Abstract
Objective
To describe the direct and indirect cost estimates of dry eye disease (DED), stratified by disease severity, and the impact of DED on quality of life (QoL) in Canadian patients.
Methods and analysis
A prospective, multicentre, observational, cross-sectional study was conducted at six sites across Canada. Eligible patients completed a 20 min survey on demography, general health, disease severity, QoL and direct (resource utilisation and out-of-pocket expenses for the past 3–24 months) and indirect costs (absenteeism and presenteeism based on Work Productivity and Activity Impairment questionnaire responses). Subgroup analyses were performed according to DED severity and presence of Sjögren’s syndrome.
Results
Responses from 146 of 151 participants were included in the analysis. DED was rated as moderate or severe by 19.2% and 69.2% of patients, respectively. Total mean annual costs of DED were $C24 331 (Canadian dollars) per patient and increased with patient-reported disease severity. Mean (standard deviation [SD]) indirect costs for mild, moderate and severe disease were $C5961 ($C6275), $C16 525 ($C11 607), and $C25 485 ($C22,879), respectively. Mean (SD) direct costs were $C958 ($C1216), $C1303 ($C1574) and $C2766 ($C7161), respectively. QoL scores were lowest in patients with Sjögren’s syndrome (8.2% of cohort) and those with severe DED.
Conclusion
This study provides important insights into the negative impact of DED in a Canadian setting. Severe DED was associated with higher direct and indirect costs and lower QoL compared with those with mild or moderate disease. Increased costs and poorer QoL were also evident for patients with DED plus Sjögren’s syndrome versus DED alone.
Keywords: ocular surface, clinical trial
Key messages.
What is already known about this subject?
Dry eye disease (DED) is associated with high direct and indirect socioeconomic costs as well as a considerable reduction in the patient’s quality of life (QoL). However, there is a paucity of these data for DED in the Canadian healthcare system.
What are the new findings?
This multisite study provides further data and confirms the particular socioeconomic and QoL impacts on Canadian patients with DED. We found that indirect costs to Canadian patients represent the largest proportion of overall costs.
How might these results change the focus of research or clinical practice?
These data support the importance of effective management of DED to offset the substantial indirect costs and QoL burden on patients. Further research should focus on more detailed economic and QoL implications as well as the impact of pharmacological therapies and other interventions such as air quality adjustments or regular work breaks.
Introduction
Dry eye disease (DED) is a complex, multifactorial disorder of the periocular tear film that may result in damage to the ocular surface and is associated with symptoms of ocular discomfort.1 Globally, the estimated prevalence of DED ranges between 5% and 50%.2 In Canada, the prevalence of DED has been estimated at 21%.3
Diagnosis of DED should consider symptomatology, visual disturbance, tear film stability and composition, tear volume, damage and/or inflammation of the ocular surface and eyelid aspects (eg, blepharitis, lid wiper epitheliopathy).4 5 In particular, the major cause of DED needs to be recognised before treatment plans can be developed.
The Tear Film and Ocular Surface Society (TFOS) Dry Eye Workshop (DEWS) II proposed a stepwise treatment approach with follow-up to monitor signs and symptoms of DED, in order to ensure that other comorbid ocular surface diseases are also treated appropriately.1 6 Initially, DED is often treated with ocular lubricants. If this approach proves inadequate, prescribed topical drugs (eg, antibiotics, corticosteroids, secretagogues and immunomodulatory or lymphocyte function-associated antigen-1 antagonist drugs) or oral antibiotics can be considered. Additional options include overnight chamber devices and tear conservation measures.6 Where treatment remains ineffective, the next step is to use serum eye drops, therapeutic contact lenses or oral secretagogues. If the above measures fail to control the disease, the TFOS DEWS II report identifies further options as lacrimal duct occlusion or longer term topical corticosteroids (monitored closely to minimise potential adverse side effects such as cataract formation and glaucoma).6
DED can incur very high socioeconomic costs, both direct (eg, medical fees, prescribed and over-the-counter drugs) and indirect (eg, unemployment, work absenteeism, presenteeism (at work but impaired productivity)).7 Indirect costs comprise most of the overall economic burden. For example, in the USA, the mean average indirect cost to society was US$55.4 billion compared with US$3.8 billion for direct costs.8 Furthermore, DED can place a substantial burden on the quality of life (QoL) of patients, impacting their physical, social and psychological well-being and affecting their workplace productivity.7 9 For example, blurred and/or fluctuating vision may restrict activities of daily living such as reading, driving, watching television and using smartphones.7
The physical impact of DED pain can be likened to a type of chronic pain syndrome, negatively affecting patients’ psychological and physical well-being and QoL.7 Several studies, as reviewed by McDonald et al,9 have demonstrated the impact that DED has on QoL. However, there are few publications describing the impact of DED on socioeconomic costs and QoL in Canada.2 3 10 The present study aimed to contribute to the literature on DED by capturing the direct and indirect cost estimates of DED, stratified by the severity of DED and to understand the impact on QoL for Canadian patients with DED.
Materials and methods
Study design
This was a prospective, multicentre, observational, cross-sectional study conducted at six optometry and ophthalmology sites across Canada. Eligible patients had a diagnosis of mild to severe DED by an accredited healthcare provider (HCP). The investigators prioritised patients with moderate to severe DED, and target recruitment for each site was set as 10%, 45% and 45% of patients with mild, moderate or severe DED, respectively, based on HCP classification.
Patients
Investigators recruited patients for the study according to their diagnosis of DED. Eligible patients were aged 18 to 64 years and were required to meet all of the following criteria: a current diagnosis of mild-to-severe DED made by an accredited healthcare professional; a routine visit to a recruiting healthcare professional; symptoms of dry eye for at least 1 year before the date of recruitment; literacy in English, ability to read and complete surveys in English and ability to read, understand and sign the informed consent form on a voluntary basis. Patients were excluded if they were already participating in another clinical trial.
