Abstract
Background
MBL-producing strains of Enterobacteriaceae are a major public health concern. We sought to define optimal combination regimens of ceftazidime/avibactam with aztreonam in a hollow-fibre infection model (HFIM) of MBL-producing strains of Escherichia coli and Klebsiella pneumoniae.
Methods
E. coli ARLG-1013 (blaNDM-1, blaCTX-M, blaCMY, blaTEM) and K. pneumoniae ARLG-1002 (blaNDM-1, blaCTXM-15, blaDHA, blaSHV, blaTEM) were studied in the HFIM using simulated human dosing regimens of ceftazidime/avibactam and aztreonam. Experiments were designed to evaluate the effect of staggered versus simultaneous administration, infusion duration and aztreonam daily dose (6 g/day versus 8 g/day) on bacterial killing and resistance suppression. Prospective validation experiments for the most active combination regimens were performed in triplicate to ensure reproducibility.
Results
Staggered administration of the combination (ceftazidime/avibactam followed by aztreonam) was found to be inferior to simultaneous administration. Longer infusion durations (2 h and continuous infusion) also resulted in enhanced bacterial killing relative to 30 min infusions. The rate of killing was more pronounced with 8 g/day versus 6 g/day aztreonam combination regimens for both tested strains. In the prospective validation experiments, ceftazidime/avibactam with aztreonam dosed every 8 and 6 h, respectively (ceftazidime/avibactam 2/0.5 g every 8 h + aztreonam 2 g every 6 h), or ceftazidime/avibactam with aztreonam as continuous infusions resulted in maximal bacterial killing and resistance suppression over 7 days.
Conclusions
Simultaneous administration of aztreonam 8 g/day given as a continuous or 2 h infusion with ceftazidime/avibactam resulted in complete bacterial eradication and resistance suppression. Further study of this combination is needed with additional MBL-producing Gram-negative pathogens. The safety of this double β-lactam strategy also warrants further study in Phase 1 clinical trials.
Introduction
While several new antibiotics with efficacy against ESBL- and KPC-producing Gram-negative infections have been approved, few have in vitro activity against MBL-producing strains of Enterobacteriaceae.1,2 Polymyxins are often active against MBL-producing Enterobacteriaceae but toxicity and emergence of resistance concerns limit their use.3,4 One strategy that may serve as a ‘bridge’ treatment for MBL-producing Gram-negative infections is the combination of two currently available antibiotics: ceftazidime/avibactam and aztreonam.5–8 Although aztreonam is not hydrolysed by MBLs, most MBL-producing Gram-negative bacteria also harbour ESBL or KPC serine β-lactamases that inactivate aztreonam.5,6,9–12 Avibactam, which inhibits serine β-lactamases, protects aztreonam from degradation, preserving its activity against MBL-producing Gram-negative bacteria. The aztreonam/avibactam combination is being tested in clinical trials. However, it will be several years before this new treatment option will be considered for approval by the FDA for treating patients infected with MBL-producing Gram-negative bacteria.13
Although the combination of ceftazidime/avibactam with aztreonam has been shown to be efficacious against MBL-producing Enterobacteriaceae in pre-clinical studies and clinical case reports, the optimal dose and schedule remain unknown.6–9 Given this critical gap in the literature, hollow-fibre infection model (HFIM) studies were conducted using clinical isolates of Escherichia coli and Klebsiella pneumoniae that co-produced NDM and ESBL (CTX-M-15) to identify optimal combination regimens that result in maximal bacterial killing and resistance suppression. We opted to conduct HFIM studies as they are an integral part of the drug development process and are used to inform dose and schedule selection for Phase III clinical trials.14–16 They are particularly useful in situations when there are limited clinical data available to define optimal therapy, especially when there is interest in studying humanized drug exposure profiles, treatment durations and starting bacterial burdens that mirror clinical practice.17,18
When designing the study, we sought to answer several important clinical questions. Firstly, we were interested in determining whether administration of ceftazidime/avibactam and aztreonam should be simultaneous or staggered, as it has been postulated that avibactam is needed to be present first to protect aztreonam from enzymatic degradation. Secondly, we sought to determine the optimal dosing of aztreonam in the presence of standard and continuous infusion (CI) ceftazidime/avibactam.19–22
Methods
Bacterial strains and antimicrobial agents and susceptibility testing
Two NDM-constitutive clinical isolates of Enterobacteriaceae, E. coli ARLG-1013 (blaNDM-1, blaCTX-M, blaCMY, blaTEM) and K. pneumoniae ARLG-1002 (blaNDM-1, blaCTXM-15, blaDHA, blaSHV, blaTEM), were selected for the HFIM studies. ARLG-1013 and ARLG 1002 were obtained from PRIMERS I and II (Platforms for Rapid Identification of MDR Gram-negative bacteria and Evaluation of Resistance Studies), with details of these strains found elsewhere.23 Aztreonam and ceftazidime analytic powders were obtained from Sigma Chemical Company (St Louis, MO, USA). Avibactam analytical powder was obtained from Allergan. MICs of ceftazidime/avibactam and aztreonam were determined by broth microdilution in accordance with CLSI standards.24 For combinations, MICs of aztreonam were determined in the presence of 8/4 mg/L of ceftazidime/avibactam. The 8/4 ceftazidime/avibactam concentration was selected from the current breakpoint and derived from three-drug chequerboard studies involving fixed concentrations of ceftazidime/avibactam and varying aztreonam concentrations.
HFIM
The HFIM studies were conducted over 7 days using a two-compartment model system with cellulosic cartridges (cartridge C3008; FiberCell Systems, Inc., Frederick, MD, USA) for bacterial growth and containment of all bacterial populations, as described previously.25 Simulated drug exposures for ceftazidime, avibactam and aztreonam were based on human population pharmacokinetic data and a half-life of 2 h.26–29
An initial bacterial inoculum of 7.5 log10 cfu/mL was selected based on quantitative bronchoalveolar lavage results observed in patients with ventilator-associated bacterial pneumonia (VABP).30,31 Samples were withdrawn from the cartridge before the first dose (0 h sample) and 1, 2, 4, 8, 24, 26, 28, 30, 48, 50, 52, 54, 72, 120 and 168 h after the initial dose for determination of total bacterial counts. Total bacterial counts were quantified by plating 50 μL aliquots onto Mueller–Hinton Agar (MHA) plates (BD Co., Franklin Lakes, NJ, USA) without drug, with a limit of detection of 2.0 log10 cfu/mL. Resistant subpopulations were quantified at 0, 24, 48, 72, 120 and 168 h by plating 50 μL aliquots onto MHA plates, each containing the following antimicrobial(s): aztreonam alone (2, 8 and 32 mg/L); ceftazidime/avibactam alone (2/4, 8/4 and 32/4 mg/L); and aztreonam/ceftazidime/avibactam (2/2/4, 8/8/4 and 32/32/4 mg/L). All cultures were incubated at 37°C for 24 h prior to quantification. Additional details for preparation of antibiotic-containing MHA plates can be found in the Supplementary data (available at JAC Online). Bactericidal activity was defined as >3 log10 cfu/mL reduction in bacterial burden from baseline.
