Abstract
KRASG12C inhibitors, including MRTX849, are promising treatment options for KRAS-mutant non–small cell lung cancer (NSCLC). PD-1 inhibitors are approved in NSCLC; however, strategies to enhance checkpoint inhibitor therapy (CIT) are needed. KRASG12C mutations are smoking-associated transversion mutations associated with high tumor mutation burden, PD-L1 positivity, and an immunosuppressive tumor microenvironment. To evaluate the potential of MRTX849 to augment CIT, its impact on immune signaling and response to CIT was evaluated. In human tumor xenograft models, MRTX849 increased MHC class I protein expression and decreased RNA and/or plasma protein levels of immunosuppressive factors. In a KrasG12C-mutant CT26 syngeneic mouse model, MRTX849 decreased intratumoral myeloid-derived suppressor cells and increased M1-polarized macrophages, dendritic cells, CD4+, and CD8+ T cells. Similar results were observed in lung KrasG12C-mutant syngeneic and a genetically engineered mouse (GEM) model. In the CT26 KrasG12C model, MRTX849 demonstrated marked tumor regression when tumors were established in immune-competent BALB/c mice; however, the effect was diminished when tumors were grown in T-cell–deficient nu/nu mice. Tumors progressed following anti–PD-1 or MRTX849 single-agent treatment in immune-competent mice; however, combination treatment demonstrated durable, complete responses (CRs). Tumors did not reestablish in the same mice that exhibited durable CRs when rechallenged with tumor cell inoculum, demonstrating these mice developed adaptive antitumor immunity. In a GEM model, treatment with MRTX849 plus anti–PD-1 led to increased progression-free survival compared with either single agent alone. These data demonstrate KRAS inhibition reverses an immunosuppressive tumor microenvironment and sensitizes tumors to CIT through multiple mechanisms.
Introduction
The discovery of clinically active KRASG12C inhibitors provides a much-needed targeted therapy for patients with this mutation (1, 2). KRASG12C mutations are present in approximately 14% of non–small cell lung cancer (NSCLC), approximately 4% of colorectal cancer and several other cancers at lower frequency. The treatment paradigm for several cancers has recently been transformed by checkpoint inhibitor therapy (CIT) targeting the PD-1/PD-L1 pathway which now constitutes a front-line treatment option as a single agent or in combination with chemotherapy and/or other therapies in NSCLC (3-6). In NSCLC, initial data demonstrated single-agent CIT was effective and durable; however, only in a subset of patients, which triggered a major effort to identify patients most likely to respond and to identify combinations with increased activity.
KRASG12C mutations are smoking-induced transversion mutations found in tumors with high tumor mutation burden (TMB), a genomic biomarker associated with increased response to CIT (7, 8). Mutant KRAS is linked to PD-L1 expression and oncogenic RAS signaling promotes immune resistance by upregulating PD-L1 expression (9). Available clinical data suggest CIT is effective in KRAS-mutant NSCLC; however, additional strategies are needed to improve the activity of this therapeutic modality (10, 11). These data highlight the molecular features and tumor-immune biology driven by KRASG12C mutations in NSCLC and suggest inhibition of KRAS may work together with PD-1–targeted therapy to improve activity of either single agent.
Although combinations involving CIT plus targeted therapies against other oncogenic drivers have not been successful in the clinic to date, there is strong biological rationale to suggest KRAS inhibition may complement CIT when utilized in combination. Studies conducted in GEM models exhibiting spontaneous tumors driven by mutated KRAS demonstrated oncogenic KRAS orchestrates an immunosuppressive tumor microenvironment and is responsible for CIT resistance (12, 13). In these models, KRAS activation leads to the increased secretion of IL23, CCL9, VEGFA, and CXCL3 by tumor cells which recruits immunosuppressive macrophages and myeloid-derived suppressor cells (MDSC) into the tumor microenvironment resulting in the exclusion of adaptive T and B cells in a PD-L1–dependent manner. Resistance to anti–PD-1 therapy in KRAS-mutant tumors was overcome through inhibition of downstream KRAS signaling through the IRF2 and Myc pathways (12, 13). The regulation of VEGF by RAS has been primarily characterized as a mechanism to upregulate tumor angiogenesis (14-16). However, the ability of VEGF to regulate immune suppression via recruitment of immature dendritic cells and MDSCs is another mechanism of tumor promotion and the VEGF pathway is currently being targeted for the treatment of several cancers, including in combination with CIT (17-19). The regulation of the cytokines IL6 and IL8 by RAS further contribute to angiogenesis and an aggressive phenotype via paracrine signaling (20, 21). Blocking oncogenic RAS was also shown to enhance tumor cell surface expression of MHC class I expression (22). Reexpression of antigen presentation machinery is likely required to elicit a maximal antitumor immune response.
In this study, various mouse models were used to elucidate the molecular and cellular effects of KRASG12C inhibition on tumor and immune biology. These data demonstrate inhibition of KRASG12C indeed reverses immunosuppressive signaling through regulation of key immune signaling factors, and, in addition, induces dramatic reconditioning of the tumor immune microenvironment. Importantly, KRASG12C inhibition and anti–PD-1 therapy induced durable tumor regressions and endowed animals with antitumor immunity, providing strong rationale to test this combination clinically.
Material and Methods
Compounds and reagents
MRTX849 was synthesized at WuXi AppTec (lot No. EW5243-1094-P1). MRTX849 in powder form was stored at room temperature and protected from light. MRTX849 was formulated at 100 mg/kg with 10% Captisol (Cydex Pharmaceuticals lot No. NC-04A-180185) in 50 mmol/L citrate buffer pH 5.0 once per week and the dosing solution was stored at 4°C protected from light. MRTX849 was administered by oral gavage (orally) daily.
Anti-mouse PD-1 antibody antagonist clone 29F.1A12 (catalog No. BP0273, lot No. 737319J2) and the isotype control antibody, rat IgG2a (catalog No. BP0089, lot No. 686318F1B) were obtained from Bio X Cell. Both reagents were diluted in In VivoPure Dilution Buffer pH 7.0 (catalog No. IP0070, lot No. 708919F1) obtained from Bio X Cell and administered at 10 mg/kg. Dosing solutions were made fresh for each dose. Both the anti-mouse PD-1 antibody and the rat IgG2a isotype control antibody was administered intraperitoneally.
The CT26.WT (CT26) cell line (ATCC CRL-2638) was maintained in vitro with RPMI1640 medium (No. 11875-093), penicillin/streptomycin (No. 15070-063), HEPES [(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), No. 15630-080], Sodium Pyruvate (No. 11360-070) obtained from Gibco/Thermo Fisher Scientific and FBS obtained from Corning (No. 35-011-CV, Corning) and Nucleus Biologics (1824-001). The LL/2 cell line (ATCC CRL-1642) was maintained in DMEM and supplemented with FBS. The lab tested for Mycoplasma every few months using a commercially available test [MycoAlert Detection kit (Lonza); catalog No. LT07-418). CRISPR-engineered cell lines underwent single-cell cloning therefore cell line clones underwent several passages during scale up and during the course of these studies.
