Executive Summary
The diagnosis and treatment of cancer patients requires access to imaging to ensure accurate management decisions and optimal outcomes. Our global assessment of imaging and nuclear medicine resources identified major shortages of equipment and workforce, particularly in low- and middle-income countries (LMIC). A microsimulation model of 11 cancers showed scale-up of imaging would avert 3.2% (2.46 million) of all cancer deaths caused by the modeled cancers between 2020-2030, saving 54.92 million life years. Scale-up of imaging, treatment and care quality would avert 9.55 million (12.5%) of all deaths caused by the modeled cancers, saving 232.30 million life years. Scale-up of imaging would cost $6.84 billion in 2020-2030 but yield global lifetime productivity gains of $1.23 trillion, a net return of $179.19 per $1 invested. Using a conservative human capital approach, scale-up of imaging would provide a net benefit of $209.46 billion and net return of $31.61 per $1 invested. Using the same model and a full income approach, combining scale-up of imaging and treatment and quality of care would provide a net benefit of $2.66 trillion, and a net return of $12.43 per $1 invested. These improved health and economic outcomes were seen across all countries and geographical regions. We propose actions and investment that would enhance access to imaging equipment, workforce capacity, digital technology, radiopharmaceuticals, and research and training programs in LMIC, to realise massive health and economic benefits and reduce the burden of cancer globally.
Introduction
The global cancer burden is increasing at an alarming rate. From 2012 to 2018, the estimated number of new cancer cases worldwide grew by more than 28%, from 14.1 million to 18.1 million, while the estimated number of cancer deaths rose more than 16%, from 8.2 million to 9.6 million. 1,2 By 2030, the numbers of new cancer cases and cancer deaths are expected to reach 22 million and 13.2 million, respectively.3,4 These statistics are all the more concerning because approximately 80% of the disability-adjusted life years (DALYs) are lost to cancer in low- and middle-income countries (LMICs), where only about 5% of the global funding for cancer control and care are applied.3,5
In 2015, The Lancet Oncology published the results of two commissions which assessed the gaps in access to cancer surgery and radiotherapy and proposed actions to address the growing burden of cancer in LMICs.6,7 The commission reports provided specific recommendations for increasing access to these treatment modalities and showed that doing so could prevent avoidable human suffering and reduce preventable deaths, while also providing substantial economic benefits. Both reports noted that cancer care is a multidisciplinary endeavour and that effective use of surgery and radiotherapy requires, among other resources, medical imaging.
In high-income countries, imaging plays essential roles in the management of almost all cancers. It is used throughout the care continuum, from detection, diagnosis and staging, to treatment planning (especially in radiation oncology), assessment of treatment response, and long-term follow-up. Moreover, interventional radiology, which relies on imaging, is increasingly integral to cancer diagnostics and treatment. Though the direct impact of imaging on overall survival is very difficult to quantify because of the complexity of cancer biology, cancer care, and lack of data, numerous studies have shown that the appropriate use of imaging for indications such as cancer staging or the assessment of treatment response can improve management decisions and reduce costs of cancer care (e.g., by obviating the need for other tests or invasive diagnostic procedures, demonstrating the need for neoadjuvant therapy, improving surgical or radiotherapy planning, preventing unnecessary surgery and discontinuing ineffective therapies).8-16
Despite the ubiquity of imaging in modern cancer care in high-income countries, the importance of imaging in oncology is frequently overlooked in efforts aimed at improving cancer care in LMICs. Many LMICs have severe shortages of imaging and nuclear medicine equipment and personnel. Data on imaging equipment available in LMICs have not been gathered systematically. There is scant data on the numbers and distribution of health professionals involved in providing imaging services—including radiologists and nuclear medicine physicians, imaging radiographers and technologists, medical physicists and, among others, radiochemists. There are few reliable studies which quantify the number and mix of health professionals needed to operate, optimally utilize and maintain imaging equipment.17 Furthermore, even in high-income countries with ready access to imaging services, there is a lack of appreciation for the importance of specialized training and expertise to the optimal interpretation and reporting of cancer imaging.17 Without data on these crucial elements, it is not possible to appropriately plan the introduction and scale-up of cancer services whose efficacy depends on effective and efficient imaging and nuclear medicine services.
At the suggestion and with the help of the International Atomic Energy Agency (IAEA), The Lancet Oncology Commission on Imaging and Nuclear Medicine was established in 2018 with the charge of examining global access to imaging and nuclear medicine for cancer care. It was also charged with analysing barriers to access to imaging for cancer care, providing new evidence to demonstrate the benefits of imaging in improving cancer care and cancer survival, and providing recommendations on how best to introduce and scale up imaging services in order to expand access to imaging and nuclear medicine services in LMICs. To produce this Commission, the health benefits of cancer imaging were analysed at a global level, using data from high-, middle- and low-income countries. The financial return on investment in cancer imaging was also investigated. Finally, given the vast imbalances in cancer burden and cancer control resources between LMICs and high-income countries, recommendations for scaling up cancer imaging resources were crafted with a specific focus on LMICs.
This Commission is organised into eight sections. Section One discusses the evolving role of cancer imaging in LMICs and the main challenges countries with limited resources must consider when tailoring the adoption and use of imaging and nuclear medicine services to the continuum of cancer care resources available to them. Section Two expands on the barriers to increasing access to cancer imaging in LMICs, presenting new data on the global availability of imaging technologies and human resources and identifying specific gaps that need to be addressed. Section Three presents an analysis of the costs, benefits and returns on investment that could be realised by investing in the global scale-up of imaging technologies and human resource capabilities, alone or in tandem with treatment modalities, care quality or both. Section Four discusses financing for a global scale-up of imaging diagnostics. Section Five discusses the important issue of ensuring radiation protection and safety for patients, workers and the public as well as quality systems when scaling up imaging and nuclear medicine capabilities globally. Section Six provides an overview of innovations in digital science technologies and novel analytical tools such as artificial intelligence and machine learning that will transform the availability of and access to imaging diagnostics and decision-making. Section Seven outlines the critical importance of teaching, training and research to ensuring adequate capabilities and quality of imaging sites and staff in LMICs. Section Eight, the conclusion, discusses the success factors critical to enabling global expansion of access to imaging for cancer, and calls for action toward this goal.
Section 1: The evolving role of cancer imaging in low- and middle-income countries: opportunities and obstacles
As described above, cancer burden is increasing rapidly—particularly in LMICs, where funding for cancer care is low and capacity to manage this rising burden lacking.18,19 As a result, huge inequities exist among countries in access to effective services for cancer care. In addition to intercountry inequities, there are also large inequities within countries, with lower levels of access for lower-income and lower-education groups compared to those with higher income and higher education levels. Such intra-country inequities exist even in the wealthy United States and are also found in LMICs, where any available highly trained personnel and advanced healthcare infrastructure—including imaging equipment—may be confined largely to private practices.17,20,21 The inequities in access to cancer services are reflected in inequities in health outcomes. Although worldwide the overall survival rates for cancer are improving, the improvement is much less evident in LMICs.17-19 Indeed, even though the incidence of cancer in LMICs remains lower than that in high-income countries, cancer-related mortality rates are significantly higher in LMICs, particularly in people under the age of 65. These circumstances are due, at least in part, to delays in diagnosis (affected by lack of access to imaging and other diagnostic tools), lack of access to optimal local and systemic treatments, and greater numbers infection-associated cancers in LMICs.22,23
It is important to recognize that cancer care is a continuum and requires parallel investments in imaging and other diagnostics, as well as in treatments. Socioeconomic benefits of investments in improvements to cancer surgery7 and radiotherapy6 infrastructure have been demonstrated, and cancer imaging is required for diagnosis, staging, and effective treatment with either surgery or radiotherapy. For example, radiotherapy patients require imaging for treatment planning, and quantitative imaging affects radiotherapy outcomes and survival.24-26 Similarly, pre-operative imaging bolsters the safety, appropriateness, quality, and effectiveness of cancer surgery. Likewise, image guidance of biopsies and minimally-invasive interventions (e.g. image-guided central venous catheter placement for the administration of medicines, or image-guided tumor ablations) are associated with higher quality, decreased morbidity, and enhanced affordability.27-31 Moreover, lack of staging information from imaging can lead to inadequate or inappropriate use of medical therapies, surgery, or radiotherapy and increase morbidity and mortality. Selection of the most appropriate antineoplastic regimen for cancer patients frequently hinges upon imaging results.32
Utilization of cancer imaging and its benefits: a review of the literature
Though imaging plays pivotal roles in cancer care, because of the complexity of the care process, the direct effects of imaging on patient outcomes have historically been difficult to quantify. Nevertheless, we undertook a review of the (albeit limited) published peer-reviewed literature and reports aimed at quantifying on a large scale the utilization of imaging, and its benefits, for cancer patients. One study from Canada, based on a survey of centres providing imaging services, examined utilization levels and the reasons for imaging; it found that about 23.1% of computed tomography (CT) examinations, 80.2% of positron emission tomography (PET)/CT examinations and 20.8% of magnetic resonance imaging (MRI) examinations were performed for cancer indications.33 However, the survey relied on subjective assessments of the distribution of indications rather than direct analysis of claims data, and the response rate regarding this issue was low.33 While CT scans are used to image a broad spectrum of conditions, a report for the UK National Health System suggests that around 95% of the CT scanners in the UK National Health Service are used for cancer staging in addition to their use for non-cancer indications, though it does not provide details into the oncologic share of imaging at the examination level.34,35 A recent study of imaging studies in the U.S. using data from the Centers for Medicare & Medicaid Services found that 9.5% of all advanced imaging studies (i.e., CT, MRI and PET studies) were performed in oncology patients.35
Imaging tests are included in oncology clinical practice guidelines by every major professional group as well as the US National Comprehensive Cancer Network (NCCN) and the UK National Institute for Health and Care Excellence (NICE), and evidence-based studies being used for reimbursement decisions for imaging studies in oncology patients demonstrate the impact of such imaging studies in clinical practice. Data from large prospective studies have demonstrated how imaging can assist in management decisions; for example, the US National Oncologic PET Registry (NOPR) collated data from over 300,000 patients over several years and indicated that the use of PET led to major changes in clinical management in 30% of patients across a wide variety of cancers.36,37
Our literature review did not turn up any relevant large-scale studies from LMICs.
Strengthening cancer care in LMICs: The need for a systems approach
Cancer control and care is complex and requires multi-disciplinary teams for successful delivery. It encompasses prevention, screening, diagnostics (including imaging, pathology and laboratory services), treatments (including, surgery, radiation and systemic therapies), survivorship, palliative care and end-of-life care. Any cancer programme would ideally include services to support all these areas at the appropriate times during the patient’s journey. Optimal cancer control also hinges on access to vaccines for common infections that can lead to cancer (e.g., human papillomavirus and hepatitis). In addition, successful delivery of cancer care requires co-ordination of the overall health system, including public and private healthcare facilities. Education of the public is necessary to promote cancer awareness and accessing of care. Furthermore, the families and careers of those affected by cancer also require support. While each of these needs demands focused attention, the process of cancer control must be viewed holistically and as consisting of a dynamic, interlinked and interdependent chain of activities, where weak links may cause a breakdown in the system of care, and where the links must match each other in order to provide value.
The shortage of a well-trained health workforce and the poor availability of health technologies in LMICs require the adoption of suitable approaches to diagnostics, including disease staging and management during treatment, that differ from those used in high-income countries. Cancer control and care in LMICs will be improved by the adoption of novel approaches to the management of cancer, implemented by way of the progressive expansion of human resources, health technologies and healthcare services for prevention, diagnosis, treatment, and palliative care. For example, in LMICs, women with locally advanced breast cancer may undergo a staging work-up for metastatic disease that includes chest x-ray and liver ultrasound but not CT, single photon emission computed tomography (SPECT) or PET combined with CT, which would typically be used in high-income countries. While an adapted approach in LMICs will miss metastatic disease in some patients whose disease might have been detected with more advanced technologies, this systematic approach will still benefit many patients. If one were to wait to initiate evaluation and treatment of patients until more advanced imaging (and potential treatment options) were available, it would mean that in the interval, which might be years, patients would go without any treatment at all.
Matching the imaging technologies to the treatments available in LMICs is critical. One must go through this optimization exercise in a systematic and evidence-informed way for a multitude of cancers, considering diagnostics, including pathology and imaging, as well as surgery, systemic therapy and radiation. The specifics for each of the imaging and treatment modalities used will differ for each cancer. Investment in cancer detection and control also must take into account health-care system complexity and ensure equitable patient access.22 Furthermore, over time, technology improvements and evidence-based cost–benefit assessments of imaging and treatment modalities will result in changes in imaging recommendations for different cancers depending on the stage of presentation. In addition, changes in the patterns of cancer presentation likely to result from economic development will require adjustment of cancer services.
In determining which imaging modalities to adopt, it is also necessary to consider the overall resources available in a country to purchase, install, operate, maintain, and – when needed – repair the imaging equipment. In practice, governments allocate a proportion of their budgets to health, which is then apportioned to different areas of need, including for maternal and child health, communicable diseases, non-communicable diseases, and injuries.23 Some of the funds are typically allocated to cancer control and care for capital expenditures (for infrastructural needs, including clinical space and capital outlays for radiology and nuclear medicine equipment, pathology laboratories, and operating rooms with necessary equipment) and operational expenditures for salaries of health care providers (e.g., physicians, nurses, technologists, pharmacists, community health workers, as well as trained oncology providers and appropriately trained staff in radiation units who are needed to safely and effectively operate them, including, for example, physicists and dosimetrists). Appropriate medicines (including chemotherapy and biologics), technologies (e.g., for radiation therapy) and diagnostics (including imaging and pathology) must be available to balance diagnostic capabilities with subsequent treatment options. The proportion of the funds allocated to cancer care will vary across and within countries depending on priorities and the different levels of services available. For instance, urban centres may have a higher level of care and more resources than more rural settings.17 In each setting, however, all aspects of care resources must be coordinated and appropriated to ensure effective and efficient budgeting.
When allocating scarce resources, the management challenges posed by imaging capacity constraints must also be considered. For instance, in some settings one or two CT scanners may serve large populations – not just cancer patients but also patients with other conditions (e.g., trauma, infection), and as a result wait times for scanning may be very long, limiting the practical utility of CT for cancer patients. For example, if a patient with diffuse large B-cell lymphoma with extensive mediastinal involvement must wait six weeks for an initial staging CT, clinicians may need to begin treatment without the aid of the CT, which may then not be done at all. In this context, knowledge of the appropriate number of imaging units required per million population to effectively manage cancer diagnosis and treatment is necessary to allow resource planning at a country level. More data on the utilization of imaging and equipment in high-income countries and LMICs would clearly assist with identifying gaps and facilitate development of strategic recommendations for expansion and use of cancer imaging at a global level.
The need for maintenance of imaging equipment must also be taken into account when planning and budgeting for improvements in cancer imaging services. In settings where there may be only one or two CT scanners, for instance, having one scanner out of service for an extended period of time will have a major clinical impact; yet equipment vendors may not have in-country service personnel, and it can be months before technicians can attend to machines in some sites. The cost of repairs and maintenance can be expensive in LMICs, leading to delays in service and prolonged down-time of equipment. Many LMICs have facilities with non-functioning imaging equipment (along with non-functioning pathology processors, linear accelerators, etc.). Unstable power grids that lead to regular interruptions in supply of electricity, among other factors, compound this issue. Loss of electrical power and power surges are common in many locations.
A further challenge relates to the absence of a reliable supply chain for imaging diagnostics, such as contrast agents and radiopharmaceuticals. Gaps in availability of critical reagents are frequent and affect the functional status of imaging modalities that depend on them. Quality management systems (QMS) are essential to ensure imaging is performed in a safe and effective manner. In addition to imaging equipment, the availability of a workforce appropriately trained to perform imaging studies is a major challenge in providing timely and equitable access to imaging for cancer. At present, in some LMICs clinicians may be able to get their patients scanned in a timely fashion, but a paucity of radiologists may delay scan reporting to a degree that affects patient care.
To help address the multiple challenges experienced by LMICs in relation to cancer imaging, there is a need for comprehensive, global mapping of medical imaging and nuclear medicine resources to identify existing gaps and inform strategies to mitigate them. In addition, given the contextual differences in cancer burden and funding levels as well as technical and human resource capacity, to enable strategic planning for optimal cancer care in LMICs, there is a need for evidence on how investments in expansion of imaging could yield clear improvements in patient outcomes in different countries and health systems. These gaps and needs are addressed in more detail, and by the provision and analysis of new data, in the next two sections of this report.
Section 2: Overcoming barriers to access: mapping gaps in imaging and nuclear medicine resources to facilitate progressive expansion of cancer care
There is a need for greater guidance to progressively expand access in LMICs to cost-effective, affordable technologies, which include diagnostic imaging and nuclear medicine, required to address the rising burden of cancer.
Applying this paradigm to the contemporary example of radiotherapy, The Lancet Oncology Commission ‘Expanding global access to radiotherapy’ demonstrated that the cost of upscaling radiotherapy from 2015 to 2035 “across all low-income and middle-income countries” is matched by “compelling evidence that investment in radiotherapy not only enables treatment of large numbers of cancer cases to save lives, but also brings positive economic benefits.”6 Similarly, The Lancet Oncology Commission on Sustainable Care for Children with Cancer has demonstrated substantial health and economic benefits of scaling up high-quality cancer services and treatment for childhood cancers.38 The study estimated $2 trillion of net benefits with an average investment of $30 billion each year in LMICs over a 30-year period (2020-2050). Both Commissions were able to demonstrate a clear investment case with estimated returns of up to $1-6 for radiotherapy and $3 for childhood cancers for every dollar invested.
Just decades ago, the possibility of extending the benefits of technologies like radiotherapy to those without access was deemed unachievable. Since then, many LMICs have made progress in primary care, which enables them to embark on the integration of technologies. For example, the World Health Organization (WHO) Global Action Plan for the Prevention and Control of Non-communicable Diseases 2013–2020 includes radiotherapy for cervical cancer and colorectal cancer.39 Improvements in economic evaluation methods, applied as part of Health Technology Assessment (HTA), have enabled more effective and transparent priority setting and paved the way for inclusion of new health technologies in Universal Health Coverage.40
In the incremental development of cancer imaging capacity, modalities including ultrasound, conventional x-ray, CT, and mammography should be given priority due to their role in the initial assessment of patients, as well as their impact throughout the disease course.41 In view of the complex nature of cancer management for certain patient groups, the type of imaging equipment that should be installed and operational at health care facilities should be based primarily on established, prioritized recommendations by the WHO.42 Our Commission’s composite recommendations for new imaging technologies are intended to complement and support these (Table 1).42 Our aim is to promote the effective and efficient delivery of multidisciplinary cancer care, with resources implemented and progressively provided in a strategic manner. This approach may be challenging in LMICs with restricted funding for health care, but the framework bolsters the capacity of countries to develop facilities in an informed, contemporary, and sustainable manner.
Table 1.
Commission-recommended Imaging Technologies for Cancer Care Facilities, Adapted for WHO Health Care Levels42*
| Level | Imaging Modality |
|---|---|
| WHO Health Care Level I | Level 1 (Primary Health Care) does not have the level of equipment or facilities to undertake cancer care; it may have a triage role to the next level. |
| WHO Health Care Level II |
|
| WHO Health Care Level III |
|
The Commission recommendation for this Table comes from a consensus development process that involved discussion at LOCI meetings, where input from imaging experts into this topic was obtained.
The differences in the recommendations from the WHO Health Care Level imaging equipment (as identified in the reference 42) are as follows:
1) The Commission suggests explicitly that Health Care Level 1 should not be a site where cancer care should be performed, as the full range of imaging equipment (including CT as a minimum) is not adequate for appropriate diagnosis and staging, and likely does not have the medical expertise or services required for complete cancer care.
2) The Commission recommends the inclusion of SPECT/CT (compared to SPECT) in Health Care Level II, as the use of these modalities is now standard at this level.
3) The Commission recommends the inclusion of Theranostics in Health Care Level III, as this replaces Radioimmunoscintigraphy.
The barriers restricting access to imaging and nuclear medicine for cancer in LMICs, many of which were touched on above, include (i) lack of equipment, (ii) lack of human resources, (iii) inadequate government funding for cancer care and health systems in general, (iv) lack of reliable data on the availability of equipment and skilled human resources needed for imaging, (v) few studies which quantify patient imaging needs (for both cancer and non-cancer indications), (vi) absence of evidence-based guidance on investments in imaging required to achieve optimal patient management, (vii) inadequate and insufficient programs for training personnel for cancer imaging, (viii) the dearth of an evidence-based, step-wise procurement process to enable selection of the most appropriate equipment (including appropriate technical specifications and requirements for maintenance and repair for the level of services and training available), (ix) insufficient expertise in medical imaging and nuclear medicine architectural planning (including radiation safety), (x) inadequate systems for appropriate patient referral and follow-up, (xi) insufficient requisite clinical resources (such as laboratory, pathology, and supplies of consumables like syringes, gloves, biopsy devices, catheters, contrast media, local anaesthetic and other medicines, like radiopharmaceuticals) and (xii) lack of safe waste disposal (including biohazards and radiopharmaceuticals).43 The barriers for implementation of imaging equipment at appropriate levels of access, as well as workforce, training and education are similar across LMIC, although differences will always exist between countries.
In addition, compatibility of equipment with local realities, such as the availability and reliability of electricity and clean water, optimal lighting in image interpretation and procedural areas, sustainable infrastructure (including temperature control, or equipment which functions durably without it), and digital linkages to patient information are issues that need to be overcome to ensure access to effective and reliable cancer imaging services.44,45 To safeguard sustainability, it is likewise essential to guarantee adequate maintenance coverage, including service contracts, warranties, availability of spare parts, and an understanding of anticipated software updates.
Furthermore, relevant patient-centred processes should include assessment of patient satisfaction, adequate communication pathways (including patient access to phone services), and available transportation to facilities for the entire target population. As well, health campaigns and community engagement can increase the awareness of the target patient population regarding cancer care, including the role of medical imaging.