Simulated regimens
Concentration–time profiles were simulated for a number of aztreonam regimens in combination with ceftazidime/avibactam (Table 1).26–29 We purposely limited the HFIM to ceftazidime/avibactam and aztreonam doses listed in the package insert to provide pragmatic solutions that could be quickly translated to the clinical setting to combat NDM-producing Enterobacteriaceae.28,29 The regimens studied were designed to simulate an array of aztreonam dosing regimens used in combination with standard and CI ceftazidime/avibactam. As part of the experiments, infusion times (0.5 and 2 h infusions and CIs), total daily dose (6 g/day versus 8 g/day) and timing of administration (simultaneous versus staggered) were varied. Model fits for all three drugs were satisfactory (see Figures S1–S3).
Table 1.
Simulated regimens in the HFIM
| Regimen | Description | Ceftazidime/avibactam regimen | Infusion | Aztreonam regimen | Infusion | Timing of administration |
|---|---|---|---|---|---|---|
| 1 | control | |||||
| 2 | monotherapy | 2/0.5 g q8h | 2 h | — | — | — |
| 3 | monotherapy | — | — | 2 g q8h | 2 h | — |
| 4 | q8h + q8h | 2/0.5 g q8h | 2 h | 2 g q8h | 2 h | simultaneous |
| 5 | q8h + q8h | 2/0.5 g q8h | 2 h | 2 g q8h | 2 h | staggered |
| 6 | CI + q8h | 6/1.5 g per day | CI | 2 g q8h | 2 h | simultaneous |
| 7 | CI + q8h | 6/1.5 g per day | CI | 2 g q8h | 2 h | staggered |
| 8 | CI + CI | 6/1.5 g per day | CI | 6 g per day | CI | simultaneous |
| 9 | q8h + q6h | 2/0.5 g q8h | 2 h | 1.5 g q6h | 2 h | simultaneous |
| 10 | CI + q6h | 6/1.5 g per day | CI | 1.5 g q6h | 2 h | simultaneous |
| 11 | q6h + q6h | 1.5/0.375 g q6h | 2 h | 1.5 g q6h | 2 h | simultaneous |
| 12 | q8h + q6h | 2/0.5 g q8h | 2 h | 2 g q6h | 2 h | simultaneous |
| 13 | CI + q6h | 6/1.5 g per day | CI | 2 g q6h | 2 h | simultaneous |
| 14 | CI + CI | 6/1.5 g per day | CI | 8 g per day | CI | simultaneous |
| 15 | q6h + q6h | 1.5/0.375 g q6h | 2 h | 2 g q6h | 2 h | simultaneous |
| 16 | q8h + q8h | 2/0.5 g q8h | 30 min | 2 g q8h | 30 min | simultaneous |
Combination administration strategies were either staggered (ceftazidime/avibactam was given first, followed by aztreonam 2 g given later) or simultaneous (ceftazidime/avibactam and aztreonam given together). Loading doses were included in the CI regimens.
Dose selection for aztreonam was performed in an iterative fashion, starting with 6 g total daily dose regimens. Mild to moderate asymptomatic serum aminotransferase elevations are common with aztreonam and appear to be dose dependent in nature.32–34 Therefore, there was interest in identifying the lowest daily dose of aztreonam that resulted in maximal bacterial killing and resistance suppression. To provide some context of the outcomes observed with the ceftazidime/avibactam with aztreonam regimens, we simulated the Phase III aztreonam/avibactam regimen currently being studied in clinical trials (aztreonam 1.5 g over 3 h, every 6 h, with avibactam 0.5 g over 3 h, every 6 h).13 Lastly, to ensure scientific rigour and reproducibility, selected regimens were completed in triplicate (n = 3 independent HFIM experimental arms) to prospectively validate the performance of leading standard and CI combination regimens against E. coli ARLG-1013.
Antibiotic assay methods
Antibiotic concentrations in the HFIM were validated. Ceftazidime concentrations were analysed via LC-MS/MS (Agilent 6460 coupled with an Agilent series 1260 UHPLC system; Agilent Technologies, Santa Clara, CA, USA). Aztreonam concentrations were quantified using LC-MS/MS with a Shimadzu LC system and a triple quadrupole tandem mass spectrometer API 4000 (Applied Biosystems/MDS Sciex, Ontario, Canada) by Keystone Bioanalytical (North Wales, PA, USA). Avibactam concentrations were quantified using LC-MS/MS with a Shimadzu LC system and a triple quadrupole tandem mass spectrometer API 5500 QTrap (Applied Biosystems/MDS Sciex by Keystone Bioanalytical), as previously described.35 Additional methods and pharmacokinetic validation are provided in the Supplementary Methods (Table S1).
Results
Isolate susceptibility
Against E. coli ARLG-1013, the following MIC values were observed: >64 mg/L for aztreonam; >64 mg/L for ceftazidime/avibactam; and 4 mg/L for aztreonam in the presence of ceftazidime/avibactam at 8/4 mg/L. Against K. pneumoniae ARLG-1002, the following MIC values were observed: >64 mg/L for aztreonam; >64 mg/L for ceftazidime/avibactam; and 2 mg/L for aztreonam in the presence of ceftazidime/avibactam at 8/4 mg/L.
