Abstract
Background
Individuals are often counseled to use behavioral weight loss strategies to reduce risk for cardiovascular disease (CVD). We examined whether any benefits for CVD risk from weight loss intervention extend uniformly to individuals across a range of underlying health states.
Methods
The time until first occurrence of a composite of fatal and nonfatal myocardial infarction and stroke, hospitalized angina, or CVD death was analyzed from 8 to 11 years of follow-up of 4,859 adults who were overweight or obese, aged 45–76 years with Type 2 diabetes. Individuals had been randomly assigned to either an intensive lifestyle intervention (ILI) or diabetes support and education (DSE). Participants were grouped by intervention assignment and a frailty index (FI) based on deficit accumulation, ordered from fewer (first tertile) to more (third tertile) deficits.
Results
Baseline FI scores were unrelated to intervention-induced weight losses and increased physical activity. The relative effectiveness of ILI on CVD incidence was inversely related to baseline FI in a graded fashion (p = .01), with relative benefit (hazard ratio = 0.73 [95% CI 0.55,0.98]) for individuals in the first FI tertile to no benefit (hazard ratio = 1.15 [0.94,1.42]) among those in the third FI tertile. This graded relationship was not seen for individuals ordered by age tertile (p = .52), and was stronger among participants aged 45–59 years (three-way interaction p = .04).
Conclusions
In overweight/obese adults with diabetes, multidomain lifestyle interventions may be most effective in reducing CVD if administered before individuals have accrued many age-related health deficits. However, these exploratory analyses require confirmation by other studies.
Clinical Trial Registration
Keywords: Multidomain lifestyle intervention, Aging, Diabetes mellitus, Obesity, Frailty index
There is controversy whether behavioral weight loss programs should be prescribed to older adults with obesity to reduce risks for cardiovascular disease (CVD). While benefits might be expected based on improvements in cardiovascular risk factors (1), meta-analyses pooled across all ages find little evidence of benefit for CVD morbidity and mortality (2). There are concerns that intentional weight loss may have serious consequences later in life such as sarcopenia, osteopenia, and nutritional deficiencies, in general (3), and, in particular, for those with diabetes (4). Such concerns must be weighed against expected benefits, including improved physical functioning, reduced risks for neuropathy and nephropathy, and lower overall healthcare costs (5–8). There may be considerable heterogeneity in the risk to benefit balance for behavioral weight loss. Understanding the biological basis of this heterogeneity may facilitate personalized therapeutic interventions (9).
Geroscience is a field in which researchers explore individual’s progress along biological, functional, and phenotypical trajectories, viewing these age-related processes as related to but distinct from chronological age (10). From this perspective, it may be better to define opportunities for targeted interventions based on an individual’s underlying age-related health status than chronological age. However, it is not clear how best to characterize the construct of age-related health. We adopt the geroscience-based Rockwood frailty index (FI) (11,12), sometimes referred to as a deficit accumulation frailty index (13). This should not be confused with other frailty indices or the frailty phenotype (14,15).
FIs are typically composed of ≥30 symptoms, signs, and conditions associated with aging. Scores are sums of deficits ascribed to these components (with each deficit graded from 0 to 1) divided by the total number of assessed components, potentially ranging from 0 to 1 (16). FI scores are predictive of adverse health outcomes and increased healthcare utilization (17–19).
We previously have calculated FI scores for the Action for Health in Diabetes (Look AHEAD) randomized controlled clinical trial (20), and reported that its behavioral weight loss intervention, relative to a control condition, induced a significant buffer against 8-year FI increases.
The motivating hypothesis for our current analyses is that age-related health status, operationalized by deficit accumulation FI scores, identifies individuals for whom behavioral weight loss intervention differentially affects the development of CVD. To test this, we conducted exploratory analyses of data Look AHEAD.
Method
The Look AHEAD design, methods, and CONSORT diagram have been published previously (21,22). Look AHEAD was a multisite, single-masked randomized controlled clinical trial that recruited 5,145 individuals (during 2001–2004) from 16 U.S. centers. All participants had Type 2 diabetes and met the following criteria: 45–76 years of age, body mass index (BMI) >25 kg/m2 (>27 kg/m2 if on insulin), glycated hemoglobin (HbA1c) < 97 mmol/mol (11%), systolic/diastolic blood pressure <160/<100 mmHg, triglycerides <600 mg/dL, and successful passing of a maximum graded exercise test. Protocols and consent forms were approved by local Institutional Review Boards.
