Abstract
The determination of anti-SARS-CoV-2 neutralizing antibodies (NAbs) is of interest in many respects. High NAb titers, for example, are the most important criterion regarding the effectiveness of convalescent plasma therapy. However, common cell culture-based NAb assays are time-consuming and feasible only in special laboratories. Our data reveal the suitability of a novel ELISA-based surrogate virus neutralization test (sVNT) to easily measure the inhibition-capability of NAbs in the plasma of COVID-19 convalescents. We propose a combined strategy to detect plasma samples with high NAb titers (≥ 1:160) reliably and to, simultaneously, reduce the risk of erroneously identifying low-titer specimens. For this approach, results of the sVNT assay are compared to and combined with those acquired from the Euroimmun anti-SARS-CoV-2 IgG assay. Both assays are appropriate for high-throughput screening in standard BSL-2 laboratories. Our measurements further show a long-lasting humoral immunity of at least 11 months after symptom onset.
Keywords: SARS-CoV-2, COVID-19, Humoral immunity, Detection of anti-SARS-CoV-2 neutralizing antibodies
Abbreviations
- SARS-CoV-2:
Severe acute respiratory syndrome coronavirus type 2
- COVID-19:
Coronavirus Disease 2019
- NAbs:
Neutralizing antibodies
- BAbs:
Binding antibodies
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified at the end of December 2019 in Wuhan, Hubei Province, China [1]. Sequencing analysis from the lower respiratory tract revealed the new coronavirus early as a causative agent of the Coronavirus disease 2019 (COVID-19) [2]. The infectious disease became a worldwide pandemic and has claimed millions of lives so far. While most infections are mild or even asymptomatic, the estimated infection fatality rate across populations is 0.68% (0.53 – 0.82%) [3]. While vaccines are promising concerning the formation of an active immunization against the virus, passive immunization can be achieved by an early treatment of SARS-CoV-2-infected individuals with the plasma of COVID-19 convalescent donors [4]. The most important criterion regarding the effectiveness of the convalescent plasma (CP) therapy is a high concentration of anti-SARS-CoV-2-neutralizing antibodies (NAbs) [5]. However, the determination of NAbs is time-consuming and can, due to the use of live authentic SARS-CoV-2 viruses, only be performed in high safety Biosafety Level 3 (BSL3) cell culture laboratories [6]. In order to select the appropriate CP, therefore, the concentration of total anti-SARS-CoV-2-binding antibodies (BAbs) is often considered, for which different serological assays are commercially available. A previous study revealed a moderate correlation between anti-spike IgG levels and NAb titers determined in a cell culture-based assay [7]. However, no statement about the antibody functionality can be made by the determination of general BAbs. Therefore, the usage of functional NAb assays is indispensable to assess the protective humoral immunity against SARS-CoV-2 after natural infection or vaccination.
We compared the results of a novel enzyme-linked immunosorbent assay (ELISA)-based surrogate virus neutralization test (sVNT) for the detection of anti-SARS-CoV-2 NAbs with those of a cell culture assay. The results were additionally correlated with total anti-SARS-CoV-2 IgG BAb ratios determined using the serological Euroimmun test. Based on our findings, we suggest a combined strategy to reliably detect samples with high NAb titers, while strongly reducing the number of false-positive, low-titer samples.
2. Results
2.1. Assay-comparison for the determination of anti-SARS-CoV-2 NAbs
A total of 108 residual blood samples of 98 COVID-19 convalescents, donated in the period between April 2020 and January 2021, were tested for the presence of anti-SARS-CoV-2 NAbs using both, a sVNT and a cell-culture based assay.
Results of both assays show a moderate correlation (r = 0.68) and NAbs were detected in all donors, as shown in Fig. 1 . The manufacturer's specified cutoff value of 20% was used for the ELISA-based surrogate assay.
Fig. 1.
