Abstract
Ammonia is an important fertilizer feedstock and an expected next-generation hydrogen carrier. Thus, it is necessary to ensure effective production of ammonia from the waste biomass. In this regard, chicken manure was treated in an autoclave under hydrothermal reaction conditions, and the ammonia release rate was determined in the temperature range of 250–400 °C for holding times ranging from 2 to 120 min. A reaction network for ammonia production was proposed, and the reaction rate constants were determined. A nitrogen yield as high as 0.8 was obtained, corresponding to a hydrogen potential of 88.1 billion m3/year from chicken manure. Consequently, chicken manure was identified as a potentially favorable feedstock for ammonium production.
1. Introduction
With increased consumption of chicken and egg worldwide, large amounts of chicken manure are being generated.1 Unlike other manures, chicken manure contains a substantially large amount of nitrogen (about 0.035 kg/kg-dry).2 However, when nitrogen-containing compound is fed to a methane fermentation reactor, nitrogen is converted into ammonia and methane fermentation is inhibited.3,4 Ammonia is toxic to microorganisms, making the application of biomethanation in chicken manure a challenging task.5 Thus, energy recovery from chicken manure is not always possible, and a large portion of chicken manure is converted into compost.6 However, the composting process releases a large amount of ammonia and carbon dioxide. The associated bad smell problem and low energy efficiency are significant energy and environmental issues.
However, the high nitrogen content of chicken manure suggests that recovery of ammonia from chicken manure can be achieved efficiently.7 Ammonia is primarily produced by using fossil fuels for fertilizer production; the process contributes significantly to carbon dioxide emissions in the agricultural sector as indicated by the life cycle analysis. The recovery of ammonia from waste biomass, such as chicken manure, can contribute to the reduction of carbon dioxide emissions from agriculture. Additionally, ammonia is expected to be a next-generation hydrogen carrier. It can be easily decomposed into nitrogen and hydrogen molecules indicating its potential applicability as a green fuel.8 It is also possible to use ammonia as fuel itself.9 Thus, ammonia can be considered a green fuel when produced from biomass.
Decomposition of nitrogen-containing molecules is required to realize ammonia generation from biomass decomposition.10 This decomposition can be conducted by microorganisms through ammonia fermentation or using thermochemical conversion, such as hydrothermal pretreatment.11 In particular, hydrothermal treatment is suitable for the rapid conversion of wet biomass. The product of hydrothermal pretreatment is known to enhance the reaction rate of the biomass conversion steps such as biomethanation and supercritical water gasification.12
The behavior of nitrogen-containing organic compounds under hydrothermal conditions using both subcritical and supercritical water has been investigated by many researchers. The study by Sato et al. was a pioneering one wherein glycine was treated in subcritical water to determine the reaction rate.13 Furthermore, Samanmulya and Matsumura gasified glycine in supercritical water.14 He et al. demonstrated the comprehensive behavior of nitrogen-containing organics. According to their study, ammonia is produced from inorganic compounds and labile proteins at temperatures <300 °C. The treatment of actual biomass includes sewage sludge,10 soy protein,15 algae,16,17 and corn stover.18 In all the aforementioned studies, ammonia production was observed depending on the treatment temperature and time. A few studies also focused on manure treatment; Huang et al. treated chicken manure at temperatures <220 °C.19 Dai et al. treated cattle manure at 190 °C.20 Lu et al. investigated hydrothermal liquefaction (HTL) of pig manure.21
However, information regarding the reaction rate of ammonia production required for plant design is limited in existing literature, especially ammonia production from actual biomass. Most studies employed autoclave reactors, and the kinetic analysis for ammonia generation was difficult. To design a plant that can produce ammonia from chicken manure under hydrothermal conditions, systematic analysis of the effect of treatment conditions on ammonia yield is required to determine the reaction characteristics of chicken manure. The purpose of this study was to conduct a hydrothermal treatment of chicken manure under systematically changing conditions to elucidate the reaction parameters.
