Abstract
We present a formulation of the multiconfigurational (MC) wave function symmetry-adapted perturbation theory (SAPT). The method is applicable to noncovalent interactions between monomers which require a multiconfigurational description, in particular when the interacting system is strongly correlated or in an electronically excited state. SAPT(MC) is based on one- and two-particle reduced density matrices of the monomers and assumes the single-exchange approximation for the exchange energy contributions. Second-order terms are expressed through response properties from extended random phase approximation (ERPA). The dispersion components of SAPT(MC) have been introduced in our previous works [Hapka M.et al. J. Chem. Theory Comput. 2019, 15, 1016−1027; Hapka M.et al. J. Chem. Theory Comput. 2019, 15, 6712–6723]. SAPT(MC) is applied either with generalized valence bond perfect pairing (GVB) or with complete active space self-consistent field (CASSCF) treatment of the monomers. We discuss two model multireference systems: the H2 ··· H2 dimer in out-of-equilibrium geometries and interaction between the argon atom and excited state of ethylene. Using the C2H4* ··· Ar complex as an example, we examine second-order terms arising from negative transitions in the linear response function of an excited monomer. We demonstrate that the negative-transition terms must be accounted for to ensure qualitative prediction of induction and dispersion energies and develop a procedure allowing for their computation. Factors limiting the accuracy of SAPT(MC) are discussed in comparison with other second-order SAPT schemes on a data set of small single-reference dimers.
1. Introduction
Quantum chemistry offers two complementary approaches to noncovalent interactions, the supermolecular approach and energy decomposition methods. The former is conceptually simple and capable of providing the most accurate potential energy surfaces, e.g., for interpretation of experiments carried out in the cold- and ultracold regimes.1−3 The latter, decomposition methods, allow insight into the nature of the interaction by partitioning the interaction energy into well-defined contributions. The symmetry-adapted perturbation theory (SAPT)4,5 can be considered one of decomposition methods—it provides representation of the interaction energy as a sum of directly calculated components with a clear physical interpretation. Modern SAPT methods not only serve as interpretative tools for systems as large as enzymes exceeding 3000 atoms,6 but have also been applied to generate potential energy surfaces for quantitative predictions, e.g., calculations of scattering cross-sections, predictions of spectra and bulk matter properties, as well as the development of force fields for biomolecules (see, e.g., refs (7−11)).
In contrast to the rich toolbox dedicated to single-determinantal wave functions,12,13 describing intermolecular interactions in complexes that demand multiconfigurational (MC) wave functions presents a challenge. The multiconfigurational treatment is often mandatory for transition-metal complexes, open-shell systems, electronically excited states, or systems dominated by static correlation effects. From the standpoint of weak intermolecular forces, proper representation of static correlation, warranted by expansion in multiple electron configurations, is not sufficient. The main difficulty lies in the recovery of the remaining dynamic correlation both within and between the interacting molecules. The latter effect, giving rise to the attractive dispersion interaction, poses a particular challenge due to its highly nonlocal and long-range nature. Although many multireference methods restoring dynamic correlation effects have been developed, neither has yet managed to combine the accuracy and efficiency required for noncovalent interactions.
Application of multireference approaches in supermolecular calculations is often difficult due to the limitations of the methods themselves. For instance, the accuracy of the popular multireference configuration interaction (MRCI) approach14 and multireference perturbation theories15,16 is limited by the lack of triple excitations and truncation of the perturbation series at the second-order, respectively. Moreover, MRCI is not size-consistent and requires approximate corrections added a posteriori.17,18 In perturbation theories, the fulfillment of the strict separability condition depends on the choice of the zeroth-order Hamiltonian.19,20 A separate problem encountered in the perturbation theories, including complete active space (CAS) perturbation theory (CASPT2)15 and multireference variants of the Møller–Plesset perturbation theory,21 is the presence of intruder states,22 which have to be removed using one of the available shift techniques.23 Intruder states also present a significant difficulty in the development of multireference coupled-cluster theories,24,25 next to numerical instabilities and algebraic complexity. Single-reference coupled-cluster (CC) approaches introduced by Piecuch and co-workers, e.g., the CC(P;Q) formalism,26,27 may be a viable alternative, as indicated by studies of interactions involving stretched intramonomer covalent bonds.28,29 Encouraging results have recently been obtained for strongly correlated interacting systems from multiconfigurational random phase approximation theory combined with generalized valence bond method.29−31 The multiconfiguration density functional theory (MC DFT)32,33 methods corrected to include long-range dynamic correlation via perturbation theory,34 the adiabatic connection formalism,35 or semiempirical dispersion models36 are also worth mentioning, but their accuracy for noncovalent interactions remains to be rigorously assessed.
The SAPT formalism offers several important advantages, which make it one of the most widely used and actively developed approaches to noncovalently bound complexes.11 Compared to the supermolecular approach, SAPT avoids the basis set superposition error since the interaction energy is computed directly based only on monomer properties. The most accurate variants of SAPT predict interaction energies closely matching the coupled-cluster singles-and-doubles with perturbative triples [CCSD(T)37,38] results. Last but not least, the interaction energy represented as a sum of energy contributions is per se size-consistent.
In more than 40 years spanning the development of SAPT, applications going beyond the single-reference treatment of the monomers have been scarce. Exact full configuration interaction (FCI) wave functions are feasible only for model, few-electron dimers and have been employed in studies of SAPT convergence.7,39−42 Reinhardt43 used valence bond (VB) wave functions to represent the electrostatic interaction between monomers of a multireference character and proposed an approximate, VB-based approach for dispersion energy calculations. The spin-flip SAPT (SF-SAPT)44,45 formalism introduced by Patkowski and co-workers opened the possibility to treat multireference, low-spin states based on single-reference description of the subsystems. First-order spin-flip exchange energy expressions for high-spin restricted open-shell Hartree–Fock (ROHF) wave function have already been derived and implemented,44,45 while extension to the second-order is underway.11
The purpose of the present paper is to present a complete SAPT formalism applicable to interactions involving multireference systems. First steps in these directions have already been taken. Recently, we have devised multiconfigurational approaches for second-order dispersion46 and exchange–dispersion47 energy calculations. In this work, we use the same methodology to derive the second-order induction energy expressions and present formulas for the first-order electrostatic and exchange energies. The energy contributions up to the second-order in the intermolecular interaction operator constitute a variant of SAPT based on MC wave functions, which we refer to as SAPT(MC). The method can be applied with any wave function model, which gives access to one- and two-electron reduced density matrices of the monomers. Following the developments of refs (46) and (47), the linear response properties required for second-order terms are accessed by solving extended random phase approximation (ERPA)48,49 equations. Both first- and second-order exchange terms are derived assuming the single-exchange approximation,50 also known as the S2 approximation. We discuss the performance of SAPT(MC) combined with either generalized valence bond perfect pairing (GVB) or complete active space self-consistent field (CASSCF) description of the monomers.
The presented SAPT(MC) formulation is valid for the interaction between monomers in spin singlet states. The formalism may be extended to monomers with nonzero spins, which couple to the high-spin state of the dimer. For single-reference wave functions, the open-shell SAPT(MC) will become equivalent to the SAPT(ROHF) method of Żuchowski and co-workers.51 As all currently available many-electron SAPT approaches, SAPT(MC) is based on nondegenerate perturbation theory and is not applicable to dimers in degenerate states.
This work is organized in five sections. In Section 2, we present formulas for first- and second-order energy contributions in the ERPA-based variant of multiconfigurational SAPT. Special attention is paid to calculations of induction and dispersion energies for complexes involving electronically excited molecules. Section 3 contains details of our implementation and computations. Results for the model multireference and single-reference dimers are presented in Section 4. In Section 5, we summarize our findings.
2. Theory
Consider a weakly interacting dimer AB, which dissociates into monomer A in state I described with the |ΨIA⟩ wave function and monomer B in state J described with the |ΨJ⟩ wave function (I and J refer to either ground or excited states of the monomers). When the unperturbed Hamiltonian is chosen as the sum of Hamiltonians of the isolated monomers, Ĥ0 = ĤA + ĤB, the zeroth-order wave function takes a product form |Ψ0⟩ = |ΨIAΨJ⟩. In this work, we assume that |Ψ0⟩ is nondegenerate.
The intermolecular interaction operator, V̂, represents the perturbation and gathers all Coulombic interactions between electrons and nuclei of the interacting partners
 |
1 |
where i and j run over NA and NB electrons in monomers A and B, respectively, vA and vB are one-electron potentials, and VAB is the nuclear–nuclear repulsion term.
In the symmetrized Rayleigh–Schrödinger (SRS) formulation52 of SAPT, the interaction energy is expanded with respect to V̂ while enforcing the antisymmetry of |ΨIA⟩ and |ΨJ⟩ wave function products. The general expression for energy contribution in the nth order in V̂ takes the form
| 2 |
where 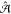 is the antisymmetrizer exchanging electrons
between the monomers; |ΨRS(n)⟩ denotes the nth order component of the wave function expansion, which
is identical in both SRS and the conventional Rayleigh–Schrödinger
(RS) perturbation theory, i.e., the expansion is based only on simple
products of zero-order functions. The difference between the SRS and
RS energies is defined as the exchange energy. The RS energy contributions
are often referred to as polarization components. For convenience,
we use the SAPT acronym when referring to SRS.
is the antisymmetrizer exchanging electrons
between the monomers; |ΨRS(n)⟩ denotes the nth order component of the wave function expansion, which
is identical in both SRS and the conventional Rayleigh–Schrödinger
(RS) perturbation theory, i.e., the expansion is based only on simple
products of zero-order functions. The difference between the SRS and
RS energies is defined as the exchange energy. The RS energy contributions
are often referred to as polarization components. For convenience,
we use the SAPT acronym when referring to SRS.
The SAPT(MC) formalism presented in this work includes interaction energy components through the second-order in V̂
| 3 |
where Eelst(1) and Eexch are first-order electrostatic and exchange energy contributions, respectively, Eind(2) and Eexch–ind are the second-order induction and exchange–induction energies, respectively, and Edisp(2) and Eexch–disp denote the dispersion energy and its exchange counterpart, respectively.
All formulas are in the natural orbital (NO) representation. We use the following index convention: greek μ and ν indices denote electronic states of monomers, pσqσrσsσ denotes natural spin orbitals, while pqrs pertains to natural orbitals denoted by φ(r). Throughout the work, the NOs are assumed to be real-valued. In the representation of natural orbitals, the one-electron reduced density matrix (1-RDM) is diagonal
| 4 |
where {âpσ†} and {âpσ} are the creation and annihilation operators, respectively, and np are the natural occupation numbers from the ⟨0,1⟩ range, summing up to half a number of electrons, ∑pnp = N/2.
All presented SAPT(MC) energy contributions are given in a spin-summed form. The expressions for the polarization energy components are valid for arbitrary spin states of the monomers. The exchange energy contributions are presented assuming singlet spin states of the monomers, which implies that αα and ββ blocks of 1-RDM are equal.
2.1. First-Order Energy Contributions
The polarization component of the first-order SAPT energy is the electrostatic energy, Eelst(1) = ⟨ΨIΨJB|V̂|ΨIΨJB⟩. This energy contribution expressed in terms of 1-RDMs takes the form
| 5 |
where vpqA(B) = ⟨φp|vA(B)|φq⟩ are the matrix elements of one-electron potentials and vpq denotes the regular two-electron Coulomb integrals vpqrs = ⟨φp(r1)φq(r2)|r12|φr(r1)φs(r2)⟩.