It is also essential to ensure the availability not just of affordable imaging, but of affordable treatment after a cancer is diagnosed. In some LMICs, current and projected estimates of patient resources (including the national Universal Health Coverage strategy) are necessary, taking into consideration financial toxicity for those marginalized by the overall cost of cancer care.46-48
Identifying the global gaps in the availability of imaging diagnostics and human resources for imaging diagnostics
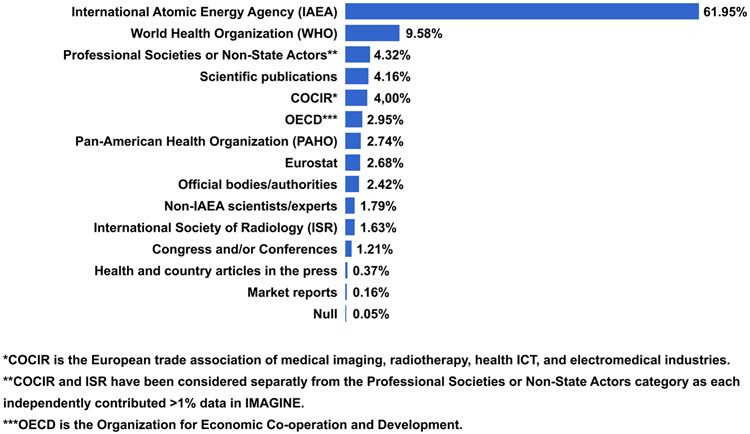
To address the data gaps identified as part of The Lancet Oncology Commission on Imaging and Nuclear Medicine, we collected new data to comprehensively analyse and map medical imaging and nuclear medicine resources globally. The survey and analysis were led by the IAEA. The data were used to construct a new database, IMAGINE (the IAEA Medical imAGIng and Nuclear mEdicine global resources database).49 The sources of data for the IMAGINE database are included in Panel 1 and summarized in Figure 1; sources for, and access to, the database are also discussed further on page 1 of the Web appendix.49
Panel 1: Data Collection for IMAGINE.
The IAEA Medical imAGIng and Nuclear mEdicine global resources database49 (see also Figure 1 and page 1 of the Web appendix)
Launched in 2019, IMAGINE is being dynamically updated. A total of 1857 datapoints in profiles of 211 countries, territories, and principalities have been collected, with dominant sources depicted in Figure 1.
Primary sources for the IMAGINE database were as follows:
The IAEA (from IAEA staff and experts; reports of national, regional and interregional meetings; fact-finding missions; countries’ authorities and counterparts to IAEA projects) and U.N. partner organizations and agencies such as the WHO, WHO regional offices, and International Agency for Research on Cancer (IARC); the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR); the United Nations Development Programme (UNDP); the World Bank; as well as Ministries of Health, Eurostat and the Organization for Economic Cooperation and Development (OECD)
National, regional, and global medical imaging and nuclear medicine professional organizations and societies such as the Arab Society of Nuclear Medicine (ARSNM), the Asia Oceania Federation of Nuclear Medicine and Biology (AOFNMB), Asociación Latinoamericana de Sociedades de Biología y Medicina Nuclear (ALASBIMN), European trade association representing the medical imaging radiotherapy, health ICT and electromedical industries (COCIR), European Association of Nuclear Medicine (EANM), European Society of Radiology (ESR), Global Diagnostic Imaging, Healthcare IT & Radiation Therapy Trade Organization (DITTA), International Organization for Medical Physics (IOMP), International Society of Radiographers and Radiation Technologists (ISRRT), International Society of Radiology (ISR), RAD-AID International, Society of Nuclear Medicine and Molecular Imaging (SNMMI), and World Federation of Nuclear Medicine and Biology (WFNMB)
A comprehensive review of published studies and reports on medical imaging and nuclear medicine resources in countries, particularly from WHO, UNSCEAR, OECD and EUROSTAT.
A survey of individual experts to address gaps in data, including ministry of health representatives and radiation authority experts in countries who work with the IAEA and agreed to share their respective country numbers for equipment and human resources
Figure 1. Major data sources for the IMAGINE database.
IMAGINE=IAEA medical imaging and nuclear medicine global resources database.
IMAGINE data were stratified into high-income, upper-middle-income, lower-middle-income and low-income countries, according to World Bank country income classifications.
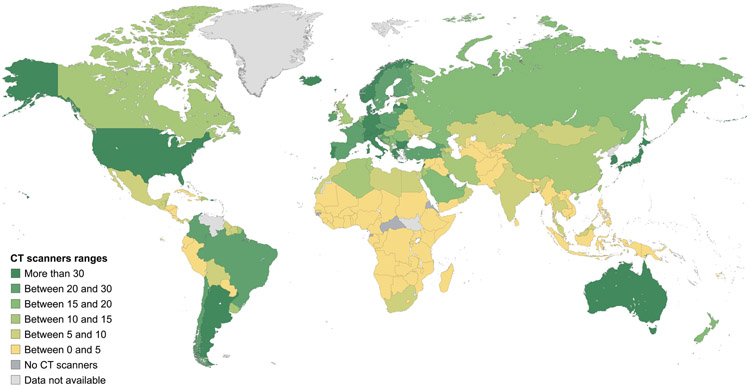
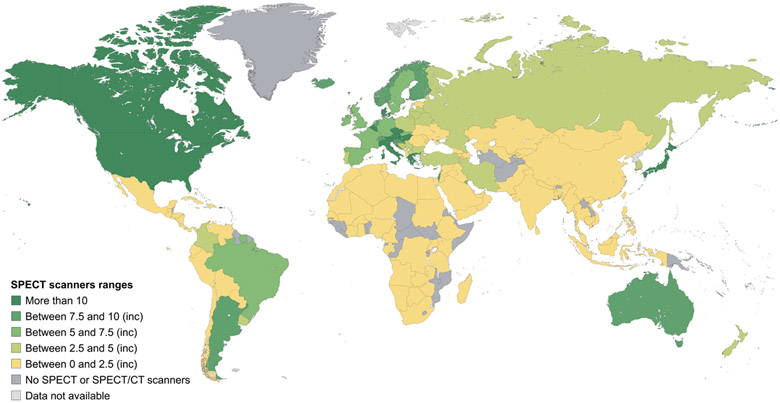
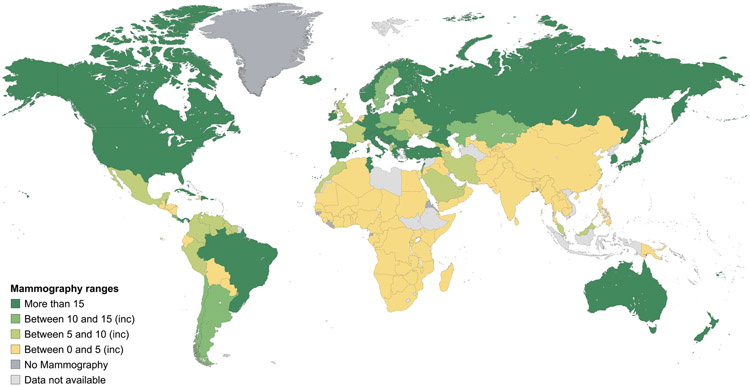
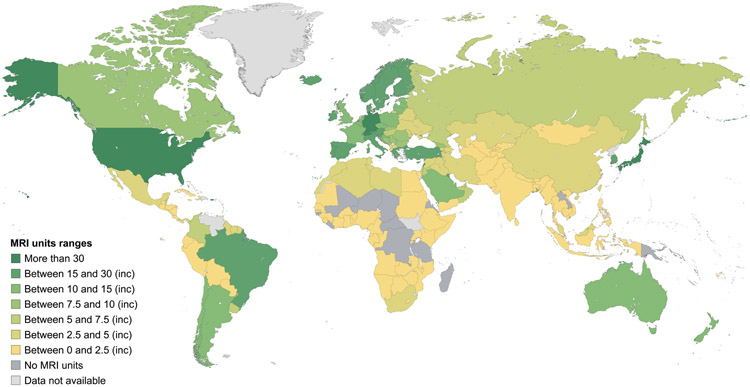
Data on mammography, CT, MRI, SPECT and PET equipment at a country level and according to the income stratification of countries are shown in Figures 2-6, and more detailed interactive information is available via the IAEA IMAGINE database website.49 Information on the numbers of x-ray and ultrasound equipment per country could not be accurately assessed due to the broad range of healthcare facilities, including small health clinics, where they may be installed.
Figure 2. Estimates of the number of CT scanners per million inhabitants.
Data are from the International Atomic Energy Agency medical imaging and nuclear medicine global resources database (IMAGINE). The map was produced by the International Atomic Energy Agency (Vienna, Austria) and is included here with permission.
Figure 6. Estimates of the number of SPECT units per million inhabitants.
Data are from the International Atomic Energy Agency medical imaging and nuclear medicine global resources database (IMAGINE). The map was produced by the International Atomic Energy Agency (Vienna, Austria) and is included here with permission. SPECT=single photon emission CT.
The survey results display a marked difference in the numbers of scanners per million population between high-income countries and LMICs (Table 2).49 For example, the mean number of people served by 1 CT scanner in high-income, upper-middle-income, lower-middle-income, and low-income countries is, respectively, 25,000, 79,000, 227,000 and 1,694,000. The difference in numbers of scanners per million inhabitants is greater for MRI (170%), and much greater for PET (530%) and SPECT (658%). While formal recommendations for numbers of scanners per million population are lacking, the information obtained from the IMAGINE database (Table 2) can be utilized to obtain estimates of installed imaging equipment to provide a range by different country income groups, enabling projection of requirements in different settings. Additionally, evidence-based tools like Health Technology Assessment (HTA) can enable nations to rationally set their own benchmarks. One relevant HTA-based country example is the Framework for the Development of Positron Emission Tomography (PET) Services in England.50 Nations may adopt and adapt such pre-existing templates from other nations to set benchmarks for themselves, in support of rational, achievable planning.
Table 2.
Number of scanners per million inhabitants by country income group
| CT | MRI | SPECT | PET | |
|---|---|---|---|---|
| High-Income Countries | ||||
| Highest value | 42.3 | 34.3 | 20.5 | 4.3 |
| Upper Quartile (75th percentile) | 32.7 | 19.2 | 9.7 | 2.5 |
| Mean | 38.8 | 27.3 | 18.2 | 3.6 |
| Median | 20.5 | 12.6 | 5.4 | 1.2 |
| Lower Quartile (25th percentile) | 14.4 | 8.5 | 2.4 | 0.6 |
| Minimum value | 6.3 | 0 | 0 | 0 |
| Interquartile range (IQR) | 24.4 | 18.8 | 15.8 | 3 |
| Standard deviation (SD) | 16 | 10.4 | 7.5 | 3.4 |
| Upper-Middle-Income Countries | ||||
| Highest value | 29.8 | 16 | 5.2 | 0.7 |
| Upper Quartile (75th percentile) | 16.2 | 7.2 | 2.5 | 0.4 |
| Mean | 12.1 | 5.4 | 1.6 | 0.3 |
| Median | 7.8 | 3.4 | 0.9 | 0.2 |
| Lower Quartile (25th percentile) | 4.8 | 1.3 | 0 | 0 |
| Minimum value | 0 | 0 | 0 | 0 |
| Interquartile range (IQR) | 11.4 | 5.9 | 2.5 | 0.4 |
| Standard deviation (SD) | 10.1 | 4.8 | 1.8 | 0.5 |
| Lower-Middle-Income Countries | ||||
| Highest value | 7.8 | 3.3 | 0.9 | 0.2 |
| Upper Quartile (75th percentile) | 3.9 | 1.4 | 0.4 | 0.1 |
| Mean | 4.3 | 1.1 | 0.3 | 0.2 |
| Median | 1.4 | 0.4 | 0.1 | 0 |
| Lower Quartile (25th percentile) | 0.9 | 0.1 | 0 | 0 |
| Minimum value | 0 | 0 | 0 | 0 |
| Interquartile range (IQR) | 3 | 1.3 | 0.4 | 0.1 |
| Standard deviation (SD) | 3.2 | 1.2 | 0.3 | 0.3 |
| Low-Income Countries | ||||
| Highest value | 1.1 | 0.3 | 0.05 | 0 |
| Upper Quartile (75th percentile) | 0.9 | 0.2 | 0.03 | 0 |
| Mean | 0.69 | 0.2 | 0.04 | 0 |
| Median | 0.4 | 0.07 | 0 | 0 |
| Lower Quartile (25th percentile) | 0.2 | 0 | 0 | 0 |
| Minimum value | 0 | 0 | 0 | 0 |
| Interquartile range (IQR) | 0.7 | 0.2 | 0.03 | 0 |
| Standard deviation (SD) | 0.8 | 0.5 | 0.1 | 0 |
Data source: IMAGINE Database49
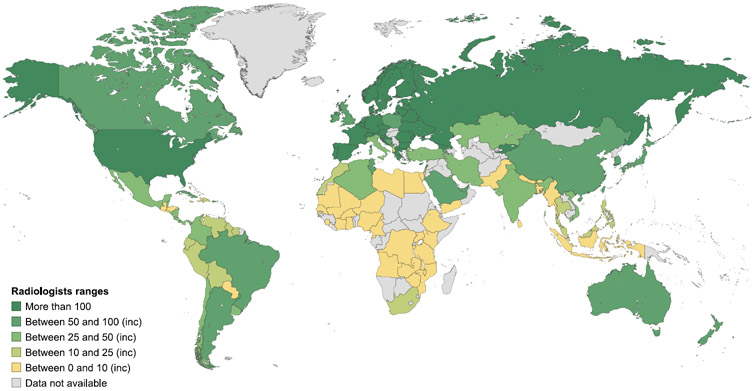
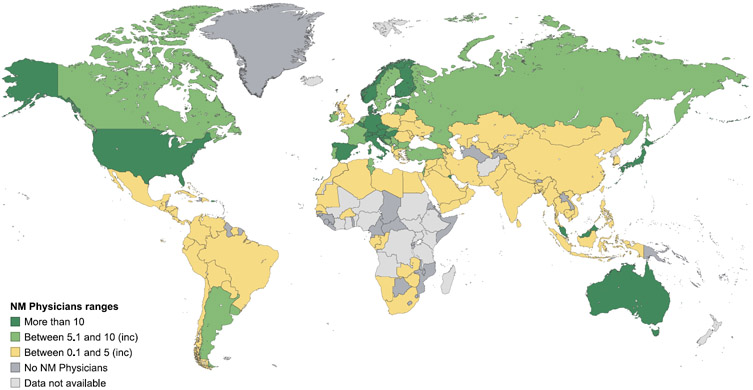
As with the availability and coverage of imaging equipment, little information exists at a global level on the number of radiologists and nuclear medicine physicians. The IMAGINE database revealed marked differences in the numbers of trained radiologists and nuclear medicine physicians between countries (Figures 7 and 8), with strikingly fewer trained professionals in low-income countries (Table 3).49 While in some countries nuclear medicine scans are read by radiologists, the survey data suggests that use of nuclear medicine scans is limited in countries where access to radiopharmaceuticals and trained professionals are additional confounding factors in appropriate scan utilization.
Figure 7. Estimated number of radiologists per million inhabitants.
Data are from the International Atomic Energy Agency medical imaging and nuclear medicine global resources database (IMAGINE). The map was produced by the International Atomic Energy Agency (Vienna, Austria) and is included here with permission.
Figure 8. Estimated number of nuclear medicine physicians per million inhabitants.
Data are from the International Atomic Energy Agency medical imaging and nuclear medicine global resources database (IMAGINE). The map was produced by the International Atomic Energy Agency (Vienna, Austria) and is included here with permission.
Table 3.
Radiologists and Nuclear Medicine Physicians per million population, by country income group
| Nuclear Medicine Physicians |
Radiologists | |
|---|---|---|
| High-Income Countries | ||
| Highest value | 26.2 | 194.0 |
| Upper Quartile (75th percentile) | 11.8 | 129.3 |
| Mean | 10.9 | 97.9 |
| Median | 6.5 | 93.1 |
| Lower Quartile (25th percentile) | 1.8 | 51.3 |
| Minimum Value | 0 | 13.9 |
| Interquartile range (IQR) | 9.1 | 46.6 |
| Standard deviation (SD) | 10.5 | 56.2 |
| Upper-Middle-Income Countries | ||
| Highest value | 6.5 | 118 |
| Upper Quartile (75th percentile) | 3 | 61 |
| Mean | 2.7 | 66.8 |
| Median | 1.5 | 30.6 |
| Lower Quartile (25th percentile) | 0.2 | 15.6 |
| Minimum Value | 0 | 1.5 |
| Interquartile range (IQR) | 2.8 | 45.4 |
| Standard deviation (SD) | 3.4 | 65.3 |
| Lower-Middle-Income Countries | ||
| Highest value | 1.2 | 68.4 |
| Upper Quartile (75th percentile) | 0.6 | 30.9 |
| Mean | 0.5 | 22.3 |
| Median | 0.1 | 6.9 |
| Lower Quartile (25th percentile) | 0 | 3 |
| Minimum Value | 0 | 0.4 |
| Interquartile range (IQR) | 0.6 | 27.9 |
| Standard deviation (SD) | 0.9 | 36.4 |
| Low-Income Countries | ||
| Highest value | 0.09 | 3.9 |
| Upper Quartile (75th percentile) | 0.04 | 3.3 |
| Mean | 0.06 | 1.9 |
| Median | 0 | 1.1 |
| Lower Quartile (25th percentile) | 0 | 0.5 |
| Minimum Value | 0 | 0.1 |
| Interquartile range (IQR) | 0.04 | 2.8 |
| Standard deviation (SD) | 0.1 | 2.5 |
Data source: IMAGINE Database49
While imaging utilization data in oncology patients in LMICs is lacking, the data from the IMAGINE database project would indicate that for many LMICs, the availability of imaging for oncology patients is quite limited. As such, the main impact of imaging in LMICs is likely to be on establishing accurate staging information to guide initial treatment decisions. As noted earlier, the lack of such information can lead to inadequate or inappropriate use of medicines, surgery, or radiotherapy, and increase morbidity and mortality.51 In this context, the health outcome and economic case for improving access to imaging in LMICs for oncology patients—detailed in the next section—is of great practical relevance.
Section 3: Costs, and health and economic benefits of scaling up diagnostic imaging for cancer: A case for investment
Section 2 of this report presents new data on the current gaps in LMICs on the availability of imaging modalities for cancer. The expansion of cancer imaging capacity could help to improve the diagnosis, treatment and care of cancer worldwide. However, analysis of the IMAGINE Database reveals not only a major shortage of imaging modalities but also a large variation among countries within and across country income groups. For example, in high-income countries there is a two-fold variation in the lower-quartile and upper-quartile levels in the availability of CT scanners, but a four-fold difference for SPECT scanners. The variation for upper-middle-income countries, lower-middle-income countries and low-income countries is larger than that observed for high-income countries (Table 2).
Recent research undertaken in conjunction with this Commission included modelling studies that estimated the potential effect on cancer survival of scaling up treatment (chemotherapy, surgery, radiotherapy, and targeted therapy) and imaging modalities (ultrasound, x-ray, CT, MRI, PET, and SPECT). These studies estimated the net survival benefit of scaling up treatment and imaging, both individually and in combination, in 200 countries and territories to the mean level of high-income countries, for 11 cancers (oesophagus, stomach, colon, rectum, anus, liver, pancreas, lung, breast, cervix uteri, and prostate).52,53 These cancers account for 60% of global diagnosed cases of cancer.53
These studies revealed substantial health benefits of scaling up imaging modalities in the management of cancer by improving 5-year net survival. The studies showed that simultaneous expansion of treatment, imaging modalities, and quality of care could improve 5-year net survival by more than ten times in low-income countries from 3·8% (95% UI 0·5–9·20) to 45·2% (40·2–52·1) and could more than double 5-year net survival in lower-middle-income countries from 20·1% (7·2–31·70) to 47·1% (42·8–50·8). There was benefit in improved survival for all country income groups with scale-up, with traditional modalities estimated to provide the largest gains in low-income countries, and MRI and PET estimated to yield the largest gains in higher-income countries. It was demonstrated that investing in medical imaging would be necessary to achieve substantial survival gains.53
However, these studies did not estimate the cost of scale up and the potential economic benefits. Therefore, in order to demonstrate health and economic benefits and costs of scale-up of imaging modalities worldwide and to ascertain whether a worldwide scale-up would generate positive and substantial rates of return on these investments, we developed and extended a modelling approach that was conceived initially for the Lancet Oncology Commission on expanding global access to radiotherapy and developed for the Lancet Oncology Commission on Sustainable Care for Children with Cancer.38
Briefly, we extended the microsimulation model of cancer survival for 11 cancers in 200 countries and territories, described above,53 to include a module on lifetime survival, treatment costs, and economic benefits. We used observed data from the Concord-3 study18 to calibrate our microsimulation model and to estimate 5-year net survival for 200 countries. We provide a detailed description of the methodology in the Web appendix (pages 2-7).
We simulated the clinical course of each individual cancer patient diagnosed between 2020–30 over their lifetime until death (from any cause), accounting for (net) cancer survival and competing mortality risks based on country-specific lifetable projections with and without scale-up. In our model we did not estimate the impact of screening, but modelled cancer cases conditional on diagnosis and stage.
We estimated the economic benefits of improving cancer survival using the full income approach (also called ‘value-of-life-year approach’). The full income approach recognizes the intrinsic societal value of a life-year. We followed the methodology used in the Lancet Commission on Global Health in 2035,54 which estimated the willingness to pay for a one-year increase in life expectancy in countries with different income levels and applied a value of 2·3 times GDP per capita per year in low-income and middle-income countries and 1·4 times GDP in high-income countries.
For sensitivity analysis we used a more conservative ‘human capital approach’. With the human capital approach, the economic value of a life-year is based on the economic contribution of an individual and is valued at 1-times per capita GDP. We accrued productivity benefits only to individuals aged 18 to 64 years in the model when using the human capital approach to reflect typical working ages.
As the human capital approach only values productivity and economic contribution and not the intrinsic value of health and an additional year of life, we used the full-income approach as our base case, which better reflects the value of an additional year to a society.
Cancer treatment costs were estimated using a modelled relationship between costs and per capita GDP based on empirical data obtained from a targeted literature review. (See pages 2-7 of Web appendix and Ward, et al.55 for more details on the model specifications, model assumptions, estimations of costs, projected health and economic benefits and limitations with data and model).
Using the model, we estimated the global costs and benefits of four different packages of scale-up, in which we improved the availability of imaging and/or treatment modalities, and quality of care to the mean level of high-income countries under different scenarios: (1) Imaging only – Scale up all imaging modalities (ultrasound, x-ray, CT, MRI, PET, SPECT); (2) Treatment only – Scale up all treatment modalities (chemotherapy, radiotherapy, surgery, targeted therapy); (3) Treatment + quality – Scale up all treatment modalities and quality of care; and (4) Comprehensive – Scale up all imaging and treatment modalities and quality of care. We compared the potential gains from scaling up all imaging vs. all treatment modalities. We also estimated the potential gains foregone from not including imaging as part of comprehensive scale-up (i.e. Treatment + quality [no imaging] vs. Comprehensive).
We include a parameter for ‘quality of care’ to control for health-system and facility-level factors not explicitly included in the model, which covers health service capabilities which also impact cancer survival, such as adequate laboratory and pathology diagnostics, infection control, nursing standards and coordination of care (see Web appendix page 4).