2. Experimental Section
2.1. Chicken Manure
Chicken manure was obtained from caged chickens. The cage was located in Higashi-Hiroshima, Japan, and the manure was from various Japanese grown-up chickens. Chicken manure obtained from uncaged broilers often contains sawdust mixed into the manure to protect the chickens from injury. However, using manure from caged chickens ensures the compositional integrity of the manure. Ammonia was found in the liquid phase when the chicken manure was suspended in water. Chicken manure initially contained some ammonia corresponding to 0.0033 kg-N/kg-wet. The ammonia and water were unavoidably removed by drying the chicken manure for further analysis. Elementary analysis was then conducted for this dried manure, and the obtained elementary composition of chicken manure is shown in Table 1. The analysis was conducted by Okayama University using a elementary analyzer (PerkinElmer, 2400II). Unfortunately, we did not analyze the ash composition because it is beyond the scope of this study. Some previous study analyzed chicken manure ash component, and Ca, K, P, S, Cl, and Si were observed.22 The moisture content of the original manure was 0.742 kg-H2O/kg-wet and the ash content was 0.249 kg-ash/kg-dry. The moisture content was determined by the weight change after drying the manure in an oven, and the initial ammonia content was determined using ion chromatography after filtrating the chicken manure suspended in water.
Table 1. Elementary Composition of Chicken Manure (kg/kg-Dry).
| C | H | N | S | ash | O (balance) |
|---|---|---|---|---|---|
| 0.378 | 0.048 | 0.035 | 0.010 | 0.249 | 0.280 |
2.2. Apparatus
An autoclave reactor (Toyo Koatsu Co., Ltd., Japan) with an inner volume of 300 cm3 was used for the hydrothermal treatment of chicken manure. It was made of stainless steel, and rod heaters, inserted into the reactor wall, heated the reactor and its contents. The maximum allowable temperature and pressure were 600 °C and 50 MPa, respectively; in this study, the highest treatment temperature was 350 °C, and the pressure was the corresponding saturation pressure or water vapor at the temperature.
Chicken manure and water were placed in an autoclave so that the dry matter content of chicken manure was 0.1 kg/kg-wet. Then, the lid was closed tightly and rod heaters were turned on. The temperature inside the reactor was measured using a K-type thermocouple and was maintained at 300 or 350 °C. The temperature was held for the desired time, and the rod heater was turned off. The reactor was opened when the temperature became lower than 30 °C to avoid ammonia loss in the gas phase. First, the product gas was collected by water replacement; then, the product slurry in the reactor was filtered to separate the solid and liquid products.
As a preliminary experiment, a smaller autoclave (96 mL) was used to observe the behavior of nitrogen in the wider temperature range of 250–400 °C for holding times of 10 and 60 min.
2.3. Analysis
The solid product was dried and weighed using an electric balance. The liquid product was analyzed using ion chromatography to determine the ammonia concentration. The ion chromatography apparatus was the product of Shimadzu Co., Ltd., Japan, and comprised a high-pressure pump LC-20AD, a column oven CTO-20A, and an electric conductivity detector CDD-10Avp. A Shim-pack IC-C4 column was used, and 2.5 mmol/dm3 oxalic acid solution was used as the eluent with a flow rate of 1.0 cm3/min.
The liquid phase included other nitrogen-containing compounds. The amount of nitrogen in the liquid product was determined by the Kjeldahl method using ion chromatography instead of titration to quantify the recovered ammonia.
The nitrogen yield of ammonia and that of liquid-phase nitrogen other than ammonia was defined as the ratio of the molar amount of nitrogen in these compounds to the molar amount of nitrogen in feedstock chicken manure, which was the sum of nitrogen in the solid phase and nitrogen in ammonia. The ratio of the amount of nitrogen in the original ammonia to total nitrogen in the feedstock was 0.270 mol-N/mol-N. This value was considered to be the initial nitrogen yield of ammonia. Hydrothermal treatment increased this value shown in the following sections by the decomposition of nitrogen in the solid.