Evaluation of the exact expression for the first-order exchange energy
| 6 |
requires access to many-particle density matrices of the monomers. For the Hartree–Fock wave function, many-particle density matrices are readily available as antisymmetrized products of the one-particle density matrix.53 At the SAPT(DFT) level of theory, one uses approximate 1-RDMs of the monomers based on the Kohn–Sham determinants and employs the same exchange expression as in the wave function SAPT.54,55
It is possible to significantly simplify the structure of eq 6 by allowing only for single exchange of electrons between the monomers in the antisymmetrizer50,56
| 7 |
where
the single-exchange operator  collects
all permutations, P̂ij, interchanging the coordinates of
electrons i and j
collects
all permutations, P̂ij, interchanging the coordinates of
electrons i and j
| 8 |
Neglecting multiple exchange of electrons is known as the S2 approximation and allows one to express the first-order exchange energy using only 1-RDMs and two-electron reduced density matrices (2-RDMs) of the monomers.57 Following the density-matrix-based formulation of ref (57), we obtain
 |
9 |
where Spq = ⟨φp|φq⟩ denotes the overlap integral, and we have introduced intermediates containing contractions of the 2-RDM, Γpσqσ′rσ″sσ‴ = ⟨Ψ|ârσ″âsσ‴†âqσ′âpσ|Ψ⟩, with the overlap integrals
| 10 |
where Γ̅pqrs is the spin-summed 2-RDM, Γ̅pqrs = Γpαqαrαsα + Γpβqαrβsα. Since we assume monomers in singlet states, the ββββ + αβαβ block is equal to its αααα + βαβα counterpart.
2.2. Second-Order Energy Contributions
2.2.1. Transition Properties from Extended Random Phase Approximation
Second-order SAPT energy components may be expressed through transition properties of the interacting monomers. The induction and dispersion energies involve transition energies and one-electron reduced transition density matrices (1-TRDMs). The SRS components, exchange–induction and exchange–dispersion energies, require both 1-TRDMs and two-electron reduced transition density matrices (2-TRDMs).
In this work, we approximate the transition properties of the interacting monomers by solving the extended random phase approximation46,48,58 equations (independently for each monomer)
| 11 |
where, in the representation of the natural spin orbitals, one obtains
| 12 |
thus, the metric matrix is
diagonal and given by the occupation numbers {np} (of a given monomer). For a system described
with a Hamiltonian Ĥ and a wave function Ψ
approximating a state of interest (ground or excited), the matrices 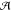 and
and 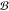 read
read
| 13 |
| 14 |
and they
are determined solely
by one- and two-particle reduced density matrices of a given system.
The ERPA equations may be formed as a symmetric real eigenproblem
using electronic Hessian matrices,  and
and  . For ground-state
calculations, the Hessian
matrices are positive definite (see, e.g., refs (59) and (60) for explicit ERPA equations
in the GVB and CAS frameworks, respectively). In the case of excited-state
wave functions, the Hessian matrices may have negative eigenvalues
corresponding to de-excitation modes in the ERPA propagator61 (see a more detailed discussion in Section 2.2.3).
. For ground-state
calculations, the Hessian
matrices are positive definite (see, e.g., refs (59) and (60) for explicit ERPA equations
in the GVB and CAS frameworks, respectively). In the case of excited-state
wave functions, the Hessian matrices may have negative eigenvalues
corresponding to de-excitation modes in the ERPA propagator61 (see a more detailed discussion in Section 2.2.3).
Apart from transition energies ων, which correspond to the poles of the ERPA eigenproblem, two quantities that are required in second-order SAPT are 1- and 2-TRDMs of the monomers. The 1-TRDM is defined as
| 15 |
Note that for singlet states αα and ββ blocks are equal: γpαqαν = γpβqβ = γpqν. The general definition of 2-TRDM reads
| 16 |
The 1-TRDM is expressed through the ERPA eigenvectors as60,62
| 17 |
| 18 |
and the formula for the half of spin-summed 2-TRDM reads47
 |
19 |
2.2.2. Induction and Dispersion Energies
The polarization components of SAPT in the second-order are the induction and dispersion energies. The induction energy is given as
 |
20 |
where ωμA (ων) are the transition energies from the state I (J for the monomer B) to μ (ν)
| 21 |
The Eind(2)(A ← B) term arises from the permanent multipole moments on B changing the wave function of monomer A. The Eind(B ← A) term describes the corresponding change in monomer B due to the perturbing field of A.
Equation 20 may be recast using contractions between 1-TRDMs of one monomer and the electrostatic potential of its unperturbed interacting partner, the latter defined as
| 22 |
where ρB is the one-electron density of the monomer B (analogous expression holds for Ω̂A). The total induction energy formula is now conveniently expressed as
| 23 |
where Ωpq = ⟨φp|Ω̂|φq⟩.
In the ERPA approximation, the spin-summed formula for Eind(2) takes the form
| 24 |
where
| 25 |
The pertinent expression for the dispersion energy is63,64
 |
26 |
which, in the ERPA form, reads46
 |
27 |
2.2.3. Excited-State Case: Explicit Contributions to Dispersion and Induction Energies from De-Excitations
Consider a dimer AIB0 in an excited state, which dissociates into a monomer A in the state I > 0 denoted in this section as AI (for simplicity, it is assumed that states of A are not degenerate) and a monomer B in the ground state, denoted as B0. While all transition energies, cf. eq 21, corresponding to B0 are positive
| 28 |
for the monomer A, they take either negative or positive values for transitions to states lower or higher than I, respectively
| 29 |
| 30 |
Let us rewrite the dispersion energy expression, eq 26, in a form in which we explicitly isolate terms involving negative transitions (de-excitations)
 |
31 |
where, by Edisp+(2)(AIB0), we denote the dispersion energy arising from the positive part of the monomer A linear response function spectrum
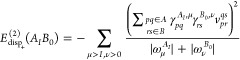 |
32 |
[to emphasize that monomers A and B are, respectively, in the excited and ground states, a notation for transition properties in this subsection is changed, compared to other sections: quantities pertaining to monomers A and B are denoted as AI and B0]. In eqs 31 and 32, signs of the transition energies in the denominators are written explicitly. Note that for excited states the dispersion energy may become positive-valued. This can occur either for a sufficiently high state of the monomer A (high I) or if the spectrum of the dimer contains states, such that |ωμAI| – |ων| ≪ 1 and |ωμAI| > |ων|.
Approximate methods, which are based on single-excitation operators and for which the linear response is directly related to an orbital Hessian matrix, are likely to miss de-excitations in the linear response function computed for the excited state of interest.65 Consequently, the second term in eq 31 would not be accounted for. Since this term involves transitions to the low-lying states, it is anticipated to give a non-negligible contribution to the dispersion energy.
A viable way to account for the de-excitations from the AI state in dispersion energy calculations is by considering linear response properties of states J lower than I. After exploiting the relations connecting response properties of the states I and J
| 33 |
| 34 |
one immediately writes the μ = J component of eq 31 as
 |
35 |
The dispersion energy for the AIB0 dimer can now be written as
| 36 |
It should be emphasized that eq 36 is fully equivalent to eq 26 if exact response properties are employed. The crucial difference between the expressions in eqs 31 and 36 is that in the former contributions to the dispersion energy from negative excitations follow from the linear response of the state AI, while in the latter, they are obtained from the response of states AJ, which are lower in energy than AI.
The ERPA model applied to excited-state reference wave function either completely misses negative excitations or reproduces them with poor accuracy. As a result, ERPA-approximated dispersion energy, eq 27, computed for the excited-state dimer AIB0 will lack important contributions from de-excitations. The way around this problem is to employ the alternative formula for the dispersion energy presented in eq 36 in the ERPA approximation. This requires computing the Edisp+(2)(AIB0) term according to eq 27 and expressing the approximated εdisp(AJB0) terms through ERPA transition properties
 |
37 |
The ZIAJ and ωI are the Ith eigenvector and eigenvalue, respectively, of the ERPA equations solved for the monomer A in the Jth state
| 38 |
| 39 |
To reiterate, the negative-energy transition I → J, which is either absent or erroneous in ERPA, is easily accessed through a positive-energy transition J → I computation carried out for the states J < I. A similar approach has recently been applied to improve the description of the correlation energy for excited states within the adiabatic connection ERPA method.65 Notice that for the lowest excited states, which are usually of interest, the εdispI→J(AJB0) terms have a negative sign, but could, in principle, be positive for the highly excited state I.
The second-order induction energy for a dimer in the excited state, obtained with the ERPA approximation, eq 24, has to be corrected for the missing de-excitations in an analogous manner
| 40 |
The Eind+(2)(AIB0) term is obtained from eq 24, where the sum with respect to μ runs through positive transitions (ωμ > 0). The εindI→J (AJB0) terms are given as
 |
41 |
and follow from solving ERPA equations for the monomer A in states lower than I (from the ground state, J = 0, up to J = I – 1). Evidently, contributions to the induction energy from negative excitations always take a positive sign.
2.2.4. Second-Order Exchange Energy Contributions
We begin with the general expressions for the second-order induction and exchange–dispersion energies in the S2 approximation66,67
 |
42 |
where |Ψind(1)⟩ and |Ψdisp⟩ are the first-order induction and dispersion wave functions, respectively
 |
43 |
First calculations of second-order exchange contributions in the single-exchange approximation for many-electron systems were performed by Chałasiński and Jeziorski.67 The authors derived general expressions in the form of a many-orbital cluster expansion based on the induction and dispersion pair functions. Expressions in terms of a one-electron orbital basis set are given in ref (68) for the exchange–dispersion energy and in ref (69) for the exchange–induction contributions. During the development of the SAPT(CCSD) approach, Korona presented the density-matrix formulation of both second-order exchange components.70,71
For ground-state single-determinant wave function or Kohn–Sham determinant, is it possible to calculate second-order exchange terms through all orders in the intermolecular overlap, as proven by Schäffer and Jansen.72,73 Recently, Waldrop and Patkowski have derived expressions for the third-order exchange–induction.74
The exchange–induction energy written in terms of density matrices and transition energies reads (the S2 notation is dropped for convenience)
 |
44 |
where υ̃(r,r′) is the generalized interaction potential
| 45 |
and γintA,μ stands for the interaction density matrix57,70
 |
46 |
The 1- and 2-TRDMs in the position representation are defined as
| 47 |
and
| 48 |
The pertinent expressions for the Eexch–ind(2)(B ← A) component follow by interchanging A and B indices.
In ref (47), we have derived the density-matrix formula for the exchange–dispersion energy based on transition properties in the ERPA framework. The corresponding expression for the exchange–induction energy component takes the form
 |
49 |
The intermediates in eq 49 read
 |
50 |
 |
51 |
and
 |
52 |
 |
53 |
where the effective two-electron potential (eq 45) in the matrix representation is
 |
54 |
(a φp orbital may belong to either monomer Xp = A or B).