We estimated the cancer deaths averted, life-years gained, cancer treatment costs, productivity gains, and lifetime return on investment for the cancer cases diagnosed in 2020-30, compared to a baseline scenario or status quo of no scale-up. We computed health and economic benefits, costs and return on investment for the 200 countries and territories and by world regions. We discounted costs and benefits at 3%. The detailed description of the data sources, methods and the approach for the modelling are provided in other publications.53,55
The results show that in the Comprehensive scenario, with scale-up of all imaging modalities, treatment and quality of care in 2020–30 would avert 9,549,500 deaths worldwide (95% uncertainty interval [UI] 6,677,800-12,743,800), accounting for 12.5% (95% UI 9.0-16.3) of the projected total of 76.0 million (95% UI 73.9-78.6) worldwide deaths from the 11 modeled cancers in this period and 133.71 million (95% UI 91.94-179.03) life years saved. Scale-up of imaging alone would avert 2,463,500 deaths (95% UI 1,154,900-4,073,900), accounting for 3.2% (95% UI 1.6-5.3) of worldwide deaths and 33.17 million (95% UI 15.18-54.93) life years saved (Both discounted and undiscounted estimates of life years saved are included in Table 4.)55
Table 4.
Health benefits among cancer cases diagnosed between 2020 and 2030 under various scenarios of scale-up for the 11 modelled cancers
| Cancer deaths averted (95%
uncertainty interval) |
Projected life-years saved
across the lifetime, millions (95% uncertainty interval) |
|||
|---|---|---|---|---|
| Number | Proportion of total deaths |
Undiscounted | Discounted (3%) | |
| Global | ||||
| Imaging only | 2,463,500 (1,154,900-4,073,900) | 3.2% (1.6-5.3) | 54.92 (25.15-91.40) | 33.17 (15.18-54.93) |
| Treatment only | 4,095,600 (1,632,300-7,093,400) | 5.4% (2.2-9.1) | 103.28 (40.37-184.19) | 58.36 (22.71-102.73) |
| Treatment + quality | 5,369,100 (2,894,300-8,032,800) | 7.0% (3.9-10.3) | 134.96 (72.84-208.11) | 76.13 (40.94-116.06) |
| Comprehensive | 9,549,500 (6,677,800-12,743,800) | 12.5% (9.0-16.3) | 232.30 (157.29-311.3) | 133.71 (91.94-179.03) |
| Africa | ||||
| Imaging only | 207,800 (78,700-579,100) | 3.0% (1.1-8.3) | 4.64 (1.65-13.76) | 2.72 (0.99-7.89) |
| Treatment only | 984,300 (299,900-1,926,700) | 14.1% (4.3-26.9) | 23.99 (7.11-47.13) | 13.50 (4.06-26.43) |
| Treatment + quality | 1,569,400 (925,500-2,211,400) | 22.3% (14.1-30.5) | 38.54 (22.47-54.77) | 21.62 (12.63-30.37) |
| Comprehensive | 2,508,100 (2,004,500-2,932,800) | 35.7% (29.8-41.7) | 61.27 (49.52-72.07) | 34.58 (27.86-40.30) |
| Asia | ||||
| Imaging only | 1,420,600 (381,700-2,784,800) | 3.2% (0.9-6.3) | 33.47 (9.16-67.14) | 20.12 (5.43-39.85) |
| Treatment only | 2,509,100 (399,600-4,813,600) | 5.6% (0.9-10.4) | 65.74 (10.72-124.31) | 36.93 (6.09-69.93) |
| Treatment + quality | 3,038,000 (822,900-5,402,900) | 6.8% (1.9-11.7) | 79.56 (21.62-142.02) | 44.64 (12.03-79.77) |
| Comprehensive | 5,282,200 (3,203,400-7,616,800) | 11.9% (7.4-16.5) | 133.99 (79.09-191.59) | 76.88 (45.7-110.17) |
| Europe | ||||
| Imaging only | 435,700 (158,600-769,700) | 3.2% (1.1-5.6) | 8.18 (2.97-14.76) | 5.16 (1.90-9.13) |
| Treatment only | 350,500 (91,800-709,800) | 2.6% (0.7-5.2) | 7.40 (1.98-14.62) | 4.45 (1.22-8.81) |
| Treatment + quality | 455,800 (116,800-971,100) | 3.3% (0.9-7.0) | 9.46 (2.41-19.98) | 5.68 (1.44-11.98) |
| Comprehensive | 982,400 (610,700-1,366,200) | 7.2% (4.6-9.8) | 19.38 (12.02-27.12) | 11.95 (7.48-16.50) |
| Latin America and the Caribbean | ||||
| Imaging only | 354,900 (26,900-633,700) | 7.0% (0.6-12.6) | 7.64 (0.55-14.04) | 4.57 (0.33-8.36) |
| Treatment only | 210,700 (28,600-610,400) | 4.1% (0.6-12.1) | 5.19 (0.77-15.17) | 2.93 (0.41-8.50) |
| Treatment + quality | 247,600 (53,400-728,300) | 4.9% (1.1-13.8) | 6.08 (1.36-17.04) | 3.42 (0.75-9.77) |
| Comprehensive | 665,000 (370,300-1,039,000) | 13.1% (7.5-19.5) | 15.13 (8.08-24.02) | 8.84 (4.81-13.85) |
| Northern America | ||||
| Imaging only | 29,700 (0-219,500) | 0.5% (0.0-4.0) | 0.67 (0.00-4.88) | 0.40 (0.00-2.94) |
| Treatment only | 15,300 (0-119,600) | 0.3% (0.0-2.2) | 0.35 (0.00-2.83) | 0.20 (0.00-1.72) |
| Treatment + quality | 21,100 (0-129,400) | 0.4% (0.0-2.4) | 0.47 (0.00-2.85) | 0.27 (0.00-1.72) |
| Comprehensive | 50,900 (0-235,800) | 0.9% (0.0-4.3) | 1.14 (0.00-5.27) | 0.68 (0.00-3.15) |
| Oceania | ||||
| Imaging only | 14,700 (700-53,900) | 2.7% (0.1-9.7) | 0.33 (0.01-1.23) | 0.19 (0.01-0.72) |
| Treatment only | 25,700 (800-73,300) | 4.7% (0.2-12.3) | 0.60 (0.02-1.70) | 0.34 (0.01-0.98) |
| Treatment + quality | 37,300 (3,000-79,800) | 6.8% (0.6-14.2) | 0.86 (0.07-1.87) | 0.49 (0.04-1.06) |
| Comprehensive | 61,000 (22,800-95,800) | 11.1% (4.4-17.1) | 1.38 (0.50-2.27) | 0.80 (0.30-1.30) |
Estimates are from the global cancer survival microsimulation model.55
The vast majority of the deaths averted would be in Asia (5,282,200 million [95% UI 3,203,400-7,616,800]) accounting for the 11.9% (95% UI 7.4-16.5) projected cancer deaths in Asia in 2020–30 and 76.88 million life years saved (95% UI 45.7-110.17). In Asia, scale-up of imaging alone would avert 1,420,600 million deaths (95% UI 381,700-2,784,800), accounting for 3.2% (95% UI 0.9-6.3) projected cancer deaths in Asia, and would result in 20.12 million life years saved (95% UI 5.43-39.85) (Table 4).55
Similarly, there would be major health gains in Africa where the Comprehensive scale-up would avert 2,508,100 cancer deaths (95% UI 2,004,500-2,932,800) amounting to 35.7% of total projected cancer deaths in Africa (95% UI 29.8-41.7), and result in 34.58 million life years saved (95% UI 27.86-40.30). Scale-up of imaging alone would avert 207,800 cancer deaths (95% UI 78,700-579,100) (3.0% of the projected total cancer deaths in Africa [95% UI1.1-8.3]) and result in 2.72 million life years saved (95%UI 0.99-7.89) in Africa (Table 4).55
Worldwide scale-up of imaging alone or in conjunction with treatment and improved quality of care produces very substantial economic benefits and return on investment (Table 5).55
Table 5.
Economic costs and benefits among cancer cases diagnosed between 2020 and 2030 (11 modelled cancers) (all results discounted 3% annually)
| Incremental cancer
treatment costs (2020-2030), $ billion (95% uncertainty interval) |
Lifetime return on
investment: Full Income [2.3x GDP], (95% uncertainty interval) |
||||
|---|---|---|---|---|---|
| Difference | Percent increase |
Productivity gains, $ billion |
Net benefit, $ billion | Return per $ invested |
|
| Global | |||||
| Imaging only | 6.84 (1.77-15.86) | 0.2% (0.1-0.3) | 1,226.21 (540.05-2,161.8) | 1,219.37 (535.47-2,157.29) | 179.19 (84.71-625.09) |
| Treatment only | 50.72 (14.92-111.88) | 1.5% (0.8-2.4) | 1,183.24 (504.9-2,206.54) | 1,132.51 (489.13-2,114.69) | 23.33 (12.40-60.40) |
| Treatment + quality | 225.50 (83.87-408.34) | 6.7% (5.7-7.8) | 1,386.07 (726.42-2,342.19) | 1,160.56 (484.04-2,053.7) | 6.15 (2.66-16.71) |
| Comprehensive | 232.88 (85.92-421.97) | 6.9% (6.0-8.0) | 2,894.41 (1,794.55-4025.16) | 2,661.54 (1,631.20-3,775.64) | 12.43 (6.47-33.23) |
| Africa | |||||
| Imaging only | 0.46 (0.23-0.79) | 1.9% (1.2-3.0) | 27.38 (9.61-65.80) | 26.93 (9.29-65.34) | 59.97 (22.11-128.14) |
| Treatment only | 6.85 (3.82-11.22) | 29.4% (17.6-42.2) | 120.97 (52.46-210.96) | 114.12 (44.51-203.06) | 17.67 (8.09-33.93) |
| Treatment + quality | 11.14 (6.64-16.98) | 47.8% (34.1-63.1) | 164.86 (88.57-237.47) | 153.72 (79.95-225.41) | 14.80 (8.05-25.71) |
| Comprehensive | 11.67 (7.01-17.70) | 50.1% (36.2-66.4) | 249.66 (187.61-303.31) | 237.99 (177.71-291.8) | 21.39 (14.15-34.34) |
| Asia | |||||
| Imaging only | 3.42 (0.66-9.37) | 0.4% (0.1-0.6) | 713.38 (86.71-1,616.35) | 709.96 (86.03-1,610.45) | 208.70 (77.77-850.18) |
| Treatment only | 24.58 (4.35-69.42) | 2.7% (0.5-6.2) | 679.76 (107.85-1,681.10) | 655.17 (103.01-1,621.55) | 27.65 (12.89-68.97) |
| Treatment + quality | 37.98 (13.16-86.15) | 4.4% (1.9-8.5) | 772.73 (182.13-1,686.61) | 734.75 (164.77-1,613.12) | 20.35 (8.10-49.52) |
| Comprehensive | 41.59 (14.76-91.25) | 4.7% (2.3-8.9) | 1,653.82 (828.58-2,458.01) | 1,612.22 (802.55-2,410.54) | 39.76 (17.99-101.74) |
| Europe | |||||
| Imaging only | 1.95 (0.23-5.52) | 0.2% (0.0-0.4) | 281.15 (77.79-612.65) | 279.20 (76.86-605.35) | 144.32 (71.07-686.83) |
| Treatment only | 14.73 (1.88-38.95) | 1.2% (0.2-2.6) | 257.18 (82.05-517.31) | 242.45 (72.14-493.25) | 17.46 (8.28-66.89) |
| Treatment + quality | 171.39 (59.5-314.06) | 14.5% (13.3-16.0) | 301.80 (114.77-602.30) | 130.41 (−119.56-444.47) | 1.76 (0.49-6.02) |
| Comprehensive | 173.59 (59.79-315.94) | 14.7% (13.6-16.1) | 618.57 (367.27-884.37) | 444.98 (160.23-737.88) | 3.56 (1.64-10.47) |
| Latin America and the Caribbean | |||||
| Imaging only | 0.52 (0.03-1.31) | 0.6% (0.0-1.1) | 138.85 (8.89-259.83) | 138.33 (8.85-259.06) | 266.38 (109.69-1,351.47) |
| Treatment only | 2.21 (0.20-7.03) | 2.9% (0.3-7.4) | 79.99 (8.78-241.17) | 77.79 (8.54-237.43) | 36.28 (14.10-152.10) |
| Treatment + quality | 2.56 (0.45-7.42) | 3.4% (0.7-8.0) | 87.66 (9.42-264.11) | 85.10 (8.85-260.56) | 34.27 (12.16-124.16) |
| Comprehensive | 3.08 (0.61-8.04) | 4.1% (1.3-8.7) | 245.96 (123.82-403.20) | 242.88 (122.20-397.69) | 79.77 (30.36-384.86) |
| Northern America | |||||
| Imaging only | 0.37 (0.00-3.26) | 0.0% (0.0-0.2) | 47.48 (0.00-348.01) | 47.12 (0.00-345.16) | 128.94 (64.85-361.54) |
| Treatment only | 1.22 (0.00-11.54) | 0.1% (0.0-0.8) | 24.24 (0.00-202.14) | 23.02 (0.00-181.52) | 19.83 (7.95-72.25) |
| Treatment + quality | 1.22 (0.00-11.54) | 0.1% (0.0-0.8) | 32.60 (0.00-202.14) | 31.37 (0.00-190.39) | 26.66 (8.18-1,398.67) |
| Comprehensive | 1.59 (0.00-11.58) | 0.1% (0.0-0.8) | 80.12 (0.00-373.7) | 78.53 (0.00-371.43) | 50.36 (8.42-984.28) |
| Oceania | |||||
| Imaging only | 0.13 (0.00-0.59) | 0.1% (0.0-0.6) | 17.96 (0.13-77.95) | 17.83 (0.13-77.42) | 137.36 (24.94-338.03) |
| Treatment only | 1.14 (0.02-4.59) | 1.2% (0.0-4.4) | 21.09 (0.12-86.53) | 19.96 (0.11-83.31) | 18.56 (5.28-51.96) |
| Treatment + quality | 1.21 (0.09-4.68) | 1.3% (0.1-4.5) | 26.42 (0.67-93.98) | 25.21 (0.57-91.45) | 21.77 (5.70-191.78) |
| Comprehensive | 1.35 (0.13-4.83) | 1.4% (0.2-4.5) | 46.29 (9.13-112.39) | 44.95 (8.92-109.14) | 34.41 (11.48-244.48) |
Estimates are from the global cancer survival microsimulation model.55
Incremental costs in 2020–30 of scaling-up imaging would be $6.84 billion (95% UI 1.77-15.86), but this investment would result in productivity gains of $1.23 trillion (95% UI 0.54-2.2) and a net benefit of $1.22 trillion (95% UI 0.54-2.2), yielding a return per dollar invested of 179.19 (95% UI 84.71-625.09). The very substantial returns realised on investment are because the scale-up of most of the cancer imaging modalities is not costly. However, the absolute numbers of deaths averted would be modest (2,463,500 deaths [95% UI 1,154,900-4,073,900], accounting for 3.2% [95% UI 1.6-5.3] of worldwide deaths and 33.17 million [95% UI 15.18-54.93] life years saved) relative to what could be achieved with the Comprehensive scale-up scenario (9,549,500 deaths worldwide [95% UI 6,677,800-12,743,800], accounting for 12.5% [95% UI 9.0-16.3] of the total worldwide deaths and 133.71 million [95% UI 91.94-179.03] life years saved).
The estimated incremental cost of Comprehensive scale-up would be $232.88 billion (95% UI 85.92-421.97), amounting to a 6.9% (95% UI 6.0-8.0) increase in current global cost of cancer treatment and care. However, the benefits of this scale up would be very substantial, with lifetime productivity gains of $2.89 trillion (95% UI 1.79-4.03) for the cancer cases diagnosed in 2020-30. This would produce a net economic benefit of $2.66 trillion (95% UI 1.63-3.78) and a return on investment of $12.43 (95% UI 6.47-33.23) for every dollar invested. Scale-up of just treatment and quality of care without imaging would produce far lower net economic benefit of $1.16 trillion (95% UI 0.48-2.05) and a return on investment of $6.15 (95% UI 2.66-16.71), less than half of what would be achieved if imaging were included in the scale-up (Table 5).55
To provide a specific example, we compared our model estimates to reported costs from Ethiopia using data from Ethiopia’s national health accounts (see case study in Panel 2).56,57
Panel 2: Incremental cost of cost and benefits of comprehensive scale-up – Ethiopia case study.
As a specific example, we compare our model estimates to reported costs from Ethiopia, for which national health accounts data are available. According to the Ethiopian Ministry of Health, national health expenditures were $US 3.10 billion for 2016/17 (about 4.2% of GDP), of which an estimated 1.8% ($55.8 million) was spent on cancers.56 Our model estimates that cancer treatment costs in Ethiopia for the baseline scenario (no scale-up) are $90.55 million (95% UI 64.51-124.12) per year on average between 2020-2030, similar to the reported estimates after accounting for population growth (UN population projections estimate that the over-50 population in Ethiopia will grow by 40% between 2015-2025).57
We estimate that with comprehensive scale-up cancer treatment costs would rise to $171.17 million (95% UI 125.55-224.80), accounting for an additional $80.6 million of spending per year on average – a 90% increase in cancer costs. Although this is a large increase in cancer spending, it is a relatively small proportion of total health expenditures, comprising about 2.8% of current total health expenditures. In return we estimate that comprehensive scale-up would yield large economic benefits over the lifetime of cancer survivors, yielding an estimated return of $18.44 per dollar invested in Ethiopia.
The net economic benefits of Comprehensive scale-up would be very substantial for all world regions (Table 5).55
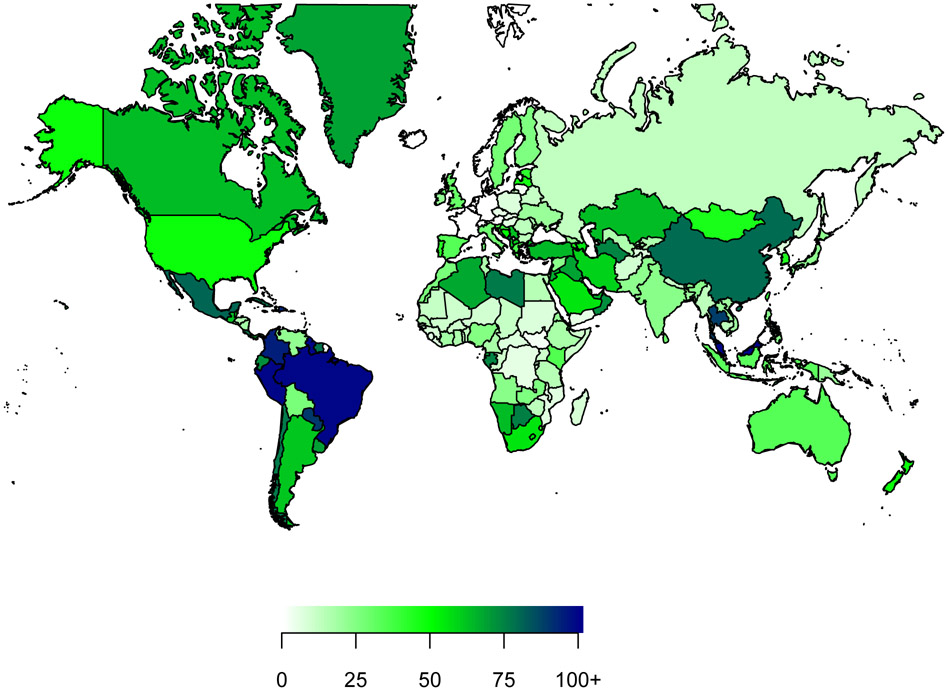
All countries worldwide would realise substantial positive returns on investment in scale-up of imaging alone or in combination with treatment and quality care (Table 5). Figure 9 presents a world map that shows lifetime returns on investment accrued to countries worldwide.
Figure 9. Estimated return on investment (comprehensive scale-up of imaging, treatment and quality of care) by country for 11 cancer types.
Comprehensive scale-up refers to scale-up of all imaging and treatment modalities and quality of care to the mean amount of that of high-income countries. Returns per US$ invested are estimated for patients diagnosed with cancer in 2020-30, compared with a baseline scenario of no scale-up. Estimates are presented in US$ in 2018 and discounted at 3% annually.
The estimated variation on the return on investment between countries is mainly due to differences in the availability of imaging modalities in different countries. Regional differences are largely due to (i) differences in baseline availability of surgery, radiotherapy and medicines and imaging modalities, (ii) quality of care, (iii) differences in income levels in countries, which influences productivity estimates, and (iv) the fact that the value placed on of a life-year using the Full Income approach varies by income group, where the value is 2·3 times GDP per capita per year in low-income and middle-income countries, and 1·4 times in high-income countries. New data compiled by the Commission on coverage of imaging modalities by country and presented in this report (Table 2) reveals substantial variation in the availability of imaging modalities.49 The range of per capita income between and without country income categories is very substantial. The Gross National Income (GNI) per capita (using Atlas methodology and purchasing power parity) ranges from $280 to $1,035 in low-income counties, from $1,036 to $4,045 in lower-middle income countries, from $4,046 to $12,535 in upper-middle income countries and from $12,536 to more than $100,000 in high-income countries.58
We present in the Web appendix (Table, page 8, based on estimates of the global cancer survival microsimulation model55) a sensitivity analysis of costs, productivity gains, net benefits and return on investments that uses the more conservative human capital approach. The Web appendix Table shows a net benefit of $209.46 billion (95% UI 94.96-394.72) and a return of 31.61 (95% UI 15.09-110.14) for scaling up imaging alone. With comprehensive scale-up, the worldwide net benefit is $340.42 billion (95% UI 99.37-592.59) and the return per dollar invested is $2.46 (95% UI 1.29-6.52), as costs of comprehensive scale-up are much higher than scaling up imaging alone. There are substantial net benefits and returns to scaling up imaging in all world regions and, with the exception of Europe, considerable net benefits and return on investment with Comprehensive scale-up (Web appendix Table).
The modelling, using the full income and human capital approaches, demonstrates very substantial health and economic benefits with considerable returns on investments when scaling up imaging diagnostics alone or as part of a comprehensive scale-up that involves simultaneous scale-up of treatment and quality of care.
Modelling suggests synergistic benefits when all of these are scaled-up simultaneously. Hence, the results are not additive. The model estimates suggest that simultaneous scale-up of multiple imaging modalities with treatment and quality of care yields synergistic returns as one might expect. Scaling up imaging without scale up in treatment is not likely to lead to major improvements as treatment capacity is soon reached and additional cases will not be adequately treated. Similarly, scaling-up quality of care without scaling up diagnostics or improving treatment availability will likely have little impact in LMIC, as many cases will not be diagnosed, and even when diagnosed will not receive surgery, radiotherapy or medicines. Hence, the results establish a compelling case for investing in worldwide comprehensive scale-up of diagnostic imaging for cancer.