2.4. Reaction Modeling
Nitrogen in chicken manure is contained in amines, proteins, nucleic acids, adenosine phosphates, etc. There, nitrogen atoms take the form of amino group, imino group, aromatic ring member, etc. Main reactions are expected to be hydrolysis of protein into amino acids followed by ammonia production by reaction with water and thermal decomposition of other molecules that also leads to ammonia. Overall, the reaction, in terms of nitrogen, starts from the conversion of nitrogen in the solid to nitrogen other than ammonia, such as amino acids in the liquid phase. Then, this liquid-phase nitrogen is converted into ammonia, as shown in eq 1.
| 1 |
Considering that these reactions are decomposition reactions and the amount of water is in excess, it is reasonable to assume that both reactions are of first order. The change in concentration with time for these compounds can be expressed as:
| 2 |
| 3 |
| 4 |
where Cs, Cl, CNH3, k1, k2, and t denote the concentrations of solid nitrogen, liquid-phase nitrogen and ammonia, reaction rate constant of the first and second reactions, and time, respectively. The solution for this set of differential equations is as follows:
| 5 |
| 6 |
| 7 |
where Cs0, Cl0, and CNH3,0 denote the initial concentrations of solid nitrogen, liquid-phase nitrogen, and ammonia, respectively. These equations were employed to fit the experimental data to determine the reaction rate constants.
3. Results and Discussion
3.1. Result of Preliminary Study
As a preliminary study, the effects of temperature and holding time were investigated using an autoclave of 96 cm3. Figure 1 shows the effect of temperature on the nitrogen yield of ammonia during hydrothermal treatment with a 10 min holding time. The experimental runs were repeated under the same conditions at least two times to check the reproducibility. All data points are shown in the figure and indicate the data scattering pattern. Although a rather large scattering of data was obtained in this preliminary study, it can be observed that a higher temperature results in a higher nitrogen yield of ammonia. The increase in yield with increasing temperature was rather monotonous, and no stepwise change was observed. This result suggests that the nitrogen in chicken manure exhibits a single behavior and is not a mixture of compounds with largely different decomposition characteristics.
Figure 1.
Effect of temperature on ammonia recovery yield (holding time 10 min).
Figure 2 shows the effect of the holding time investigated using an autoclave of 96 cm3. When the holding time was increased from 10 to 60 min at 300 °C, an increase in the nitrogen yield of ammonia was observed. This result supports the notion that nitrogen reactivity in chicken manure is homogeneous. If it is a mixture of easily decomposed compounds and recalcitrant compounds, elongation of the reaction time at low temperatures should not result in a large increase in ammonia yield.
Figure 2.
Effect of holding time on ammonia recovery yield (300 °C).
3.2. Ammonia Generation Characteristics of Chicken Manure at 300 °C
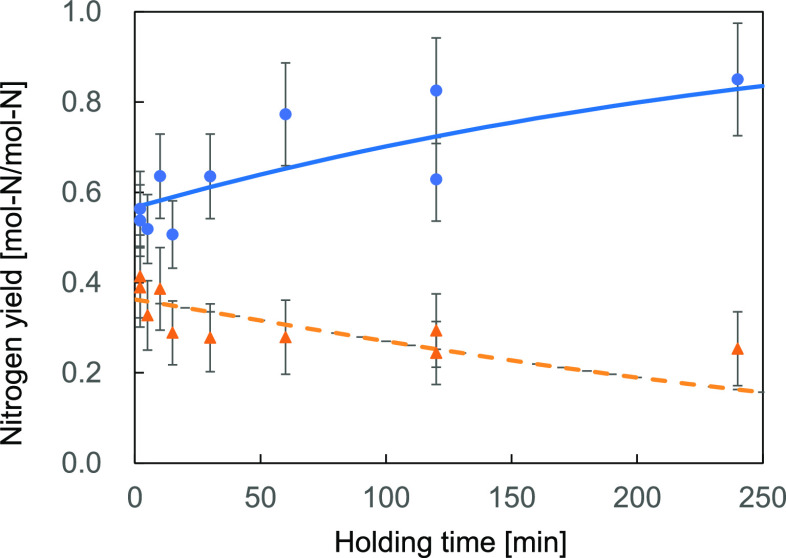
A detailed study of the increase in ammonia yield with holding time was conducted using a 300 cm3 autoclave. Liquid-phase nitrogen was also analyzed to obtain its yield. The results obtained at 300 °C are shown in Figure 3. Again, all the data points were shown for repeated experimental runs under the same conditions for reproducibility. Sufficient reproducibility was obtained, and a distinct increase in ammonia yield and a decrease in the yield of liquid-phase nitrogen (except ammonia) were observed. The ammonia yield at a holding time of 0 min is approximately equal to 0.5 mol-N/mol-N. As shown in the previous section, before treatment, the chicken manure contained ammonia in 0.270 mol-N/mol-N. The other 0.23 mol-N/mol-N should have been generated during the heating period.
