Abstract
Objectives
To report characteristics, treatment and overall survival (OS) trends, by stage and pathology, of patients diagnosed with non-small cell lung cancer (NSCLC) at Leeds Teaching Hospital NHS Trust in 2007–2018.
Design
Retrospective cohort study based on electronic medical records.
Setting
Large NHS university hospital in Leeds.
Participants
3739 adult patients diagnosed with incident NSCLC from January 2007 to August 2017, followed up until March 2018.
Main outcome measures
Patient characteristics at diagnosis, treatment patterns and OS.
Results
34.3% of patients with NSCLC were clinically diagnosed (without pathological confirmation). Among patients with known pathology, 45.2% had non-squamous cell carcinoma (NSQ) and 33.3% had squamous cell carcinoma (SQ). The proportion of patients diagnosed at stage I increased (16.4%–27.7% in 2010–2017); those diagnosed at stage IV decreased (57.0%–39.1%). Surgery was the most common initial treatment for patients with pathologically confirmed stage I NSCLC. Use of radiotherapy alone increased over time in patients with clinically diagnosed stage I NSCLC (39.1%–60.3%); chemoradiation increased in patients with stage IIIA NSQ (21.6%–33.3%) and SQ (24.2%–31.9%). Initial treatment with systemic anticancer therapy (SACT) increased in patients with stages IIIB–IV NSQ (49.0%–67.5%); the proportion of untreated patients decreased (30.6%–15.0%). Median OS improved for patients diagnosed with stage I NSQ and SQ and stage IIIA NSQ over time. Median OS for patients with stages IIIB–IV NSQ and SQ remained stable, <10% patients were alive 3 years after diagnosis. Median OS for clinically diagnosed stages IIIB–IV patients was 1.2 months in both periods.
Conclusions
OS for stage I and IIIA patients improved over time, likely due to increased use of stereotactic ablative radiation, surgery (stage I) and chemoradiation (stage IIIA). Conversely, OS outcomes remained poor for stage IIIB–IV patients despite increasing use of SACT for NSQ. Many patients with advanced-stage disease remained untreated.
Keywords: epidemiology, chemotherapy, radiation oncology, oncology, respiratory tract tumours
Strengths and limitations of this study.
These data reflect outcomes and trends for a single site in the UK; however, the REAL-Oncology study represents an unselected population, which is relevant to real-world practice and enables long-term (>10 years) analyses across numerous subgroups.
This analysis included patients with a clinical diagnosis of non-small cell lung cancer (NSCLC) who, despite representing a large proportion of patients with NSCLC, are often not captured in real-world studies.
Limited information on radiotherapy was available at the time of the analysis; thus, it was not possible to formally differentiate radiotherapy with palliative intent from that with curative intent.
The follow-up duration was relatively short for patients diagnosed at the end of the study period, at 7 months.
Data on comorbidities that might have explained why a significant proportion of patients with advanced disease did not receive any systemic anticancer therapy were not available.
Introduction
In the UK, lung cancer is the third most common type of cancer and the leading cause of cancer death.1 Around 85% of patients with lung cancer have non-small cell lung cancer (NSCLC), which consists predominantly of non-squamous cell carcinoma (NSQ) and squamous cell carcinoma (SQ).2 Early diagnosis of lung cancer can be challenging.3 Consequently, approximately two-thirds of patients present with advanced or metastatic NSCLC (stages III and IV), for which treatment options are limited and prognosis is poor;4 5 5-year survival rates for patients with metastatic disease are less than 5%.5 6
Surgery and radiotherapy can be used successfully in patients diagnosed with early-stage NSCLC; adjuvant chemotherapy is also indicated for selected patients who have undergone resection for stage II and III disease and can improve outcomes.7 For advanced NSCLC (stages IIIB–IV), chemotherapy with platinum-based agents has long been the standard of care for patients with good performance status (PS) and vascular endothelial growth factor-targeting therapies have been used in the first-line setting in patients with NSQ.8 However, increased understanding of NSCLC driver mutations, such as those in the epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) genes, has led to the development of targeted therapies, including tyrosine kinase inhibitors (TKIs). These allow for more personalised treatment approaches in selected patients with actionable driver mutations.8
The development of immunotherapeutic agents has transformed the NSCLC treatment landscape. Since 2015, immune checkpoint inhibitors (ICIs) targeting the programmed death-1/programmed death ligand 1 axis have been approved in Europe and are now recommended for first-line or second-line treatment of patients with metastatic NSCLC.9 In addition, ongoing clinical trials are investigating neoadjuvant and adjuvant use of ICIs for patients diagnosed at earlier stages of NSCLC.10–12 As with any new treatment, there is a need to assess how ICIs impact patient survival in real-world clinical practice to help inform future treatment decisions, which requires an understanding of the NSCLC landscape prior to their availability. Real-world databases include a wealth of information that can be used to complement data from clinical trials and are a valuable source of evidence in a rapidly changing treatment landscape.
We report the characteristics, treatment and overall survival (OS) trends for patients diagnosed with NSCLC at a large teaching hospital in England prior to routine availability of ICIs. This study, based on the REAL-Oncology database, is part of the I-O Optimise programme, an ongoing initiative leveraging real-world data sources to provide insights into the evolving landscape of thoracic malignancies, including NSCLC.13
Methods
Study setting
REAL-Oncology is a research partnership between Leeds Cancer Centre (LCC), the University of Leeds and IQVIA, using NHS oncology patient data to answer various research questions. LCC is a major NHS cancer centre that serves a metropolitan catchment area of 750 000 people for secondary care and over 5 million for tertiary care.
