Abstract
Aim
This review aims to summarize and discuss some of the most relevant clinical trials in epidemiology, diagnostics, and treatment of hypertension published in 2020 and 2021.
Data synthesis
The trials included in this review are related to hypertension onset age and risk for future cardiovascular disease, reliability of different blood pressure monitoring methods, role of exercise-induced hypertension, treatment of hypertension in patients with SARS-CoV-2 infection, management of hypertension high-risk patient groups, e.g., in the elderly (≥80 years) and patients with atrial fibrillation, and the interplay between nutrition and hypertension, as well as recent insights into renal denervation for treatment of hypertension.
Conclusions
Hypertension onset age, nighttime blood pressure levels and a riser pattern are relevant for the prognosis of future cardiovascular diseases. The risk of coronary heart disease appears to increase linearly with increasing exercise systolic blood pressure. Renin-angiotensin system blockers are not associated with an increased risk for a severe course of COVID-19. In elderly patients, a risk-benefit assessment of intensified blood pressure control should be individually evaluated. A J-shaped association between cardiovascular disease and achieved blood pressure could also be demonstrated in patients with atrial fibrillation on anticoagulation. Salt restriction and lifestyle modification remain effective options in treating hypertensive patients at low cardiovascular risk. Sodium glucose co-transporter 2 inhibitors and Glucagon-like peptide-1 receptor agonists show BP-lowering effects. Renal denervation should be considered as an additional or alternative treatment option in selected patients with uncontrolled hypertension.
Keywords: Arterial hypertension, Cardiovascular risk, Exercise-induced hypertension, COVID-19, Renal denervation
Abbreviations: ABP, ambulatory blood pressure; ABPM, ambulatory blood pressure monitoring; ACE2, angiotensin-converting enzyme-2; ACE-I, Angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; aHR, adjusted hazard ratio; ARBs, angiotensin receptor blockers; BP, blood pressure; CI, confidence interval; COVID-19, coronavirus disease 2019; CVD, cardiovascular diseases; DASH, dietary approaches to stop hypertension; GLP1-RA, Glucagon-like peptide-1 receptor agonists; HBP, home blood pressure; HBPM, home blood pressure monitoring; HR, hazard ratio; LVMI, left ventricular mass index; OBP, office blood pressure; RAS, renin-angiotensin system; T2DM, diabetes mellitus type 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2; SBP, systolic blood pressure; SGLT-2, Sodium glucose co-transporter 2; 24-h, 24-hour; RDN, renal denervation; W, Watt
Introduction
Hypertension remains the most prevalent risk factor for cardiovascular diseases (CVD) [1]. Blood pressure (BP) control to guideline-recommended target levels is frequently not achieved, even with the use of various treatment modalities [2]. This review sought to discuss some of the most relevant clinical trials published in 2020 and 2021 in major journals in the field of hypertension research. The studies discussed herein deal with the association between age of hypertension onset and future CVD, the reliability of office, home, and ambulatory BP, the prognostic value of nighttime BP, new insights into exercise-induced hypertension and management of hypertension in high-risk patient groups, as patients ≥80 years and patients with atrial fibrillation (AF) requiring oral anticoagulation. With the outbreak of the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) pandemic, challenges in the management of patients with SARS-CoV-2 and concomitant hypertension arose. Since the SARS-CoV-2 cell entry depends on the angiotensin-converting-enzyme 2 and potentially involved in blood pressure regulation and cardiovascular outcomes, we critically discuss trials investigating the impact of renin-angiotensin system- (RAS)-inhibitor treatment on outcomes of coronavirus disease 2019 (COVID-19) in patients with hypertension. Finally, we deal with the most recent trials in device-based hypertension treatments, above all results of the first pivotal study of catheter-based renal denervation.
Epidemiology
Association of hypertension onset age and risk for future CVD
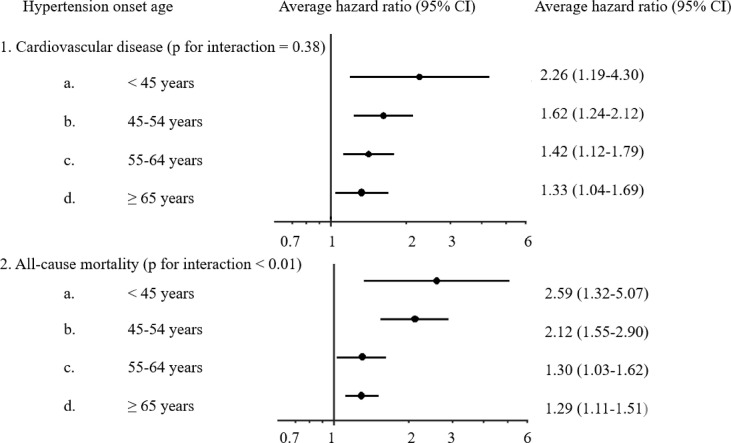
The relationship between age of hypertension onset and risk for future cardiovascular events and all-cause mortality was studied in the Kailuan trial [3]. Cardiovascular events were defined as myocardial infarction, and ischemic or hemorrhagic stroke [3]. Between July 2006 and October 2007, a total of 71,245 participants without preexisting hypertension or CVD were enrolled and followed for ten years. A total of 20,221 cases of new-onset hypertension were documented. Each case was matched with a control subject of the same age (±1 year) and sex resulting in 19,887 case-control-pairs. During a median follow-up of 6.5 years, 1672 CVDs (387 myocardial infarctions, 1116 ischemic strokes and 182 hemorrhagic strokes) and 2008 deaths were documented. Hypertension was associated with an elevated risk for CVD and all-cause mortality. The younger the age of hypertension onset was, the stronger was the association. Participants ≤45 years demonstrated the highest risk for all the endpoints: adjusted hazard ratio (aHR) 2.26 (95% confidence interval (CI) 1.19 to 4.30) for CVDs and 2.59 (95% CI 1.32 to 5.07) for death (Fig. 1 ) when compared with older individuals [3].