Figure 3.
Change in nitrogen yield with holding time (300 °C) (circle: ammonia, triangle: other liquid-phase nitrogen).
This change in yield was fitted using the model shown in eqs 5–7. The fitted curve is shown as a solid line. A good agreement between the experimental results and the model was obtained with reaction rate constants k1 = 0.000540 min–1 and k2 = 0.00408 min–1.
3.3. Ammonia Generation Characteristics of Chicken Manure at 350 °C
Figure 4 shows the change in nitrogen yields for ammonia and liquid-phase nitrogen, except for ammonia. A similar trend was obtained at 300 °C, with higher yields of ammonia and lower yields of liquid-phase nitrogen. The initial ammonia yield is approximately 0.55 mol-N/mol-N, slightly higher than 0.5 mol-N/mol-N for 300 °C. Heating the reactor to 350 °C takes a longer time, and more conversion during the heating period is reasonable.
Figure 4.
Change in nitrogen yield with holding time (350 °C) (circle: ammonia, triangle: other liquid-phase nitrogen).
Fitting with the model again provided good agreement between the data and model calculation results. The reaction rate constants were k1 = 0.00918 min–1 and k2 = 0.00426 min–1. These values are higher than those at 300 °C, which are reasonable considering the Arrhenius law; however, the value of k2 did not change significantly.
3.4. Overall Discussion
The obtained results indicate that the material being decomposed during the hydrothermal treatment holding time is homogeneous, and the serial reaction model expresses the behavior well. A higher temperature results in a faster reaction rate, which is also a reasonable observation based on the Arrhenius law. Initial nitrogen yield of ammonia is 0.27 mol/mol and during heating time, increase in nitrogen yield by 0.23 mol/mol for 300 °C and by 0.28 mol/mol for 350 °C was observed for ammonia.
Notably, this decomposition during the heating period was much larger than the amount expected from the obtained reaction rate. Approximately 20 min was required to heat up to the target temperature. Considering that the initial temperature of this heating period is room temperature lower than 30 °C and no reaction should take place, the yield increase should be smaller than 0.05 mol/mol. Thus, although the material decomposed during the holding time is homogeneous, the material being decomposed during the heating period should be decomposed much more easily. Thus, it can be deduced that chicken manure contains more than two types of nitrogen. One is easily decomposed and converted into ammonia even during the heating-up period. The other is much more difficult to decompose and is gradually converted into ammonia during the holding time.
The form of nitrogen in organics varies; some, such as urea, are easily decomposed to nitrogen in cyclic carbon structures. Ammonia production rates of compounds with the same nitrogen-containing group, for example, glycine, alanine, ethylamine, etc., should be similar; however, other nitrogen forms such as nitrogen in deoxyribonucleic acid can exhibit a much slower rate of ammonia production.
An ammonia yield as high as 0.8 was obtained in this study. When moisture content is 0.742 kg-H2O/kg-wet and nitrogen content is 0.035 kg-N/kg-dry, 1 kg-wet of chicken manure has 9.03 g/kg-wet of solid nitrogen. In addition, 3.34 g/kg-wet of nitrogen exists in the form of ammonia. A conversion of 0.8 mol/mol of nitrogen results in 9.89 g/kg-wet of nitrogen converted into ammonia. Ammonia can be easily stripped, and separation of this 9.89 g/kg-wet leaves the remaining 2.47 g/kg-wet of nitrogen.
Conventionally, chicken manure cannot be used as a feedstock for methane fermentation. This is because the ammonia produced from nitrogen-containing compounds inhibits fermentation. The tolerable ammonia concentration was 3 g/kg-wet for high-temperature methane fermentation.23 The above 2.47 g/kg-wet of nitrogen allows methane fermentation of chicken manure. Thus, the results of this study, validating efficient conversion of nitrogen to ammonia, indicate the possibility of hydrothermal pretreatment and subsequent ammonia stripping as an effective measure to suppress ammonia inhibition during methane fermentation.
The ammonia produced can be easily converted into hydrogen. There are 30.8 billion heads of chickens worldwide.24 One head produces 0.084 kg/d of manure.25 Considering this, the conversion of 0.8 mol/mol of nitrogen suggests an ammonia potential of 44.5 million t/year or hydrogen potential of 88.1 billion m3/year.