Study design
This retrospective analysis extracted data on prescribed chemotherapy, and pathology and radiology records that were entered into electronic medical records (EMRs) at the Leeds Teaching Hospitals NHS Trust as part of routine clinical practice. The study included patients aged ≥18 years with an incident diagnosis of NSCLC (International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) code for malignant neoplasm of the trachea (C33) or malignant neoplasm of bronchus and lung (C34)) between January 2007 and August 2017 at Leeds Teaching Hospital. All patients diagnosed by the lung multidisciplinary team were included, including those clinically identified solely on the basis of history, clinical examination and CT scan and those with confirmed morphology (ie, International Classification of Diseases for Oncology, third Edition (ICD-O-3) code for NSCLC pathology, online supplemental appendix table S1). Patients were excluded if their first diagnosis of NSCLC was confirmed in another NHS hospital trust, they had missing data on age or sex, their ICD-O-3 morphology codes indicated small cell lung cancer (80 413–80 459) or they had a concomitant (within 5 years prior to NSCLC diagnosis) primary tumour at the time of diagnosis, except for non-metastatic non-melanoma skin cancers or in situ or benign tumours. Patients with missing data on tumour, node and metastasis (TNM) classification were also excluded from the present analyses.
bmjopen-2020-046396supp001.pdf (124.6KB, pdf)
The end of follow-up was the date of death or end of study (April 2018). The date of death was confirmed by reconciliation of EMRs with Office for National Statistics death certifications. Patient sociodemographic (age, sex, WHO PS) and clinical characteristics (TNM stage, tumour pathology) were extracted on/at the nearest date to NSCLC diagnosis (index date). TNM classification at diagnosis was recorded according to the sixth edition of the TNM classification up to 31 December 2009,14 the seventh edition from 1 January 20106 and the eighth edition from 1 January 2017.15 Tumour pathology was defined as NSQ (including adenocarcinoma and large cell carcinoma), SQ, NSCLC not otherwise specified (NOS), ‘other’ (neuroendocrine carcinoma and other miscellaneous carcinoma) or ‘unconfirmed’ (clinically diagnosed unknown pathology).
The date of initial treatment was defined as the first instance of lung surgery, radiotherapy or systemic anticancer therapy (SACT) occurring within 6 months of diagnosis and initial treatment categories were defined using all treatments received within a specified time period following this date (online supplemental appendix table S2). A line of therapy (LoT) was defined as one or more cycles of chemotherapy or continuous oral treatment for targeted agents in patients with incident stages IIIB–IV NSCLC. An algorithm based on the sequencing of SACT treatments received was developed to determine first and subsequent LoTs. LoT outputs were validated by clinicians.
Analyses
Patient characteristics at diagnosis are described using summary statistics. The evolution of treatment patterns and OS over time were investigated in two subcohorts defined by date of diagnosis: January 2007 to December 2012 and January 2013 to August 2017. Therapy received and treatment duration are described by LoT for advanced-stage patients using the same time periods. OS was estimated using Kaplan-Meier methods. The proportions of patients surviving to 1, 2 or 3 years after the date of diagnosis are reported with corresponding two-sided 95% CIs. Differences in OS between time periods were compared using log-rank hypothesis tests.
To comply with patient confidentiality requirements, data outputs relating to groups of fewer than five patients were masked. In some circumstances, data relating to larger patient subgroups were also masked to avoid extrapolation of counts of fewer than five patients.
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Results
Patients
Overall, 4225 patients diagnosed with NSCLC between January 2007 and August 2017 were included in the study. Of these, 486 were excluded because of missing TNM staging information, resulting in an analysis cohort of 3739 patients. Patients had a median (IQR) age of 73 (65–80) years and were evenly split by sex (table 1). Pathology findings were available for 2458 patients (65.7%), with the remaining 1281 (34.3%) being clinically diagnosed without pathological confirmation. Where pathology was available, NSQ was the most frequent subtype (45.2%), followed by SQ (33.3%), NOS (17.9%) and ‘other’ NSCLC (3.6%; table 1).
Table 1.
Demographic and clinical characteristics of full patient population*
| All stages | All NSCLC | NSQ | SQ | NSCLC NOS | Other NSCLC | Clinically diagnosed unknown pathology |
| N=3739 | n=1112 | n=819 | n=439 | n=88 | n=1281 | |
| Age, years | ||||||
| Mean (SD) | 72.3 (10.9) | 68.6 (11.0) | 70.8 (9.4) | 68.9 (10.6) | 70.1 (10.7) | 78.0 (9.3) |
| Median (Q1–Q3) | 73 (65–80) | 69 (62–77) | 71 (64–77) | 69 (63–77) | 71 (63–78) | 79 (72–85) |
| Range | 18–101 | 31–101 | 33–96 | 18–92 | 42–91 | 43–99 |
| Male, n (%) | 1881 (50.3) | 519 (46.7) | 505 (61.7) | 220 (50.1) | 49 (55.7) | 588 (45.9) |
| TNM stage, n (%) | ||||||
| I | 717 (19.2) | 223 (20.1) | 127 (15.5) | 30 (6.8) | 19 (21.6) | 318 (24.8) |
| II | 434 (11.6) | 113 (10.2) | 132 (16.1) | <40 (<9.1) | <13 (<14.8) | 137 (10.7) |
| IIIA | 469 (12.5) | 110 (9.9) | 164 (20.0) | 54 (12.3) | 8 (9.1) | 133 (10.4) |
| IIIB | 337 (9.0) | 89 (8.0) | 117 (14.3) | <55 (<12.3) | <5 (<6.0) | 77 (6.0) |
| IV | 1782 (47.7) | 577 (51.9) | 279 (34.1) | 263 (59.9) | 47 (53.4) | 616 (48.1) |
| Pathology, n (%) | ||||||
| Adenocarcinoma | 1019 (27.3) | 1019 (91.6) | 0 | 0 | 0 | 0 |
| SQ | 819 (21.9) | 0 | 819 (100.0) | 0 | 0 | 0 |
| NSCLC NOS | 439 (11.7) | 0 | 0 | 439 (100.0) | 0 | 0 |
| Large cell carcinoma | 93 (2.5) | 93 (8.4) | 0 | 0 | 0 | 0 |
| Other NSCLC | 88 (2.4) | 0 | 0 | 0 | 88 (100.0) | 0 |
| Clinically diagnosed, unknown pathology | 1281 (34.3) | 0 | 0 | 0 | 0 | 1281 (100.0) |
| WHO performance score, n (%) | ||||||
| 0 | 292 (7.8) | 149 (13.4) | 70 (8.6) | 38 (8.7) | 12 (13.6) | 23 (1.8) |
| 1 | 1031 (27.6) | 445 (40.0) | 319 (39.0) | 144 (32.8) | 37 (42.2) | 86 (6.7) |
| 2 | 758 (20.3) | 230 (20.7) | 230 (28.1) | 80 (18.2) | 25 (28.4) | 193 (15.1) |
| 3 | 933 (25.0) | 154 (13.9) | 118 (14.4) | 97 (22.1) | <15 (<17.0) | 553 (43.2) |
| 4 | 372 (10.0) | 39 (3.5) | 16 (2.0) | 26 (5.9) | 0 | 291 (22.7) |
| Missing | 353 (9.4) | 95 (8.5) | 66 (8.1) | <55 (<12.5) | <5 (<5.7) | 135 (10.5) |
*For some categories including low numbers of patients, data have been masked to conceal patient identities. Includes six patients diagnosed in 2006.