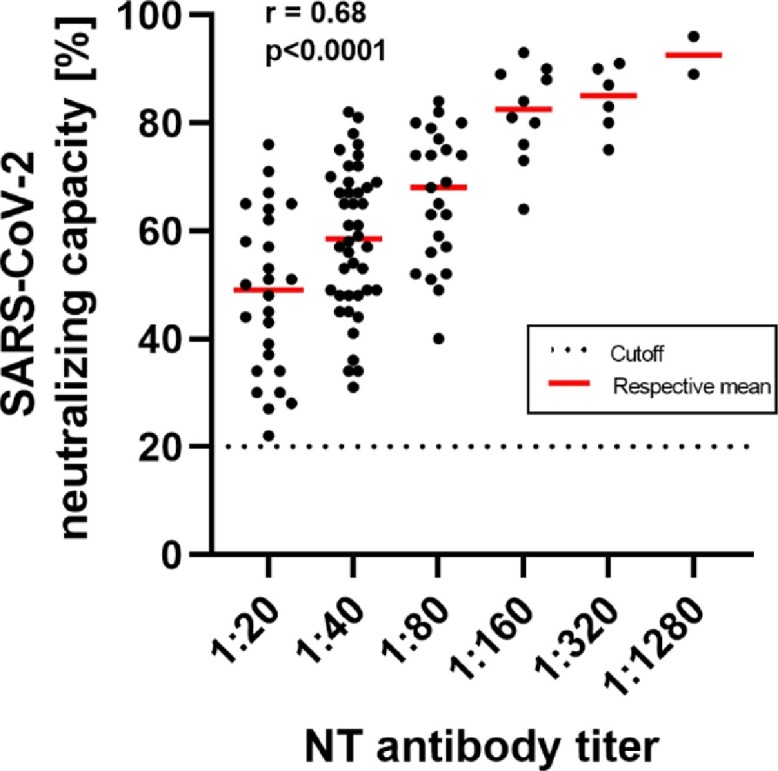
Comparison of the results obtained from the sVNT ELISA and the cell culture assay for the determination of anti-SARS-CoV-2 neutralizing antibodies. Neutralizing antibody-capacities are indicated as a percentage for the sVNT assay or expressed as an antibody-titer for the cell-culture based assay, respectively. The dotted horizontal line symbolizes the positive cutoff (20%) of the sVNT assay specified by the manufacturer. The correlation coefficient was determined using one-way ANOVA.
2.2. Correlation of anti-SARS-CoV-2 igG NAbs and BAbs
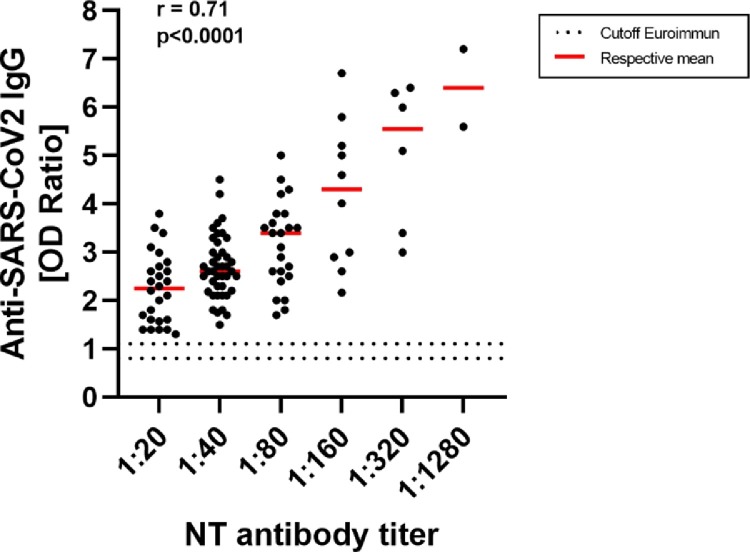
Residual blood samples were additionally tested for the presence of total anti-SARS-CoV-2 IgG BAbs directed against domain S1 of the viral spike protein using the serological ELISA of Euroimmun (Lübeck, Germany). A moderate correlation of the values determined in the cell culture NAb and Euroimmun assay was generally observed (r = 0.71), with occasional samples revealing high NAbs despite comparatively low anti-SARS-CoV-2 IgG ratios. All convalescents tested showed SARS-CoV-2 IgG seroconversion (Fig. 2 ).
Fig. 2.
Comparison of the cell culture neutralizing antibody (NAb) assay and the semiquantitative Euroimmun assay for the detection of anti-SARS-CoV-2 IgG binding antibodies (BAbs). Results of the Euroimmun anti-SARS-CoV2 IgG assay are expressed as a ratio. Values of the cell-culture based NAb assay are expressed as antibody-titers. The dotted horizontal lines symbolize the positive (OD ratio: 1.1) and the equivocal (OD ratio: 0.8) cutoff of the Euroimmun assay specified by the manufacturer. All convalescents included showed SARS-CoV-2 seroconversion. The correlation coefficient was determined using one-way ANOVA.