When 1- and 2-TRDMs in eqs 52 and 53 are expanded according to eqs 17 and 18, and eq 19, respectively, one arrives at the matrix representation of the VμA and Vν terms
 |
55 |
 |
56 |
where
| 57 |
| 58 |
| 59 |
| 60 |
| 61 |
| 62 |
with NA and NB intermediates given in eq 10 and the remaining intermediates defined as
| 63 |
 |
64 |
 |
65 |
Both induction and exchange–induction terms in SAPT are routinely calculated in the coupled approximation,75 so that the response of monomer orbitals due to the perturbation field of its interacting partner is accounted for. The uncoupled approach, which neglects the influence of the perturbing field, is used in calculations of the dispersion and exchange–dispersion energies in the wave function SAPT5 including the popular SAPT0 model.76,77 In both SAPT(DFT) and SAPT(CCSD), the coupled level of theory has been shown to give highly accurate second-order energy contributions.55,70,71,78−81
Evaluation of the exchange–induction energy requires construction of the T and W intermediates (eqs 64 and 65, respectively), which has the nOCC6 scaling (nOCC are the orbitals with nonzero occupancy). Since the 2-RDM matrix elements factorize unless all four indices correspond to fractionally occupied orbitals, the formal scaling with the sixth power is only with respect to the number of such orbitals. In comparison, the exchange–dispersion energy is more expensive, as it requires steps with an nOCCnSEC3 scaling (nSEC are active and unoccupied orbitals).47 It should also be noted that the bottleneck step in evaluation of the first-order exchange energy (eq 9) engages three four-index quantities (the NA, NB intermediates, and integrals), which amounts to scaling with the 6th power of the number of active orbitals. Note that for GVB the 2-RDMs factorize also in the active block,48 which results in identical scaling as in the SAPT(HF) method.
Recently, we have demonstrated that the uncoupled approximation in the ERPA framework combined with either CASSCF or GVB description of the monomers leads to a poor quality of the second-order dispersion energy.46,47 A more accurate dispersion energy is obtained if the monomer response properties are expanded up to the first order in the coupling parameter, which we refer to as the semicoupled approximation.46 The fully coupled ERPA scheme gives the best results for both dispersion and exchange–dispersion energies. In this work, all second-order energy components were obtained with the coupled approximation.
In Section 2.2.3, we demonstrated how to account for contributions from the negative transitions in second-order dispersion and induction energies calculated in the ERPA framework. Extension of this procedure to second-order exchange terms would involve first computing and storing both transition density matrices and (positive) transition energies to higher states (J) in the calculation for the monomer in the lower state (I) and then using them in a computation of the second-order exchange energies for a monomer in the higher state (J). The expected effect of accounting for negative transitions in the exchange–polarization energy is smaller compared to that of the polarization counterparts, and the afore-sketched procedure has not been implemented.
The multiconfigurational SAPT method, comprising first- and second-order energy components, is based on the chosen wave function theory applied to description of monomers. It is important to notice that the computation of all SAPT terms requires only knowledge of the corresponding one- and two-electron reduced density matrices of monomers. In the rest of this work, we use the notation SAPT(MC) for the proposed method, where MC indicates the underlying multiconfigurational wave function model employed to obtain reduced density matrices. The results will be presented for two multiconfigurational wave functions: CASSCF and GVB approximations. For comparison, we also include the SAPT results following from the single-determinantal description of monomers, denoted as SAPT(HF).
3. Computational Details
The ERPA equations applied to GVB or CAS wave functions require dividing the orbital space of each monomer into three disjoint subsets referred to as s1, s2, and s3. The cardinalities of the subsets are represented by the Ms1, Ms2, and Ms3 notations. For wave functions of the CAS type, the s1 set contains all inactive orbitals, whereas s2 and s3 correspond to the active and virtual orbitals, respectively. When ERPA is applied with the GVB reference, the s1 set is defined as all orbitals that occupation numbers fulfill the np > 0.992 condition. The s2 set includes all active orbitals, i.e., strongly occupied orbitals with occupation numbers 0.992 ≥ np ≥ 0.5 and their weakly occupied partners from the same geminal.46 The remaining orbitals are grouped in the s3 set. The p and q indices of the [Xν]pq and [Yν]pq vectors span the following range
 |
66 |
(analogous range is assumed
for the pq and rs indices of 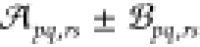 ).
).
In ERPA, the presence of degeneracies and near-degeneracies in the p ∈ s2 ∧ q ∈ s2 space (cf. eq 66) may lead to numerical instabilities. To circumvent this, we discarded pairs of orbitals [in practical terms, it means discarding corresponding rows and columns in the ERPA matrices (see eq 11)], applying the |np – nq|/np < 10–2 condition for the GVB wave function and |np – nq|/np < 10–6 for the CAS wave function.
The results obtained with CASSCF and GVB treatments of the monomers are denoted as SAPT(CAS) and SAPT(GVB), respectively. Pertinent calculations were performed in the locally developed code. The necessary integrals, 1- and 2-RDMs for CASSCF wave functions, were obtained from a developer version of the Molpro program.82 The GVB calculations were carried out in the locally modified Dalton program83 and interfaced with our code. The MP2 natural orbitals were used as the starting guess in both CASSCF and GVB calculations.
For the H2 ··· H2 dimer, discussed in Section 4.1, we carried out reference calculations exactly up to the second-order in SAPT, using an in-house code developed for interactions between two-electron monomers and based on the direct projection onto irreducible representations of the symmetric S4 group.84 The pertinent results are denoted as SAPT(FCI) in this work.
The augmented correlation-consistent orbital basis sets of double- and triple-zeta qualities (aug-cc-pVXZ, X = D,T)85,86 were employed throughout the work. Monomer calculations were carried out in the dimer-centered basis set.
In Section 4.3, we present results of SAPT(GVB) and SAPT(CAS) calculations for benchmark data set of noncovalently bound complexes introduced by Korona,87 which we refer to as the TK21 data set. The accuracy of individual SAPT energy components and interaction energies is verified against the SAPT(CCSD) benchmark. All CCSD calculations were performed with frozen core electrons. The SAPT(HF), SAPT(DFT), and SAPT2+(CCD) results are also reported. The exchange–correlation PBE088,89 functional employed in SAPT(DFT) was asymptotically corrected using the GRAC scheme90 applied with the experimental values of the ionization potentials. The SAPT(HF), SAPT(DFT), and SAPT(CCSD) calculations were performed in Molpro.82 The SAPT2+(CCD) results were obtained with the Psi491 program. In the latter variant of SAPT, the interaction energy is represented as
 |
67 |
where the (ij) superscript refers to the ith- and jth-order expansions in the intermolecular interaction operator and intramolecular correlation operator, respectively; the energy terms marked with the “resp” index account for the orbital relaxation effects. Except for the Edisp,CCD(2) term, the interaction energy components grouped in eq 67 are identical to the SAPT292 approach. The “+(CCD)” notation indicates that the dispersion energy is obtained in the coupled pair approximation including noniterative contributions from single and triple excitations, here referred to as the CCD+ST(CCD)93,94 approach.
The accuracy of SAPT interaction energies discussed in Section 4.3 is verified against counterpoise-corrected95 (CP) supermolecular CCSD(T) results. To this end, we approximate higher-order induction contributions at the Hartree–Fock level of theory96,97
| 68 |
where EintHF is the supermolecular Hartree–Fock interaction energy. The δHF component was added to the SAPT interaction energy provided that the ratio of the sum of the induction and exchange–induction energies to the total interaction energy was larger than 12.5%, in agreement with the criterion selected in ref (98). Note that in Section 4.3 error statistics for total interaction energies are reported for the S2 subset of the TK21 data set, which excludes six largest dimers (see refs (87) and (99)).
As an additional test, we performed SAPT calculations for the A24 data set100 of Řezáč and Hobza. Since we observed the same qualitative trends as in the TK21 case, results for the A24 data set are given in the Supporting Information.
4. Results
4.1. Multireference Ground-State System: H2 ··· H2
We begin the analysis of multiconfigurational SAPT with a model H2 ··· H2 dimer. We monitor the change of the interaction energy upon bond dissociation in one of the hydrogen molecules. A quantitative description of this system is challenging as it has to capture the balance between long-range dynamic correlation and increasing nondynamic correlation effects.29,101
We examine the T-shaped structure of the H2 ··· H2 complex in which one of the covalent H–H bonds is stretched from 1.37 a0 to 7.2 a0 (see Figure 1 for a detailed description). In SAPT(CAS) calculations, each monomer is described with a CAS(2,5) wave function. Note that for two-electron monomers SAPT(GVB) is equivalent to SAPT(CAS) based on CAS(2,2) wave functions.
Figure 1.

SAPT energy contributions at the Hartree–Fock (HF), GVB, CASSCF (CAS), CCSD, DFT, and FCI levels of theory for the H2 ··· H2 dimer in the T-shaped configuration. The intermolecular distance is fixed at 6.21 a0. In one of the monomers, the RH–H distance is varied from 1.37 to 7.20 a0, while in the other monomer, the H–H bond is kept at a fixed distance of 1.44 a0. The basis set is aug-cc-pVTZ.
SAPT schemes based on either Hartree–Fock or Kohn–Sham description of the monomers fail to predict the behavior of individual interaction energy components as the H–H bond is elongated and the complex gains a multireference character (Figure 1). Although one could resort to spin-unrestricted variants of SAPT,102,103 breaking of the spin symmetry leads to an erroneous shape of the interaction energy components (see Figure S1 in the Supporting Information). The SAPT(CCSD) approach initially remains in excellent agreement with the SAPT(FCI) benchmark. The largest relative percent errors in SAPT(CCSD) near the equilibrium geometry (RH–H = 1.41 a0) occur for the exchange–induction and exchange–dispersion energies, which are overestimated by ca. 4 and 7%, respectively. These discrepancies in the single-reference regime result from exclusion of certain cumulant contributions in the second-order exchange expressions.70,71 After the H–H bond length exceeds 3.0 a0, the XCCSD-3 approximation underlying SAPT(CCSD)104,105 starts to break down, which translates into qualitative errors in all interaction energy components.29
Both SAPT(GVB) and SAPT(CAS) predict the correct shape of the interaction energy curves (Figures 1 and 2). The GVB-based variant systematically underestimates the magnitude of all SAPT contributions and SAPT interaction energy. The exchange–induction energy deviates most from the benchmark with relative percent errors in the 10–20% range. Errors for the remaining components stay below 12% near the equilibrium, and the accuracy improves together with the increasing share of the nondynamic correlation in the system (see also Tables S1–S3 in the Supporting Information). SAPT(CAS) is more accurate; errors with respect to the SAPT(FCI) benchmark do not exceed 3% not only in individual components, but also in the total interaction energy. The error of the SAPT(FCI) interaction energy with respect to counterpoise-corrected95 supermolecular FCI (denoted as EintFCI in Figure 2) increases from 1.1% in the equilibrium geometry to 15% at RH–H = 7.2 a0. The remaining discrepancy between SAPT(FCI) and supermolecular FCI reflects the role of interaction energy terms higher than second-order in the perturbation operator.
Figure 2.
SAPT interaction energy for the H2 ··· H2 dimer in the T-shaped configuration (see Figure 1 for geometry description). EintFCI denotes the supermolecular FCI interaction energy. The basis set is aug-cc-pVTZ.
As discussed in ref (46), further extension of the active space in SAPT(CAS) is of little benefit for this system. Instead, to reach higher accuracy, one needs to move beyond the ERPA scheme and solve full linear response equations, i.e., include response not only from the orbitals but also from the wave function expansion coefficients.
4.2. Excited-State System: C2H4* ··· Ar
In this section, we present SAPT(CAS) calculations for the C2H4 ··· Ar dimer in which the ethylene molecule is either in the ground or in the electronically excited state. We focus on the singlet excitation of a valence character with the largest contribution from the π → π* transition.106 The C2H4 ··· Ar complex is kept in the C2v symmetry with the Ar atom located on the axis perpendicular to the C2H4 plane and bisecting its C–C bond (see also Table S4 in the Supporting Information for geometry of the C2H4 molecule). The interaction energy curves presented in Figure 3 and Table 1 are obtained by varying the distance R between the Ar atom and the center of the mass of ethylene. Counterpoise correction95 has been applied to all supermolecular interaction energies presented in this section.