NOS, not otherwise specified; NSCLC, non-small cell lung cancer; NSQ, non-squamous cell carcinoma; Q, quartile; SQ, squamous cell carcinoma;; TNM, tumour, node and metastasis.
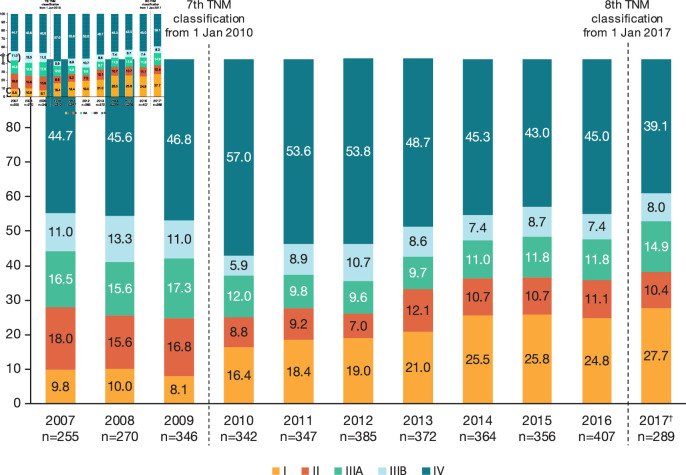
Over the study period, 717 patients (19.2%) were diagnosed with stage I disease, 434 (11.6%) with stage II and 806 (21.6%) with stage III; almost half of patients (47.7%) were diagnosed with stage IV disease. TNM classification evolved during the study period, which might have contributed to some of the changes observed over time in the stage distribution at diagnosis. However, over the period when the seventh TNM classification was used (2010–2016), the proportion of patients diagnosed with stage I disease increased from 16.4% in 2010 to 24.8% in 2016 (figure 1), while diagnoses of stages IIIA and IIIB NSCLC remained stable over time. There was an overall reduction in the proportion of patients diagnosed at stage IV, from 57.0% in 2010 to 45.0% in 2016.
Figure 1.
TNM stage at non-small cell lung cancer incident diagnosis, by year of diagnosis. †Diagnosed up to 31 August 2017. TNM, tumour, node and metastasis.
Treatments
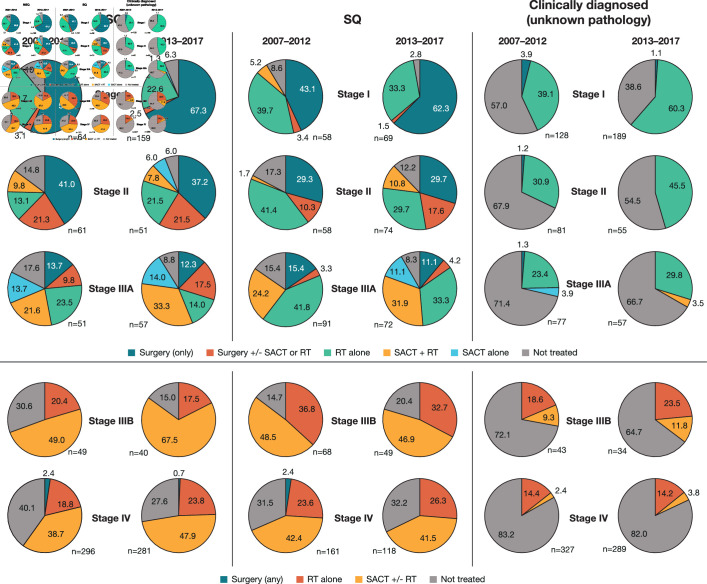
Over the study period, 2337 patients (62.5%) received an initial treatment within 6 months after diagnosis. As expected, treatment rates declined with increasing disease stage, from 78.2% for patients diagnosed with stage I disease to 49.8% for those diagnosed with stage IV. The proportion of patients with early-stage (stages I–IIIA) NSCLC who did not receive treatment decreased over time; 21.2% of patients with stages I–IIIA disease remained untreated 6 months after diagnosis in 2013–2017 compared with 32.3% in 2007–2012 (data not shown). The proportion of patients with stage IV disease remaining untreated decreased among those with NSQ, while no changes were observed among those with SQ (figure 2).
Figure 2.
Initial treatment by TNM stage and time period (2007–2012 vs 2013–2017) in patients with NSQ, SQ or CDUP*,†. *Time periods for receipt of initial treatment are based on the date of diagnosis during two consecutive time periods (January 2007–December 2012 and January 2013–August 2017). †Where analytical groups included fewer than five patients, percentages are not shown as labels. CDUP, clinically diagnosed with unknown pathology; NSQ, non-squamous cell carcinoma; RT, radiotherapy; SACT, systemic anticancer therapy; SQ, squamous cell carcinoma; TNM, tumour, node and metastasis.
Initial treatments over time (stages I–IV)
Figure 2 shows the evolution of initial treatments by TNM stage between 2007–2012 and 2013–2017 in patients with NSQ and SQ and in those who were clinically diagnosed. Over the study period, patients diagnosed with pathologically confirmed stage I disease were most commonly treated with curative surgery alone or, to a lesser extent, curative radiotherapy alone. The proportion receiving surgery alone as initial treatment increased between 2007–2012 and 2013–2017; conversely, the proportion receiving radiotherapy alone decreased. Among patients with clinically diagnosed stage I disease, the proportion receiving radiotherapy increased between 2007–2012 and 2013–2017 (from 39.1% to 60.3%; figure 2).
For patients with pathologically confirmed stage II disease, there was no notable difference in the use of surgery (alone or with adjuvant therapy) between 2007–2012 and 2013–2017. In 2013–2017, among patients with NSQ and SQ, respectively, 37.3% and 29.7% received surgery alone and 21.6% and 17.6% received surgery associated with (neo)adjuvant therapy (mostly adjuvant SACT). Radiotherapy alone was the most common treatment for patients with clinically diagnosed stage II disease, with 30.9% treated in 2007–2012 compared with 45.5% in 2013–2017.
Among the patients diagnosed with pathologically confirmed stage IIIA disease, the proportion receiving SACT plus concurrent radiotherapy (chemoradiation) increased to around one-third in 2013–2017. Some differences in the use of surgery were observed according to histology. In 2013–2017, one-third of patients with NSQ received surgery (surgery alone, 12.3%; surgery associated with adjuvant therapy, 17.5%), and only around 15% of patients with SQ disease received surgery (mostly surgery alone).