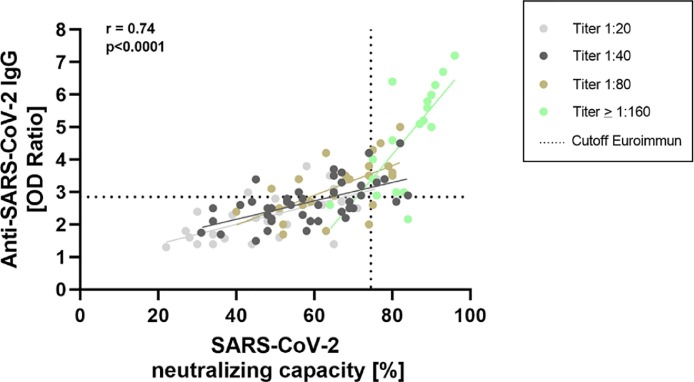
The percentage neutralization values determined using the sVNT assay also showed a moderate correlation with anti-SARS-CoV-2 IgG ratios (r = 0.74, Fig. 3 ). Using ROC-analysis, appropriate cutoffs for the Euroimmun IgG- and sVNT assay were determined to reliably identify high-titer plasmas. The analysis indicated an optimal cutoff of 74.5% and 2.85 for the sVNT- and Euroimmun assay, respectively. Using these cutoff-values leads to a reliable identification (sensitivity: 88.89%, specificity: 87.78%) of high-titer plasmas of COVID-19 convalescent donors.
Fig. 3.
Comparison of the ELISA-based sVNT NAb assay and the serological Euroimmun assay for the detection of anti-SARS-CoV-2 IgG BAbs. Neutralizing antibody-capacities are indicated as a percentage determined using the sVNT assay. Results of the Euroimmun anti-SARS-CoV-2 IgG assay are expressed as a ratio. We color-coded the different NAb titers determined in the cell culture assay for a better overview. The dashed vertical line symbolizes the positive cutoff for the sVNT assay of 74.5%. The dashed horizontal line symbolizes the cutoff for the Euroimmun assay (OD-ration: 2.85). Both cutoffs were determined using ROC-analysis. Considering both cutoff-values leads to a reliable detection (sensitivity: 88.89%, specificity: 87.78%) of high-titer plasmas of COVID-19 convalescents. The correlation coefficient was calculated using simple linear regression.
3. Discussion
Passive immunization is a promising approach to protect SARS-CoV-2-infected individuals from a severe COVID-19 course. Data from a prospective study suggest that early treatment of infected adults with CP can prevent severe COVID-19 by up to 73% [8]. The effectiveness of therapy depends crucially on the NAb concentration of the plasma transfused. Duan et al. showed that transfusing one dose (200 ml) of COVID-19 CP with a NAb titer of ≥ 1:160 significantly improved the clinical outcomes of ten patients suffering from COVID-19 disease [9]. The NAbs are determined standardly using cell culture-based assays, which can only be performed in special BSL3 laboratories and are very time-consuming (several days). An alternative is the novel ELISA-based sVNT neutralization assay, which has some practical advantages: It can be performed in any standard Biosafety Level 2 (BSL2) laboratory within a few hours, does not require special equipment and is feasible for high-throughput testing [10]. In addition to some standardized seroassays for the identification of total anti-SARS-CoV-2 IgG BAbs, which do not address the functionality of the antibody response, the sVNT assay has been approved by the U.S. Food and Drug Administration (FDA) as being “acceptable for use in the manufacture of high titer COVID-19 convalescent plasma.” As a qualifying criterion for therapeutic CP, the FDA recommends a ratio of ≥ 3.5 concerning the anti-SARS-CoV-2 IgG Euroimmun assay and an inhibition-value ≥ 68% for the sVNT neutralization test. According to the FDA, NAb titers should be ≥ 1:160 when using a common cell culture assay to be adequate for therapy [11]. All 98 convalescents included in our cohort expressed anti-SARS-CoV-2 NAbs detectable in both assays, whereby results show a moderate correlation (Fig. 1). Assay correlation (r = 0.68) was comparable to those reported in previous studies [12, 13]. It is of note that our data also suggest long-lasting humoral immunity of at least 11 months against the new coronavirus, as the maximum period between symptom onset and donation was 323 days (mean: 89.53 days, 95% CI: 74.98 days to 104.07 days, median: 62 days). This is, to the best of our knowledge, the longest reported persistence of humoral immunity against SARS-CoV-2 so far.