Figure 1.
The association of hypertension onset age and risk for future cardiovascular disease. The average hazard ratios (95% confidence interval) of incident cardiovascular disease (myocardial infarction, ischemic stroke, and hemorrhagic stroke) and all-cause mortality of new-onset hypertensive participants across age groups in 39,744 Chinese participants [3].
Perspective
The Kailuan trial confirmed once more the association between arterial hypertension and risk for CVD and all-cause mortality. The younger the age of hypertension onset was, the stronger was this association [3].
Diagnosis
Reliability of office, home, and ambulatory blood pressure
The 2018 European Guidelines on the management of hypertension recommend that the diagnosis of hypertension should not only be based on office BP (OBP) but also on “out-of-office” measurements, such as ambulatory blood pressure monitoring (ABPM) or home blood pressure monitoring (HBPM) [2]. This should enable a more accurate diagnosis, especially white-coat and masked hypertension. The reliability and prognostic value of OBP, ambulatory BP (ABP), and home BP (HBP) and their associations with left ventricular mass index (LVMI) in untreated subjects were analyzed in the Improving the Detection of Hypertension (IDH) trial. The study enrolled 408 participants without CVD who had their OBP assessed at three visits and completed three weeks of HBPM (measured twice in the morning and twice in the evening), two 24-hour (24-h) ABPM recordings, and a 2-dimensional echocardiogram [4]. For SBP and DBP, the highest reliability has been observed to be present in HBP, OBP and 24-h ABP respectively. Likewise, the strongest correlation with elevated LVMI as a hypertension-mediated organ damage was found for HBP [4].
Perspective
The Improving the Detection of Hypertension trial suggested that 1 week of HBP monitoring (measured twice in the morning and twice in the evening) may be the most reliable method for diagnosing hypertension. The use of “out-of-office” methods can help to diagnose various phenotypes of hypertension, such as white-coat and masked hypertension [5].
Importance of nighttime blood pressure
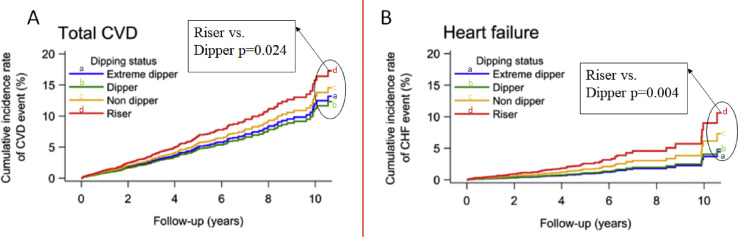
24-h ABPM plays an incremental role in the management of hypertension as it provides information on circadian variations in BP and can identify white-coat and masked hypertension [6]. A reduction in SBP by 10–20% during nighttime is considered physiological dipping. An absence of (“non-dipping”), or reverse (“rising“) SBP-dipping is associated with higher CVD risk [7,8]. The prospective JAMP-trial (Japan Ambulatory Blood Pressure Monitoring Prospective) included 6359 asymptomatic patients with at least one cardiovascular risk factor (mainly hypertension) between 2008 and 2017. This study aimed to determine associations between both, nocturnal hypertension and BP dipping patterns and the risk of CVD, including heart failure [9]. During a follow-up period of 4.5 years, there were 306 CVDs (119 stroke, 99 coronary artery disease, 88 heart failure events). Nighttime SBP was significantly associated with higher risk of CVD and in particular heart failure (HR per 20-mmHg increase 1.21; 95% CI 1.03 to 1.41; p = 0.017 and 1.36; 95% CI 1.08 to 1.71; p = 0.009). The worst outcomes were found in patients with rising pattern in nighttime BP (HR for CVD 1.48; 95% CI 1.05 to 2.08; p = 0.924 and 2.45 for heart failure; 95% CI 1.34 to 4.48; p = 0.004) (Fig. 2 ) [9].
Figure 2.
