Abstract
A straightforward, user-friendly, efficient protocol for the one pot, ZnI2-catalyzed allenylation of terminal alkynes with pyrrolidine and ketones, toward trisubstituted allenes, is described. Trisubstituted allenes can be obtained under either conventional heating or microwave irradiation conditions, which significantly reduces the reaction time. A sustainable, widely available, and low-cost metal salt catalyst is employed, and the reactions are carried out under solvent-free conditions. Among others, synthetically valuable allenes bearing functionalities such as amide, hydroxyl, or phthalimide can be efficiently prepared. Mechanistic experiments, including kinetic isotope effect measurements and density functional theory (DFT) calculations, suggest a rate-determining [1,5]-hydride transfer during the transformation of the intermediate propargylamine to the final allene.
1. Introduction
The chemistry of allenes has captivated the scientific community, over the past few decades, and is now regarded as one of the hot topics in Organic Chemistry.1 Once regarded as too reactive to bear any synthetic value, allenes have proven to be relatively stable moieties, also found in many natural products.2 Allenes exhibit unique chemical, conformational, and structural characteristics, as well as important applications in synthetic Organic Chemistry, catalysis, diastereoselective synthesis, and pharmaceuticals.1a−1c,1e−1g,2,3 Their synthetic value is easily realized, considering the plethora of useful organic transformations they can undergo. These include cyclization and cycloaddition reactions,2c,3k,4 hydroarylations,5 hydroaminations,6 hydrocyanations,7 hydroalcoxylations,8 and hydroborations.9 In particular, when allenes bear carbonyl, amide, carboxyl, amine, or hydroxyl groups, at certain positions with regard to the allenic moiety, cyclization reactions toward heterocycles such as furans, nitrogen-containing cyclic compounds, or oxazoles take place, all having a wide range of synthetic utility.1e,6a,10
Due to the synthetic importance of allenes, a number of protocols have been reported in the literature. To this end, allenes can be approached by employing a variety of transformations,11 including 1,2-elimination,12 addition,13 SN2′ substitution,14 Wittig-type, and related reactions,15 as well as coupling with diazo compounds.16 By carefully examining most of these synthetic approaches, one realizes that the propargylic moiety comprises a key intermediate.9c,11,16f,17 Along these lines, a strategy that has received a lot of attention lately, due to its experimental simplicity and wide substrate scope, is the synthesis of allenes from amines, carbonyl compounds, and alkynes, usually mediated by transition metal catalysts (Scheme 1). This transformation was first reported by Crabbé and co-workers in their seminal work on the synthesis of monosubstituted allenes from paraformaldehyde, diisopropylamine, and terminal alkynes (Scheme 1).18 The reaction (Crabbé homologation) is catalyzed by CuBr. Diisopropylamine, formaldehyde, and the alkyne initially yield the corresponding propargylamine, which undergoes an intramolecular transformation to form the allene product.
Scheme 1. Selected Examples for the Allenylation of Terminal Alkynes with Amines and Carbonyl Compounds.
Several related reports have been published ever since. The research group of Ma has significantly contributed to the field.19 Specifically, in 2002, Ma and co-workers reported a chiral approach for the synthesis of 2,3-allenoles with high ee% (enantiomeric excess) and good to very good yields (64–79%), under conditions analogous to those developed by Crabbé (Scheme 1).20 Later on, Nakamura and co-workers showed that the homologation of propargyl benzyl ethers to monosubstituted allenes can be performed under microwave (MW) conditions, using CuBr and employing dicyclohexylamine as a hydride donor.21 Ma and co-workers developed two additional modified versions of the Crabbé homologation by replacing CuBr with CuI, allowing the formation of monosubstituted allenes bearing amide, ether, mesylate, or hydroxyl moieties (Scheme 1).22 The use of aldehydes other than formaldehyde, for the synthesis of 1,3-disubstituted allenes, was realized only a decade ago, more than 30 years after the first report on the use of paraformaldehyde. In particular, Ma and co-workers reported on the synthesis of 1,3-disubstituted allenes from alkynes, morpholine, and mainly aryl-substituted aldehydes, under ZnI2 catalysis (Scheme 1).23 Subsequently, Kitagaki, Mukai, and co-workers reported that CuI is also capable of performing the Crabbé homologation toward 1,3-disubstituted allenes.24 In addition to the low catalyst loading employed, the reaction was carried out under microwave irradiation; however, the reaction conditions were relatively harsh (200 °C) and the isolated yields were moderate. Moreover, Ma and co-workers developed a CuI-catalyzed protocol, under conventional heating, utilizing aliphatic aldehydes,25 against which the previously reported ZnI2 protocol was not as efficient. This modified copper-catalyzed strategy also allowed the synthesis of hydroxyl-substituted allenes at the α- or β-position. A related, two-step approach was introduced by Yu and co-workers, furnishing 1,3-disubstituted allenes bearing sensitivity to high-temperature functionalities.26 Although the corresponding allenes are obtained in high yields under relatively low temperatures, the reaction conditions require stoichiometric amounts of ZnI2 (1.5 equiv), in addition to the fact that two catalysts are needed. An NHC-coordinated Au catalyst was also shown to be efficient, toward 1,3-disubstituted allenes, under low catalyst loading (2.5 mol %) and mild conditions (70 °C, Scheme 1).27 Minor drawbacks of this catalytic system are the prolonged reaction times (48 h), in addition to its focus on aryl-substituted aldehydes.
A study focusing on the capability of a variety of secondary amines to facilitate the [1,5]-hydride shift in propargylamines, leading to terminal or 1,3-disubstituted allenes, showed that allyl tert-butylamine (for terminal and 1,3-substituted allenes) and 1,2,3,6-tetrahydropyridine (for 1,3-disubstituted allenes) afford the best results.28 The α-hydrogens of the amine are allylic in both cases, a fact that could rationalize the corresponding efficient hydride transfer ability.
Early reports on Au- and Ag-based catalytic protocols, developed by Che and co-workers, provide access to chiral 1,3-disubstituted allenes from preformed chiral propargylamines.29 Chiral allenes are synthetically valuable and biologically relevant and have been found in many natural products.1b,1c,11b,30 Therefore, it comes as no surprise that the asymmetric version of the allenylation of terminal alkynes has attracted great interest. Along these lines, Cu-catalyzed, Zn-catalyzed, and dual catalytic systems comprising Cu/Zn or Cu/Cd have been developed for the asymmetric synthesis of 1,3-disubstituted allenes in either one- or two-step approaches.31 Chirality is achieved using a chiral amine, inducing the enantioselective formation of the in situ generated propargylamine intermediates, which are then converted to the axially chiral allenes. In some cases, a hydroxyl group residing at the α-carbon of the alkyne moiety has been shown to positively influence the ee% in this regard.31a
The one-pot synthesis of trisubstituted allenes was achieved about 8 years ago,32 3 years after the allenylation of terminal alkynes to 1,3-disubstituted allenes was reported for the first time.23 Given that 1,3-disubstituted allenes are furnished by employing aldehydes, trisubstituted allenes should be in principle accessible by employing ketones as the carbonyl counterparts. Moreover, the in situ generated intermediate from the reaction of aldehydes is proposed to be a propargylamine; therefore, the analogous tetrasubstituted propargylamines should be the key intermediate species when ketones are applied. However, ketones are more challenging substrates than aldehydes, in this transformation, due to the increased steric protection of the carbonyl center and electronic effects.33 Therefore, the synthesis of propargylamines employing ketones was reported only a decade ago.34
In a seminal work, the research group of Ma reported the ability of CdI2 to catalyze the one-pot synthesis of trisubstituted allenes from alkynes, employing pyrrolidine as the amine, though with a relatively limited ketone scope (Scheme 1).32 Notably, ZnI2 was unable to mediate this transformation above traceless amounts in the presence of toluene as a solvent, whereas although CuI was highly reactive for the synthesis of the precursor propargylamines, it could not conclude the transformation to the desired allenes. Very few reports have been published toward trisubstituted allenes by exploiting this kind of transformation ever since.19,35 Ma and co-workers developed a two-step procedure, employing CuI to facilitate the first step of the reaction, toward propargylamines, followed by filtration of the crude mixture and further reaction with ZnBr2, to yield the final allene product.35a A dual catalytic system, employing CuI and ZnBr2 in a one-pot approach, was developed by the same research group; however, 2 equiv of Ti(OEt)4 are also necessary for the reaction to proceed efficiently (Scheme 1).35b
Our continuous interest in the application of sustainable metal catalysis in useful organic transformations,36 as well as the above-described importance of allenes in organic synthesis, prompted us to develop an efficient, single-catalyst protocol for the synthesis of trisubstituted allenes. In a recent work of ours, we reported the synthesis of tetrasubstituted propargylamines from amines, alkynes, and ketones under Zn(OAc)2 catalysis.37 During the exploration of this transformation, using a variety of metal salts, we found that under certain conditions, ZnI2 alone can mediate the allenylation of terminal alkynes to trisubstituted allenes, though in poor yields. Given that this transformation had not been satisfactorily developed, with the reported methods using toxic metals under near stoichiometric loadings or a cocktail of catalysts, additives, and solvents, we were interested to further study the use of zinc salts. Ideally, the reaction would be catalyzed by a single, nontoxic, and inexpensive catalyst, employing stoichiometric amounts of the starting amines, alkynes, and ketones. Moreover, the protocol would preferably avoid the use of a solvent to minimize waste, also avoiding prolonged reaction times. Herein, we report our findings on such an efficient and user-friendly catalytic protocol, employing ZnI2 in the absence of the solvent, operating under either conventional heating or microwave irradiation conditions, thus substantially reducing reaction time from 16 to 1 h (under microwave conditions).
2. Results and Discussion
The optimization of the reaction began using pyrrolidine (1 mmol), cyclohexanone (1 equiv), phenylacetylene (1 equiv), and 60 mol % ZnI2, by heating the reaction mixture for 16 h at 120 °C. Based on the gas chromatography-mass spectrometry (GC-MS) analysis of the crude mixture, the conversion of starting materials was complete, but less than 25% yield of the corresponding trisubstituted allene was obtained (Table 1). A one-pot two-step approach was also probed, initially employing 20 mol % of ZnI2 at 120 °C, followed, after 16 h, by an addition of 60 mol % ZnI2 and heating the reaction at the same temperature for an additional 1 h, either in the absence or in the presence of dry toluene; however, both attempts yielded poor results. When phenylacetylene was replaced with 1-octyne, slightly better results were obtained. A common strategy to activate the carbonyl moiety by rendering it more electrophilic is to use Ti(OEt)4 as an additive. In fact, this reagent has been used in both KA2 (ketone-amine-alkyne coupling)32,38 and allenylation reactions employing carbonyl compounds35b as an activating reagent. Besides increasing the electrophilicity of the carbonyl groups, Ti(OEt)4 also serves as a drying agent, abstracting the water produced during the course of the reaction. On this basis, when Ti(OEt)4 was used as an additive (1 equiv), after 16 h at 120 °C and following chromatographic purification, 5a was obtained in 64% isolated yield (Table 1, entry 8).
Table 1. Optimization of the Reaction Conditionsa.
| entry | catalyst (mol %) | additive | solvent | T (°C) | time (h) | 5af yield | 5a:4a ratio |
|---|---|---|---|---|---|---|---|
| 1 | ZnI2 (60) | neat | 120 | 16 | (24) | ||
| 2b | ZnI2 (60) | Ti(OEt)4 | neat | 120 | 16 | 58 | 77:23 |
| 3b | ZnBr2 (60) | Ti(OEt)4 | neat | 120 | 16 | 45 | 94:6 |
| 4b | ZnCl2 (60) | Ti(OEt)4 | neat | 120 | 16 | 36 | 89:11 |
| 5b | Ti(OEt)4 | neat | 120 | 16 | 0 | ||
| 6b | ZnI2 (80) | Ti(OEt)4 | neat | 120 | 16 | 64 | 90:10 |
| 7b | ZnI2 (60) | Ti(OEt)4 | p-cymene | 120 | 16 | 16 | 19:81 |
| 8 | ZnI2(60) | Ti(OEt)4 | neat | 120 | 16 | 77 (64) | 91:9 |
| 9c | ZnI2 (60) | Ti(OEt)4 | neat | 120 | 16 | 43 | 54:46 |
| 10 | ZnI2 (40) | Ti(OEt)4 | neat | 120 | 16 | 67 | 85:15 |
| 11 | ZnI2 (60) | Ti(OEt)4 NaI | neat | 120 | 16 | 55 | 73:27 |
| 12 | ZnI2 (60) | Ti(OEt)4 Bu4NI | neat | 120 | 16 | 17 | 29:71 |
| 13 | ZnI2 (60) | Ti(OEt)4 | neat | 120 | 8 | 16 | 41:59 |
| 13 | ZnI2 (60) | Ti(OEt)4 | neat | 110 | 16 | 44 | 83:17 |
| 14 | ZnI2 (60) | Ti(OEt)4 | neat | 130 | 16 | 65 | 97:3 |
| 15d | ZnI2(60) | Ti(OEt)4 | neat | 120 | 1 | 86 (71) | 95:5 |
| 16e | ZnI2 (60) | Ti(OEt)4 | neat | 120 | 1 | 74 | 96:4 |
| 17d | ZnI2 (60) | Ti(OEt)4 | neat | 110 | 1 | 46 | 67:33 |
| 18d | ZnI2 (60) | Ti(OEt)4 | neat | 130 | 1 | 79 | 95:5 |
| 19d | ZnI2 (60) | Ti(O-i-Pr)4 | neat | 120 | 1 | (18) | |
| 20 | ZnI2 (60) | Ti(O-i-Pr)4 | neat | 120 | 18 | 7 |
Unless otherwise mentioned, all reagents and additives were employed in 1 equiv.
2 equiv of Ti(OEt)4 were used.
0.5 equiv of Ti(OEt)4 were used (conventional heating).
The reaction was performed under microwave irradiation (MW) at 300 W.
The reaction was performed under microwave irradiation (MW) at 200 W.
Yield of allene 5a in the crude mixture (isolated yields in brackets).
A number of zinc salts were then evaluated for their catalytic activity, with ZnI2 providing the best results (Table 1). Reduction or increase of Ti(OEt)4 equivalents to half or two, respectively, led to lower 5a yields. Upon decreasing the catalyst loading to 40 mol %, the formation of 5a decreased slightly. The presence of NaI or tBu4NI as additional iodine sources had a negative impact on the formation of the desired allene too. We then focused on optimizing the reaction temperature and time. A decrease of the reaction time to 8 h lowered the allene yield, whereas the reduction or increase of the temperature to 110 or 130 °C, respectively, had a negative impact on the yield of the reaction as well. In the absence of a zinc catalyst, the reaction does not take place. A series of amines were also studied for their efficiency in the formation of the desired allenes. Morpholine, piperidine, di-n-propylamine, N-allyl-N-tert-butylamine, 1-octylamine, cyclohexylamine, and benzylamine were tested, besides pyrrolidine. Piperidine and N-allyl-N-tert-butylamine afforded the best results; however, both were outperformed by pyrrolidine.
Microwave irradiation (MW) has become very attractive, over the past few decades, as an alternative means of heating up reactions, which are thus heated more efficiently, with reaction times often being substantially reduced.39 Given that alkyne allenylation under MW irradiation conditions has been reported in the past, with paraformaldehyde or substituted aldehydes as the carbonyl moieties,21,24 we tested our reaction protocol under MW conditions, resulting in the isolation of allene 5a in 71% yield after chromatographic purification. Not only was the desired product obtained in higher yield, but, equally important, the reaction time was substantially reduced to 1 h. Prompted by the positive result, we decided to pursue further both the conventional heating and the MW irradiation protocols. Therefore, slightly more than half of the substrate scope experiments were conducted under conventional heating, and the rest of the reactions were carried out under MW irradiation, while a few reactions were set up under both protocols for comparison purposes.
Finally, upon replacing Ti(OEt)4 with Ti(O-i-Pr)4, the formation of 5a was not satisfactory under microwave irradiation conditions (18% isolated yield, Table 1). When the same reaction was conducted under conventional heating, the isolated yield decreased even further (7%).
