV/AIDS outpatients. Soc Sci Med 2006;62:2420–2433. [DOI] [PubMed] [Google Scholar]
- 25.Wagner GJ, Remien RH, Carballo-Dieguez A, Dolezal C. Correlates of adherence to combination antiretroviral therapy among members of HIV-positive mixed status couples. AIDS Care 2002;14:105–109. [DOI] [PubMed] [Google Scholar]
- 26.Holt R, Court P, Vedhara K, et al. The role of disclosure in coping with HIV infection. AIDS Care 1998; 10:49–60. [DOI] [PubMed] [Google Scholar]
- 27.Klitzman RL, Kirshenbaum B, Dodge B, et al. Intricacies and inter-relationships between HIV disclosure and HAART: A qualitative study. AIDS Care 2004;16: 628–640. [DOI] [PubMed] [Google Scholar]
- 28.Hinkin CH, Hardy DJ, Mason KI, et al. Medication adherence in HIV-infected adults: Effect of patient age, cognitive status, and substance abuse. AIDS 2004;18:S19–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maggiolo F, Ripamonti D, Arici C, et al. Simpler regimens may enhance adherence to antiretrovirals in HIV-infected patients. HIV Clin Trials 2002;3:371–378. [DOI] [PubMed] [Google Scholar]
- 30.Puigventos F, Riera M, Delibes C, et al. Adherence to antiretroviral drug therapy: A systematic review. Med Clin 2002;119:130–137. [DOI] [PubMed] [Google Scholar]
- 31.Kastrissios H, Suarez JR, Katzenstein D, et al. Characterizing patterns of drug-taking behavior with a multiple drug regimen in an AIDS clinical trial. AIDS 1998;12:2295–2303. [DOI] [PubMed] [Google Scholar]
- 32.Wellons MF, Sanders L, Edwards LJ, et al. HIV infection: Treatment outcomes in older and younger adults. J Am Geriat Soc 2002;50:603–607. [DOI] [PubMed] [Google Scholar]
- 33.Wutoh AK, Brown CM, Kumoji EK, et al. Antiretroviral adherence and use of alternative therapies among older HIV-infected adults. J Natl Med Assoc 2001;93:243–250. [PMC free article] [PubMed] [Google Scholar]
- 34.Goodkin K, Shapshak P, Asthana D, et al. Older age and plasma viral load in HIV-1 infection. AIDS 2004; 18:S87–S98. [PubMed] [Google Scholar]
- 35.Halkitis PN, Kutnick AH, Slater S. The social realities of adherence to protease inhibitor regimens: Substance use, health care and psychological states. J Health Psychol 2005;10:545–558. [DOI] [PubMed] [Google Scholar]
- 36.Turner CF, Ku L, Rogers SM, et al. Adolescent sexual behavior, drug use, and violence: Increased reporting with computer survey technology. Science 1998;280: 867–873. [DOI] [PubMed] [Google Scholar]
- 37.Haubrich RH, Little SJ, Currier JS, et al. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response. AIDS 1999;13:1099–1107. [DOI] [PubMed] [Google Scholar]
- 38.Bondi MW, Drake AI, Grand I. Verbal learning and memory in alcohol abusers and poly-substance abusers with concurrent alcohol abuse. J Int Neuropsychol Soc 1998;4:319–328. [PubMed] [Google Scholar]
- 39.Waldrop-Valverde D, Ownby RL, Wilkie FL, et al. Neurocognitive aspects of medication adherence in HIV-positive in injecting drug users. AIDS Behav 2006;10:287–297. [DOI] [PubMed] [Google Scholar]
- 40.Witteveen E, van Ameijden EJC. Drug users and HIV-combination therapy (HAART): Factors which impede or facilitate adherence. Subst Use Misuse 2002; 37:1905–1925. [DOI] [PubMed] [Google Scholar]
- 41.Murphy DA, Roberts KJ, Martine DJ, Marelich W, Hoffman D. Barriers to antiretroviral adherence among HIV-infected adults. AIDS Patient Care STDs 2000;14:47–58. [DOI] [PubMed] [Google Scholar]
- 42.Peretti-Watel P, Spire B, Lert F, Obadia Y, VESPA Group. Drug use patterns and adherence to treatment among HIV-positive patients: Evidence from a large sample of French outpatients. Drug Alcohol Depend 2006;82(suppl 1):S71–S79. [DOI] [PubMed] [Google Scholar]
- 43.Braithwaite RS, McGinnis KA, Conigliaro J, et al. Atemporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res 2005;29: 1190–1197. [DOI] [PubMed] [Google Scholar]
- 44.Back DJ, Burger DM, Flexner CW, Gerber JG. The pharmacology of antiretroviral nucleoside and nucleotide reverse transcriptase inhibitors: Implications for once-daily dosing. JAIDS 2005;39:S1–S23. [DOI] [PubMed] [Google Scholar]
- 45.Portsmouth SD, Osorio J, McCormick K, Gazzard BG, Moyle GJ. Better maintained adherence on switching from twice-daily to once-daily therapy for HIV: A 24-week randomized trial of treatment simplification using stavudine prolonged-release capsules. HIV Med 2005;6:185–190. [DOI] [PubMed] [Google Scholar]
- 46.Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med 2001;134:968–977. [DOI] [PubMed] [Google Scholar]
- 47.Holzemer WL, Bakken S, Portillo CJ, Grimes R, Welch J. Testing a nurse-tailored HIV medication adherence intervention. Nurs Res 2006;55:189–197. [DOI] [PubMed] [Google Scholar]