Results of both NAb assays showed a moderate correlation to those of the Euroimmun anti-SARS-CoV-2 IgG assay (cell culture assay: r = 0.71, sVNT assay: r = 0.74). By contrast, only a fair correlation between NAbs and anti-SARS-CoV-2 IgA antibodies (Euroimmun, Lübeck) was detected for both assays (cell culture assay: r = 0.55, sVNT assay: r = 0.32, see supplement figure S1 and S2). While data for the sVNT assay are lacking, a recent study of Müller et al. has already shown a moderate correlation between a cell culture-based NAb assay and anti-SARS-CoV-2 IgG BAb ratios determined by using the Euroimmun ELISA assay [14]. As opposed to our data, the authors also showed a moderate correlation to anti-SARS-CoV-2 IgA antibody results. This might be explainable by the fact that IgA-antibody levels seem to drop rapidly, whereby IgG antibodies against the virus are stably detectable for several months in individuals who have recovered from COVID-19 [15, 16]. Therefore, the correlation between NAbs and IgA antibodies depends strongly on the time post-infection.
Using high anti-SARS-CoV-2 IgG ratios (e.g. ≥3.5 as recommended by the FDA) as the only CP-qualification criterion would result in some plasmas with high NAbs not being identified, as shown in Figs. 2 and 3. Importantly, this approach also detects occasional plasmas showing relatively low NAb concentrations and would, therefore, not be appropriate for CP therapy. By using an inhibition value of ≥ 64% as a positive cutoff for the sVNT assay, we detected 100% (18/18) of high-titer plasmas (titer ≥1:160). However, this approach also results in the identification of a considerable number of plasmas showing lower NAb titers in the cell culture assay (Fig. 3). To reduce the number of low-titer plasmas being identified, we propose a combined approach concerning qualification of the CP, which can be performed in any standard laboratory. Based on the results of a ROC-analysis, using a positive cutoff of ratio ≥2.85 for the Euroimmun IgG assay and an inhibition value ≥74.5% for the sVNT assay reduces the identification of “false-positive” low-titer plasmas, while further detecting 88.89% (sensitivity) of high-titer specimens (titer ≥ 1:160). Of note, this approach also yields a specificity of 87.78%.
In summary, based on our results, we propose a combined strategy to detect plasma samples showing high NAb titers reliably and additionally reduce the risk of identifying false-positive, low-titer specimens. Our data further reveal a long-lasting humoral immunity against SARS-CoV-2 of at least 11 months.
3.1. Study limitation
With a larger cohort size, the cutoff values calculated in the ROC-analysis to identify high-titer plasmas could have been determined even more precisely. However, most of the data were collected in the early phase of the COVID-19 pandemic, when a low SARS-CoV-2 seroprevalence prevailed in Germany. As a result, the availability of adequate donors was limited.
4. Methods
4.1. Human donors
The convalescents included in our study had a mild or moderate disease course not requiring hospitalization and SARS-CoV-2 RNA was initially detected by PCR. All donors underwent a medical examination before donation. Samples were collected in accordance with the German Act on Medical Devices for the collection of human residual material. Ethical approval was obtained from the ethical committee of the HDZ NRW in Bad Oeynhausen (Reg. No. 670/2020).
4.1.1. Determination of neutralizing anti-SARS-CoV-2 antibodies
The sVNT cPass ELISA from GenScript (Piscataway Township, USA) is designed to mimic the virus–host interaction using a purified receptor-binding domain (RBD) protein and immobilized cell surface receptor, angiotensin converting enzyme-2 (ACE2). Due to horseradish peroxidase-conjugated RBD, the absorbance of a sample can be measured at 450 nm and is inversely proportional to the NAb titer of the respective specimen. The experimental procedure was performed as specified by the manufacturer [10].