With the optimized conditions in hand, we explored the scope of the reaction against a variety of ketones and alkynes. The reaction of cyclohexanone with 1-octyne gave product 5a in 64 or 71% isolated yield, under conventional heating or MW irradiation, respectively (Scheme 2). When the ring of the cyclic ketone was shortened by one methylenic group, the isolated yield decreased to 28 or 24%, respectively (5b, Scheme 2), most probably because of the increased stabilization of the ketimine cation derived from pyrrolidine and cyclopentanone, compared to that formed from pyrrolidine and cyclohexanone. Replacing cyclopentanone with cycloheptanone increased the yield of the desired allene (5c) to 50% (conventional heating), while the use of a cyclic ketone bearing an even larger ring (cyclododecanone) gave 5d in 29 or 50% isolated yield, under conventional heating or MW irradiation, respectively. Replacing cyclic ketones with linear aliphatic ketones resulted in a reduction of the allene yield (5e and 5f, Scheme 2). This was anticipated, given that linear ketones lack the strain release driving force related to the cyclic ketones when nucleophilically attacked by the amine.
Scheme 2. Scope of Ketones.
All reactions were carried out at a 1 mmol scale for all reagents, 60 mol % ZnI2, and 1 mmol Ti(OEt)4. Reaction under conventional heating.
Reaction under microwave irradiation.
Allene 5h was isolated as a 2.7:1 dr diastereomeric mixture (determined by GC-MS).
The influence of ketone’s stereochemical environment/hindrance on the efficiency of the transformation was also investigated by employing two ketones bearing a methyl group at the α- or β-position in relation to the carbonyl moiety. When 2-methyl-cyclohexanone reacted with 1-octyne, a 35 or 33% yield of the corresponding allene 5g was obtained (conventional heating or MW irradiation, respectively). On the other hand, when 3-methyl-cyclohexanone reacted under MW irradiation conditions, a 54% yield of 5h was obtained as a diastereomeric mixture (2.7:1 dr). The decreased efficiency of allenylation, especially in the case of 2-methyl-cyclohexanone, can be attributed to the increased nonfavorable stereochemical interactions between the ketiminium cation and the zinc acetylide during the nucleophilic attack of the latter, leading to the propargylamine intermediate. In the case of 3-methyl-cyclohexanone, the methyl group is moved one carbon atom further away from the carbonyl, thus inducing a less significant stereochemical congestion to the overall outcome. When 1,4-dioxaspiro[4.5]decan-8-one was subjected to the MW condition protocol, a 37% yield of allene 5i was obtained (Scheme 2). This allene compound encompasses a useful handle for additional elaboration, which can be done by removing the 1,4-dioxaspiro group and further functionalization.
3-Pentanone and 4-decanone did not allow the formation of the corresponding allenes, being essentially unreactive under our thermal condition to protocol. Interestingly, when 2,4-dimethyl-3-pentanone 2b or dicyclohexylmethanone 2c were employed, the allene product obtained did not contain the ketone fragment. Instead, allene 5f was isolated, in 45 or 73% yield, respectively (Scheme 3). This observation suggests that bulkier ketones are not compatible with our protocol, instead leading to the dimerization and hydroamination of the terminal alkyne, and the subsequent formation of the corresponding allene structure, via a [1,5]-hydride shift, a transformation reported in the literature.40 This transformation was not observed with the other substrates studied herein, most probably due to the fact that all other ketones used are way more reactive due to their decreased steric protection. Finally, none of the aryl ketones employed (acetophenone, p-MeO-, m-MeO-, p-Cl-, and p-NO2-acetophenone, 2-acetylpyridine, and benzophenone) afforded the desired allene, even when these were highly electrophilic, such as p-NO2-acetophenone. In the case of p-MeO-, m-MeO-, and p-Cl-acetophenone, no allene or propargylamine species were obtained. Instead, starting materials and unidentified byproducts were observed. In the case of p-NO2-acetophenone, only starting materials were identified, whereas in the case of 2-acetylpyridine, we observed starting materials and 6% of the allene product, based on GC-MS analysis, at the end of the reaction.
Scheme 3. Hydroamination of 1-Octyne Observed when Bulky Ketones are Employed.
Τhe scope of the alkynes was probed next (Scheme 4). 1-Octyne can efficiently react with a number of ketones, providing the corresponding allenes, as discussed above and is shown in Scheme 2. Replacing 1-octyne with 1-pentyne yielded 42% of allene 5j under conventional heating conditions (Scheme 4). This decrease in the isolated yield obtained for 5j, in comparison to that for 5k, can be attributed to the low boiling point of 1-pentyne in relation to the temperature of the reaction. The transformation is highly efficient with 4-phenyl-1-butyne, providing allene 5k in 73% yield for either conventional heating or MW conditions. When 3-phenyl-1-propyne was used, the yield for 5l decreased to 18% (conventional heating conditions). Interestingly, upon replacing the aryl group of 3-phenyl-1-propyne with a cyclohexyl group, the yield for the corresponding allene (5m) increased to 45%. A compound bearing a phthalimide group at the a-position in relation to the alkyne moiety furnished allene 5n in 51% yield, whereas an amide group at the same position led to a 32% isolated yield for the desired allene 5o, as well as to a 30% yield of the intramolecular cyclization product of the alkyne, that is, the corresponding oxazole product.
Scheme 4. Scope of Alkynes.
Unless otherwise mentioned, all reagents were employed in a 1 mmol scale, as well as the additive, the catalyst loading was 60 mol %, and the reaction was performed under conventional heating; 5 equiv of 1-pentyne were used.
The reaction afforded 73% of 5k when performed under conventional and MW conditions.
The reaction was performed at a 0.8 mmol scale.
A total of 1.6 mmol of 4-phenyl-1-butyne and pyrrolidine 1 was used.
Allene 5t was isolated at 70% under conventional heating.
Allene 5t was isolated at 72% under MW conditions.
The reaction was performed under microwave conditions.
Allene 5x was obtained as a mixture of diastereomers with 1.9:1 dr (determined by GC-MS).
A total of 1.6 mmol of pyrrolidine 1 was used.
Unfortunately, the presence of an ester group, instead of an amide, when 3b was used (Scheme 5), did not allow the formation of the desired allene. In fact, ethyl benzoate (6b) was isolated in 90% yield, originating from the nucleophilic attack of ethoxide, deriving from Ti(OEt)4, to the carbonyl group of 3b, as well as 7% of allene 6b (Scheme 5). The formation of 6b can be rationalized by the ZnI2-catalyzed reaction between pyrrolidine, cyclohexanone, and alkyne 3b, followed by hydrolysis of the ester group. Alternatively, or simultaneously, alkyne 3b can hydrolyze first, yielding the corresponding propargylic alcohol, which is then involved in the three-component reaction with pyrrolidine and cyclohexanone, toward 6b.
Scheme 5. Performance of the Ester-Substituted Alkyne 3b.
Allenes 5p, 5q, and 5r were also isolated in 38, 35, and 26% yield, respectively (Scheme 4). These allenes show that our protocol can tolerate a number of functional groups, besides amides, phthalimides, and 1,4-dioxaspiro compounds, and can be used in late-stage functionalization strategies. Moreover, such allenes can be modified further, either on the allene moiety or the free hydroxyl group (or even at the bromide in 5q and 5r), providing access to a variety of synthetically useful scaffolds.
The combination of the aforementioned alkynes with ketones other than cyclohexanone resulted in the synthesis of the corresponding allenes in moderate to good yields (Scheme 4). 4-Phenyl-1-butyne gave the best results, allowing the isolation of allenes 5s and 5t in 60 and 70% yield, respectively, under conventional heating conditions. The isolated yield of 5t under MW conditions was essentially the same as that obtained under conventional heating (72%). Given that the phthalimide moiety provides an important handle for further functionalization, we studied the performance of N-propargylphthalimide with a variety of ketones other than cyclohexanone under MW irradiation conditions. Cyclic or linear ketones allowed the isolation of allenes 5u, 5v, 5w, and 5x, in 46, 51, 62, and 44% yield, respectively (Scheme 4). Due to the fact that both the ketone and the alkyne leading to 5y are solids at room temperature, this reaction was difficult to operate under MW conditions; therefore, conventional heating was used in this case, leading to a 51% isolated yield. Finally, we note that our findings described herein suggest that MW irradiation conditions, when compared to conventional heating, have either a positive or an insignificant impact on the reaction outcome, besides, of course, the greatly shortened reaction times.
In some cases of our substrate scope studies, a byproduct of dienic nature was also generated. When aliphatic alkynes were employed, the yield of this diene byproduct was very low, usually insignificant. However, when aromatic alkynes were used, diene formation became a major drawback for the isolation of the desired allene products. Phenylacetylene allowed the formation of the allenes in low yield, in addition to byproducts. Aryl-substituted alkynes bearing electron-withdrawing substituents (p-Cl- and p-CF3-phenylacetylene) allowed limited formation of the desired allene, with the majority of products being the enamine deriving from cyclohexanone and pyrrolidine, as well as a number of unidentified byproducts. On the other hand, when p-OMe-phenylacetylene (3c, Scheme 6) was employed, a mixture of the desired allene 5z and diene 7 was obtained, in a 0.55:1 ratio, respectively. The diene product is obtained in a higher ratio when a more strongly electron-donating substituent is introduced on the aryl alkyne, as in N,N-dimethylamino-phenylacetylene 3d (Scheme 6). In this case, diene 8 (trans diastereoisomer) was exclusively obtained in 25% isolated yield. Other phenyl acetylenes, such as p-Me- and p-Cl-substituted, gave a mixture of inseparable additional byproducts, in addition to the mixture of the corresponding allenes and dienes.
Scheme 6. Synthesis of Dienes via the Allenylation of Terminal Alkynes.
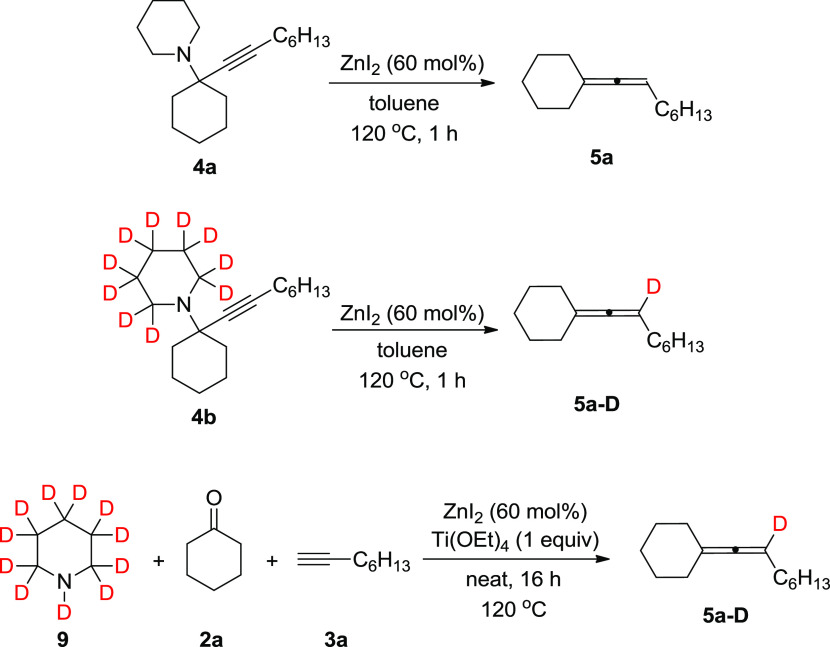
The mechanism of terminal alkyne allenylation has been proposed to begin with the formation of a propargylamine moiety, followed by a hydride transfer that yields the allene structure.19−22,24−32,35 To identify the propargylamine species as a possible intermediate in our protocol, propargylamine 4a was heated at 120 °C for 1 h in the presence of ZnI2, yielding allene 5a (Scheme 7). Next, propargylamine-d10 4b was synthesized. Upon its reaction with ZnI2 for 1 h at 120 °C, allene 5a-D was obtained, with full deuterium incorporation on the allenic carbon (Scheme 7). When piperidine-d11 9 was used as the amine, under our protocol conditions, allene 5a-D was furnished, again with full deuterium incorporation at the allenic carbon (Scheme 7). These observations suggest that propargylamines are indeed the intermediate species en route to the allenes. Moreover, the allenic hydrogen originates from the amine moiety of 4b, which is the product of an initial KA2 reaction between piperidine-d11 9, cyclohexanone, and 1-octyne.
Scheme 7. Mechanistic Experiments.
To find the rate-determining step of our protocol’s transformation, a reaction was set up using a 1:1 mixture of piperidine-h11 (10) and piperidine-d11 (9), along with 1-octyne and cyclohexanone, under our standard, conventional heating conditions. It is worth mentioning that the reliable measurement of the corresponding kinetic isotope effect required a very long delay time during our 1H NMR studies. This is because in many of the allenes isolated, allenic protons give integrations lower than 1 (usually in the range 0.83–0.98) per proton nuclei, an effect known in the literature.32,35 Although these prolonged relaxation times are not really problematic with regards to the characterization of the products, they comprise a significant problem when one wants to precisely measure a kinetic isotope effect. Fortunately, by increasing the relaxation delay (d1) of the 1H NMR experiment for allene 5a from the typical 1–100 s, we found that the integration ratio was substantially improved. By increasing the relaxation delay time further, to 300 s, the ratio of the allylic methylenic protons vs the allenic proton in 5a became 2.03:1.00. Using this relaxation delay times in our 1H NMR measurements, for the intermolecular competition between piperidine-h11 and piperidine-d11, the ratio of the corresponding allenes (5a/5a-D) was found to be 2.25 (Scheme 8). This measurement suggests the existence of a primary kinetic isotope effect in the overall transformation, with a value of kH/kD = 2.25 ± 0.15. This can be rationalized with a C–H/C–D bond breaking at the rate-determining step of the transformation. This finding is in agreement with analogous results in transformations leading to disubstituted allenes,18a,31k as well as our density functional theory (DFT) calculations’ results (vide infra).
Scheme 8. Kinetic Isotope Effect Results.
To study the formation of the diene byproduct, a reaction was set up, employing pyrrolidine, 2,2,6,6-tetradeuterated-cyclohexanone (11), and N,N-dimethylamino-phenylacetylene (3d) under our standard, conventional heating reaction conditions (Scheme 9). A mixture of diene products 12a–c was obtained. Deuterium was incorporated into two of the vinylic carbons, with the vinylic/benzylic carbon atom bearing only protons in all cases. In product 12a, obtained in a 62% relative ratio, the deuterium atom was located at the olefinic site of the cyclohexene ring. In diene 12b, obtained in a 35% relative ratio, the deuterium atom was located at the sp2 carbon in the α-position with regards to the cyclohexene ring. Finally, diene 12c, in which both the above-mentioned carbon centers are connected with protons, was obtained in a 3% relative ratio (Scheme 9). The “loss” of deuterium nuclei from the vinylic carbon of the cyclohexene ring can be rationalized by the enole–ketone equilibrium of deuterated ketone 11, during the course of the reaction, due to their exchange by protons deriving from the amine and the terminal alkyne. The fact that no deuterium incorporation was observed on the vinylic/benzylic carbon suggests that this proton may originate from the amine due to the [1,5]-hydride shift. Notably, the other carbon in 12, previously α to the cyclohexanone carbonyl carbon (in the product, allylic on the cyclohexane ring), was found to have a 0.76(H)/1.24(D) ratio. Based on these findings and related literature precedence,41 we hypothesize that the allene product is a precursor to the diene byproduct, which is most probably obtained via the activation of the allene moiety by ZnI2.
Scheme 9. Deuterium Labeling Experiment for the Diene Byproduct.
Reaction of ketone 11 (with initial 96% deuterium atoms on the α-carbons of the carbonyl group) toward the diene byproduct 12.