Cardiovascular disease risk by dipping status. Cumulative incidence rate of A-total cardiovascular events and B- heart failure events by different dipping patterns (extreme dipper, dipper, non-dipper, and riser). Riser pattern is associated with higher risk of cardiovascular risk, in particular heart failure [9]. CVD: cardiovascular disease, CHF: chronic heart failure.
Perspective
The JAMP trial is among the largest, prospective ABPM studies consistently using the same device and monitoring protocol. The results suggest that nighttime BP levels and a riser pattern were independently associated with higher risk of CVD, in particular heart failure, highlighting the importance of antihypertensive approaches targeting elevated nighttime BP.
Exercise-induced hypertension
Cardio-pulmonary exercise testing provides the opportunity to assess the behavior of BP under reproducible, standardized physical exercise conditions. There is an association between exaggerated exercise SBP and risk of CVD during follow-up [10]. However, there is no uniform definition for exercise-induced hypertension [11]. The German Society of Hypertension, for instance, recommends to use an upper threshold of 200/100 mmHg for diagnosing exercise-induced hypertension in middle-aged women and men during submaximal effort at 100 Watts (W) [11].
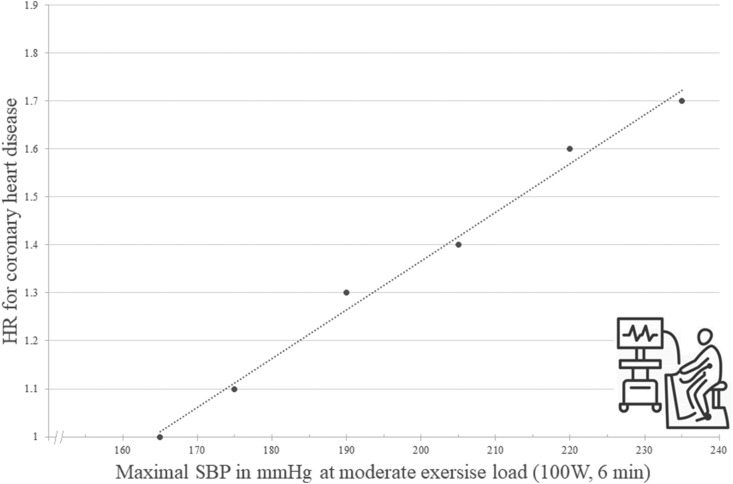
A recently published study investigated the relationship between a sustained elevation of exercise SBP at moderate workload of 100 W for 6 minutes and future risk of coronary artery disease in healthy men using repeated exercise testing [11]. In the 1970s, 2014 healthy, white men were enrolled in the OSLO-ISCHEMIA-STUDY [11]. After 7 years of follow up, 1392 men remained in the study. Each participant underwent bicycle exercise testing at two visits at 100 W for 6 minutes, followed by an increase of workload by 50 W every sixth minute until near exhaustion. Based on the results, participants were divided into three groups: 1st group with SBP <165 mmHg at 100 W at both visits, 2nd group with SBP <165 mmHg at 100 W in one of two visits and 3rd group with SBP >165 mmHg at 100 W at both visits [11]. During the median follow up of 24.7 years, 452 coronary heart disease events occurred of which 186 were death. The risk of CVD increased almost linearly in participants with increasing exercise SBP (Fig. 3 ) [11].
Figure 3.
Risk of future coronary events depending on exercise blood pressure. There is an increasing risk of coronary heart disease with increasing exercise systolic blood pressure at moderate exercise workload (100 W for 6 minutes) independent of systolic blood pressure value at rest. The association appears to be linear [11]. HR: hazard ratio, SBP systolic blood pressure, W: Watt.
Perspective
There is an increasing risk of coronary heart disease with increasing exercise SBP independent of SBP at rest. The association appears to be linear from the low range of exercise SBP, and there is no sign of a distinct SBP threshold level for increased coronary disease risk. When interpreting the results of this study, it must be considered that only healthy, Norwegian middle-aged men were included. Therefore, these findings may not be applicable to women, young or elderly patients, or patients of other ethnicities [11].
Therapy
Hypertension and COVID-19
It has been suggested that hypertension is among the major risk factors for severe complications of COVID-19 [12,13]. However, it remains elusive whether this association is due to hypertension per se or attributed to confounding factors (such as older age, diabetes and other comorbidities typically associated with hypertension). A retrospective analysis on 2877 patients with COVID-19 in China showed an increased adjusted risk for death in patients with hypertension compared to those without hypertension (4.0% vs. 1.1%, aHR 2.12; 95% CI 1.17 to 3.82; p = 0.013) [14]. On the contrary, the prevalence of hypertension among these patients appears to be lower than the estimated prevalence of hypertension seen with other viral infections [15] and in the general population in China [[16], [17], [18]].