Section 4: Financing the global scale-up of diagnostics
New financing will be needed to scale up capacity for cancer imaging diagnostics in order to expand access to effective and affordable services in LMICs. But where will new financing come from?
In most LMICs, the largest proportion of funding will likely come from domestic sources – namely, public financing (government budget allocated to health) and complementary financing from the private sector. In addition, there is potential for funding from external private companies, Overseas Development Assistance (ODA), or development banks that provide loans or invest in health infrastructure projects, for example, to establish new diagnostic imaging facilities or upgrade existing ones. Examples of development banks include the World Bank Group, a conglomerate of five institutions, as well as the European Investment Bank, African Development Bank, InterAmerican Development Bank, Islamic Development Bank, and Asian Development Bank.
Donations can also come from or be facilitated by non-state actors (NSAs) or non-governmental organizations (NGOs) and U.N. organizations like the WHO and IAEA. For example, the IAEA allocated €5.74 million in 2019 for support of nuclear medicine and diagnostic imaging, including procurement of medical imaging equipment and expansion of capacity. The beneficiaries of cooperation are member state LMICs.59
The level of public financing for any sector is determined by the ‘fiscal space’ available to the government, which is defined as “…the availability of budgetary room that allows a government to provide resources for a desired purpose without any prejudice to the sustainability of a government’s financial position.”60 Fiscal space depends on the sources of finances from (i) economic growth that creates favourable macroeconomic conditions for increased government revenues and budget, (ii) strengthening tax administration, (iii) reprioritization of health within the government budget, (iv) borrowing from domestic and international sources or ODA to invest in health, (v) more effective and efficient allocation of available health resources and (vi) innovative domestic and international financing.61,62 We describe below the main sources of financing that could be used to expand fiscal space and present a table summarising the potential magnitude of funds and the suitability of different funding sources for investing in scale-up of imaging diagnostics and cancer care.
(i) Improved economic growth. The International Monetary Fund (IMF) projects positive economic growth in LMICs between 2020 and 2025.63 Other estimates suggest that in 2015–40, continued growth of GDP and higher government revenues could help increase per capita government spending on health by around 5.3% each year in upper-middle-income countries, 4.2% in middle-income countries and 1.8% in low-income countries.64 These estimates are based on pre-COVID-19 economic parameters. An investment case for imaging diagnostics is critical to harness new funding.
(ii) Generation of revenues by strengthening tax administration. In LMICs, government revenues from tax are low, on average 15% of GDP in low-income countries, 25% in lower-middle-income countries, 30% in upper-middle-income countries and 40% in high-income countries.65 Modelling studies estimate that an increase in tax revenue, where at least one-third of newly raised revenues is allocated to health, could on average increase public expenditure on health in LMICs by 78% (95% Confidence Interval (CI) 60-90%).66
Increased taxes on tobacco and alcohol are highly cost-effective public policies. Egypt, the Philippines and Thailand have successfully used tobacco taxes to generate funding for the health sector.67 A 20% and 50% price increase in tobacco prices could generate over 50 years-worth of additional tax revenues, approximately US$1987 billion (Uncertainty Interval (UI): US$1613 to US$2297 billion) and US$3625 billion (UI: US$2534 to US$4599 billion) respectively, and in low-income countries an average of additional revenue of 0.17% of GDP each year in the 50% scenario.68
(iii) Reprioritization of health within government budget. Evidence on health and economic benefits for new health investments could be used to persuade governments to reprioritize their investments. Modelling estimates that budget reprioritization could potentially increase funds allocated in LMICs to health by 72% (95% CI, 57-87%).65
(iv) Borrowing from domestic and international sources and ODA. Concessional financing with low interest rates and generous grace periods for repayments could be mobilized from international development banks to invest in the expansion of diagnostics capacity. In 2017, the World Bank had 45 active projects for a total sum of $470 million for medical equipment procurement.69 The African Development Bank has recently approved an equity investment in a new fund for health infrastructure that will raise $100 million to fund health infrastructure projects in Africa.70
Investment in diagnostic imaging is particularly attractive for development banks as these are infrastructure investments that can generate an income stream for the investors to service the loans over time and also provide an opportunity for public-private partnerships or private sector investments for provision of public services that can be outsourced by governments.
In addition to loans, guarantees provided by development banks can be used to encourage mobilization of private financing by mitigating investment risks in LMICs for projects to establish or develop facilities for imaging diagnostics.
In 2013, the World Bank Group provided US$4.5 billion of guarantees across 30 countries.71 The guarantees were structured as partial risk guarantees, partial credit guarantees, or policy-based guarantees.72 Partial risk guarantees support private sector investment, including public–private partnerships. Partial credit guarantees enable commercial borrowing in support of public investment projects, and policy-based guarantees support commercial borrowing for budget financing or reform programs. Guarantees offer several benefits to borrowers. The reduced risk of default improves the country’s ability to borrow for investment. Guarantees can reduce the cost of capital as a result of lower interest rates that the borrowing government must pay, because these rates are moderated by the guarantor’s credit worthiness (the World Bank has AAA rating). Guarantees also allow governments to share the risk of projects with the private sector. Such guarantees would be very suited to investments in expanding capacity for imaging diagnostics in LMICs.
(v) More effective and efficient allocation of available health resources in health systems. With a priority setting, more efficient allocation and use of resources, governments could generate a 26% (95% CI, 21–31%) increase in public expenditure on health.65
(vi) Innovative financing. Funding mobilized from non-traditional sources is another potential source of financing for diagnostic imaging. Innovative financing mechanisms such as the Global Fund, Gavi and Unitaid73,74 (which link different elements of the financing value chain—namely, resource mobilization, pooling, channelling, resource allocation, and implementation) have channelled more than $55 billion to LMICs for health.
Social or Development Impact Bonds are promising innovative financing instruments that could be used to finance expansion of diagnostics capability in LMICs. A Social (Development) Impact Bond is created by a government agency (or External Funder such as a development agency or a charitable foundation) that wishes to achieve a desired social or health outcome.75,76 The government/agency engages an external organization to achieve the outcome. A third-party investor provides upfront working capital to the external organization as an at-risk investment. If the desired social outcome is achieved, the government/agency releases payment to the external organization, based on terms specified in an upfront contract, which repays its investors their principal, plus a return on the investment. If the outcome is not met, the government/agency disburses no payment.
The potential new funding from multiple sources to expand fiscal space (Table 6).63-72,74-77 far exceeds the financing needed globally for comprehensive scale-up of interventions for cancer care.
Table 6:
Potential funding sources for expanding fiscal space for health and investment in scale-up of imaging diagnostics and cancer care in low-income and middle-income countries
| Potential funding source | Potential additional fiscal space that could be created | Feasibility of creating additional fiscal space | Suitability for funding scale-up of imaging diagnostics for cancer |
|---|---|---|---|
| Improved economic growth. | Substantial. Could help increase each year per capita government spending on health by around 5.3% in upper-middle-income countries, 4.2% in middle-income countries and 1.8% in low-income countries.64 | Feasible. LMICs are projected to achieve robust economic growth.63 despite COVID-19 many have returned to positive growth trajectories. (see footnote) | Would generate sustainable general revenue income for allocation to health. |
| Generation of revenues by strengthening tax administration | Allocating at least one-third of newly raised revenues to health, could on average increase public expenditure on health in low-income and middle-income countries by 78% (95% CI 60-90%).66 | Feasible. Tax revenues in LMICs are 15-30% of GDP compared to 40% in high-income countries but would require stronger tax collection systems that would take time to implement.65 | Additional revenues would need to be allocated to health. Sustainable funding. |
| Increased taxes on tobacco, alcohol and sugary beverages | Substantial. In low-income countries 50% increase in tobacco prices could generate on average additional revenue of 0.17% of GDP each year.68 | Feasible, but would require political will to fight opposition. Highly cost-effective.67 | Sustainable funding with additional health and economic benefits. Could be earmarked for health. |
| Reprioritization of health within government budget | Substantial. In LMICs could increase funds allocated to health by 72% (95% CI, 57-87%).66 | Would require strong political capital to achieve reprioritization. | Sustainable funding. |
| Borrowing from domestic and international sources and ODA | Substantial, but under-utilized. Could be in the form of hybrid financing – mix of loan and equity from public and private sectors. | Feasible. Low interest rates make this an attractive option. Infrastructure loans available from World Bank and Regional Development Banks. Export guarantees would substantially reduce borrowing costs.69,70,72,71 | Encourage public-private partnerships to reduce capital investment requirements for government. Could provide revenue stream to investors to offset costs. |
| Innovative financing | Substantial. Large potential. | Social or Development Impact Bonds could be used to invest in scale up.74,75,76 Easily measurable results with investment in imaging diagnostics. | Encourage public-private partnerships to reduce capital investment requirements for government. Provides revenue stream to investors to offset costs. |
Sources for Table 6: Authors’ analysis synthesis of evidence from references in table and the International Monetary Fund 2020 report, “World Economic Outlook: A Long and Difficult Ascent.”77
With measurable performance indicators, the investment in population-based health can be a tool towards a nation’s development rather than a mere by-product of it. Medical imaging is a cornerstone of the strengthening of health systems to address the disability-adjusted life years (DALYs) lost to cancer, a burden that falls disproportionately (80%) on LMICs, though these nations receive only ~5% of current global funding for cancer control.3,5
Section 5: Radiation protection and safety and quality systems
The safe utilization of medical imaging in cancer care requires appropriate standards for radiation protection and safety with regard to patients, families, workers and the public, irrespective of the level of economic development of a country. To ensure that appropriate standards are met, there are responsibilities at the national, institutional and individual levels. Whether the imaging modality utilizes ionizing or non-ionizing radiation, there must be adequate safety infrastructure, education and training of staff, appropriate staffing levels and effective quality assurance systems.
Protecting patients and workers when using ionizing radiation in medicine
The latest figures published by the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR)78 indicate that approximately 3.6 billion diagnostic radiology X-ray examinations and 33 million diagnostic nuclear medicine examinations are performed each year internationally. Imaging frequency during cancer care is not explicitly considered in these figures, however. 78 Medical uses of ionizing radiation (excluding therapeutic uses) constitute more than 98% of the world population’s exposure to radiation from man-made sources. Between the global surveys for 1991-1996 and 1997-2007, the total annual number of diagnostic medical examinations (both medical and dental) was estimated to have risen by 50%.78 However, more recent national figures for the United States79 suggest that the largest contributor to radiation dose, CT scanning, has stabilized in numbers. The second-largest contributor, imaging using nuclear medicine, has shown similar numbers per year in the last five years for SPECT/CT procedures, and continued increase in PET/CT studies (mainly in patients with cancer) globally, in both high-income countries and LMICs.80, 81,82 In relation to occupational radiation exposure, according to UNSCEAR,78 worldwide, the estimated number of health care workers involved in the medical uses of radiation is 7.4 million, which is considered to be increasing with time.
Radiation protection for patients and workers needs to be approached systematically when using ionizing radiation in medicine.83 Remarkable progress has been made in understanding the health effects of radiation over the last century. There is a need to increase awareness among the medical community of the amount of radiation received by patients in imaging procedures.84 However, there is a lack of qualified medical physics support, in particular in diagnostic radiology and nuclear medicine theranostics, in LMICs.85 This shortfall poses major risks for patients and healthcare workers as radiation safety, quality systems and maintenance are insufficiently guaranteed. Further, in many LMICs the medical radiation devices and their use are not sufficiently governed by appropriate governmental, legal and regulatory frameworks for safety. The rapid evolution of technology for imaging involving radiation exposure poses challenges for maintaining the safety of patients and healthcare workers, as this maintenance requires the education and training of health professionals and regulatory staff; furthermore, the rapid evolution of technology makes it challenging to keep regulations up to date. Regulation of the use of ionizing radiation in medicine is uneven when comparing countries globally.86
The radiation exposure of patients for diagnosis, intervention or therapy differs from other uses of radiation in that it is done for the direct benefit of the individual, who also incurs the radiation risk and other risks of the procedure.87 The guidelines which justify the use of a procedure should be developed by health authorities together with professional bodies and should be reviewed from time to time to ensure radiological procedures that are no longer justified are removed from guidelines and medical practice.88 Optimization of radiation protection in imaging means that the level of protection and safety should be the best possible under the prevailing circumstances, and should be implemented in all scenarios. It should be noted that this pertains not only to radiation doses that are excessive for the given image but also to doses too low to generate images of suitable diagnostic quality for accurate interpretation. This trade-off between radiation exposure and suitable diagnostic quality is a challenging issue in cancer care, as repeated exposure to radiation over short and long intervals is common. Dose limits apply to occupational exposure and public exposure arising from medical uses of ionizing radiation, not to the exposure of patients. For some areas of medical uses of ionizing radiation, such as image-guided interventional procedures, good radiation protection practice for staff must be followed in order not to exceed occupational dose limits.88
Responsibilities at a national level for safe operation of facilities and use of radiation sources
For safe operation of facilities and use of radiation sources, it is necessary for a country to have appropriate governmental, legal and regulatory frameworks for safety.89 The government establishes laws and adopts policies relating to safety as well as the responsibilities and functions of different governmental bodies in respect of safety. Among the important responsibilities of a government is the establishment of an independent regulatory body with the necessary legal authority, competence and resources. In the health sector, according to international safety standards,90 it is the responsibility of the government to ensure that a country’s diagnostic reference levels, an optimization tool for diagnostic imaging, are established through consultation between the relevant health authorities, professional bodies and the regulatory agencies. The regulatory agency has different means of ensuring compliance, such as authorization and inspection of facilities and activities and enforcement of regulatory requirements.89 At a national level, other organizations have an important role for the safety of patients, workers and public, such as health authorities, professional bodies, technical standards associations, regulatory agencies involved in approval of medical devices, and agencies involved in health technology assessment.90 Many countries are lacking an adequate radiation safety infrastructure. For LMICs and other countries that may need to strengthen this at a national level, there is guidance published by the IAEA on overcoming this challenge.91
Responsibilities at a facility level and individual level for safe operation of facilities and use of radiation sources
Hospitals and other healthcare institutions performing radiological and nuclear medicine imaging procedures should have appropriate equipment (with planned replacement cycles), maintenance and quality systems, and staffing to perform studies in an optimal manner. Health professionals working in such facilities should have appropriate training and qualifications in clinical practice and adhere to relevant radiation safety standards. Optimization of radiation protection is lacking in facilities in many countries and can be improved using simple and inexpensive techniques.92
Clinical Imaging Guidelines and Appropriate Use Criteria are the imaging referral guidelines developed by international expert groups that facilitate the choice of “best test first” and help strengthen the justification of exposure to radiation in imaging procedures.93 Justified procedures, by definition, bring individual patients more benefit than risk. This means that the proposed overall increase of imaging using ionizing radiation will bring the global population more benefit than risk as long as generic justification of the radiological procedure has been carried out by the health authority in conjunction with appropriate professional bodies, and the justification of the medical exposure for the individual patient has been carried out by means of consultation between the radiological medical practitioner and the referring medical practitioner. Improving the appropriate use of imaging is important for the radiation protection of patients and for overall patient care. According to the international basic safety standards developed by the International Atomic Energy Agency (IAEA),90 relevant national or international referral guidelines shall be taken into account when justifying the medical exposure of an individual patient in a radiological or nuclear medicine procedure. These guidelines are produced, maintained and disseminated by a number of international organizations,94-99 are for the use of referring physicians, radiologists and nuclear medicine physicians, and are important for the radiation protection of patients. However, it should be noted that knowledge in cancer care, especially for new therapeutic drugs, is evolving rapidly, which makes it challenging to keep guidelines up to date.
Quality Systems
The provision of safe, high-quality imaging services depends on the control of several variables, including infrastructure, staffing, regulatory environment, quality control of instruments, compliance with national regulations for patients’ and workers’ safety, and the basis in evidence of a medical practice. This framework requires the identification of quality policies and objectives, and the production of a documented system with clearly defined processes, procedures, and responsibilities. Such a system is usually referred to as a Quality Management System (QMS), and its purpose is to help direct activities to meet customer and regulatory requirements and to continually improve effectiveness and efficiency. Typically, a QMS also provides a platform to identify areas for improvement. The IAEA has developed quality management audit methodologies for nuclear medicine (QUANUM).100,101 and radiology (QUAADRIL)102 that facilitate the adoption of quality policies in medical imaging departments. The programmes cover all aspects of medical imaging, including management, radiation regulations and safety, radiation protection of patients, quality control of instruments, operations and services, diagnostic clinical service and radiopharmacy. The European Society of Radiology (ESR) has also published guidance on clinical audit performance.103
Radiopharmaceuticals and targeted therapy
Radiopharmaceuticals are radiolabelled compounds that, once administered to the patient, are incorporated into cells or tissues to provide diagnostic information or to result in a therapeutic effect. These unique molecular tools, which are indispensable for the practice of nuclear medicine, need to be prepared shortly before being administered to patients, due to the short physical half-life of the radionuclides used. The vast majority of radiopharmaceuticals used for diagnostic and therapeutic purposes are dosed in sub-pharmacologic quantities of ligand attached to radioisotope, thereby avoiding clinically relevant drug-related side-effects. According to the international pharmacopoeia, radiopharmaceuticals are “medicinal formulations” and, therefore, their production must be carried out in facilities that have appropriate QMSs in place. This production can be undertaken by a licensed commercial organization, or alternatively using hospital-based facilities that comply with appropriate domestic or international standards.104-106 Testing of final product and radiation safety are essential in ensuring safe and appropriate use.
Access to and availability of radiopharmaceuticals are a major factor in the provision of clinically necessary nuclear medicine procedures. Barriers to accessing radiopharmaceuticals include a lack of co-ordinated supply (particularly in low- and middle-income countries), transportation issues, facility infrastructure, and appropriate staff training and availability. Provision of essential nuclear medicine procedures for cancer patients therefore requires a health system and regulatory framework which facilitates access to radiopharmaceuticals, as well as the infrastructure and trained staff to perform these procedures.105 In this context, local production of radiopharmaceuticals for immediate injection should not necessarily require facilities that meet Current Good Manufacturing Practice (cGMP) standards in full, but the radiopharmaceuticals should undergo appropriate quality control prior to administration.105
With regard to the radiation protection of patients and workers, the safety of the public and of family members should also be considered.107 Many nuclear medicine procedures are done on an out-patient basis and the exposure to the public and patient families following a procedure needs to be considered.31 This includes educating the patient on how to reduce exposure to the public and family members from the ionizing radiation from the radiopharmaceuticals that have been administered to the patient for the diagnostic test or for radionuclide therapy.108
Protecting patients and healthcare workers when using Magnetic Resonance Imaging (MRI)
In contrast to imaging procedures using ionizing radiation, there is a lack of comprehensive data in the field of MRI. The global number of workers involved in MRI is unknown, although the safety of healthcare workers involved with MRI is an area that needs to be considered. In particular, for some types of MRI procedures, occupational exposure to the magnetic fields of health professionals can be substantive, and requires considerable protective measures, especially for high and very high magnetic fields. Workers’ protection has been comprehensively addressed recently in Directive 2013/35/EU109 on the health and safety requirements regarding the exposure of workers to the risks arising from physical agents (electromagnetic fields) and is also mentioned in some national and professional guidelines.110
MRI safety is mostly dominated by the interaction of implanted devices with the different magnetic fields which are used to make the images. Therefore, it is of utmost importance to have a policy to assess the safety of medical implants and devices prior to MRI (e.g. cardiac pacemakers, vascular clips in the brain, neurostimulators, cochlear implants, medication patches and delivery pumps); access to an updated list of device magnetic compatibility is necessary. Currently this information is provided by multiple different institutions (websites, articles), and there is a need to develop a resource under the umbrella of international or professional organisations. In addition, there is also a need to develop international guidelines for the safety of MRI facilities, although some exist in the literature and in the regulatory frameworks of some countries.
MRI protocols should be integrated within clinical sites performing MRI studies. Further, safety culture developed in the field of ionizing radiation should be expanded to the use of MRI, even if the health effects of ionizing radiation and MRI are fundamentally different.
Responsibilities at a national and institutional level for protecting patients and healthcare workers when using Magnetic Resonance Imaging
Currently, radiation regulatory bodies are not always considering MRI and, in general, the safety of MRI is mostly a concern of labour organisations in the general context of medical and non-medical magnetic fields. The establishment of a legal and regulatory framework for magnetic fields would be helpful, provided medical applications are looked at separately from non-medical usage. The involvement of medical professional bodies in this is considered essential. The potential benefits for low- and middle-income countries would be significant.
Safety processes are fundamental in the daily life of MRI facilities, and mostly involve screening of patients for implanted devices and avoiding missile effects of ferromagnetic objects in the MR scanner room, which can harm both patients and staff members. The use of QMSs should be increased and incentivised.
Specific attention should be paid to pregnant women. Although no harmful foetal effects for pregnant workers are known, some national authorities recommend avoiding any magnetic exposure during pregnancy. Staff at MRI facilities should be educated and incentivized to develop a safety-oriented culture, based on published guidelines, so that near-miss events are shared and used for process improvement.111 A self-commitment approach to maintain knowledge, skills and competences should also be stimulated and facilitated.
Section 6: The potential of advances in digital sciences and device engineering for improving cancer care in LMICs
Unprecedented advances in computing, data science, information technology (IT) and engineering are affecting all aspects of healthcare, including radiology and nuclear medicine.112,113 For example, in cancer imaging specifically, artificial intelligence (AI) and its subfields, machine learning (ML) and natural language processing (NLP), have been used to assist in clinical diagnosis and outcome prediction in various ways, including tumor detection and characterization and identifying cohorts of patients who require vigilant monitoring.114-118 Novel analytical techniques based on AI are also being implemented to tackle unmet needs in patient workflow and logistics. Furthermore, the growth of wireless technologies (cellular phones and other wireless devices that acquire and transmit data) is opening new horizons for innovation in healthcare delivery. Indeed, according to the World Health Organization, mobile health (m-Health), which may be defined as the application of mobile phones or other wireless devices for medical or public health purposes, could potentially transform health service delivery around the world.119 Advances in digital sciences promise to reduce the cost and improve the deployment of cancer imaging in high-income countries and LMICs.