4. Conclusions
Chicken manure was successfully treated under hydrothermal conditions to convert more than 80% of constituent nitrogen into ammonia. The reaction was expressed using a series reaction model, and the reaction rate constants were k1 = 0.000540 min–1 and k2 = 0.00408 min–1 at 300 °C and k1 = 0.00918 min–1 and k2 = 0.00426 min–1 at 350 °C. This result indicates the suitability of chicken manure as feedstock for producing ammonia, which is a potentially favorable hydrogen carrier and suggests a hydrogen potential of 88.1 billion m3/year from chicken manure.
Acknowledgments
This research was performed by the Environment Research and Technology Development Fund (JPMEERF20203001) of the Environmental Restoration and Conservation Agency of Japan. Chicken manure was supplied from Japanese Avian Bioresource Project Research Center, Hiroshima University. Elementary analysis was made by Advanced Research Science Center, Okayama University.
The authors declare no competing financial interest.
References
- Food and Agriculture Organization of the United Nations , The future of food and agriculture – Alternative pathways to 2050. Supplementary material. 2018.
- Kafle G. K.; Chen L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manage. 2016, 48, 492–502. 10.1016/j.wasman.2015.10.021. [DOI] [PubMed] [Google Scholar]
- Gallert C.; Winter J. Mesophilic and thermophilic anaerobic digestion of source-sorted organic wastes: effect of ammonia on glucose degradation and methane production. Appl. Microbiol. Biotechnol. 1997, 48, 405–410. 10.1007/s002530051071. [DOI] [Google Scholar]
- Chen J. L.; Ortiz R.; Steele T. W. J.; Stuckey D. C. Toxicants inhibiting anaerobic digestion: A review. Biotechnol. Adv. 2014, 32, 1523–1534. 10.1016/j.biotechadv.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Ulusoy Y.; Ulukardesler A. H.; Arslan R.; Tekin Y. Energy and emission benefits of chicken manure biogas production: a case study. Environ. Sci. Pollut. Res. 2021, 28, 12351–12356. 10.1007/s11356-018-3466-0. [DOI] [PubMed] [Google Scholar]
- Kelleher B. P.; Leahy J. J.; Henihan A. M.; O’Dwyer T. F.; Sutton D.; Leahy M. J. Advances in poultry litter disposal technology - a review. Bioresour. Technol. 2002, 83, 27–36. 10.1016/S0960-8524(01)00133-X. [DOI] [PubMed] [Google Scholar]
- Liu X.; Elgowainy A.; Wang M. Life cycle energy use and greenhouse gas emissions of ammonia production from renewable resources and industrial by-products. Green Chem. 2020, 22, 5751–5761. 10.1039/D0GC02301A. [DOI] [Google Scholar]
- Feng J.; Liu L.; Ju X.; Wang J.; Zhang X.; He T.; Chen P. Highly Dispersed Ruthenium Nanoparticles on Y2O3 as Superior Catalyst for Ammonia Decomposition. ChemCatChem 2021, 13, 1552–1558. 10.1002/cctc.202001930. [DOI] [Google Scholar]
- Valera-Medina A.; Xiao H.; Owen-Jones M.; David W. I. F.; Bowen P. J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. 10.1016/j.pecs.2018.07.001. [DOI] [Google Scholar]
- He C.; Wang K.; Yang Y. H.; Amaniampong P. N.; Wang J. Y. Effective Nitrogen Removal and Recovery from Dewatered Sewage Sludge Using a Novel Integrated System of Accelerated Hydrothermal Deamination and Air Stripping. Environ. Sci. Technol. 2015, 49, 6872–6880. 10.1021/acs.est.5b00652. [DOI] [PubMed] [Google Scholar]
- Nishio N.; Nakashimada Y. Recent development of anaerobic digestion processes for energy recovery from wastes. J. Biosci. Bioeng. 2007, 103, 105–112. 10.1263/jbb.103.105. [DOI] [PubMed] [Google Scholar]