CRISPR/Cas9 engineering mouse syngeneic KrasG12C-mutant tumor cell lines
The mouse colon cancer cell line CT26 was genetically modified to express KRASG12C. The CT26 cell line is triallelic for the Kras gene and each allele harbors a mutation at codon 12 that changes a glycine residue (G) to an aspartic acid residue (D; ref. 23). To create a CT26 cell line derivative harboring the KrasG12C mutation, the G12D codon was changed to a G12C codon using a Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated 9 (CRISPR/Cas9) system (Synthego) in the following manner. A synthetically modified single-guide ribonucleic acid (sgRNA) targeting the region near the Kras 12 codon was designed and synthesized on the basis of high specificity and propensity to create double strand breaks when complexed with the Cas9 DNA endonuclease. A single-stranded oligodeoxynucleotide (ssODN) was designed to enable homologous donor repair at the site of the sgRNA cut site and introduce the desired cysteine codon (GAT: D to TGT: C) at position 12 while also introducing silent mutations to prevent recutting. Cas9/sgRNA riboprotein complexes and ssODN were transfected into CT26 cells. sgRNA and ssODN sequences are provided in Supplementary Data. Single-cell CT26 clones were isolated, and their genotypes were screened by Sanger DNA sequencing and Inference of CRISPR Edits (ICE) analysis (Synthego) to identify homozygous G12C-targeted clones. The CT26 KrasG12C E3 clone was selected for in vivo studies.
The mouse lung cancer cell line LL/2 harbors KrasG12C and NrasQ61H mutations and was genetically modified to no longer express functional Nras. Nras-targeting sgRNAs complexed with Cas9 protein were transfected into LL/2 cells and single-cell clones were grown up and screened to identify clones that harbored inactivating mutations in Nras.
Immunoblotting
Cells and tumors were lysed with either RIPA (Thermo Fisher Scientific, No. 8990) or Lysis/Binding Buffer AM11 (Active Motif, No. 52097) with protease and phosphatase inhibitors added fresh before use. Cells in 10-cm plates were treated as described previously, or tumor fragments were snap frozen in homogenization tubes (Omni, No. 19-628-3) after treatment as described previously. Tumor fragments were lysed with approximately 500-μL lysis buffer added and homogenized with three rounds of shaking, 20 seconds each, on ice (MPBio, FastPrep-24 system). Cell pellets were collected by scraping on ice with PBS with protease and phosphatase inhibitors before transferring to a 1.5-mL microcentrifuge tube and spun in a centrifuge at 14,000 × g for 20 seconds at 4°C. Cell pellets were then snap frozen on dry ice before lysing with lysis buffer. Cell lysates were left on ice for 30 minutes and vortexed four times to ensure complete lysis. Cell and tumor lysates were spun in a centrifuge at 14,000 × g for 15 minutes at 4°C. All sample supernatants were stored at −80°C. For immunoblotting, 30 μg of protein were loaded onto a 12% Bis-Tris gel (Bio-Rad, No. 3450118) and run according to manufacturer instructions with the Precision Plus Protein Kaleidoscope Prestained Protein Standards (Bio-Rad, No. 1610375). Proteins were transferred onto Nitrocellulose membrane (Life Technologies, No. IB23001) using the IBlot Dry Blotting system (Life Technologies). Primary antibodies were added overnight at 4°C with rocking. KRAS antibody was purchased from Sigma-Aldrich (product No. SAB1404011). pERK (9101L), pS6 (2211L), ERK (9102L), S6 (2217L), and Alpha tubulin (3873S) antibodies were purchased from Cell Signaling Technology. The next day, the primary antibodies were removed, blots were washed five times with 1× TBS + 0.1% Tween-20, and solutions consisting of LI-COR IRDye secondary antibodies were added for 2 hours at room temperature with shaking. After secondary incubation, the wells were washed again and imaged using the LI-COR Odyssey CLx Imaging system (LI-COR) set to the AutoScan channel for both the 700 and 800 wavelength channels to measure the signal intensity from the IRDye 680RD goat anti-rabbit and IRDye 800CW goat anti-mouse secondary antibodies, respectively. Images were imported into LiCor's Image Studio software version 4.0 and then .tif files were exported to Windows Photo Viewer.
Cell proliferation assays
Cells were plated in 96-well white-walled plates (No. 3917, Corning) for cell viability determination in standard tissue culture conditions. The day after plating, serial dilutions of MRTX849 to evaluate a concentration-dependent effect on cell viability were made using Rainin multichannel pipettes. A total of 72 hours later, the CellTiter-Glo Luminescent Cell Viability Assay (No. G7573, Promega) was used to measure cell viability effects of MRTX849. Plates were read where luminescence was measured using a ClarioStar plate reader (BMG Labtech). Data were analyzed and IC50 values were determined using GraphPad Prism (GraphPad). Viable cells were counted at the beginning of the assay using a Cellometer Mini (Nexcelom Bioscience) using Trypan Blue exclusion so as to plate the same number of viable cells per well at the beginning of the experiment.
Generation and therapeutic treatment of KrasG12C-mutant genetically engineered mouse models
All genetically engineered mouse model (GEMM) studies, including breeding and treatment studies, were performed with approval of the Institutional Animal Care and Use Committee (IACUC) at New York University School of Medicine (New York City, NY). The GEM harbors a conditional activating mutation of hKRASG12C at the collagen I locus (Li and colleagues, 2018). p53R270H (mutant omologous to human p53R273H) and Msh2tm2.1Rak (MSH2 deletions for studying DNA mismatch repair) were obtained from The Jackson laboratory. CRE recombinase was induced in 6-week-old littermates through intranasal inhalation of 2.5 × 106 pfu adeno-Cre (University of Iowa adenoviral core) for LSL-KRASG12C Trp53R270H GEMM (KCP) and 1 × 107 pfu for the LSL-KRASG12C Msh2fl/f (KCM). Lung adenocarcinoma appeared 6 weeks after induction for KCP, and 12 weeks for KCM. Once MRI showed the tumor size reached 300 to 400 mm3, mice were randomly assigned to treatment groups. No gender bias between male and female mice was observed regarding to the tumor growth and response to drug treatment. Mice were treated with MRTX1257 50 mg/kg once daily by oral gavage, either alone or in combination with anti-PD1 10 mg/kg three times a week by intraperitoneally. Vehicle control and isotype control were included as well. For efficacy studies, GEMMs were dosed with a low dose of 10 mg/kg MRTX849 and dosed continuously for long term and monitored by MRI every 2 weeks. For immune profiling studies, GEMMs were dosed for 6 days. Progression-free survival (PFS) was analyzed based on the standard criteria in clinical trials. Briefly, PFS was the duration between treatment start and progression, which was defined by increase of tumor size compared with the previous scan of MRI and the appearance of new lesions. Survival data were calculated using Kaplan–Meier log-rank test.