Interventions
Participants were randomly assigned to intensive lifestyle intervention (ILI) or diabetes support and education (DSE). The multidomain ILI targeted reducing caloric intake and increasing physical activity to induce weight loss to average >7% at year 1 and to maintain this over time (23). Consumption of 1,200–1,800 kilocalories/d based on initial weight was targeted. Physical activity of >175 min/wk through activities similar in intensity to brisk walking was also targeted, as was improved diet (<30% calories from fat, <10% calories from saturated fat, and >15% calories from protein). Cardiometabolic risk factors (lipids, HbA1c, and blood pressure) were monitored: participants were provided results and these were shared with their clinicians (with participant’s consent). During the first 6 mo, ILI participants attended three group meetings and one individual session per month. For the remainder of the first year, they were provided two group and one individual meeting per month. The intensity of the intervention gradually decreased thereafter (23).
DSE participants were invited to attend group sessions focused on diet, physical activity, and social support (24). Four meetings were offered during year 1, three per year in years 2–4, and one annually thereafter. Participants did not receive specific diet, activity, or weight goals or information on behavioral strategies; however, the protocol for sharing risk factor information with participants and their physicians was the same as for ILI.
Interventions were terminated September 2012, when all participants’ planned follow-up was at least 8 years.
Weight and Fitness
Measures were obtained by certified staff, masked to intervention assignment (23). Weights were measured annually. A maximal graded exercise test was administered at baseline and a submaximal test at years 1 and 4 (25). Changes in fitness were computed as differences between estimated metabolic equivalents (METS) when the participants achieved or exceeded 80% of age-predicted maximal heart rate or Borg Rating of Perceived Exertion of >16, at baseline and subsequently.
Frailty Index
We constructed a 38-item FI for Look AHEAD participants modeled after the index of the Systolic Blood Pressure Intervention Trial (17) and augmented to include nine additional deficits related to diabetes and obesity (Supplemental Exhibit S1) (20).
Cardiovascular Disease Events
The primary outcome for the Look AHEAD trial was the first postrandomization occurrence of a composite CVD outcome (fatal and nonfatal myocardial infarction and stroke, hospitalized angina, or CVD death) (22). A masked expert panel centrally adjudicated events based on hospital records, death certificates, and study data.
Baseline Risk Factors
Blood pressure was measured in duplicate using an automated device. Blood specimens were collected after ≥12-h of fasting and analyzed centrally (Northwest Lipid Research Laboratories, University of Washington) using standard procedures. Other self-reported characteristics and conditions were assessed with questionnaires and staff interviews. Baseline CVD history was self-report of prior myocardial infarction, coronary artery bypass, angioplasty/stent procedures, peripheral vascular disease, stroke, stable angina, or class I/II heart failure. Hypertension was defined by current treatment or measured blood pressure >140/90 mmHg. Depression symptoms were assessed with the Beck Depression Index.
Statistical Analysis
We analyzed de-identified data developed for investigators outside the core Look AHEAD study group. We adopted an intention-to-treat approach, and used data from all evaluable participants who were grouped by random intervention assignment (intention-to-treat). Of the 5,145 Look AHEAD participants, 4,901 (95.3%) provided consent for data sharing; data were sufficient to compute baseline FI scores for 4,859 (99.1%) of these participants. We grouped participants into tertiles based on baseline FI, with participants ranked from least to greatest numbers of accrued deficits, an approach we prespecified in an analysis plan. Baseline characteristics among tertiles were compared using chi-squared tests and analyses of variance.
We adopted the approach used for the Look AHEAD primary results paper, in which follow-up was censored as of September 11, 2012 (22). For our cohort, this resulted in an average [range] of planned follow-up time of 9.8 [8.4,11.1] years. Outcomes were assessed by phone calls every 6 mo and at the end of follow-up. The percentages of individuals for whom outcome status had not been ascertained within the past 6 mo and who had not earlier reached the endpoint were 8.7% (tertile 1), 10.3% (tertile 2), and 11.6% (tertile 3). These percentages differed significantly among the tertiles (p = .03), but not between intervention groups (p = .11) or by age (p = .20), and there was not a significant interaction between tertiles and intervention groups (p = .60). A CONSORT diagram for the full cohort has been previously published providing greater detail (22).
Within each intervention group, we modeled longitudinal trajectories for percent change in BMI and change in fitness (METS from graded exercise tests), using mixed-effects models, with interaction terms to compare intervention effects across baseline FI tertiles. We used proportional hazards regression and interaction terms to assess the consistency of intervention effects on CVD incidence among baseline FI tertiles. We repeated this approach among participants grouped by tertile of chronological age. The consistency of intervention effects on CVD incidence among predefined subgroups (sex and age group) and by baseline FI was evaluated using proportional hazards regression and interactions terms.
Sensitivity Analyses
We examined whether omitting self-reported history of myocardial infarction and stroke from the FI impacted our results, repeating the proportional hazards regression with the reduced FI.