To gain further insight into the overall terminal alkyne allenylation reaction mechanism, we carried out DFT calculations with the Gaussian 16 suite of programs, using B3LYP functional, together with the 6-31G(d,p) basis set for the structure optimizations and M06-2X/def2tzvpp for the single-point energy refinements. To mimic the reaction conditions, we used an implicit solvent model (IEFPCM) with toluene as the solvent. As reagent models for the calculations, pyrrolidine (1), cyclohexanone (2a), 1-octyne (3a), and ZnI2 were used. As previously computed in a related system,36f the initial deprotonation of 1-octyne generates alkynyl-Zn complex I, and its attack to iminium electrophile II presents an activation energy of only 10.3 kcal/mol (TS1, Scheme 10), forming neutral species III in an exothermic process. Next, the critical step of H transfer was calculated, finding the transition step TS2, with an energy of 12.1 kcal/mol, relative to the starting materials I + II, which corresponds to an activation of 19.8 kcal/mol from III.
Scheme 10. Energy Profile for the ZnI2 Catalyzed Allenylation of 1-Octyne with Pyrrolidine and Cyclohexanone.
According to the activation energies in Scheme 10, the H transfer is rate limiting (TS2, ΔG‡ = 19.8 kcal/mol), but the elimination step could in principle compete (ΔG‡ = 19.1 kcal/mol) in certain circumstances (vide infra). Also, the energy of TS2 seems too low for the experimental reaction temperature (120 °C), and thus, the agreement between the experiments and calculations was not complete at this point. The zwitterionic Zn-alkenyl species IV presents two main conformers depending on the relative anti or syn disposition of the imminium and zinc moieties. Not surprisingly, the energetically lowest conformation of IV is syn (as shown in Scheme 10), placing the negative and positive charges close to each other, whilst the elimination through TS3 prefers an anti-orientation. Indeed, the activation energies for the anti (9.4 kcal/mol, TS3-anti) and syn (12.1 kcal/mol) elimination pathways differ substantially, allowing to safely discard the syn option. In this regard, it is interesting to note that both the triple bond and the amine can coordinate the ZnI2 salt in complex III, but, as shown in Scheme 11, the N-Zn coordination in III-N is stronger by about 5 kcal/mol than the alkyne coordination in III-yne. This observation is crucial since III-N can be considered the steady state of the reaction, increasing the computed activation energy of the rate-limiting TS2 to 25.5 kcal/mol, which perfectly explains the KIE and the reaction temperature. This scenario also led us to consider an alternative H-transfer mechanism from III-N, where the H-shift and ZnI2-elimination would occur in a concerted manner through a cyclic transition state (TS4), with concomitant cleavage of the C–N bond and H transfer from the pyrrolidine ring to the alkyne fragment. However, TS4 presents a very large activation energy (ΔG‡ > 45 kcal/mol), being unable to compete with the relatively lower energies of the two-step process in TS2 and TS3-anti.
Scheme 11. Zn-yne vs Zn-N Coordination Modes during the 1,5-Hydride Transfer.
Based on the above-described mechanistic studies and theoretical calculations, as well as literature precedence on related transformations,32,35 we propose a possible mechanism shown in Scheme 12. Initially, the amine reacts with the ketone providing a ketiminium cation, a process assisted by the preformed ammonium cation (proton donor) and enhanced by the presence of the Lewis acid Ti(OEt)4, which interacts with the carbonyl group, effectively increasing its electrophilicity. The alkyne reagent reacts with the ZnI2 catalyst, forming the zinc acetylide, a process most probably supported by the amine. This in situ generated zinc acetylide nucleophilically attacks the ketiminium cation, forming the propargylamine intermediate, in addition to one molecule of water, which reacts with Ti(OEt)4, to give ethanol. Activation of the triple bond of the propargylamine intermediate by ZnI2 enables a [1,5]-hydride transfer, which is the rate-determining step of the reaction, leading to the removal of the amine component, as well as the zinc catalyst, furnishing the final allene.
Scheme 12. Proposed Mechanism.
3. Conclusions
Herein, we are presenting a straightforward synthetic protocol for the allenylation of terminal alkynes with ketones and pyrrolidine, toward trisubstituted allenes, under inexpensive, sustainable, and widely available ZnI2 catalysis. The one-pot reaction requires stoichiometric amounts for all three reactants, as well as more sustainable conditions and reduced catalyst loading, compared to all analogous protocols reported thus far in the literature. Ti(OEt)4 is also used to activate the carbonyl group and scavenge water. Our protocol does not require the use of solvent and is efficient either under conventional heating or MW irradiation conditions, which substantially reduce reaction time. A variety of alkynes and aliphatic ketones have been successfully employed. Equally important, the protocol is functional-group tolerant and, therefore, can be employed in late-stage functionalization steps. Mechanistic investigations revealed that the allenic proton originates from the amine utilized. Moreover, the key intermediate to the allenes is the corresponding propargylamine compound. Kinetic isotope effect measurements and DFT calculations suggest that the 1,5-hydride transfer, transforming the intermediate propargylamines to the corresponding allenes, is the rate-limiting step of the overall transformation. We also present a brief study on the related formation of 1,3-dienes, which are the byproducts for some specific substrates utilized. These findings may prove helpful toward designing a new method for the synthesis of 1,3-dienes.
4. Experimental Section
4.1. General Information
All chemicals, starting materials, and catalysts were received from commercial sources, and the majority of these were used without further purification, with the exception of cyclohexanone and pyrrolidine, which were distilled prior to their use. All reactions were carried out under an argon atmosphere in flame-dried, Teflon-sealed screw-cap pressure tubes or Schlenk tubes. The course of the reactions was monitored via GC-MS or thin layer chromatography (TLC), using silica gel 60 coated aluminum sheets (0.2 mm), absorbing at 254 nm (silica gel 60 F254), as well as using a potassium permanganate solution for visualization. All products were isolated by high-pressure gradient column chromatography, using silica gel 60 (230–400 mesh) and mixtures of hexanes/ethyl acetate as the eluent.
NMR spectra were recorded on Bruker Avance-400 MHz or Varian Mercury 200 MHz instruments, using CDCl3 as a solvent and its residual solvent peak as a reference. NMR spectroscopic data are given in the order: chemical shift, multiplicity (s, singlet, br, broad, d, doublet, t, triplet, q, quartet, dd, doublet of doublets, dt, doublet of triplets, m, multiplet), coupling constant in hertz (Hz), and a number of protons. High-resolution mass spectrometry (HRMS) spectra were recorded using a QTOF maxis Impact (Bruker) spectrometer with electron spray ionization (ESI). GC-MS spectra were recorded with a Shimadzu GCMS-QP2010 Plus Chromatograph Mass Spectrometer using a MEGA (MEGA-5, FT: 0.25 μm, ID: 0.25 mm, L: 30 m, Tmax: 350 °C, Column ID no. 11475) column, using chloroform as a solvent.
4.2. General Procedure for the Synthesis of Alkynes
4.2.1. N-(Prop-2-yn-1-yl)benzamide (Used for Allene 5o)
To a two-necked flask, flame-dried and purged with Ar, benzoic acid (1.221 g, 10 mmol, 1 equiv), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) hydrochloride (2.75 g, 15 mmol, 1.5 equiv), HOBt (2.027 g, 15 mmol, 1.5 equiv), and 15 mL of dry dichloromethane (DCM) were added. The reaction mixture was stirred for 10 min and then cooled to 0 °C. Afterward, 2-propynylamine dissolved in 5 mL of dry DCM was added, and the reaction was allowed to return to room temperature and react under these conditions for 18 h. The reaction mixture was washed with a 5% aqueous citric acid solution (twice) and, afterward, with a 10% aqueous K2CO3 (twice). The organic layer was then dried over MgSO4, filtered, and the solvent was removed in vacuo to furnish a white solid, which was purified by gradient column chromatography, allowing the isolation of N-(Prop-2-yn-1-yl)benzamide as a white solid. Spectral analysis for N-(Prop-2-yn-1-yl)benzamide agrees with the reported spectral data found in the literature.42
4.2.2. Prop-2-yn-1-yl Benzoate (3b)
To a two-necked flask, flame-dried and purged with Ar, containing benzoic acid (1 g, 8.2 mmol, 1 equiv) and 10 mL of dry dimethylformamide (DMF), potassium carbonate (2.263 g, 16.4 mmol, 2 equiv) and propargyl bromide (1.461 g, 12.3 mmol, 1.5 equiv) dissolved in 5 mL of dry DMF were added at 0 °C. The reaction was allowed to return to room temperature and was left to react at these conditions for 18 h. The mixture was quenched using a saturated aqueous solution of NH4CO3, followed by extraction (three times) with ethyl acetate. The organic phase was washed with water and a saturated NaCl aqueous solution, dried over MgSO4 and, after removal of the solvents in vacuo, alkyne 3b was isolated as an orange oil. Spectral analysis for 3b is in accordance with the reported spectra in the literature.43
4.2.3. 1-Phenylbut-3-yn-1-ol (Used for Allene 5p)
To a two-necked flask, flame-dried and purged with Ar, connected with a reflux condenser and a dropping funnel, and containing granulated magnesium (486 mg, 20 mmol, 4 equiv), mercury hydrochloride (13.6 mg, 0.05 mmol, 0.01 equiv) in 5 mL dry diethyl ether and a solution of propargyl bromide (595 mg, 5 mmol, 1 equiv) in 5 mL dry diethyl ether were added dropwise. After the mixture turned gray, the second mixture of benzaldehyde (530 mg, 5 mmol, 1 equiv) in 10 mL of dry diethyl ether was added dropwise at 0 °C. The reaction mixture was allowed to return to room temperature and was left under stirring at these conditions for 18 h. The mixture was neutralized by the addition of an aqueous solution of hydrochloric acid (1 M), followed by filtration through a Buchner funnel through a short silica gel pad. Upon removing the solvent in vacuo, the resulting residue was purified by gradient column chromatography using a mixture of hexanes/ethyl acetate to yield 83% of alkyne 1-Phenylbut-3-yn-1-ol. Spectral analysis for 1-Phenylbut-3-yn-1-ol is in agreement with the reported spectra in the literature.44
4.2.4. 1-(4-Bromophenyl)but-3-yn-1-ol (Used for Allenes 5q and 5r)
To a two-necked flask, flame-dried and purged with Ar, connected with a reflux condenser and a dropping funnel, and containing granulated magnesium (486 mg, 20 mmol, 4 equiv), mercury hydrochloride (13.6 mg, 0.05 mmol, 0.01 equiv) in 5 mL dry diethyl ether and a solution of propargyl bromide (595 mg, 5 mmol, 1 equiv) in 5 mL diethyl ether were added dropwise. After the mixture turned gray, a mixture of 4-bromo-benzaldehyde (995 mg, 5 mmol, 1 equiv) in 10 mL of dry diethyl ether was added dropwise at 0 °C. The reaction mixture was allowed to return to room temperature and was left at these conditions for 18 h. The mixture was neutralized by the addition of an aqueous solution of hydrochloric acid (1 M), followed by filtration through a Buchner funnel through a short silica gel pad. The resulting residue, after removing the solvents in vacuo, was identified as 1-(4-bromophenyl)but-3-yn-1-ol and required no further purification. Spectral analysis for 1-(4-bromophenyl)but-3-yn-1-ol is in agreement with the reported spectra in the literature.44
4.3. General Procedure for the Synthesis of 2.2.6.6-Tetradeuterated-cyclohexanone (11)
To a round bottom flask, flame-dried and purged with Ar, equipped with a reflux condenser, cyclohexanone (393 mg, 4 mmol, 1 equiv), K2CO3 (44 mg, 0.32 mmol, 0.08 equiv), and 6 mL D2O were added. The reaction mixture is heated at reflux conditions for 108 h. The resulting solution was extracted with diethyl ether (three times) and dried over MgSO4, filtered, and the solvent was removed under reduced pressure, leading to the isolation of ketone 11 in 61% yield, with 96% deuterium incorporation at the α-carbons of the carbonyl group.45
4.4. General Procedure for the Synthesis of Propargylamine 4a
To a Teflon-sealed screw-cap pressure tube, flame-dried and purged with Ar, CuI (76 mg, 0.4 mmol, 0.2 equiv), cyclohexanone (196 mg, 2 mmol, 1 equiv), 1-octyne (220 mg, 2 mmol, 1 equiv), and piperidine-h11 (170 mg, 2 mmol, 1 equiv) were added. The reaction vessel was sealed with a screw cap and was heated at 120 °C for 18 h. The reaction mixture was filtered through a silica-coated pad and was purified via gradient column chromatography, using a mixture of hexanes/ethyl acetate as an eluent, yielding 31% of 4a as an orange oil.46
4.5. General Procedure for the Synthesis of Propargylamine 4b
To a Schlenk tube, flame-dried and purged with Ar, CuCl2 (6.7 mg, 0.05 mmol, 0.1 equiv), cyclohexanone (50 mg, 0.5 mmol, 1 equiv), 1-octyne (55 mg, 0.5 mmol, 1 equiv), and piperidine-d11 (48 mg, 0.5 mmol, 1 equiv) were added. The reaction tube was sealed with a screw cap and was heated at 110 °C for 20 h. The reaction mixture was filtered through a Buchner funnel having a short silica gel pad, and, following the removal of the solvent in vacuo, was purified via gradient column chromatography, using a mixture of hexanes/ethyl acetate as an eluent, yielding 42% of 4b as an orange oil.47
4.6. General Procedure for the Kinetic Isotope Effect Studies
To a Teflon-sealed screw-cap pressure tube, flame-dried and argon purged, containing a magnetic stirring bar, 60 mol % of ZnI2 (0.6 mmol, 0.6 equiv), Ti(OEt)4 (1 mmol, 1 equiv), 1-octyne (1 mmol, 1 equiv), cyclohexanone (1 mmol, 1 equiv), and an equimolar mixture of piperidine-h11 and piperidine-d11 (0.5 mmol piperidine-h11 and 0.5 mmol piperidine-d11) were added, respectively. The reaction tube was then sealed and left to react for 3 h at 120 °C in a preheated oil bath. Afterward, the reaction mixture was cooled, diluted with CHCl3, and filtered through a short silica gel pad, followed by the removal of the solvent in vacuo, leading to the crude reaction mixture. This was dry loaded on a SiO2 column and was purified via flash column chromatography, using hexane as the eluent. This procedure was repeated three times, yielding allenes 5a and 5a-D with a relative ratio of 2.25:1.
4.7. General Procedures for the Synthesis of Allenes 5
All reactions were set up according to one of the following two experimental procedures:
Procedure A: To a Teflon-sealed screw-cap pressure tube or a Schlenk tube, flame-dried and purged with Ar, containing a magnetic stirring bar, 60 mol % of ZnI2 (0.6 mmol, 0.6 equiv) was added, followed by the addition of Ti(OEt)4 (1 mmol, 1 equiv). Afterward, the alkyne (1 mmol, 1 equiv), ketone (1 mmol, 1 equiv), and pyrrolidine (1 mmol, 1 equiv) were added sequentially. The reaction tube was then sealed and left to react for 16 h at 120 °C in a preheated oil bath. The reaction mixture was then cooled to room temperature. The addition of chloroform or ethyl acetate and filtration through a short silica gel pad, followed by the removal of the solvent in vacuo, led to the crude reaction mixture, which was purified via gradient column chromatography, using a mixture of hexanes/ethyl acetate as the eluent. When the crude mixture was not solid, dry loading on the column chromatography is more efficient. All products were characterized by 1H NMR and 13C NMR, and, for the compounds not reported in the literature HRMS as well. All spectra obtained were in agreement with the assigned structures.
Procedure B: To a microwave pressure tube, flame-dried and purged with Ar, containing a magnetic stirring bar, 60 mol % of ZnI2 (0.6 mmol, 0.6 equiv) was added, followed by the addition of Ti(OEt)4 (1 mmol, 1 equiv). Afterward, the alkyne (1 mmol, 1 equiv), ketone (1 mmol, 1 equiv), and pyrrolidine (1 mmol, 1 mmol) were added sequentially. The reaction tube was then sealed and irradiated for 1 h, at 120 °C, at 300 W. The reaction mixture was then cooled to room temperature. The addition of chloroform or ethyl acetate and filtration through a short silica gel pad followed, and, after the removal of the solvent in vacuo, the crude mixture was purified via gradient column chromatography, where a mixture of hexanes/ethyl acetate was used as the eluent. When the crude mixture was not solid, the dry loading of the crude mixture was more efficient. All products were characterized by 1H NMR and 13C NMR, and for the compounds not reported in the literature HRMS as well. All spectra obtained were in agreement with the assigned structures.