Objectives
The two primary objectives of the study were to describe the direct out-of-pocket costs and indirect costs (absenteeism and presenteeism) attributable to DED and to determine the QoL impact attributable to DED. The four secondary objectives comprised the above analyses conducted in groups stratified by patient-reported DED severity (mild, moderate or severe) and by Sjögren’s syndrome status (diagnosed vs no known Sjögren’s syndrome).
Data collection and assessments
All patients who provided consent were asked to complete a survey lasting approximately 20 min (on their own or with help) during their routine optometrist/ophthalmologist visit. Study data were collected from the survey, which was composed of six sections: demographic data, general health data, DED severity data, QoL data, indirect costs and direct costs (online supplemental table S1). Demographic data (age, sex, ethnicity, education, employment status) and clinical characteristics data (smoking status, contact lens use, screen exposure, DED treatment, DED risk factors, DED duration, ocular and non-ocular comorbidities) over the past 12 months were collected. Patient-reported DED severity and presence of Sjögren’s syndrome were captured. Patients were asked whether they had been formally diagnosed with Sjögren’s syndrome. Possible answers were ‘yes’, ‘no’, and ‘I don't know’; surveys with this question left unanswered were listed as ‘unknown’. Formal testing (eg, serology or minor salivary gland biopsy) was not performed to rule out Sjögren’s syndrome in patients who did not receive a positive diagnosis.
bmjophth-2021-000709supp001.pdf (234.8KB, pdf)
Current DED severity was assessed according to patient self-rating of their discomfort with eye dryness using the Eye Dryness Score Visual Analog Scale (EDS VAS; score range 0–100 (no discomfort to maximum discomfort)). Scores of <40 were considered mild, 40 to <60 moderate and ≥60 severe DED. The EDS VAS has not been validated for the evaluation of DED severity; however, inclusion of a VAS as a key component of a DED questionnaire has been shown as having consistently good repeatability.11
All costs are reported in 2018 Canadian Dollars. Annual direct costs were calculated using patient-reported resource utilisation and out-of-pocket expenses for the past 3–24 months. Five categories of treatments were used to assess out-of-pocket costs to patients with DED: ocular lubricants, cyclosporine, punctal plugs, optometrist or ophthalmologist visits and nutritional supplements. A patient’s direct annual cost was the sum of their annualised cost types. Population and subgroup direct costs were calculated using the average of each patient’s direct annual cost values.
Annual indirect costs were calculated with the human capital approach based on each patient’s annual salary and their Work Productivity and Activity Impairment (WPAI) questionnaire12 scores (online supplemental table S2). Average salary was estimated according to age group, sex, employment status and highest level of education from Statistics Canada,13 adjusted to 2018 levels using the Bank of Canada consumer price index inflation calculator.14 The WPAI questionnaire comprises questions on how many hours at work were missed over the past 7 days due to DED (absenteeism) and the percentage of DED-related impairment in productivity while working over the past 7 days (presenteeism). The WPAI questionnaire has been validated to quantify work impairment for several diseases and to compare impairment between study treatment groups or individuals with different disease severity levels.15–21 It has also been used to evaluate impairment related to DED.22–24
bmjophth-2021-000709supp002.pdf (24.3KB, pdf)
Current QoL was evaluated according to the National Eye Institute Visual Function Questionnaire (NEI VFQ 25).25 The 25 questions of the VFQ were recoded into 12 subscale variables: general health, general vision, ocular pain, near activities, distance activities, social functioning, mental health, role difficulties, dependency, driving, colour vision and peripheral vision. For each subscale, numeric responses were converted according to scoring rules to values on a 0–100 scale, and the averages of the responses were calculated for each. Lower scores indicate poorer QoL. Overall QoL scores were calculated by averaging vision-targeted subscale scores, excluding general health.
Sample size and statistical analysis
The study aimed to recruit approximately 150 patients from six sites over 6 months; assuming a SD-to-mean ratio of 0.14, this would give a relative precision in the primary analysis of up to 7%, with the smallest cohort estimated to contain 15 surveys. This SD-to-mean ratio was derived from a 2013 US survey of patients with DED,26 which determined mean and SD for Short Form-6 dimension (SF-6D) Health Utility Index results to be 0.7 and 0.1, respectively, representing an SD-to-mean ratio of 0.14 and a precision of 2.29%. According to Yu et al, the SD-to-mean cost ratio from the payer’s perspective was closer to 0.1.8 For our sample size calculations, we assumed the ratio between SD and sample mean to be 0.1, resulting in a precision estimate of approximately 1.6% (1.96×0.1√150, where 1.96 represents the value of the 97.5th percentile of the normal distribution to calculate a two-sided 95% CI).
Data analysis was purely descriptive and this study did not explore any associations. Summary statistics for categorical variables included frequency and percentage of each category or modality. Summary statistics for continuous variables are reported as mean and SD.
Results
Patients
In total, 151 patients from six Canadian optometrist and/or ophthalmologist sites consented to participate and the study lasted for 7 months (from August 2018 to March 2019). Data from 146 patients were analysed. Data from the remaining five patients were excluded from the analysis, mainly because they had not experienced DED symptoms for at least 1 year prior to recruitment. Demographic characteristics are shown in table 1. The mean (SD) age was 49.8 (11.4) years. Most patients were women (89.7%), of whom almost half (62/131 (47.3%)) were aged ≥55 years. The majority of patients were Caucasian (71.2%), followed by East Asians (17.8%), and there were only a few patients with mixed ethnicities (2.1%). Most patients (71.9%) held a college degree or a higher qualification, with approximately half of the group (45.9%) educated to university degree level (at or above bachelor level). The remaining patients (28.1%) did not hold a college or higher degree. Most patients were employed (65.1%) and only 2.1% were on disability leave as a result of DED. The estimated mean (SD) annual income was $C68 781 ($C22 983), with almost half (49.3%) of patients expected to earn >$C40 000 but ≤$C60 000.