Results
Baseline Characteristics by FI
FI scores at baseline ranged from 0.066 to 0.588, with median 0.202. The upper boundaries of the first and second tertiles were 0.178 and 0.230, respectively. Supplemental Exhibit S2 describes baseline characteristics of the cohort by FI tertile. While some differences among tertiles reflect contributions of individual components (eg, obesity, hypertension, and smoking), the overall patterns are not unexpected. Higher FI scores were associated with male sex, current smoking, CVD history, and greater mean BMI, systolic blood pressure, triglycerides levels, HbA1c, and diabetes durations. Intervention assignment was balanced across tertiles of FI.
Intervention-Related Changes in Weight and Fitness by FI Tertile
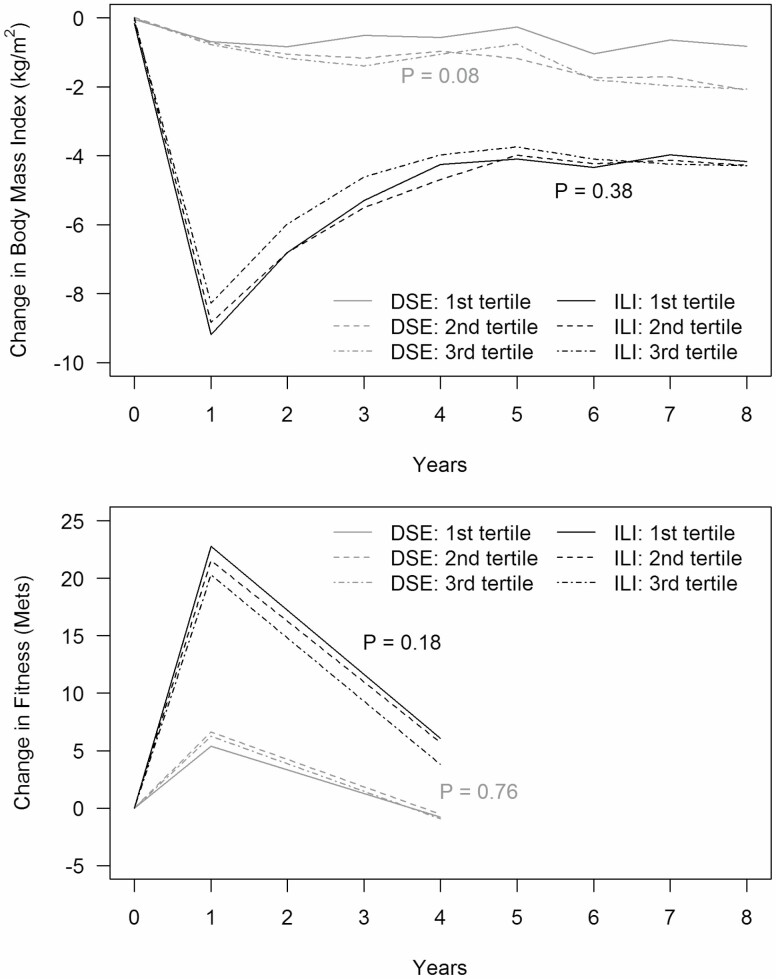
Figure 1 portrays mean percent changes from baseline of BMI over time by FI tertile and intervention assignment. ILI participants had significantly greater reductions in BMI than DSE participants (overall p < .0001). Within the ILI cohort, the mean BMI dropped markedly from baseline to year 1 and rose gradually through the remainder of follow-up. There were no statistically significant differences in the percent change in BMI over follow-up by FI tertile groups for ILI participants (p = .38). BMI gradually declined for DSE participants in all three FI tertiles, with slightly (but not significantly) less decline among those in the first tertile (p = .08). Supplement S3 provides a similar portrayal of mean percent weight changes.
Figure 1.
Mean percent changes in body mass index (kg/m2) and fitness (METS) by baseline frailty index tertile over time, with adjustment for age, gender, and race/ethnicity.
Figure 1 also portrays mean changes over time in fitness by FI tertile. Compared to DSE, fitness increased significantly in ILI participants in all FI tertiles (overall p < .001). The largest increases were at year 1, which were partially attenuated at year 4. There was little difference in average changes in fitness for ILI participants across the FI tertiles (p = .18). Among DSE participants, there were also no differences in changes in fitness over time among FI tertiles (p = .76).