Figure 3.

Interaction energy curves for the C2H4 ··· Ar dimer in the ground state (left) and in the π → π* state (right). Results marked with an asterisk do not include contributions from the negative energy transitions defined in eqs 37 and 41. The basis set is aug-cc-pVTZ.
Table 1. Components of Interaction Energy for the C2H4 ··· Ar Dimer in the Ground (g.s.) and Excited Statesa.
| g.s. | π → π* | π → π* | |
|---|---|---|---|
| component | R = 4.00 Å | R = 3.30 Å | R = 3.60 Å |
| Eelst(1) | –0.257 | –2.486 | –1.106 |
| Eexch(1) | 0.723 | 6.054 | 2.719 |
| Eind+(2) | –0.474 | –3.987 | –1.551 |
| ∑JεindI→J | 0.000 | 0.000 | 0.000 |
| Eexch–ind(2) | 0.457 | 4.434 | 1.813 |
| Edisp+(2) | –1.026 | –4.561 | –2.783 |
| ∑JεdispI→J | 0.000 | –1.087 | –0.744 |
| Eexch–disp(2) | 0.090 | 0.844 | 0.421 |
| EintSAPT* | –0.487 | 0.298 | –0.487 |
| EintSAPT | –0.487 | –0.789 | –1.231 |
To access the excited-state wave functions of both the dimer and the ethylene molecule, we carried out three-state state-averaged CAS calculations (SA-CAS) in the CAS(2,3) active space, i.e., two active electrons distributed on π, σ, and π* active orbitals. In these calculations, the targeted π → π* state is the third state in the SA ensemble. Note that in both SAPT(CAS) and supermolecular CASSCF calculations the Ar atom is represented with a single determinant.
For ground-state calculations, we used supermolecular CCSD(T) results as a benchmark. To obtain reference values for excited states, we adopted the procedure of ref (107), which combines the CCSD(T) description of the ground state with excitation energies calculated at the EOM-CCSD108 level of theory
 |
69 |
where the asterisk indicates a molecule in the excited state, ωEOM–CCSD denotes the pertinent EOM-CCSD excitation energy and EintCCSD(T) is a ground-state interaction energy.
In Figure 3, we compare SAPT(CAS) interaction energy curves with supermolecular CAS(2,3) results and a coupled-cluster benchmark. As it has been rigorously shown in ref (99), supermolecular CAS interaction energy misses dispersion contributions if active orbitals are assigned only to one monomer, which is the case here. The CAS+DISP curves in Figure 3 represent CAS interaction energy supplemented with the dispersion component taken from SAPT(CAS) calculations, EDISP(2) = Edisp + Eexch–disp(2). For the π → π* state, both SAPT(CAS) and CAS+DISP interaction energies were computed by explicitly accounting for the de-excitation-energy terms according to eqs 36 and 40. The SAPT(CAS)* and CAS+DISP* curves were obtained by neglecting the εdisp and εindI→J terms.
Inspection of Figure 3 and Table 1 shows that the C2H4 ··· Ar complex in the ground state is bound by the dispersion forces. The CAS interaction curve is mainly repulsive and features only a shallow minimum located at ca. 5.0 Å and 0.03 mEh deep. Addition of the dispersion energy in CAS+DISP builds up a van der Waals minimum 0.56 mEh deep localized at 4.0 Å, which is in reasonable agreement with the CCSD(T) reference (0.48 mEh at Req = 4.0 Å). The performance of SAPT(CAS) is excellent. The total SAPT(CAS) interaction energy at the optimal monomer separation is equal to −0.49 mEh, and the entire interaction curve almost coincides with the benchmark. The dispersion energy is clearly the dominating attractive contribution amounting to −1.03 mEh in the minimum (see Table 1).
Computation of the second-order SAPT components in the proposed SAPT(CAS) approach involves solving the ERPA equations. When the monomer reduced density matrices entering ERPA equations correspond to an unstable CAS solution, either near-instabilities or instabilities may occur in the linear response. In general, the SA-CAS calculation in a small active space bears the risk that wave functions describing higher excited states are not stable in ERPA. This is what we have encountered for the SA-CAS π → π* state of the studied C2H4 ··· Ar dimer (see Figures S2–S4). To avoid instabilities in the ERPA equations, which manifest in discontinuous interaction energy curves, we applied a three-point cubic extrapolation of second-order energy contributions based on the Dyall partitioning of the monomer Hamiltonian109,110 and expansion of the ERPA response properties in the coupling parameter.46,111 The details on the cubic extrapolation model are provided in the Supporting Information.
The interaction energy curves for the π → π* state are shown in Figure 3. At the CASSCF level of theory, the interaction has a purely repulsive character. The CAS+DISP model gives a binding curve, which remains in excellent agreement with the coupled-cluster reference. The employed CC method (eq 69) predicts a 1.61 mEh deep minimum at an intermonomer separation of 3.3 Å. The CAS+DISP minimum occurs at a slightly shorter distance of 3.2 Å and is 1.60 mEh deep. Note that the nearly perfect agreement with CC has to rest partially on error cancellation since CAS+DISP neglects contributions from negative excitations in the second-order exchange–dispersion energy (only εdispI→J terms are included in the model). The interaction energy curve from SAPT(CAS) calculations deviates from both CAS+DISP and CC results at the intermediate and short ranges. SAPT(CAS) localizes the minimum at 3.6 Å and underbinds by as much as 0.4 mEh compared to the CC reference (Table 1). The large discrepancy between second-order SAPT and the hybrid CAS+DISP approach reflects that both higher-order induction terms and exchange contributions beyond the S2 approximation, present in the supermolecular CAS and absent in SAPT, become important already for the low-lying π → π* valence state.
Contributions from the negative-energy transitions in the linear response are essential for a quantitative description of the C2H4* ··· Ar interaction. Neglecting the εI→J terms in SAPT reduces the well depth by a factor of 2 (cf. SAPT(CAS)* results in Figure 3). Similarly, comparing CAS+DISP with CAS+DISP* reveals that a good agreement of CAS+DISP with the coupled-cluster reference is possible only after inclusion of the de-excitation part of the spectrum. The observed energy lowering comes solely from the εdisp terms, as the induction counterparts vanish due to symmetry (Table 1). In the van der Waals minimum, the two dispersion terms (εdisp2→0 and εdisp (cf. eq 36)) sum up to −1.1 mEh, which is a sizable effect considering that positive-energy transitions amount to −4.6 mEh.
4.3. Single-Reference Systems
In this section, we analyze the performance of the multiconfigurational SAPT schemes for many-electron dimers of the TK21 data set of Korona87 against benchmark SAPT(CCSD) results. Additionally, we present SAPT(PBE0) and SAPT2+(CCD) results. Although TK21 includes systems governed by the dynamic rather than static correlation effects, our aim is to determine the level accuracy, which could be expected of the studied multiconfigurational SAPT if applied to multireference systems. Note that in all SAPT calculations the exchange terms were obtained in the S2 approximation. The first-order exchange and second-order exchange–induction contributions in SAPT(CCSD) include the cumulant contributions.70,112
Figure 4 shows relative percent errors of the individual SAPT energy components with respect to the SAPT(CCSD) reference (see also Tables S8–S11 in the Supporting Information). Let us begin with the first-order energy terms. Both SAPT(GVB) and SAPT(CAS) recover the electrostatic and exchange energies with similar accuracy—the mean absolute errors (Δ̅abs) for these contributions fall in the 6–8% range. In contrast to the electrostatic energy, the first-order exchange is systematically underestimated with mean errors of −6.4 and −5.4% obtained with GVB and CAS wave functions, respectively. SAPT(GVB) affords a smaller spread of errors compared to SAPT(CAS), in particular, for the Eelst(1) component. The multireference treatment of the monomers constitutes an improvement over the Hartree–Fock (single-determinantal) description (the Δ̅abs values from the Hartree–Fock-based SAPT calculations amount to ca. 13% for both components) but it remains inferior to both SAPT(DFT) and SAPT2+(CCD).
Figure 4.

Box plots of relative
percent errors (Δ) in the polarization
SAPT components (top: electrostatic, induction, and dispersion energies)
and in the exchange SAPT components (bottom: exchange, exchange–induction,
and exchange–dispersion, all in the S2 approximation) for dimers of the TK21 data set. HF, GVB,
and CAS denote wave function description of the monomers. PBE0 stands
for the asymptotically corrected PBE0 functional. Relative percent
errors are calculated according to the 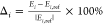 formula. Errors are given with respect
to the SAPT(CCSD) reference. Errors for the Eexch–disp(2) energy are reported for the S2 subset of TK21. The box
and outer fences encompass 50 and 95% of the distribution, respectively.
The outliers are marked as single points.
formula. Errors are given with respect
to the SAPT(CCSD) reference. Errors for the Eexch–disp(2) energy are reported for the S2 subset of TK21. The box
and outer fences encompass 50 and 95% of the distribution, respectively.
The outliers are marked as single points.
The second-order SAPT energy contributions obtained with the SAPT(CAS) variant are consistently more accurate than their SAPT(GVB) counterparts. In the TK21 data set, the largest difference occurs for the induction energy, where SAPT(GVB) deviates from the benchmark by 14.6 and 20.1% in terms of Δ̅abs and standard deviation, respectively, whereas the respective errors for SAPT(CAS) amount to 7.8 and 9.5%. This confirms that the CAS-based ERPA provides a better approximation for both transition density matrices and transition energies than when GVB density matrices are used.46,47 Similar as in the first order, the polarization terms (Eind(2) and Edisp) from multiconfigurational SAPT compare favorably with SAPT(HF), but do not match the quality of SAPT(DFT) or SAPT2+(CCD) results. The discrepancy is more pronounced for the dispersion energy where the mean absolute errors from CCD+ST(CCD) and SAPT(DFT) calculations are equal to 2.2 and 2.9%, respectively, compared to 6.4% obtained with SAPT(CAS) and 9.9% at the SAPT(GVB) level of theory.
The second-order exchange–induction and exchange–dispersion energies are more challenging than the polarization terms. SAPT(DFT) performs best, recovering the Eexch–ind(2) and Eexch–disp contributions with the Δ̅abs values of 7.5 and 4.3%, respectively. Both SAPT(GVB) and SAPT(CAS) tend to underestimate the second-order exchange (Δ̅abs values fall in the 10–14% range). Note that SAPT2+(CCD) provides more accurate exchange–induction energy than SAPT(HF) due to the inclusion of intramonomer correlation effects via the tEexch–ind(22) term. In contrast, the exchange–dispersion energy obtained at the uncoupled level in SAPT2+(CCD) is less accurate compared to its coupled counterpart included in the SAPT(HF) approach.
In Table 2, we examine the accuracy of total SAPT interaction energies with respect to the SAPT(CCSD) reference evaluated for the S2 subset of the TK21 data set. Error statistics is given in terms of the mean error Δ̅, the standard deviation σ, the mean absolute error Δ̅abs, and the maximum absolute error Δmax. Both multiconfigurational SAPT approaches reach similar accuracy. With Δ̅abs = 6.0% and σ = 7.4%, SAPT(GVB) remains in slightly better agreement with the benchmark than SAPT(CAS) (the respective values for the latter are Δ̅abs = 6.9% and σ = 8.9%). The error statistics for multiconfigurational SAPT matches SAPT(DFT) results, where Δ̅abs and σ amount to 5.6 and 7.5%, respectively. This indicates a systematic error cancellation between attractive and repulsive energy contributions in the ERPA-based SAPT since for the individual energy components, SAPT(DFT) is clearly closest to SAPT(CCSD).