Syngeneic mouse model studies
All mouse studies were conducted in compliance with all applicable regulations and guidelines of the IACUC from the NIH. Mice were maintained under pathogen-free conditions, and food and water were provided ad libitum. Eight- to 10-week-old female BALB/c mice (Envigo) were injected subcutaneously with CT26 KrasG12C tumor cells in 100 μL of PBS and Matrigel matrix (Corning No. 356237; Discovery Labware) in the right hind flank with 1.0e6 cells 50:50 cells: Matrigel. Rechallenge mice were injected subcutaneously with tumor cells in 100 μL of PBS and Matrigel matrix in the left hind flank with 1.0e6 cells 50:50 cells:Matrigel. Six-week-old female C57Bl/6 mice (Charles River Laboratories) were injected subcutaneously with LL/2 KrasG12C cells in 100 μL of PBS and Matrigel matrix (Corning No. 356237; Discovery Labware) in the right hind flank with 1.0e6 cells 50:50 cells:Matrigel. Mouse health was monitored daily, and caliper measurements began when tumors were palpable. Tumor volume measurements were determined utilizing the formula 0.5 × L × W2 in which L refers to length and W refers to width of each tumor. For the combination efficacy study, when tumors reached an average tumor volume of approximately 220 mm3, mice were randomized into four treatment groups, with 10 mice per treatment group. MRTX849 was administered orally, daily and the PD-1 antibody was administered intraperitoneally, every 3 days for three doses. Both MRTX849 and PD-1 were administered alone and in combination. Control mice were dosed by oral gavage with the Captisol oral vehicle and dosed intraperitoneally with the isotype control antibody, every 3 days for three doses. Tumors were measured three times per week and body weights were measured two times per week. For the pharmacodynamic FACS study, when CT26 KrasG12C tumors reached an average tumor volume between 220 and 730 mm3, mice were randomized into treatment groups, with four mice per group. MRTX849 was administered orally, daily for four or eight doses depending on the treatment group and the PD-1 antibody was administered intraperitoneally, every 3 days for two or three doses depending on the treatment group. Both MRTX849 and PD-1 were administered alone and in combination. Control mice were dosed by oral gavage with the Captisol oral vehicle and dosed intraperitoneally with the isotype control antibody. Tumors were measured at randomization and termination; body weights were measured twice weekly.
Flow cytometry
Tumors were removed 3 hours after the last dose on day 4 or day 8 and placed into Vitacor (formerly Lifor Solution; Detraxi) then shipped to MI Bioresearch overnight on wet ice and processed using a mouse Tumor Dissociation Kit form Miltenyi Biotec. Ice-cold solid tissues were weighed, minced, and placed into a gentleMACS C Tube containing a dissociation enzyme cocktail in culture medium. The cells were resuspended in cell staining buffer and transferred to a new 96-well plate containing prediluted antibodies. The samples were incubated for 30 minutes protected from light at 4°C. The cells were resuspended in ice-cold cell staining buffer and acquired on the Attune NxT Acoustic Focusing Cytometer. Antibodies used targeted CD45, CD3, CD4, CD8, PD-1, CD49b/CD335, CD69, CD25, FoxP3, CD11b, Ly-6C, Ly-6G, F4/80, MHCII, CD24, CD206, CD19, CD11c, Absolute Counting Beads, Ki-67 Viability.
For GEMM studies, mice were euthanized after 6 days of dosing, tumors microdissected, and tissues fixed for IHC or dissociated to single-cell suspensions for flow cytometry analysis. Tumors were mechanically separated and enzymatically dissociated with collagenase/DNAase for 30 minutes at 37°C. Cells were filtered through a 70 μm cell strainer and red blood cells were lysed with RBC Lysis Buffer (BioLegend) for 5 minutes at room temperature. Cells were resuspended in PBS/2% FBS/1 mmol/LEDTA staining buffer and incubated with surface staining antibodies for 1 hour at room temperature. Cells were washed with staining buffer and resuspended in Fixation/Permeabilization Buffer (BioLegend) for 30 minutes, washed, and incubated with intracellular staining antibodies in 1 × Permeabilization Buffer (BioLegend) for 1 hour at room temperature. Cells were washed and resuspended with staining buffer, and acquired on the BD LSRII Fortessa Cytometer. Antibodies used targeted CD45, CD3, CD4, CD8, CD44, CD62L, PD-1, TIM-3, FOXP3, CTLA4, Ki67, CD11b, CD11c, Ly-6C, Ly-6G, F4/80, Dx5, CD19, and Zombie Aqua Live/Dead Viability stain.
Results
KRASG12C inhibition modifies tumor cell–intrinsic factors that regulate antigen presentation and an immunosuppressive tumor microenvironment
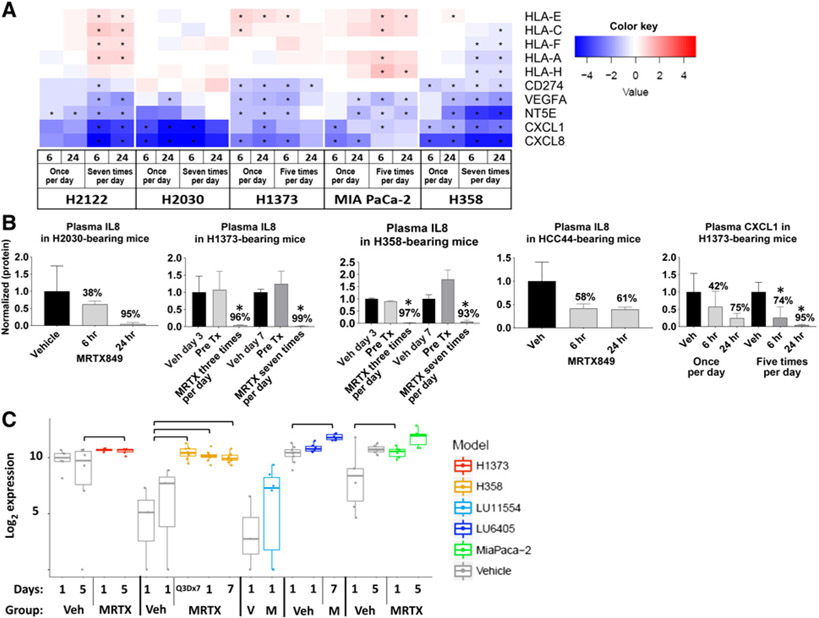
To determine the effects of KRASG12C inhibition on expression of key genes implicated in the response to PD-1 inhibition by tumor cells, RNA sequencing (RNA-seq) was performed on bulk tumor and human RNA expression was assessed in five KRASG12C-mutant human xenograft tumor models grown in immune-compromised mice treated with vehicle or 100 mg/kg of MRTX849. In a previous study, repeat dose administration of MRTX849 demonstrated significant tumor growth inhibition in each of these models (2). VEGFA, NT5E, CXCL1, and CXCL8 were significantly downregulated in MRTX849-treated tumors in all five models compared with vehicle-treated tumors following a single or repeat dosing after 6 and 24 hours after last dose (Fig. 1A). In addition, MHC genes including HLA-E, HLA-C, HLA-F, HLA-A and HLA-H were significantly upregulated at one or more timepoints in a subset of models, while CD274 was significantly downregulated in three models. Single or repeat dose administration of MRTX849 also significantly decreased the plasma concentration of IL8 (CXCL8 gene) and CXCL1 protein in mice bearing selected xenograft models (Fig. 1B) demonstrating reduced concentration of key immune signaling molecules in the tumor microenvironment as well as systemic circulation. As MRTX849 is in active clinical development, these circulating factors may represent a noninvasive method of monitoring KRAS inhibition in the clinic. The expression of MHC class I protein levels (MHC class I antibody raised against amino acids 63–362 of human HLA-B) was also increased following MRTX849 treatment in several xenograft models as measured by reverse phase protein array analysis across a panel of models (Fig. 1C; ref. 24). Increased expression of MHC class I protein expression is predicted to enable recognition of tumor cell antigens by the immune system.
Figure 1.