Intervention-Related CVD Event Rates by FI Tertile
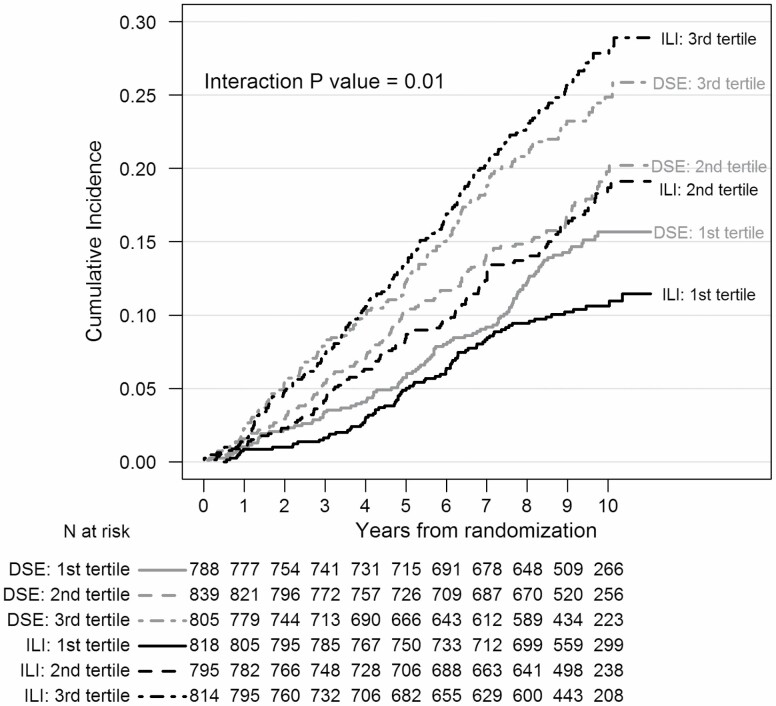
Figure 2 portrays the incidence of the CVD composite outcome over time, stratified by intervention assignment and baseline FI tertile. Compared to those in the first FI tertile, CVD incidence was greater for participants in the second tertile (HR = 1.48 [95% confidence interval 1.23, 1.79]) and third tertile (HR = 2.18 [1.83, 2.62]) groups, with significant overall differences among the FI tertiles after adjustment for age, sex, and race/ethnicity (p < .0001).
Figure 2.
Kaplan–Meier plots of times until incidence of the Look AHEAD primary cardiovascular outcome by intervention assignment and baseline frailty index tertile. Ranges: First tertile [0.066,0.178], Second tertile [0.178,0.230], and Third tertile [0.230,0.588].
Differences in the incidence of CVD across intervention groups varied by FI tertile in a graded manner (interaction p < .0001). As seen in Table 1, the relative impact of random assignment to ILI on CVD incidence trended toward relative benefit among individuals in the first FI tertile (HR = 0.73 [0.55, 0.98]) to no benefit among individuals in the third tertile (HR = 1.15 [0.94, 1.42] (interaction p = .01). Also in Table 1 are results from separate analyses for each component of the composite outcome. While none are statistically significant, the trends for benefit of ILI for those with lowest FI scores are seen for each component except CVD death, which occurred infrequently. Including percent weight loss at 1 year, which may potentially serve as an imprecise marker for intentionality of longer term weight loss, did not alter findings (the interaction p value remains = .01).
Table 1.
Relative Intervention Effects by Baseline Frailty Index Tertile on the Overall and Component-Specific Incidence of the Look AHEAD Primary Composite Cardiovascular Outcome: Results from Proportional Hazards Regression with Adjustment for Age, Gender, and Race/Ethnicity
| Outcome | FI Tertile | Number of Events | Hazard Ratio [95% CI] | Interaction p value | |
|---|---|---|---|---|---|
| DSE (N = 2,324) | ILI (N = 2,427) | ||||
| Composite | First | 106 | 80 | 0.73 [0.55,0.98] | |
| Second | 136 | 124 | 0.97 [0.72,1.17] | .01 | |
| Third | 164 | 186 | 1.15 [0.94,1.42] | ||
| CVD Death | First | 8 | 10 | 1.24 [0.49,3.15] | |
| Second | 22 | 18 | 0.82 [0.44,1.53] | .67 | |
| Third | 26 | 22 | 0.98 [0.50,1.56] | ||
| MI | First | 49 | 33 | 0.67 [0.40,1.14] | |
| Second | 66 | 53 | 0.82 [0.57,1.17] | .15 | |
| Third | 72 | 70 | 1.00 [0.72,1.39] | ||
| Stroke | First | 16 | 13 | 0.79 [0.38,1.64] | |
| Second | 21 | 25 | 1.23 [0.69,2.20] | .41 | |
| Third | 38 | 44 | 1.20 [0.78,1.86] | ||
| Hospitalized Angina | First | 18 | 12 | 0.66 [0.32,1.37] | |
| Second | 29 | 28 | 0.98 [0.58,1.64] | .88 | |
| Third | 69 | 55 | 0.80 [0.56,1.14] |
We performed a parallel analysis, grouping individuals according to tertile of baseline age (Table 2). There was no evidence that differences between ILI and DSE on the incidence of composite CVD varied by age (p = .52).