Table 1.
Patient demographics
| All values are n (%) unless stated otherwise | Patients (N=146) |
| Mean (SD) age, years | 49.8 (11.4) |
| Female | 131 (89.7) |
| Age group, years | |
| 18–24 | 1 (0.7) |
| 25–34 | 19 (13.0) |
| 35–44 | 29 (19.9) |
| 45–54 | 35 (24.0) |
| 55–64 | 62 (42.5) |
| Ethnicity* | |
| Caucasian | 104 (71.2) |
| East Asian | 26 (17.8) |
| South Asian | 8 (5.5) |
| Latino/Hispanic | 3 (2.1) |
| African | 3 (2.1) |
| Middle Eastern | 2 (1.4) |
| Caribbean | 2 (1.4) |
| Other† | 4 (2.7) |
| Highest level of education | |
| University certificate, diploma or degree | |
| Above bachelor level | 22 (15.1) |
| At bachelor level | 45 (30.8) |
| Below bachelor level | 4 (2.7) |
| College, CEGEP or other non-university certificate or diploma | 34 (23.3) |
| Trades | |
| Certificate of apprenticeship or qualification | 2 (1.4) |
| Certificate or diploma (excluding certificate of apprenticeship) | 9 (6.2) |
| High school diploma or equivalency certificate | 26 (17.8) |
| No certificate, diploma, or degree | 4 (2.7) |
| Employment status | |
| Employed | 95 (65.1) |
| Disability leave | |
| Due to DED | 3 (2.1) |
| Other reason | 9 (6.2) |
| Unemployed | |
| Job seeking | 9 (6.2) |
| Not job seeking | 9 (6.2) |
| Retired/pre-pension plan | 15 (10.3) |
| None of the above (‘I do something else’) | 6 (4.1) |
| Estimated income,‡ CAD | |
| Mean (SD) | $C68 781 ($C22 983) |
| Median (Q1, Q3) | $C58 755 ($C50 513, $C87 415) |
| Estimated annual income,‡ CAD | |
| ≤$C40 000 | 4 (2.7) |
| >$C40 000–≤$C60 000 | 72 (49.3) |
| >$C60 000–≤$C80 000 | 24 (16.4) |
| >$C80 000–≤$C100 000 | 32 (21.9) |
| >$C100 000 | 14 (9.6) |
*As three patients were of mixed ethnicity the total does not add up to 100%.
†Includes one Filipino; the remaining three patients did not specify their ethnicity.
‡Average salary was estimated according to age group, sex, employment status, and highest level of education from Statistics Canada,13 adjusted to 2018 levels using the Bank of Canada consumer price index inflation calculator.14
CAD, Canadian dollars; CEGEP, College of General and Vocation Education; DED, dry eye disease.
Clinical characteristics are shown in table 2. Most patients (64.4%) had never smoked, 29.5% had smoked previously and only 6.2% were current smokers. A minority of patients used contact lenses (13.7%). Most patients (82.3%) spent more than 3 hours per day looking at screens, with 24.7% looking at screens for at least 7 hours per day. DED was rated on the EDS VAS as moderate or severe by 19.2% and 69.2% of patients, respectively. The analysis population only included patients who had been diagnosed with DED for at least 1 year: 90 patients (61.6%) had experienced DED for 1–5 years and the remainder had a disease duration of ≥6 years.
Table 2.
Clinical characteristics
| All values are n (%) unless stated otherwise | Patients (N=146) |
| Use contact lenses | 20 (13.7) |
| Screen exposure, hours | |
| 0–<1 | 10 (6.8) |
| 1–2 | 16 (11.0) |
| 3–4 | 48 (32.9) |
| 5–6 | 36 (24.7) |
| 7–15 | 36 (24.7) |
| Current type of treatment | |
| Preserved | 33 (22.6) |
| Non-preserved (eg, hydroxypropyl cellulose ophthalmic insert) | 52 (35.6) |
| Prescription eye drops (eg, lifitegrast, cyclosporine) | 73 (50.0) |
| Specialty drugs | 11 (7.5) |
| Compounded ointments | 12 (8.2) |
| Gel (eg, loteprednol etabonate) | 21 (21.2) |
| Other | 39 (26.7) |
| None | 8 (5.5) |
| Sjögren’s syndrome | |
| No | 122 (83.6) |
| Yes | 12 (8.2) |
| I do not know | 11 (7.5) |
| Unknownb | 1 (0.7) |
| Patient-reported severity of DED | |
| Mild | 17 (11.6) |
| Moderate | 28 (19.2) |
| Severe | 101 (69.2) |
| DED duration, years | |
| 1–5 | 90 (61.6) |
| 6–10 | 28 (26.0) |
| 10–35 | 18 (12.3) |
*'Unknown’ indicates that no answer was provided.
†Including hypertension, antidiabetic and analgesic medications.
‡For example, Sjögren’s syndrome, rheumatoid arthritis, lupus.
§For example, mood, depression, anxiety.
¶Including laser-assisted in situ keratomileusis or photorefractive keratectomy.
DED, dry eye disease; GvHD, graft versus host disease; HRT, hormone replacement therapy; HSCT, haematopoietic stem cell transplantation.
An absence of ocular comorbidities and/or surgeries/injections accounted for 52.7% and 67.8% of patients, respectively. The remainder reported a variety of ocular comorbidities, of which the most frequent were seasonal allergies with itchy eyes (21.9%) and Meibomian gland dysfunction (18.5%). The most frequent ocular surgery was refractive eye surgery, which had been conducted in 15.8% of patients. The most frequently used treatment reported by patients was eye drops (preserved, 22.6%; non-preserved, 35.6%). Concomitant medications included multivitamins, which were being taken by 40.4% of patients and antidepressants (used by 15.1% of patients). A total of 21.9% of patients were not taking any concomitant medications.