HFIM experiments
Timing of combination administration: simultaneous versus staggered
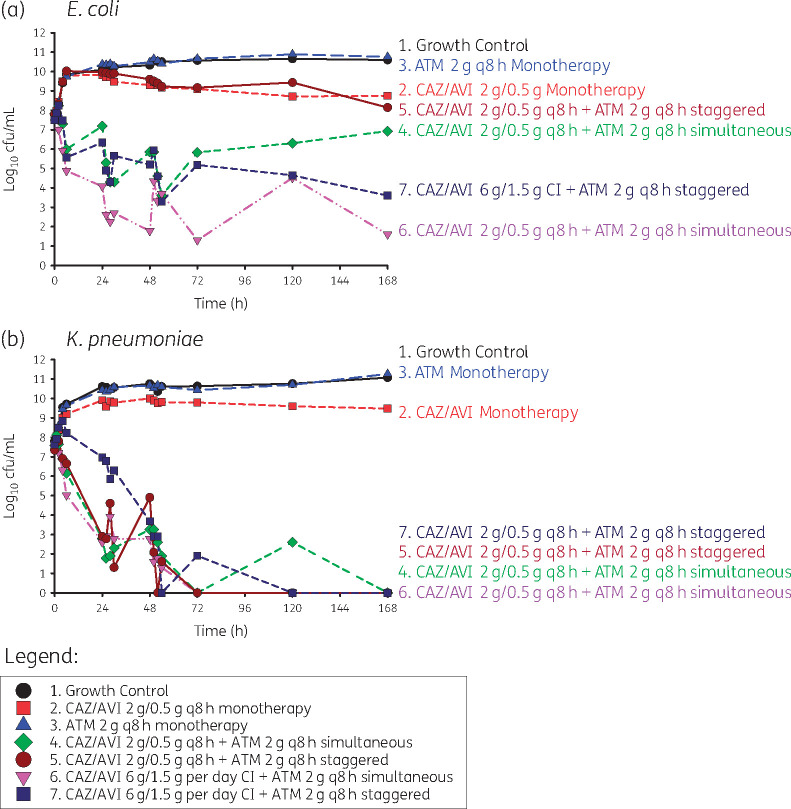
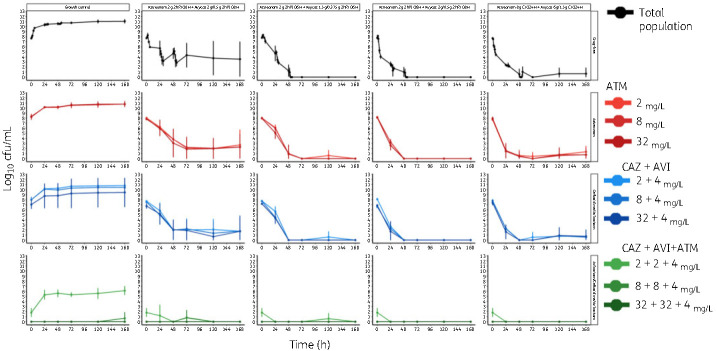
Against E. coli ARLG-1013 (Figure 1a), there was no bacterial killing with staggered administration of standard dosing of ceftazidime/avibactam followed by aztreonam; bacterial counts were similar to those observed with the monotherapy regimens and growth control. With simultaneous administration of aztreonam with standard dosing of ceftazidime/avibactam, there was initial bactericidal activity (4.23 log10 cfu/mL reduction by 54 h) followed by steady regrowth over the course of the experiment. Initial bacterial killing was observed with both simultaneous and staggered administration of aztreonam in the presence of CI ceftazidime/avibactam. However, there were ∼4 log10 cfu/mL of bacteria present at 168 h with staggered administration of aztreonam with CI ceftazidime/avibactam. In contrast, bacterial counts were lower than the limit of detection at 168 h with simultaneous infusion of aztreonam and CI ceftazidime/avibactam.
Figure 1.
Staggered versus simultaneous dosing regimens for ceftazidime/avibactam (CAZ/AVI) in combination with aztreonam (ATM) against E. coli and K. pneumoniae. Combination administration strategies were either staggered (ceftazidime/avibactam was given first, followed by aztreonam 2 g given later) or simultaneous (ceftazidime/avibactam and aztreonam given together). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Against K. pneumoniae ARLG-1002 (Figure 1b), both staggered and simultaneous administration of aztreonam with ceftazidime/avibactam resulted in a rapid initial bacterial killing of −5.04 and −4.44 log10 cfu/mL reduction, respectively, and with no detectable bacterial counts below the limit of detection by 168 h. Time to the limit of detection was longer with staggered versus simultaneous administration of aztreonam with ceftazidime/avibactam, at 50 h versus 30 h.
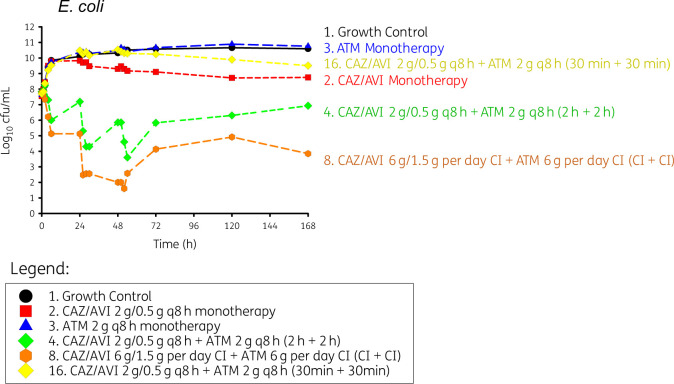
Duration of infusion with simultaneous dosing
The 30 min infusion time of the aztreonam and ceftazidime/avibactam combination, the shortest infusion time studied, resulted in no bacterial killing of E. coli ARLG-1013 (Figure 2). Additionally, the 30 min infusion time had a similar rate of growth within the first 24 h compared with the growth control [0.105 (log10 cfu/mL)/h for 30 min infusion versus 0.0864 (log10 cfu/mL)/h for growth control as determined by simple linear regression, P = 0.368]. Initial bacterial killing was observed with both 2 h infusion and CI of aztreonam and ceftazidime/avibactam. However, for both bacteria, the rate and extent of bacterial killing was more pronounced with CIs of aztreonam and ceftazidime/avibactam compared with the 2 h infusion regimen, with bacterial counts reaching the limit of quantification by 26 h for both species. Both the 2 h infusion and CI failed to achieve eradication; however, bacterial counts were consistently lower and at the limit of detection for the CI regimen.