Table 2.
Graded Relationships of Relative Intervention Effect, Intensive Lifestyle Intervention (ILI) versus Diabetes Support and Education (DSE) on CVD risk for Participants Grouped by Tertiles of Deficit Accumulation Frailty Index and Chronological Age
| Tertile | Deficit Accumulation Frailty Index | Chronological Age | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio: ILI vs DSE | 95% Confidence Interval | p Value | Hazard Ratio: ILI vs DSE | 95% Confidence Interval | p Value | |
| First | 0.73 | [0.55,0.98]* | 1.12 | [0.83,1.53] | ||
| Second | 1.00 | [0.72,1.17] | .01 | 0.92 | [0.72,1.18] | .32 |
| Third | 1.29 | [0.94,1.42] | 0.92 | [0.75,1.12] |
*95% confidence interval excludes 1.0.
The interaction between FI and intervention assignment remained significant (p = .04) after adjustment for an interaction between baseline CVD history and intervention assignment. Omitting history of myocardial infarction and stroke from the FI did not materially alter results. With the FI recalculated without these two components, proportional hazards regression yielded HR [95% confidence intervals] HR=0.74 [0.56,0.98] for the first tertile; HR = 0.88 [0.68,1.13] for the second tertile; and HR = 1.20 [0.97,1.48] for the third tertile (interaction p = .004), thus a slightly more significant finding than from the full FI.
Interactions Between Intervention Assignment, FI, and Sex or Age
Table 3 portrays differences in intervention effects by baseline FI for participants grouped by sex and age. There was a significant graded inverse relationship in the intervention-related HR for CVD events among women (p = .01) and younger participants (p < .01), but not for men (p = .29) or older participants (p = .79). The three-way interaction among sex, intervention assignment, and FI was not statistically significant (p = .39). However, the three-way interaction among age, intervention assignment, and FI was (p = .04). For participants aged 45–59 years at baseline, 95% confidence intervals for relative ILI benefit for individuals with the lowest FI scores and for relative harm for individuals with the highest FI scores both excluded 1.0.
Table 3.
Differences in Relative Intervention Effects by Baseline Frailty Index According to Gender and Age: Intensive Lifestyle Intervention (ILI) versus Diabetes Support and Education (DSE)
| FI Tertile | Hazard Ratio [95% Confidence Interval] | |
|---|---|---|
| Females | Males | |
| First | 0.62 [0.38,1.02] | 0.81 [0.56,1.15] |
| Second | 1.00 [0.68,1.48] | 0.86 [0.63,1.18] |
| Third | 1.29 [0.95,1.78] | 1.05 [0.79,1.40] |
| p = .01 | p = .23 | |
| Age 45–59 y | Age 60–76 y | |
| First | 0.54 [0.34,0.86]* | 0.91 [0.62,1.32] |
| Second | 0.96 [0.65,1.42] | 0.89 [0.65,1.21] |
| Third | 1.46 [1.05,2.03]* | 0.97 [0.73,1.79] |
| p < .01 | p = .75 |
*95% confidence interval excludes 1.
Discussion
We hypothesized that deficit accumulation, a geroscience-guided construct of age-related health status, would be useful for identifying individuals for whom behavioral weight loss interventions may be most effective lowering the CVD risk. We further hypothesized that a deficit accumulation FI would be a better metric for tailoring intervention prescription than chronological age. We discuss three principle findings. First, individuals assigned to ILI achieved relatively similar decreases in weight and increases in physical fitness, irrespective of baseline FI score. Second, the effect of ILI versus DSE on CVD varied depending on participant’s baseline frailty level, ranging from reduced risk among individuals with low baseline FI to no benefit among those with the highest FI scores. Even through FI and age were correlated, chronological age did not show this inverse relationship. Third, the interaction between FI and the intervention appeared to be stronger among relatively younger participants and potentially among women.
Deficit Accumulation, Weight Loss, and Increases in Physical Activity
There is considerable evidence that adults with health-related deficits can successfully adhere to lifestyle interventions. Bibas, and colleagues systematically reviewed clinical trials conducted in older cohorts with deficits in physical function and health (26). Targeted outcomes included lean body mass, strength, physical performance, and bone mineral density; interventions included exercise training and nutritional supplements. While none of the inclusion criteria of trials they examined were based specifically on FIs, they found general support that individuals with age-related health deficits were able to adhere to physical activity and dietary regimens, which resulted in weight loss and overall improvements in physical function. For example, Villareal, and colleagues conducted a randomized clinical trial of weight loss and physical activity interventions in 107 individuals with obesity, aged ≥65 and older, who were frail, sedentary, and had deficits in at least two of the following: physical performance tasks, two or more activities of daily living, or one activity of daily living and peak oxygen consumption (27,28). Over 1 year, individuals assigned to active interventions achieved marked increases in physical activity and weight loss that were associated with improvements in components of the cardiometabolic syndrome.