Table 2. Summary of Error Statistics (in Percent) for the SAPT Interaction Energy for Dimers of the TK21/S2 Data Seta.
Errors of the SAPT interaction energy (EintSAPT) are given with respect to SAPT(CCSD) results. Errors of the SAPT interaction energies corrected for the δHF term (Eint) are given with respect to CP-corrected95 supermolecular CCSD(T) results calculated in the same basis set. The 2+(CCD) notation refers to the SAPT2+(CCD) scheme. All exchange energy components are computed in the S2 approximation. The basis set is aug-cc-pVTZ.
Errors with respect to SAPT(CCSD).
Errors with respect to supermolecular CCSD(T).
It is interesting to compare SAPT interaction energies against the supermolecular CCSD(T) reference. To this end, we approximate higher-order induction effects with the δHF term (eq 68). As expected, SAPT(CCSD) is the front runner (Δ̅abs = 3.2%) followed by SAPT(DFT) with a mean absolute error of 4.7% (lower section of Table 2). Both SAPT(GVB) and SAPT(CAS) are less accurate—the Δ̅abs value for the former reaches 5.9%, while for the latter, it amounts to 6.4%. Still, the multiconfigurational SAPT variants outperform not only the Hartree–Fock-based scheme (Δ̅abs = 11.3%) but also the SAPT2 model with the CCD+ST(CCD) dispersion (Δ̅abs = 8.7%). The relatively large errors of SAPT2+(CCD) can be traced to the poor representation of the second-order exchange components. Recall that the presented SAPT results neglect exchange effects beyond the S2 approximation, which is expected to worsen the agreement between SAPT and CCSD(T) interaction energies.
To summarize, the examined SAPT(GVB) and SAPT(CAS) methods benefit from a partial recovery of the intramonomer correlation effects by the underlying multiconfigurational wave function, as evidenced by a systematic improvement of all energy components with respect to the SAPT(HF) results. Nevertheless, the observed effect is small and relatively large errors compared to fully correlated SAPT schemes persist. This is best exemplified by first-order energies, which probe the quality of the monomer density (Eelst(1)) and density matrices (Eexch). In the second-order, the accuracy of SAPT(MC) is affected by both the missing intramonomer correlation and approximations in the ERPA response equations (see also discussion in refs (46) and (47)). For the TK21 data set, we observed that both SAPT(GVB) and SAPT(CAS) tend to underestimate second-order contributions, which leads to a fortuitous error cancellation in the total interaction energy. When both TK21 and A24 data sets are considered (Table S24), SAPT(MC) predicts interaction energies with mean absolute errors and standard deviation below 8 and 10%, respectively, which is significantly better than the Hartree–Fock-based SAPT (Δ̅abs ≤ 18% and σ ≤ 20%) and comparable to the SAPT2+(CCD) model (Δ̅abs ≤ 10% and σ ≤ 10%).
5. Conclusions
We have proposed a SAPT(MC) formalism applicable to dimers in which at least one of the monomers warrants a multireference description. In the approach, the interaction energy is expanded through the second-order terms in the intermolecular interaction operator. Formulas for the exchange energy contributions are given in the single-exchange approximation (the S2 approximation) and are valid for ground and nondegenerate excited states of the monomers in spin singlet states. While singlet states require spin-free reduced density matrices, extension to high-spin dimers is straightforward and involves spin-resolved components of RDMs. Response properties that enter the density-matrix-based SAPT formulas are obtained by solving the extended random phase approximation (ERPA) eigenproblems for each subsystem. Combined with ERPA equations, the presented variant of SAPT requires access only to one- and two-electron reduced density matrices of the monomers. Note that, contrary to the supermolecular method, in SAPT the dimer wave function is never computed which is advantageous for multiconfigurational systems. In this work, we applied SAPT(MC) with either CASSCF or GVB wave functions.
Based on the model H2 ··· H2 dimer in which one of the monomers undergoes dissociation, we have verified that SAPT(MC) is capable of describing interactions in systems dominated by nondynamic correlation. The interaction energy curve from SAPT(GVB) calculations has the correct shape, and the largest deviation from the FCI benchmark does not exceed 13%. In the H2 ··· H2 dimer, several active orbitals are sufficient to recover both intra- and intermonomer correlation effects. SAPT(CAS), with only five active orbitals per monomer, predicts the total interaction energy, as well as individual energy contributions, with errors below 3% with respect to the FCI results. In contrast, SAPT schemes based on the single-reference description of the monomers, SAPT(HF) and SAPT(DFT), fail dramatically when entering the strongly correlated regime.
The proposed multiconfigurational SAPT method is the only one among the existing SAPT approaches that offers the analysis of noncovalent interactions in systems involving electronically excited molecules in singlet states. In this work, we examined the role of negative transitions in the linear response function of an excited subsystem in the description of the second-order components of SAPT. In Section 2.2.3, a general protocol for direct evaluation of negative-transition terms has been proposed and its implementation in the ERPA approximation framework has been presented. As an example of an excited-state complex, we have selected the C2H4 ··· Ar dimer and described it with a small CAS wave function. While for the ground state of the system, SAPT(CAS) remains in excellent agreement with supermolecular CCSD(T) results, the excited π → π* state of ethylene poses a significant challenge. First, we have demonstrated that second-order energy contributions related to negative excitation energies are sizable and must be accounted for in SAPT. Second, even for the low-lying valence state of ethylene, the lack of higher-order induction terms and restriction to the S2 approximation significantly limit the accuracy of SAPT(CAS) results. To illustrate this, we have presented interaction energy curves obtained in a hybrid approach, which recovers induction terms up to infinite order in V̂. Indeed, a combination of supermolecular CASSCF and second-order dispersion energy from SAPT(CAS) calculations, which we refer to as the CAS+DISP approach,99 outperforms SAPT for the π → π* state and remains in excellent agreement with the coupled-cluster reference.
The CAS+DISP hybrid can be viewed as SAPT(CAS) supplemented with a CASSCF analogue of the δHF term, i.e., the δCAS correction. These two methods become equivalent if the δCAS term is computed from a formula similar to eq 68, with CAS supermolecular energy and SAPT(CAS) energy components. While there is no advantage of SAPT(CAS) + δCAS procedure over CAS+DISP when both employ the same CAS wave functions, using CAS functions of different levels could be beneficial. Such an approach would employ CAS in the minimal active space to evaluate the δCAS term and higher-level CAS for description of monomers in SAPT(CAS). Similar to δHF correction,113−115 addition of δCAS would be recommended not only for excited-state complexes but also for ground-state polar systems.
To better characterize the performance of SAPT(MC) for many-electron systems, we compared different SAPT schemes against a standard single-reference data set of noncovalently bound dimers. The individual energy components from both SAPT(GVB) and SAPT(CAS) calculations are more accurate than their SAPT(HF) counterparts. This holds also for total interaction energies, where we observe a partial error cancellation between polarization and exchange terms in the second-order. The correlated SAPT schemes included in the comparison, i.e., SAPT(DFT) and SAPT2+(CCD), are systematically better than our multiconfigurational SAPT, which should be attributed to two factors. One is that ERPA-based SAPT misses the majority of dynamical correlation within the monomers as a result of employing GVB or CAS wave functions with small active spaces. Second is the quality of response properties (excitation energies and transition density matrices) from ERPA equations. Unlike the full linear response (LR-MCSCF,116 equivalent to MC-RPA117), ERPA includes response of the orbitals only.
The proposed formulation of multireference SAPT can be applied with wave function methods capable of handling large active spaces, such as density-matrix renormalization group (DMRG),118,119 generalized active space (GAS),120,121 or v2RDM-driven CAS.122 An efficient alternative is offered by range-separated multiconfigurational DFT.123,124
Without additional approximations, SAPT(MC) scales with the sixth power of molecular size. The computational bottlenecks are the solution of the full ERPA eigenproblem and evaluation of the exchange–dispersion energy formula,47 both involving steps with an nOCC3nSEC cost, where nOCC are inactive and active and nSEC are active and unoccupied orbitals.
A feasible path to reduce the scaling and increase the efficiency of the method with no damage to the accuracy involves density fitting or Cholesky decomposition techniques routinely applied in single-reference SAPT approaches.76,80,92,125−128
Acknowledgments
The authors would like to thank Grzegorz Chałasiński for helpful discussions and comments on the manuscript. K.P. and M.H. were supported by the National Science Centre of Poland under Grant no. 2016/23/B/ST4/02848.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jctc.1c00344.