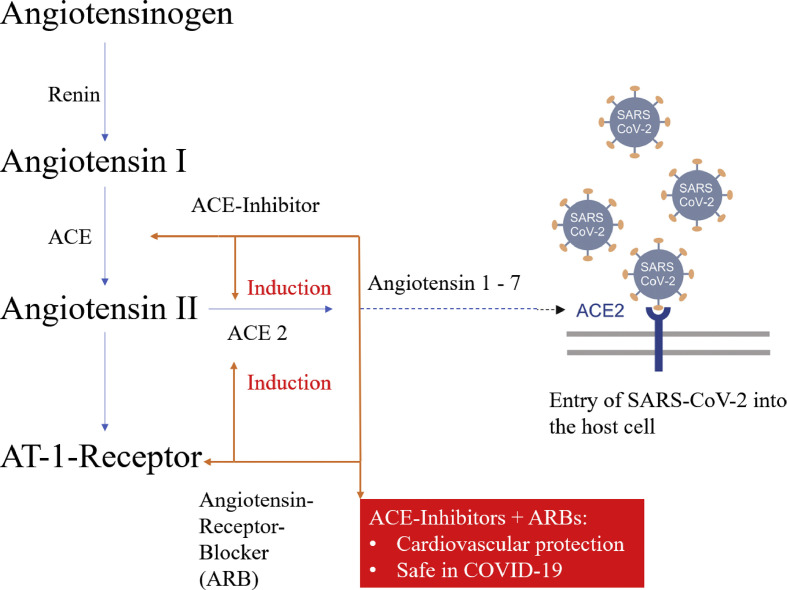
It is known that angiotensin-converting enzyme-2 (ACE2) expression can be increased by angiotensin-converting enzyme inhibitors (ACE-I) and angiotensin receptor blockers (ARBs) [18]. Since ACE2 facilitates SARS-CoV-2 entry into cells, concerns have been expressed that treatment with these drugs could increase the risk of severe COVID-19 (Fig. 4 ) [[2], [18], [19]]. Although the impact of discontinuing ACE-I and ARBs in patients with COVID-19 was uncertain, some authors suggested switching antihypertensive therapy with RAS blockers to calcium antagonists [18,19]. In March 2020, the European Society of Cardiology recommended to continue treatment with their usual anti-hypertensive therapy because there is no solid evidence suggesting that treatment with ACE-I or ARBs during SARS-CoV-2 infection is deleterious [20].
Figure 4.
The interaction of the renin-angiotensin-system-blockers with SARS-CoV-2. Angiotensin-converting enzyme-2 facilitates SARS-CoV-2 entry into cells. Its expression can be increased by angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. ARB: angiotensin receptor blocker, AT: angiotensin, SARS-CoV-2: severe acute respiratory syndrome coronavirus type 2, ACE: angiotensin-converting enzyme.
In the meantime, more robust evidence on this topic has become available. A recent study combined clinical data (n = 144) and single-cell sequencing data of airway samples (n = 48) with in vitro experiments [21]. A distinct inflammatory predisposition of immune cells was correlated with critical COVID-19 progression in patients with hypertension [21]. Treatment with ACE-I was associated with dampened COVID-19-related hyperinflammation [21]. These processes could, in part, explain the worse outcomes observed in hypertensive patients. The limitations of this study are the relatively small sample size and lack of reproducibility by other trials [21].
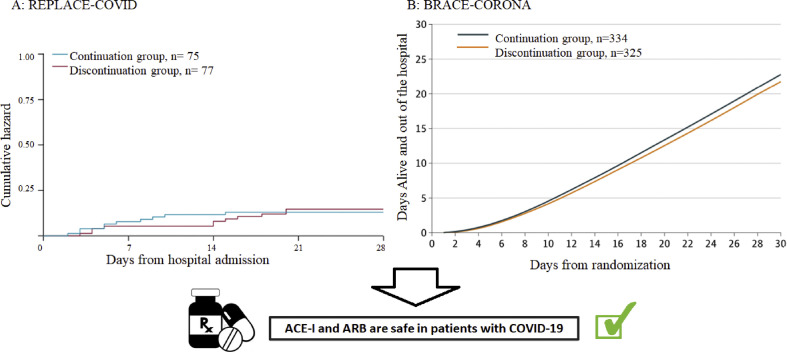
Several observational-studies demonstrated that neither the risk of SARS-CoV-2 infection nor the risk of a severe course of COVID-19 were associated with ACE-I or ARB therapy[[22], [23], [24]] These observational data were recently supported by the findings of two randomized, controlled trials [25,26]. The open-label REPLACE-COVID-Study randomized 152 patients, who were already treated with ARB or ACE-I in a 1:1 pattern to continuation or discontinuation of this treatment [25]. No significant differences between the two groups in the rank score for clinical outcome during hospitalization (days of survival, duration of mechanical ventilation, duration of dialysis, SOFA-score) were detected (73; 95% CI 40 to 110 vs. 81; 95% CI 38 to 117; p = 0.61) (Fig. 5 A) [25].
Figure 5.