4.7.1. Oct-1-en-1-ylidenecyclohexane (5a)35b
Allene 5a was synthesized via procedures A and B and was obtained as a colorless oil in 64% (123 mg) and 71% (136 mg) yield, respectively. 1H NMR (400 MHz, CDCl3): δ 5.00–4.90 (m, 1H), 2.18–2.03 (m, 4H), 1.95 (q, J = 7.0 Hz, 2H), 1.67–1.46 (m, 6H), 1.44–1.21 (m, 8H), 0.93–0.84 (t, J = 6.5 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3): δ 198.5, 102.4, 88.9, 32.0, 31.9, 29.5, 29.3, 28.8, 27.7, 26.4, 22.9, 14.3.
4.7.2. Oct-1-en-1-ylidenecyclopentane (5b)
Allene 5b was synthesized via procedures A and B and was obtained as a colorless oil in 28% (50 mg) and 24% (43 mg) yield, respectively. 1H NMR (200 MHz, CDCl3): δ 5.14–4.96 (m, 1H), 2.43–2.25 (m, 4H), 1.96 (q, J = 6.5 Hz, 2H), 1.72–1.59 (m, 4H), 1.44–1.10 (m, 8H), 0.88 (t, J = 6.5 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3): δ 197.2, 103.6, 91.6, 31.9, 31.4, 29.5, 29.3, 28.9, 27.2, 22.8, 14.3. HRMS calcd for C13H22 (M+): 178.1722; found: 178.1732.
4.7.3. Oct-1-en-1-ylidenecycloheptane (5c)
Allene 5c was synthesized via procedure A and was obtained as a colorless oil in 50% (103 mg) yield. 1H NMR (200 MHz, CDCl3): δ 5.00–4.88 (m, 1H), 2.34–2.08 (m, 4H), 1.95 (q, J = 6.5 Hz, 2H), 1.70–1.45 (m, 8H), 1.44–1.17 (m, 8H), 0.89 (t, J = 6.5 Hz, 3H); 13C{1H} NMR (50 MHz, CDCl3): δ 202.0, 104.3, 88.6, 32.9, 31.9, 29.5, 29.4, 29.3, 28.9, 28.8, 22.9, 14.3. HRMS calcd for C15H26 (M+): 206.2035; found: 206.2045.
4.7.4. Oct-1-en-1-ylidenecyclododecane (5d)
Allene 5d was synthesized via procedures A and B and was obtained as a colorless oil in 29% (80 mg) and 50% (138 mg) yield, respectively. 1H NMR (200 MHz, CDCl3): δ 5.09–4.90 (m, 1H), 2.12–1.82 (m, 6H), 1.61–1.09 (m, 26H), 0.88 (t, J = 6.5 Hz, 3H); 13C{1H} NMR (50 MHz, CDCl3): δ 202.24, 100.87, 91.01, 31.92, 29.94, 29.88, 29.66, 29.00, 24.73, 24.59, 24.27, 23.24, 22.82, 22.43, 14.28. HRMS calcd for C20H36 (M+): 276.2817; found: 276.2789.
4.7.5. 4-Methyldodeca-4,5-diene (5e)
Allene 5e was synthesized via procedure A and was obtained as a colorless oil in 49% (88 mg) yield. 1H NMR (200 MHz, CDCl3): δ 5.06–4.91 (m, 1H), 2.02–1.82 (m, 4H), 1.66 (d, J = 3.0 Hz, 3H), 1.53–1.09 (m, 10H), 1.00–0.73 (m, 6H); 13C{1H} NMR (50 MHz, CDCl3): δ 201.4, 99.1, 90.2, 36.5, 31.9, 29.6, 29.5, 29.0, 22.8, 20.9, 19.4, 14.3, 14.0. HRMS calcd for C13H24 (M+): 180.1878; found: 180.1898.
4.7.6. 7-Methylpentadeca-7,8-diene (5f)35a
Allene 5f was synthesized via procedure A and was obtained as a colorless oil in 43% (96 mg) yield. 1H NMR (200 MHz, CDCl3): δ 5.07–4.88 (m, 1H), 2.01–1.85 (m, 4H), 1.66 (d, J = 3.0 Hz, 3H), 1.47–1.13 (m, 16H), 0.89 (t, J = 6.5 Hz, 6H); 13C{1H} NMR (50 MHz, CDCl3): δ 201.3, 99.3, 90.2, 34.3, 32.0, 31.9, 29.6, 29.5, 29.2, 29.0, 27.7, 22.9, 19.5, 14.3.
4.7.7. 1-Methyl-2-(oct-1-en-1-ylidene)cyclohexane (5g)48
Allene 5g was synthesized via procedures A and B and was obtained as a colorless oil in 35% (72 mg) and 33% (68 mg) yield, respectively. 1H NMR (400 MHz, CDCl3): δ 5.16–4.97 (m, 1H), 2.35–2.18 (m, 1H), 2.06–1.86 (m, 4H), 1.85–1.65 (m, 3H), 1.48–1.18 (m, 10H), 1.16–1.00 (m, 1H), 0.96 (d, J = 6.5 Hz, 3H), 0.88 (t, J = 6.5, 3H); 13C{1H} NMR (50 MHz, CDCl3): δ 197.99, 108.41, 108.02, 91.34, 91.30, 36.60, 36.26, 34.68, 34.61, 32.47, 32.42, 31.96, 31.94, 29.76, 29.62, 29.58, 29.27, 29.02, 29.00, 27.89, 27.59, 26.43, 26.38, 22.87, 22.85, 19.90, 19.87, 14.27.
4.7.8. 1-Methyl-3-(oct-1-en-1-ylidene)cyclohexane (5h)
Allene 5h was synthesized via procedure B and was obtained as a colorless oil in 54% (111 mg) yield. 1H NMR (400 MHz, CDCl3): δ 5.00–4.88 (m, 1H), 2.27–2.11 (m, 2H), 2.00–1.85 (m, 3H), 1.83–1.47 (m, 4H), 1.47–1.19 (m, 9H), 1.07–0.94 (m, 1H), 0.92 (d, J = 6.5 Hz, 3H), 0.88 (t, J = 7.0, 3H); 13C{1H} NMR (101 MHz, CDCl3): δ 198.69, 198.65, 102.16, 101.70, 88.85, 88.76, 40.15, 40.05, 34.82, 34.65, 33.85, 33.26, 31.91, 31.49, 31.45, 29.57, 29.43, 29.39, 29.08, 28.85, 27.01, 26.48, 22.89, 22.84, 22.44, 22.26, 14.27. HRMS calcd for C15H26 (M+): 206.2035; found: 206.2035.
4.7.9. 8-(Oct-1-en-1-ylidene)-1,4-dioxaspiro[4.5]decane (5i)
Allene 5i was synthesized via procedures A and was obtained as a yellow oil in 37% (93 mg) yield. 1H NMR (200 MHz, CDCl3): δ 5.06–4.90 (m, 1H), 3.95 (s, 4H), 2.25 (dt, J1 = 7.5 Hz, J2 = 2.0 Hz, 4H), 2.03–1.84 (m, 2H), 1.72 (t, J = 6.5 Hz, 4H), 1.47–1.08 (m, 8H), 0.87 (t, J = 6.5 Hz, 3H); 13C{1H} NMR (50 MHz, CDCl3): δ 198.7, 108.6, 99.9, 89.4, 64.4, 35.6, 31.9, 29.3, 29.1, 28.9, 28.8, 22.8, 14.2. HRMS calcd for C16H26O2 (M+): 250.1933; found: 250.1908.
4.7.10. Pent-1-en-1-ylidenecyclohexane (5j)49
Allene 5j was synthesized via procedure A, using 5 equiv of 1-pentyne, and was obtained as a colorless oil in 42% (63 mg) yield. 1H NMR (200 MHz, CDCl3): δ 5.02–4.86 (m, 1H), 2.15–2.01 (m, 4H), 1.92 (q, J = 7.0 Hz, 2H), 1.73–1.18 (m, 8H), 0.91 (t, J = 7.0 Hz, 3H); 13C{1H} NMR (50 MHz, CDCl3): δ 198.5, 102.4, 88.6, 32.0, 31.6, 27.7, 26.4, 22.4, 13.7.
4.7.11. (4-Cyclohexylidenebut-3-en-1-yl)benzene (5k)49
Allene 5k was synthesized via procedure A and was obtained as a colorless to yellowish oil in 73% (155 mg) yield. 1H NMR (200 MHz, CDCl3): δ 7.40–7.07 (m, 5H), 5.11–4.93 (m, 1H), 2.73 (t, J = 7.0 Hz, 2H), 2.43–2.19 (m, 2H), 2.19–1.95 (m, 4H), 1.73–1.35 (m, 6H); 13C{1H} NMR (50 MHz, CDCl3): δ 198.6, 142.3, 128.6, 128.3, 125.8, 103.1, 88.2, 35.5, 31.8, 31.1, 27.6, 26.3.
4.7.12. (3-Cyclohexylideneallyl)benzene (5l)50
Allene 5l was synthesized via procedure A and was obtained as a colorless to yellowish oil in 18% (36 mg) yield. 1H NMR (200 MHz, CDCl3): δ 7.39–7.10 (m, 5H), 5.23–5.04 (m, 1H), 3.32 (d, J = 7.0 Hz, 2H), 2.21–1.98 (m, 4H), 1.71–1.35 (m, 6H); 13C{1H} NMR (101 MHz, CDCl3): δ 199.3, 141.2, 128.8, 128.4, 126.0, 103.2, 88.5, 36.5, 31.8, 27.5, 26.3.
4.7.13. (3-Cyclohexylideneallyl)cyclohexane (5m)
Allene 5m was synthesized via procedure A and was obtained as a colorless to yellowish oil in 45% (92 mg) yield. 1H NMR (200 MHz, CDCl3): δ 4.99–4.80 (m, 1H), 2.23–1.93 (m, 4H), 1.84 (t, J = 7.0 Hz, 2H), 1.77–1.43 (m, 11H), 1.39–1.03 (m, 4H), 1.03–0.78 (m, 2H); 13C{1H} NMR (50 MHz, CDCl3): δ 198.9, 101.7, 87.2, 38.1, 37.7, 33.2, 32.0, 27.7, 26.8, 26.5, 26.4. HRMS calcd for C15H26 (M+): 204.1878; found: 204.1883.
4.7.14. 2-(3-Cyclohexylideneallyl)isoindoline-1,3-dione (5n)35b
Allene 5n was synthesized via procedure A and was obtained as a white solid in 51% (136 mg) yield. 1H NMR (200 MHz, CDCl3): δ 7.92–7.79 (m, 2H), 7.78–7.65 (m, 2H), 5.10–4.97 (m, 1H), 4.27 (d, J = 4.5 Hz, 2H), 2.05–1.81 (m, 4H), 1.55–1.37 (m, 2H), 1.34–1.21 (m, 2H), 1.10–0.91 (m, 2H); 13C{1H} NMR (50 MHz, CDCl3): δ 197.6, 168.0, 134.0, 132.4, 123.3, 107.0, 84.5, 37.1, 31.1, 27.0, 25.8.
4.7.15. N-(3-Cyclohexylideneallyl)benzamide (5o)51
Allene 5o was synthesized via procedure A and was obtained as an orange solid in 32% (77 mg) yield. 1H NMR (400 MHz, CDCl3): δ 7.77 (d, J = 7.5 Hz, 2H), 7.47 (t, J = 7.5 Hz, 1H), 7.41 (t, J = 7.5 Hz, 2H), 6.37 (s, 1H), 5.20–5.11 (m, 1H), 3.97 (t, J = 5.0 Hz, 2H), 2.20–2.03 (m, 4H), 1.67–1.38 (m, 6H); 13C{1H} NMR (50 MHz, CDCl3): δ 197.5, 167.3, 134.7, 131.5, 128.6, 126.9, 106.5, 86.5, 38.9, 31.5, 27.5, 26.0. HRMS calcd for C16H19NO (M+): 241.1467; found: 241.1448.
4.7.16. 4-Cyclohexylidene-1-phenylbut-3-en-1-ol (5p)
Allene 5p was synthesized via procedure A and was obtained as a yellowish oil in 38% (87 mg) yield. 1H NMR (400 MHz, CDCl3): δ 7.43–7.23 (m, 5H), 5.04–4.92 (m, 1H), 4.76 (t, J = 6.5 Hz, 1H), 2.49–2.38 (m, 2H), 2.35–2.27 (m, 1H), 2.17–2.01 (m, 4H), 1.65–1.46 (m, 6H); 13C{1H} NMR (101 MHz, CDCl3): δ 200.0, 143.9, 128.4, 127.5, 126.1, 103.2, 84.7, 73.7, 39.7, 31.8, 31.6, 27.5, 26.2. HRMS calcd for C16H20O (M+): 228.1514; found: 228.1502.
4.7.17. 1-(4-Bromophenyl)-4-cyclohexylidenebut-3-en-1-ol (5q)
Allene 5q was synthesized via procedure A and was obtained as a yellowish oil in 35% (108 mg) yield. 1H NMR (200 MHz, CDCl3): δ 7.46 (d, J = 8.0 Hz, 2H), 7.24 (d, J = 8.0 Hz, 2H), 5.02–4.85 (m, 1H), 4.71 (t, J = 6.5 Hz, 1H), 2.52–2.22 (m, 2H), 2.18–1.91 (m, 4H), 1.77–1.37 (m, 6H); 13C{1H} NMR (50 MHz, CDCl3): δ 200.0, 142.9, 131.5, 127.9, 121.2, 103.4, 84.3, 73.0, 39.7, 31.7, 31.6, 27.4, 26.1. HRMS calcd for C16H19BrO (M+): 306.0619; found: 306.0604.
4.7.18. 1-(4-Bromophenyl)-4-(1,4-dioxaspiro[4.5]decan-8-ylidene)but-3-en-1-ol (5r)
Allene 5r was synthesized via procedure A, at a 0.8 mmol scale, and was obtained as a yellow oil in 26% (95 mg) yield. 1H NMR (200 MHz, CDCl3): δ 7.45 (d, J = 8.0 Hz, 2H), 7.22 (d, J = 8.0 Hz, 2H), 5.04–4.85 (m, 1H), 4.78–4.56 (m, 1H), 3.94 (s, 4H), 2.46–2.27 (m, 3H), 2.27–2.07 (m, 4H), 1.81–1.58 (m, 4H); 13C{1H} NMR (50 MHz, CDCl3): δ 200.3, 142.8, 131.5, 127.9, 121.3, 108.3, 100.6, 84.8, 73.1, 64.4, 39.5, 35.3, 28.6, 28.5. HRMS calcd for C18H21BrO3 (M+): 364.0674; found: 364.0692.
4.7.19. (5-Methylocta-3,4-dien-1-yl)benzene (5s)
Allene 5s was synthesized via procedure A and was obtained as a yellowish oil in 60% (120 mg) yield. 1H NMR (200 MHz, CDCl3): δ 7.41–7.03 (m, 5H), 5.17–4.96 (m, 1H), 2.73 (t, J = 7.5 Hz, 2H), 2.42–2.19 (m, 2H), 1.90 (dt, J1 = 7 Hz, J2 = 3 Hz, 2H), 1.64 (d, J = 3 Hz, 3H), 1.56–1.24 (m, 2H), 0.91 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (50 MHz, CDCl3): δ 201.6, 142.3, 128.7, 128.3, 125.8, 99.8, 89.5, 36.4, 35.8, 31.2, 20.9, 19.3, 14.0. HRMS calcd for C15H20 (M+): 200.1565; found: 200.1564.