For patients diagnosed with stage IIIB or IV NSQ, initial treatment with SACT (with or without radiotherapy) increased between 2007–2012 and 2013–2017, largely as the proportion of untreated patients decreased; it is likely that some patients with stage IIIB disease received chemoradiation with curative intent. Treatment of patients with stage IIIB or IV SQ disease remained similar between the two time periods.
Patterns of SACT use in advanced NSCLC (stages IIIB–IV)
Of the 2119 patients diagnosed with stages IIIB–IV NSCLC during the study period, 648 (30.6%) received a first LoT, 223 (10.5%) received a second LoT and 60 (2.8%) received a third LoT. Similar proportions of patients with stages IIIB–IV NSQ and SQ received a first LoT (45.0% and 45.5%, respectively). Higher proportions of patients with stage IIIB or IV NSQ received second and third LoTs (17.9% and 5.6%, respectively) compared with SQ NSCLC (13.6% and 2.8%, respectively).
The most common first LoT regimens for patients with stages IIIB–IV NSCLC were platinum-based chemotherapy doublets; in 2007–2012, carboplatin plus gemcitabine was the most common (39.9% of treated patients; data not shown); in 2013–2017, carboplatin plus pemetrexed was the most common (28.7% of treated patients; table 2). The proportions of patients with NSQ receiving cisplatin-based and pemetrexed-based regimens increased between 2007–2012 and 2013–2017 (table 2).
Table 2.
First-line and second-line SACT in patients with stages IIIB–IV NSQ or SQ carcinoma*
| NSQ | SQ | |||
| First-line SACT† | 2007–2012 | 2013–2017 | 2007–2012 | 2013–2017 |
| Patients receiving first-line SACT, N | 139 | 161 | 104 | 76 |
| Platinum-based chemotherapy, n (%)‡ | 109 (78.4) | 119 (73.9) | 97 (93.3) | 73 (96.1) |
| Carboplatin based | 93 (66.9) | 78 (48.4) | 88 (84.6) | 68 (65.4) |
| Cisplatin based | 11 (7.9) | 33 (20.5) | 9 (8.7) | <5 |
| Pemetrexed included | 58 (41.7) | 107 (77.0) | <5 | <5 |
| Non-platinum-based chemotherapy, n (%) | <5 | 0 | <5 | <5 |
| TKI, n (%) | 17 (12.2) | 34 (21.1) | 0 | <5 |
| Anti-PD-1/PD-L1 checkpoint inhibitors, n (%) | 0 | <5 | 0 | <5 |
| Clinical trial—unknown treatment, n (%) | 8 (5.8) | <5 | 5 (4.8) | 0 |
| Second-line SACT§ | 2007–2012 | 2013–2018 | 2007–2012 | 2013–2018 |
| Patients receiving second-line SACT, N | 53 | 66 | 31 | 23 |
| Platinum-based therapy, n (%)‡ | <5 | 13 (19.7) | 5 (16.1) | 8 (34.8) |
| Non-platinum-based chemotherapy, n (%) | <5 | 7 (10.6) | <5 | <5 |
| TKI, n (%) | 47 (88.7) | 31 (47.0) | 23 (74.2) | 6 (26.1) |
| Anti-PD-1/PD-L1 checkpoint inhibitors, n (%) | 0 | 10 (15.2) | 0 | 5 (21.7) |
| Clinical trial—unknown treatment, n (%) | 0 | <5 | 0 | 0 |
*For some categories including low numbers of patients, data have been masked to conceal patient identities.
†Time periods for receipt of initial SACT are based on the date of diagnosis: January 2007–December 2012 and January 2013–August 2017.
‡Platinum based is defined as any regimen including a platinum agent (monotherapy or in combination) and is further defined as ‘carboplatin based’, ‘cisplatin based’ (including regimens in which carboplatin and cisplatin were both used) and ‘pemetrexed included’ (any platinum-based regimen also including pemetrexed).
§Time periods for receipt of second-line SACT are based on the start date for second-line treatment: January 2007–December 2012 and January 2013–April 2018.
NSQ, non-squamous cell carcinoma; PD-1, programmed death-1; PD-L1, programmed death ligand 1; SACT, systemic anticancer therapy; SQ, squamous cell carcinoma; TKI, tyrosine kinase inhibitor.
Among 119 patients with NSQ receiving a second LoT, the most common treatment was a TKI (65.5%); however, use of TKIs in second line decreased over time. Among 54 patients with SQ receiving a second LoT, the most common treatment was also a TKI (53.7%); use of TKIs in second line also decreased over time in this subcohort, concomitant with an increase in use of platinum-based chemotherapies. Given the late introduction of ICIs with respect to the study cohort (January 2017), a relatively small proportion of patients with stages IIIB–IV NSQ and SQ received second-line treatment with an ICI in 2013–2018.
Duration of SACT treatment (stages IIIB–IV)
For patients with stages IIIB–IV NSCLC who received first-line platinum-based chemotherapy, treatment duration was similar over the analysis period. In 2013–2017, the median (IQR) treatment duration was 2.8 (1.4–3.2) months for patients with NSQ and 2.2 (1.4–2.8) months for patients with SQ.
Among the small number of patients with stages IIIB–IV NSQ who received a TKI in the first line, median treatment durations were consistent over time at around 5 months. For patients with stages IIIB–IV NSCLC who received a second LoT, the median (IQR) treatment duration for those treated in 2013–2018 was 2.5 (1.4–5.4) months for those with NSQ and 2.1 (1.4–2.4) months for those with SQ.