Figure 2.
Effect of infusion duration on bacterial killing and regrowth against E. coli (ARLG 1013). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
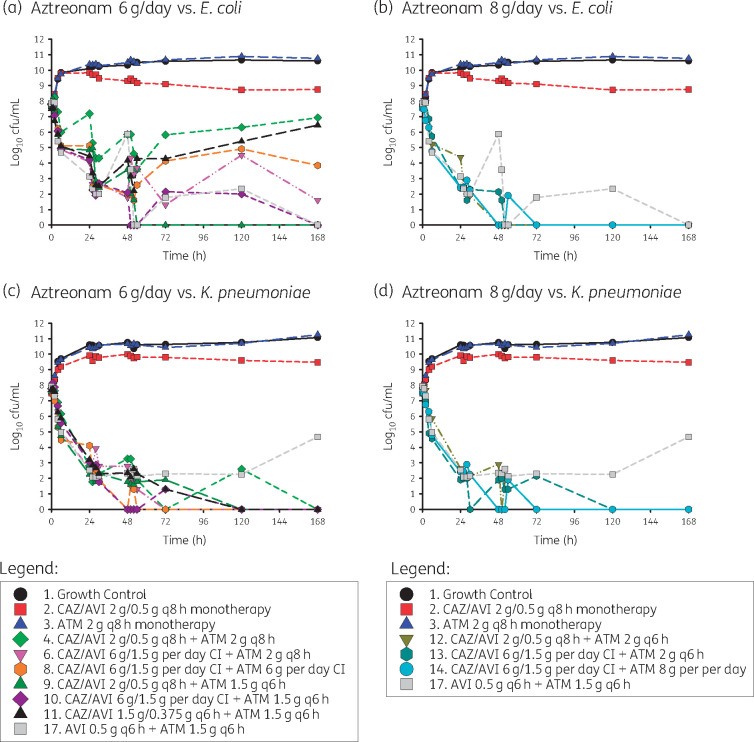
Daily aztreonam dose (6 g versus 8 g daily) with ceftazidime/avibactam and aztreonam/avibactam
Against E. coli ARLG-1013, initial bactericidal activity was observed with both aztreonam 6 g/day and 8 g/day in combination with ceftazidime/avibactam (Figure 3a) but the rate and extent of killing was more pronounced with 8 g/day aztreonam combination regimens (Figure 3b). Only three of the six experiments with aztreonam 6 g/day achieved bacterial counts below the lower limits of detection by the end of the experiment (i.e. 168 h), whereas all experiments using 8 g/day achieved complete eradication by 72 h. For the three aztreonam 6 g/day combination regimens that achieved bacterial counts below the lower limits of detection by 168 h, two were combined with CI ceftazidime/avibactam and one was aztreonam 1.5 g IV every 6 h with standard dosing of ceftazidime/avibactam. Compared with aztreonam 2 g IV every 6 h with standard dosing of ceftazidime/avibactam, aztreonam 1.5 g IV every 6 h with standard dosing of ceftazidime/avibactam had a longer time to complete bacterial eradication and exhibited a sawtooth bacterial killing effect.
Figure 3.
Effects of altering aztreonam dose from 6 g/day to 8 g/day in combination with standard and CI ceftazidime/avibactam. Other regimens included a growth control, aztreonam monotherapy, ceftazidime/avibactam monotherapy and the aztreonam/avibactam regimen [aztreonam/avibactam 1.5/0.5 g IV q6h (2 h infusion)], which is currently being evaluated in a Phase III clinical trial. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Rapid initial bacterial killing and complete bacterial eradication with resistance suppression was observed across all aztreonam 6 g/day and 8 g/day regimens against K. pneumoniae ARLG-1002 (Figure 3c and d). Most notably, the initial rate of bacterial killing was similar between aztreonam 1.5 g IV every 6 h with standard dosing ceftazidime/avibactam and aztreonam 2 g IV every 6 h with standard dosing ceftazidime/avibactam, with rates of −0.222 (log10 cfu/mL)/h and −0.217 (log10 cfu/mL)/h, respectively. However, the time to complete bacterial eradication was longer with aztreonam 6 g/day, including the aztreonam 1.5 g IV every 6 h with standard dosing ceftazidime/avibactam, relative to the 8 g/day aztreonam combination regimen.
Results from the HFIM runs simulating the aztreonam/avibactam dosing regimen currently being studied in clinical trials (i.e. aztreonam 1.5 g with avibactam 0.5 g every 6 h) are also shown in Figure 3. Against K. pneumoniae ARLG-1002, rapid bacterial killing was noted with avibactam/aztreonam, followed by bacterial regrowth. For E. coli ARLG 1013, bacterial growth was still detected at 120 h but suppression of bacterial counts was observed by 168 h.