- Kato A.; Matsumura Y. Hydrothermal pulping of wet biomass as pretreatment for supercritical water gasification studied using cabbage as a model compound. J. Jpn. Inst. Energy 2003, 82, 97–102. 10.3775/jie.82.97. [DOI] [Google Scholar]
- Sato N.; Daimon H.; Fujie K. Decomposition of glycine in high temperature and high pressure water. Kagaku Kogaku Ronbunshu 2002, 28, 113–117. 10.1252/kakoronbunshu.28.113. [DOI] [Google Scholar]
- Samanmulya T.; Matsumura Y. Effect of activated carbon catalytic on supercritical water gasification of glycine as a model compound of protein. J. Jpn. Inst. Energy 2013, 92, 894–899. 10.3775/jie.92.894. [DOI] [Google Scholar]
- Sheehan J. D.; Savage P. E. Products, Pathways, and Kinetics for the Fast Hydrothermal Liquefaction of Soy Protein Isolate. ACS Sustain. Chem. Eng. 2016, 4, 6931–6939. 10.1021/acssuschemeng.6b01857. [DOI] [Google Scholar]
- Cheng F.; Mallick K.; Henkanatte Gedara S. M.; Jarvis J. M.; Schaub T.; Jena U.; Nirmalakhandan N.; Brewer C. E. Hydrothermal liquefaction of Galdieria sulphuraria grown on municipal wastewater. Bioresour. Technol. 2019, 292, 121884–121884. 10.1016/j.biortech.2019.121884. [DOI] [PubMed] [Google Scholar]
- Kruse A.; Koch F.; Stelzl K.; Wüst D.; Zeller M. Fate of Nitrogen during Hydrothermal Carbonization. Energy Fuels 2016, 30, 8037–8042. 10.1021/acs.energyfuels.6b01312. [DOI] [Google Scholar]
- Zhang Y.; Jiang Q.; Xie W.; Wang Y.; Kang J. Effects of temperature, time and acidity of hydrothermal carbonization on the hydrochar properties and nitrogen recovery from corn stover. Biomass Bioenergy 2019, 122, 175–182. 10.1016/j.biombioe.2019.01.035. [DOI] [Google Scholar]
- Huang W.; Yuan T.; Zhao Z.; Yang X. I.; Huang W.; Zhang Z.; Lei Z. Coupling Hydrothermal Treatment with Stripping Technology for Fast Ammonia Release and Effective Nitrogen Recovery from Chicken Manure. ACS Sustain. Chem. Eng. 2016, 4, 3704–3711. 10.1021/acssuschemeng.6b00315. [DOI] [Google Scholar]
- Dai L.; Yang B.; Li H.; Tan F.; Zhu N.; Zhu Q.; He M.; Ran Y.; Hu G. A synergistic combination of nutrient reclamation from manure and resultant hydrochar upgradation by acid-supported hydrothermal carbonization. Bioresour. Technol. 2017, 243, 860–866. 10.1016/j.biortech.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Lu J.; Li H.; Zhang Y.; Liu Z. Nitrogen Migration and Transformation during Hydrothermal Liquefaction of Livestock Manures. ACS Sustain. Chem. Eng. 2018, 6, 13570–13578. 10.1021/acssuschemeng.8b03810. [DOI] [Google Scholar]
- Yanagida T.; Minowa T.; Nakamura A.; Matsumura Y.; Noda Y. Behavior of Inorganic Elements in Poultry Manure during Supercritical Water Gasification. J. Jpn. Inst. Energy 2008, 87, 731–736. 10.3775/jie.87.731. [DOI] [Google Scholar]
- Abouelenien F.; Fujiwara W.; Namba Y.; Kosseva M.; Nishio N.; Nakashimada Y. Improved methane fermentation of chicken manure via ammonia removal by biogas recycle. Bioresour. Technol. 2010, 101, 6368–6373. 10.1016/j.biortech.2010.03.071. [DOI] [PubMed] [Google Scholar]
- FAOSTAT website for statistics. http://www.fao.org/faostat/en/#data (accessed Apr. 2).
- Chávez-Fuentes J. J.; Capobianco A.; Barbušová J.; Hutňan M. Manure from Our Agricultural Animals: A Quantitative and Qualitative Analysis Focused on Biogas Production. Waste Biomass Valor. 2017, 8, 1749–1757. 10.1007/s12649-017-9970-5. [DOI] [Google Scholar]