The cell-culture based assay for detection of anti-SARS-CoV-2 NAbs was performed as previously described [14]. In brief, a virus stock solution with the SARS-CoV-2 NRW-42 isolate EPI ISL 425,126 [17] was added to a final concentration of 1000 TCID50/well to heat-inactivated and diluted plasma samples. The plasma neutralization titer was determined by microscopic inspection as the highest plasma dilution without a virus-induced cytopathic effect. All samples were tested in duplicate.
4.1.2. Determination of binding anti-SARS-CoV-2 igG and igA antibodies
Two commercial ELISAs (Euroimmun, Lübeck, Germany) targeting the viral spike-protein were used for the determination of anti-SARS-CoV-2 IgG and IgA antibodies. Semiquantitative results were calculated as a ratio of the extinction of samples over the extinction of a calibrator. Measurements were fully automated, according to the manufacturer's protocol, using the Euroimmun Analyzer I system.
4.1.3. Statistical analysis
The software GraphPad Prism 9.0 was used for statistical analysis of data. The respective correlation coefficient was calculated by using either one-way ANOVA (dataset Figs. 1 and 2) or simple linear regression (dataset Fig. 3). p-values of 0.05 or less were considered statistically significant. For the cutoff determination in Fig. 3, a ROC analysis was performed, which also yields the reported values for sensitivity and specificity.
Table 1.
Donor_characteristics.
| NT antibody titer | SARS-CoV-2 neutralizing capacity [%] | Anti-SARS-CoV-2 IgG [OD Ratio] | Symptom onset | Day of donation | Period post symptom onset [days] |
|---|---|---|---|---|---|
| 20 | 22 | 1.3 | 03-03-2020 | 24-04-2020 | 52 |
| 20 | 27 | 1.8 | 07-03-2020 | 28-04-2020 | 52 |
| 20 | 28 | 1.6 | 13-03-2020 | 19-05-2020 | 67 |
| 20 | 30 | 1.4 | 11-03-2020 | 04-05-2020 | 54 |
| 20 | 30 | 2.4 | 19-03-2020 | 19-05-2020 | 61 |
| 20 | 34 | 1.7 | 08-03-2020 | 29-04-2020 | 52 |
| 20 | 34 | 1.4 | 08-03-2020 | 06-05-2020 | 59 |
| 20 | 37 | 1.57 | 08-03-2020 | 23-04-2020 | 46 |
| 20 | 39 | 2.3 | 26-03-2020 | 28-05-2020 | 63 |
| 20 | 43 | 2.99 | 27-03-2020 | 27-04-2020 | 31 |
| 20 | 44 | 1.4 | 15-03-2020 | 19-01-2021 | 310 |
| 20 | 45 | 2.2 | 17-03-2020 | 19-05-2020 | 63 |
| 20 | 48 | 2.1 | 17-03-2020 | 13-05-2020 | 57 |
| 20 | 50 | 2.8 | 16-03-2020 | 16-11-2020 | 245 |
| 20 | 51 | 2.4 | 27-03-2020 | 13-05-2020 | 47 |
| 20 | 51 | 1.6 | 17-03-2020 | 26-05-2020 | 70 |