Twelve patients (8.2%) reported having Sjögren’s syndrome and the remaining 134 (91.8%) had no known Sjögren’s syndrome, including 122 (83.6%) who indicated that they had not received this diagnosis and 12 patients (8.2%) in whom the status was unknown. Among other diagnostic risk factors, thyroid disorders were reported by 15.1% of patients, whereas 32.2% did not report any diagnostic risk factors.
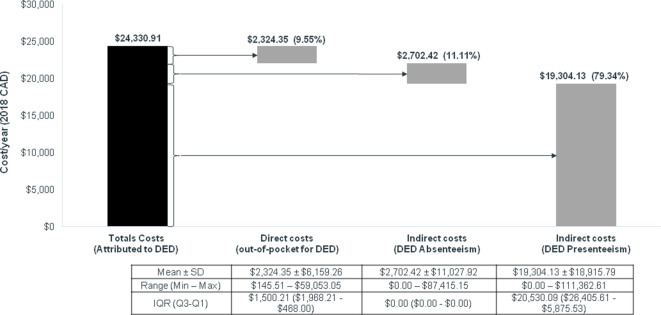
Annual costs of DED
The total annual costs of DED per patient were on average $C24 331 (figure 1); the majority was related to the indirect costs of DED presenteeism (79.3%). The mean (SD) patient-reported indirect cost attributable to DED was $C21 052 ($C20 812)/year (figure 1). Mean (SD) indirect costs of presenteeism and absenteeism were $C19 304 ($C18 916)/year and $C2702 ($C11 028)/year, respectively. The mean (SD) total patient-reported direct cost attributable to DED was $C2324 ($6159)/year (figure 1).
Figure 1.
Mean annual costs due to DED. CAD, Canadian dollars; DED, dry eye disease.
When average annual costs were stratified by patient-reported DED severity, the mean (SD) indirect costs of absenteeism and presenteeism attributable to severe DED totaled $C25 485 ($C22 879)/year, representing an increase of 54% versus the costs of moderate DED ($C16 525 ($C11 607)/year) and 328% vs mild DED ($C5961 ($C6275)/year) (figure 2). Direct out-of-pocket costs per year were also highest for severe DED (mean (SD): $C2766 ($C7161)), representing an increase in 112% versus the costs of moderate DED ($C1303 ($C1574)) and 189% versus mild DED ($C958 ($C1216)).
Figure 2.
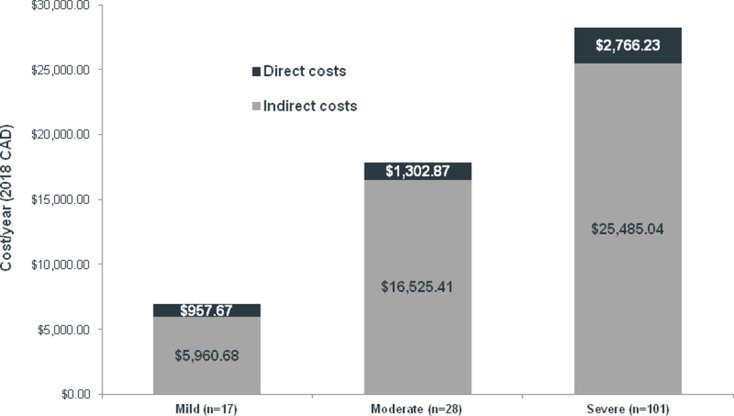
Mean annual costs according to patient-reported DED severity. CAD, Canadian dollars; DED, dry eye disease.
The mean (SD) indirect costs per year of absenteeism and presenteeism attributable to Sjögren’s syndrome totalled $C41 094 ($C15 720), representing an increase in 132% versus the costs for patients with no known Sjögren’s syndrome ($C17694 ($C17 153)) (figure 3). Direct out-of-pocket costs were also higher for Sjögren’s syndrome (mean (SD): $C2689 ($C2430)), representing an increase in 22% versus the costs for patients with no known Sjögren’s syndrome ($C2203 ($C6660)).
Figure 3.
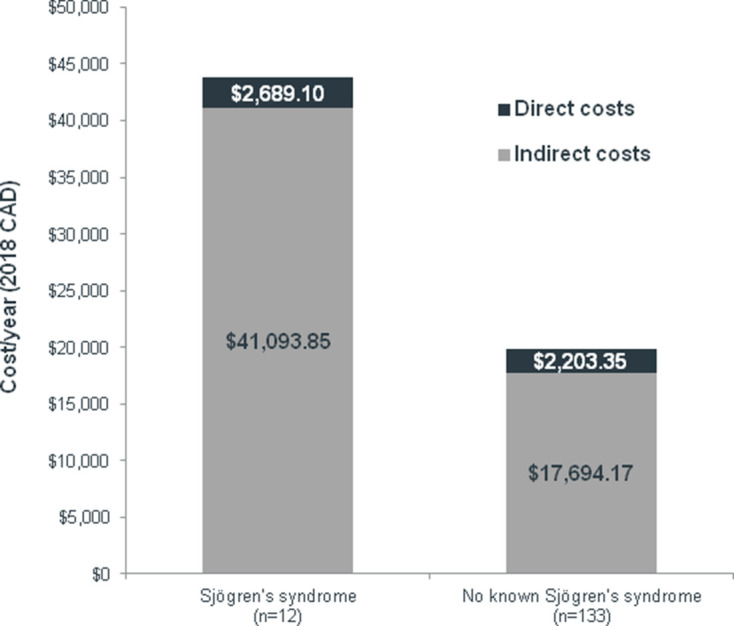
Mean annual costs according to Sjögren’s syndrome status. CAD, Canadian dollars.