KRASG12C regulates antigen presentation and an immunosuppressive tumor microenvironment in a tumor cell–intrinsic manner. A, Heatmap of log2 fold changes in RNA expression from RNA-seq data of selected immune-modulating genes in human tumor xenografts established in immune-compromised mice dosed orally with MRTX849 once every day, five times per day or seven times per day and harvested 6 or 24 hours after administration relative to expression in vehicle-treated tumors. “*” indicates FDR < 0.05, n = 3 per treatment group. B, Plasma concentration of selected secreted immune-modulating proteins in immune-compromised mice harboring human tumor xenografts orally administered vehicle or MRTX849 every day or for the number of days indicated. Plasma was collected 24 hours after last dose for the H1373 and H358 models and as indicated for other models. “*” indicates Student t test, P = 0.05, MRTX849-treated plasma compared with vehicle-treated plasma, n = 3 per treatment group. Veh = vehicle; Pre Tx = pretreatment. C, log2 expression of human MHC class I protein in human xenograft models established in immune-compromisedmice orallyadministered vehicle or 100 mg/kg MRTX849 once every day, five times per day or seven times per day. Presence of a bar indicates statistically significant difference between indicated groups by Student t test, P < 0.05, n = 3 per treatment group. Two different vehicle-treated tumor volume groups (300 and 500 mm3) were used for the H358 study.
MRTX849 covalently modifies KRASG12C, inhibits KRAS-dependent signaling, and demonstrates tumor regression in CRISPR/Cas9-engineered CT26 KrasG12C tumors
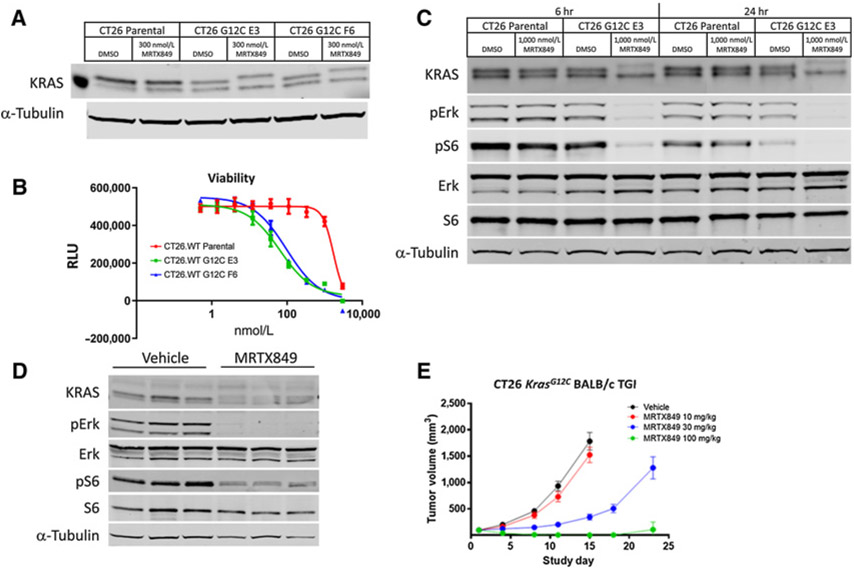
To explore the impact of KRASG12C inhibition by MRTX849 and to interrogate the mechanism of response to treatment in an immune-competent tumor model, the CT26.WT cell line was engineered using CRISPR/Cas9 to replace the endogenous KrasG12D alleles with KrasG12C (Supplementary Fig. S1). The KRASG12C protein in two CT26 KrasG12C cell clones exhibited an upward shift on a Western blot analysis when treated with 300 nmol/L MRTX849 for 24 hours confirming the KRASG12C protein was expressed and covalently modified by MRTX849 treatment (Fig. 2A). In a 3-day viability assay, MRTX849 demonstrated growth inhibition of the two CT26 KrasG12C cell clones with IC50 values of 65 and 96 nmol/L compared with parental cells, which were relatively insensitive (IC50 = 1.7 μmol/L; Fig. 2B). Clone E3 was selected for further characterization in vitro and in vivo. Treatment of CT26 KrasG12C E3 clone cells with 1 μmol/L MRTX849 for 6 or 24 hours in vitro correlated with strong inhibition of phosphorylated ERK (pERK, Thr202/Tyr204) and phosphorylated S6 (pS6, Ser235/236) as expected following near complete modification of KRASG12C (Fig. 2C). To evaluate whether antigen presentation machinery were upregulated following KRASG12C inhibition, CT26 KrasG12C cell were treated with 300 nmol/L MRTX849 and H-2Dd surface protein was increased as assessed by flow cytometry (Supplementary Fig. S2A).
Figure 2.
CRISPR/Cas9-engineered CT26 KrasG12C cells and tumors are sensitive to MRTX849 treatment. A, Parental CT26.WT and G12C-engineered CT26.WT cell lines (CT26 KrasG12C) derived from single-cell clones (clone E3 and F6) were incubated with 300 nmol/L of MRTX849 for 24 hours and probed via immunoblot with total anti-KRAS and anti-α-tubulin antibodies. The increased molecular weight of KRASG12C in the MRTX849-treated lane is indicative of covalent KRASG12C modification. The molecular weight of the standard protein to the left of the DMSO-treated parental lysate is 20 kD. B, A Cell Titer-Glo viability assay was performed on parental and CT26 KrasG12C E3 and F6 cell line clones treated with MRTX849 in vitro for 3 days. C, Parental CT26.WT and CT26 KrasG12C clone E3 cells were incubated with 1,000 nmol/L of MRTX849 for 6 or 24 hours and lysates were probed via immunoblot with anti-KRAS, pERK (Thr202/Tyr204), ERK, pS6 (Ser235/236), S6 or α-tubulin. D, 100 mg/kg MRTX849 was administered orally to mice bearing CT26 KrasG12C E3 tumors. Tumors were harvested 6 hours after dosing and lysates were probed via immunoblot with anti-KRAS, pERK (Thr202/Tyr204), ERK, pS6 (Ser235/236), S6 or α-tubulin. E, 10, 30, and 100 mg/kg MRTX849 was administered orally every day to BALB/cmice bearing established CT26 KrasG12C tumors (n = 7 per treatment group; average starting tumor volume ~100mm3) and tumor volume was measured over the study at indicated days. Average tumor volume ± SEM shown.
CT26 KrasG12C E3 tumors were grown in immune competent BALB/c mice and dramatic KRASG12C modification and inhibition of pERK (Thr202/Tyr204) and pS6 (Ser235/236) was observed 6 hours following a single oral dose of 100 mg/kg MRTX849 (Fig. 2D). In repeat dose efficacy studies in immune-competent BALB/c mice, treatment with 100 mg/kg/day MRTX849 led to complete responses and treatment with 30 mg/kg/day led to strong tumor growth inhibition confirming CT26 KrasG12C tumors were highly dependent on KRAS signaling in vivo (Fig. 2E). H-2Dd expression was also upregulated in CT26 KrasG12C tumors from mice dosed for 4 days with MRTX849 100 mg/kg compared with vehicle treated mice (Supplementary Fig. S2B).