We previously reported that relatively older Look AHEAD ILI participants achieved at least as large weight losses and increases in physical activity and fitness as younger participants (29,30). Their success in this was independent of measures of general and physical health, CVD history, and hypertension (30). Our current finding that the impact of the intervention on weight loss and fitness gain was independent of FI builds on these prior results.
Age-Related Health Status and CVD Risk Reduction
Greater baseline FI scores were strongly associated with increased risk for CVD incidence. The hazard for CVD was more than doubled for participants in the third compared with first FI tertile. This underscores the clinical significance of FIs as prognostic factors.
To the best of our knowledge, few randomized trials have examined whether the efficacy of interventions designed to reduce CVD morbidity and mortality varies according to frailty measured by deficit accumulation. Previous studies have focused on pharmacological interventions and cohorts older than the Look AHEAD participants. In The Hypertension in the Very Elderly Trial of adults 80 years or older, baseline FI levels did not moderate the association between antihypertensive therapy and CVD incidence among individuals with hypertension, in a cohort with 50% individuals who were overweight or obese. Only 4% of these individuals had diabetes (31). Similarly, in a subgroup of participants 75 years or older who were free of diabetes in the Systolic Blood Pressure Intervention Trial, there was no evidence of heterogeneity in the effect of antihypertensive therapy on incident CVD by FI (32). In the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial, baseline FI did not moderate the impact of pharmacological treatment of heart failure in a cohort of predominantly older adults with obesity (mean age 72 years; 45% with diabetes) (33). Our finding that the Look AHEAD multidomain intervention may only reduce CVD risk for individuals with lower FI is unprecedented. While evidence that ILI may increase CVD risks among individuals with greater baseline FI burdens is not strong, it is of concern and requires additional study.
De Vries and colleagues also examined factors associated with differences in how the Look AHEAD ILI was associated with risks for CVD (34). Their approach was data-driven, and involved fitting a proportional hazards model for CVD incidence that simultaneously included many risk factors and their interactions with intervention assignment. Their resulting model, which predicted individual’s overall risks well, was then used to rank participants according to predicted differences in risks had they been assigned to ILI versus DSE, that is, the estimated treatment effect they were predicted to receive from ILI relative to DSE. These differences ranged from positive to negative, and were conceptualized to reflect potential benefit or harm. Characteristics among individuals grouped according to where they fell along the distribution of predicted treatment effects, from benefit to harm, were examined. The characteristics most strongly associated with predicted treatment effect included sociodemographic (age, sex, race/ethnicity, and income), medical (CVD history, duration of diabetes, and insulin use), physical (weight, waist girth, and blood pressure), and blood-based (HbA1c and lipid levels) factors. The primary limitation with this approach is that the CVD outcome is used twice, both to derive the initial model and to examine CVD incidence by the predicted difference in risk. This raises concerns about over-fitting and limitations to external validity. Another limitation with this work (shared by our current study) is that CVD is examined in isolation. We recognize that clinical treatment decisions with respect to weight loss are not solely based on CVD risk.
Our hypothesis-based analyses point to a more personalized recommendation for intentional weight loss in older individuals that draws from geroscience, which builds on the paradigm of deficit accumulation. Our results suggest that some individuals may lower their risk for CVD through lifestyle intervention whereas others may not, and that age-related health status may be one of the factors that predict the likelihood of cardiovascular benefit. There is considerable literature questioning whether weight loss reduces CVD risk in older individuals or persons with diabetes (35). Our findings add nuance to this, suggesting that for adults with Type 2 diabetes intentional weight loss may reduce CVD risk for those with fewer health deficits.
The finding that the interaction between FI and intervention assignment was independent of CVD history suggests that FI captures an underlying construct distinct from CVD.
Findings for Age and Gender
We found no evidence that CVD risk reduction from lifestyle intervention varied by chronological age. Geroscientists posit that biological aging is very different from chronological aging, and can depend on many factors including genetics, exposures, resilience, and healthcare (10).
The relationship between intervention effects and baseline FI appeared to be stronger among women compared with men (however, the three-way interaction was not statistically significant). It was, however, significantly stronger among relatively younger compared with older participants. Overall, FI scores tended to be lower among women and individuals who were relatively younger, which suggests that lifestyle intervention may be most effective in women and those individuals who are 60 years of age or younger (before significant deficits in age-related health status have occurred).