While in high-income countries, digital technologies are gradually replacing existing established structures, LMICs with less developed digital infrastructures, are in a unique position to implement digital technologies from the start, and therefore possibly at a faster pace. For example, in some LMICs, cellular phone systems have already superseded communication using traditional landlines for health telecommunications119 and m-Health is already used for cancer screening.120 Mobile teleradiology, in particular, is a branch of m-Health that utilizes mobile phone technology to provide specialist care in image interpretation. This refers not only to radiology and nuclear medicine specialists providing services remotely, but also to communication with the patient via telemedicine visits – a strategy that has been used in high-income countries and has markedly expanded during the COVID-19 crisis. In LMICs, the dissemination of technology for telemedicine (including teleradiology) would not only help with the COVID-19 crisis and future pandemics, but would also help more generally to provide country-wide care, lessening the need for travel to medical centres. Hospital stakeholders in LMICs need to overcome a number of hurdles, as they must first assess IT infrastructure, internet access and electricity supply to establish appropriate regional goals that leverage technologies that are easily accessible, affordable and user-friendly, while at the same time guaranteeing patient privacy. According to a 2016 WHO survey, only 28% of lower-middle-income countries and 30% of low-income countries had legislation for protection of eHealth data, as opposed to > 80% of high-income countries.121 Nevertheless, progress is being made, at least in some eHealth areas: the implementation of e-learning, for example, has already enhanced access to medical information in numerous LMICs.122
What follows is a discussion of various digital technologies that hold particular promise for advancing cancer imaging in LMICs, now or in the future. It should be noted that the infrastructure required to implement many of these technologies includes electronic medical records (EMR) systems. While EMR are widely used in high-income countries, their distribution in LMICs is less pervasive. While > 50% of upper-middle- and high-income countries have adopted national electronic health record (EHR) systems that are based on EMRs, adoption rates in lower-middle and low-income countries are much lower: 35% and 15%, respectively.121 However, open-source EMR platforms have been used in dozens of countries in Africa, Asia and Latin America,123 and as the implementation of eHealth solutions in LMICs is a key factor in improving health outcomes, novel approaches for providing low-cost, easily accessible electronic health records are a major focus of governments, and international bodies (e.g., WHO) and industry.121,124,125
Imaging technology and image acquisition: Mobile and low-cost imaging equipment
The acquisition of high-quality digital image data is a prerequisite for accurate diagnosis with any of the imaging technologies used in the management of cancer patients. In many LMICs, hospital systems continue to function in the analogue world, with digital image data often only available in private practices. However, where hospital systems in LMICs are able to invest in high-quality digital image data, then connectivity between imaging sites can assist with technical queries and enhance the quality of acquired image data.119 It is important that the imaging systems be installed according to protocols that meet the standard of care in high-income countries and that local health care professionals like technologists, nurses, pharmacists etc. be adequately trained.
The availability of any imaging in LMICs is often restricted by cost, hence innovative technologies have been used to create next-generation scanners that are less expensive to purchase and operate and have mobile capabilities. The development of these technologies has required interaction between industry and academia, and has immediate relevance for LMIC implementation. The average hospital-grade ultrasound (US) unit can cost more than a hospital’s capital budget and often serves as the primary diagnostic imaging modality in many LMICs. The above-referenced, over 65-fold disparity factor between high- and low-income countries in terms of CT installations, as indicated by the IAEA IMAGINE data,49 is therefore unsurprising. A relevant factor in this context may be that the majority of high-income countries (> 90%) rely chiefly on public funding of eHealth programmes, whereas in the majority of low- and lower-middle-income countries (70%), donor funding is the dominant source of support.121 This difference in commitment by governments might affect middle- and long term strategic goals and investment decisions by stakeholders. This infrastructural deficit also significantly limits the use of available scanners for image-guided procedures, which is one reason why many LMICs continue to rely on blind or surgical biopsies for cancer diagnosis. New, innovative, low-cost solutions such as hand-held point-of-care (POC) m-Health US devices now offer a safe, simple and sustainable solution toward building capacity for cancer control in LMICs. New US transducer technologies mitigating the frequency limitations of piezoelectric crystals126 permit a single low-cost, portable transducer to be used for multiple clinical applications (Panel 3).127, 128
Panel 3: Use of ultrasound in low-income settings.
In the last two decades widespread adoption of smartphone technology has facilitated the ubiquitous availability of powerful computation and high-resolution displays. Ultrasound manufacturers have leveraged the availability of these technologies to create a new class of low-cost mHealth portable devices that connect directly to consumer electronic devices. New ultrasound transducer technologies mitigating the frequency limitations of piezoelectric crystals permit a single transducer to be used for multiple clinical applications.126 In combination, these technologies have vastly increased the availability of medical ultrasound while reducing its cost. Medical ultrasound is routinely available in LMICs, where it plays a central role in oncologic diagnosis and monitoring in the female pelvis, thyroid, liver, breast, peritoneal cavity, and kidneys and is commonly utilized for biopsy and tumor ablation guidance. For example, mHealth devices are facilitating a competency-based training program for Nigerian radiologists to perform and clinically implement US-guided breast biopsies, which are the standard of care in high-income countries and recommended by the Breast Health Global Initiative for many LMICs.127 This project was started in Nigeria because it is the most populous country in Africa, with the highest breast cancer mortality rate.128Further, the Nigerian government is committed to cancer control, with more than 350 available radiologists. This work was done with the African Research Group for Oncology (ARGO), a National Cancer Institute-recognized cancer consortium that aims to improve outcomes for cancer patients in Nigeria. In 2017, none of the ARGO radiologists could perform an US-guided breast biopsy.
The project’s first step was a multidisciplinary assessment of the needs of local stakeholders, which identified a need for and favourability toward an mHealth-based US-guided biopsy training program in Nigeria.127, 128 The local stakeholders included surgeons, radiologists and pathologists, as the change in practice was feasible only with multidisciplinary support. The training program approach was competency-based and included instructor-led and e-learning modules as well as simulation-based training. The mHealth device enabled independent learning and provided users access to newly developed AI applications that helped in the successful training and clinical implementation. The training program is self-propagating and the assessment metrics are currently being validated.
Advances in the design of x-ray sources, detectors, and reconstruction algorithms have made possible the potential for motion-free, completely solid-state CT scanners.129 Compared to standard scanners, these scanners promise to be less expensive as well as more easily transported, assembled, and serviced owing to the elimination of moving parts in the CT gantry, which will be ideal for LMIC use. Specialized MRI systems have been developed that use permanent magnets instead of super conducting or resistive electromagnets will enable low-cost, portable, and point-of-care MRI.130 Although the resulting field strength (less than 0.3T) is lower than that of standard 1.5T scanners, advances in hardware design and reconstruction algorithms have made the use of low-field MRI scanners possible, particularly for niche applications.131 Such scanners promise to be light-weight, low-cost, and portable, enabling more ready deployment in LMICs. Similarly, recent technology advances include PET systems with scalable ring configuration that reduce costs while maintaining diagnostic capabilities.102 LMICs looking to invest in these new technologies need to be informed about the type of regional support that is available, and partnerships between manufacturers, governments and private providers in LMIC will be required to ensure equipment can be maintained and operational for routine patient access and avoid scenarios where prolonged downtime may occur. New AI-based approaches will improve or in some cases eliminate the need for in-person equipment services, will monitor quality and safety, and will also allow more information to be extracted from imaging examinations, as digital imaging data can be analysed not just qualitatively but also quantitatively. AI-based approaches for optimizing imaging include the use of biosensors (e.g., for MRI and PET scanners) that automatically adjust for patient weight and anatomy, optimize coil positions, and analyse heartbeat and breathing rhythm to correct for body motion.132 Furthermore, AI-based image reconstruction algorithms are fast and can suppress noise and artefacts and produce higher-quality images, as demonstrated in CT,133 MRI134 and PET.135 Because quantitative imaging features are influenced by the vendor-specific settings and image acquisition protocols, AI-based approaches for standardization are currently being investigated.136 Using MRI as an example, Figure 10 presents a vision of a streamlined, AI-driven workflow, in which digital technologies enable automation, standardization, and optimization of every step, from patient registration through imaging acquisition and interpretation (Panel 4).
Figure 10. Artificial intelligence-driven workflow for imaging in patients with cancer.
An illustration of a streamlined, artificial intelligence-driven imaging workflow, in which digital technologies enable the automation, standardisation, and optimisation of every step, from patient registration to imaging acquisition and interpretation.
Panel 4: Implications of advances in digital sciences and low-cost imaging technologies for improving diagnostic imaging in LMICs.
Next-generation forms of imaging technologies (including US, CT, MRI and PET) are being developed that promise to be less expensive to purchase and operate and have mobile capabilities, thus helping to overcome workforce and other resource limitations in LMICs.
New, lower-cost mobile health (m-Health) devices can directly connect healthcare workers in remote and underserved areas to central expert sites via wireless telecommunication. This will allow remote experts to directly assist in patient care (e.g., by guiding nurses through the performance of basic radiologic procedures, or by providing review of and consultation regarding imaging results). In addition, it will allow experts to remotely provide ongoing education, training and mentorship.
Radio frequency identification (RFID) technology can automatically inform healthcare personnel about patient diseases (including COVID-19), risk factors and contraindications, as well as medications, improving overall safety and quality.
AI will allow automatic protocoling of imaging exams tailored to the individual patient’s clinical indication, risk factors and body type.
AI-based image analysis algorithms can directly process diagnostic images and aid in the triage of patients with significant findings in need of urgent care.
AI-based image processing can also reduce the radiologist’s workload by serving as a virtual “second reader” that assists in lesion detection and characterization and improves diagnostic quality and confidence.
Linking of images with information from a patient's electronic medical record can assist radiologists with timely and accurate reporting and also help ensure treating clinicians have access to all results when making management decisions.
Natural language processing may aggregate and logically combine qualitative information from different sources, such as imaging tests and pathology.
AI-based integration of qualitative and quantitative data from multiple disciplines, including radiology and pathology, could allow “integrated reporting” by radiologists and nuclear medicine physicians and, in the future, access to “integrated diagnostics” dashboards that would provide clinicians with precise prognostic and predictive information for patient management in a condensed format.
Patient registration and protocolling: Improvement of patient safety through radio frequency technology
Radio frequency identification (RFID) technology has been commercially available in one form or another since the 1970s, but it has only recently been introduced into healthcare. RFID is a wireless system of communication, whereby tags containing patient data transmit that data through radio waves that can be picked up or “read” by stationary or portable devices.137 A number of health care device manufacturers are incorporating RFID technology into their workflow solutions. Much as the contactless payment services that have become standard in the consumer economy allow efficient, convenient and safe financial transactions, contactless patient identification and registration by means of RFID is expected to improve workflow as well as patient safety and patient experience.138 Prerequisites for the use of RFIDs are a compatible Hospital Information System (HIS) and electronic medical record (EMR). A key advantage to using RFIDs and accessible EMRs is the improvement of patient safety through prevention of human error,139 including the failure to recognize a predisposition to contrast media reaction, the need for premedication, or the presence of an implantable medical device that precludes a patient from undergoing high-field MRI examinations. Another advantage is that, with the help of RFID technology, amendments to national or global safety guidelines can be automatically implemented after approval by a central healthcare authority, thereby enabling the application of country-wide, uniform safety standards. An additional important benefit of modern digital technology is the potential of AI to manage, predict and reduce patient exposure to ionizing radiation and thus further contribute to patient safety.140
Further advantages can be found in the use of RFIDs and EMR information to directly guide image acquisition in order to tailor imaging protocols to a particular type of cancer or clinical question, without the need for manual interaction by a radiologist or a nuclear medicine physician. This approach enables country-wide standardization of imaging protocols that adhere to the latest versions of published expert guidelines and ensures that state-of-the-art imaging can also be performed in areas and at institutions that lack relevant specialists. Finally, the use of RFIDs may reduce physical interaction between patients and healthcare personnel, depending on the imaging test being performed – a benefit that is particularly valuable during the current COVID-19 crisis, with its obligatory social distancing rules. Notably, implementation of this type of technology is facilitated by a supporting legal framework, which is frequently lacking in the majority of LMIC. As the WHO survey confirms, policies or legislation to address patient safety and quality of care are only in place in 10-20% of low-income and lower middle-income countries, compared to almost 80% of high-income countries.121
Image analysis and interpretation: AI and Machine Learning to bring tertiary care image interpretation to LMIC community hospitals
State-of-the-art diagnostic image analysis and interpretation require digital imaging as well as lossless compression and transfer using picture archiving and communication system (PACS) technology. In addition, advanced workstations and screens are needed for viewing radiology and nuclear medicine images, which most facilities in LMICs do not have141 (very often, a laptop serves as the diagnostic workstation and the radiology report is hand-written and placed in the patient’s paper chart). In addition, the availability of an EMR system is highly desirable for effective management of imaging data, but again, most LMICs do not have this either. Access to an open-source PACS that is integrated with an open EMR would provide critical information for clinical decision-making and possibly help to reduce costs. Advanced AI-based image analysis and interpretation are currently among the most extensively investigated topics in radiology and nuclear medicine as well as computer science, with the main goals being automation, improved accuracy, and decision support.142-147
Computer-aided detection (CAD) systems have been applied in different cancers and organs/tissues, most extensively for lung nodules and breast cancer.117,118,144,147-150 While commercial solutions have been available for several years, widespread clinical implementation is still pending. This is likely to change as positive and negative predictive values improve with the level of model complexity and generalizability, as offered by novel AI-driven approaches that utilize mathematical patterns extracted from imaging data—the so-called radiomic features. Much as the application of deep learning algorithms to cranial CT has been shown to allow expert-level identification of findings that require urgent attention (e.g., haemorrhage and fractures),151,152 ML algorithms could be used for the triage of cancer patients. For instance, ML algorithms could be applied in lung cancer and breast cancer screening programs in high-risk populations, or in the follow-up of cancer patients undergoing surveillance after complete remission. In LMICs, such an approach could help address the gaps in expertise and availability in rural, hard-to-access areas where very few trained radiologists are available to provide care,123,153 as well as in areas where radiologists are overwhelmed by the volumes of images they are required to interpret.141 The same applies to ultrasound, which, for instance, is utilized extensively in LMICs to stage cervical cancer.154 The high operator dependence of ultrasound makes the lack of sufficiently trained experts even more critical, so that deep learning algorithms, such as have been used to interpret thyroid, breast, and abdominal ultrasound,147,155,156 are expected to have considerable impact. For example, AI could be used as a “second reader” to confirm accuracy or serve as a reference standard. This application of AI could have immediate applicability in LMICs where there are few radiologists and ultrasounds are frequently performed by technicians and nurses.141
Decision support represents another application of computer-assisted image analysis, which is, however, still experimental and therefore not yet in clinical use.157 Based on radiomic data, diagnostic confidence could be improved for the interpretation of equivocal lesions that are difficult to characterize by human visual perception. For instance, studies have suggested that radiomics can help differentiate CNS lymphoma and atypical glioblastoma multiforme on PET158 and MRI,159 or different types of gastric malignancies on CT.160 Notably, radiomic features can be extracted not only after selection of a lesion by the radiologist, but also fully automatically by AI algorithms such as the convolutional neural network (CNN) U-Net, which segments lesions without the need for human interaction.161 This, however, requires powerful computing infrastructure, especially graphics processing units (GPU). In view of the reported association between molecular tumor phenotypes and radiomic features, the latter could possibly have a role as surrogate markers in LMICs where genomic and molecular biomarkers are not readily available and accessible.162-164 This is also an area of ongoing research, and further validation will be required before it becomes part of the standard of care.
Integrated reporting and the promise of integrated diagnostics
An important goal in cancer imaging is the efficient production of integrated imaging reports, in which all pertinent imaging and other patient data is accounted for and combined. This process can be enhanced by AI. For example, the use of NLP for qualitative content extraction from routine clinical reports could provide radiologists and nuclear medicine physicians with relevant clinical information that can be readily utilized during image interpretation.115,143 Automated extraction of quantitative metrics (e.g., PET standardized uptake values) and derivation of changes over time could also enhance and speed up image interpretation. Radiologists and nuclear medicine physicians may then integrate all of this information into final reports to better assist referring clinicians with patient management decisions.165
There is currently an unmet need to condense the wealth of medical diagnostic data produced during routine patient workup into a form that retains and emphasizes all clinically relevant information. Efforts to develop this novel, holistic approach, termed “integrated diagnostics,” strive to provide a digital framework for combining imaging, pathology, laboratory, genomic, and other diagnostic and clinical data to give clinicians easy access to aggregated information. A prerequisite for integrated diagnostics is the collection and aggregation of digitally structured “big” data113—for example, through electronic health records. In practice, the first step in applying integrated diagnostics to an individual patient would be extraction of all the relevant types of clinical and diagnostic data from that patient in digitized form. The second step would be visualization and integrated display of the data on a single dashboard. Finally, the last step would be the use of computational data analytics to integrate the patient’s data in light of insights drawn from big data and offer precise predictive and prognostic information on which to base clinical decisions and patient counselling. Currently, one of the substantial hurdles to the implementation of this vision of integrated diagnostics, even at elite institutions in high-income countries, is the need to be able to mine clinical notes digitally—a process for which NLP will be a key tool. However, with NLP technology quickly evolving, and with the growing need to streamline information resulting from the rapid increase in the complexity and volume of patient data, integrated diagnostics is our best hope for ensuring consistently personalized, evidence-based cancer management and optimized patient outcomes.
Section 7: Research and Training
Research (basic and clinical) is essential to the formation of practices and policies in cancer care; in fact, it has been shown that integrating research and teaching in clinical practice ultimately leads to improved care and better patient outcomes.166 Hence, research must also be considered essential to elevating practice standards and driving training and education in any institution. Although available resources, socioeconomic issues and health systems in high-income countries differ vastly in LMICs, the integration of research into clinical practice is no less important. The creation and support of LMIC-based research groups is a precondition for setting research priorities that address local contexts, developing evidence-based practices uniquely suited to LMICs, and adapting evidence developed in high-income countries to an LMIC context. It must be remembered that research requires data, and the acquisition of prospective, complete and accurate data is a challenge in many settings. Provision of cancer care, including the imaging services that go along with it, in LMICs must be continually assessed to determine patient outcomes and gaps in care. Many of these gaps could relate to imaging, either lack of availability, or suboptimal quality, but continual prospective data collection can help design interventions to overcome these challenges. This can be viewed as part of the spectrum of implementation research, and is critically important in these settings.
Evidence-based research
Clinical trials are crucial to the evolution and development of cancer treatment. Clinical trials are increasingly being performed for novel radionuclide therapy, interventional radiology and diagnostic imaging studies, and these imaging approaches also serve to evaluate treatment response and disease progression as study end-points for treatment efficacy and decision making.167-170 For cancer trials of solid tumours (Phase III especially), conventional CT size measurements by RECIST criteria are used in the vast majority of evaluations, although modern technologies, including hybrid-PET (e.g. PERCIST, Deauville criteria) may be included for some trials.171 Clinical trials can be extended to LMICs to evaluate LMIC-specific pathologies or to perform multi-centre, multinational trials. An innovative approach could also be to pool data from multiple separate trials, including sites in LMICs, as has been proposed for data obtained from trials in COVID-19 patients.172 High-income countries are working on major training programs, e.g. in nuclear medicine, to establish co-operative trial networks and site validation processes.173 Such programs, extending from developed countries to LMICs, advance the goal of population-based evidence for new indications and data registries, which is essential for health technology assessments (HTA).
The introduction of new health care technology, including imaging, should be evidence-based, and systematic evaluation of its impact and cost-effectiveness should inform technology-related policy-making in health care.173 HTA can be initiated in developed countries, but LMICs should be included to account for scenarios where population differences may impact results. It could be argued that evidence-based assessments of new imaging (and radionuclide therapy) indications arising from developed countries should be made available for regulatory approval and funding in LMICs to avoid duplicating trials or HTAs in multiple countries. In addition, policies that have found success in high-income countries should be evaluated in the context of LMICs and subject to relevant science and research. It is possible that different approaches for the integration of imaging into cancer care will be needed, particularly in the context of very low-resource settings.
Global health research
LMICs carry the highest burden of cancer globally.39 However, most of the world’s research funding originates in and is distributed to high-income countries, both for adult and childhood cancers.5,174,175 This influences the development of new imaging technologies, radiopharmaceutical innovation, and analytic approaches (e.g. AI), which require critical infrastructure and expertise to generate and implement novel approaches to imaging. Global health research fosters collaboration between high-income countries and LMICs and provides opportunities to address global health disparities, accelerating the development of therapeutics and building research capacity in LMICs. The overarching goal is to foster independence and promote professional development in LMICs in order to sustainably develop resources and capacity, expand access to cancer imaging, and provide affordable and high-quality cancer care. In addition, global research initiatives provide an opportunity to not only assess resource-sparing approaches, but also to implement new techniques in LMICs in a real-world research setting that is controlled to allow for in-depth and unbiased assessment. Multiple grant funding bodies have dedicated funds to global health research, for example The National Institutes of Health (NIH) offer international research training grants that support research training programs that develop and strengthen the scientific leadership and expertise needed for research in LMICs. Global research from patterns of care studies to randomized phase III studies are funded and conducted through the IAEA Coordinated research programme.176 The programme facilitates research collaboration between high-income countries (HICs) and LMICs in medical disciplines that use radiation (such as nuclear medicine, radiology, radiotherapy and medical physics) and supports the development of quality-assured clinical research in LMICs. In addition, the program allows cross-specialty research collaborations (Panel 5; Figure 11).177 Other grant funding bodies include the Medical Research Council (UK), The Bill and Melinda Gates Foundation, and the Welcome Trust (UK).
Panel 5: Research and Training Support for LMICs.
To improve outcomes for cancer patients, LMICs must support the development of workforces suited to contemporary practice in imaging and nuclear medicine. Numerous meaningful initiatives by governments and professional organizations around the world have been implemented, with the most comprehensive global coordination of such programs having been undertaken by the International Atomic Energy Agency (IAEA) since 1987. A primary mission of the IAEA is to promote and support research on the practical applications of atomic energy and related techniques for peaceful purposes across the world, including in healthcare, with a special focus upon LMIC member states. The challenges of conducting such work in LMICs include insufficient resources (human and infrastructural), lack of training in conducting clinical research, and underestimation of participants’ own capabilities to support projects. Through the IAEA Coordinated Research Activities (CRA) platform, pertinent activities and plans to strengthen health systems are initiated, supported, and coordinated between LMICs and HICs. Through well-designed multicenter international research protocols, participants are supported in their work to develop and contribute to local research and autonomously implement quality improvements.
To date, about 100 Coordinated Research Projects (CRPs) in the field of nuclear medicine and diagnostic imaging have been initiated, with more than 1000 research institutions participating. These collaborative strategies aim to engage LMICs in well-designed international multicenter clinical trials in order to address scientific questions most relevant and in some cases specific to LMICs and to improve daily clinical practice. In nuclear medicine and diagnostic imaging, projects range from workforce training for advanced imaging modalities to scaling up the local applications of advanced imaging modalities such as PET to addressing specific types of cancer prevalent in LMICs. The worldwide distribution of participants currently active in IAEA CRPs devoted to addressing health conditions is illustrated in Figure 11.