4.7.20. 8-(4-Phenylbut-1-en-1-ylidene)-1,4-dioxaspiro[4.5]decane (5t)
Allene 5t was synthesized via procedures A and B, using 1.6 equiv for alkyne 4-phenyl-1-butyne and pyrrolidine 1, and was obtained as a yellow oil in 70% (189 mg) and 72% (195 mg) yield, respectively. 1H NMR (200 MHz, CDCl3): δ 7.35–7.09 (m, 5H), 5.11–4.95 (m, 1H), 3.95 (s, 3H), 2.72 (t, J = 7.0 Hz, 2H), 2.39–2.09 (m, 6H), 1.77–1.59 (m, 4H); 13C{1H} NMR (50 MHz, CDCl3): δ 198.9, 142.0, 128.6, 128.3, 125.8, 108.5, 100.5, 88.7, 64.4, 35.5, 35.4, 30.8, 28.7. HRMS calcd for C18H22O2 (M+): 270.1620; found: 270.1594.
4.7.21. 2-(3-Cyclododecylideneallyl)isoindoline-1,3-dione (5u)
Allene 5u was synthesized via procedure B and was obtained as a white-yellow solid in 46% (162 mg) yield. 1H NMR (200 MHz, CDCl3): δ 7.92–7.61 (m, 4H), 5.25–5.07 (m, 1H), 4.26 (d, J = 5.0 Hz, 2H), 2.02–1.75 (m, 4H), 1.49–0.81 (m, 18H); 13C{1H} NMR (50 MHz, CDCl3): δ 201.5, 168.0, 134.0, 132.5, 123.3, 106.1, 86.8, 36.9, 29.5, 24.6, 24.2, 24.1, 23.4, 22.5. HRMS calcd for C23H29NO2 (M+): 351.2198; found: 351.2192.
4.7.22. 2-(4-Methylhepta-2,3-dien-1-yl)isoindoline-1,3-dione (5v)
Allene 5v was synthesized via procedure B and was obtained as a yellow oil in 51% (130 mg) yield. 1H NMR (200 MHz, CDCl3): δ 7.89–7.78 (m, 2H), 7.75–7.66 (m, 2H), 5.16–5.01 (m, 1H), 4.24 (d, J = 4.0 Hz, 2H), 1.83–1.68 (m, 2H), 1.53 (d, J = 3.0 Hz, 3H), 1.38–1.10 (m, 3H), 0.79 (t, J = 7.0 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3): δ 201.3, 169.2, 134.6, 132.4, 122.9, 103.6, 85.4, 37.3, 36.4, 20.6, 18.7, 13.9. HRMS calcd for C16H17NO2 (M+): 255.1259; found: 255.1233.
4.7.23. 2-(4-Methyldeca-2,3-dien-1-yl)isoindoline-1,3-dione (5w)
Allene 5w was synthesized via procedure B and was obtained as a white-yellow solid in 62% (184 mg) yield. 1H NMR (200 MHz, CDCl3): δ 7.91–7.63 (m, 4H), 5.18–5.00 (m, 1H), 4.24 (d, J = 5.0 Hz, 2H), 1.84–1.69 (m, 2H), 1.54 (d, J = 3.0 Hz, 3H), 1.34–1.02 (m, 8H), 0.83 (d, J = 6.5, 3H); 13C{1H} NMR (50 MHz, CDCl3): δ 201.1, 167.9, 133.9, 132.3, 123.2, 103.8, 85.8, 37.2, 33.8, 31.7, 29.0, 27.3, 22.6, 18.7, 14.2. HRMS calcd for C19H23NO2 (M+): 297.1729; found: 297.1701.
4.7.24. 2-(3-(3-Methylcyclohexylidene)allyl)isoindoline-1,3-dione (5x)
Allene 5x was synthesized via procedure B and was obtained as a white-yellowish paste in 44% (123 mg) yield. 1H NMR (200 MHz, CDCl3): δ 7.93–7.62 (m, 4H), 5.11–4.96 (m, 1H), 4.32–4.16 (m, 2H), 2.20–1.98 (m, 2H), 1.77–1.10 (m, 6H), 0.97–0.68 (m, 4H); 13C{1H} NMR (101 MHz, CDCl3): δ 198.52, 197.78, 167.99, 167.92, 133.99, 133.97, 132.52, 132.42, 123.23, 106.78, 105.89, 84.63, 84.37, 39.17, 39.05, 37.40, 37.04, 34.35, 34.29, 33.29, 33.02, 30.65, 30.60, 26.40, 26.13, 22.31, 22.03. HRMS calcd for C18H19NO2 (M+): 281.1416; found: 281.1391.
4.7.25. 2-(3-(1,4-Dioxaspiro[4.5]decan-8-ylidene)allyl)isoindoline-1,3-dione (5y)
Allene 5y was synthesized via procedure A, using 1.6 equiv of pyrrolidine 1, and was obtained as a white-yellowish solid in 51% (166 mg) yield. 1H NMR (200 MHz, CDCl3): δ 7.91–7.62 (m, 4H), 5.13–5.00 (m, 1H), 4.25 (d, J = 4.5 Hz, 2H), 3.84 (s, 4H), 2.26–1.99 (m, 4H), 1.65–1.45 (m, 2H), 1.33–1.09 (m, 2H); 13C{1H} NMR (50 MHz, CDCl3): δ 198.0, 167.9, 134.1, 132.3, 123.3, 108.0, 104.4, 85.2, 64.3, 37.0, 34.9, 28.1. HRMS calcd for C19H19NO4 (M+): 325.1314; found: 325.1294.
Acknowledgments
The research project was supported by the Hellenic Foundation for Research and Innovation (H.F.R.I.) under the “1st Call for H.F.R.I. Research Projects to support Faculty Members & Researchers and the procurement of high-cost research equipment grant” (Project Number: 16—Acronym: SUSTAIN). We thank Professor Thomas Mavromoustakos for his advice and support concerning the calculation of the relaxation delay times for the NMR analysis related to the kinetic isotope effect measurements. We also acknowledge the contribution of COST Action CA15106 (C–H Activation in Organic Synthesis—CHAOS). We also thank the Spanish Ministerio de Ciencia e Innovación (PID2019-110008GB-I00) and IZO-SGI SGIker of UPV/EHU for financial and human support. The Special Account for Research Grants of the National and Kapodistrian University of Athens is also gratefully acknowledged for funding (research program 70/4/17454).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03092.
Spectroscopic data for compounds 5, 5a-D, and 12; computational methods and Cartesian coordinates (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Taylor D. R. The Chemistry of Allenes. Chem. Rev. 1967, 67, 317–359. 10.1021/cr60247a004. [DOI] [Google Scholar]; b Hoffmann-Röder A.; Krause N. Synthesis and Properties of Allenic Natural Products and Pharmaceuticals. Angew. Chem., Int. Ed. 2004, 43, 1196–1216. 10.1002/anie.200300628. [DOI] [PubMed] [Google Scholar]; c Krause N.; Hashmi A. S. K.. Modern Allene Chemistry; Wiley-VCH, Verlag GmbH: Weinheim, Germany, 2004. [Google Scholar]; d Brummond K. M. Allene Chemistry. Beilstein J. Org. Chem. 2011, 7, 394–395. 10.3762/bjoc.7.50. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Krause N.; Winter C. Gold-Catalyzed Nucleophilic Cyclization of Functionalized Allenes: A Powerful Access to Carbo-and Heterocycles. Chem. Rev. 2011, 111, 1994–2009. 10.1021/cr1004088. [DOI] [PubMed] [Google Scholar]; f Yu S.; Ma S. Allenes in Catalytic Asymmetric Synthesis and Natural Product Syntheses. Angew. Chem., Int. Ed. 2012, 51, 3074–3112. 10.1002/anie.201101460. [DOI] [PubMed] [Google Scholar]; g Soriano E.; Fernández I. Allenes and Computational Chemistry: From Bonding Situations to Reaction Mechanisms. Chem. Soc. Rev. 2014, 43, 3041–3105. 10.1039/c3cs60457h. [DOI] [PubMed] [Google Scholar]
- a Ohno H.; Chiba H.; Inuki S.; Oishi S.; Fujii N. The Synthesis of Alkaloids Using Transition-Metal-Catalyzed Intramolecular Amination Reactions. Synlett 2014, 25, 179–192. 10.1055/s-0033-1340165. [DOI] [Google Scholar]; b Reissig H. U.; Zimmer R.. Allenes in Multicomponent Synthesis of Heterocycles. Multicomponent Reactions in Organic Synthesis; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2014; pp 301–332. [Google Scholar]; c Alonso J. M.; Paz M. When Indoles Meet Allene and Its Derivatives. Eur. J. Org. Chem. 2020, 7197–7213. 10.1002/ejoc.202001269. [DOI] [Google Scholar]; d Fernandes R. A.; Pathare R. S.; Gorve D. A. Advances in Total Synthesis of Some 2, 3, 5-Trisubstituted Tetrahydrofuran Natural Products. Chem. Asian J. 2020, 2815–2837. 10.1002/asia.202000753. [DOI] [PubMed] [Google Scholar]
- a Lechel T.; Pfrengle F.; Reissig H. U.; Zimmer R. Three Carbons for Complexity! Recent Developments of Palladium-Catalyzed Reactions of Allenes. ChemCatChem 2013, 5, 2100–2130. 10.1002/cctc.201200875. [DOI] [Google Scholar]; b López F.; Mascareñas J. L. And [4+3] Catalytic Cycloadditions of Allenes. Chem. Soc. Rev. 2014, 43, 2904–2915. 10.1039/C4CS00024B. [DOI] [PubMed] [Google Scholar]; c Kitagaki S.; Inagaki F.; Mukai C. Cyclization of Allenes. Chem. Soc. Rev. 2014, 43, 2956–2978. 10.1039/c3cs60382b. [DOI] [PubMed] [Google Scholar]; d Alcaide B.; Almendros P.; Aragoncillo C. Cyclization Reactions of Bis(Allenes) for the Synthesis of Polycarbo(Hetero)Cycles. Chem. Soc. Rev. 2014, 43, 3106–3135. 10.1039/C3CS60462D. [DOI] [PubMed] [Google Scholar]; e Adams C. S.; Weatherly C. D.; Burke E. G.; Schomaker J. M. The Conversion of Allenes to Strained Three-Membered Heterocycles. Chem. Soc. Rev. 2014, 43, 3136–3163. 10.1039/C3CS60416K. [DOI] [PubMed] [Google Scholar]; f Lledó A.; Pla-Quintana A.; Roglans A. Allenes, Versatile Unsaturated Motifs in Transition-Metal-Catalysed [2+2+2] Cycloaddition Reactions. Chem. Soc. Rev. 2016, 45, 2010–2023. 10.1039/C5CS00535C. [DOI] [PubMed] [Google Scholar]; g Swamy K. C. K.; Anitha M.; Gangadhararao G.; Rama Suresh R. Exploring Allene Chemistry Using Phosphorus-Based Allenes as Scaffolds. Pure Appl. Chem. 2017, 89, 367–377. 10.1515/pac-2016-0907. [DOI] [Google Scholar]; h Santhoshkumar R.; Cheng C. H. Fickle Reactivity of Allenes in Transition-Metal-Catalyzed C–H Functionalizations. Asian J. Org. Chem. 2018, 7, 1151–1163. 10.1002/ajoc.201800133. [DOI] [Google Scholar]; i Holmes M.; Schwartz L. A.; Krische M. J. Intermolecular Metal-Catalyzed Reductive Coupling of Dienes, Allenes, and Enynes with Carbonyl Compounds and Imines. Chem. Rev. 2018, 118, 6026–6052. 10.1021/acs.chemrev.8b00213. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Michalak M.; Kosni W. Chiral N -Heterocyclic Carbene Gold Complexes: Synthesis and Applications in Catalysis. Catalysts 2019, 9, 890 10.3390/catal9110890. [DOI] [Google Scholar]; k Zhang W.; Guanlin L.; Huo X.; Xieyang J. Asymmetric Synthesis of Allylic Compounds via Hydrofunctionalisation and Difunctionalisation of Dienes, Allenes, and Alkynes. Chem. Soc. Rev. 2020, 49, 2060–2118. 10.1039/C9CS00400A. [DOI] [PubMed] [Google Scholar]; l Lozovskiy S. V. Synthesis of Heterocycles from Allenes Containing Electron-Withdrawing Substituents under the Conditions of Electrophilic Activation: Recent Advances. Chem. Heterocycl. Compd. 2020, 56, 848–853. 10.1007/s10593-020-02741-1. [DOI] [Google Scholar]; m Cadierno V. Gold-Catalyzed Addition of Carboxylic Acids to Alkynes and Allenes: Valuable Tools for Organic Synthesis. Catalysts 2020, 10, 1–37. 10.3390/catal10101206. [DOI] [Google Scholar]; n Hoveyda A. H.; Zhou Y.; Shi Y.; Brown M. K.; Wu H.; Torker S. Sulfonate N-Heterocyclic Carbene–Copper Complexes: Uniquely Effective Catalysts for Enantioselective Synthesis of C–C, C–B, C–H, and C–Si Bonds. Angew. Chem., Int. Ed. 2020, 59, 21304–21359. 10.1002/anie.202003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Yang Y.; Petersen J. L.; Wang K. K. Polycyclic Aromatic Compounds via Radical Cyclizations of Benzannulated Enyne-Allenes Derived from Ireland-Claisen Rearrangement. J. Org. Chem. 2003, 68, 8545–8549. 10.1021/jo035036z. [DOI] [PubMed] [Google Scholar]; b Brummond K. M.; You L. Consecutive Rh(I)-Catalyzed Alder-Ene/Diels-Alder/Diels-Alder Reaction Sequence Affording Rapid Entry to Polycyclic Compounds. Tetrahedron 2005, 61, 6180–6185. 10.1016/j.tet.2005.03.141. [DOI] [Google Scholar]; c Luzung M. R.; Mauleón P.; Toste F. D. Gold(I)-Catalyzed [2 + 2]-Cycloaddition of Allenenes. J. Am. Chem. Soc. 2007, 129, 12402–12403. 10.1021/ja075412n. [DOI] [PubMed] [Google Scholar]; d Krause N.; Aksin-Artok Ö.; Breker V.; Deutsch C.; Gockel B.; Poonoth M.; Sawama Y.; Sawama Y.; Sun T.; Winter C. Combined Coinage Metal Catalysis for the Synthesis of Bioactive Molecules. Pure Appl. Chem. 2010, 82, 1529–1536. 10.1351/PAC-CON-09-09-23. [DOI] [Google Scholar]; e Boobalan R.; Kuppusamy R.; Santhoshkumar R.; Gandeepan P.; Cheng C. H. Access to Isoquinolin-1(2 H)-Ones and Pyridones by Cobalt-Catalyzed Oxidative Annulation of Amides with Allenes. ChemCatChem 2017, 9, 273–277. 10.1002/cctc.201601190. [DOI] [Google Scholar]; f Han Y.; Ma S. Rhodium-Catalyzed Highly Diastereoselective Intramolecular [4 + 2] Cycloaddition of 1,3-Disubstituted Allene-1,3-Dienes. Org. Chem. Front. 2018, 5, 2680–2684. 10.1039/C8QO00650D. [DOI] [Google Scholar]; g Huang W.; Zhang Y. C.; Jin R.; Chen B. L.; Chen Z. Synthesis of Axially Chiral 1,2,3-Triazol-5-Ylidene-Au(I) Complex and Its Application in Enantioselective [2 + 2] Cycloaddition of Alleneamides with Alkenes. Organometallics 2018, 37, 3196–3209. 10.1021/acs.organomet.8b00524. [DOI] [Google Scholar]; h Sala R.; Broggini G. Palladium-Catalyzed Domino Carbopalladation/Cyclization of Allenes. Targets Heterocycl. Syst. 2018, 22, 139–164. [Google Scholar]; i Yu S.; Vermeeren P.; van Dommelen K.; Bickelhaupt F. M.; Hamlin T. A. Understanding the 1,3-Dipolar Cycloadditions of Allenes. Chem. - Eur. J. 2020, 26, 11529–11539. 10.1002/chem.202000857. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Nelson R.; Calvelo M.; García-Fandiño R.; Lledós A.; Ujaque G.; Mascareñas J. L.; López F. Skeletal Diversity in Pt- And Au-Catalyzed Annulations of Allenedienes: Dissecting Unconventional Mechanistic Pathways. Chem. Sci. 2020, 11, 4209–4220. 10.1039/D0SC00650E. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Jadhav P.; Chen J.; Liu R.; Jadhav P. D.; Chen J.; Liu R.. Letterof Cyclopentadienes with Nitrosoarenes via Nitroso- Povarov versus OxidativeNitroso-Povarov Reactions Gold (I) -Catalyzed Highly Enantioselective [4 + 2]-Annulations of Cy- Clopentadienes with Nitrosoarenes via Nitroso-Povarovversus Oxi- Dat. 2020, No. I.