Overall survival
OS over time (stages I, II and IIIa)
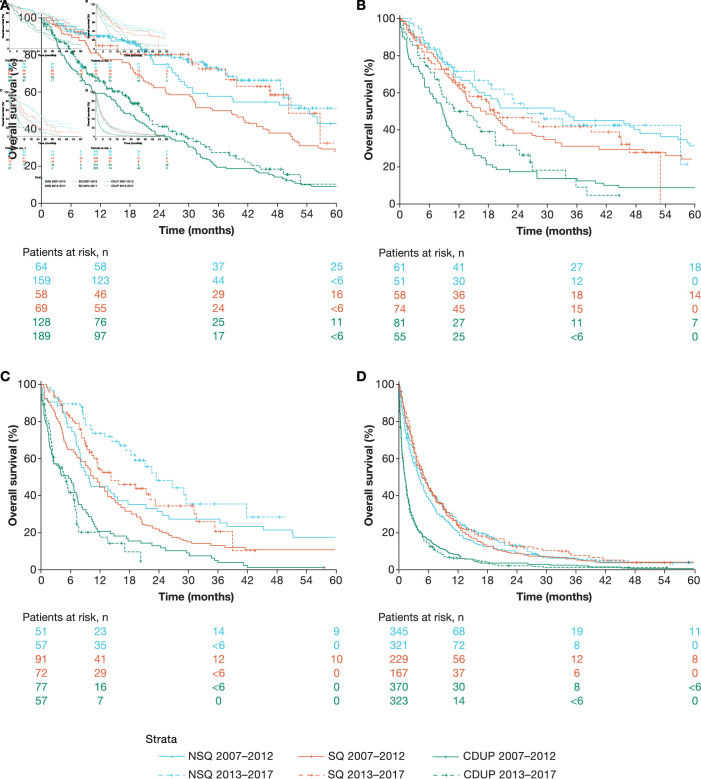
For patients with stage I NSQ, median (IQR) OS from diagnosis was 55.3 (24.8–98.5) months for those diagnosed in 2007–2012 and was not reached (NR; 34.2 months–NR) for those diagnosed in 2013–2017; median OS increased among patients with stage I SQ (from 37.3 (18.5–66.8) to 51.1 (32.6–NR) months) (figure 3A, online supplemental appendix table S3). Median (IQR) OS for patients with stage I NSCLC without pathological diagnosis increased slightly from 16.7 (5.8–33.1) to 20.9 (8.0–40.3) months between 2007–2012 and 2013–2017, respectively.
Figure 3.
Evolution of OS in patients diagnosed with stage I (A), stage II (B), stage IIIA (C) or stages IIIB–IV (D) NSCLC with NSQ, SQ or CDUP. CDUP, clinically diagnosed with unknown pathology; NSCLC, non-small cell lung cancer; NSQ, non-squamous cell carcinoma; OS, overall survival; SQ, squamous cell carcinoma.
Median (IQR) OS for patients with stage II NSQ was 34.3 (10.6–80.0) months for those diagnosed in 2007–2012 and 26.4 (10.2–58.0) months for those diagnosed in 2013–2017; in patients with stage II SQ, the respective median OS was 17.2 (8.6–58.2) and 19.9 (7.2–53.9) months (figure 3B, online supplemental appendix table S3). For patients without pathological diagnosis and stage II disease, median (IQR) OS increased slightly from 8.9 (2.9–16.8) to 11.3 (5.4–26.9) months, respectively, over the same periods.
Among patients with stage IIIA NSQ, median (IQR) OS increased from 9.9 (6.5–38.6) months for those diagnosed in 2007–2012 to 24.0 (10.6–NR) months for those diagnosed in 2013–2017; median (IQR) OS also increased among patients with stage IIIA SQ (from 10.7 (4.4–21.1) to 14.5 (8.4–36.0) months) (figure 3C, online supplemental appendix table S3). Significant improvement in 1-year OS was observed in patients with NSQ, which increased from 45% (33%–61%) to 74% (63%–87%). Median (IQR) OS among patients with stage IIIA NSCLC without confirmed pathology remained low over the study period at around 5 months.
OS over time (stages IIIB–IV)
Median OS and landmark OS rates for patients with stages IIIB–IV NSQ or SQ were similar for those diagnosed in 2007–2012 and 2013–2017, with no notable changes over time (figure 3D, online supplemental appendix table S3). During both periods, less than 10% of patients with stages IIIB–IV NSQ or SQ were alive 3 years after diagnosis. Median OS for clinically diagnosed patients with stages IIIB–IV NSCLC was 1.2 months for both time periods (figure 3D, online supplemental appendix table S3).
Discussion
These data from the REAL-Oncology database, part of I-O Optimise, provide insight into NSCLC management prior to the reimbursement of immunotherapies in the UK. Over the analysis period (2007–2017, with follow-up to 2018), most patients with NSCLC in this database were diagnosed with advanced disease. This is consistent with the overall proportion of patients with NSCLC and available TNM staging diagnosed in England in 2017, of whom around 50% had stage IV disease,16 and with real-world evidence across Europe from the same period.17 18
Nevertheless, in our analysis, there was an 11.6% increase in the proportion of patients diagnosed with stage I NSCLC over 2010–2017. The Cancer Reform Strategy,19 implemented in England in 2007, aimed to build on advances made following the introduction of the NHS Cancer Plan in 2000,19 which was designed to close the survival gap for patients with cancer in England compared with those in countries with similar healthcare systems. The strategy further aimed to improve cancer prevention, early diagnosis and patient management and led to the establishment of the National Cancer Equality Initiative (2008)20 and the National Awareness and Early Diagnosis Initiative (NAEDI)21 in collaboration with Cancer Research UK (2008).22 Consequently, the increased proportion of patients diagnosed with stage I NSCLC in the present analysis may partly reflect the impact of these reforms on cancer diagnosis in England during the study period. Notably, the proportion of patients diagnosed with early-stage NSCLC in the REAL-Oncology database was slightly higher than that reported for all lung cancers in the 2017 National Audit for England (20% diagnosed at stage I; 8% at stage II).16
At the time of our analysis, no national lung cancer screening programme existed in the UK. However, a pilot programme originally funded by NAEDI began in Leeds in 2011 aiming to assess lung cancer outcomes in response to a range of public health interventions.23 Consequently, between 2008–2010 and 2013–2015, there was an 80.8% increase in community referrals for chest X-rays and a significant stage shift in diagnosis, with an 8.8% increase in patients diagnosed at stages I and II and a 9.3% reduction in those diagnosed at stages III and IV.23 While these results are promising, lack of a concurrent control population over the same period meant that the relative contribution of other factors impacting diagnosis could not be determined. Final results from the NELSON Study reported a significant reduction in 10-year mortality from lung cancer among male smokers who received regular CT screening compared with those who did not.24 Similarly, the large US National Lung Screening Trial reported a 20% decreased risk of death from lung cancer among high-risk individuals screened with CT compared with those screened with radiography.25 These findings support the introduction of a UK-wide lung cancer screening programme, which could decrease the number of patients diagnosed with advanced NSCLC. In addition to the impact of screening, transition from the sixth to the seventh edition of TNM classification for NSCLC in 2010 is likely to have impacted tumour staging at diagnosis,26 as reported in Sweden and Denmark over the same period based on national registries data (Ekman et al27). Specifically, in the seventh edition, tumour size cut-offs for the T descriptor were revised and the importance of pleural effusions and mediastinal invasion for the M descriptor was acknowledged, resulting in the upstaging of some tumours and the downstaging of others.6
Consistent with National Audit data from England and with real-world evidence from Europe, the largest proportion of patients with available pathological data in the REAL-Oncology database had NSQ, mostly adenocarcinoma.16 17 28 Our database also allowed the identification of clinically diagnosed patients who accounted for 34% of the analysis population and tended to be older and have higher PS compared with those with confirmed pathology.29 These patients were either not deemed suitable for treatment or had an early stage peripheral tumour invisible on bronchoscopy and compromised respiratory function; therefore, biopsy confirmation was not justified in either case. Our findings are consistent with an International Cancer Benchmarking Partnership study showing that the rate of clinical diagnosis (ie, no pathological confirmation) for lung cancer over 2004–2007 was higher in the UK (26.0%) compared with Australia (14.4%), Canada (18.2%), Denmark (13.5%), Norway (10.1%) and Sweden (5.2%).30 Although the National Lung Cancer Audit in England set a target of 75% for pathological confirmation, there remains wide variation. For example, Khakwani et al found that the rates of pathologically confirmed lung cancer in England varied widely according to age, sex, PS, comorbidity and the method of referral to a specialist. The two most important patient features were age and PS, with less than 50% of patients aged ≥75 years with PS >2 having a pathological confirmation.31