| 20 | 53 | 2 | 18-03-2020 | 11-05-2020 | 54 |
| 20 | 58 | 3.8 | 14-03-2020 | 07-05-2020 | 54 |
| 20 | 62 | 2.6 | 19-03-2020 | 12-05-2020 | 54 |
| 20 | 64 | 3.5 | 08-03-2020 | 28-04-2020 | 51 |
| 20 | 65 | 1.4 | 10-03-2020 | 11-05-2020 | 62 |
| 20 | 65 | 3.1 | 17-03-2020 | 27-05-2020 | 71 |
| 20 | 67 | 2.7 | 27-03-2020 | 08-05-2020 | 42 |
| 20 | 71 | 2.5 | 14-03-2020 | 19-05-2020 | 66 |
| 20 | 76 | 3.4 | 09-03-2020 | 19-05-2020 | 71 |
| 40 | 31 | 1.75 | 08-03-2020 | 22-04-2020 | 45 |
| 40 | 34 | 2.5 | 09-03-2020 | 18-11-2020 | 254 |
| 40 | 34 | 2.1 | 25-03-2020 | 19-11-2020 | 239 |
| 40 | 36 | 1.7 | 11-03-2020 | 28-04-2020 | 48 |
| 40 | 41 | 2.6 | 09-03-2020 | 11-11-2020 | 247 |
| 40 | 44 | 2.7 | 16-03-2020 | 03-11-2020 | 232 |
| 40 | 45 | 3.39 | 11-03-2020 | 27-04-2020 | 47 |
| 40 | 45 | 1.5 | 07-03-2020 | 06-05-2020 | 60 |
| 40 | 48 | 2.5 | 07-03-2020 | 28-05-2020 | 82 |
| 40 | 48 | 2.3 | 18-03-2020 | 02-06-2020 | 76 |
| 40 | 48 | 1.8 | 24-03-2020 | 02-06-2020 | 70 |
| 40 | 49 | 2.18 | 10-03-2020 | 23-04-2020 | 44 |
| 40 | 49 | 2.5 | 27-03-2020 | 19-05-2020 | 53 |
| 40 | 49 | 2.1 | 17-03-2020 | 06-05-2020 | 50 |
| 40 | 53 | 2.8 | 19-03-2020 | 29-04-2020 | 41 |
| 40 | 53 | 2.7 | 27-03-2020 | 05-05-2020 | 39 |
| 40 | 54 | 2.6 | 28-03-2020 | 09-11-2020 | 226 |
| 40 | 56 | 3 | 10-03-2020 | 29-04-2020 | 50 |
| 40 | 57 | 2.8 | 10-03-2020 | 28-04-2020 | 49 |
| 40 | 57 | 2.3 | 06-04-2020 | 28-01-2021 | 297 |
| 40 | 58 | 1.8 | 27-03-2020 | 27-05-2020 | 61 |
| 40 | 59 | 2.1 | 09-03-2020 | 11-05-2020 | 63 |
| 40 | 61 | 2.1 | 10-03-2020 | 27-05-2020 | 78 |
| 40 | 61 | 2.6 | 12-03-2020 | 27-05-2020 | 76 |
| 40 | 65 | 3 | 13-03-2020 | 05-11-2020 | 237 |
| 40 | 65 | 3.5 | 16-03-2020 | 27-01-2021 | 317 |
| 40 | 65 | 3.7 | 17-03-2020 | 20-05-2020 | 64 |
| 40 | 67 | 3.6 | 12-03-2020 | 06-05-2020 | 55 |
| 40 | 67 | 3.3 | 14-03-2020 | 25-05-2020 | 72 |
| 40 | 67 | 2.4 | 13-03-2020 | 11-05-2020 | 59 |
| 40 | 68 | 2.2 | 23-03-2020 | 19-05-2020 | 57 |
| 40 | 69 | 2.7 | 23-03-2020 | 14-05-2020 | 52 |
| 40 | 69 | 2.5 | 11-03-2020 | 12-05-2020 | 62 |
| 40 | 70 | 2.5 | 13-03-2020 | 22-05-2020 | 70 |
| 40 | 72 | 3.2 | 12-03-2020 | 28-01-2021 | 322 |
| 40 | 72 | 2.9 | 14-03-2020 | 27-01-2021 | 319 |
| 40 | 74 | 4.2 | 13-03-2020 | 13-05-2020 | 61 |
| 40 | 76 | 3.3 | 20-03-2020 | 27-05-2020 | 68 |
| 40 | 78 | 3.4 | 15-03-2020 | 25-05-2020 | 71 |
| 40 | 81 | 2.7 | 13-03-2020 | 27-05-2020 | 75 |