Notes on the extrapolation scheme for the second-order energy terms together with numerical illustration for C2H4* ··· Ar (Figures S2–S4); SAPT(MC) interaction energy components for the H2 ··· H2 and C2H4 ··· Ar dimers (Tables S1–S3 and S5, respectively); geometry of the C2H4 molecule (Table S4); interaction energies at SAPT(CAS), CASSCF, CAS+DISP, and CC levels of theory for the Ar ··· C2H4 complex (Table S6); specification of active spaces chosen for CASSCF calculations (Table S7); individual SAPT(MC), SAPT(HF), SAPT(PBE0), SAPT2+(CCD), and SAPT(CCSD) interaction energy components together with error statistics for systems of TK21 and A24 data sets in aug-cc-pVDZ and aug-cc-pVTZ basis sets (Tables S8–S24); and Box plots of relative percent errors in the calculated SAPT energies with respect to the SAPT(CCSD) benchmark (Figures S5–S11) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Balakrishnan N. Perspective: Ultracold molecules and the dawn of cold controlled chemistry. J. Chem. Phys. 2016, 145, 150901 10.1063/1.4964096. [DOI] [PubMed] [Google Scholar]
- Cold Chemistry; Dulieu O.; Osterwalder A., Eds.; Theoretical and Computational Chemistry Series; The Royal Society of Chemistry, 2018; pp P001–P670. [Google Scholar]
- Pawlak M.; Żuchowski P. S.; Jankowski P. Kinetic Isotope Effect in Low-Energy Collisions between Hydrogen Isotopologues and Metastable Helium Atoms: Theoretical Calculations Including the Vibrational Excitation of the Molecule. J. Chem. Theory Comput. 2021, 17, 1008–1016. 10.1021/acs.jctc.0c01122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalewicz K.; Jeziorski B. Symmetry-adapted double-perturbation analysis of intramolecular correlation effects in weak intermolecular interactions. Mol. Phys. 1979, 38, 191–208. 10.1080/00268977900101601. [DOI] [Google Scholar]
- Jeziorski B.; Moszynski R.; Szalewicz K. Perturbation theory approach to intermolecular potential energy surfaces of van der Waals complexes. Chem. Rev. 1994, 94, 1887–1930. 10.1021/cr00031a008. [DOI] [Google Scholar]
- Parrish R. M.; Thompson K. C.; Martínez T. J. Large-Scale Functional Group Symmetry-Adapted Perturbation Theory on Graphical Processing Units. J. Chem. Theory Comput. 2018, 14, 1737–1753. 10.1021/acs.jctc.7b01053. [DOI] [PubMed] [Google Scholar]
- Szalewicz K.; Patkowski K.; Jeziorski B.. Intermolecular Forces and Clusters II; Wales D. J., Ed.; Springer Berlin Heidelberg: Berlin, 2005; pp 43–117. [Google Scholar]
- Szalewicz K.; Bukowski R.; Jeziorski B.. Theory and Applications of Computational Chemistry; Dykstra C. E.; Frenking G.; Kim K. S.; Scuseria G. E., Eds.; Elsevier: Amsterdam, 2005; pp 919–962. [Google Scholar]
- Szalewicz K. Symmetry-adapted perturbation theory of intermolecular forces. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2012, 2, 254–272. 10.1002/wcms.86. [DOI] [Google Scholar]
- Jansen G. Symmetry-adapted perturbation theory based on density functional theory for noncovalent interactions. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2014, 4, 127–144. 10.1002/wcms.1164. [DOI] [Google Scholar]
- Patkowski K. Recent developments in symmetry-adapted perturbation theory. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2020, 10, e1452 10.1002/wcms.1452. [DOI] [Google Scholar]
- Řezáč J.; Hobza P. Benchmark Calculations of Interaction Energies in Noncovalent Complexes and Their Applications. Chem. Rev. 2016, 116, 5038–5071. 10.1021/acs.chemrev.5b00526. [DOI] [PubMed] [Google Scholar]
- Patkowski K.Chapter One - Benchmark Databases of Intermolecular Interaction Energies: Design, Construction, and Significance; Dixon D. A., Ed.; Annual Reports in Computational Chemistry; Elsevier, 2017; Vol. 13, pp 3–91. [Google Scholar]
- Werner H.; Knowles P. J. An efficient internally contracted multiconfiguration-reference configuration interaction method. J. Chem. Phys. 1988, 89, 5803–5814. 10.1063/1.455556. [DOI] [Google Scholar]
- Andersson K.; Malmqvist P. A.; Roos B. O.; Sadlej A. J.; Wolinski K. Second-order perturbation theory with a CASSCF reference function. J. Chem. Phys. 1990, 94, 5483–5488. 10.1021/j100377a012. [DOI] [Google Scholar]
- Angeli C.; Cimiraglia R.; Malrieu J.-P. n-electron valence state perturbation theory: A spinless formulation and an efficient implementation of the strongly contracted and of the partially contracted variants. J. Chem. Phys. 2002, 117, 9138–9153. 10.1063/1.1515317. [DOI] [Google Scholar]
- Langhoff S. R.; Davidson E. R. Configuration interaction calculations on the nitrogen molecule. Int. J. Quant. Chem. 1974, 8, 61–72. 10.1002/qua.560080106. [DOI] [Google Scholar]
- Pople J. A.; Seeger R.; Krishnan R. Variational configuration interaction methods and comparison with perturbation theory. Int. J. Quant. Chem. 1977, 12, 149–163. 10.1002/qua.560120820. [DOI] [Google Scholar]
- van Dam H. J. J.; van Lenthe J. H.; Ruttink P. J. A. Exact size consistency of multireference Møller-Plesset perturbation theory. Int. J. Quant. Chem. 1999, 72, 549–558. . [DOI] [Google Scholar]
- Rintelman J. M.; Adamovic I.; Varganov S.; Gordon M. S. Multireference second-order perturbation theory: How size consistent is “almost size consistent”. J. Chem. Phys. 2005, 122, 044105 10.1063/1.1817891. [DOI] [PubMed] [Google Scholar]
- Hirao K. Multireference Møller–Plesset method. Chem. Phys. Lett. 1992, 190, 374–380. 10.1016/0009-2614(92)85354-D. [DOI] [Google Scholar]
- Camacho C.; Cimiraglia R.; Witek H. A. Multireference perturbation theory can predict a false ground state. Phys. Chem. Chem. Phys. 2010, 12, 5058–5060. 10.1039/c000196a. [DOI] [PubMed] [Google Scholar]
- Chang S.-W.; Witek H. A. Choice of Optimal Shift Parameter for the Intruder State Removal Techniques in Multireference Perturbation Theory. J. Chem. Theory Comput. 2012, 8, 4053–4061. 10.1021/ct2006924. [DOI] [PubMed] [Google Scholar]
- Jeziorski B. Multireference coupled-cluster Ansatz. Mol. Phys. 2010, 108, 3043–3054. 10.1080/00268976.2010.524169. [DOI] [Google Scholar]
- Evangelista F. A. Perspective: Multireference coupled cluster theories of dynamical electron correlation. J. Chem. Phys. 2018, 149, 030901 10.1063/1.5039496. [DOI] [PubMed] [Google Scholar]
- Shen J.; Piecuch P. Biorthogonal moment expansions in coupled-cluster theory: Review of key concepts and merging the renormalized and active-space coupled-cluster methods. Chem. Phys. 2012, 401, 180–202. 10.1016/j.chemphys.2011.11.033. [DOI] [Google Scholar]; Recent advances in electron correlation methods and applications.
- Shen J.; Piecuch P. Combining active-space coupled-cluster methods with moment energy corrections via the CC(P;Q) methodology, with benchmark calculations for biradical transition states. J. Chem. Phys. 2012, 136, 144104 10.1063/1.3700802. [DOI] [PubMed] [Google Scholar]
- Chipman D. M. Stretching of hydrogen-bonded OH in the lowest singlet excited electronic state of water dimer. J. Chem. Phys. 2006, 124, 044305 10.1063/1.2162542. [DOI] [PubMed] [Google Scholar]
- Pastorczak E.; Shen J.; Hapka M.; Piecuch P.; Pernal K. Intricacies of van der Waals Interactions in Systems with Elongated Bonds Revealed by Electron-Groups Embedding and High-Level Coupled-Cluster Approaches. J. Chem. Theory Comput. 2017, 13, 5404–5419. 10.1021/acs.jctc.7b00797. [DOI] [PubMed] [Google Scholar]
- Pastorczak E.; Jensen H. J. A.; Kowalski P. H.; Pernal K. Generalized Valence Bond Perfect-Pairing Made Versatile Through Electron-Pairs Embedding. J. Chem. Theory Comput. 2019, 15, 4430–4439. 10.1021/acs.jctc.9b00384. [DOI] [PubMed] [Google Scholar]
- Pastorczak E.; Pernal K. Molecular interactions in electron-groups embedding generalized valence bond picture. Theor. Chem. Acc. 2018, 137, 1–10. 10.1007/s00214-018-2378-x. [DOI] [Google Scholar]
- Stoll H.; Savin A.. Density Functional Methods in Physics; Dreizler R.; da Providencia J., Eds.; Plenum: New York, 1985; pp 177–207. [Google Scholar]
- Savin A.Recent Developments of Modern Density Functional Theory; Seminario J. M., Ed.; Elsevier: Amsterdam, 1996; pp 327–357. [Google Scholar]
- Fromager E.; Cimiraglia R.; Jensen H. J. A. Merging multireference perturbation and density-functional theories by means of range separation: Potential curves for Be2, Mg2, and Ca2. Phys. Rev. A 2010, 81, 024502 10.1103/PhysRevA.81.024502. [DOI] [Google Scholar]
- Hapka M.; Pastorczak E.; Krzemińska A.; Pernal K. Long-range-corrected multiconfiguration density functional with the on-top pair density. J. Chem. Phys. 2020, 152, 094102 10.1063/1.5138980. [DOI] [PubMed] [Google Scholar]
- Stein C. J.; Reiher M. Semiclassical Dispersion Corrections Efficiently Improve Multiconfigurational Theory with Short-Range Density-Functional Dynamic Correlation. J. Phys. Chem. A 2020, 124, 2834–2841. 10.1021/acs.jpca.0c02130. [DOI] [PubMed] [Google Scholar]
- Raghavachari K.; Trucks G. W.; Pople J. A.; Head-Gordon M. A fifth-order perturbation comparison of electron correlation theories. Chem. Phys. Lett. 1989, 157, 479–483. 10.1016/S0009-2614(89)87395-6. [DOI] [Google Scholar]
- Bartlett R. J.; Watts J.; Kucharski S.; Noga J. Non-iterative fifth-order triple and quadruple excitation energy corrections in correlated methods. Chem. Phys. Lett. 1990, 165, 513–522. 10.1016/0009-2614(90)87031-L. [DOI] [Google Scholar]
- Korona T.; Jeziorski B.; Moszynski R.; Diercksen G. H. F. Degenerate symmetry-adapted perturbation theory of weak interactions between closed- and open-shell monomers: application to Rydberg states of helium hydride. Theor. Chem. Acc. 1999, 101, 282–291. 10.1007/s002140050442. [DOI] [Google Scholar]
- Patkowski K.; Korona T.; Jeziorski B. Convergence behavior of the symmetry-adapted perturbation theory for states submerged in Pauli forbidden continuum. J. Chem. Phys. 2001, 115, 1137–1152. 10.1063/1.1379330. [DOI] [Google Scholar]
- Patkowski K.; Jeziorski B.; Szalewicz K. Unified treatment of chemical and van der Waals forces via symmetry-adapted perturbation expansion. J. Chem. Phys. 2004, 120, 6849–6862. 10.1063/1.1676119. [DOI] [PubMed] [Google Scholar]
- Przybytek M.; Patkowski K.; Jeziorski B. Convergence behavior of symmetry-adapted perturbation expansions for excited states. A model study of interactions involving a triplet helium atom. Collect. Czech. Chem. Commun. 2004, 69, 141–176. 10.1135/cccc20040141. [DOI] [Google Scholar]
- Reinhardt P. A possible valence-bond approach to symmetry-adapted perturbation theory. Comput. Theor. Chem. 2017, 1116, 174–183. 10.1016/j.comptc.2017.03.008. [DOI] [Google Scholar]