MRTX849 treatment reconditions the tumor microenvironment in KrasG12C syngeneic models
To characterize the effects of MRTX849 on tumor immune cell populations, CT26 KrasG12C tumor-bearing BALB/c mice were treated with vehicle or MRTX849 at 100 mg/kg every day for 4 or 8 days. Intact tumors were dissociated and then analyzed for cells of myeloid and lymphoid lineage using flow cytometry. The relative abundance of intratumoral monocytic and granulocytic myeloid-derived suppressor cells (MDSCs) was decreased whereas the fraction of immune-promoting M1-polarized macrophages was increased in both MRTX849 and MRTX849 plus anti–PD-1–treated tumors compared with vehicle at 4- and/or 8-day time points (Fig. 3A). An increase in antigen-presenting dendritic cells was also observed in MRTX849 and MRTX849 plus anti–PD-1–treated tumors at 4 days. In addition, several intratumoral lymphoid populations of T-cell origin were increased at both 4 and 8 days including CD3+ lymphocytes, CD4+ T cells, CD4+ Th cells, T regulatory cells (Tregs), and natural killer (NK) T cells in MRTX849 and MRTX849 plus anti–PD-1–treated tumors compared with vehicle (Fig. 3B). CD19+ B cells were increased in the MRTX849 groups at 8 days of treatment. CD8+ T cells were also significantly increased by MRTX849 at 4 days and a trend toward increased CD8+ T cells were observed at 8 days and in PD-1 and combination-treated mice from both days. The magnitude of the effects on immune cell populations between MRTX849 single agent and MRTX849 plus anti–PD-1–treated tumors were similar and demonstrate significant immune cell changes following combination treatment appear as mostly driven through blockade of KRASG12C. These data demonstrate mutant KRASG12C is responsible for actively driving immune suppression and that inhibition of KRAS markedly shifts the immune cell repertoire from an immunosuppressive state toward an antitumor immune supportive response.
Figure 3.
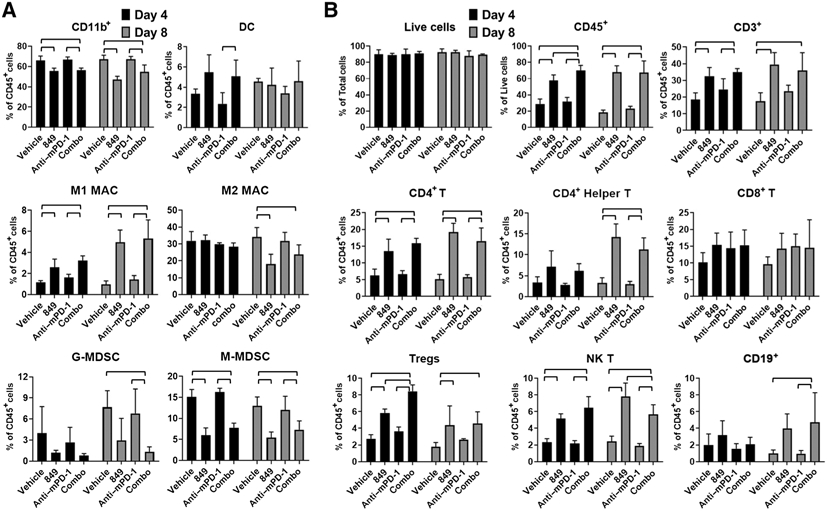
MRTX849 and combined MRTX849 and anti–PD-1 administration markedly alters immune cell populations in the tumor microenvironment. MRTX849 (100 mg/kg), rat IgG2a isotype Control antibody (10 mg/kg, Bio X cell lot No. 686318F1B), and mouse PD-1 antibody at 10 mg/kg (Bio X cell clone 29F.1A12) were administered orally, daily (MRTX849) or intraperitoneally, every 3 days (PD-1 and isotype control) or in combination to mice bearing established, subcutaneous CT26 KrasG12C tumors (n = 4 per treatment group; average starting tumor volume approximately 220 mm3 (vehicle and PD-1–treated cohorts) or approximately 730 mm3 (MRTX849 or combination-treated cohorts. Intratumoral myeloid (A) and lymphoid (B) populations were analyzed in excised disaggregated tumors by flow cytometry following 4 or 8 days of treatment administration. Analyzed cell populations include DC—dendritic cell, M1MAC—M1-polarized macrophage, M2MAC—M2-polarized macrophage, G-MDSC—granulocytic myeloid-derived suppressor cell, M-MDSC—monocytic MDSC, Tregs—T regulatory cells, NK T—natural killer T cells denoted by cell lineage–specific markers including CD11b, CD45, CD3, CD4, CD8, etc. (see Supplementary Methods). Flow cytometry analysis on single-cell suspensions of CT26 KRASG12C tumors treated with vehicle or MRTX849 was run twice and data were similar between experiments. Bars denotes statistical significance by one-way ANOVA with post hoc Tukey test.
To evaluate the effect of KRASG12C inhibition on immune response in an additional tumor model, the LL/2 mouse syngeneic lung cancer cell line harboring KrasG12C and NrasQ61H mutations was employed (25). After deleting the Nras gene using CRISPR, LL/2 tumors were grown in immune-competent C57Bl/6 mice and treated with MRTX849 at 100 mg/kg every day or vehicle or 4 or 8 days and tumors were disaggregated and collected for flow cytometry. Tumors treated with MRTX849 decreased in volume over this sample collection time course (Supplementary Fig. S3A). From the myeloid cell flow cytometry analysis, M1-polarized macrophages were increased at each time point, while monocytic MDSCs were reduced at day 4 and granulocytic MDSCs were reduced at day 8 (Supplementary Fig. S3B). Furthermore, populations of T-cell origin including CD3+, CD4+, CD4+ Th, CD8+, Treg, NK, and NKT cell populations were all increased at day 4 and/or day 8 (Supplementary Fig. S3C). The alterations in immune cell populations in KrasG12C-mutant LL/2 tumors following treatment with MRTX849 are similar to the effects observed in the CT26 KrasG12C model and further suggest KRAS inhibition leads to a marked reversal of the immunosuppressive program mediated by oncogenic KrasG12C.
To further characterize T-cell populations in CT26 KrasG12C tumors following single-agent and combination therapy, T-cell receptor beta (TCRB) sequencing was performed on tumors from vehicle, MRTX849, anti–PD-1 antibody and combination-treated mice. A highly significant increase in T-cell frequency and diversity was observed in MRTX849 and combination-treated tumors compared with PD-1–treated or vehicle-treated tumors (Supplementary Fig. S4). An increase in T-cell frequency was also observed in PD-1 monotherapy treated tumors relative to vehicle-treated tumors. These data corroborate the increase in T-cell abundance observed by flow cytometry by MRTX849 monotherapy and in combination and demonstrate T-cell diversity was expanded accordingly. The increase in T-cell diversity is likely directly related to an increase in the number of tumor antigens recognized by the immune system following the tumor cell killing and reversal of immune suppression induced by MRTX849 treatment. Although T-cell clonality was not increased by MRTX849 or combination treatment, the dramatic increase in T-cell infiltration (frequency) and diversity provides strong evidence of a mounting adaptive cell-mediated, antitumor immune response.