- a Nakanowatari S.; Mei R.; Feldt M.; Ackermann L. Cobalt(III)-Catalyzed Hydroarylation of Allenes via C-H Activation. ACS Catal. 2017, 7, 2511–2515. 10.1021/acscatal.7b00207. [DOI] [Google Scholar]; b Han X.; Lin P.; Li Q. Recent Advances of Allenes in the First-Row Transition Metals Catalyzed C-H Activation Reactions. Chin. Chem. Lett. 2019, 30, 1495–1502. 10.1016/j.cclet.2019.04.027. [DOI] [Google Scholar]
- a Alcaide B.; Almendros P. Novel Cyclization Reactions of Aminoallenes. Adv. Synth. Catal. 2011, 353, 2561–2576. 10.1002/adsc.201100160. [DOI] [Google Scholar]; b Regás D.; Afonso M. M.; Palenzuela J. A. Pyridines and Pyridine Derivatives from Vinyl Allenes and Imines. Tetrahedron 2012, 68, 9345–9349. 10.1016/j.tet.2012.09.057. [DOI] [Google Scholar]; c Kim H.; Rhee Y. H. Stereodefined N,O-Acetals: Pd-Catalyzed Synthesis from Homopropargylic Amines and Utility in the Flexible Synthesis of 2,6-Substituted Piperidines. J. Am. Chem. Soc. 2012, 134, 4011–4014. 10.1021/ja2116298. [DOI] [PubMed] [Google Scholar]; d Thieme N.; Breit B. Enantioselective and Regiodivergent Addition of Purines to Terminal Allenes: Synthesis of Abacavir. Angew. Chem., Int. Ed. 2017, 56, 1520–1524. 10.1002/anie.201610876. [DOI] [PubMed] [Google Scholar]; e Bernar I.; Fiser B.; Blanco-Ania D.; Gómez-Bengoa E.; Rutjes F. P. J. T. Pd-Catalyzed Hydroamination of Alkoxyallenes with Azole Heterocycles: Examples and Mechanistic Proposal. Org. Lett. 2017, 19, 4211–4214. 10.1021/acs.orglett.7b01826. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Schmidt J. P.; Breit B. Transition Metal Catalyzed Stereodivergent Synthesis of: syn- and anti-δ-Vinyl-lactams: Formal Total Synthesis of (-)-Cermizine C and (-)-Senepodine G. Chem. Sci. 2019, 10, 3074–3079. 10.1039/C8SC05502E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Amako Y.; Arai S.; Nishida A. Transfer of Axial Chirality through the Nickel-Catalysed Hydrocyanation of Chiral Allenes. Org. Biomol. Chem. 2017, 15, 1612–1617. 10.1039/C7OB00047B. [DOI] [PubMed] [Google Scholar]; b Hori H.; Arai S.; Nishida A. Olefin-Migrative Cleavage of Cyclopropane Rings through the Nickel-Catalyzed Hydrocyanation of Allenes and Alkenes. Adv. Synth. Catal. 2017, 359, 1170–1176. 10.1002/adsc.201601400. [DOI] [Google Scholar]; c Long J.; Gao J.; Fang X. Nickel-Catalyzed Asymmetric Hydrocyanation of Allenes. Org. Lett. 2020, 22, 376–380. 10.1021/acs.orglett.9b03938. [DOI] [PubMed] [Google Scholar]
- a Zhang Z.; Widenhoefer R. A. Regio- And Stereoselective Synthesis of Alkyl Allylic Ethers via Gold(l)-Catalyzed Intermolecular Hydroalkoxylation of Allenes with Alcohols. Org. Lett. 2008, 10, 2079–2081. 10.1021/ol800646h. [DOI] [PubMed] [Google Scholar]; b Webster S.; Sutherland D. R.; Lee A.-L. Chirality Transfer in Gold(I)-Catalysed Hydroalkoxylation of 1,3-Disubstituted Allenes. Chem. - Eur. J. 2016, 22, 18593–18600. 10.1002/chem.201603918. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Tsukamoto H.; Ito K.; Doi T. Synthesis of Multi-Substituted Dihydrofurans via Palladium-Catalysed Coupling between 2,3-Alkadienols and Pronucleophiles. Chem. Commun. 2018, 54, 5102–5105. 10.1039/C8CC02589D. [DOI] [PubMed] [Google Scholar]; d Li Z.; Xie W.-B. Asymmetric Synthesis of Ethers by Catalytic Alkene Hydroalkoxylation. Synthesis 2020, 52, 2127 10.1055/s-0039-1690874. [DOI] [Google Scholar]
- a Tao X.; Daniliuc C. G.; Dittrich D.; Kehr G.; Erker G. Borane-Induced Dimerization of Arylallenes. Angew. Chem., Int. Ed. 2018, 57, 13922–13926. 10.1002/anie.201808436. [DOI] [PubMed] [Google Scholar]; b Nagashima Y.; Sasaki K.; Suto T.; Sato T.; Chida N. Stereodivergent Hydroboration of Allenes. Chem. Asian J. 2018, 13, 1024–1028. 10.1002/asia.201800134. [DOI] [PubMed] [Google Scholar]; c Qin A.; Qian H.; Chen Q.; Ma S. Palladium-Catalyzed Coupling of Propargylic Alcohols with Boronic Acids under Ambient Conditions. Chin. J. Chem. 2020, 38, 372–382. 10.1002/cjoc.201900442. [DOI] [Google Scholar]
- a Hoffmann-Röder A.; Krause N. Gold(III) Chloride Catalyzed Cyclization of a-Hydroxyallenes to 2,5-Dihydrofurans. Org. Lett. 2001, 3, 2537–2538. 10.1021/ol016205+. [DOI] [PubMed] [Google Scholar]; b Zhou C.-Y.; Chan P. W. H.; Che C.-M. Gold(III) Porphyrin-Catalyzed Cycloisomerization of Allenones. Org. Lett. 2006, 8, 325–328. 10.1021/ol052696c. [DOI] [PubMed] [Google Scholar]; c Zhang Z.; Widenhoefer R. A. Gold(I)-Catalyzed Intramolecular Enantioselective Hydroalkoxylation of Allenes. Angew. Chem., Int. Ed. 2006, 46, 283–285. 10.1002/anie.200603260. [DOI] [PubMed] [Google Scholar]; d Dudnik A. S.; Sromek A. W.; Rubina M.; Kim J. T.; Kel’i A. V.; Gevorgyan V. Metal-Catalyzed 1,2-Shift of Diverse Migrating Groups in Allenyl Systems as a New Paradigm toward Densely Functionalized Heterocycles. J. Am. Chem. Soc. 2008, 130, 1440–1452. 10.1021/ja0773507. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Poonoth M.; Krause N. Stereoselective Synthesis of Conjugated Bisallenols as Precursors of Novel Bis(2,5-Dihydrofuran) Derivatives. Adv. Synth. Catal. 2009, 351, 117–122. 10.1002/adsc.200800469. [DOI] [Google Scholar]; f Lalonde R. L.; Wang Z. J.; Mba M.; Lackner A. D.; Toste D. F. Gold(I)-Catalyzed Enantioselective Synthesis of Pyrazolidines, Isoxazolidines, and Tetrahydrooxazines. Angew. Chem., Int. Ed. 2010, 49, 598–601. 10.1002/anie.200905000. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Okada T.; Sakaguchi K.; Shinada T.; Ohfune Y. Au-Catalyzed Cyclization of Allenylsilanes. Regioselective Conversion to 2-Amino-4-Silylmethylene γ-Butyrolactone. Tetrahedron Lett. 2011, 52, 5740–5743. 10.1016/j.tetlet.2011.07.144. [DOI] [Google Scholar]; h Miles D. H.; Veguillas M.; Toste F. D. Gold(I)-Catalyzed Enantioselective Bromocyclization Reactions of Allenes. Chem. Sci. 2013, 4, 3427–3431. 10.1039/c3sc50811k. [DOI] [Google Scholar]; i Muñoz M. P. Silver and Platinum-Catalysed Addition of O-H and N-H Bonds to Allenes. Chem. Soc. Rev. 2014, 43, 3164–3183. 10.1039/c3cs60408j. [DOI] [PubMed] [Google Scholar]; j Hu F.; Xia Y.; Ma C.; Zhang Y.; Wang J. Cu(I)-Catalyzed Synthesis of Furan-Substituted Allenes by Use of Conjugated Ene-yne Ketones as Carbene Precursors. J. Org. Chem. 2016, 81, 3275–3285. 10.1021/acs.joc.6b00236. [DOI] [PubMed] [Google Scholar]; k Bogachenkov A. S.; Dogadina A. V.; Boyarskaya I. A.; Boyarskiy V. P.; Vasilyev A. V. Synthesis of 1,4-Dihydrophosphinoline 1-Oxides by Acid-Promoted Cyclization of 1-(Diphenylphosphoryl)Allenes. Org. Biomol. Chem. 2016, 14, 1370–1381. 10.1039/C5OB02143J. [DOI] [PubMed] [Google Scholar]; l Zhou J.; Fu C.; Ma S. Gold-Catalyzed Stereoselective Cycloisomerization of Allenoic Acids for Two Types of Common Natural γ-Butyrolactones. Nat. Commun. 2018, 9, 1654 10.1038/s41467-018-03894-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; m Zorba L.; Kidonakis M.; Saridakis I.; Stratakis M. Cycloisomerization of Conjugated Allenones into Furans under Mild Conditions Catalyzed by Ligandless Au Nanoparticles. Org. Lett. 2019, 21, 5552–5555. 10.1021/acs.orglett.9b01869. [DOI] [PubMed] [Google Scholar]; n Bernhard Y.; Gilbert J.; Bousquet T.; Favrelle-Huret A.; Zinck P.; Pellegrini S.; Pelinski L. One-Pot Synthesis of 2,5-Disubstituted Furans through In Situ Formation of Allenes and Enolization Cascade. Eur. J. Org. Chem. 2019, 7870–7873. 10.1002/ejoc.201901669. [DOI] [Google Scholar]
- a Yu S.; Ma S. How Easy Are the Syntheses of Allenes?. Chem. Commun. 2011, 47, 5384–5418. 10.1039/C0CC05640E. [DOI] [PubMed] [Google Scholar]; b Neff R. K.; Frantz D. E. Recent Advances in the Catalytic Syntheses of Allenes: A Critical Assessment. ACS Catal. 2014, 4, 519–528. 10.1021/cs401007. [DOI] [Google Scholar]; c Chu W.-D.; Zhang Y.; Wang J. Recent Advances in Catalytic Asymmetric Synthesis of Allenes. Catal. Sci. Technol. 2017, 7, 4570–4579. 10.1039/C7CY01319A. [DOI] [Google Scholar]; d Armstrong R. J. Synthesis of Allenes by 1,2-Elimination. Curr. Org. Chem. 2019, 23, 3027–3039. 10.2174/1385272823666191121122011. [DOI] [Google Scholar]; e Fu L.; Greßies S.; Chen P.; Liu G. Recent Advances and Perspectives in Transition Metal-Catalyzed 1,4-Functionalizations of Unactivated 1,3-Enynes for the Synthesis of Allenes. Chin. J. Chem. 2020, 38, 91–100. 10.1002/cjoc.201900277. [DOI] [Google Scholar]; f Shruthi K. S.; Singh P.; Prasad K. R. Stereoselective Synthesis of Functionalized Allenes from Tartaric Acid. Tetrahedron 2020, 76, 131706 10.1016/j.tet.2020.131706. [DOI] [Google Scholar]
- a Yokota M.; Fuchibe K.; Ueda M.; Mayumi Y.; Ichikawa J. Facile Synthesis of 1,1-Difluoroallenes via the Difluorovinylidenation of Aldehydes and Ketones. Org. Lett. 2009, 11, 3994–3997. 10.1021/ol9016673. [DOI] [PubMed] [Google Scholar]; b Mori N.; Obuchi K.; Katae T.; Sakurada J.; Satoh T. Alkenylation of Thiophenes and Furans at the 2-Position and a Synthesis of Allenes Conjugated with α,β-Unsaturated Ester with Magnesium Alkylidene Carbenoids. Tetrahedron 2009, 65, 3509–3517. 10.1016/j.tet.2009.02.019. [DOI] [Google Scholar]; c Satoh T.; Kaneta H.; Matsushima A.; Yajima M. A New Synthesis of β,γ-Unsaturated Esters and Allenic Esters with Construction of a Carbon-Carbon Bond between α- and β-Positions by the Reaction of Magnesium Alkylidene Carbenoids with Lithium Ester Enolates. Tetrahedron Lett. 2009, 50, 6280–6285. 10.1016/j.tetlet.2009.08.102. [DOI] [Google Scholar]; d Zhang Y.; Hao H.-D.; Wu Y. An 1,2-Elimination Approach to the Enantioselective Synthesis of 1,3-Disubstituted Linear Allenes. Synlett 2010, 905–908. 10.1055/s-0029-1219542. [DOI] [Google Scholar]
- a Yu X.; Ren H.; Xiao Y.; Zhang J. Efficient Assembly of Allenes, 1,3-Dienes, and 4H-Pyrans by Catalytic Regioselective Nucleophilic Addition to Electron-Deficient 1,3-Conjugated Enynes. Chem. - Eur. J. 2008, 14, 8481–8485. 10.1002/chem.200801004. [DOI] [PubMed] [Google Scholar]; b Todo H.; Terao J.; Watanabe H.; Kuniyasu H.; Kambe N. Cu-Catalyzed Regioselective Carbomagnesiation of Dienes and Enynes with Sec- and Tert-Alkyl Grignard Reagents. Chem. Commun. 2008, 1332–1334. 10.1039/b716678h. [DOI] [PubMed] [Google Scholar]; c Ma Z.; Zeng R.; Yu Y.; Ma S. Highly Stereoselective Synthesis of 6-Perfluoroalkyl-6-Fluoroalka-2,3,5-(Z)-Trienols through Carbometallation-Elimination of 5-Perfluoroalkyl-substituted 4(E)-Alken-2-Ynols with Grignard Reagents. Tetrahedron Lett. 2009, 50, 6472–6475. 10.1016/j.tetlet.2009.09.003. [DOI] [Google Scholar]; d Zhang W.; Zheng S.; Liu N.; Werness J. B.; Guzei I. A.; Tang W. Enantioselective Bromolactonization of Conjugated (Z)-Enynes. J. Am. Chem. Soc. 2010, 132, 3664–3665. 10.1021/ja100173w. [DOI] [PubMed] [Google Scholar]; e Nishimura T.; Makino H.; Nagaosa M.; Hayashi T. Rhodium-Catalyzed Enantioselective 1,6-Addition of Arylboronic Acids to Enynamides: Asymmetric Synthesis of Axially Chiral Allenylsilanes. J. Am. Chem. Soc. 2010, 132, 12865–12867. 10.1021/ja1066509. [DOI] [PubMed] [Google Scholar]; f Xiao Y.; Zhang J. Tetrasubstituted Allenes by Pd0-Catalyzed Three-Component Tandem Michael Addition/Cross-Coupling Reaction. Chem. Commun. 2010, 46, 752–754. 10.1039/B919060K. [DOI] [PubMed] [Google Scholar]; g Mömming Cornelia M.; Kehr G.; Wibbeling B.; Fröhlich R.; Schirmer B.; Grimme S.; Erker G. Formation of Cyclic Allenes and Cumulenes by Cooperative Addition of Frustrated Lewis Pairs to Conjugated Enynes and Diynes. Angew. Chem., Int. Ed. 2010, 49, 2414–2417. 10.1002/anie.200906697. [DOI] [PubMed] [Google Scholar]; h Yao Q.; Liao Y.; Lin L.; Lin X.; Ji J.; Liu X.; Feng X. Efficient Synthesis of Chiral Trisubstituted 1,2-Allenyl Ketones by Catalytic Asymmetric Conjugate Addition of Malonic Esters to Enynes. Angew. Chem., Int. Ed. 2016, 55, 1859–1863. 10.1002/anie.201509455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Sanz R.; Gohain M.; Miguel D.; Martínez A.; Rodríguez F. Synthesis of 3-Allenylindoles and 3-Dienylindoles by Brønsted Acid Catalyzed Allenylation of 2-Arylindoles with Tertiary Propargylic Alcohols. Synlett 2009, 1985–1989. 10.1055/s-0029-1217543. [DOI] [Google Scholar]; b Fandrick D. R.; Reeves J. T.; Tan Z.; Lee H.; Song J. J.; Yee N. K.; Senanayake C. H. Regioselective Allene Synthesis and Propargylations with Propargyl Diethanolamine Boronates. Org. Lett. 2009, 11, 5458–5461. 10.1021/ol9022529. [DOI] [PubMed] [Google Scholar]; c Jiang H.; Wang W.; Yin B.; Liu W. Facile Synthesis of Trisubstituted Allenynes by Phosphane-Mediated Deoxygenation of 2,4-Pentadiyn-1-ol. Eur. J. Org. Chem. 2010, 4450–4453. 10.1002/ejoc.201000483. [DOI] [Google Scholar]