- Patkowski K.; Żuchowski P. S.; Smith D. G. A. First-order symmetry-adapted perturbation theory for multiplet splittings. J. Chem. Phys. 2018, 148, 164110 10.1063/1.5021891. [DOI] [PubMed] [Google Scholar]
- Waldrop J. M.; Patkowski K. Spin splittings from first-order symmetry-adapted perturbation theory without single-exchange approximation. J. Chem. Phys. 2019, 150, 074109 10.1063/1.5086079. [DOI] [PubMed] [Google Scholar]
- Hapka M.; Przybytek M.; Pernal K. Second-Order Dispersion Energy Based on Multireference Description of Monomers. J. Chem. Theory Comput. 2019, 15, 1016–1027. 10.1021/acs.jctc.8b01058. [DOI] [PubMed] [Google Scholar]
- Hapka M.; Przybytek M.; Pernal K. Second-Order Exchange-Dispersion Energy Based on a Multireference Description of Monomers. J. Chem. Theory Comput. 2019, 15, 6712–6723. 10.1021/acs.jctc.9b00925. [DOI] [PubMed] [Google Scholar]
- Chatterjee K.; Pernal K. Excitation energies from extended random phase approximation employed with approximate one- and two-electron reduced density matrices. J. Chem. Phys. 2012, 137, 204109 10.1063/1.4766934. [DOI] [PubMed] [Google Scholar]
- Pernal K.; Chatterjee K.; Kowalski P. H. How accurate is the strongly orthogonal geminal theory in predicting excitation energies? Comparison of the extended random phase approximation and the linear response theory approaches. J. Chem. Phys. 2014, 140, 014101 10.1063/1.4855275. [DOI] [PubMed] [Google Scholar]
- Murrell J. N.; Randić M.; Williams D. The theory of intermolecular forces in the region of small orbital overlap. Proc. Roy. Soc. A. 1965, 284, 566–581. 10.1098/rspa.1965.0081. [DOI] [Google Scholar]
- Żuchowski P. S.; Podeszwa R.; Moszynski R.; Jeziorski B.; Szalewicz K. Symmetry-adapted perturbation theory utilizing density functional description of monomers for high-spin open-shell complexes. J. Chem. Phys. 2008, 129, 084101 10.1063/1.2968556. [DOI] [PubMed] [Google Scholar]
- Jeziorski B.; Szalewicz K.; Chalasinski G. Symmetry forcing and convergence properties of perturbation expansions for molecular interaction energies. Int. J. Quant. Chem. 1978, 14, 271–287. 10.1002/qua.560140306. [DOI] [Google Scholar]
- Jeziorski B.; Bulski M.; Piela L. First-Order perturbation treatment of the short-range repulsion in a system of many closed-shell atoms or molecules. Int. J. Quant. Chem. 1976, 10, 281–297. 10.1002/qua.560100208. [DOI] [Google Scholar]
- Heßelmann A.; Jansen G. First-order intermolecular interaction energies from Kohn-Sham orbitals. Chem. Phys. Lett. 2002, 357, 464–470. 10.1016/S0009-2614(02)00538-9. [DOI] [Google Scholar]
- Misquitta A. J.; Szalewicz K. Intermolecular forces from asymptotically corrected density functional description of monomers. Chem. Phys. Lett. 2002, 357, 301–306. 10.1016/S0009-2614(02)00533-X. [DOI] [Google Scholar]
- Bulski M.; Chałasiński G.; Jeziorski B. Many-orbital cluster expansion for the exchange-repulsion energy in the interaction of closed-shell systems. Theor. Chim. Acta 1979, 52, 93–101. 10.1007/BF00634785. [DOI] [Google Scholar]
- Moszynski R.; Jeziorski B.; Rybak S.; Szalewicz K.; Williams H. L. Many-body theory of exchange effects in intermolecular interactions. Density matrix approach and applications to He-F–, He-HF, H2-HF, and Ar-H2 dimers. J. Chem. Phys. 1994, 100, 5080–5092. 10.1063/1.467225. [DOI] [Google Scholar]
- Rowe D. J. Equations-of-Motion Method and the Extended Shell Model. Rev. Mod. Phys. 1968, 40, 153–166. 10.1103/RevModPhys.40.153. [DOI] [Google Scholar]
- Pastorczak E.; Pernal K. ERPA-APSG: a computationally efficient geminal-based method for accurate description of chemical systems. Phys. Chem. Chem. Phys. 2015, 17, 8622–8626. 10.1039/C4CP05958A. [DOI] [PubMed] [Google Scholar]
- Pastorczak E.; Pernal K. Correlation Energy from the Adiabatic Connection Formalism for Complete Active Space Wave Functions. J. Chem. Theory Comput. 2018, 14, 3493–3503. 10.1021/acs.jctc.8b00213. [DOI] [PubMed] [Google Scholar]
- Golab J. T.; Yeager D. L.; Jørgensen P. Proper characterization of MC SCF stationary points. Chem. Phys. 1983, 78, 175–199. 10.1016/0301-0104(83)85106-4. [DOI] [Google Scholar]
- Pernal K. Intergeminal Correction to the Antisymmetrized Product of Strongly Orthogonal Geminals Derived from the Extended Random Phase Approximation. J. Chem. Theory Comput. 2014, 10, 4332–4341. 10.1021/ct500478t. [DOI] [PubMed] [Google Scholar]
- McWeeny R. The density matrix in many-electron quantum mechanics I. Generalized product functions. Factorization and physical interpretation of the density matrices. Proc. R. Soc. A 1959, 253, 242–259. [Google Scholar]
- Jaszunski M.; McWeeny R. Time-dependent Hartree-Fock calculations of dispersion energy. Mol. Phys. 1985, 55, 1275–1286. 10.1080/00268978500102021. [DOI] [Google Scholar]
- Drwal D.; Pastorczak E.; Pernal K. Excited states in the adiabatic connection fluctuation-dissipation theory: Recovering missing correlation energy from the negative part of the density response spectrum. J. Phys. Chem. A 2021, 154, 164102 10.1063/5.0046852. [DOI] [PubMed] [Google Scholar]
- Chałasiński G.; Jeziorski B.; Andzelm J.; Szalewicz K. On the multipole structure of exchange dispersion energy in the interaction of two helium atoms. Mol. Phys. 1977, 33, 971–977. 10.1080/00268977700100881. [DOI] [Google Scholar]
- Chałasiński G.; Jeziorski B. Exchange polarization effects in the interaction of closed-shell systems. Theor. Chem. Acc. 1977, 46, 277–290. 10.1007/BF00554513. [DOI] [Google Scholar]
- Rybak S.; Jeziorski B.; Szalewicz K. Many-body symmetry-adapted perturbation theory of intermolecular interactions. H2O and HF dimers. J. Chem. Phys. 1991, 95, 6576–6601. 10.1063/1.461528. [DOI] [Google Scholar]
- Jeziorski B.; Moszynski R.; Ratkiewicz A.; Rybak S.; Szalewicz K.; Williams H. L. In SAPT: A Program for Many-body Symmetry-adapted Perturbation Theory Calculations of Intermolecular Interaction Energies, Methods and Techniques in Computational Chemistry: METECC, 1993; p 79.
- Korona T. Second-order exchange-induction energy of intermolecular interactions from coupled cluster density matrices and their cumulants. Phys. Chem. Chem. Phys. 2008, 10, 6509–6519. 10.1039/b807329e. [DOI] [PubMed] [Google Scholar]
- Korona T. Exchange-Dispersion Energy: A Formulation in Terms of Monomer Properties and Coupled Cluster Treatment of Intramonomer Correlation. J. Chem. Theory Comput. 2009, 5, 2663–2678. 10.1021/ct900232j. [DOI] [PubMed] [Google Scholar]
- Schäffer R.; Jansen G. Intermolecular exchange-induction energies without overlap expansion. Theor. Chem. Acc. 2012, 131, 1235 10.1007/s00214-012-1235-6. [DOI] [Google Scholar]
- Schäffer R.; Jansen G. Single-determinant-based symmetry-adapted perturbation theory without single-exchange approximation. Mol. Phys. 2013, 111, 2570–2584. 10.1080/00268976.2013.827253. [DOI] [Google Scholar]
- Waldrop J. M.; Patkowski K. Nonapproximated third-order exchange induction energy in symmetry-adapted perturbation theory. J. Chem. Phys. 2021, 154, 024103 10.1063/5.0035050. [DOI] [PubMed] [Google Scholar]
- Jaszuński M. Coupled Hartree-Fock calculation of the induction energy. Mol. Phys. 1980, 39, 777–780. 10.1080/00268978000100661. [DOI] [Google Scholar]
- Hohenstein E. G.; Sherrill C. D. Density fitting and Cholesky decomposition approximations in symmetry-adapted perturbation theory: Implementation and application to probe the nature of π–π interactions in linear acenes. J. Chem. Phys. 2010, 132, 184111 10.1063/1.3426316. [DOI] [Google Scholar]
- Parker T. M.; Burns L. A.; Parrish R. M.; Ryno A. G.; Sherrill C. D. Levels of symmetry adapted perturbation theory (SAPT). I. Efficiency and performance for interaction energies. J. Chem. Phys. 2014, 140, 094106 10.1063/1.4867135. [DOI] [PubMed] [Google Scholar]
- Heßelmann A.; Jansen G. Intermolecular induction and exchange-induction energies from coupled-perturbed Kohn-Sham density functional theory. Chem. Phys. Lett. 2002, 362, 319–325. 10.1016/S0009-2614(02)01097-7. [DOI] [Google Scholar]
- Heßelmann A.; Jansen G. Intermolecular dispersion energies from time-dependent density functional theory. Chem. Phys. Lett. 2003, 367, 778–784. 10.1016/S0009-2614(02)01796-7. [DOI] [Google Scholar]
- Misquitta A. J.; Jeziorski B.; Szalewicz K. Dispersion Energy from Density-Functional Theory Description of Monomers. Phys. Rev. Lett. 2003, 91, 033201 10.1103/PhysRevLett.91.033201. [DOI] [PubMed] [Google Scholar]
- Korona T.; Jeziorski B. Dispersion energy from density-fitted density susceptibilities of singles and doubles coupled cluster theory. J. Chem. Phys. 2008, 128, 144107 10.1063/1.2889006. [DOI] [PubMed] [Google Scholar]
- Werner H.-J.; Knowles P. J.; Knizia G.; Manby F. R.; Schütz M. Molpro: a general-purpose quantum chemistry program package. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2012, 2, 242–253. 10.1002/wcms.82. [DOI] [Google Scholar]
- Aidas K.; Angeli C.; Bak K. L.; Bakken V.; Bast R.; Boman L.; Christiansen O.; Cimiraglia R.; Coriani S.; Dahle P.; Dalskov E. K.; Ekström U.; Enevoldsen T.; Eriksen J. J.; Ettenhuber P.; Fernández B.; Ferrighi L.; Fliegl H.; Frediani L.; Hald K.; Halkier A.; Hättig C.; Heiberg H.; Helgaker T.; Hennum A. C.; Hettema H.; Hjertenæs E.; Høst S.; Høyvik I.-M.; Iozzi M. F.; Jansík B.; Jensen H. J. A.; Jonsson D.; Jørgensen P.; Kauczor J.; Kirpekar S.; Kjærgaard T.; Klopper W.; Knecht S.; Kobayashi R.; Koch H.; Kongsted J.; Krapp A.; Kristensen K.; Ligabue A.; Lutnæs O. B.; Melo J. I.; Mikkelsen K. V.; Myhre R. H.; Neiss C.; Nielsen C. B.; Norman P.; Olsen J.; Olsen J. M. H.; Osted A.; Packer M. J.; Pawlowski F.; Pedersen T. B.; Provasi P. F.; Reine S.; Rinkevicius Z.; Ruden T. A.; Ruud K.; Rybkin V. V.; Sałek P.; Samson C. C. M.; de Merás A. S.; Saue T.; Sauer S. P. A.; Schimmelpfennig B.; Sneskov K.; Steindal A. H.; Sylvester-Hvid K. O.; Taylor P. R.; Teale A. M.; Tellgren E. I.; Tew D. P.; Thorvaldsen A. J.; Thøgersen L.; Vahtras O.; Watson M. A.; Wilson D. J. D.; Ziolkowski M.; Ågren H. The Dalton quantum chemistry program system. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2013, 4, 269–284. 10.1002/wcms.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korona T.; Moszynski R.; Jeziorski B.. Convergence of Symmetry-Adapted Perturbation Theory for the Interaction between Helium Atoms and between a Hydrogen Molecule and a Helium Atom. In Advancesin Quantum Chemistry; Löwdin P.-O.; Sabin J. R.; Zerner M. C.; Karwowski J.; Karelson M., Eds.; Academic Press, 1997; Vol. 28, pp 171–188. [Google Scholar]