| 40 | 82 | 4.5 | 12-03-2020 | 22-05-2020 | 71 |
| 40 | 84 | 2.9 | 17-03-2020 | 13-05-2020 | 57 |
| 80 | 40 | 2.4 | 17-03-2020 | 10-11-2020 | 238 |
| 80 | 49 | 3.1 | 19-03-2020 | 28-04-2020 | 40 |
| 80 | 51 | 2.5 | 17-03-2020 | 29-04-2020 | 43 |
| 80 | 52 | 2 | 10-03-2020 | 06-05-2020 | 57 |
| 80 | 52 | 1.7 | 08-03-2020 | 06-05-2020 | 59 |
| 80 | 56 | 3.4 | 17-03-2020 | 25-05-2020 | 69 |
| 80 | 57 | 2.6 | 14-03-2020 | 02-06-2020 | 80 |
| 80 | 59 | 2.7 | 13-03-2020 | 07-05-2020 | 55 |
| 80 | 63 | 1.8 | 22-03-2020 | 11-05-2020 | 50 |
| 80 | 63 | 4.2 | 11-03-2020 | 08-05-2020 | 58 |
| 80 | 65 | 2.9 | 10-03-2020 | 06-05-2020 | 57 |
| 80 | 68 | 3.5 | 15-03-2020 | 28-04-2020 | 44 |
| 80 | 69 | 3.4 | 14-03-2020 | 08-05-2020 | 55 |
| 80 | 74 | 3.8 | 10-03-2020 | 04-05-2020 | 55 |
| 80 | 74 | 2 | 12-03-2020 | 12-01-2021 | 306 |
| 80 | 74 | 3.5 | 11-03-2020 | 29-05-2020 | 79 |
| 80 | 75 | 2.6 | 16-03-2020 | 25-05-2020 | 70 |
| 80 | 75 | 4.3 | 10-03-2020 | 13-05-2020 | 64 |
| 80 | 77 | 4.5 | 16-03-2020 | 27-05-2020 | 72 |
| 80 | 79 | 3.8 | 11-03-2020 | 25-05-2020 | 75 |
| 80 | 80 | 3.6 | 15-03-2020 | 22-05-2020 | 68 |
| 80 | 80 | 3.5 | 13-03-2020 | 06-05-2020 | 54 |
| 80 | 82 | 5 | 18-03-2020 | 12-05-2020 | 55 |
| 160 | 64 | 2.6 | 10-03-2020 | 06-05-2020 | 57 |
| 160 | 75 | 4.01 | 10-03-2020 | 22-04-2020 | 43 |
| 160 | 76 | 2.9 | 10-03-2020 | 26-05-2020 | 77 |
| 160 | 80 | 4.6 | 13-03-2020 | 07-05-2020 | 55 |
| 160 | 81 | 3 | 14-03-2020 | 20-05-2020 | 67 |
| 160 | 84 | 2.16 | 14-03-2020 | 24-04-2020 | 41 |
| 160 | 88 | 5.2 | 10-03-2020 | 25-05-2020 | 76 |
| 160 | 89 | 5.8 | 01-03-2020 | 14-05-2020 | 74 |
| 160 | 90 | 5 | 13-03-2020 | 27-05-2020 | 75 |
| 160 | 93 | 6.7 | 16-03-2020 | 26-05-2020 | 71 |
| 320 | 75 | 3.4 | 14-03-2020 | 12-05-2020 | 59 |
| 320 | 80 | 6.4 | 10-03-2020 | 05-05-2020 | 56 |
| 320 | 83 | 3 | 10-03-2020 | 11-05-2020 | 62 |
| 320 | 87 | 5.1 | 18-03-2020 | 26-05-2020 | 69 |
| 320 | 90 | 6 | 10-03-2020 | 19-01-2021 | 315 |
| 320 | 91 | 6.3 | 21-03-2020 | 22-05-2020 | 62 |
| 1280 | 89 | 5.6 | 15-03-2020 | 18-05-2020 | 64 |
| 1280 | 96 | 7.2 | 15-03-2020 | 27-05-2020 | 73 |
| Mean: 93.52, 95% CI: 59.38 – 127.66, Median: 40 | Mean: 61.85, 95% CI: 58.54 – 65.16, Median: 64 | Mean: 3.0245, 95% CI: 2.7893 – 3.2598, Median: 2.7 | Mean: 89.53, 95% CI: 74.98 – 104.07, Median: 62 |
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2021.104984.