- a Shono T.; Ito K.; Tsubouchi A.; Takeda T. Titanocene(II)-Promoted Carbonyl Allenation Utilizing 1,1-Dichloroalk-1-enes. Org. Biomol. Chem. 2005, 16, 2914–2916. 10.1039/b508820h. [DOI] [PubMed] [Google Scholar]; b Zhou H.; Liu G.; Zeng C. Bismetalated Carbon for Tandem Wittig-Type Reaction via Allylgallation of Magnesium Acetylides: A Convenient and Efficient Method to Allyl Allenes. J. Organomet. Chem. 2008, 693, 787–791. 10.1016/j.jorganchem.2007.12.003. [DOI] [Google Scholar]; c Mundal D. A.; Lutz K. E.; Thomson R. J. A Direct Synthesis of Allenes by a Traceless Petasis Reaction. J. Am. Chem. Soc. 2012, 134, 5782–5785. 10.1021/ja301489n. [DOI] [PubMed] [Google Scholar]
- a Poh J.-S.; Tran D. N.; Battilocchio C.; Hawkins J. M.; Ley S. V. A Versatile Room-Temperature Route to Di- and Trisubstituted Allenes Using Flow-Generated Diazo Compounds. Angew. Chem., Int. Ed. 2015, 54, 7920–7923. 10.1002/anie.201501538. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wu C.; Hu F.; Liu Z.; Deng G.; Ye F.; Zhang Y.; Wang J. Cu(I)-Catalyzed Coupling of Diaryldiazomethanes with Terminal Alkynes: An Efficient Synthesis of Tri-Aryl-Substituted Allenes. Tetrahedron 2015, 71, 9196–9201. 10.1016/j.tet.2015.10.043. [DOI] [Google Scholar]; c Ye F.; Wang C.; Ma X.; Hossain M. L.; Xia Y.; Zhang Y.; Wang J. Synthesis of Terminal Allenes through Copper-Mediated Cross-Coupling of Ethyne with N-Tosylhydrazones or α-Diazoesters. J. Org. Chem. 2015, 80, 647–652. 10.1021/jo502316q. [DOI] [PubMed] [Google Scholar]; d Chu W.-D.; Zhang L.; Zhang Z.; Zhou Q.; Mo F.; Zhang Y.; Wang J. Enantioselective Synthesis of Trisubstituted Allenes via Cu(I)-Catalyzed Coupling of Diazoalkanes with Terminal Alkynes. J. Am. Chem. Soc. 2016, 138, 14558–14561. 10.1021/jacs.6b09674. [DOI] [PubMed] [Google Scholar]; e Poh J.-S.; Makai S.; von Keutz T.; Tran D. N.; Battilocchio C.; Pasau P.; Ley S. V. Rapid Asymmetric Synthesis of Disubstituted Allenes by Coupling of Flow-Generated Diazo Compounds and Propargylated Amines. Angew. Chem., Int. Ed. 2017, 56, 1864–1868. 10.1002/anie.201611067. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Hossain M. L.; Wang J. Cu(I)-Catalyzed Cross-Coupling of Diazo Compounds with Terminal Alkynes: An Efficient Access to Allenes. Chem. Rec. 2018, 18, 1548–1559. 10.1002/tcr.201800023. [DOI] [PubMed] [Google Scholar]
- a Lavallo V.; Frey G. D.; Kousar S.; Donnadieu B.; Bertrand G. Allene Formation by Gold Catalyzed Cross-Coupling of Masked Carbenes and Vinylidenes. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 13569–13573. 10.1073/pnas.0705809104. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chen B.; Wang N.; Fan W.; Ma S. Efficient Synthesis of N-(Buta-2,3-Dienyl) Amides from Terminal N-Propargyl Amides and Their Synthetic Potential towards Oxazoline Derivatives. Org. Biomol. Chem. 2012, 10, 8465–8470. 10.1039/c2ob26291f. [DOI] [PubMed] [Google Scholar]; c Li H.; Grassi D.; Guénée L.; Bürgi T.; Alexakis A. Copper-Catalyzed Propargylic Substitution of Dichloro Substrates: Enantioselective Synthesis of Trisubstituted Allenes and Formation of Propargylic Quaternary Stereogenic Centers. Chem. - Eur. J. 2014, 20, 16694–16706. 10.1002/chem.201404668. [DOI] [PubMed] [Google Scholar]; d Yang Z.; Hao W.-J.; Wang S.-L.; Zhang J.-P.; Jiang B.; Li G.; Tu S.-J. Synthesis of Allenyl Sulfones via a TBHP/TBAI-Mediated Reaction of Propargyl Alcohols with Sulfonyl Hydrazides. J. Org. Chem. 2015, 80, 9224–9230. 10.1021/acs.joc.5b01684. [DOI] [PubMed] [Google Scholar]; e Luo H.; Yu Y.; Ma S. Suzuki Coupling for Preparation of Allenes - Ligand Effects and Chirality Transfer. Org. Chem. Front. 2016, 3, 1705–1710. 10.1039/C6QO00489J. [DOI] [Google Scholar]; f Kessler S. N.; Bäckvall J.-E. Iron-Catalyzed Cross-Coupling of Propargyl Carboxylates and Grignard Reagents: Synthesis of Substituted Allenes. Angew. Chem., Int. Ed. 2016, 55, 3734–3738. 10.1002/anie.201511139. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Ruchti J.; Carreira E. M. Rh-Catalyzed Stereospecific Synthesis of Allenes from Propargylic Benzoates and Arylboronic Acids. Org. Lett. 2016, 18, 2174–2176. 10.1021/acs.orglett.6b00793. [DOI] [PubMed] [Google Scholar]; h Ma S.; Liu Q.; Tang X.; Cai Y. Copper-Catalyzed Synthesis of Tetrasubstituted Allenes from Quaternary Ammonium Salts and Grignard Reagents. Asian J. Org. Chem. 2017, 6, 1209–1212. 10.1002/ajoc.201600620. [DOI] [Google Scholar]; i Zhang Z.; Shao X.; Zhang G.; Li Q.; Li X. Highly Efficient Synthesis of Multi-Substituted Allenes from Propargyl Acetates and Organoaluminum Reagents Mediated by Palladium. Synthesis 2017, 49, 3643–3653. 10.1055/s-0036-1588177. [DOI] [Google Scholar]; j Zhang W.; Huang C.; Yuan Y.; Ma S. Catalytic Transient Leaving Group for Atom-Economic Synthesis of Allenes from 2-Alkynols. Chem. Commun. 2017, 53, 12430–12433. 10.1039/C7CC06866B. [DOI] [PubMed] [Google Scholar]; k Yang Y.; Liu Z.; Porta A.; Zanoni G.; Bi X. Alkynyl N-Nosylhydrazones: Easy Decomposition to Alknynl Diazomethanes and Application in Allene Synthesis. Chem. - Eur. J. 2017, 23, 9009–9013. 10.1002/chem.201701462. [DOI] [PubMed] [Google Scholar]; l Domingo-Legarda P.; Soler-Yanes R.; Quirós-López M. T.; Buñuel E.; Cárdenas D. J. Iron-Catalyzed Coupling of Propargyl Bromides and Alkyl Grignard Reagents. Eur. J. Org. Chem. 2018, 4900–4904. 10.1002/ejoc.201800849. [DOI] [Google Scholar]; m Guisán-Ceinos M.; Martín-Heras V.; Soler-Yanes R.; Cárdenas D. J.; Tortosa M. Copper-Catalysed Cross-Coupling of Alkyl Grignard Reagents and Propargylic Ammonium Salts: Stereospecific Synthesis of Allenes. Chem. Commun. 2018, 54, 8343–8346. 10.1039/C8CC03760D. [DOI] [PubMed] [Google Scholar]; n Shao X. B.; Zhang Z.; Li Q. H.; Zhao Z. G. Synthesis of Multi-Substituted Allenes from Organoalane Reagents and Propargyl Esters by Using a Nickel Catalyst. Org. Biomol. Chem. 2018, 16, 4797–4806. 10.1039/C8OB00781K. [DOI] [PubMed] [Google Scholar]; o Wang H.; Luo H.; Zhang Z.-M.; Zheng W.-F.; Yin Y.; Qian H.; Zhang J.; Ma S. Pd-Catalyzed Enantioselective Syntheses of Trisubstituted Allenes via Coupling of Propargylic Benzoates with Organoboronic Acids. J. Am. Chem. Soc. 2020, 142, 9763–9771. 10.1021/jacs.0c02876. [DOI] [PubMed] [Google Scholar]; p Taj Muhammad M.; Jiao Y.; Ye C.; Chiou M.-F.; Israr M.; Zhu X.; Li Y.; Wen Z.; Studer A.; Bao H. Synthesis of Difluoromethylated Allenes through Trifunctionalization of 1,3-Enynes. Nat. Commun. 2020, 11, 1881 10.1038/s41467-019-14254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Crabbé P.; Fillion H.; André D.; Luche J.-L. Efficient Homologation of Acetylenes to Allenes. J. Chem. Soc. Chem. Commun. 1979, 859–860. 10.1039/C39790000859. [DOI] [Google Scholar]; b Searles S.; Li Y.; Nassim B.; Lopes M.-T. R.; Tran P. T.; Crabbé P. Observation on the Synthesis of Allenes by Homologation of Alk-1-Ynes. J. Chem. Soc., Perkin Trans. 1 1984, 1, 747–751. 10.1039/P19840000747. [DOI] [Google Scholar]
- Huang X.; Ma S. Allenation of Terminal Alkynes with Aldehydes and Ketones. Acc. Chem. Res. 2019, 52, 1301–1312. 10.1021/acs.accounts.9b00023. [DOI] [PubMed] [Google Scholar]
- Ma S.; Hou H.; Zhao S.; Wang G. Efficient Synthesis of Optically Active 2,3-Allenols via the Simple CuBr-Mediated Reaction of Optically Active Propargylic Alcohols with Paraformaldehyde. Synthesis 2002, 1643–1645. 10.1055/s-2002-33656. [DOI] [Google Scholar]
- Nakamura H.; Sugiishi T.; Tanaka Y. Synthesis of Allenes via CuBr-Catalyzed Homologation of Alk-1-Ynes Accelerated by Microwave. Tetrahedron Lett. 2008, 49, 7230–7233. 10.1016/j.tetlet.2008.10.019. [DOI] [Google Scholar]
- a Kuang J.; Ma S. An Efficient Synthesis of Terminal Allenes from Terminal 1-Alkynes. J. Org. Chem. 2009, 74, 1763–1765. 10.1021/jo802391x. [DOI] [PubMed] [Google Scholar]; b Luo H.; Ma S. CuI-Catalyzed Synthesis of Functionalized Terminal Allenes from 1-Alkynes. Eur. J. Org. Chem. 2013, 3041–3048. 10.1002/ejoc.201201696. [DOI] [Google Scholar]
- Kuang J.; Ma S. One-Pot Synthesis of 1,3-Disubstituted Allenes from 1-Alkynes, Aldehydes, and Morpholine. J. Am. Chem. Soc. 2010, 132, 1786–1787. 10.1021/ja910503k. [DOI] [PubMed] [Google Scholar]
- Kitagaki S.; Komizu M.; Mukai C. Can the Crabbé Homologation Be Successfully Applied to the Synthesis of 1,3-Disubstituted Allenes?. Synlett 2011, 1129–1132. 10.1055/s-0030-1259936. [DOI] [Google Scholar]
- Kuang J.; Luo H.; Ma S. Copper (I) Iodide-Catalyzed One-Step Preparation of Functionalized Allenes from Terminal Alkynes: Amine Effect. Adv. Synth. Catal. 2012, 354, 933–944. 10.1002/adsc.201100772. [DOI] [Google Scholar]
- Jiang G.-J.; Zheng Q.-H.; Dou M.; Zhuo L.-G.; Meng W.; Yu Z.-X. Mild-Condition Synthesis of Allenes from Alkynes and Aldehydes Mediated by Tetrahydroisoquinoline (THIQ). J. Org. Chem. 2013, 78, 11783–11793. 10.1021/jo4018183. [DOI] [PubMed] [Google Scholar]
- Lustosa D. M.; Clemens S.; Rudolph M.; Hashmi A. S. K. Gold-Catalyzed One-Pot Synthesis of 1,3-Disubstituted Allenes from Benzaldehydes and Terminal Alkynes. Adv. Synth. Catal. 2019, 361, 5050–5056. 10.1002/adsc.201900824. [DOI] [Google Scholar]
- Schaarschmidt M.; Wanner K. T. Synthesis of Allene Substituted Nipecotic Acids by Allenylation of Terminal Alkynes. J. Org. Chem. 2017, 82, 8371–8388. 10.1021/acs.joc.7b00630. [DOI] [PubMed] [Google Scholar]
- a Lo V. K.-Y.; Wong M.-K.; Che C.-M. Gold-Catalyzed Highly Enantioselective Synthesis of Axially Chiral Allenes. Org. Lett. 2008, 10, 517–519. 10.1021/ol702970r. [DOI] [PubMed] [Google Scholar]; b Lo V. K.-Y.; Zhou C.-Y.; Wong M.-K.; Che C.-M. Silver(I)-Mediated Highly Enantioselective Synthesis of Axially Chiral Allenes under Thermal and Microwave-Assisted Conditions. Chem. Commun. 2010, 46, 213–215. 10.1039/B914516H. [DOI] [PubMed] [Google Scholar]
- a Ma S. Some Typical Advances in the Synthetic Applications of Allenes. Chem. Rev. 2005, 105, 2829–2872. 10.1021/cr020024j. [DOI] [PubMed] [Google Scholar]; b Neff R. K.; Frantz D. E. Recent Applications of Chiral Allenes in Axial-to-Central Chirality Transfer Reactions. Tetrahedron 2015, 71, 7–18. 10.1016/j.tet.2014.08.030. [DOI] [Google Scholar]