Limitations
Our analyses are exploratory and post hoc, and should be interpreted with caution. While the interaction between the FI and the Look AHEAD lifestyle intervention was statistically significant, our estimates were not sufficiently precise. For example, we should not exclude potential benefit for participants in middle and highest FI tertiles. We grouped individuals by baseline FI tertiles; other cutpoints could be adopted and may yield different results. Deficit accumulation is a dynamic process, and it may be that relationships vary depending on its current composition and one’s chronological age. The Look AHEAD cohort, as comprised of volunteers for a clinical trial of a behavioral intervention, may not represent general clinical populations, and it is not clear whether our results may extend to older adults without diabetes. It is possible that the relationship between frailty and CVD risk is driven by the components of CVD that are known risk factors for CVD rather than by aging; however, it is difficult to assess this. At baseline, the FI components most strongly and independently associated with history of CVD included both traditional risk factors (angina, sleep apnea, and cholesterol) and others not included in traditional risk factor models (hearing loss, vision loss, and chronic pain).
Summary
Our findings support the importance of administering lifestyle interventions to reduce risks for CVD events early in the process of age-related declines in overall health.
Funding
This work was supported by two diversity supplements to the Action for Health in Diabetes Extension Study Biostatistics Research Center (grant numbers 3U01DK057136-19S1 and 3U01DK057136-19S2). The Action for Health in Diabetes is supported through the following cooperative agreements from the National Institutes of Health (grant numbers DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992). The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; Office of Research on Women’s Health; the Centers for Disease Control and Prevention; and the Department of Veterans Affairs. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (IHS) provided personnel, medical oversight, and use of facilities. The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the IHS or other funding sources. Additional support was received from the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); Frederic C. Bartter General Clinical Research Center (M01RR01346); Wake Forest Alzheimer’s Disease Core Center (P30-AG049638)99; and the Wake Forest Claude D. Pepper Older Americans Independence Center (P30-AG021332). The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America. This manuscript is based on a subset of the Look AHEAD cohort: participants from the Southwest Native American sites are not included. The complete cohort has been described (The Look AHEAD Research Group. Baseline characteristics of the randomized cohort from the Look AHEAD (Action for Health in Diabetes) research study. Diabetes Vasc Dis Res 2006;3:202–215 NIH Registration: NIHMS81811). The analyses performed herein were not conducted at the Look AHEAD Data Coordinating Center. This does not represent the work of the Look AHEAD study group.
Conflict of Interest
SK and MAE are members of the journal’s editorial board. No other conflicts of interest are noted.
Supplementary Material
Acknowledgments
FRS conducted analyses and collaborated on drafting the manuscript. NMP, KMB, SK, JM, BJN, RRW, AB, FI, and DO collaborated on drafting the manuscript and reviewed several interim drafts and the final manuscript. MAE conceived of this work, obtained funding, oversaw analyses, and collaborated on drafting the manuscript.
References
- 1. Haywood C, Sumithran P. Treatment of obesity in older persons—a systematic review. Obes Rev. 2019;20:588–598. doi: 10.1111/obr.12815 [DOI] [PubMed] [Google Scholar]
- 2. Ma C, Avenell A, Bolland M, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. Br Med J. 2017;359:j4849. doi: 10.1136/bmj.j4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coker RH, Wolfe RR. Weight loss strategies in the elderly: a clinical conundrum. Obesity (Silver Spring). 2018;26:22–28. doi: 10.1002/oby.21961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kyrou I, Tsigos C. Obesity in the elderly diabetic patient: is weight loss beneficial? No. Diabetes Care. 2009;32Suppl 2:S403–S409. doi: 10.2337/dc09-S348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The Look AHEAD Research Group. Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2014;2:801–809. Doi: 10.1016/S2213-8587(14)70156-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Houston DK, Neiberg RH, Miller ME, et al. Physical function following a long-term lifestyle intervention among middle aged and older adults with type 2 diabetes: the look AHEAD study. J Gerontol A Biol Sci Med Sci. 2018;73:1552–1559. doi: 10.1093/gerona/glx204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. The Look AHEAD Research Group. Effects of a long-term lifestyle modification program on peripheral neuropathy in overweight or obese adults with type 2 diabetes in the Look AHEAD Study. Diabetologia 2017;60:980–988. doi: 10.1016/S2213-8587(14)70156-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Espeland MA, Glick HA, Bertoni A, et al. Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the action for health in diabetes. Diabetes Care. 2014;37:2548–2556. doi: 10.2337/dc14-0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mutie PM, Giordano GN, Franks PW. Lifestyle precision medicine: the next generation in type 2 diabetes prevention? BMC Med. 2017;15:171. doi: 10.1186/s12916-017-0938-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrucci L, Levine ME, Kuo P-L, et al. Time and metrics of aging. Circ Res 2018;123:740–744. doi: 10.1161/CIRCRESAHA.118.312816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738–743. doi: 10.1093/gerona/62.7.738 [DOI] [PubMed] [Google Scholar]