Primary outcomes of REPLACE-COVID [25] BRACE-CORONA [26] trials. A shows the cumulative hazard for all-cause death of the REPLACE-COVID trial [25]. B demonstrate the mean number of days alive and out of the hospital of the BRACE-CORONA [26] trail. In both trials, no significant difference between the groups was observed. ACE-I: angiotensin-converting enzyme inhibitors, ARB: angiotensin receptor blocker.
The BRACE-CORONA-Study showed similar results [26]. In this trial, 659 patients were randomized either to continuation or discontinuation of antihypertensive treatment with either ARB or ACE-I [26]. No significant differences regarding the primary endpoint, days of survival or days of hospital stay, were found between the two populations (continuation: 21.9 days; 95% CI 13.9 to 29.9 vs. discontinuation 22.9 days; 95% CI 15.8 to 30) (Fig. 5B) [26].
Perspective
ACE-I and ARBs are not associated with an increased risk for a severe course of COVID-19. It can be assumed that the use of these drugs leads to a long-term benefit, also during COVID-19, mediated via their beneficial cardiovascular effects.
Hypertension therapy in the elderly
Elderly patients with hypertension often have other comorbidities and therefore frequently receive polypharmacotherapy, which can lead to adverse events and increased morbidity [27]. Therefore, “deprescribing” has been proposed as a strategy of reduction and optimization of pharmacotherapy in the elderly [27].
The unblinded, non-inferiority OPTIMISE-trial examined whether antihypertensive medication reduction is feasible, safe, and not associated with loss of SBP control [28]. A total of 569 patients ≥80 years with SBP <150 mmHg, taking at least two different antihypertensive agents were randomized to a strategy of medication reduction (removal of one hypertension drug, n = 282) or usual care (no changes in medication, n = 287) [28]. The primary endpoint was maintaining SBP <150 mmHg at 12-week follow-up; 86% of the participants in the medication reduction group had a SBP <150 mmHg vs. 88% in the usual care group, indicating no difference between the groups (adjusted risk reduction: 0.98; 97.5% 1-sited CI 0.92 to ∞; p = 0.01 for non-inferiority). Expectedly, SBP and DBP increased significantly by 3.4 mmHg (95% CI 1.0 to 5.8; p = 0.005) and 2.2 mmHg (95% CI 0.9 to 3.6; p = 0.001) in the medication reduction group, respectively. Of the 7 prespecified secondary endpoints, 5 showed no significant difference between the strategies. However, medication reduction was only sustainable in 187 (66.3%) participants at 12 weeks.
Perspective
This study suggested that antihypertensive medication reduction can be achieved without substantial changes in BP control in certain elderly patients but was maintained in only 2 out of 3 patients at 12 weeks. In elderly patients, a risk-benefit assessment of intensified BP control should be critically and individually evaluated.
Anticoagulation in atrial fibrillation and hypertension
The current European Guidelines recommend BP target values of 120–129/≤80 mmHg in patients <65 years [2,6]. These recommendations are based on J-shaped associations between BP and cardiovascular outcomes [[29], [30], [31]]. Recently, new findings from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial illustrated a J-curve phenomenon also in patients with atrial fibrillation under oral anticoagulation [32]. In this study, Dabigatran compared with warfarin was tested in 18,113 patients with non-valvular atrial fibrillation (6012 patients received dabigatran 110 mg, 6075 dabigatran 150 mg and 6020 warfarin). The median follow up was 2 years [32].
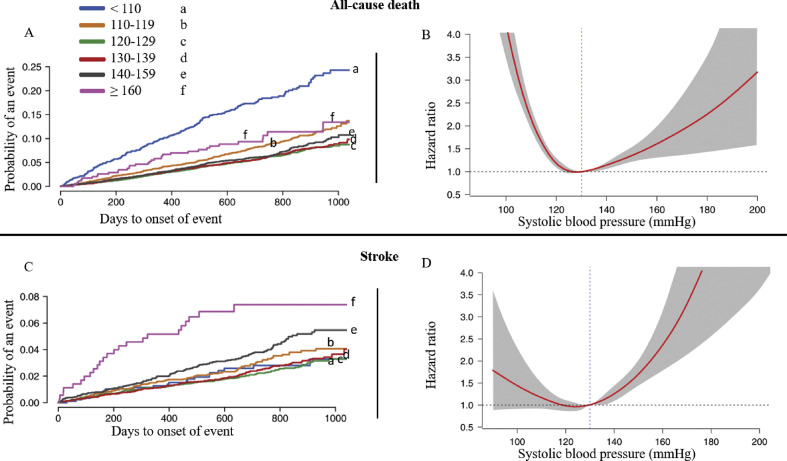
Compared with the reference range 120–129 mmHg, J-shaped associations were detectable. In patients with SBP of 140–160 mmHg the risk of mortality increased by 19% (HR 1.19; 95% CI 1.00 to 1.42) and in patients with SBP of 110–119 mmHg by 48% (HR 1.48; 95% CI 1.25 to 1.75) (Fig. 6 ) [32]. However, in patients with low SBP, the incidence of stroke and systemic embolism was not increased. On the other hand, in patients with SBP above the target range, the risk of systemic embolism increased as expected (HR 1.81; 95% CI 1.40 to 2.33) (Fig. 6) [32]. High dose dabigatran protected from systemic embolism compared with warfarin within the reference range (HR 0.54; 95% CI 0.35 to 0.85) and lower dose dabigatran protected from severe bleeding when compared with warfarin (HR 0.70; 95% CI 0.52 to 0.94). There was an increase in severe bleeding if SBP was <110 mmHg, compared with SBP within reference range (HR 2.17; 95% CI 1.7 to 2.75) [32].