- a Ye J.; Li S.; Chen B.; Fan W.; Kuang J.; Liu J.; Liu Y.; Miao B.; Wan B.; Wang Y.; Xie X.; Yu Q.; Yuan W.; Ma S. Catalytic Asymmetric Synthesis of Optically Active Allenes from Terminal Alkynes. Org. Lett. 2012, 14, 1346–1349. 10.1021/ol300296k. [DOI] [PubMed] [Google Scholar]; b Periasamy M.; Sanjeevakumar N.; Dalai M.; Gurubrahamam R.; Reddy P. O. Highly Enantioselective Synthesis of Chiral Allenes by Sequential Creation of Stereogenic Center and Chirality Transfer in a Single Pot Operation. Org. Lett. 2012, 14, 2932–2935. 10.1021/ol300717e. [DOI] [PubMed] [Google Scholar]; c Ye J.; Lü R.; Fan W.; Ma S. Studies on ZnBr2-Mediated Synthesis of Axially Chiral Aryl-Substituted Allenes from Terminal Alkynes, Aromatic Aldehydes and (S)-α,α-Diphenylprolinol. Tetrahedron 2013, 69, 8959–8963. 10.1016/j.tet.2013.07.021. [DOI] [Google Scholar]; d Lü R.; Ye J.; Cao T.; Chen B.; Fan W.; Lin W.; Liu J.; Luo H.; Miao B.; Ni S.; Tang X.; Wang N.; Wang Y.; Xie X.; Yu Q.; Yuan W.; Zhang W.; Zhu C.; Ma S. Bimetallic Enantioselective Approach to Axially Chiral Allenes. Org. Lett. 2013, 15, 2254–2257. 10.1021/ol400822m. [DOI] [PubMed] [Google Scholar]; e Gurubrahamam R.; Periasamy M. Copper(I) Halide Promoted Diastereoselective Synthesis of Chiral Propargylamines and Chiral Allenes using 2-Dialkylaminomethylpyrrolidine, Aldehydes, and 1-Alkynes. J. Org. Chem. 2013, 78, 1463–1470. 10.1021/jo302534f. [DOI] [PubMed] [Google Scholar]; f Ye J.; Ma S. Conquering Three-Carbon Axial Chirality of Allenes. Org. Chem. Front. 2014, 1, 1210–1224. 10.1039/C4QO00208C. [DOI] [Google Scholar]; g Tang X.; Huang X.; Cao T.; Han Y.; Jiang X.; Lin W.; Tang Y.; Zhang J.; Yu Q.; Fu C.; Ma S. CuBr2-Catalyzed Enantioselective Routes to Highly Functionalized and Naturally Occurring Allenes. Org. Chem. Front. 2015, 2, 688–691. 10.1039/C5QO00084J. [DOI] [Google Scholar]; h Periasamy M.; Reddy P. O.; Sanjeevakumar N. Convenient Methods for the Synthesis of Highly Functionalized and Naturally Occurring Chiral Allenes. Tetrahedron: Asymmetry 2014, 25, 1634–1646. 10.1016/j.tetasy.2014.11.002. [DOI] [Google Scholar]; i Periasamy M.; Reddy P. O.; Edukondalu A.; Dalai M.; Alakonda L. M.; Udaykumar B. Zinc Salt Promoted Diastereoselective Synthesis of Chiral Propargylamines Using Chiral Piperazines and Their Enantioselective Conversion into Chiral Allenes. Eur. J. Org. Chem. 2014, 6067–6076. 10.1002/ejoc.201402766. [DOI] [Google Scholar]; j Zhang J.; Ye J.; Ma S. Harmony of CdI2 with CuBr for the One-Pot Synthesis of Optically Active α-Allenols. Org. Biomol. Chem. 2015, 13, 4080–4089. 10.1039/C4OB02673J. [DOI] [PubMed] [Google Scholar]; k Huang X.; Cao T.; Han Y.; Jiang X.; Lin W.; Zhang J.; Ma S. General CuBr2-Catalyzed Highly Enantioselective Approach for Optically Active Allenols from Terminal Alkynols. Chem. Commun. 2015, 51, 6956–6959. 10.1039/C5CC00697J. [DOI] [PubMed] [Google Scholar]; l Periasamy M.; Edukondalu A.; Ramesh E. Synthesis and Desymmetrization of meso-2,3-Diphenylpiperazine for Application in Asymmetric Transformations. ChemistrySelect 2017, 2, 3937–3942. 10.1002/slct.201700488. [DOI] [Google Scholar]; m Periasamy M.; Mohan L.; Satyanarayana I.; Reddy P. O. Enantioselective Synthesis of β-Allenoates via Phosphine-Catalyzed and ZnI2-Promoted Preparation of Oxazolidines and Propargylamines Using Chiral Amines, 1-Alkynes, and Propiolates. J. Org. Chem. 2018, 83, 267–274. 10.1021/acs.joc.7b02632. [DOI] [PubMed] [Google Scholar]; n Ma D.; Duan X.; Fu C.; Huang X.; Ma S. Dimethylprolinol Versus Diphenylprolinol in CuBr2-Catalyzed Enantioselective Allenylation of Terminal Alkynols. Synthesis 2018, 50, 2533–2545. 10.1055/s-0036-1592007. [DOI] [Google Scholar]
- Tang X.; Zhu C.; Cao T.; Kuang J.; Lin W.; Ni S.; Zhang J.; Ma S. Cadmium Iodide-Mediated Allenylation of Terminal Alkynes with Ketones. Nat. Commun. 2013, 4, 2450 10.1038/ncomms3450. [DOI] [PubMed] [Google Scholar]
- a Zhuang W.; Saaby S.; Jørgensen K. A. Direct Organocatalytic Enantioselective Mannich Reactions of Ketimines: An Approach to Optically Active Quaternary α -Amino Acid Derivatives. Angew. Chem., Int. Ed. 2004, 43, 4476–4478. 10.1002/anie.200460158. [DOI] [PubMed] [Google Scholar]; b Wada R.; Shibuguchi T.; Makino S.; Oisaki K.; Kanai M.; Shibasaki M. Catalytic Enantioselective Allylation of Ketoimines. J. Am. Chem. Soc. 2006, 128, 7687–7691. 10.1021/ja061510h. [DOI] [PubMed] [Google Scholar]; c Zorba L. P.; Vougioukalakis G. C. The Ketone-Amine-Alkyne (KA2) Coupling Reaction: Transition Metal-Catalyzed Synthesis of Quaternary Propargylamines. Coord. Chem. Rev. 2020, 429, 213603 10.1016/j.ccr.2020.213603. [DOI] [Google Scholar]
- Pereshivko O. P.; Peshkov V. A.; Van Der Eycken E. V. Unprecedented Cu(I)-Catalyzed Microwave-Assisted Three-Component Coupling of a Ketone, an Alkyne, and a Primary Amine. Org. Lett. 2010, 12, 2638–2641. 10.1021/ol1008312. [DOI] [PubMed] [Google Scholar]
- a Kuang J.; Tang X.; Ma S. Zinc Diiodide-Promoted Synthesis of Trisubstituted Allenes from Propargylic Amines. Org. Chem. Front. 2015, 2, 470–475. 10.1039/C5QO00047E. [DOI] [Google Scholar]; b Liu Q.; Tang X.; Cai Y.; Ma S. Catalytic One-Pot Synthesis of Trisubstituted Allenes from Terminal Alkynes and Ketones. Org. Lett. 2017, 19, 5174–5177. 10.1021/acs.orglett.7b02443. [DOI] [PubMed] [Google Scholar]
- a Pinaka A.; Vougioukalakis G. C. Using Sustainable Metals to Carry out “Green” Transformations: Fe- and Cu-Catalyzed CO2 Monetization. Coord. Chem. Rev. 2015, 288, 69–97. 10.1016/j.ccr.2015.01.010. [DOI] [Google Scholar]; b Tzouras N. V.; Stamatopoulos I. K.; Papastavrou A. T.; Liori A. A.; Vougioukalakis G. C. Sustainable Metal Catalysis in C–H Activation. Coord. Chem. Rev. 2017, 343, 25–138. 10.1016/j.ccr.2017.04.012. [DOI] [Google Scholar]; c Liori A. A.; Stamatopoulos I. K.; Papastavrou A. T.; Pinaka A.; Vougioukalakis G. C. A Sustainable, User-Friendly Protocol for the Pd-Free Sonogashira Coupling Reaction. Eur. J. Org. Chem. 2018, 6134–6139. 10.1002/ejoc.201800827. [DOI] [Google Scholar]; d Papastavrou A. T.; Pauze M.; Gomez-Bengoa E.; Vougioukalakis G. C. Unprecedented Multicomponent Organocatalytic Synthesis of Propargylic Esters via CO2 Activcation. ChemCatChem 2019, 11, 5379–5386. 10.1002/cctc.201900207. [DOI] [Google Scholar]; e Adejumo T. T.; Tzouras N. V.; Zorba L. P.; Radanović D.; Pevec A.; Grubišić S.; Mitić D.; Anđelković K. K.; Vougioukalakis G. C.; Čobeljić B.; Turel I. Synthesis, Characterization, Catalytic Activity, and DFT Calculations of Zn(II) Hydrazone Complexes. Molecules 2020, 25, 4043 10.3390/molecules25184043. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Neofotistos S. P.; Tzouras N. V.; Pauze M.; Gómez-Bengoa E.; Vougioukalakis G. C. Manganese-Catalyzed Multicomponent Synthesis of Tetrasubstituted Propargylamines: System Development and Theoretical Study. Adv. Synth. Catal. 2020, 362, 3872–3885. 10.1002/adsc.202000566. [DOI] [Google Scholar]; g Tonis E.; Stein F.; Stamatopoulos I. K.; Stubbe J.; Zarkadoulas A.; Sarkar B.; Vougioukalakis G. C. A Pd-Free Sonogashira Coupling Protocol Employing an In-Situ-Prepared Copper/Chelating 1,2,3-Triazolylidene System. Synlett 2021, 32, 616–620. 10.1055/a-1290-8469. [DOI] [Google Scholar]
- Tzouras N. V.; Neofotistos S. P.; Vougioukalakis G. C. Zn-Catalyzed Multicomponent KA2 Coupling: One-Pot Assembly of Propargylamines Bearing Tetrasubstituted Carbon Centers. ACS Omega 2019, 4, 10279–10292. 10.1021/acsomega.9b01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce C. J.; Nguyen M.; Larsen C. H. Copper/Titanium Catalysis Forms Fully Substituted Carbon Centers from the Direct Coupling of Acyclic Ketones, Amines, and Alkynes. Angew. Chem., Int. Ed. 2012, 51, 12289–12292. 10.1002/anie.201206674. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Xu W.; You Z.; Wang Z.; Luo Y.; Gao L.; Yin C.; Peng R.; Lan L. A New Type of Power Energy for Accelerating Chemical Reactions: The Nature of a Microwave-Driving Force for Accelerating Chemical Reactions. Sci. Rep. 2016, 6, 25149 10.1038/srep25149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy M.; Reddy P. O.; Satyanarayana I.; Mohan L.; Edukondalu A. Diastereoselective Synthesis of Tetrasubstituted Propargylamines via Hydroamination and Metalation of 1-Alkynes and Their Enantioselective Conversion to Trisubstituted Chiral Allenes. J. Org. Chem. 2016, 81, 987–999. 10.1021/acs.joc.5b02554. [DOI] [PubMed] [Google Scholar]
- a Price J. D.; Johnson R. P. Cumulene Photochemistry: Photoreactions of a Strained 1.2-Cyclooctadiene. J. Org. Chem. 1991, 56, 6372–6376. 10.1021/jo00022a029. [DOI] [Google Scholar]; b Hayashi R.; Hsung R.; Feltenberger J. B.; Lohse A. G. Regio- and Stereoselective Isomerizations of Allenamides: Synthesis of 2-Amido-Dienes and Their Tandem Isomerization-Electrocyclic Ring-Closure. Org. Lett. 2009, 11, 2125–2128. 10.1021/ol900647s. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Kim J. H.; Kim S. W.; Jung S. M.; Ahn K.-H.; Kang E. J. Regioselectivities in Fe(III)-catalyzed Cycloisomerization Reactions of γ-Allenyl Alcohol. Bull. Korean Chem. Soc. 2015, 36, 2846–2850. 10.1002/bkcs.10581. [DOI] [Google Scholar]; d Titov A. A.; Kobzev M. S.; Borisova T. N.; Listratova A. V.; Evenko T. V.; Varlamov A. V.; Voskressensky L. G. Facile Methods for the Synthesis of 8-Ylidene-1.2.3.8-tetrahydrobenzazecines. Eur. J. Org. Chem. 2020, 3041–3049. 10.1002/ejoc.202000203. [DOI] [Google Scholar]
- Derosa J.; Cantu A. L.; Boulous M. N.; O’Duill M. L.; Turnbull J. L.; Liu Z.; De La Torre D. M.; Engle K. M. Palladium(II)-Catalyzed Directed anti-Hydrochlorination of Unactivated Alkynes with HCl. J. Am. Chem. Soc. 2017, 139, 5183–5193. 10.1021/jacs.7b00892. [DOI] [PubMed] [Google Scholar]
- Chamduang C.; Pingaew R.; Prachayasittikul V.; Prachayasittikul S.; Ruchirawat S.; Prachayasittikul V. Novel Triazole-Tetrahydroisoquinoline Hybrids as Human Aromatase Inhibitors. Bioorg. Chem. 2019, 93, 103327 10.1016/j.bioorg.2019.103327. [DOI] [PubMed] [Google Scholar]
- Cheng X.; Jiang X.; Yu Y.; Ma S. Efficient Synthesis of 3-Chloromethyl-2(5H)-furanones and 3-Chloromethyl- 5,6-dihydropyran-2-ones via the PdCl2-Catalyzed Chlorocyclocarbo-nylation of 2,3- or 3,4-Allenols. J. Org. Chem. 2008, 73, 8960–8965. 10.1021/jo8015677. [DOI] [PubMed] [Google Scholar]
- Pellicciari R.; Natalini B.; Sadeghpour B. M.; Marinozzi M.; Snyder J. P.; Williamson B. L.; Kuethe J. T.; Padwa A. The Reaction of α-Diazo-β-Hydroxy Esters with Boron Trifluoride Etherate: Generation and Rearrangement of Destabilized Vinyl Cations. A Detailed Experimental and Theoretical Study. J. Am. Chem. Soc. 1996, 118, 1–12. 10.1021/ja950971s. [DOI] [Google Scholar]
- Cai Y.; Tang X.; Ma S. Identifying a Highly Active Copper Catalyst for KA2 Reaction of Aromatic Ketones. Chem. - Eur. J. 2016, 22, 2266–2269. 10.1002/chem.201504823. [DOI] [PubMed] [Google Scholar]
- Palchak Z. L.; Lussier D. J.; Pierce C. J.; Larsen C. H. Synthesis of Tetrasubstituted Propargylamines from Cyclohexanone by Solvent-Free Copper(II) Catalysis. Green Chem. 2015, 17, 1802–1810. 10.1039/C4GC02318H. [DOI] [Google Scholar]
- Abrams S. R.; Shaw A. C. On the Mechanism of 1,3-Prototropic Shifts in Acetylene-Allene Isomerizations. J. Org. Chem. 1987, 52, 1835–1839. 10.1021/jo00385a033. [DOI] [Google Scholar]
- Takagi K.; Fukuda H.; Shuto S.; Otaka A.; Arisawa M. Safe Removal of the Allyl Protecting Groups of Allyl Esters Using a Recyclable, Low-Leaching and Ligand-Free Palladium Nanoparticle Catalyst. Adv. Synth. Catal. 2015, 357, 2119–2124. 10.1002/adsc.201500055. [DOI] [Google Scholar]
- Xu M.; Ren T.-T.; Li C.-Y. Gold-Catalyzed Oxidative Rearrangement of Homopropargylic Ether via Oxonium Ylide. Org. Lett. 2012, 14, 4902–4905. 10.1021/ol302238t. [DOI] [PubMed] [Google Scholar]
- Okamoto S.; Sato H.; Sato F. Highly Efficient Synthesis of Alka-1,3-dien-2-yltitanium Compounds from Alka-2.3-dienylcarbonates. A New, Practical Synthesis of 1.3-dienes and 2-iodo-1.3-dienes. Tetrahedron Lett. 1996, 37, 8865–8868. 10.1016/S0040-4039(96)02126-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.