MRTX849 demonstrates acquired adaptive tumor immunity and durable complete responses in most mice treated with MRTX849 in combination with an anti–PD-1 antibody
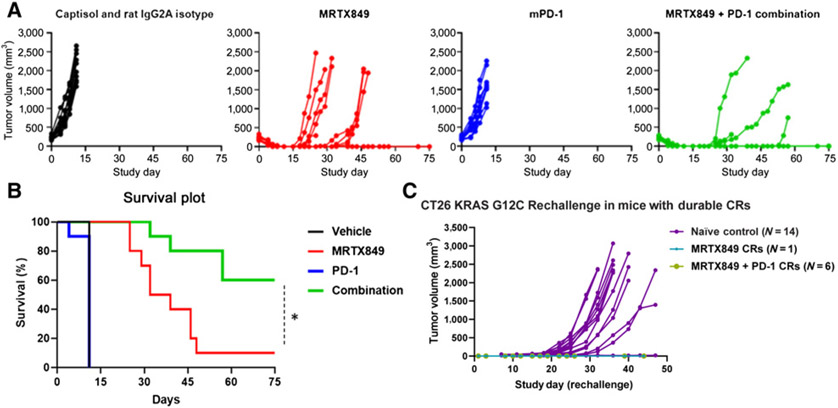
To evaluate the ability of MRTX849 to augment the antitumor activity of anti–PD-1 therapy, CT26 KrasG12C tumor-bearing BALB/c mice were treated with an isotype control antibody, MRTX849, anti–PD-1 antibody, or the combination in a study designed to assess antitumor response and survival of tumor bearing mice (Fig. 4A and B). Treatment of CT26 KrasG12C tumor-bearing mice with an anti–PD-1 antibody demonstrated minimal tumor growth inhibition compared with isotype control treatment. Treatment with MRTX849 as a single agent resulted in rapid tumor regression, including complete responses; however, tumor regrowth despite continued treatment was observed in nine of 10 mice. In contrast, treatment with the combination of MRTX849 and the anti–PD-1 antibody also resulted in rapid regression of tumors in all mice; however, a durable complete tumor response without evidence of regrowth was observed in six of 10 mice. This translated to increased survival benefit in the combination group compared with all other groups.
Figure 4.
MRTX849 and anti–PD-1 combination treatment leads to durable complete responses in the majority of treated mice. A, MRTX849 (100 mg/kg), rat IgG2a isotype control antibody (10 mg/kg, Bio X cell lot No. 686318F1B), and mouse PD-1 antibody (10 mg/kg, Bio X cell clone 29F.1A12) were administered orally, daily (MRTX849) and intraperitoneally, every 3 days × three doses (PD-1 and isotype control) alone or in combination as denoted to mice bearing established, subcutaneous CT26 KrasG12C tumors on the right flank (n = 10/treatment group, average starting tumor volume of ~220 mm3). Vehicle was dosed through study day 10, MRTX849 was dosed through study day 25 and PD-1 was dosed on study days 1, 4, and 7. Data are illustrated as individual tumor volumes over the measured time course for the efficacy and rechallenge studies. B, Survival in the combination-treated cohort was statistically significant compared with all other treatment cohorts by the Mantel-Cox test. “*” denotes adjusted P < 0.05. C, Data in the rechallenge graph depict individual tumor volumes from reimplantation of 1e6 CT26 KrasG12C cells into the left flank in the one MRTX849-treated mouse and the six combination-treated mice each of which exhibited durable complete tumor responses (CR) in the first implant/study (A). A cohort of naïve mice were also implanted as a control and tumors developed normally in 13 of 14 mice (denoted in purple).
To determine whether adaptive antitumor immunity was acquired in the six combination-treated mice and the one MRTX849-treated mouse that exhibited durable complete responses, CT26 KrasG12C cells were reimplanted on the opposite flank of these animals and tumors did not form in any of the mice (Fig. 4C). As a control, a cohort of treatment-naïve mice were implanted at the same time and tumors formed normally in 13 of 14 mice. These data provide clear evidence MRTX849 augmented PD-1 therapy and that the combination resulted in durable adaptive antitumor immunity.
To characterize the growth and evaluate the response to therapy in an immune compromised mouse strain, CT26 KrasG12C tumors were established in nu/nu mice and treated with MRTX849 at 100 mg/kg every day. Tumors also regressed following MRTX849 treatment; however, the magnitude and duration of the antitumor response was diminished compared with the complete responses observed in the same tumor model established in the BALB/c strain (Supplementary Fig. S5; Fig. 4A). These data suggest part of the mechanism of action of single-agent MRTX849 includes engagement of the adaptive immune response elicit enhanced antitumor activity.
In KrasG12C-mutant GEM lung cancer models, KRASG12C inhibition reconditions the tumor immune microenvironment and anti–PD-1 combination therapy extends PFS
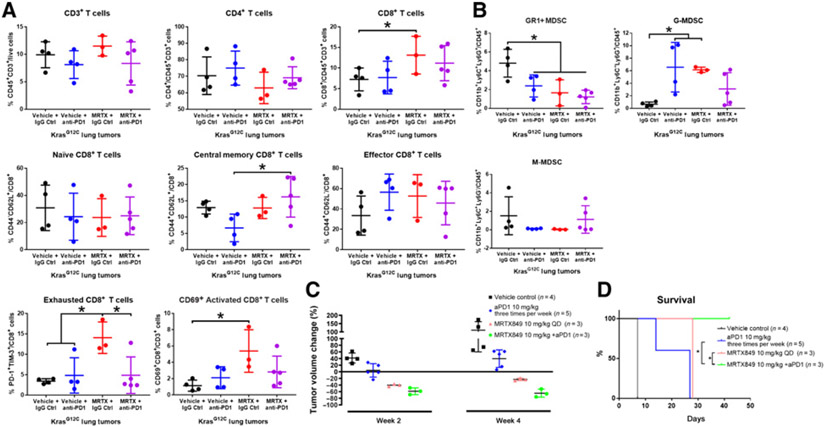
MRTX1257 (Supplementary Fig. S6), a close analog of MRTX849 that is similarly efficacious in selected tumor models in vivo, was used to evaluate the effects of KRASG12C inhibition in GEM lung cancer models. Lung tumor-bearing LSL-KrasG12C Trp53R270H GEM mice were administered either vehicle plus isotype control, vehicle plus anti-mouse PD-1 antibody, MRTX1257 at 50 mg/kg every day, or MRTX1257 plus anti-mouse PD-1 for 6 days followed by tumor harvest and flow cytometry for lymphocyte and myeloid cell populations. Several CD8+ T-cell populations (CD8+, exhausted CD8+ and CD69+ activated T cells) were increased, Gr1+ MDSCs were decreased whereas granulocytic myeloid-derived suppressor cells (G-MDSC) were increased (Fig. 5A and B) following MRTX1257 treatment. Combination treatment with MRTX1257 plus an anti–PD-1 antibody led to trends that were similar compared with MRTX1257-treated tumors, whereas treatment with an anti–PD-1 antibody resulted in little change in the CD8+ T-cell populations (Fig. 5A and B) but similar changes in the MDSC populations compared with the MRTX1257 single-agent–treated tumors.
Figure 5.
MRTX1257 reconditions the tumor immune microenvironment and MRTX849 plus anti–PD-1 therapy prolongs survival in KrasG12C-mutant GEMMs. A and B, Lung tumor-bearing LSL-KrasG12C Trp53R270H GEM mice were administered vehicle plus isotype control, vehicle plus anti-mouse PD-1 antibody (10 mg/kg three times per week, Bio X cell clone 29F.1A12), MRTX1257 (50 mg/kg) or MRTX1257 plus anti-mouse PD-1 for 6 days. Mice were euthanized on day 7 and lung tumor nodules were harvested and disaggregated for immune profiling for lymphoid (A) or myeloid (B) populations by flow cytometry. Flow cytometry data are expressed for individual tumors using scatter plots along with mean ± SD for selected cell markers as defined in the Supplementary Materials and Methods. C, Tumor volume was monitored in repeat dose efficacy studies in the LSL-KrasG12C Msh2fl/f lung GEM model at baseline and at week 2 and week 4. See Supplementary Fig. S7 for statistical analysis. D, PFS analysis demonstrates combination treatment led to a statistically significant improvement in survival compare with MRTX849 or anti–PD-1 single-agent treatment using the log-rank (Mantel-Cox) test (P < 0.05).