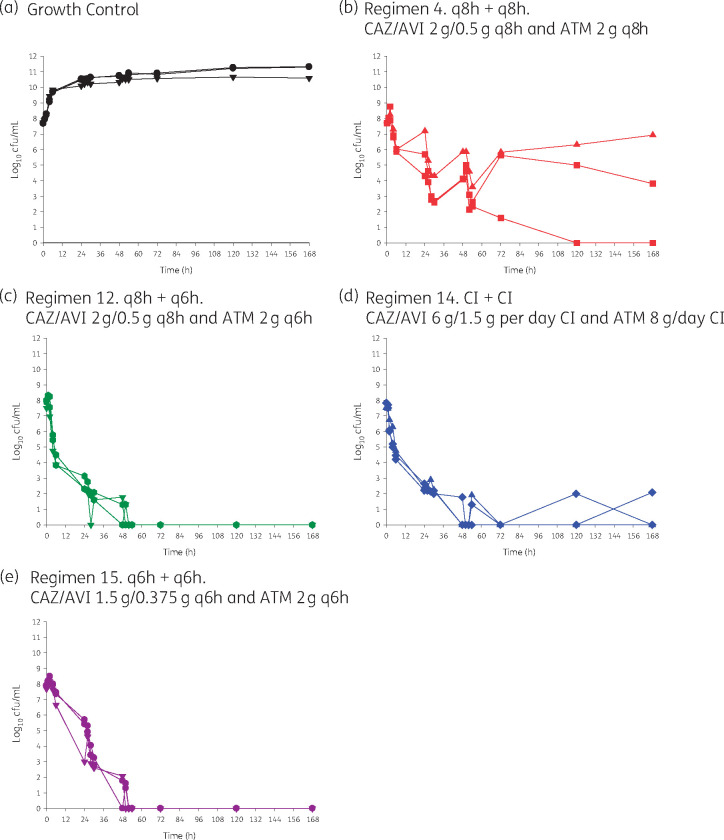
Validation of HFIM studies with E. coli ARLG-1013
The following regimens were completed in triplicate: growth control; ceftazidime/avibactam 2/0.5 g every 8 h (2 h infusion) and aztreonam 2 g every 8 h (2 h infusion); ceftazidime/avibactam 2/0.5 g every 8 h (2 h infusion) and aztreonam 2 g every 6 h (2 h infusion); ceftazidime/avibactam 6/1.5 g per day CI and aztreonam 8 g/day CI; and ceftazidime/avibactam 1.5/0.375 g every 6 h (2 h infusion) and aztreonam 2 g every 6 h (2 h infusion). We selected the higher intensity 8 g/day of aztreonam regimens because they exhibited more rapid and sustained killing and less of a sawtooth killing pattern. We included ceftazidime/avibactam 2/0.5 g every 8 h (2 h infusion) and aztreonam 2 g every 8 h (2 h infusion) as a positive control since it included the most commonly used standalone regimens in clinical practice. The results of the triplicate prospective validation HFIM experiments with E. coli ARLG-1013 are shown in Figure 4. When aztreonam 8 g/day was administered with CI ceftazidime/avibactam, rapid initial bacterial killing was observed by 24 h and suppression of the resistant subpopulation over 168 h. Comparing all aztreonam 8 g/day combination regimens, bacterial reductions in the first 24 h were: >5.75 log10 cfu/mL for ceftazidime/avibactam CI and aztreonam CI, >5.84 log10 cfu/mL for ceftazidime/avibactam every 6 h and aztreonam every 6 h; and >5.82 log10 cfu/mL for ceftazidime/avibactam every 8 h and aztreonam 2 g every 6 h. These reductions were sustained at 168 h, with bacterial counts below the limit of detection. Given the logistical challenges associated with dosing aztreonam every 6 h and ceftazidime/avibactam every 8 h, we also tested aztreonam 2 g every 6 h with ceftazidime/avibactam 1.875 g every 6 h. Overall, complete bacterial eradication was achieved by 48 h with this regimen, but the rate and extent of bacterial killing was less pronounced relative to the other two aztreonam 8 g/day with ceftazidime/avibactam regimens.
Figure 4.
Prospective validation of optimal regimens identified in HFIM studies. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Population analysis profiles (PAPs)
PAPs showed that in all cases the resistant subpopulations capable of growing on agar containing aztreonam or ceftazidime/avibactam were largely represented by the total counts. There was little heterogeneity in bacterial resistance with either aztreonam or ceftazidime/avibactam. The triple-drug PAPs showed complete suppression compared with the growth control, indicating that there was no proliferation of resistance due to the treatment, even in the least successful regimen of ceftazidime/avibactam 2/0.5 g every 8 h (2 h infusion) and aztreonam 2 g every 8 h (2 h infusion) (Figure 5).
Figure 5.
PAPs of E. coli ARLG-1013 from prospective validation studies. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Discussion
The most compelling findings in this HFIM study are the rate and extent of bactericidal activity and resistance suppression observed with aztreonam in combination with ceftazidime/avibactam. Across most tested combination regimens, a bactericidal effect was observed within the first 8 h, complete bacterial eradication was achieved within 48 h, and there was no bacterial regrowth or resistance emergence over the 7 day study duration. This level of rapid bactericidal activity has only been reported with high-dose polymyxin-based triple combination regimens.25 However, regrowth and resistance development are commonplace with polymyxin-based combination regimens, especially those employing physiological concentrations of polymyxins.25 The combination of aztreonam 8 g/day with ceftazidime/avibactam also outperformed the aztreonam/avibactam regimen currently in clinical development (1.5/0.5 g every 6 h) in the HFIM experiments.13 While the mechanism of rapid and sustained bacterial killing with aztreonam and ceftazidime/avibactam has not been established, it is likely attributable to maximal saturation of the diverse PBPs present in Gram-negative bacteria, flooding of the periplasm with β-lactams and inhibition of available β-lactamases.9,36
Another clinically relevant finding from the HFIM experiments was the daily dose of aztreonam required for maximal effect with ceftazidime/avibactam. Aztreonam 6 g/day regimens are most commonly used in clinical practice. However, most of the combination regimens that included aztreonam 6 g/day did not perform as well as those using total daily doses of 8 g. The enhanced activity of aztreonam 8 g/day versus 6 g/day was largely consistent across both ceftazidime/avibactam dosing strategies (standard and CI) and tested isolates. While this was observed with both strains, the benefits of aztreonam 8 g/day versus 6 g/day in combination with ceftazidime/avibactam were most pronounced with E. coli. Of note, there were only subtle differences in bacterial killing and complete bacterial eradication between aztreonam 1.5 g IV every 6 h with standard dosing ceftazidime/avibactam and aztreonam 2 g IV every 6 h with standard dosing ceftazidime/avibactam. Furthermore, both regimens outperformed standard dosing (aztreonam 2 g IV every 8 h) in combination with standard dosing ceftazidime/avibactam. Future dose-ranging and fractionation HFIM studies are needed to determine whether there are any appreciable differences in these two regimens. For now, the decision to use aztreonam 1.5 or 2 g IV every 6 h in combination with standard dosing ceftazidime/avibactam in clinical practice requires a detailed risk versus benefit analysis on a case-by-case basis.
Despite the notion that avibactam must be present prior to the administration of aztreonam to effectively inhibit β-lactamases, we did not find this to be the case. For both E. coli and K. pneumoniae, staggered administration of ceftazidime/avibactam followed by aztreonam resulted in less bacterial killing and greater regrowth than simultaneous administration of the two together. Against K. pneumoniae, these effects were less pronounced than for E. coli. However, bacterial killing was not as rapid, nor was bacterial killing as extensive as was observed with the simultaneously administered combination regimens. These findings suggest that both agents must be administered simultaneously to maximize protection against circulating β-lactamases and achieve maximal killing.