Figure 6.
Blood pressure and complications in patients on therapeutic anticoagulation. A and C show the Kaplan–Meier event curves according to mean-achieved systolic blood pressure for all-cause death and stroke, respectively. B and D illustrate a J-shape associations between the mean-achieved systolic blood pressure and all-cause death and stroke, respectively [32].
Perspective
A J-shaped association between CVD and achieved SBP and DBP was demonstrated in anticoagulated patients with atrial fibrillation. Low SBP levels were associated with an increased risk of mortality, systemic embolism, and bleeding in patients with atrial fibrillation on oral anticoagulation and may identify patients at elevated risk.
Dietary approaches to treat hypertension
Effective lifestyle changes may be sufficient to delay or even prevent initiation of drug therapy in patients with grade 1 hypertension [2]. Lifestyle modification may also enhance the effect of BP-lowering therapy but should not delay the initiation of drug therapy in hypertensive patients at high cardiovascular risk. Hence, the guidelines of the ESC/ESH on hypertension management recommend lifestyle modifications, i.e. limiting salt intake, moderating alcohol consumption, high intake of vegetables and fruits, etc. [2].
In the crossover, parallel DASH (Dietary Approaches to Stop Hypertension)-Sodium trial, 412 adults with SBP/DBP 120–159/80-95 mmHg not taking antihypertensive medications were randomized to DASH or control diet [33]. Participants consumed each of the 3 sodium levels (low, medium, and high) for 4 weeks and received all meals but were free to drink calorie-free beverages (e.g., water). After each period, energy intake, weight, self-reported thirst, and 24-h urine volume were assessed. On both diets, weight was slightly but significantly reduced with low sodium intake in participants on DASH diet (p trend = 0.049) and in participants on control diet (p trend = 0.001). Furthermore, higher sodium levels were associated with more thirst (p trend 0.001 for both diets), whereas urine volume was higher in the control diet group (p trend = 0.007). Moreover, lower sodium intake decreased both office SBP and DBP regardless of diet (−3.1/-1.5 vs. −6.4/-3.2 mmHg, all p-trends <0.001). Additionally, sodium reduction did not increase energy requirements to maintain a stable weight, but decreased thirst (in both diets) and urine volume (control diet only), respectively [33].
Another randomized trial investigated the effect of a 6-month DASH-focused nutrition compared with a diet consistent with pediatric guideline recommendations established by the American National High Blood Pressure Education Program in 159 adolescents (aged 11–18) with newly diagnosed elevated BP or stage 1 hypertension [34]. To promote DASH adherence, participants were frequently counseled. The office SBP change was greater in participants with DASH diet than those in the control group −2.7 mmHg (95% CI -5.2 to −0.1; p = 0.03) and −1.7 mmHg (95% CI -4.2 to 0.9; p = 0.20) vs. −0.3 mmHg (95% CI -0.5 to 0.03) and −0.2 mmHg (95% CI -0.4 to −0.03) at 6 months and 18 months, respectively [34].
Perspective
Two recent DASH studies reiterated the traditional understanding of sodium physiology in BP regulation. Therefore, salt restriction and lifestyle modification remain effective options in treating hypertensive patients at low cardiovascular risk.
Diabetes and hypertension
Sodium glucose co-transporter 2 (SGLT2) inhibitors and Glucagon-like peptide-1 receptor agonists (GLP1-RA) are newer classes of antihyperglycemic drugs used for treating diabetes mellitus type 2 (T2DM) [35]. Moreover, regardless of the presence of diabetes, they appear to be advantageous in the treatment of CVD, especially heart failure [36].
Additionally, recent studies showed that SGLT2 inhibitors and GLP1-RAs exhibit BP lowering effects [37,38]. Thus, the 2019 European Guidelines on diabetes management recommend that reduction of BP should be considered while treating with GLP1-RAs and SGLT-2 inhibitors [35].
The BP-lowering effect of dapagliflozin was examined in a double blind, randomized trial, in which 85 patients with diabetes on mono- or combination therapy were randomized 1:1 to dapagliflozin or placebo for 12 weeks [39]. All participants underwent 24-h ABPM. The 24-h SBP change was significantly reduced in the dapagliflozin group but not in the placebo group (−5.8 ± 9.5 vs. 0.1 ± 8.7 mmHg, p = 0.005) [39].