Around 60% of analysed patients received at least one treatment, consistent with the 2017 National Audit (59%) for all lung cancers in England.32 The initial treatment rate declined sequentially with increasing disease stage, a pattern previously observed in Europe.17 There was a notable increase in the proportion of patients with stage I NSCLC who received surgery alone, possibly due to the aforementioned pilot programme in Leeds during that time. In England and Wales, the proportion of patients undergoing resection for histologically confirmed NSCLC increased from 14% in 2008 to 22% in 2012. This may reflect both improvements in earlier diagnosis and changes in surgical practice.29 33–35
The proportion of patients with clinically diagnosed NSCLC receiving radiotherapy alone increased markedly, concomitant with a decrease in the proportion of untreated patients. This may reflect the increased use of stereotactic body radiation therapy as an alternative to surgery for patients with early-stage disease and contraindications for surgery. There was also a notable increase in the use of chemoradiation for patients with stage IIIA NSCLC over the study period, similar to reports from other European population-based studies.17 36 This followed the publication of data from several clinical trials, as well as a meta-analysis, demonstrating a significant survival benefit with concomitant versus sequential chemoradiation for patients with locally advanced NSCLC.37 Additionally, advances in staging procedures, such as the use of positron emission tomography (PET)-CT, have enabled the identification of stage III patients with low nodal involvement who may benefit from chemoradiation.38
The observed changes in treatment patterns among patients with stages I–IIIA NSCLC in the REAL-Oncology database were mirrored by changes in survival. Survival outcomes for patients diagnosed with stage I NSCLC tended to improve over time. Again, the pilot screening programme in Leeds and/or the increase in surgical interventions among patients with NSQ and SQ and in radiotherapy use in patients who were clinically diagnosed could have driven these improvements. These findings are consistent with marginal improvements in survival among patients diagnosed with early-stage NSCLC in England during the period of our study.31 Additionally, this may reflect the effects of super staging, with the introduction of PET scanning and endoscopic sampling of lymph nodes.39 Improved survival outcomes for patients diagnosed with stage IIIA NSCLC may be related to the increased use of surgery with SACT or radiotherapy and chemoradiation during the latter diagnostic period. Furthermore, transition to the seventh edition of TNM classification for NSCLC in 2010 may have influenced subsequent treatment allocations and survival outcomes for some patients.26
Changes in the recommended management of advanced NSCLC in Europe from 2005, including the use of pemetrexed as maintenance therapy in the first-line setting for platinum-treated NSQ patients and the advent of new TKIs for patients with EGFR and ALK mutations, likely influenced the observed treatment patterns.40 41 Additionally, during the course of our study, some patients with advanced NSCLC in England were granted access to ICIs via the Early Access to Medicines Scheme,42 43 which is reflected by the small proportions of patients who received these treatments. Nevertheless, despite changes in treatment patterns, there was little change in survival outcomes for patients with advanced NSCLC during the analysis period; the prognosis for these patients, particularly those with SQ, remained poor. Indeed, less than 10% of patients diagnosed with advanced NSCLC remained alive 3 years after diagnosis. This is in contrast to reports of temporal improvements in OS among patients with stage IIIB/IV NSCLC based on registry data from Sweden and Denmark over 2005–2015 (Ekman et al27), and a recent study showing a decline in mortality due to NSCLC in the USA over 2013–2016.44 While previous real-world studies have demonstrated similarly poor survival outcomes for patients with stages IIIB and IV NSCLC,17 45 survival rates for patients with advanced lung cancer in the UK have historically been low compared with other developed countries. This has led to the implementation of several healthcare reforms and initiatives since 2000, which have so far made only limited progress at closing this survival gap, as reflected here.30 46
Despite some improvements in patient outcomes over time, real-world estimates of OS among patients with stage IIIB/IV NSCLC are often below those reported in randomised controlled trials (RCTs). In a systematic review of 23 RCTs published over 2001–2010 comparing first-line chemotherapy for patients with stage IIIB/IV NSCLC, median OS was 6.2–11.8 months for those with SQ, 7.5–11.8 months for NSQ and 21.6–30.9 months for EGFR+NSCLC.47 These values are substantially higher than the median OS reported here for patients with advanced disease. Notably, median patient age was lower in the RCTs at 56–67 years (vs 73 years in our analysis) and the majority of patients had a PS of 1 (vs 35.4% of patients with a PS 0–1 in our analysis). Thus, real-world data from patients treated in routine clinical practice are important to supplement clinical trial data, which may overestimate real-world outcomes.48
The REAL-Oncology database represents an unselected population, which is relevant to real-world practice and enables robust analyses across numerous subgroups over a long timeframe (>10 years). Furthermore, this data source allowed the identification of clinically diagnosed patients, a population not often captured and representing here more than one-third of patients with NSCLC. However, the current study includes only data from Leeds trust and may not be representative of clinical practice elsewhere in England. Additionally, limited information was available regarding radiotherapy at the time of this analysis (date of administration, dose and type of radiotherapy) and it was therefore neither possible to formally differentiate palliative radiotherapy from radiotherapy with curative intent nor to identify the use of stereotactic ablative radiotherapy (SABR) in early-stage patients. However, the increased use of radiotherapy over time in early-stage patients with clinically diagnosed NSCLC was almost certainly due to SABR, which was available in our centre from May 2009. It is hoped that improvements to the algorithm used and the subsequent availability of more detailed data regarding radiotherapy will address this limitation. It is also acknowledged that the follow-up duration was relatively short, at 7 months, for patients diagnosed at the end of the study period. Finally, data on biomarkers and comorbidities were not available.