- Dunning T. H. Jr Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. 10.1063/1.456153. [DOI] [Google Scholar]
- Kendall R. A.; Dunning T. H. Jr; Harrison R. J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. 10.1063/1.462569. [DOI] [Google Scholar]
- Korona T. A coupled cluster treatment of intramonomer electron correlation within symmetry-adapted perturbation theory: benchmark calculations and a comparison with a density-functional theory description. Mol. Phys. 2013, 111, 3705–3715. 10.1080/00268976.2012.746478. [DOI] [Google Scholar]
- Perdew J. P.; Burke K.; Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- Adamo C.; Barone V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. 10.1063/1.478522. [DOI] [Google Scholar]
- Grüning M.; Gritsenko O. V.; van Gisbergen S. J. A.; Baerends E. J. Shape corrections to exchange-correlation potentials by gradient-regulated seamless connection of model potentials for inner and outer region. J. Chem. Phys. 2001, 114, 652–660. 10.1063/1.1327260. [DOI] [Google Scholar]
- Parrish R. M.; Burns L. A.; Smith D. G. A.; Simmonett A. C.; DePrince A. E.; Hohenstein E. G.; Bozkaya U.; Sokolov A. Y.; Di Remigio R.; Richard R. M.; Gonthier J. F.; James A. M.; McAlexander H. R.; Kumar A.; Saitow M.; Wang X.; Pritchard B. P.; Verma P.; Schaefer H. F.; Patkowski K.; King R. A.; Valeev E. F.; Evangelista F. A.; Turney J. M.; Crawford T. D.; Sherrill C. D. Psi4 1.1: An Open-Source Electronic Structure Program Emphasizing Automation, Advanced Libraries, and Interoperability. J. Chem. Theory Comput. 2017, 13, 3185–3197. 10.1021/acs.jctc.7b00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenstein E. G.; Sherrill C. D. Density fitting of intramonomer correlation effects in symmetry-adapted perturbation theory. J. Chem. Phys. 2010, 133, 014101 10.1063/1.3451077. [DOI] [PubMed] [Google Scholar]
- Williams H. L.; Szalewicz K.; Moszynski R.; Jeziorski B. Dispersion energy in the coupled pair approximation with noniterative inclusion of single and triple excitations. J. Chem. Phys. 1995, 103, 4586–4599. 10.1063/1.470646. [DOI] [Google Scholar]
- Parrish R. M.; Hohenstein E. G.; Sherrill C. D. Tractability gains in symmetry-adapted perturbation theory including coupled double excitations: CCD+ST(CCD) dispersion with natural orbital truncations. J. Chem. Phys. 2013, 139, 174102 10.1063/1.4826520. [DOI] [PubMed] [Google Scholar]
- Boys S.; Bernardi F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. 10.1080/00268977000101561. [DOI] [Google Scholar]
- Jeziorska M.; Jeziorski B.; Čížek J. Direct calculation of the Hartree-Fock interaction energy via exchange-perturbation expansion. The He-He interaction. Int. J. Quant. Chem. 1987, 32, 149–164. 10.1002/qua.560320202. [DOI] [Google Scholar]
- Moszynski R.; Heijmen T.; Jeziorski B. Symmetry-adapted perturbation theory for the calculation of Hartree-Fock interaction energies. Mol. Phys. 1996, 88, 741–758. 10.1080/00268979650026262. [DOI] [Google Scholar]
- Taylor D. E.; Ángyán J. G.; Galli G.; Zhang C.; Gygi F.; Hirao K.; Song J. W.; Rahul K.; Anatole von Lilienfeld O.; Podeszwa R.; Bulik I. W.; Henderson T. M.; Scuseria G. E.; Toulouse J.; Peverati R.; Truhlar D. G.; Szalewicz K. Blind test of density-functional-based methods on intermolecular interaction energies. J. Chem. Phys. 2016, 145, 124105 10.1063/1.4961095. [DOI] [PubMed] [Google Scholar]
- Hapka M.; Krzemińska A.; Pernal K. How Much Dispersion Energy Is Included in the Multiconfigurational Interaction Energy?. J. Chem. Theory Comput. 2020, 16, 6280–6293. 10.1021/acs.jctc.0c00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Řezáč J.; Hobza P. Describing Noncovalent Interactions beyond the Common Approximations: How Accurate Is the “Gold Standard,” CCSD(T) at the Complete Basis Set Limit?. J. Chem. Theory Comput. 2013, 9, 2151–2155. 10.1021/ct400057w. [DOI] [PubMed] [Google Scholar]
- Brzȩk F.; Boguslawski K.; Tecmer P.; Żuchowski P. S. Benchmarking the accuracy of seniority-zero wavefunction methods for non-covalent interactions. J. Chem. Theory Comput. 2019, 15, 4021–4035. 10.1021/acs.jctc.9b00189. [DOI] [PubMed] [Google Scholar]
- Hapka M.; Żuchowski P. S.; Szczȩśniak M. M.; Chałasiński G. Symmetry-adapted perturbation theory based on unrestricted Kohn-Sham orbitals for high-spin open-shell van der Waals complexes. J. Chem. Phys. 2012, 137, 164104 10.1063/1.4758455. [DOI] [PubMed] [Google Scholar]
- Gonthier J. F.; Sherrill C. D. Density-fitted open-shell symmetry-adapted perturbation theory and application to π-stacking in benzene dimer cation and ionized DNA base pair steps. J. Chem. Phys. 2016, 145, 134106 10.1063/1.4963385. [DOI] [PubMed] [Google Scholar]
- Korona T.; Jeziorski B. One-electron properties and electrostatic interaction energies from the expectation value expression and wave function of singles and doubles coupled cluster theory. J. Chem. Phys. 2006, 125, 184109 10.1063/1.2364489. [DOI] [PubMed] [Google Scholar]
- Korona T.; Przybytek M.; Jeziorski B. Time-independent coupled cluster theory of the polarization propagator. Implementation and application of the singles and doubles model to dynamic polarizabilities and van der Waals constants. Mol. Phys. 2006, 104, 2303–2316. 10.1080/00268970600673975. [DOI] [Google Scholar]
- Briggs E. A.; Besley N. A. Modelling excited states of weakly bound complexes with density functional theory. Phys. Chem. Chem. Phys. 2014, 16, 14455–14462. 10.1039/C3CP55361B. [DOI] [PubMed] [Google Scholar]
- Ikabata Y.; Nakai H. Extension of local response dispersion method to excited-state calculation based on time-dependent density functional theory. J. Chem. Phys. 2012, 137, 124106 10.1063/1.4754508. [DOI] [PubMed] [Google Scholar]
- Monkhorst H. J. Calculation of properties with the coupled-cluster method. Int. J. Quant. Chem. 1977, 12, 421–432. 10.1002/qua.560120850. [DOI] [Google Scholar]
- Dyall K. G. The choice of a zeroth-order Hamiltonian for second-order perturbation theory with a complete active space self-consistent-field reference function. J. Chem. Phys. 1995, 102, 4909 10.1063/1.469539. [DOI] [Google Scholar]
- Rosta E.; Surján P. R. Two-body zeroth order Hamiltonians in multireference perturbation theory: The APSG reference state. J. Chem. Phys. 2002, 116, 878–890. 10.1063/1.1427918. [DOI] [Google Scholar]
- Pernal K. Electron Correlation from the Adiabatic Connection for Multireference Wave Functions. Phys. Rev. Lett. 2018, 120, 013001 10.1103/PhysRevLett.120.013001. [DOI] [PubMed] [Google Scholar]
- Korona T. First-order exchange energy of intermolecular interactions from coupled cluster density matrices and their cumulants. J. Chem. Phys. 2008, 128, 224104 10.1063/1.2889006. [DOI] [PubMed] [Google Scholar]
- Patkowski K.; Szalewicz K.; Jeziorski B. Third-order interactions in symmetry-adapted perturbation theory. J. Chem. Phys. 2006, 125, 154107 10.1063/1.2358353. [DOI] [PubMed] [Google Scholar]
- Patkowski K.; Szalewicz K.; Jeziorski B. Orbital relaxation and the third-order induction energy in symmetry-adapted perturbation theory. Theor. Chem. Acc. 2010, 127, 211–221. [Google Scholar]
- Lao K. U.; Schäffer R.; Jansen G.; Herbert J. M. Accurate Description of Intermolecular Interactions Involving Ions Using Symmetry-Adapted Perturbation Theory. J. Chem. Theory Comput. 2015, 11, 2473–2486. 10.1021/ct5010593. [DOI] [PubMed] [Google Scholar]
- Olsen J.; Jørgensen P. Linear and nonlinear response functions for an exact state and for an MCSCF state. J. Chem. Phys. 1985, 82, 3235–3264. 10.1063/1.448223. [DOI] [Google Scholar]
- Oddershede J.; Jørgensen P.; Yeager D. L. Polarization propagator methods in atomic and molecular calculations. Comput. Phys. Rep. 1984, 2, 33–92. 10.1016/0167-7977(84)90003-0. [DOI] [Google Scholar]
- Legeza Ö.; Noack R.; Sólyom J.; Tincani L.. Computational Many-Particle Physics; Fehske H.; Schneider R.; Weiße A., Eds.; Springer Berlin Heidelberg: Berlin, 2008; pp 653–664. [Google Scholar]
- Chan G. K.-L.; Sharma S. The Density Matrix Renormalization Group in Quantum Chemistry. Ann. Rev. Phys. Chem. 2011, 62, 465–481. 10.1146/annurev-physchem-032210-103338. [DOI] [PubMed] [Google Scholar]
- Olsen J.; Roos B. O.; Jørgensen P.; Jensen H. J. A. Determinant based configuration interaction algorithms for complete and restricted configuration interaction spaces. J. Chem. Phys. 1988, 89, 2185–2192. 10.1063/1.455063. [DOI] [Google Scholar]
- Ma D.; Li Manni G.; Gagliardi L. The generalized active space concept in multiconfigurational self-consistent field methods. J. Chem. Phys. 2011, 135, 044128 10.1063/1.3611401. [DOI] [PubMed] [Google Scholar]
- Fosso-Tande J.; Nascimento D. R.; DePrince A. E. III Accuracy of two-particle N-representability conditions for describing different spin states and the singlet-triplet gap in the linear acene series. Mol. Phys. 2016, 114, 423–430. 10.1080/00268976.2015.1078008. [DOI] [Google Scholar]
- Savin A.; Flad H.-J. Density functionals for the Yukawa electron-electron interaction. Int. J. Quant. Chem. 1995, 56, 327–332. 10.1002/qua.560560417. [DOI] [Google Scholar]
- Fromager E.; Knecht S.; Jensen H. J. A. Multi-configuration time-dependent density-functional theory based on range separation. J. Chem. Phys. 2013, 138, 084101 10.1063/1.4792199. [DOI] [PubMed] [Google Scholar]
- Hesselmann A.; Jansen G.; Schütz M. Density-functional theory-symmetry-adapted intermolecular perturbation theory with density fitting: A new efficient method to study intermolecular interaction energies. J. Chem. Phys. 2005, 122, 014103 10.1063/1.1824898. [DOI] [PubMed] [Google Scholar]
- Bukowski R.; Podeszwa R.; Szalewicz K. Efficient calculation of coupled Kohn-Sham dynamic susceptibility functions and dispersion energies with density fitting. Chem. Phys. Lett. 2005, 414, 111–116. 10.1016/j.cplett.2005.08.048. [DOI] [Google Scholar]
- Podeszwa R.; Bukowski R.; Szalewicz K. Density-Fitting Method in Symmetry-Adapted Perturbation Theory Based on Kohn-Sham Description of Monomers. J. Chem. Theory Comput. 2006, 2, 400–412. 10.1021/ct050304h. [DOI] [PubMed] [Google Scholar]
- Garcia J.; Podeszwa R.; Szalewicz K. SAPT codes for calculations of intermolecular interaction energies. J. Chem. Phys. 2020, 152, 184109 10.1063/5.0005093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