To evaluate the effect of PD-1 blockade therapy on KrasG12C GEMMs in combination with MRTX849, we crossed KrasG12C mice with a Msh2tm2.1Rak strain to generate KRASG12C;Msh2fl/fl lung GEM (KCM). Tumors from this model are genomically unstable due to MSH2 loss of function and are likely more susceptible to immune checkpoint blockade due to the presence of an increased mutation/neoantigen burden (26). Treatment with either 10 mg/kg MRTX849 or MRTX849 plus anti–PD-1 resulted in tumor regression at 2 and 4 weeks (Fig. 5C; Supplementary Fig. S7). Although the tumor volume of combination-treated mice did not reach statistical significance compared to MRTX849 single-agent–treated tumors using ANOVA multiple comparisons, there was a trend of greater regression in combination-treated tumors. Moreover, PFS was significantly improved in MRTX849 plus a-PD-1 antibody–treated versus MRTX849 single-agent–treated mice (Fig. 5D). The marked alterations in the tumor immune cell composition and the increased antitumor effects of KRASG12C inhibition plus anti–PD-1 therapy in GEM lung cancer models demonstrate KRASG12C inhibition reverses immunosuppressive programs in KRAS-driven spontaneous tumor model systems and provide strong preclinical evidence supporting the combination of KRASG12C and PD-1 inhibitors to treat KRASG12C-mutant cancers.
Discussion
Understanding the mechanism of action of covalent KRASG12C inhibitors represents an important step to maximize the full potential of this novel class of therapeutics. In particular, a thorough understanding of the molecular effects and immune sequelae of KRASG12C inhibition in the context of CIT is of high interest. On one hand, KRASG12C mutations are transversion mutations associated with smoking, a high TMB and PD-L1 positivity, each of which are factors that correlate with response to CIT. On the other hand, KRAS activation results in an immunosuppressive state related to immune evasion through decreased antigen presentation and increased expression of immunosuppressive cytokines that aid recruitment of immunosuppressive cell types. These phenomena are implicated in resistance to CIT. The observation that CIT is effective in a subset of KRASG12C-mutant NSCLC demonstrate evasion of the immune system via the PD-1 pathway is critical for progression of KRASG12C-mutant cancers, but also that further augmentation of an immune response in treatment of these cancers may be harnessed to improve treatment outcomes (4, 10, 11). These data highlight the need to better understand the pathways regulated by KRAS and PD-1 to develop strategies to improve outcomes for KRASG12C-mutant patients. Several mechanisms have previously been identified that demonstrate KRAS actively suppresses antitumor immunity including reduction of tumor MHC class I expression and inducing an immunosuppressive tumor microenvironment in part through regulation of VEGFA, IL8, and CXCL family cytokines (12-15, 22). By analyzing the effects of MRTX849 on immune signaling pathways in human cell line xenograft models grown in immune compromised mice, several tumor cell–centric immune effects were identified. The changes in RNA and protein levels of key immune signaling molecules shortly after MRTX849 treatment demonstrate reversal of multiple immunosuppressive programs is one of the first consequences of KRASG12C inhibition in vivo.
The reduction of MDSC and immunosuppressive M2-polarized macrophage populations with a concomitant increase in key lymphocyte populations following treatment with single-agent MRTX849 suggests KRASG12C inhibitors may trigger an antitumor immune response as a key component of their antitumor mechanism of action. Notably, these effects were observed in syngeneic mouse cancer cell line–derived lung and colon cancer tumor models as well as a GEM model of lung cancer. Each of these models are reflective of cancer types with the highest frequency of KRASG12C mutations. The diminished antitumor effect observed by MRTX849 in the tumors grown in the T-cell–deficient nu/nu strain further supports the hypothesis that changes in immune cell populations following KRAS inhibition contribute to the antitumor effects of the drug.
Although single-agent MRTX849 led to significant regression in immune-competent tumor models, durable complete responses were only observed with anti–PD-1 combination therapy suggesting that further enhancement of an antitumor immune response through blocking the PD-1 pathway may be critical to provide longer-lasting disease control in patients. In the CT26 KrasG12C model, in addition to the reduction of granulocytic and monocytic MDSC populations, macrophage phenotypes were shifted away from an immunosuppressive M2 phenotype toward an M1-polarized phenotype. Together, these observations indicated that every intratumoral myeloid population was essentially shifted toward an immunestimulated state. These changes were likely related to and may have created a favorable microenvironment to enable an increase in major lymphocytic cell populations, including CD4+ T, CD8+ T, CD19+ B, and NK cells. Therefore, the further enhancement of a T-cell–dependent adaptive immune response through addition of PD-1 inhibition would be predicted to induce the elimination of the remainder of antigen-positive tumor cells. The increase in Tregs may be a consequence of a productive antitumor immune response and represent a mechanism whereby the immune system limits the response. Finally, not only did cotargeting the PD-1 pathway in concert with KRASG12C inhibition induce durable complete responses, the combination endowed the cured mice with durable immunity against subsequent tumor engraftment. This combination was therefore required to markedly reverse the profound immune suppression present in KRAS-mutant tumors (27).
Suppression of MHC class I by KRAS signaling may also be a key molecular feature of KRAS-mutant cancers allowing these cancers to evade the immune system and grow unchecked (22). In addition to the increased tumor antigens available for presentation following direct tumor cell killing by MRTX849, the reversal of tumor antigen suppression on tumor cells through increase of MHC protein expression coupled with the increase in dendritic cells following MRTX849 treatment likely work in concert to further expose tumor antigens previously not recognized by the immune system. Together, these factors would be predicted to facilitate an enhanced tumor cell–directed immune response. In addition, the reduction of intratumoral immunosuppressive cell populations creates a tumor microenvironment conducive to antigen presentation. Finally, the increase in T cells and T-cell diversity in the tumor provides strong evidence that an adaptive antitumor immune response is induced following MRTX849 and combination treatment.
Of note, similar immune cell changes have been observed following MEK inhibition and MEK plus PD-1 pathway inhibition supporting the role of the KRAS/MEK/ERK pathway in mediating an enhanced adaptive immune response (28). However, in contrast to MEK inhibition, treatment with a KRASG12C inhibitor is poised to enable more complete inhibition of the primary oncogenic driver and is clearly advantageous compared with MEK inhibition which has been challenging to deliver to KRAS-mutant patients due to incomplete target inhibition and dose-limiting toxicities related to the importance of the RAS/MEK/ERK pathways in normal cells. These data also exhibit several parallels with BRAF-mutant melanoma. In this disease, BRAF mutations have been shown to induce an immune-suppressed tumor microenvironment via suppression of tumor antigen expression, secretion of immune inhibitory cytokines and the presence of immune inhibitory cells including MDSCs, tumor-associated macrophages, and Tregs (29-32). Treatment with BRAF inhibitors result in increased antigen expression, decreased expression of VEGF and immunosuppressive cytokines IL6 and IL8 and enriched T-cell infiltrates.