Impact of DED on QoL
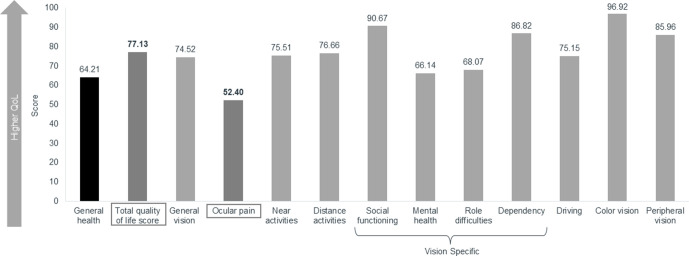
The total mean (SD) VFQ-25 QoL score was 77.13 (15.69) (figure 4). Ocular pain had the lowest score of all subscales (mean (SD) score 52.40 (21.99)), indicating that it had the greatest negative effect on QoL. DED also impacted mental health (66.14 (25.84)), role difficulties (at work and for other activities, 68.07 (26.47)) and driving (75.15 (19.38)).
Figure 4.
Average QoL subscale scores. Scores for the 12 VFQ 25 QoL subscales and the total QoL score, based on score ranges of 0–100, with lower scores indicating lower QoL. DED, dry eye disease; QoL, quality of life, VFQ, Visual Function Questionnaire.
On average, self-reported severe DED was associated with a lower total VFQ-25 QoL score (mean 72.42 (SD 16.19)) than moderate (86.60 (6.98)) or mild (89.55 (7.14)) DED. The mean (SD) total VFQ-25 QoL score was lower in patients with Sjögren’s syndrome compared with those without the condition (59.81 (14.16) vs 79.21 (15.04)), respectively.
Discussion
This study provides an analysis of the economic burden and effects on QoL in Canadian patients with DED. Patients with self-reported severe DED reported lower QoL, higher medical fees and impaired work productivity compared with mild and moderate cases. Annual indirect costs increased from a mean (SD) of $C5961 ($C6275) in mild DED to $C25 485 ($C22 879) in severe DED. Direct costs followed a similar pattern, with a mean (SD) of $C958 ($C1216)/year in mild DED rising to $C2766 ($C7161)/year in severe DED. Patients with Sjögren’s syndrome also incurred higher costs than those without Sjögren’s syndrome. The mean total QoL score was 77, with ocular pain impacting patients’ QoL the most (mean NEI VFQ-25 score 52); DED also negatively affected patients’ mental health, role difficulties and driving. Unsurprisingly, QoL was worse in patients with severe DED versus those with mild/moderate disease and in those with versus those without Sjögren’s syndrome.
Much of the previous literature have evaluated the costs and burden of DED from a payers’ or healthcare systems perspective and have found that DED confers a substantial economic burden. In a systematic literature review, the majority of economic data was found to be from the USA (9/12 articles), and health-related QoL data were predominantly from Europe (11/20 articles) and the USA (8/20 articles).9 McDonald et al found that indirect costs comprised the largest proportion of overall DED costs, due to significant loss in work productivity.9 In the USA, for example, indirect costs were US$11 302/year per patient (with the overall burden to society being US$55 billion), and the numbers of days lost per year for patient-reported mild, moderate and severe DED were 91, 95 and 128, respectively.8 None of the 12 economic burden articles included Canadian data, although one article reported Canadian QoL data.
Our research provides additional insights into the burden of DED in a Canadian setting, taking the patients’ direct costs into account and estimating indirect costs to society. We have shown that in Canada, indirect costs comprise the largest proportion of overall costs compared with direct costs. We found that DED impacts both indirect and direct costs. In addition, direct costs increased with disease severity. Our data are supported by an analysis of 2005 data from 2171 US patients, where indirect versus direct costs were estimated as US$11 302/year versus US$783/year per patient.8 Likewise, direct costs increased with patient-reported disease severity (US$678/year for mild DED, US$771 for moderate DED and US$1267 for severe DED). In this study, both prescription and non-prescription medications were considered in the five categories of healthcare resource use (ocular lubricant treatment, cyclosporine, punctal plugs, physician visits and nutritional supplements).
The WPAI questionnaire is a validated questionnaire for the evaluation of work impairment.15–21 It was associated with higher construct validity and fewer omissions when administered by an interviewer rather than self-administered.15 The WPAI was also found to provide higher mean estimates of productivity loss and related cost compared with other questionnaires.27 28 To evaluate ixekizumab therapy on work productivity among patients with chronic plaque psoriasis (PSO), Armstrong et al used the WPAI-PSO questionnaire.29 Results were represented as observed mean percentages of absenteeism, presenteeism, work productivity loss (overall work impairment associated with absenteeism and presenteeism) and activity impairment, as derived from patient assessment of each factor on a scale of 0–10.
Direct costs determined in our study were considerably higher than those found in an analysis of 2003–2004 DED management data from six European countries (France, Germany, Italy, Spain, Sweden and the UK).30 To treat 1000 patients with DED, estimated direct medical costs (patient examinations, diagnostic tests, prescription medications and surgical procedures) ranged from US$270 000 (France) to US$1.1 million (UK); the per-patient average amounts converted to 2018 Canadian dollars (estimated $C1.13 conversion rate31 inflated with the Consumer Price Index14) would be approximately $C360–$1460. However, these figures reflect the data captured through management by an ophthalmologist only; self-treatment with over-the-counter agents and therapies prescribed by a general practitioner were not included.