CRPs also support research into optimal post-graduate student supervision in LMICs. For example, a ‘Doctoral CRP in Advances in Medical Imaging Techniques’ linked PhD students in Medical Physics from LMICs with faculty supervisors from degree-conferring institutions in high-income countries. Students were selected from LMICs across the globe: Bangladesh, Bulgaria, Mexico, Montenegro, Morocco, Thailand, and others. The related core research projects assessed the effectiveness, applications, quality, optimization, and safe use of advanced imaging techniques. The students learned how to perform advanced clinical research and implement practice and quality improvement strategies. The research measurably enhanced local and national training programs and improved the clinical practice of advanced imaging in radiology and nuclear medicine in the researchers’ home countries.
Another CRP aimed to improve clinical applications of PET/CT in LMICs. It included an international study on the use of ‘PET/CT for Stage III non-small cell lung cancer (NSCLC) radiotherapy planning (IAEA-PERTAIN study) that involved more than 350 patients in LMICs including Brazil, Estonia, India, Jordan, Pakistan, Turkey, Uruguay, and Vietnam. Following rigorous and comprehensive training through hands-on courses, webinars and participant feedback, knowledge and skills were successfully transferred for the delineation of radiotherapy target volumes, and a study on the impact of PET/CT in radiotherapy planning upon 2-year survival rates was performed. Additional outcomes included the development of guidelines for PET/CT in image acquisition and target volume delineation, the adoption of new protocols, and changes in clinical practice. Instrumental to the CRP’s success was the accreditation of the FDG-PET/CT studies by means of quality control and quality assurance measures by the European Association of Nuclear Medicine (EANM) Research Ltd. (EARL). This was provided through EANM collaboration with the facilities of target countries. Local trainers were trained, and their experience and expertise were subsequently disseminated through seminars and conferences. The initiative also resulted in the publication of several high-impact peer-reviewed articles. This CRP also fostered multidisciplinary training and skill development on contouring using PET/CT for radiation oncologists and medical imaging specialists alike. Successful CRP examples like this are amenable to being applied in other LMICs and tailored to their local contexts. Future programs will address areas of unmet need including updates on the use of diagnostic imaging in LMICs, application of digital connectivity and AI, and theranostic techniques.
Figure 11. Active International Atomic Energy Agency coordinated research projects in human health.
CRPs = coordinated research projects. The map was produced by the International Atomic Energy Agency (Vienna, Austria) and is included here with permission. The color gray indicates the presence of no coordinated research projects.
Research, education and training
The establishment of a research culture in imaging departments is essential, and requires institutional commitment, dedicated leadership, and exemplary role models; this is highly relevant in both high-income countries and LMICs. Research should be integrated into training programs. Research structures within LMICs should include a well-organised policy framework that facilitates research, and the provision of appropriate infrastructure for research. The provision of protected research time, while challenging in a busy clinical practice environment, should be prioritized in LMICs, where time constraints represent a significant barrier to research activities. A special priority should be placed on implementation research, which is essential to translate research from high-income countries to clinical practice in LMICs. Currently, there is weak or no research infrastructure in many LMICs. There is frequently little or no in-country expertise in clinical and implementation science research, and while growing funding sources are encouraging, personnel must be hired and dedicated to cancer research to begin the process. Reference should be made to the recommendations and guidelines of the GCRP (Good Clinical Research Practice, WHO).175 Continuing reviews and quality assurance and audit programmes should be integrated into the routine activity of imaging departments. These can form an important research activity that is often underemphasized and may include assessing the accuracy and consistency of reports, quality and safety studies, workflow and unique practices in order to improve the quality of imaging services and cancer care in general.
Education and training activities in LMIC can extend from country based programs, to overseas attachments, distance learning, online didactic lectures, and workshops. With the support of digital technologies (Section 6), transmission of images for training in image interpretation can also be facilitated in LMIC, and this may be combined as blended learning with practical training in local facilities. For example, it has been found that tele-ultrasound training by real-time image interpretation and guidance from experts from afar is feasible and of value in training and patient management in the LMIC setting.178 A number of international professional imaging societies have organised outreach programs to LMICs for this purpose, including the Society of Nuclear Medicine and Molecular imaging (SNMMI), European Association of Nuclear Medicine (EANM), Radiological Society of North America (RSNA), European Society of Radiology (ESR), World Federation of Paediatric Imaging (WFPI), etc. Online education is available on the websites of these and other societies. Furthermore, international organizations, including the World Health Organization (WHO) and International Atomic Energy Agency (IAEA), regularly reach out to LMICs to provide training and education in radiation safety and skillsets required for establishing imaging facilities. These activities are essential to ensuring radiologists and nuclear medicine physicians and other imaging professionals gain practical education and training, and enhance the quality of imaging studies performed in LMIC.
Section 8: Scaling up capacity for sustainable access to cancer imaging diagnostics – Call to action
The Commission has identified several important challenges hindering access to effective services for cancer imaging diagnostics, including investment in imaging equipment, workforce capacity, digital technology including electronic clinical data, access to radiopharmaceuticals, and research and training. The Commission has also presented new compelling evidence on the very substantial health and economic benefits of scaling up cancer imaging diagnostics in LMICs, where they are most needed and where wide inequities exist in access to effective cancer services and in cancer outcomes. These benefits will be greatest with comprehensive approach to scale-up, where the scale-up of diagnostic capacity is aligned with treatment capacity and where there is simultaneous improvement in quality of care.
In this section, we explore critical success factors for scaling up, the roles key stakeholders could play in the scale-up process, and targets that will help translate ambition into actions and realise the vision of effective, equitable scale-up of cancer imaging diagnostics in LMICs.
Critical success factors for scaling up cancer imaging diagnostics
The challenges and opportunities in the global fight against cancer and critical success factors for an effective response with comprehensive scale-up have been outlined in earlier studies.6,38,179
The first critical success factor is strong and visible global and country-level leadership. International development agencies, global leaders and governments with commensurate funding should firmly commit to scaling up imaging diagnostics capabilities. In addition, inclusion of medical imaging and nuclear medicine metrics in global health statistics and country progress monitoring is essential.
The second critical success factor relates to the development of a compelling case for investing in the scale-up of cancer imaging diagnostics. The Commission results show substantial health and economic benefits for such investments. Now that clear evidence of an investment case exists, a straightforward narrative should communicate the benefits of investment for individuals, households, and countries, and the potential opportunities provided by imaging diagnostics for cancer patients worldwide.
The third critical success factor relates to alignment. Activities aimed at scale-up of services for cancer imaging diagnostics align with global efforts to achieve Sustainable Development Goals (SDGs). In particular, the health-related goal SDG 3, “Ensure healthy lives and promote well-being at all ages,” has set achievement of Universal Health Coverage (UHC) by 2030 as the target.180 Global efforts to scale up cancer imaging diagnostics should be fully aligned and integrated with actions aimed at achieving UHC. Alignment of expansion of imaging diagnostics with UHC will require a comprehensive approach to scale-up, where the scale-up of diagnostic capacity is aligned with scale-up in treatment capacity. This alignment will optimize use of available resources in countries, help strengthen health systems, ensure a more strategic approach to the provision of diagnostic services for cancer, and help with the sustainability of scale-up.
The fourth critical success factor is the creation of inclusive coalitions of partnerships and networks to drive scale-up of cancer imaging diagnostics (Panel 6).41,100,181,182,183 Such coalitions should involve, among others, civil society, individuals affected by cancer, professional associations, health professionals, researchers, funders, international agencies, the private sector, and innovators.
Panel 6: An inclusive global coalition to scale up diagnostic cancer imaging capabilities in LMICs.
An inclusive coalition of partnerships and networks is critical for the development of an effective global- and country-level response to scale-up of cancer imaging diagnostics. All actors – such as governments, civil society, affected individuals, health professionals, professional associations, researchers, funders, international agencies, the private sector and innovators – bring capabilities that can be harnessed to create synergies in the scale-up process.
Governments
Governments can use the evidence generated by the Commission to convene relevant stakeholders and coordinate investments in diagnostic imaging services for cancer patients as part of efforts aimed at expansion of UHC. Governments play a critical role in providing leadership and making political and fiscal decisions to invest in health systems that generate health and economic returns for their citizens and economies.
International Agencies
International agencies such as WHO can play a vital role in the incorporation of cost-effective imaging diagnostics in essential diagnostics lists to support their inclusion as part of benefits packages for UHC. The WHO ‘Best Buys” for NCDs,181 and the WHO priority medical devices list41 include diagnostic imaging, and imaging is also included in the publication, “WHO report on cancer: setting priorities, investing wisely and providing care for all.”182 WHO provides leadership in the establishment of guidelines and policies on human health, including cancer, and in the implementation of programs aimed at improving access to essential diagnostics and treatment, aimed at reducing the burden of disease globally and particularly in LMICs.
Global and regional development banks have a critical role in working with government and the private sector to develop innovative financing solutions (see Section 4) to enable the expansion of cancer imaging diagnostics in LMICs.
The IAEA (https://www.iaea.org), an independent, intergovernmental technology-based organization within the United Nations family, is an important stakeholder in the scale-up of cancer imaging diagnostics in LMICs. As the focal point for nuclear cooperation worldwide, IAEA works to promote safe, secure and peaceful use of nuclear technologies, including diagnostic imaging and nuclear medicine. A wide range of support encompasses provision of equipment, education and training, quality and safety of clinical practice through guidance documents, equipment calibration, and support of clinical and health economics research. Working with the WHO and its International Agency for Research on Cancer (IARC https://www.iarc.fr), the IAEA has undertaken fact-finding missions and imPACT reviews183 in more than 100 countries to assess their cancer control, from national registries to palliation, including diagnostic imaging. In addition, IAEA quality assurance methodologies such as QUANUM (for nuclear medicine) and QUAADRIL (for radiology) have been instrumental in supporting quality programs in many countries, including LMICs.100
Civil Society
Civil society involvement is critical for bringing a voice to those affected by cancer, building awareness at the global and country levels, and mobilising support for concerted action. Civil society has an important role to play in articulating health rights and influencing global actors and country-level policies to help include cancer imaging diagnostics as an integral part of UHC expansion. The Union for International Cancer Control (UICC) (https://www.uicc.org), which has brought together more than 1,000 non-governmental organizations involved in cancer, is well-positioned to strengthen civil society and help mobilise global leaders through the World Cancer Summit and the World Cancer Declaration.
Professional Associations
Professional associations play an important role in establishing professional standards, developing capacity, expanding access to high-quality healthcare services for cancer patients, and for imaging technologies (e.g. American College of Radiology (ACR), American Society of Clinical Oncology (ASCO), American Society for Radiation Oncology (ASTRO), European Society for Medical Oncology (ESMO), Radiological Society of North America (RSNA), European Society of Radiology (ESR), International Society of Radiology (ISR), International Society for Strategic Studies in Radiology (IS3R), European Society for Radiotherapy and Oncology (ESTRO), Society of Nuclear Medicine & Molecular Imaging (SNMMI), European Association of Nuclear Medicine (EANM), Asia Oceania Federation of Nuclear Medicine and Biology (AOFNMB), World Federation of Nuclear Medicine and Biology (WFNMB)). These groups could effectively contribute to and accelerate the scale-up of capacity for imaging diagnostics and access to effective services in LMICs by working with international and country-level partners to expand human resource capacity through education and training, by providing clinical guidelines adapted to the LMIC setting for optimal use of imaging resources and by establishing or strengthening regional collaborations in R&D and innovation.
Philanthropic Organizations
In LMICs, philanthropic organisations have played an important role in mobilising donations and public funding to establish Academic Cancer Centres that provide high-quality services to select populations. Many of these centres have twinning arrangements with cancer centres in high-income countries and provide an opportunity to integrate operations with publicly funded elements of health systems to establish integrated cancer networks. Such integration will help to create synergies to more optimally expand access to care for cancer patients.
The private sector
In LMICs, the private for-profit sector has created considerable capacity for cancer imaging diagnostics, but generally for those who can afford to pay for the services. The private sector can use this experience to work with governments, international agencies and philanthropic organizations to develop innovative financing and service delivery models to scale-up imaging diagnostics and expand access to effective services.
However, the private for-profit healthcare provider sector is not well-regulated in many LMICs, and there is limited data on the quality of services provided or the outcomes achieved. The private sector is also a major funder of R&D and innovation for diagnostics, medicines and health technologies for the management of cancer, but much of this effort is likewise targeted for high-income countries. Novel collaborations of public-private institutions, universities, philanthropic organizations and international development agencies could help harness private sector capability to develop affordable imaging diagnostics solutions for cancer in LMICs.
Wide-ranging initiatives have emerged over the years to expand capacity for cancer care in LMICs by improving clinical know-how, increasing the amount and quality of cancer care, and establishing research activities. These initiatives have been underpinned by collaborations involving multiple stakeholders from LMICs and high-income countries, typically through academic institutions that have established twinning arrangements. However, though beneficial to those involved in the collaborations and patients accessing the institutions involved in these collaborations, many of these initiatives have remained projects of limited scope; as such, they have not always produced noticeable differences in access to cancer services for large numbers of citizens in LMICs, or made cancer outcomes more equitable at a population-level.
The implementation of multidisciplinary teams including oncologists, surgeons, radiologists, nuclear medicine physicians, and pathologists is critical to ensure quality cancer patient care. The establishment of collaborative networks in LMICs that bring together experts in cancer imaging diagnostics with oncologists and other health professionals to ensure quality standards and appropriate use of medical imaging and nuclear medicine in clinical care is a key driver of improved outcomes of cancer patients.
Currently there is no clear, overarching global strategy for scaling up cancer imaging diagnostics in many LMICs, and efforts are often fragmented as a result. A multi-stakeholder coalition should develop a global strategy for scaling up imaging diagnostics to ensure alignment with and coordination of the many short-term initiatives and ‘pilot projects,’ which do not sustainably address the shortcomings in access to effective cancer imaging diagnostics.
The fifth critical success factor is investment in research, development, and innovation to develop novel technological solutions and service delivery models that can rapidly address shortages in human resources, infrastructure, affordable diagnostics, care models and financing. For example, this could involve expanding the use of new lower-cost scanner technologies, through the wider application of digital connectivity solutions that can enable radiologists in-country or internationally to interpret scans remotely, and through the use of virtual digital learning platforms to train and support health professionals. Investment in research, development and innovation will also enable better application of evidence-based solutions, best practices and transfer of know-how. The application of these innovative approaches can provide opportunities for rapid and more affordable scale-up of capacity for imaging diagnostics and digital health solutions in LMICs.
The sixth critical success factor is the mobilisation and better use of existing resources by optimising the use of existing health workforce, equipment and infrastructure assets in countries through networks or collaboratives for cancer imaging diagnostics. These networks or collaboratives could be operationally aligned with cancer networks and include public, private and philanthropic institutions. Development of such networks or collaboratives would require careful national and sub-national level planning to ensure appropriate investment to address capacity gaps. Planning could be augmented with the strategic purchasing of imaging diagnostic services by national authorities to achieve economies of scale and equitable allocation of available funds.
The findings of this Commission show the substantial health and economic benefits of successful scale-up of capacity for cancer imaging diagnostics in LMICs and high-income countries. These benefits will be greatest with a comprehensive approach to scale-up, where the scale-up of diagnostic capacity is aligned with treatment capacity. The pathway to scale-up and the speed of expansion of imaging diagnostics for cancer in each country will necessarily vary, given that the political will, infrastructure, the availability of radiotherapy, surgery, medical treatment, imaging modalities, human resources and financing will vary in each country. However, there are a set of actions each country could take to enable scale-up.
We propose six major actions, with targets, to achieve the important goal of equitable access to imaging diagnostics worldwide.
Action 1: Incorporate imaging diagnostics in essential benefits packages when expanding universal health coverage in LMICs
Cancer imaging diagnostics should be incorporated into national essential benefits packages for diagnostics when expanding UHC, with explicit targets for scale-up of capacity in health systems to expand the coverage of effective services.
Target:
By 2030, as part of the efforts to expand UHC, at least 80% of LMICs should incorporate appropriate cancer imaging diagnostics in their essential benefits packages to expand access to effective services.
Action 2: Incorporate in national cancer control plans costed actions to scale up cancer imaging diagnostics
Predictable financing is critical for the scale-up of cancer imaging diagnostics and to sustain these services. LMIC should develop fully costed national cancer plans that articulate how sustainable cancer care could be progressively developed and funded.
Target:
By 2030, 60% of LMICs should have national cancer control plans that articulate specific actions for the scale-up of cancer imaging diagnostics, with the necessary fiscal space for funding this expansion.
Action 3: Expand access to effective services for imaging diagnostics by scaling up the current capacity of human resources and imaging equipment
The ability of LMICs to improve health outcomes for cancer patients depends on their ability to expand the availability of imaging equipment and a suitable trained workforce to a level that provides appropriate access for cancer patients. Levels of imaging equipment and human resources per million population vary substantially in countries of similar and different income groups. Average and median levels of imaging equipment and human resources per million population range from three-fold to ten-fold between low-income and lower-middle-income countries, between lower-middle-income countries and upper-middle-income countries, and between upper-middle-income countries and high-income countries (see Sections 2 and 3).
Target:
By 2040, at least 50% of low-income, lower-middle-income, and upper-middle-income countries should expand capacity of human resources and availability of imaging equipment to reach or exceed median levels per million population currently achieved by countries of the next income group adjusted for cancer incidence.
Action 4: Ensure provision of optimal access to effective imaging diagnostics by establishing collaboratives for cancer imaging diagnostics
Countries should work with stakeholder coalitions to create national and regional collaboratives focused on cancer imaging diagnostics, or expand them where they exist, in order to better use available capacity for providing packages of effective cancer services. These collaboratives could be enabled through virtual digital linkages.
Target:
By 2030, establish in 50% of LMICs collaborative networks of imaging diagnostics to expand coverage of effective imaging diagnostics services for cancer.
Action 5: Invest in education and training to expand human resources
The establishment of a trained workforce of radiologists, nuclear medicine physicians, radiographers and technologists, nurses, physicists and radiochemists is critical for ensuring that safe and effective imaging and nuclear medicine services can be provided and that quality systems make available accurate and reliable information for cancer care. Digital solutions and virtual platforms that facilitate the development of workforce planning and training could enable rapid scale-up of training in LMICs.
Target:
By 2030, 80% of LMICs should establish plans for workforce development and for the use of digital platforms for workforce training.
Action 6: Invest in training, research, development and innovation to develop affordable cancer imaging diagnostics in LMICs
Research funding related to cancer imaging diagnostics in LMICs is small, fragmented and largely inaccessible to researchers outside high-income countries. The lack of affordable solutions for imaging diagnostics hinders the achievement of improved health outcomes for cancer patients. Investments are needed in research and innovation in LMICs to ensure better use of available interventions and create affordable and accessible imaging solutions and new care delivery models for cancer patients appropriate for LMICs.
Target:
By 2025, the establishment of a $100 million ‘Innovation Fund for Cancer Imaging Diagnostics' to improve coordination of funding for education, training, research and development, and innovation in LMICs, with a target of mobilising and investing thereafter at least $25 million per year.
Conclusions
There is compelling evidence of the substantial health benefits of scaling up medical imaging and nuclear medicine access for cancer patients. Improvements in science have enabled rapid developments in affordable imaging technologies and solutions and flexible low-cost digital platforms for virtual training. Science and technology are not the barriers to worldwide equitable scale-up of effective cancer imaging diagnostics; rather, achieving equitable scale-up is a matter of vision and will. Success will be realised through political leadership, active participation from all major stakeholders, and alignment of country-level and global efforts to expand access to medical imaging and nuclear medicine, leading to better outcomes for cancer patients.
Supplementary Material
Figure 3. Estimates of the number of PET scanners per million inhabitants.
Data are from the International Atomic Energy Agency medical imaging and nuclear medicine global resources database (IMAGINE). The map was produced by the International Atomic Energy Agency (Vienna, Austria) and is included here with permission.
Figure 4. Estimates of the number of mammography units per million inhabitants.
Data are from the International Atomic Energy Agency medical imaging and nuclear medicine global resources database (IMAGINE). The map was produced by the International Atomic Energy Agency (Vienna, Austria) and is included here with permission.
Figure 5. Estimates of the number of MRI units per million inhabitants.
Data are from the International Atomic Energy Agency medical imaging and nuclear medicine global resources database (IMAGINE). The map was produced by the International Atomic Energy Agency (Vienna, Austria) and is included here with permission.
Acknowledgments:
Garon Scott and Ada Muellner, MS, both medical editors in the Department of Radiology, Memorial Sloan Kettering Cancer Center, edited portions of the manuscript. HH and JSL were supported by NIH NCI Cancer Center Support Grant P30 CA008748. JSL was supported by NIH NCI grant R35 CA232130. AMS was supported by NHMRC grant 1177837. Harvard TH Chan School of Public Health also provided funding support for this study.
Appendix
The work in this report was enhanced by the generous financial or in-kind support we received from the following organizations:
AANM African Association of Nuclear Medicine
ACR American College of Radiology
ALASBIMN Association of Latin American Societies of Biology and Nuclear Medicine
ANZSNM Australian and New Zealand Society of Nuclear Medicine
AOFNMB Asia Oceania Federation of Nuclear Medicine and Biology
AORTIC African Organization for Research & Training in Cancer
ASCO American Society of Clinical Oncology
ARSNM Arab Society of Nuclear Medicine
ASR African Society of Radiology
ASTRO American Society for Radiation Oncology
BCRF Breast Cancer Research Foundation
EANM European Association of Nuclear Medicine
ESMO European Society for Medical Oncology
ESR European Society of Radiology
ESTRO European Society for Radiotherapy and Oncology
HKCR Hong Kong College of Radiologists
IAEA International Atomic Energy Agency
IS3R International Society for Strategic Studies in Radiology
ISR International Society of Radiology
NCI National Cancer Institute
PAARS Pan-Arab Association of Radiological Societies
RSNA Radiological Society of North America
SASNM South African Society of Nuclear Medicine
SNMMI Society of Nuclear Medicine & Molecular Imaging
UICC Union for International Cancer Control
WFNMB World Federation of Nuclear Medicine and Biology
WMIS World Molecular Imaging Society
Commissioners at large
(Participated in meetings and contributed to the planning and outline of the paper, but do not meet all authorship criteria.)