- 12. Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903. doi: 10.1111/j.1532-5415.2008.01656.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ortolá R, Struijk EA, García-Esquinas E, Rodríguez-Artalejo F, Lopez-Garcia E. Changes in dietary intake of animal and vegetable protein and unhealthy aging. Am J Med 2020;133:231–239. doi: 10.1016/j.amjmed.2019.06.051. [DOI] [PubMed] [Google Scholar]
- 14. Paulson D, Lichtenberg PA. The Paulson-Lichtenberg frailty index: evidence for a self-report measure of frailty. Aging Ment Health. 2015;19:892–901. doi: 10.1080/13607863.2014.986645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43:10–12. doi: 10.1093/ageing/aft160 [DOI] [PubMed] [Google Scholar]
- 16. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pajewski NM, Williamson JD, Applegate WB, et al. Characterizing frailty status in the systolic blood pressure intervention trial. J Gerontol A Biol Sci Med Sci. 2016;71:649–655. doi: 10.1093/gerona/glv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drubbel I, de Wit NJ, Bleijenberg N, Eijkemans RJ, Schuurmans MJ, Numans ME. Prediction of adverse health outcomes in older people using a frailty index based on routine primary care data. J Gerontol A Biol Sci Med Sci. 2013;68:301–308. doi: 10.1093/gerona/gls161 [DOI] [PubMed] [Google Scholar]
- 19. Roe L, Normand C, Wren MA, Browne J, O’Halloran AM. The impact of frailty on healthcare utilisation in Ireland: evidence from the Irish longitudinal study on ageing. BMC Geriatr. 2017;17:203. doi: 10.1186/s12877-017-0579-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simpson F, Pajewski NM, Nicklas B, et al. Impact of multidomain lifestyle intervention on frailty through the lens of deficit accumulation in adults with Type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci 2019. doi: org/10.1093/gerona/glz197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. The Look AHEAD Research Group. Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–628. doi: 10.1016/S0197-2456(03)00064-3 [DOI] [PubMed] [Google Scholar]
- 22. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. New Eng J Med 2013;369:145–154. doi: 10.1056/NEJMoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The Look AHEAD Research Group. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity 2006;14:737–752. doi: 10.1038/oby.2006.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. The Look AHEAD Research Group. The development and description of the diabetes support and education (comparison group) intervention for the Action for Health in Diabetes (Look AHEAD) Trial. Clin Trials 2011;8:320–329. doi: 10.1177/1740774511405858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jakicic JM, Jaramillo SA, Balasubramanyam A, et al. Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: results from the Look AHEAD Study. Int J Obes (Lond). 2009;33:305–316. doi: 10.1038/ijo.2008.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bibas L, Levi M, Bendayan M, Mullie L, et al. Therapeutic interventions for frail elderly patients: Part 1. Published randomized trials. Prog Cardiovasc Dis. 2014;57: 134–143. doi: 10.1016/j.pcad.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 27. Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bouchonville M, Armamento-Villareal R, Shah K, et al. Weight loss, exercise, or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int J Obesity. 2014;38:423–431. doi: 10.1038/ijo.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring). 2011;19:1987–1998. doi: 10.1038/oby.2011.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Espeland MA, Rejeski WJ, West DS, et al. Intensive weight loss intervention in older individuals: results from the action for health in diabetes type 2 diabetes mellitus clinical trial. J Am Geriatr Soc. 2013:61:912–922. doi: 10.1111/jgs.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med. 2015;13:78. doi: 10.1186/s12916-015-0328-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673–2682. doi: 10.1001/jama.2016.7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanders NA, Supiano MA, Lewis EF, et al. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail. 2018;20:1570–1577. doi: 10.1002/ejhf.1308 [DOI] [PubMed] [Google Scholar]
- 34. de Vries TI, Dorresteijn JAN, van der Graaf Y, Visseren FLJ, Westerink J. Heterogeneity of treatment effects from an intensive weight loss intervention on cardiovascular events in patients with type 2 diabetes: data from the Look AHEAD trial. Diabetes Care 2019;42:1988–1994. doi: 10.2337/dc19-0776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xing Z, Pei J, Huang J, et al. Weight change is associated with increased all-cause mortality and non-cardiac mortality among patients with type 2 diabetes. Endocrine 2019;64:82–89. doi: 10.1007/s12020-019-01892-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.