Similar to our data, in which patients ranked ocular pain as the lowest favourable item in terms of QoL, impaired QoL in patients with active primary Sjögren’s syndrome is mostly caused by ocular pain and dryness.32 In the USA, the mean ocular pain subscale score was significantly lower for patients with moderate-to-severe DED compared with patients with milder DED.33 Additionally, assessment of the relative burden of DED in the USA showed that DED consistently caused bodily pain (effect size −0.08) and decreased role-physical (defined as limitations due to physical problems, −0.07) and vitality scores (−0.11) when compared with people without DED. However, the difference was only clinically significant for moderate or severe DED.34 QoL was impaired to a greater extent in patients with Sjögren’s syndrome versus those with non-Sjögren’s syndrome DED.33 Our data are in agreement with these observations.
Although outside the scope of this analysis, additional factors in the severity of DED should be taken into consideration. The overwhelming majority (89.7%) of patients were women, and nearly half (42.5%) of the patients in this study were aged 55 years or older. Both of these characteristics are significantly associated with not only increased prevalence but also greater severity of DED. According to a study by Tellefsen Nøland et al, women had significant increased osmolarity, shorter tear break-up time (TBUT), decreased meibum quality, reduced meibum expressibility and decreased corneal sensitivity than men.35 Increased age was associated with significantly worse Ocular Surface Disease Index (OSDI) score, shorter TBUT, lower Schirmer I test score and reduced meibum expressibility. Postmenopausal women were found to have higher scores related to ocular symptoms, vision-related functions and environmental triggers than perimenopausal women, and OSDI score increased with age.36 Screen exposure has also been shown to have a significant effect on DED. One-half of subjects in this study reported at least 5 hours of daily screen time. Prolonged digital exposure time was a significant risk factor for DED in several studies,37–40 and significantly shorter TBUT times were observed in patients with ≥8 hours of daily screen time.40 Smartphone usage was strongly associated with paediatric DED.41
While our findings provide important evidence on the economic and QoL burden associated with DED in a Canadian population, they must be interpreted in context. Canada has a distinct climate with the potential to worsen the symptoms of DED as a result of dry heat indoors in the winter, a requirement for air conditioning during the summer and the effect of spring and fall allergy seasons. As this was a survey-based study, it relied on human recall to evaluate productivity loss, QoL impact and out-of-pocket costs. Recall bias was minimised by using validated recall periods for the VFQ-25 and WPAI questionnaires.15 42 Selection bias is a typical limitation of cross-sectional surveys as recruitment of patients with DED who had visited an optometrist/ophthalmologist could lead to an underestimation of productivity losses and their costs (due to patients being too sick to attend a routine visit). An overestimation of losses is also possible because patients with mild DED may not have required a routine visit during the study period. Direct and indirect costs were limited to those pertaining to the patient, and the impact of overall direct costs to the insurer or the overall economy was not considered. Indirect cost according to time lost at work may not account for additional variables such as paid sick leave. The 7-day period over which absenteeism and presenteeism were both estimated and may not accurately reflect patients’ actual impairment. The findings of our study are also limited by the sample size and subsequent stratification, particularly for the subgroup with a diagnosis of Sjögren’s syndrome (n=12). Data related to Sjögren’s syndrome should be interpreted with particular caution, as patient-reported absence of this diagnosis was not verified with serology testing or minor salivary gland biopsy. Beyond non-responses to the diagnosis of Sjögren’s syndrome, missing or invalid data were minimal, as the investigators checked completed surveys prior to patient discharge. As our analysis was descriptive, and no hypothesis was tested, confounding is not expected to impact our findings.
In conclusion, a cross-sectional survey assessed the economic burden and QoL associated with DED in a sample of patients living in Canada. The annual cost of DED averaged $C24 330, with indirect costs accounting for 90% of the total. Direct and indirect costs, and the negative impact of DED on QoL, were higher in patients with self-reported severe DED compared with those with mild or moderate disease. Sjögren’s syndrome was also associated with higher costs and lower QoL than in patients without Sjögren’s syndrome. This study highlights the considerable burdens associated with DED in terms of patient costs (direct and indirect) and reduced QoL, particularly with increased DED severity. These findings in a Canadian population are consistent with those of studies in other countries with different healthcare structures. Further research will be required to establish effective methods of reducing these burdens through expanded use of pharmacological therapies and other interventions such as air quality adjustments or regular work breaks.
Acknowledgments
The authors would like to thank the Vector Eye Centre, Calgary, Alberta (Jamie Bhamra, Lead Physician; Alison Endsin, Study Coordinator), Rosedale Medical Centre, Toronto, Ontario (Clara C. Chan, Lead Physician; Paul Lam, Study Coordinator), Ivey Eye Institute, London, Ontario (Rookaya Mather, Lead Physician; Bobbi Smuck and Julie Duncan, Study Coordinators), Clarity Eye Institute, Scarborough, Ontario (King Chow, Lead Physician; Heather Coulter, Study Coordinator), Valley Family Optometry (Krista Flynn, Lead Physician; Shyanne Jenkins, Study Coordinator), and Elmsdale Vision Centre, Elmsdale, Nova Scotia (Andrew Webber, Lead Physician; Dolly Chippett and Stacey MacKenzie, Study Coordinators), and the patients who participated in this study. The authors also wish to thank Johanna Mancini, Megan Naeini and Deepi Minhas of IQVIA for their help in the design, management and analysis of the study, Jeff Alexander of SNELL Medical Communication for writing assistance, and Christopher Reaume of Takeda.
Footnotes
Contributors: All authors contributed to the completion of this study and to the preparation of this manuscript.
Funding: The study was supported by Takeda and Novartis. The sponsor provided a formal review of the publication; however, the authors had final authority over the content. Medical writing support was provided by Envision Pharma Group, which was supported by Takeda and Novartis.