In another study, the BP-lowering effect of dapagliflozin was compared with placebo in 1205 patients with heart failure and reduced ejection fraction from the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial [40]. The BP-lowering effect of dapagliflozin was examined considering office SBP as both a continuous and categorical variable. The placebo-corrected reduction in office SBP from baseline to 2 weeks with dapagliflozin was −2.54 mmHg (95% CI -3.33 to −1.76; p < 0.001). The between-treatment difference was greater in patients with highest compared with lowest SBP category [40].
Perspective
SGLT-2 inhibitors and GLP-1 RAs show BP-lowering effects. More randomized controlled trials should be conducted to further evaluate their role in hypertension therapy.
Device-based therapies – catheter-based renal denervation
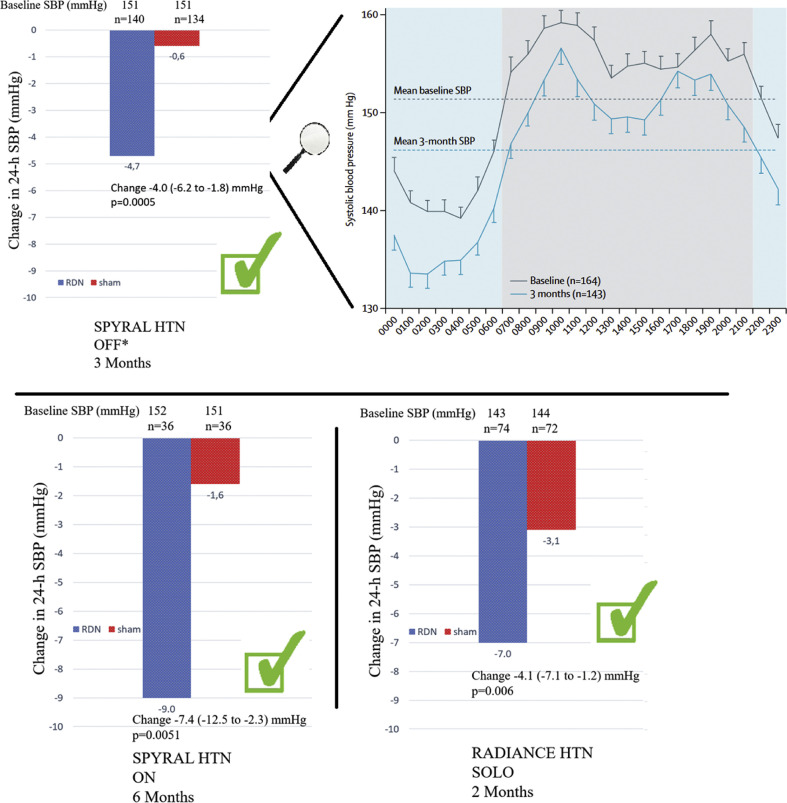
In 2020, the results of the SPYRAL HTN- OFF-MED Pivotal trial [41] were published. In this trial data from the pilot and pivotal trials were combined using a Bayesian design. The trial was powered for change in mean 24-h and office SBP between baseline and three months [41]. A total of 331 patients were randomized either to renal denervation (RDN) (n = 166) or sham (n = 165) treatment. While mean 24-h SBP did not change significantly in the sham group (−0.6 mmHg; 95% CI −2.1 to 0.9), there was a significant reduction in the RDN group (−4.7 mmHg; 95% CI −6.4 to −2.9). The primary endpoint, which was a baseline-adjusted change in mean 24-h SBP after 3 months, was reached. The between-group change of mean SBP was −3.9 mmHg (Bayesian 95% credible interval: −6.2 to −1.6). This study is one of three randomized, sham-controlled studies demonstrating that radiofrequency- (SPYRAL-HTN)[[41], [42], [43]] and ultrasound-based (RADIANCE-HTN) [43] RDN can safely and significantly reduce BP in patients with and without antihypertensive medication (Fig. 7 )[6, [41], [42], [43], [44], [45]].
Figure 7.
Change in 24-h systolic blood pressure after renal denervation. Three randomized, sham-controlled trials demonstrating that radiofrequency- (SPYRAL HTN OFF and SPYRAL HTN ON) [[41], [42], [43]] and ultrasound-based (RADIANCE-HTN) [43] renal denervation can significantly reduce systolic blood pressure. ∗ including the pilot trial, SBP: systolic blood pressure, 24-h SBP: 24-h systolic blood pressure.