Although we postulated that the CI combination would outperform the standard dosing combination regimen, we did not find this to be the case with aztreonam 8 g/day combination regimens. Given the similar bactericidal activity of high-dose aztreonam paired with either standard-dose or CI ceftazidime/avibactam, practical concerns should drive combination regimen selection. The principal advantages of CI are fewer daily administrations and reduced costs for labour, supplies and administration. The major disadvantages of CI are the need for a dedicated line for infusion (which often leads to drug compatibility and administration issues), issues of drug stability and waste, and lack of ambulation for the patient. CI often requires insertion of a central line, which places patients at unnecessary risk of secondary catheter-related infection.19 Additionally, safety of CIs of aztreonam and ceftazidime/avibactam have not been formally established in a regulatory study. However, there are considerable published data on the safety of β-lactams when given as CIs.20–22
Several factors should be considered when interpreting these findings. Firstly, these HFIM experiments represent a conservative estimate of bactericidal activity, as the model does not account for the effect of the native immune system.17 Despite this limitation, multiple studies have demonstrated parity between results of HFIM studies and animal studies.17,37 We acknowledge the use of two bacterial isolates (one each of E. coli and K. pneumoniae). However, we have observed similar findings demonstrating the bactericidal activity of the ceftazidime/avibactam and aztreonam combination over 48 h against other strains of K. pneumoniae producing NDM and CTX-M-15 (data not shown). Total daily doses were limited to those approved by the FDA as the goal was to identify optimal regimens for immediate uptake into clinical practice, given the dearth of available agents with activity against MBL-producing Enterobacteriaceae.28,29 We did not believe it prudent to alter the daily ceftazidime/avibactam dose as data suggest that the amount of avibactam in the current FDA-approved dosing is minimally sufficient for effect and a lesser amount may lead to suboptimal outcomes.11,38 Therefore, we chose to alter the aztreonam dose and interval in the presence of standard FDA-approved and CI dosing of ceftazidime/avibactam when defining optimal ceftazidime/avibactam with aztreonam combination regimens.
This study was not designed as a pharmacodynamics target-defining study. It is possible that optimal dosing of ceftazidime/avibactam with aztreonam could be further improved with a more quantitative understanding of the pharmacokinetic/pharmacodynamic drivers for effect when they are used in combination. Our results, however, are consistent with other studies that have shown time-over-threshold pharmacodynamic targets for β-lactam/β-lactamase inhibitor combinations, given the benefit observed with prolonged infusions.11,39,40 Quantification of bacterial inoculum was stopped at 7 days, designed to mimic a typical treatment course for VABP.30 In more severe infections, a longer treatment course may be necessary. Most combination regimens, especially those containing 8 g of aztreonam per day, resulted in bacterial counts below the limit of detection by 48 h. This effect was sustained throughout the remainder of the experiment, making it unlikely that a longer study duration would have altered the results.
Like all studies of this nature, the results need to be interpreted with extreme caution and additional work is still needed to properly characterize the effectiveness of ceftazidime/avibactam with aztreonam against MBL-producing Gram-negative pathogens. Although this study had good validity (42 HFIM arms, including prospective validation experiments), only two NDM-1-producing K. pneumoniae and E. coli isolates were tested and further work with other NDM-producing Enterobacteriaceae, especially those that co-harbour an OXA-48-like β-lactamase, are needed to understand the external validity of the findings. Furthermore, it is well established that there is large variability in the hydrolytic capabilities of the various MBLs and it is unclear whether the findings are applicable to K. pneumoniae and E. coli isolates that harbour other commonly observed MBLs such as VIM or IMP. It is also unknown whether the results can be applied to other MBL-producing pathogens such as Pseudomonas aeruginosa and Acinetobacter baumannii that possess a more diverse set of intrinsic, acquired and adaptive resistance mechanisms than K. pneumoniae and E. coli.
Lastly, clinical efficacy and safety of these combination regimens still need to be established in humans. While both ceftazidime/avibactam and aztreonam are generally safe and well tolerated, asymptomatic serum aminotransferase elevations are common during high-dose IV aztreonam therapy (10% to 38%).34 Aztreonam-induced liver injury appears to be transient, mild and asymptomatic, being marked by serum enzyme elevations only. Data indicate that aztreonam is unlikely to be a cause of clinically apparent liver injury and no individual cases of frank liver injury and jaundice attributable to aztreonam have been reported. However, there are no available data on safety when these antibiotics are used in combination. It is unclear whether ceftazidime/avibactam combined with aztreonam will further exacerbate liver enzyme elevations or lead to other adverse events due to the potential for cumulative toxicity from dual-β-lactam treatment.36 From a clinical perspective, the next logical sequential steps are to conduct a Phase 1 study in healthy participants and, if successful, a Phase 1b study in the targeted-use patient population. This represents a novel post-approval pre-clinical to clinical trial design for evaluating combination therapy. Given the similar bactericidal activity of the optimal regimens identified in the validation studies [ceftazidime/avibactam 2/0.5 g every 8 h (2 h infusion) with aztreonam 2 g every 6 h (2 h infusion)] and CI (ceftazidime/avibactam 6/1.5 g per day CI with aztreonam 8 g/day CI), we would recommend studying both regimens in the proposed Phase 1 studies to determine whether there are any differential safety concerns. Furthermore, the two regimens have different practical concerns and it will be advantageous for clinicians to have safety data on both regimens. If both are found to be safe, we would advocate testing the optimal standard dosing combination in a pathogen-focused comparator clinical trial, given that both standalone regimens in the combination are already approved by the FDA and EU.
In summary, against NDM-1-producing K. pneumoniae and E. coli isolates, nearly all combination regimens of ceftazidime/avibactam 6/1.5 g per day with aztreonam 6–8 g per day resulted in rapid and sustained bactericidal activity across 7 day HFIM experiments. Combination regimens that included 8 g/day of aztreonam largely outperformed those utilizing 6 g/day with few exceptions. Additionally, prolonged (i.e. ≥2 h) and simultaneous infusions appear to be necessary for optimal dosing of this combination against the tested NDM-producing pathogens. Similar to all studies of this nature, definitive conclusions regarding the effectiveness of this combination regimen cannot be made until additional experiments are performed against an array of Gram-negative pathogens that harbour different MBLs with varying hydrolytic capabilities and other resistance mechanisms. Lastly, the clinical efficacy and safety of the optimal combination regimens identified in this study need to be established in humans and use at this time should be based on an assessment of risk versus benefit.