Competing interests: CC has been a consultant for Alcon, Allergan, Bausch+Lomb, Johnson & Johnson, Labtician, Santen, and Shire* and has received research support from Allergan, Bausch+Lomb and TearLab. SZ has received grant/research support from Shire* and honoraria/consulting fees from Allergan, Labtician, J&J, Santen, and Shire*. VM was a paid consultant to Shire* in connection with this study. JGB was an employee of Shire* at the time of the study and is a current employee of Novartis. CLP has been a consultant for Alcon Canada, Innova Medical, Shire*, Novartis and Bausch+Lomb Canada. *A Takeda company.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was performed in accordance with the Health Insurance Portability and Accountability Act 1996 (HIPAA) regulations and the principles of the Declaration of Helsinki. Institutional review board (IRB) and ethics committee approvals were obtained as follows: Rosedale Medical Centre (CCC); Advarra IRB approval SSU00064394 (26 July 2018); Vector Eye Centre (JB); Conjoint Health Research Ethics Board, University of Calgary approval; REB18-1185 (1 August 2018); Ivey Eye Institute (RM); Western University Health Science Research Ethics Board approval 112286 (20 August 2018); Clarity Eye Institute (KC) Advarra IRB approval SSU00075068 (11 January 2019); Valley Family Optometry (KF); Advarra IRB approval SSU00076123 (1 February 2019); Elmsdale Vision Centre (AW); Advarra IRB approval SSU00064416 (26 July 2018); written informed consent was obtained from all patients. Neither patients nor the public were involved in the design, conduct, reporting or dissemination plans of this study.
References
- 1.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf 2017;15:276–83. 10.1016/j.jtos.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 2.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf 2017;15:334–65. 10.1016/j.jtos.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 3.Caffery B, Srinivasan S, Reaume CJ, et al. Prevalence of dry eye disease in Ontario, Canada: a population-based survey. Ocul Surf 2019;17:526–31. 10.1016/j.jtos.2019.02.011 [DOI] [PubMed] [Google Scholar]
- 4.Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf 2017;15:539–74. 10.1016/j.jtos.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 5.Pflugfelder SC, Solomon A, Stern ME. The diagnosis and management of dry eye: a twenty-five-year review. Cornea 2000;19:644–9. 10.1097/00003226-200009000-00009 [DOI] [PubMed] [Google Scholar]
- 6.Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf 2017;15:575–628. 10.1016/j.jtos.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 7.Uchino M, Schaumberg DA. Dry eye disease: impact on quality of life and vision. Curr Ophthalmol Rep 2013;1:51–7. 10.1007/s40135-013-0009-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea 2011;30:379–87. 10.1097/ICO.0b013e3181f7f363 [DOI] [PubMed] [Google Scholar]
- 9.McDonald M, Patel DA, Keith MS, et al. Economic and Humanistic Burden of Dry Eye Disease in Europe, North America, and Asia: A Systematic Literature Review. Ocul Surf 2016;14:144–67. 10.1016/j.jtos.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 10.Abetz L, Rajagopalan K, Mertzanis P, et al. Development and validation of the impact of dry eye on everyday life (IDEEL) questionnaire, a patient-reported outcomes (pro) measure for the assessment of the burden of dry eye on patients. Health Qual Life Outcomes 2011;9:111. 10.1186/1477-7525-9-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaumberg DA, Gulati A, Mathers WD, et al. Development and validation of a short global dry eye symptom index. Ocul Surf 2007;5:50–7. 10.1016/S1542-0124(12)70053-8 [DOI] [PubMed] [Google Scholar]
- 12.Reilly Associates . WPAI:SHP v2.0 (US English), 2010. Available: http://www.reillyassociates.net/WPAI_SHP.html
- 13.Statistics Canada . 2016 Census of Population - Employment Income Statistics (table) [Internet], 2017. Available: http://www12.statcan.gc.ca/census-recensement/2016/dp-pd/dt-td/Rp-eng.cfm?LANG=E&APATH=3&DETAIL=0&DIM=0&FL=A&FREE=0&GC=0&GID=0&GK=0&GRP=1&PID=110647&PRID=10&PTYPE=109445&S=0&SHOWALL=0&SUB=0&Temporal=2017&THEME=123&VID=0&VNAMEE=&VNAMEF
- 14.Bank of Canada . Consumer Price Indexes for Canada [Internet]. Available: https://www.bankofcanada.ca/rates/related/inflation-calculator/
- 15.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4:353–65. 10.2165/00019053-199304050-00006 [DOI] [PubMed] [Google Scholar]
- 16.Reilly MC, Bracco A, Ricci J-F, et al. The validity and accuracy of the Work Productivity and Activity Impairment questionnaire--irritable bowel syndrome version (WPAI:IBS). Aliment Pharmacol Ther 2004;20:459–67. 10.1111/j.1365-2036.2004.02091.x [DOI] [PubMed] [Google Scholar]
- 17.Reilly MC, Gooch KL, Wong RL, et al. Validity, reliability and responsiveness of the work productivity and activity impairment questionnaire in ankylosing spondylitis. Rheumatology 2010;49:812–9. 10.1093/rheumatology/kep457 [DOI] [PubMed] [Google Scholar]
- 18.Reilly MC, Gerlier L, Brabant Y, et al. Validity, reliability, and responsiveness of the work productivity and activity impairment questionnaire in Crohn's disease. Clin Ther 2008;30:393–404. 10.1016/j.clinthera.2008.02.016 [DOI] [PubMed] [Google Scholar]