Our findings provide valuable insight into the real-world treatment and survival outcomes for patients in the preimmunotherapy era in Leeds and demonstrate that, irrespective of changes in treatment patterns and against a background of policy reforms, long-term survival for patients diagnosed with metastatic NSCLC remains poor. Future analyses from the REAL-Oncology database will help evaluate the impact of new TKIs and ICIs on OS for patients with NSCLC.
Supplementary Material
Acknowledgments
Professional writing and editorial assistance were provided by Lisa Jolly, PhD, of Parexel, funded by Bristol Myers Squibb.
Footnotes
Contributors: LL, CC, MMD and JRP conceived and designed the study, with contributions from MS, MT and WS. GH is the lead for the Leeds Teaching Hospital NHS Trust Real world programme, clinical lead for the Patient Pathway Manager (the Electronic Health Record) and professor of Cancer Medicine and Digital Health (University of Leeds). SC is the senior information officer working with the REAL-Oncology team at Leeds Teaching Hospital NHS Trust. MS provided expertise to REAL-Oncology regarding the management of lung cancer. MS, MT, WS and MR analysed the data. All authors contributed to the interpretation of the data, the drafting of the work and subsequent critical revision of the manuscript. MS accepts full responsibility for the work and the conduct of the study and had full access to the data.
Funding: REAL-Oncology is a collaboration between Leeds Teaching Hospital NHS Trust, the University of Leeds and IQVIA. Commercial clients of IQVIA include Bristol Myers Squibb, which funded the project this work is based on. REAL-Oncology retains all operational, scientific and communications controls.
Competing interests: GH is an employee of the University of Leeds and holds an honorary contract with Leeds Teaching Hospital NHS Trust. He leads the Leeds Teaching Hospitals NHS Trust real-world evidence team collaboration with IQVIA and, as part of this collaboration, IQVIA funds the staff who support this work. He also reports partial grant funding for a collaboration outside this study from IQVIA. MS was an employee at Leeds Teaching Hospital NHS Trust at the time of the study and he holds an honorary contract with Leeds Teaching Hospital NHS Trust. He receives consultancy fees from BMS. MS, MT and MR are employees of IQVIA. WS and SC are subcontracted to IQVIA and hold honorary contracts with Leeds Teaching Hospital NHS Trust. CC, MMD and JRP are employees of BMS. CC and JRP report stock ownership in BMS. LL was contracted (paid) as a consultant by BMS to support the I-O Optimise initiative and is an employee of Epi-Fit.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was completed with UK Health Research Authority approval through the National Institute for Health Research Integrated Research Approvals System. The research was performed in accordance with the Leeds Teaching Hospitals NHS Trust research governance framework; as a non-interventional retrospective descriptive study using existing patient records, the need for ethics approval was waived. The study was performed in accordance with the Declaration of Helsinki.
References
- 1.GLOBOCAN . Global cancer Observatory, 2018. Available: https://gco.iarc.fr/ [Accessed 16 Oct 2020].
- 2.Riessk J. Shifting paradigms in non-small cell lung cancer: an evolving therapeutic landscape. Am J Manag Care 2013;19:s390–7. [PubMed] [Google Scholar]
- 3.Birring SS, Peake MD. Symptoms and the early diagnosis of lung cancer. Thorax 2005;60:268–9. 10.1136/thx.2004.032698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmadzada T, Kao S, Reid G, et al. An update on predictive biomarkers for treatment selection in non-small cell lung cancer. J Clin Med 2018;7. 10.3390/jcm7060153. [Epub ahead of print: 15 Jun 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin H, Wang F, Liu H, et al. New advances in immunotherapy for non-small cell lung cancer. Am J Transl Res 2018;10:2234–45. [PMC free article] [PubMed] [Google Scholar]
- 6.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136:260–71. 10.1378/chest.08-0978 [DOI] [PubMed] [Google Scholar]
- 7.Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1–21. 10.1093/annonc/mdx222 [DOI] [PubMed] [Google Scholar]
- 8.Boolell V, Alamgeer M, Watkins DN, et al. The evolution of therapies in non-small cell lung cancer. Cancers 2015;7:1815–46. 10.3390/cancers7030864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2018;29:iv192–237. 10.1093/annonc/mdy275 [DOI] [PubMed] [Google Scholar]
- 10.Eichhorn F, Klotz LV, Bischoff H, et al. Neoadjuvant anti-programmed death-1 immunotherapy by pembrolizumab in resectable nodal positive stage II/IIIa non-small-cell lung cancer (NSCLC): the NEOMUN trial. BMC Cancer 2019;19:413. 10.1186/s12885-019-5624-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cascone T, William WN, Weissferdt A, et al. Neoadjuvant nivolumab (N) or nivolumab plus ipilimumab (Ni) for resectable non-small cell lung cancer (NSCLC): clinical and correlative results from the NEOSTAR study. JCO 2019;37:8504. 10.1200/JCO.2019.37.15_suppl.8504 [DOI] [Google Scholar]
- 12.Owen D, Chaft JE. Immunotherapy in surgically resectable non-small cell lung cancer. J Thorac Dis 2018;10:S404–11. 10.21037/jtd.2017.12.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekman S, Griesinger F, Baas P, et al. I-O optimise: a novel multinational real-world research platform in thoracic malignancies. Future Oncol 2019;15:1551–63. 10.2217/fon-2019-0025 [DOI] [PubMed] [Google Scholar]
- 14.American Joint Committee on Cancer . Cancer staging manual. 6th ed. Springer Science+Business Media, New York, 2002. [Google Scholar]
- 15.Detterbeck FC, Boffa DJ, Kim AW, et al. The eighth edition lung cancer stage classification. Chest 2017;151:193–203. 10.1016/j.chest.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 16.Royal College of Physicians . National lung cancer audit annual report 2017 (audit period 2016), 2018. [Google Scholar]
- 17.Verleye L, De Gendt C, Vrijens F, et al. Patterns of care for non-small cell lung cancer patients in Belgium: a population-based study. Eur J Cancer Care 2018;27. 10.1111/ecc.12747. [Epub ahead of print: 18 08 2017]. [DOI] [PubMed] [Google Scholar]
- 18.Caballero Vázquez A, García Flores P, Romero Ortiz A, et al. Changes in non-small cell lung cancer diagnosis, molecular testing and prognosis 2011-2016. J Thorac Dis 2018;10:5468–75. 10.21037/jtd.2018.08.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Department of Health . Cancer reform strategy, 2007. Available: https://www.nhs.uk/NHSEngland/NSF/Documents/Cancer%20Reform%20Strategy.pdf [Accessed 16 Oct 2020].