- 48.de Bruin M, Hospers HJ, van den Borne HW, Kok G, Prins JM. Theory- and evidence-based intervention to improve adherence to antiretroviral therapy among HIV-infected patients in the Netherlands: A pilot study. AIDS Patient Care STDs 2005;19:384–394. [DOI] [PubMed] [Google Scholar]
- 49.Rathbun RC, Farmer KC, Stephens JR, Lockhart SM. Impact of an adherence clinic on behavioral outcomes and virologic response in treatment of HIV infection: A prospective, randomized, controlled pilot study. Clin Ther 2005;27:199–209. [DOI] [PubMed] [Google Scholar]
- 50.Wagner GJ. Predictors of antiretroviral adherence as measured by self-report, electronic monitoring, and medication diaries. AIDS Patient Care STDs 2002;16:599–608. [DOI] [PubMed] [Google Scholar]
- 51.Pinheiro CA, de-Carvalho-Leite JC, Drachler ML, Silveira VL. Factors associated with adherence to antiretroviral therapy in HIV/AIDS patients: A cross-sectional study in Southern Brazil. Brazil J Med Bio Res 2002;35:1173–1181. [DOI] [PubMed] [Google Scholar]
- 52.Ammassari A, Murri R, Pezzotti P, et al. Self-reported symptoms and medication side-effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. JAIDS 2001;28:445–449. [DOI] [PubMed] [Google Scholar]
- 53.Wilson KJ, Doxanakis A, Fairley CK. Predictors for non-adherence to antiretroviral therapy. Sex Health 2004;1:251–257. [DOI] [PubMed] [Google Scholar]
- 54.Knafl GJ, Fennie KP, Bova C, Dieckhaus K, Williams AB. Electronic monitoring device event modeling on an individual-subject basis using adaptive Poisson Regression. Stat Med 2004;23:783–801. [DOI] [PubMed] [Google Scholar]
- 55.Ickovics JR, Meisler AW. Adherence in AIDS clinical trials: A framework for clinical research and clinical care. J Clin Epidemiol 1997;50:385–391. [DOI] [PubMed] [Google Scholar]
- 56.Watters JK, Biernacki P. Targeted sampling: Options for the study of hidden populations. Soc Problems 1989;36:416–430. [Google Scholar]
- 57.Skinner HA. The drug abuse screening test. Addict Behav 1982;7:363–371. [DOI] [PubMed] [Google Scholar]
- 58.Kruse W, Rampmaier J, Ullrich G, Weber E. Patterns of drug compliance with medications to be taken once and twice daily assessed by continuous electronic monitoring in primary care. Int J Clin Pharm Ther 1994;32:452–457. [PubMed] [Google Scholar]
- 59.Wagner GJ, Rabkin JG. Measuring medication adherence: Are missed doses reported more accurately then perfect adherence? AIDS Care 2000;12:405–408. [DOI] [PubMed] [Google Scholar]
- 60.Hill Z, Kendall C, Fernandez M. Patterns of adherence to antiretrovirals: Why adherence has no simple measure. AIDS Patient Care STDs 2003;17:519–525. [DOI] [PubMed] [Google Scholar]
- 61.Bryk SW, Raudenbush AS, eds. Hierarchical Linear Models: Applications and Data Analysis Methods. Thousand Oaks, CA: Sage Publications, Inc., 1992. [Google Scholar]
- 62.Bryk SW, Raudenbush AS. Examining correlates of diversity. J Edu Stat 1987;12:241–269. [Google Scholar]
- 63.Strenio JLF, Weisberg HI, Bryk AS. Empirical Bayes estimation of individual growth curves parameters and their relationship to covariates. Biometrics 1983;39:71–86. [PubMed] [Google Scholar]
- 64.Ferguson TF, Stewart KE, Funkhouser E, et al. Patient-perceived barriers to antiretroviral adherence: Associations with race. AIDS Care 2002;14:607–617. [DOI] [PubMed] [Google Scholar]
- 65.Wynn GH, Cozza KL, Zapor MJ, Wortmann GW, Armstrong SC. Antiretrovirals, Part III: Antiretrovirals and drugs of abuse. Psychosomatics 2005;46:79–87. [DOI] [PubMed] [Google Scholar]
- 66.Antoniou T, Tseng AL. Interactions between recreational drugs and antiretroviral agents. Ann Pharmacother 2002;36:1598–1613. [DOI] [PubMed] [Google Scholar]
- 67.Harrington RD, Woodward JA, Hooton TM, Horn JR. Life-threatening interactions between HIV-1 protease inhibitors and the illicit drugs MDMA and –hydroxybutyrate. Arch Intern Med 1999;159:2221–2224. [DOI] [PubMed] [Google Scholar]
- 68.Joy JE, Watson SJ Jr., Benson JA Jr., eds. The medical value of marijuana and related substances. In: Marijuana and Medicine: Assessing the Science Base. Washington, DC: National Academy Press, 1999:137–191. [PubMed] [Google Scholar]
- 69.Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy. Neurology 2007;68:515–521. [DOI] [PubMed] [Google Scholar]
- 70.Ware NC, Wyatt MA, Tugenberg T. Adherence, stereotyping and unequal HIV treatment for active users of illegal drugs. Soc Sci Med 2005;61:565–576. [DOI] [PubMed] [Google Scholar]
- 71.Miller WR, Rollnick S. Talking oneself into change: Motivational interviewing, stages of change, and therapeutic process. J Cog Psychother 2004;18:299–308. [Google Scholar]