Appendix. Supplementary materials
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu H., Stratton C.W., Tang Y.-.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J. Med. Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyerowitz-Katz G., Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int. J. Infect. Dis. 2020;101:138–148. doi: 10.1016/j.ijid.2020.09.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajendran K., Krishnasamy N., Rangarajan J., Rathinam J., Natarajan M., Ramachandran A. (2020) Convalescent plasma transfusion for the treatment of COVID-19: systematic review. J. Med. Virol. doi:10.1002/jmv.25961. [DOI] [PMC free article] [PubMed]
- 5.Harvala H., Robb M.L., Watkins N., Ijaz S., Dicks S., Patel M., Supasa P., Wanwisa D., Liu C., Mongkolsapaya J., Bown A., Bailey D., Vipond R., Grayson N., Temperton N., Gupta S., Ploeg R.J., Bolton J., Fyfe A., Gopal R., Simmonds P., Screaton G., Thompson C., Brooks T., Zambon M., Miflin G., Roberts D.J. (2020) Convalescent plasma therapy for the treatment of patients with COVID-19: assessment of methods available for antibody detection and their correlation with neutralising antibody levels. Transfus. Med. doi:10.1111/tme.12746. [DOI] [PMC free article] [PubMed]
- 6.Center of Disease Control and Prevention (2021) Interim laboratory biosafety guidelines for handling and processing specimens associated with coronavirus disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/lab/lab-biosafety-guidelines.html. Zugegriffen: 03. März 2021.
- 7.Legros V., Denolly S., Vogrig M., Boson B., Siret E., Rigaill J., Pillet S., Grattard F., Gonzalo S., Verhoeven P., Allatif O., Berthelot P., Pélissier C., Thiery G., Botelho-Nevers E., Millet G., Morel J., Paul S., Walzer T., Cosset F.-.L., Bourlet T., Pozzetto B. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell. Mol. Immunol. 2021;18(2):318–327. doi: 10.1038/s41423-020-00588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Libster R., Pérez Marc G., Wappner D., et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N. Engl. J. Med. 2021;384(7):610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan K., Liu B., Li C., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U S A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan C.W., Chia W.N., Qin X., Liu P., Chen M.I.-.C., Tiu C., Hu Z., Chen V.C.-.W., Young B.E., Sia W.R., Tan Y.-.J., Foo R., Yi Y., Lye D.C., Anderson D.E., Wang Ll-F. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 11.Food and drug administration emergency use authorization (EUA) for convalescent plasma. https://www.fda.gov/media/141477/download. Zugegriffen: 03. März 2021.
- 12.Meyer B., Reimerink J., Torriani G., Brouwer F., Godeke G.-.J., Yerly S., Hoogerwerf M., Vuilleumier N., Kaiser L., Eckerle I. Reusken C validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT) Emerg. Microbes Infect. 2021;9(1):2394–2403. doi: 10.1080/22221751.2020.1835448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valcourt E.J., Manguiat K., Robinson A., Chen J.C.-.Y., Dimitrova K., Philipson C., Lamoureux L., McLachlan E., Schiffman Z., Drebot M.A., Wood H. Evaluation of a commercially-available surrogate virus neutralization test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) Diagn. Microbiol. Infect. Dis. 2020;99(4) doi: 10.1016/j.diagmicrobio.2020.115294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller L., Ostermann P.N., Walker A., Wienemann T., Mertens A., Adams O., Andree M., Hauka S., Lübke N., Keitel V., Drexler I., Di Cristanziano V., Hermsen D.F., Kaiser R., Boege F., Klein F., Schaal H., Timm J., Senff T. (2021) Sensitivity of anti-SARS-CoV-2 serological assays in a high-prevalence setting. Eur. J. Clin. Microbiol. Infect. Dis. doi:10.1007/s10096-021-04169-7. [DOI] [PMC free article] [PubMed]
- 15.Iyer A.S., Jones F.K., Nodoushani A., et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci. Immunol. 2020;5(52) doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer B., Lindenkamp C., Lichtenberg C., Birschmann I., Knabbe C., Hendig D. (2021) Evidence of long-lasting humoral and cellular immunity against SARS-CoV-2 even in elderly COVID-19 convalescents showing a mild to moderate disease progression. [DOI] [PMC free article] [PubMed]
- 17.Walker A., Houwaart T., Wienemann T., Vasconcelos M.K., Strelow D., Senff T., Hülse L., Adams O., Andree M., Hauka S., Feldt T., Jensen B.-.E., Keitel V., Kindgen-Milles D., Timm J., Pfeffer K., Dilthey A.T. Genetic structure of SARS-CoV-2 reflects clonal superspreading and multiple independent introduction events, North-Rhine Westphalia, Germany, February and March 2020. Euro. Surveill. 2020;25(22) doi: 10.2807/1560-7917.ES.2020.25.22.2000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.