- 20.National Cancer Registration and Analysis Service . National cancer equality initiative, 2015. Available: http://www.ncin.org.uk/cancer_type_and_topic_specific_work/topic_specific_work/equality [Accessed 16 Oct 2020].
- 21.Cancer Research UK . National awareness and early diagnosis initiative (NAEDI), 2008. Available: https://www.cancerresearchuk.org/sites/default/files/health_professional_naedi_briefing_sheet.pdf [Accessed 16 Oct 2020].
- 22.Exarchakou A, Rachet B, Belot A, et al. Impact of national cancer policies on cancer survival trends and socioeconomic inequalities in England, 1996-2013: population based study. BMJ 2018;360:k764. 10.1136/bmj.k764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy MPT, Cheyne L, Darby M, et al. Lung cancer stage-shift following a symptom awareness campaign. Thorax 2018;73:1128–36. 10.1136/thoraxjnl-2018-211842 [DOI] [PubMed] [Google Scholar]
- 24.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020;382:503–13. 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 25.National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg 2009;15:4–9. [PubMed] [Google Scholar]
- 27.Ekman S, Horvat P, Rosenlund M, et al. Epidemiology and survival outcomes for patients with NSCLC in Scandinavia in the Preimmunotherapy era: a SCAN-LEAF retrospective analysis from the I-O optimise initiative. JTO Clin Res Rep 2021;2:100165. 10.1016/j.jtocrr.2021.100165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyi H, Hillerdal G, Andersson O, et al. Lung cancer among native and foreign-born Swedes: histopathology, treatment, and survival. Acta Oncol 2016;55:1344–8. 10.1080/0284186X.2016.1189095 [DOI] [PubMed] [Google Scholar]
- 29.Department of Health . The impact of patient age on clinical decision-making in oncology, 2012. Available: http://www.ncin.org.uk/cancer_type_and_topic_specific_work/topic_specific_work/equality [Accessed 16 Oct 2020].
- 30.Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004-2007. Thorax 2013;68:551–64. 10.1136/thoraxjnl-2012-202297 [DOI] [PubMed] [Google Scholar]
- 31.Khakwani A, Rich AL, Powell HA, et al. Lung cancer survival in England: trends in non-small-cell lung cancer survival over the duration of the National lung cancer audit. Br J Cancer 2013;109:2058–65. 10.1038/bjc.2013.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Royal College of Physicians . National lung cancer audit annual report 2018 (audit period 2017), 2019. [Google Scholar]
- 33.Lau KKW, Rathinam S, Waller DA, et al. The effects of increased provision of thoracic surgical specialists on the variation in lung cancer resection rate in England. J Thorac Oncol 2013;8:68–72. 10.1097/JTO.0b013e3182762315 [DOI] [PubMed] [Google Scholar]
- 34.Walters S, Benitez-Majano S, Muller P, et al. Is England closing the International gap in cancer survival? Br J Cancer 2015;113:848–60. 10.1038/bjc.2015.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Service NH . National lung cancer audit: 2012 patient cohort, 2013. [Google Scholar]
- 36.Driessen EJM, Schulkes KJG, Dingemans A-MC, et al. Patterns of treatment and survival among older patients with stage III non-small cell lung cancer. Lung Cancer 2018;116:55–61. 10.1016/j.lungcan.2017.12.013 [DOI] [PubMed] [Google Scholar]
- 37.Aupérin A, Le Péchoux C, Rolland E, et al. Meta-Analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181–90. 10.1200/JCO.2009.26.2543 [DOI] [PubMed] [Google Scholar]
- 38.Huber RM, De Ruysscher D, Hoffmann H, et al. Interdisciplinary multimodality management of stage III nonsmall cell lung cancer. Eur Respir Rev 2019;28. 10.1183/16000617.0024-2019. [Epub ahead of print: 30 Jun 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veit P, Ruehm S, Kuehl H, et al. Lymph node staging with dual-modality PET/CT: enhancing the diagnostic accuracy in oncology. Eur J Radiol 2006;58:383–9. 10.1016/j.ejrad.2005.12.042 [DOI] [PubMed] [Google Scholar]
- 40.Felip E, Stahel RA, Pavlidis N, et al. ESMO minimum clinical recommendations for diagnosis, treatment and follow-up of non-small-cell lung cancer (NSCLC). Ann Oncol 2005;16 Suppl 1:i28–9. 10.1093/annonc/mdi821 [DOI] [PubMed] [Google Scholar]
- 41.Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii27–39. 10.1093/annonc/mdu199 [DOI] [PubMed] [Google Scholar]
- 42.NHS England . National cancer drugs fund list, 2019. Available: https://www.england.nhs.uk/wp-content/uploads/2017/04/national-cdf-list-v1.148.pdf [Accessed 16 Oct 2020].
- 43.MHRA . Expired early access to medicines scheme scientific opinions, 2019. Available: https://www.gov.uk/government/publications/early-access-to-medicines-scheme-expired-scientific-opinions/expired-early-access-to-medicines-scheme-scientific-opinions [Accessed 16 Oct 2020].
- 44.Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med 2020;383:640–9. 10.1056/NEJMoa1916623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peters BJM, Cramer-Vd Welle CM, Smit AAJ, et al. Trends in prescribing systemic treatment and overall survival for non-small cell lung cancer stage IIIB/IV in the Netherlands: 2008-2012. Cancer Epidemiol 2017;51:1–6. 10.1016/j.canep.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 46.Holmberg L, Sandin F, Bray F, et al. National comparisons of lung cancer survival in England, Norway and Sweden 2001-2004: differences occur early in follow-up. Thorax 2010;65:436–41. 10.1136/thx.2009.124222 [DOI] [PubMed] [Google Scholar]
- 47.Pilkington G, Boland A, Brown T, et al. A systematic review of the clinical effectiveness of first-line chemotherapy for adult patients with locally advanced or metastatic non-small cell lung cancer. Thorax 2015;70:359–67. 10.1136/thoraxjnl-2014-205914 [DOI] [PubMed] [Google Scholar]
- 48.Lakdawalla DN, Shafrin J, Hou N, et al. Predicting real-world effectiveness of cancer therapies using overall survival and progression-free survival from clinical trials: empirical evidence for the ASCO value framework. Value Health 2017;20:866–75. 10.1016/j.jval.2017.04.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-046396supp001.pdf (124.6KB, pdf)
Data Availability Statement
No data are available.