Funding
Research reported herein was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1AI104681, to R.A.B. under Award Numbers R01AI100560, R01AI063517, and R01AI072219, and to B.T.T. under Award Number R01AI148560. This study was also supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, Award Number 1I01BX001974 to R.A.B. from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development, and the Geriatric Research Education and Clinical Center VISN 10. V.G.F. was supported by mid-career mentoring award K24-AI093969 from the NIH.
Transparency declarations
T.P.L.: Achaogen: consultant; Melinta: consultant, scientific advisor and speakers’ bureau. Motif: consultant and scientific advisor. Nabriva: consultant. Paratek: consultant and scientific advisor, consulting fee. Sunovion: Speaker. Merck & Co: consultant, grant recipient.
B.T.T.: Nabriva: consultant; Achaogen: grant. N.O.: grant from Merck. R.A.B.: grants from Allecra, Entasis, Merck, Roche, Wockhardt, Shionogi and Achaogen. J.B.B. reports the following disclosure: MicuRX: consultant. V.G.F. served as Chair of the V710 Scientific Advisory Committee (Merck); has received grant support from Cerexa/Actavis/Allergan, Pfizer, Advanced Liquid Logics, NIH, MedImmune, Basilea Pharmaceutica, Karius, ContraFect, Regeneron Pharmaceuticals, and Genentech; has NIH STTR/SBIR grants pending with Affinergy, Locus, and Medical Surface, Inc; has been a consultant for Pfizer, Novartis, Galderma, Novadigm, Durata, Debiopharm, Genentech, Achaogen, Affinium, Medicines Co., Cerexa, Tetraphase, Trius, MedImmune, Bayer, Theravance, Cubist, Basilea, Affinergy, Janssen, xBiotech, Contrafect, Regeneron, Basilea, Destiny, Amphliphi Biosciences; Integrated Biotherapeutics; C3J; and has received honoraria from Theravance Biopharma, Inc., and Green Cross, and has a patent pending in sepsis diagnostics. All other authors: none to declare.
Author contributions
T.P.L. and B.T.T. led the development of the research question, study design, implementation of the study protocol, analysis and interpretation of data, and drafting the report, along with N.M.S., N.O., A.E.E., P.N.H., K.R.B., J.Z., X.T., J.B.B., V.G.F., H.F.C. and R.A.B. All authors provided critical reviews and final approval of the manuscript.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Department of Veterans Affairs.
Data-sharing statement
Researchers interested in accessing the clinical trial data presented herewith are encouraged to submit a research proposal and publication plan. The proposal and plan will be reviewed by the ARLG publications committee and/or appropriate study team members. If approved and upon receipt and approval of a signed data access/use agreement, individual participant data necessary to complete the proposed analysis will be made available. Related documents, including the study protocol, statistical analysis plan and data dictionary, may also be shared. Access to data will only be granted to researchers who provide a methodologically and scientifically sound proposal. Proposed analyses that are duplicative of ongoing or proposed analyses may not be supported. To submit a proposal, please complete a proposal at https://arlg.org/how-to-apply/protocol-concept. Alternatively, visit dcri.org/data-sharing. There may be costs associated with data sharing that researchers would be expected to cover.
Supplementary data
Additional Methods, Table S1 and Figures S1 to S3 are available as Supplementary data at JAC Online.
Supplementary Material
References
- 1.IDSA. Antibacterial Resistance. https://www.idsociety.org/policy--advocacy/antimicrobial-resistance/.
- 2.Wright H, Bonomo RA, Paterson DL.. New agents for the treatment of infections with Gram-negative bacteria: restoring the miracle or false dawn? Clin Microbiol Infect 2017; 23: 704–12. [DOI] [PubMed] [Google Scholar]
- 3.Sader HS, Castanheira M, Duncan LR. et al. Antimicrobial susceptibility of Enterobacteriaceae and Pseudomonas aeruginosa isolates from United States medical centers stratified by infection type: results from the International Network for Optimal Resistance Monitoring (INFORM) Surveillance Program, 2015-2016. Diagn Microbiol Infect Dis 2018; 92: 69–74. [DOI] [PubMed] [Google Scholar]
- 4.Zavascki AP, Nation RL.. Nephrotoxicity of polymyxins: is there any difference between colistimethate and polymyxin B? Antimicrob Agents Chemother 2017; 61: e02319–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karlowsky JA, Kazmierczak KM, de Jonge BLM. et al. In vitro activity of aztreonam/avibactam against Enterobacteriaceae and Pseudomonas aeruginosa isolated by clinical laboratories in 40 countries from 2012 to 2015. Antimicrob Agents Chemother 2017; 61: e00472–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall S, Hujer AM, Rojas LJ. et al. Can ceftazidime/avibactam and aztreonam overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae? Antimicrob Agents Chemother 2017; 61: e02243–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mojica MF, Ouellette CP, Leber A. et al. Successful treatment of bloodstream infection due to metallo-β-lactamase-producing Stenotrophomonas maltophilia in a renal transplant patient. Antimicrob Agents Chemother 2016; 60: 5130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw E, Rombauts A, Tubau F. et al. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J Antimicrob Chemother 2018; 73: 1104–6. [DOI] [PubMed] [Google Scholar]
- 9.Davido B, Fellous L, Lawrence C. et al. Ceftazidime/avibactam and aztreonam, an interesting strategy to overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2017; 61: e01008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kazmierczak KM, Rabine S, Hackel M. et al. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2016; 60: 1067–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh R, Kim A, Tanudra MA. et al. Pharmacokinetics/pharmacodynamics of a β-lactam and β-lactamase inhibitor combination: a novel approach for aztreonam/avibactam. J Antimicrob Chemother 2015; 70: 2618–26. [DOI] [PubMed] [Google Scholar]
- 12.Wenzler E, Deraedt MF, Harrington AT. et al. Synergistic activity of ceftazidime/avibactam and aztreonam against serine and metallo-β-lactamase-producing gram-negative pathogens. Diagn Microbiol Infect Dis 2017; 88: 352–4. [DOI] [PubMed] [Google Scholar]
- 13.ClinicalTrials.gov. To Investigate the Safety and Tolerability of Aztreonam-Avibactam (ATM-AVI). https://clinicaltrials.gov/ct2/show/NCT01689207.