Our findings in the KrasG12C-engineered CT26 model are also in agreement with a previous study exploring the pharmacodynamic and antitumor effects of the KRASG12C inhibitor AMG510 alone and in combination with anti–PD-1 therapy (1). The current studies extend these data and also demonstrate that KRASG12C regulates tumor cell immune signaling, antigen presentation, and additional microenvironmental factors. Moreover, the dramatic reconditioning of the tumor immune microenvironment was also observed in additional models including a second syngeneic mouse model and autochthonous GEM model. Together, these findings provide compelling evidence that KRASG12C inhibition induces proimmunogenic effects in multiple tumor contexts.
Although targeted therapies directed at oncogenic drivers in NSCLC are highly effective in a majority of patients, unfortunately, disease progression invariably occurs for most patients. Conversely, anti–PD-1–based therapies are not effective for most patients as a single agent; however, in patients that do respond, responses are notable for their durability. The combination of a KRASG12C-targeted therapy with CIT may potentially bring the best of both therapeutic classes together—a high response rate consistent with oncogene-targeted therapies in NSCLC coupled with the durable clinical benefit observed with CIT. The high TMB and PD-L1 expression and responsiveness to CIT for KRAS-mutant NSCLC also contrasts with observations in EGFR, ALK, ROS1, and RET alteration positive cancers suggesting a unique opportunity to combine a targeted therapy and CIT in this oncogene-driven NSCLC segment (33, 34). Ultimately, therefore, this combination may provide a therapeutic strategy that provides increasingly durable clinical benefit for a large proportion of patients with KRASG12C-mutant NSCLC.
Supplementary Material
Acknowledgments
K.-K. Wong received research funding from Mirati Therapeutics. We thank Molecular Diagnostic Services (MDS) for mouse pharmacology support, MI Bioresearch for flow cytometry support, Adaptive Biotechnologies for TCRB sequencing, and Flagship Biosciences for IHC support.
Footnotes
Note: Supplementary data for this article are available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Reprints and Subscriptions
To order reprints of this article or to subscribe to the journal, contact the AACR Publications Department at pubs@aacr.org
References
- 1.Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, et al. The clinical KRAS (G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019;575: 217–23. [DOI] [PubMed] [Google Scholar]
- 2.Hallin J, Engstrom LD, Hargis L, Calinisan A, Aranda R, Briere DM, et al. The KRAS(G12C) inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov 2020;10:54–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez P, Peters S, Stammers T, Soria J-C. Immunotherapy for the first-line treatment of patients with metastatic non-small cell lung cancer. Clin Cancer Res 2019;25:2691–8. [DOI] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078–92. [DOI] [PubMed] [Google Scholar]
- 7.Dogan S, Shen R, Ang DC, Johnson ML, D'Angelo SP, Paik PK, et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res 2012;18:6169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 2018;24:1441–8. [DOI] [PubMed] [Google Scholar]
- 9.Coelho MA, de Carné Trécesson S, Rana S, Zecchin D, Moore C, Molina-Arcas M, et al. Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA. Immunity 2017;47:1083–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeanson A, Tomasini P, Souquet-Bressand M, Brandone N, Boucekine M, Grangeon M, et al. Efficacy of immune checkpoint inhibitors in KRAS-mutant non-small cell lung cancer (NSCLC). J Thorac Oncol 2019;14: 1095–101. [DOI] [PubMed] [Google Scholar]
- 11.Passiglia F, Cappuzzo F, Alabiso O, Bettini AC, Bidoli P, Chiari R, et al. Efficacy of nivolumab in pre-treated non-small-cell lung cancer patients harbouring KRAS mutations. Br J Cancer 2019;120:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kortlever RM, Sodir NM, Wilson CH, Burkhart DL, Pellegrinet L, Brown Swigart L, et al. Myc cooperates with Ras by programming inflammation and immune suppression. Cell 2017;171:1301–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao W, Overman MJ, Boutin AT, Shang X, Zhao Di, Dey P, et al. KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell 2019;35:559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okada F, Rak JW, Croix BS, Lieubeau B, Kaya M, Roncari L, et al. Impact of oncogenes in tumor angiogenesis: mutant K-ras up-regulation of vascular endothelial growth factor/vascular permeability factor is necessary, but not sufficient for tumorigenicity of human colorectal carcinoma cells. Proc Natl Acad Sci U S A 1998;95:3609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, et al. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res 1995;55:4575–80. [PubMed] [Google Scholar]
- 16.Rak J, Mitsuhashi Y, Sheehan C, Tamir A, Viloria-Petit A, Filmus J, et al. Oncogenes and tumor angiogenesis: differential modes of vascular endothelial growth factor up-regulation in ras-transformed epithelial cells and fibroblasts. Cancer Res 2000;60:490–8. [PubMed] [Google Scholar]
- 17.Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 2013;73:539–49. [DOI] [PubMed] [Google Scholar]
- 18.Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J,et al. Control of the immune response by pro-angiogenic factors. Front Oncol 2014;4:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288–301. [DOI] [PubMed] [Google Scholar]
- 20.Ancrile BB, O'Hayer KM, Counter CM. Oncogenic ras-induced expression of cytokines: a new target of anti-cancer therapeutics. Mol Interv 2008;8:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunaga N, Imai H, Shimizu K, Shames DS, Kakegawa S, Girard L, et al. Oncogenic KRAS-induced interleukin-8 overexpression promotes cell growth and migration and contributes to aggressive phenotypes of non-small cell lung cancer. Int J Cancer 2012;130:1733–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Jawhari JJ, El-Sherbiny YM, Scott GB, Morgan RSM, Prestwich R, Bowles PA, et al. Blocking oncogenic RAS enhances tumour cell surface MHC class I expression but does not alter susceptibility to cytotoxic lymphocytes. Mol Immunol 2014;58:160–8. [DOI] [PubMed] [Google Scholar]
- 23.Castle JC, Loewer M, Boegel S, de Graaf J, Bender C, Tadmor AD, et al. Immunomic, genomic and transcriptomic characterization of CT26 colorectal carcinoma. BMC Genomics 2014;15:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baldelli E, Bellezza G, Haura EB, Crinó L, Cress WD, Deng J, et al. Functional signaling pathway analysis of lung adenocarcinomas identifies novel therapeutic targets for KRAS mutant tumors. Oncotarget 2015;6:32368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannou AD, Marazioti A, Kanellakis NI, Giopanou I, Lilis I, Zazara DE, et al. NRAS destines tumor cells to the lungs. EMBO Mol Med 2017;9:672–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Downey CM, Jirik FR. DNA mismatch repair deficiency accelerates lung neoplasm development in K-ras(LA1/+) mice: a brief report. Cancer Med 2015;4:897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1–10. [DOI] [PubMed] [Google Scholar]
- 28.Loi S, Dushyanthen S, Beavis PA, Salgado R, Denkert C, Savas P, et al. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res 2016;22:1499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandalà M, De Logu F, Merelli B, Nassini R, Massi D. Immunomodulating property of MAPK inhibitors: from translational knowledge to clinical implementation. Lab Invest 2017;97:166–75. [DOI] [PubMed] [Google Scholar]
- 30.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res 2013;19:1225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res 2012;18:1386–94. [DOI] [PubMed] [Google Scholar]
- 32.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med 2006;203:1651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong Z-Yi, Zhong W-Z, Zhang Xu-C, Su J, Xie Z, Liu Si-Y, et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res 2017;23:3012–24. [DOI] [PubMed] [Google Scholar]
- 34.Scheel AH, Ansén S, Schultheis AM, Scheffler M, Fischer RN, Michels S, et al. PD-L1 expression in non-small cell lung cancer: correlations with genetic alterations. Oncoimmunology 2016;5:e1131379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.