Asante, Kwanele, African Organization for Research & Training in Cancer, Johannesburg, South Africa
Douillard, Jean-Yves, European Society for Medical Oncology, Nantes, France
El Diasty, Tarek, Urology and Nephrology Center, Mansoura University, Mansoura, Egypt
Gospodarowicz, Mary, Princess Margaret Cancer Centre, University of Toronto, Toronto, Canada
Harari, Paul, American Society for Radiation Oncology, Madison, Wisconsin, USA
Hatazawa, Jun, Asia Oceania Federation of Nuclear Medicine and Biology, Osaka, Japan
Husseiny Salama, Dina, Atomic Energy Authority, Cairo, Egypt
Mendonca, Renato Adam, DASA, Sao Paulo, Brazil
Minoshima, Satoshi, Department of Radiology and Imaging Sciences, University of Utah, Salt Lake City, Utah, USA, the Society of Nuclear Medicine and Molecular Imaging, Reston, Virginia, USA
Nass, Sharyl, Health and Medicine Division, National Academies of Sciences, Engineering, and Medicine, Washington D.C., USA
Parizel, Paul, Royal Perth Hospital & University of Western Australia, Perth, Australia
Rao, Vjay, Thomas Jefferson University, Philadelphia, USA
Shannoun, Ferid, United Nations Scientific Committee on the Effects of Atomic Radiation, Vienna, Austria
Slotman, Ben J., Amsterdam University medical centers, Amsterdam, the Netherlands
Trimble, Edward, US National Cancer Institute, Bethesda, Maryland, USA
We acknowledge contributions of the following individuals:
Boland, Giles, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA
Bucheli, Juan C, International Atomic Energy Agency, Vienna, Austria
Cerci, Juliano, Quanta – Diagnóstico e Terapia, Curitiba, Brazil
del Rosario Perez, Maria, Word Health Oganization
Giammarile, Francesco, International Atomic Energy Agency, Vienna, Austria
Herold, Christian J., Department of Medical Imaging and Image-Guided Therapy, Medical University of Vienna, Vienna General Hospital, Vienna, Austria
Ilbawi, André, Word Health Oganization, Geneva, Switzerland
Juluru, Krishna, Memorial Sloan Kettering Cancer Center, New York, USA
Kingham, Peter T., Memorial Sloan Kettering Cancer Center, New York, USA
Krestin, Gabriel P., Department of Radiology & Nuclear Medicine, Erasmus MC University Medical Center Rotterdam, Rotterdam, The Netherlands
Lacson, Ronilda, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA
Mayerhöfer, Marius, Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, USA
Mollura, Daniel, RAD-AID International, Chevy Chase, Maryland, USA
Okolielova, Tetiana, International Atomic Energy Agency, Vienna, Austria
Pellet, Olivier, International Atomic Energy Agency, Vienna, Austria
Pynda, Yaroslav, International Atomic Energy Agency, Vienna, Austria
Sathekge, Mike, Department of Nuclear Medicine, University of Pretoria and Steve Biko Academic Hospital, Pretoria, South Africa
Schlemmer, Heinz-Peter, German Cancer Research Center (DKFZ), Heidelberg, Germany
Sichizya, Veronica, University Teaching Hospitals – Adult, Lusaka, Zambia
Sutton, Elizabeth, Memorial Sloan Kettering Cancer Center, New York, USA
Vargas, Alberto H., Memorial Sloan Kettering Cancer Center, New York, USA
Varghese, Cherian, World Health Organization, Geneva, Switzerland
Footnotes
Declaration of Interests: HH reports personal fees from Ion Beam Applications (IBA) for service on its Board of Directors, outside the submitted work. She serves without compensation on the following boards and committees: External Advisory Board, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins; International Advisory Board, University of Vienna; Scientific Committee, DKFZ (German Cancer Research Center); Board of Trustees, DKFZ (German Cancer Research Center); Scientific Advisory Board, Euro-BioImaging. JAB reports personal fees from Board of Directors, Accumen, Inc., outside the submitted work. JSL reports personal fees from Clarity Pharmaceuticals, Varian Medical Systems, TPG Capital and InVicro, Inc; equity interests in Telix Pharmaceuticals and Evergreen Theragnostics; preclinical research support from Eli-Lilly, Sapience Therapeutics, Inc, MabVax Therapeutics, SibTech, Inc, Thermo Fisher Scientific, Ground Fluor Pharmaceuticals, Inc, ImaginAb, Merck & Company, AbbVie, Inc, Bristol Myers Squibb, Genentech; fee-for-service work from Y-mAbs and Regeneron Pharmaceuticals; personal fees from, and equity in, pHLIP Technologies; income from licensed intellectual property from Summit Biomedical Imaging, LLC, CheMatech, Elucida , Theragnostics Ltd., Daiichi Sankyo, and Samus Therapeutics LLC., outside the submitted work. AMS reports trial funding from Abbvie, EMD Serono, ITM, Telix and Cyclotek, research funding from Medimmune, AVID, Adalta, and Theramyc, and personal fees from Life Science Pharmaceuticals and Imagion, outside the submitted work.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6): 394–424 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol 2012; 13(8): 790–801. [DOI] [PubMed] [Google Scholar]
- 4.Stewart BW, Wild CP, eds. World Cancer Report, 2014. International Agency for Research on Cancer. Lyon, 2014. Available at: https://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014. . [Google Scholar]
- 5.Farmer P, Frenk J, Knaul FM, et al. Expansion of cancer care and control in countries of low and middle income: a call to action. Lancet 2010; 376(9747): 1186–93. [DOI] [PubMed] [Google Scholar]
- 6.Atun R, Jaffray D, Barton M., et al. Expanding global access to radiotherapy: a Lancet Oncology Commission. Lancet Oncol 2015; 16(10): 1153–86. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan R, Alatise OI, Anderson BO, et al. Global cancer surgery: delivering safe, affordable, and timely cancer surgery: a Lancet Oncology Commission. Lancet Oncol 2015; 16(11): 1193–24. [DOI] [PubMed] [Google Scholar]
- 8.Brown G, Davies S, Williams GT, et al. Effectiveness of preoperative staging in rectal cancer: digital rectal examination, endoluminal ultrasound or magnetic resonance imaging? . Br J Cancer 2004; 91(1): 23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown LC, Ahmed HU, Faria R, et al. Multiparametric MRI to improve detection of prostate cancer compared to ultrasound-guided prostate biopsy alone: the PROMIS study. Health Technol Assess 2018; 22(39): 1–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dzau VJ, Ginsburg GS, Chopra A, et al. Realizing the Full Potential of Precision Medicine in Health and Health Care. NAM Perspective September. 2016. [DOI] [PubMed] [Google Scholar]
- 11.Facey K, Bradbury I, Laking G, Payne E. Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess 2007; 11(44): iii–iv, xi-267. [DOI] [PubMed] [Google Scholar]
- 12.Fischer B, Lassen U, Mortensen J, et al. Preoperative Staging of Lung Cancer with Combined PET–CT. N Engl J Med 2009; 361: 32–9. [DOI] [PubMed] [Google Scholar]
- 13.Hricak H, Powell CB, Yu KK, et al. Invasive cervical carcinoma: role of MR imaging in pretreatment work-up--cost minimization and diagnostic efficacy analysis. 198 1996; Radiology (2): 403–9. [DOI] [PubMed] [Google Scholar]
- 14.Langer A A systematic review of PET and PET/CT in oncology: a way to personalize cancer treatment in a cost-effective manner? BMC Health Serv Res 2010; 10(283). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott AM, Gunawardana DH, Kelley B, et al. Positron Emission Tomography Changes Management and Improves Prognostic Stratification in Patients with Recurrent Colorectal Cancer: Results of a Multi-Center Prospective Study. J Nucl Med 2008; 49(9): 1451–7. [DOI] [PubMed] [Google Scholar]
- 16.Vijenthira A, Chan K, Cheung MC, Prica A. Cost-effectiveness of first-line treatment options for patients with advanced-stage Hodgkin lymphoma: a modelling study. Lancet Haematol 2020; 7(2): PE146–E56. [DOI] [PubMed] [Google Scholar]
- 17.Schlemmer HP, Bittencourt LK, D'Anastasi M, et al. Global Challenges for Cancer Imaging. J Glob Oncol 2018; (4): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allemani C, Matsuda T, Di Carlo V, et al. ; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018; 391(10125): 1023–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allemani C, Weir HK, Carreira H, et al. ; CONCORD Working Group. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015; 385(9972): 977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagley AF, Anscher MS, Choi S, et al. Association of sociodemographic and health-related factors with receipt of nondefinitive therapy among younger men with high-risk prostate cancer. JAMA Netw Open 2020;3:e201255. Published online 2020 Mar 19. doi: 10.1001/jamanetworkopen.2020.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfister DG, Rubin DM, Elkin EA, et al. Risk adjusting survival outcomes in hospitals that treat patients with cancer without information on cancer stage. JAMA Oncol. 2015;1(9):1303–1310. doi: 10.1001/jamaoncol.2015.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Editorial. Think globally about cancer. Nature Med 2019; 25(351). [DOI] [PubMed] [Google Scholar]
- 23.Shah SC, Kayamba V, Peek RM Jr, Heimburger D. Cancer Control in Low- and Middle-Income Countries: Is It Time to Consider Screening? J Glob Oncol. 2019March;5:1–8. doi: 10.1200/JGO.18.00200. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lou B, Doken S, Zhuang T, et al. An image-based deep learning framework for individualizing radiotherapy dose. Lancet Digit Health 2019; 1(3): e136–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mac Manus MP, Hicks RJ, Ball DL, et al. F-18 fluorodeoxyglucose positron emission tomography staging in radical radiotherapy candidates with non-small cell lung carcinoma: powerful correlation with survival and high impact on treatment. Cancer 2001; 92(4): 886–95. [DOI] [PubMed] [Google Scholar]
- 26.Press RH, Shu HG, Shim H, et al. The Use of Quantitative Imaging in Radiation Oncology: A Quantitative Imaging Network (QIN) Perspective. Int J Radiat Oncol Biol Phys 2018; 102(4): 1219–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Accordino MK, Wright JD, Buono D, Neugut AI, Hershman DL. Trends in use and safety of image-guided transthoracic needle biopsies in patients with cancer. J Oncol Pract 2015; 11(3): e351–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galluzzo A, Genova C, Dioguardi S, Midiri M, Cajozzo M. Current role of computed tomography-guided transthoracic needle biopsy of metastatic lung lesions. Future Oncol 2015; 11((2 Suppl)): 43–6. [DOI] [PubMed] [Google Scholar]
- 29.Huo J, Aloia TA, Xu Y, Chung TH, Sheu T, Tina Shih YC. Comparative Effectiveness of Computed Tomography- Versus Ultrasound-Guided Percutaneous Radiofrequency Ablation Among Medicare Patients 65 Years of Age or Older With Hepatocellular Carcinoma. Value Health 2019; 22(3): 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preim B, Botha C. Visual computing for medicine (Second Edition) Theory, Algorithms, and Applications. Amsterdam: Elsevier; 2014. [Google Scholar]
- 31.World Health Organization [Internet]. Communicating radiation risks in paediatric imaging: Information to support healthcare discussions about benefit and risk; 2016. [cited 2020 November 30]. Available from: https://www.who.int/ionizing_radiation/pub_meet/radiation-risks-paediatric-imaging/en/. .
- 32.Martens MH, Lambregts DMJ, Kluza E et al. Tumor Response to Treatment: Prediction and Assessment. Curr Radiol Rep 2014; 2(62). [Google Scholar]
- 33.The Canadian Medical Imaging Inventory, 2017; March2018. [cited 2020 Nov 30]. Available from: https://www.cadth.ca/canadian-medical-imaging-inventory-2017.
- 34.The Institute of Physics and Engineering in Medicine, The College of Radiographers, and The Royal College of Radiologists [Internet]. CT Equipment, Operations, Capacity and Planning in the NHS: Report from the Clinical Imaging Board; June 2015. [cited 2020 Nov 30]. Available from: https://www.rcr.ac.uk/sites/default/files/ct_equipment_in_the_nhs_report_cib_may_2015_v2_final240615.pdf.
- 35.Rosenkrantz AB, Chaves L, Hughes DR, Recht MP, Nass SJ, Hricak H. National Trends in Oncologic Diagnostic Imaging. Journal of the American College of Radiology : JACR, 04July2020, 17(9):1116–1122 DOI: 10.1016/j.jacr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hillner BE, Siegel BA, Liu D, et al. Impact of positron emission tomography/computed tomography and positron emission tomography (PET) alone on expected management of patients with cancer: initial results from the National Oncologic PET Registry J Clin Oncol 2008May; 1(13 ): 2155–61. [DOI] [PubMed] [Google Scholar]
- 37.Lindsay MJ, Siegel BA, Tunis SR, et al. The National Oncologic PET Registry: expanded medicare coverage for PET under coverage with evidence development. AJR Am J Roentgenol 2007; 188(4): 1109–13. [DOI] [PubMed] [Google Scholar]
- 38.Atun R, Bhakta N, Denburg A, et al. Sustainable care for children with cancer: a Lancet Oncology Commission. Lancet Oncology 2020; 21(4): E185–E224. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization [Internet]. Updated Appendix 3, WHO Global NCD Action Plan 2013–2020, Technical Annex; April12, 2017. [cited 2020 Nov 30]. Available from: https://www.who.int/ncds/governance/technical_annex.pdf?ua=1.
- 40.World Health Organization [Internet]. 2015 Global Survey on Health Technology Assessment by National Authorities; 2015. [cited 2020 Nov 30]. Available from: https://www.who.int/health-technology-assessment/MD_HTA_oct2015_final_web2.pdf?ua=1.
- 41.World Health Organization [Internet]. Geneva. WHO list of priority medical devices for cancer management; 2017. [cited 2020 Dec 1]. Available from: https://apps.who.int/iris/handle/10665/255262?mode=full. [Google Scholar]
- 42.Maksimovic R, Berument AV. World Health Organization. Innovative Technology in Addressing Global Health Issues: the WHO Perspective. Presentation made on April 29 – May 1, South Carolina, USA. https://www.who.int/diagnostic_imaging/imaging_modalities/InnovativeTechAddressingGlobalHealthIssues_WHOPerspective.pdf?ua=1 (accessed November 20,2020). [Google Scholar]
- 43.RAD-AID International [Internet]. RAD-AID Radiology Readiness Survey; 2013. [cited 2020 Nov 30]. Available from: https://www.rad-aid.org/resource-center/radiology-readiness.
- 44.Dodd D, Jack A. Cancer optimism tempered by ‘financial toxicity’. FT Health. Financial Times. https://www.ft.com/content/a796face-658f-11e8-a39d-4df188287fff. [Google Scholar]
- 45.IAEA. Establishing a Comprehensive Cancer Center. Under review and pending publication (2020).
- 46.National Cancer Institute [Internet]. Financial Toxicity and Cancer Treatment (PDQ®)–Health Professional Version; September. 2019. [cited 2020 Nov 30]. Available from: https://www.cancer.gov/about-cancer/managing-care/track-care-costs/financial-toxicity-hp-pdq.
- 47.Schnipper L Elephant in the Room: Financial Toxicity in Cancer Care. 2019. [Google Scholar]
- 48.World Health Organization Barcelona Office for Health Systems Strengthening. Can people afford to pay for health care? New evidence on financial protection in Europe: regional report. Available from: https://apps.who.int/iris/bitstream/handle/10665/311654/9789289054058-eng.pdf.
- 49.IMAGINE - IAEA Medical Imaging and Nuclear Medicine Global Resources Database; October2019. [cited 2020 Nov 30]. Available from: https://humanhealth.iaea.org/HHW/DBStatistics/IMAGINE.html.
- 50.Department of Health [Internet]. A Framework for the Development of Positron Emission Tomography (PET) Services in England; 10October2005. [cited 2020 Nov 30]. Available from: http://www.inahta.org/wp-content/uploads/2014/09/PET_A_framework_for_development_of_PET_services_in_England.pdf. [Google Scholar]
- 51.van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 2002; 359(9315): 1388–93. [DOI] [PubMed] [Google Scholar]
- 52.Ward ZJ, Grover S, Scott AM, et al. The role and contribution of imaging in global cervical cancer management – survival estimates from a simulation-based analysis. Lancet Oncol 2020; 21(8): 1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward ZJ, Scott AM, Hricak H, et al. Estimating the impact of treatment and imaging modalities on 5-year net survival of 11 cancers in 200 countries: a simulation-based analysis. Lancet Oncol 2020; 21(8): 1077–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jamison DT, Summers LH, Alleyne G, et al. Global Health 2035: a world converging within a generation. Lancet 2013; 382: 1898–955. . [DOI] [PubMed] [Google Scholar]
- 55.Ward ZJ, Scott AM, Hricak H, Atun R. Global costs, health, and economic benefits of scaling up treatment and imaging modalities for survival of 11 cancers: a simulation-based analysis. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ministry of Health – Federal Democratic Republic of Ethiopia. Ethiopia Health Accounts 2016/17. http://www.moh.gov.et/ejcc/sites/default/files/2020-01/Ethiopia%207th%20Health%20Accounts%20Report_2016-17.pdf.
- 57.United Nations, Department of Economic and Social Affairs, Population Division (2019). World Population Prospects 2019, Online Edition. https://population.un.org/wpp/Download/Standard/CSV/.
- 58.Serajuddin U and Hamadeh N. New World Bank country classifications by income level: 2020-2021. World Bank Blogs. Available at: https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2020-2021. Accessed November 20, 2020. [Google Scholar]
- 59.International Atomic Energy Agency (IAEA) [Internet]. IAEA Technical Cooperation Projects – Nuclear Medicine and Diagnostic Imaging Section [cited 2020 Nov 30]. Available from: https://www.iaea.org/projects/technical-cooperation-projects/3328.
- 60.Heller PS. Understanding fiscal space. IMF Policy Discussion Paper. PDP 05/4. Washington D.C., USA: International Monetary Fund; March 2005. [Google Scholar]
- 61.Reddy CL, Peters AW, Jumbam DT, et al. Innovative financing to fund surgical systems and expand surgical care in low-income and middle-income countries. BMJ Global Health 2020; 5: e002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tandon A, Cashin C. Assessing Public Expenditure on Health from a Fiscal Space Perspective. Washington D.C. USA: World Bank; 2010. [Google Scholar]
- 63.International Monetary Fund [Internet]. IMF Data Mapper. World Economic Outlook. Real GDP Growth; 2019. [cited 2020 Nov 30]. Available from: https://www.imf.org/external/datamapper/NGDP_RPCH@WEO/OEMDC/ADVEC/WEOWORLD. [Google Scholar]
- 64.Dieleman JL, Sadat N, Chang AY, et al. for Global Burden of Disease Health Financing Collaborator Network. Future and potential spending on health 2015–40: development assistance for health, and government, prepaid private, and out-of-pocket health spending in 184 countries. Lancet 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glenday G, Bharali I, Wang Z. Enhancing Domestic Revenues: Constraints and Opportunities . A cross-country comparative study of tax capacity, effort and gaps: Center for Policy Impact in Global Health Duke University, and Duke University Center for International Development, April2019. [Google Scholar]
- 66.Barroy H, Sparkes S, Dale E, Mathonnat J. Can Low- and Middle-Income Countries Increase Domestic Fiscal Space for Health: A Mixed-Methods Approach to Assess Possible Sources of Expansion. Health Systems & Reform 2018; 4(3): 214–26. [DOI] [PubMed] [Google Scholar]
- 67.World Health Organization [Internet]. Geneva. Earmarked tobacco taxes: lessons learnt from nine countries; 2015. [cited 2020 Nov 30]. Available from: http://apps.who.int/iris/bitstream/10665/206007/1/9789241510424_eng.pdf?ua=1. [Google Scholar]
- 68.Summan A, Stacey N, Birckmayer J, et al. The potential global gains in health and revenue from increased taxation of tobacco, alcohol and sugar-sweetened beverages: a modelling analysis. BMJ Global Health 2020; 5: e002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.The World Bank [Internet]. Washington DC. Industry Engagement Program. Large Medical Diagnostic Imaging Equipment Market Review and Action Plan; 2017. [cited 2020 Nov 30]. Available from: http://pubdocs.worldbank.org/en/452061518466768717/Procurement-Market-Review-and-Action-Plan.pdf. [Google Scholar]
- 70.African Development Bank [Internet]. African Development Bank Group approves $10 million equity in Razorite Healthcare Fund for Africa; 2020. July 23 [cited 2020 Dec 1]. Available from: https://www.afdb.org/en/news-and-events/press-releases/african-development-bank-group-approves-10-million-equity-razorite-healthcare-fund-africa-35206
- 71.World Bank Guarantee Program—for the consultation of “modernizing the World Bank’s operational policy on guarantees. http://siteresources.worldbank.org/INTGUARANTEES/Resources/HighlightsWBGProgramforConsulJan252012.pdf (accessed July 25, 2020).
- 72.The World Bank Group guarantee products. http://treasury.worldbank.org/bdm/pdf/Brochures/WBG_Guarantees_Matrix.pdf (accessed July 25, 2020).
- 73.Atun R, Knaul FM, Akachi Y, Frenk J. Innovative financing for health: what is truly innovative? Lancet 2012; 380: 2044–49. [DOI] [PubMed] [Google Scholar]
- 74.Atun R, Silva S, Knaul FM. Innovative financing instruments for global health 2002-15: a systematic analysis. Lancet Glob Health 2017; 5: e720–26. [DOI] [PubMed] [Google Scholar]
- 75.Social Finance. Investing in Social Outcomes: Development Impact Bonds. http://www.socialfinance.org.uk/investing-in-social-outcomes-development-impact-bonds/ (accessed June 2, 2020.
- 76.Social Finance. Social Impact Bonds. http://www.socialfinance.org.uk/wp-content/uploads/2016/07/SIBs-Early-Years_Social-Finance_2016_Final3.pdf (accessed June 2, 2020.
- 77.International Monetary Fund. 2020. World Economic Outlook: A Long and Difficult Ascent. Washington, DC, October. https://www.imf.org/en/Publications/WEO/Issues/2020/09/30/world-economic-outlook-october-2020; accessed November 27, 2020. [Google Scholar]
- 78.UNSCEAR. Sources and Effects of Ionizing Radiation. UNSCEAR 2008 Report to the General Assembly, with Scientific Annexes. United Nations, New York: 2010. [Google Scholar]
- 79.NCRP REPORT No. 184. Medical Radiation Exposure of Patients in the United States Recommendations of the NATIONAL COUNCIL ON RADIATION PROTECTION AND MEASUREMENTS. November 15, 2019.