- 19.Revicki DA, Willian MK, Menter A, et al. Impact of adalimumab treatment on patient-reported outcomes: results from a phase III clinical trial in patients with moderate to severe plaque psoriasis. J Dermatolog Treat 2007;18:341–50. 10.1080/09546630701646172 [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Blanc PD, Hayden ML, et al. Assessing productivity loss and activity impairment in severe or difficult-to-treat asthma. Value Health 2008;11:231–9. 10.1111/j.1524-4733.2007.00229.x [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Bansback N, Boonen A, et al. Validity of the work productivity and activity impairment questionnaire--general health version in patients with rheumatoid arthritis. Arthritis Res Ther 2010;12:R177. 10.1186/ar3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols KK, Bacharach J, Holland E, et al. Impact of dry eye disease on work productivity, and patients' satisfaction with over-the-counter dry eye treatments. Invest Ophthalmol Vis Sci 2016;57:2975–82. 10.1167/iovs.16-19419 [DOI] [PubMed] [Google Scholar]
- 23.Dana R, Meunier J, Markowitz JT, et al. Patient-Reported burden of dry eye disease in the United States: results of an online cross-sectional survey. Am J Ophthalmol 2020;216:7–17. 10.1016/j.ajo.2020.03.044 [DOI] [PubMed] [Google Scholar]
- 24.Patel VD, Watanabe JH, Strauss JA, et al. Work productivity loss in patients with dry eye disease: an online survey. Curr Med Res Opin 2011;27:1041–8. 10.1185/03007995.2011.566264 [DOI] [PubMed] [Google Scholar]
- 25.RAND . The National eye Institute 25-Item visual function questionnaire (VFQ-25) manual, 2000. Available: https://www.rand.org/content/dam/rand/www/external/health/surveys_tools/vfq/vfq25_manual.pdf
- 26.Farrand KF, Stillman IÖ, Fridman M, Schaumberg S, et al. Impact of dry eye disease on quality of life, work productivity, daily activities, and health care resource use in a survey of 74,095 American adults. Value Health 2016;19:A127. 10.1016/j.jval.2016.03.521 [DOI] [Google Scholar]
- 27.Gardner BT, Dale AM, Buckner-Petty S, et al. Comparison of employer productivity metrics to lost productivity estimated by commonly used questionnaires. J Occup Environ Med 2016;58:170–7. 10.1097/JOM.0000000000000587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Gignac MAM AM, Beaton D, et al. Canadian arthritis network work productivity group. productivity loss due to presenteeism among patients with arthritis: estimates from 4 instruments. J Rheumatol 2010;37:1805–14. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong AW, Lynde CW, McBride SR, et al. Effect of ixekizumab treatment on work productivity for patients with moderate-to-severe plaque psoriasis: analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatol 2016;152:661–9. 10.1001/jamadermatol.2016.0269 [DOI] [PubMed] [Google Scholar]
- 30.Clegg JP, Guest JF, Lehman A, et al. The annual cost of dry eye syndrome in France, Germany, Italy, Spain, Sweden and the United Kingdom among patients managed by ophthalmologists. Ophthalmic Epidemiol 2006;13:263–74. 10.1080/09286580600801044 [DOI] [PubMed] [Google Scholar]
- 31.Online currency converter. Available: https://freecurrencyrates.com/en/exchange-rate-history/USD-CAD/2006/cbr [Accessed 29 Apr 2021].
- 32.Cornec D, Devauchelle-Pensec V, Mariette X, et al. Severe health-related quality of life impairment in active primary Sjögren's syndrome and patient-reported outcomes: data from a large therapeutic trial. Arthritis Care Res 2017;69:528–35. 10.1002/acr.22974 [DOI] [PubMed] [Google Scholar]
- 33.Nichols KK, Mitchell GL, Zadnik K. Performance and repeatability of the NEI-VFQ-25 in patients with dry eye. Cornea 2002;21:578–83. 10.1097/00003226-200208000-00009 [DOI] [PubMed] [Google Scholar]
- 34.Mertzanis P, Abetz L, Rajagopalan K, et al. The relative burden of dry eye in patients' lives: comparisons to a U.S. normative sample. Invest Ophthalmol Vis Sci 2005;46:46–50. 10.1167/iovs.03-0915 [DOI] [PubMed] [Google Scholar]
- 35.Tellefsen Nøland S, Badian RA, Utheim TP, et al. Sex and age differences in symptoms and signs of dry eye disease in a Norwegian cohort of patients. Ocul Surf 2021;19:68–73. 10.1016/j.jtos.2020.11.009 [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Alfaro P, Garcia S, Rodriguez I, et al. Dry eye disease symptoms and quality of life in perimenopausal and postmenopausal women. Climacteric 2021;24:261–6. 10.1080/13697137.2020.1849087 [DOI] [PubMed] [Google Scholar]
- 37.Wang MTM, Muntz A, Mamidi B, et al. Modifiable lifestyle risk factors for dry eye disease. Cont Lens Anterior Eye 2021:101409. 10.1016/j.clae.2021.01.004 [DOI] [PubMed] [Google Scholar]
- 38.Sánchez-Valerio MDR, Mohamed-Noriega K, Zamora-Ginez I, et al. Dry eye disease association with computer exposure time among subjects with computer vision syndrome. Clin Ophthalmol 2020;14:4311–7. 10.2147/OPTH.S252889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uchino M, Schaumberg DA, Dogru M, et al. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology 2008;115:1982–8. 10.1016/j.ophtha.2008.06.022 [DOI] [PubMed] [Google Scholar]
- 40.Gajta A, Turkoanje D, Malaescu I. Dry eye syndrome among computer users. AIP Conference Proceedings 2015;1694:040011. [Google Scholar]
- 41.Moon JH, Kim KW, Moon NJ. Smartphone use is a risk factor for pediatric dry eye disease according to region and age: a case control study. BMC Ophthalmol 2016;16:188. 10.1186/s12886-016-0364-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mangione CM, Lee PP, Pitts J, et al. Psychometric properties of the National eye Institute visual function questionnaire (NEI-VFQ). NEI-VFQ field test Investigators. Arch Ophthalmol 1998;116:1496–504. 10.1001/archopht.116.11.1496 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjophth-2021-000709supp001.pdf (234.8KB, pdf)
bmjophth-2021-000709supp002.pdf (24.3KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available.