- 14.U.S. Department of Health and Human Services, FDA, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Limited population pathway for antibacterial and antifungal drugs: guidance for industry (draft guidance). https://www.fda.gov/files/drugs/published/Limited-Population-Pathway-for-Antibacterial-and-Antifungal-Drugs-Guidance-for-Industry.pdf.
- 15.EMA—Committee for Medicinal Products for Human Use (CHMP). 2012. Guideline on the evaluation of medicinal products indicated for treatment of bacterial infections (CPMP/EWP/558/95 rev 2). https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-medicinal-products-indicated-treatment-bacterial-infections-revision-2_en.pdf.
- 16.EMA—Committee for Medicinal Products for Human Use (CHMP). 2016. Guideline on the use of pharmacokinetics and pharmacodynamics in the development of antimicrobial medicinal products (EMA/CHMP/594085/2015). https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-use-pharmacokinetics-pharmacodynamics-development-antimicrobial-medicinal-products_en.pdf.
- 17.Drusano GL.From lead optimization to NDA approval for a new antimicrobial: use of pre-clinical effect models and pharmacokinetic/pharmacodynamic mathematical modeling. Bioorg Med Chem 2016; 24: 6401–8. [DOI] [PubMed] [Google Scholar]
- 18.Bulitta JB, Hope WW, Eakin AE. et al. Generating robust and informative nonclinical in vitro and in vivo bacterial infection model efficacy data to support translation to humans. Antimicrob Agents Chemother 2019; 63: e02307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lodise TP, Lomaestro BM, Drusano GL.. Application of antimicrobial pharmacodynamic concepts into clinical practice: focus on β-lactam antibiotics: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2006; 26: 1320–32. [DOI] [PubMed] [Google Scholar]
- 20.Abdul-Aziz MH, Sulaiman H, Mat-Nor MB. et al. β-Lactam infusion in severe sepsis (BLISS): a prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent β-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med 2016; 42: 1535–45. [DOI] [PubMed] [Google Scholar]
- 21.Dulhunty JM, Roberts JA, Davis JS. et al. Continuous infusion of β-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clin Infect Dis 2013; 56: 236–44. [DOI] [PubMed] [Google Scholar]
- 22.Dulhunty JM, Roberts JA, Davis JS. et al. A multicenter randomized trial of continuous versus intermittent β-lactam infusion in severe sepsis. Am J Respir Crit Care Med 2015; 192: 1298–305. [DOI] [PubMed] [Google Scholar]
- 23.Evans SR, Hujer AM, Jiang H. et al. Rapid molecular diagnostics, antibiotic treatment decisions, and developing approaches to inform empiric therapy: PRIMERS I and II. Clin Infect Dis 2016; 62: 181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Twenty-Ninth Edition: M100. 2019.
- 25.Bulman ZP, Chen L, Walsh TJ. et al. Polymyxin combinations combat Escherichia coli harboring mcr-1 and blaNDM-5: preparation for a postantibiotic era. Mbio 2017; 8: e00540–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bulitta JB, Landersdorfer CB, Huttner SJ. et al. Population pharmacokinetic comparison and pharmacodynamic breakpoints of ceftazidime in cystic fibrosis patients and healthy volunteers. Antimicrob Agents Chemother 2010; 54: 1275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H, Zhou W, Zhou D. et al. Evaluation of aztreonam dosing regimens in patients with normal and impaired renal function: a population pharmacokinetic modeling and Monte Carlo simulation analysis. J Clin Pharmacol 2017; 57: 336–44. [DOI] [PubMed] [Google Scholar]
- 28.AZACTAM (aztreonam) for injection, USP. Package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050580s042lbl.pdf.
- 29.AVYCAZ (ceftazidime and avibactam) for injection, for intravenous use. Package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/206494s002lbl.pdf.
- 30.Kalil AC, Metersky ML, Klompas M. et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63: e61–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaccard CR, Schell RF, Spiegel CA.. Efficacy of bilateral bronchoalveolar lavage for diagnosis of ventilator-associated pneumonia. J Clin Microbiol 2009; 47: 2918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saade E, Wilson B, El Chakhtoura NG. et al. Drug-induced liver injury (DILI) in a national cohort of hospitalized patients treated with aztreonam and ceftazidime. IDweek 2018, San Francisco, CA, USA. Abstract 2409.
- 33.DeMaria A Jr, Treadwell TL, Saunders CA. et al. Randomized clinical trial of aztreonam and aminoglycoside antibiotics in the treatment of serious infections caused by gram-negative bacilli. Antimicrob Agents Chemother 1989; 33: 1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Library of Medicine and National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: clinical and research information on drug-induced liver injury. https://livertox.nlm.nih.gov/Aztreonam.htm. [PubMed]
- 35.Wenzler E, Bunnell KL, Bleasdale SC. et al. Pharmacokinetics and dialytic clearance of ceftazidime/avibactam in a critically ill patient on continuous venovenous hemofiltration. Antimicrob Agents Chemother 2017; 61: e00464–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahme C, Butterfield JM, Nicasio AM. et al. Dual β-lactam therapy for serious Gram-negative infections: is it time to revisit? Diagn Microbiol Infect Dis 2014; 80: 239–59. [DOI] [PubMed] [Google Scholar]
- 37.Lenhard JR, Thamlikitkul V, Silveira FP. et al. Polymyxin-resistant, carbapenem-resistant Acinetobacter baumannii is eradicated by a triple combination of agents that lack individual activity. J Antimicrob Chemother 2017; 72: 1415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coleman K, Levasseur P, Girard AM. et al. Activities of ceftazidime and avibactam against β-lactamase-producing Enterobacteriaceae in a hollow-fiber pharmacodynamic model. Antimicrob Agents Chemother 2014; 58: 3366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felton TW, Goodwin J, O’Connor L. et al. Impact of bolus dosing versus continuous infusion of piperacillin and tazobactam on the development of antimicrobial resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 2013; 57: 5811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicasio AM, VanScoy BD, Mendes RE. et al. Pharmacokinetics-pharmacodynamics of tazobactam in combination with piperacillin in an in vitro infection model. Antimicrob Agents Chemother 2016; 60: 2075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.