- 80.KASHYAP R, Dondi M, PAEZ D, MARIANI G. Hybrid imaging worldwide-challenges and opportunities for the developing world: a report of a Technical Meeting organized by IAEA. Semin Nucl Med 2013; May;43((3)): 208–23. [DOI] [PubMed] [Google Scholar]
- 81.PAEZ D, BECIC T, BHONSLE U, JALILIAN AR, NUÑEZ-MILLER R, OSSO JA JR. Current Status of Nuclear Medicine Practice in the Middle East. Semin Nucl Med 2016; July;46((4)): 265–72. [DOI] [PubMed] [Google Scholar]
- 82.PAEZ D, ORELLANA P, GUTIÉRREZ C, RAMIREZ R, MUT F, TORRES L. Current Status of Nuclear Medicine Practice in Latin America and the Caribbean. J Nucl Med 2015; October;56((10)): 1629–34. [DOI] [PubMed] [Google Scholar]
- 83.HOLMBERG O, CZARWINSKI R, METTLER F The importance and unique aspects of radiation protection in medicine. Eur J Radiol 2010; 76((1)): 6–10. [DOI] [PubMed] [Google Scholar]
- 84.SHIRALKAR S, RENNIE A, SNOW M, GALLAND RB, LEWIS MH, GOWER-THOMAS K. Doctors’ knowledge of radiation exposure: questionnaire study. BMJ 2003; 327: 371–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.IAEA. Clinical Training of Medical Physicists Specializing in Diagnostic Radiology. Training Course Series No. 47 Vienna, 2011. [Google Scholar]
- 86.International Atomic Energy Agency [Internet]. Vienna. International Safety Standards [cited 2020 Dec 1]. Available from: https://www.iaea.org/resources/rpop/resources/international-safety-standards. [Google Scholar]
- 87.ICRP. Radiological Protection in Medicine. ICRP Publication 105 Ann ICRP 2007; 37(6): 1–61. [DOI] [PubMed] [Google Scholar]
- 88.INTERNATIONAL ATOMIC ENERGY AGENCY, Radiation Protection and Safety in Medical Uses of Ionizing Radiation, IAEA Safety Standards Series No. SSG-46, IAEA, Vienna: (2018). [Google Scholar]
- 89.INTERNATIONAL ATOMIC ENERGY AGENCY, Governmental, Legal and Regulatory Framework for Safety, IAEA Safety Standards Series No. GSR Part 1 (Rev. 1), IAEA, Vienna: (2016). [Google Scholar]
- 90.EUROPEAN COMMISSION FAAOOTUN, INTERNATIONAL ATOMIC ENERGY AGENCY, INTERNATIONAL LABOUR ORGANIZATION, OECD NUCLEAR ENERGY AGENCY, PAN AMERICAN HEALTH ORGANIZATION, UNITED NATIONS ENVIRONMENT PROGRAMME, WORLD HEALTH ORGANIZATION, Radiation Protection and Safety of Radiation Sources: International Basic Safety Standards, IAEA Safety Standards Series No. GSR Part 3, IAEA, Vienna: (2014). [Google Scholar]
- 91.INTERNATIONAL ATOMIC ENERGY AGENCY, Establishing the Infrastructure for Radiation Safety, IAEA Safety Standards Series No. SSG-44, IAEA, Vienna: (2018). [Google Scholar]
- 92.MUHOGORA W, REHANI MM. Review of the current status of radiation protection in diagnostic radiology in Africa. J. Med. Imag 4(3), 031202 (2017), doi: 10.1117/1.JMI.4.3.031202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.BETTMANN MA, HOLMBERG O, DEL ROSARIO PEREZ M, REMEDIOS D, MALONE J. International Collaboration on Clinical Imaging Guidelines: Many Hands Make Light Work. J Am Coll Radiol. 2015January;12(1):43–4. [DOI] [PubMed] [Google Scholar]
- 94.American College of Radiology [Internet]. ACR Appropriateness Criteria [cited 2020 Dec 1]. Available from: https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria
- 95.European Society of Radiology [Internet]. ESR iGuide: Clinical Decision Support using European Imaging Referral Guidelines [cited 2020 Dec 1]. Available from: https://www.myesr.org/esriguide
- 96.REMEDIOS D, BRINK J, HOLMBERG O, et al. Clinical imaging guidelines part 1: a proposal for uniform methodology. J Am Coll Radiol. 2015. January;12(1):45–50. [DOI] [PubMed] [Google Scholar]
- 97.The Royal College of Radiologists [Internet]. London. Introducing iRefer8 - updated diagnostic imaging guidelines for referring clinicians; 2017. May 24 [cited 2020 Dec 1]. Available from: https://www.rcr.ac.uk/posts/introducing-irefer8-updated-diagnostic-imaging-guidelines-referring-clinicians [Google Scholar]
- 98.Society of Nuclear Medicine & Medical Imaging [Internet]. Reston, Virginia. Appropriate Use Criteria (AUC) [cited 2020 Dec 1]. Available from: http://www.snmmi.org/ClinicalPractice/content.aspx?ItemNumber=15666. [Google Scholar]
- 99.European Association of Nuclear Medicine [Internet]. Guidelines [cited 2020 Dec 1]. Available from: https://www.eanm.org/publications/guidelines/
- 100.DONDI M, PAEZ D, TORRES L, et al. Implementation of Quality Systems in Nuclear Medicine: Why It Matters. An Outcome Analysis (Quality Management Audits in Nuclear Medicine Part III). Semin Nucl Med. 2018. May;48(3):299–306. doi: 10.1053/j.semnuclmed.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 101.INTERNATIONAL ATOMIC ENERGY AGENCY, Quality Management Audits in Nuclear Medicine Practices, Second Edition, Vienna: (2015). [Google Scholar]
- 102.INTERNATIONAL ATOMIC ENERGY AGENCY, Comprehensive Clinical Audits of Diagnostic Radiology Practices: A Tool for Quality Improvement, Vienna: (2010). [Google Scholar]
- 103.EUROPEAN SOCIETY OF RADIOLOGY, The ESR Audit Tool (Esperanto): genesis, contents and pilot. Insights into Imaging volume 9, 899–903 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.EUROPEAN COMMISSION, The Rules Governing Medicinal Products in the European Union Volume 4, EU Guidelines of Good Manufacturing Practice, Brussels: (2010). [Google Scholar]
- 105.INTERNATIONAL ATOMIC ENERGY AGENCY TECDOC SERIES, Good Practice for Introducing Radiopharmaceuticals for Clinical Use, Vienna: (2018). [Google Scholar]
- 106.INTERNATIONAL ATOMIC ENERGY AGENCY TECDOC SERIES, Quality Control in the Production of Radiopharmaceuticals, Vienna: (2018). [Google Scholar]
- 107.ICRP. Radiological Protection in Therapy with Radiopharmaceuticals. ICRP Publication 140. Ann ICRP 2019; 48(1):1–106. [DOI] [PubMed] [Google Scholar]
- 108.International Atomic Energy Agency [Internet]. Vienna. Nuclear Medicine Physics: A Handbook for Teachers and Students; 2014. December [cited 2020 Dec 1]. Available from: https://www-pub.iaea.org/MTCD/Publications/PDF/Pub1617web-1294055.pdf. [Google Scholar]
- 109.EUROPEAN COMMISSION, Directive 2013/35/EU on the minimum health and safety requirements regarding the exposure of workers to the risks arising from electromagnetic fields (2013).
- 110.AMERICAN COLLEGE OF RADIOLOGY, ACR Guidance Document on MR Safe Practices: 2013 Journal of magnetic resonance imaging; 37:501–530 (2013). [DOI] [PubMed] [Google Scholar]
- 111.CROSS NM, HOFF MN, KANAL KM. Avoiding MRI-Related Accidents: A Practical Approach to Implementing MR Safety. J Am Coll Radiol. 2018. 15(12):1738–1744. [DOI] [PubMed] [Google Scholar]
- 112.Panch T, Szolovits P, Atun R. Artificial intelligence, machine learning and health systems. J Glob Health 2018; 8(2): 020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pisano ED, Garnett LR. Big Data and Radiology Research. J Am Coll Radiol 2019; 16(9 Pt B): 1347–50. [DOI] [PubMed] [Google Scholar]
- 114.Liu B, Cheng J, Guo DJ, et al. Prediction of prostate cancer aggressiveness with a combination of radiomics and machine learning-based analysis of dynamic contrast-enhanced MRI. Clin Radiol 2019. [DOI] [PubMed] [Google Scholar]
- 115.Lou R, Lalevic D, Chambers C, Zafar HM, Cook TS. Automated Detection of Radiology Reports that Require Follow-up Imaging Using Natural Language Processing Feature Engineering and Machine Learning Classification. J Digit Imaging 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Munir K, Elahi H, Ayub A, Frezza F, Rizzi A. Cancer Diagnosis Using Deep Learning: A Bibliographic Review. Cancers (Basel) 2019; 11(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nasrullah N, Sang J, Alam MS, Mateen M, Cai B, Hu H. Automated Lung Nodule Detection and Classification Using Deep Learning Combined with Multiple Strategies. Sensors (Basel) 2019; 19(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shen L, Margolies LR, Rothstein JH, Fluder E, McBride R, Sieh W. Deep Learning to Improve Breast Cancer Detection on Screening Mammography. Sci Rep 2019; 9(1): 12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.World Health Organization [Internet]. Geneva. M-health: new horizons for mobile health through mobile technologies: second global survey on e-health; 2011. [cited 2020 Dec 1]. Available from: http://www.who.int/goe/publications/goe_mhealth_web.pdf. [Google Scholar]
- 120.Littman-Quinn R, Mibenge C, Antwi C, Chandra A, Kovarik CL. Implementation of m-health applications in Botswana: telemedicine and education on mobile devices in a low resource setting. J Telemed Telecare 2013; 19(2): 120–5. [DOI] [PubMed] [Google Scholar]
- 121.World Health Organisation. Global Diffusion of eHealth: Making universal health coverage achieveable. https://www.who.int/goe/publications/global_diffusion/en/.
- 122.Frehywot S, Vovides Y, Talib Z, et al. E-learning in medical education in resource constrained low- and middle-income countries. Hum Resour Health 2013; 11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wahl B, Cossy-Gantner A, Germann S, Schwalbe NR. Artificial intellegance (AI) and global health: how can AI contribute to health in resource-poor settings? . BMJ Glob Health 2018; 3: e000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Akhlaq A, McKinstry B, Muhammad KB, Sheikh A. Barriers and facilitators to health information exchange in low- and middle-income country settings: a systematic review. Health Policy and Planning 2016; 31: 1310–25. [DOI] [PubMed] [Google Scholar]
- 125.Labrique AB, Wadhwani C., Williams KA, et al. Best practices in scaling digital health in low and middle income countries. BMC Global Health 2018; 14(103). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brenn K, Ergun AS, Firouzi K, et al. Advances in Capacitative Micromachines Ultrasonic Transducers. Micromachines (Basel) 10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.National Institutes of Health [Internet]. Tablet-based Mobile Health Ultrasound for Point-of-care Breast Cancer Diagnosis in Nigeria; 2020August10 [cited 2020 Dec 1]. Available from: https://clinicaltrials.gov/ct2/show/NCT04501419.
- 128.Azubuike SO, Muirhead C, Hayes L, McNally R. Rising global burden of breast cancer: the case of sub-Saharan Africa (with emphasis on Nigeria) and implications for regional development: a review. World Journal of Surgical Oncology 2018; 16(63). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cramer A, Hecla J, Wu D, et al. Stationary Computed tomography for space and other resource-constrained environments. Sci Rep 2018; 8: 14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cooley CZ, Stockmann JP, Armstrong BD, et al. Two-dimensional imaging in a lightweight portable MRI scanner without gradient coil. Magn Reson Med 2015; 73: 872–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cooley CZ, Haskell MW, Cauley SF, et al. Design of sparse halbach magnet arrays for portable MRI using a genetic algorithm. IEEE Transactions on Magnetics 2018; 54: 1–12 2018; 54: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Runge VM, Richter JK, Heverhagen JT. Motion in Magnetic Resonance. New Paradigms for Improved Clinical Diagnosis. Invest Radiol 2019; 54: 383–95. [DOI] [PubMed] [Google Scholar]
- 133.Yang Q, Yan P, Zhang Y, et al. Low-Dose CT Image Denoising Using a Generative Adversarial Network With Wasserstein Distance and Perceptual Loss. IEEE Trans Med Imaging 2018. June; 37(6): 1348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lin DJ, Johnson PM, Knoll F, Lui YW. Artificial Intelligence for MR Image Reconstruction: An Overview for Clinicians. J Magn Reson Imaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Häggström I, Schmidtlein CR, Campanella G, Fuchs TJ. DeepPET: A deep encoder-decoder network for directly solving the PET image reconstruction inverse problem. Med Image Anal 2019. May; 54: 253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liang G, Fouladvand S, Zhang J et al. GANai:standardizing CT images using generative ad-versarial network with alternative improvement. bioRxiv 460188. [Google Scholar]
- 137.US Food and Drug Administration. Radio Frequency Identification (RFID). https://www.fda.gov/radiation-emitting-products/electromagnetic-compatibility-emc/radio-frequency-identification-rfid (accessed August 8, 2020.
- 138.Arunachalam SP, Asan O, Nestler DM, et al. Patient-Care Team Contact Patterns Impact Treatment Length of Stay in the Emergency Department. Conf Proc IEEE Eng Med Biol Soc 2019July; 2019 Jul. p. 345–8. [DOI] [PubMed] [Google Scholar]
- 139.Álvarez López Y, Franssen J, Álvarez Narciandi G, Pagnozzi J, González-Pinto Arrillaga I, Las-Heras Andrés F. RFID Technology for Management and Tracking: e-Health Applications. Sensors (Basel) 2018; 18(8): 2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shan H, Padole A, Homayounieh F, et al. Competitive performance of a modularized deep neural network compared to commercial algorithms for low-dose CT image reconstruction. Nat Mach Intell 2019; 1: 269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mollura DJ, Soroosh G, Culp MP, Group R-ACW. 2016 RAD-AID Conference on International Radiology for Developing Countries: Gaps, Growth, and United Nations Sustainable Development Goals. J Am Coll Radiol 2017; 14(6): 841–7. [DOI] [PubMed] [Google Scholar]
- 142.Lacson R, Cochon L, Ip I, et al. Classifying Safety Events Related to Diagnostic Imaging From a Safety Reporting System Using a Human Factors Framework. J Am Coll Radiol 2019; 16(3): 282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lacson R, Harris K, Brawarsky P, et al. Evaluation of an Automated Information Extraction Tool for Imaging Data Elements to Populate a Breast Cancer Screening Registry. J Digit Imaging 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Masud R, Al-Rei M, Lokker C. Computer-Aided Detection for Breast Cancer Screening in Clinical Settings: Scoping Review. JMIR Med Inform 2019; 7(3): e12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sippo DA, Warden GI, Andriole KP, et al. Automated extraction of BI-RADS final assessment categories from radiology reports with natural language processing. J Digit Imaging 2013; 26(5): 989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stolz LA, Muruganandan KM, Bisanzo MC, et al. Point-of-care ultrasound education for non-physician clinicians in a resource-limited emergency department. Trop Med Int Health 2015; 20(8): 1067–72. [DOI] [PubMed] [Google Scholar]
- 147.Yang S, Gao X, Liu L, et al. Performance and Reading Time of Automated Breast US with or without Computer-aided Detection. Radiology. 292 2019; 3: 540–9. [DOI] [PubMed] [Google Scholar]
- 148.Gu Y, Lu X, Yang L, et al. Automatic lung nodule detection using a 3D deep convolutional neural network combined with a multi-scale prediction strategy in chest CTs. Comput Biol Med; 103: 220–31. [DOI] [PubMed] [Google Scholar]
- 149.Masood A, Yang P, Sheng B, et al. Cloud-Based Automated Clinical Decision Support System for Detection and Diagnosis of Lung Cancer in Chest CT. IEEE J Transl Eng Health Med 2019; 8: 4300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu W, Gao L, Duan H, Huang G, Ye X, Nie S. Segmentation of pulmonary nodules in CT images based on 3D-UNET combined with three-dimensional conditional random field optimization Med Phys 2020. [DOI] [PubMed] [Google Scholar]
- 151.Chilamkurthy S, Ghosh R, Tanamala S, et al. Deep learning algorithms for detection of critical findings in head CT scans: a retrospective study. Lancet 2018; 392(10162): 2388–96. [DOI] [PubMed] [Google Scholar]
- 152.Kuo W, Hӓne C, Mukherjee P, Malik J, Yuh EL. Expert-level detection of acute intracranial hemorrhage on head computed tomography using deep learning. Proc Natl Acad Sci USA; 116(45): 22737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Adambounou K, Adjenou V, Salam AP, et al. A low-cost tele-imaging platform for developing countries. Front Public Health 2014; 2(135). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Olpin J, Chuang L, Berek J, Gaffney D. Imaging and cancer of the cervix in low- and middle-income countries. Gynecol Oncol Rep 2018; 25: 115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Akkus Z, Cai J, Boonrod A, et al. A Survey of Deep-Learning Applications in Ultrasound: Artificial Intelligence-Powered Ultrasound for Improving Clinical Workflow. J Am Coll Radiol 2019; 16(9 Pt B): 1318–28. [DOI] [PubMed] [Google Scholar]
- 156.Nasief HG, Rosado-Mendez IM, Zagzebski JA, Hall TJ. A Quantitative Ultrasound-Based Multi-Parameter Classifier for Breast Masses. Ultrasound Med Biol 2019; 45(7): 1603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu X, Faes L, Kale AU, et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digital Health 2019; 1: e273–97. [DOI] [PubMed] [Google Scholar]
- 158.Kong Z, Jiang C, Zhu R, et al. 18F-FDG-PET-based radiomics features to distinguish primary central nervous system lymphoma from glioblastoma. Neuroimage Clin 2019; 23: 101912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kang D, Park JE, Kim YH, et al. Diffusion radiomics as a diagnostic model for atypical manifestation of primary central nervous system lymphoma: development and multicenter external validation. Neuro Oncol; 20(9): 1251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ba-Ssalamah A, Muin D, Schernthaner R, et al. Texture-based classification of different gastric tumors at contrast-enhanced CT. Eur J Radiol 2013October; 82(10): e537–43. [DOI] [PubMed] [Google Scholar]
- 161.Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. International Conference on Medical image computing and computer-assisted intervention 2015 [Google Scholar]
- 162.Cook GJR, Goh V. What can artificial intelligence teach us about the molecular mechanisms underlying disease? Eur J Nucl Med Mol Imaging 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Du Y, Qi Y, Jin Z, Tian J. Noninvasive imaging in cancer immunotherapy: the way to precision medicine. Cancer Lett 2019. [DOI] [PubMed] [Google Scholar]
- 164.Sun R, Limkin EJ, Vakalopoulou M, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol 2018; 19(9): 1180–91. [DOI] [PubMed] [Google Scholar]
- 165.Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017; 14: 749–62. [DOI] [PubMed] [Google Scholar]
- 166.Jha AK, Li Z, Orav EJ, Epstein AM. Care in U.S. Hospitals-The Hospital Quality Alliance Program. N Engl J Med 2005; 353: 265–74. [DOI] [PubMed] [Google Scholar]
- 167.FitzGerald TJ, Bishop-Jodoin M, Followill DS, et al. Imaging and data acquisition in clinical trials for radiation therapy. Int J Radiat Oncol Biol Phys 2016; 94(2): 404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hofman MS, Violet J, Hicks RK, et al. [177Lu]-PSMA-617 radionculide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single arm, phase 2 study. Lancet Oncology 2018; 19(6): 825–33. [DOI] [PubMed] [Google Scholar]
- 169.Murphy P, Koh DM. Imaging in clinical trials. Cancer Imaging 2010; 10: S74–S82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Eng J Med 2017; 376: 125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Barrington SF, Kirkwood AA, Franceschetto, et al. PET-CT for staging and early response: results from the Results-Adapted therapy in Advancd Hodgkin Lymphoma Study. Blood 2016; 127(12): 1531–8. [DOI] [PubMed] [Google Scholar]
- 172.Petkova E, Antman EM, Troxel AB. Pooling Data From Individual Trials in the COVID-19 Era. JAMA [DOI] [PubMed] [Google Scholar]
- 173.Roach PJ, Francis R, Emmett L, et al. The impact of 68Ga-PSMA PET/CT on Management Intent in Prostate Cancer: results of an Australian Prospective Multicenter Study. J Nucl Med 2018; 59: 82–8. [DOI] [PubMed] [Google Scholar]
- 174.Loucaides EM, Fitchett EJA, Sullivan R, Atun R. Global public and philanthropic investment in childhood cancer research: systematic analysis of research funding, 2008-16. Lancet Oncology 2019; 20(12): e672–e84. [DOI] [PubMed] [Google Scholar]
- 175.Maruthappu M, Head MG, Zhou CD, et al. Investments in cancer research awarded to UK institutions and the global burden of cancer 2000–2013: a systematic analysis. BMJ Open 2017; 7(e013936). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Abdel-Wahab M, Zubizarreta E, Polo A and Meghzifene A. Improving Quality and Access to Radiation Therapy - An IAEA Perspective. Semin Radiat Oncol 2017; 27: 109–17. [DOI] [PubMed] [Google Scholar]
- 177.International Atomic Energy Agency [Internet]. Vienna. Radiation Therapy Planning of Non-small cell lung cancer based on PET/CT. Studies number E13042 and E33038 [cited 2020 Dec 1]. Available from: https://www.iaea.org/projects/crp/e13042. [Google Scholar]
- 178.Britton N, Miller MA, Safadi S, Siegel A, Levine AR, McCurdy MT. Tele-ultrasound in Resource-limited settings: A systematic Review. Frontiers in Public Health 2019; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Atun R, Cavalli F. The global fight against cancer: challenges and opportunities. Lancet 2018; 391: 412–3. [DOI] [PubMed] [Google Scholar]
- 180.United Nations [Internet]. United Nations Sustainable Development Goals. Goal 3: Ensure healthy lives and promote well-being for all at all ages [cited 2020 Dec 1]. Available from: https://www.un.org/sustainabledevelopment/health/. [Google Scholar]
- 181.World Health Organization [Internet]. Geneva. Tackling NCDs: ‘Best buys’ and other recommended interventions for the prevention and control of noncommunicable diseases; 2016. [cited 2020 Dec 1]. Available from: https://apps.who.int/iris/bitstream/handle/10665/259232/WHO-NMH-NVI-17.9-eng.pdf?sequence=1 [Google Scholar]
- 182.World Health Organization [Internet]. Geneva. WHO report on cancer: setting priorities, investing wisely and providing care for all [cited 2020 Dec 1]. Available from: https://www.who.int/publications/i/item/who-report-on-cancer-setting-priorities-investing-wisely-and-providing-care-for-all [Google Scholar]
- 183.Abdel-Wahab M, Zubizarreta E, Polo A and Meghzifene A. Assessment of cancer control capacity and readiness: the role of the International Atomic Energy Agency. Lancet Oncol 2017; 18(10): E587–E94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.