A common feature of all studies in RDN is the high variability in treatment effects [46]. From this perspective, strategies to a priori identify patients with high likelihood of BP fall are of great importance [46]. The most recent studies on RDN did not include patients with severe (resistant) but mild to moderate hypertension. In this context, 24-h heart rate in the SPYRAL HTN-OFF MED trial [47] and baseline nighttime SBP including its variability in the DENER-HTN trial [48] predicted future BP reductions after RDN [49]. These parameters were also investigated in a post-hoc analysis of the RADIANCE-HTN SOLO study. The participants were not treated with concomitant antihypertensive therapy following ultra-sound RDN or sham [49]. The 24-h heart rate ≥73.5 bpm had insufficient predictive value, whereas nighttime SBP ≥136 mmHg and SBP variability of ≥12 mmHg had good specificity (>90%) but low sensitivity [49]. Furthermore, another study evaluated the changes in plasma renin activity and aldosterone after RDN in a total of 226 patients from the SPYRAL HTN-OFF MED trial. At baseline, there was no significant difference in the plasma renin activity between the RDN- and the control group (1.0 ± 1.1 vs. 1.1 ± 1.1 mg/mL/hour; p = 0.37) [50]. After 3 months, the change in plasma renin activity was significantly greater in the RDN group than in the control group (−0.2 ± 1.0; p = 0.019 vs. 0.1 ± 0.9 mg/mL/hour; p = 0.001 for RDN). Moreover, significant reductions in plasma aldosterone were observed. Despite similar baseline BP, a significant greater reduction of 24-h and office SBP at 3 months for RDN compared with control group was observed, in patients with higher baseline plasma renin activity (≥0.65 vs.<0.65 ng/mL/hour) [50].
New study results are expected shortly, RADIANCE HTN-TRIO (NCT02649426), REQUIRE (NCT02918305) and RADIANCE II (NCT03614260) are three studies focusing on ultrasound-based RDN. Chemical-mediated RDN with the injection of alcohol in the perivascular space of the renal arteries through microneedles is under investigation in the Target BP I (NCT02910414) and TARGET BP OFF-MED (NCT03503773) studies [51].
Not only in hypertension but also in other CVDs characterized by increased sympathetic activity, RDN may represent a therapy option, e.g., atrial fibrillation (SYMPLICITY-AF, NCT02064764), chronic kidney disease (RDN-CKD, NCT04264403), heart failure with reduced ejection fraction (RE-ADAPT-HF, NCT02085668), or congestive heart failure and renal failure (SYMPLICITY-HF, NCT01392196).
Perspective
The efficacy and safety of RDN has been demonstrated in several randomized controlled trials. Therefore, RDN should be considered as an additional or alternative treatment option, beside lifestyle modification and antihypertensive therapy, in selected patients with uncontrolled hypertension.
Declaration of competing interest
Felix Mahfoud is supported by Deutsche Gesellschaft für Kardiologie (DGK), and Deutsche Forschungsgemeinschaft (SFB TRR219) and has received scientific support and speaker honoraria from Bayer, Boehringer Ingelheim, Medtronic and ReCor Medical. Michael Böhm is supported by the Deutsche Forschungsgemeinschaft (German Research Foundation; TTR 219, project number 322900939) and reports personal fees from Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Servier, Medtronic, Vifor, Novartis and Abbott. The other authors have no conflict of interest.
Handling Editor: F. Galletti
References
- 1.Stanaway J.D., Afshin A., Gakidou E., Lim S.S., Abate D., Abate K.H., et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Stu. Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams B., Mancia G., De Backer G., Dominiczak A., Cifkova R., Fagard R., et al. ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of hypertension (ESH) and of the European society of Cardiology (ESC) Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. 2018. [DOI] [PubMed] [Google Scholar]
- 3.Wang C., Yuan Y., Zheng M., Pan A., Wang M., Zhao M., et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J Am Coll Cardiol. 2020;75:2921–2930. doi: 10.1016/j.jacc.2020.04.038. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz J.E., Muntner P., Kronish I.M., Burg M.M., Pickering T.G., Bigger J.T., et al. Reliability of office, home, and ambulatory blood pressure measurements and correlation with left ventricular mass. J Am Coll Cardiol. 2020;76:2911–2922. doi: 10.1016/j.jacc.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancia G., Facchetti R., Cuspidi C., Bombelli M., Corrao Giovanni, Grassi G. Limited reproducibility of MUCH and WUCH : evidence from the ELSA study. Eur Heart J. 2020;41:1565–1571. doi: 10.1093/eurheartj/ehz651. [DOI] [PubMed] [Google Scholar]
- 6.Al Ghorani H., Kulenthiran S., Lauder L., Böhm M., Mahfoud F. Hypertension trials update. J Hum Hypertens. 2021;35:398–409. doi: 10.1038/s41371-020-00477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kario K., Pickering T.G., Matsuo T., Hoshide S., Schwartz J.E., Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. doi: 10.1161/hy1001.092640. [DOI] [PubMed] [Google Scholar]
- 8.Ingelsson E., Björklund-Bodegård K., Lind L., Ärnlöv J., Sundström J. Diurnal blood pressure pattern and risk of congestive heart failure. J Am Med Assoc. 2006;295:2859–2866. doi: 10.1001/jama.295.24.2859. [DOI] [PubMed] [Google Scholar]
- 9.Kario K., Hoshide S., Mizuno H., Kabutoya T., Nishizawa M., Yoshida T., et al. Nighttime blood pressure phenotype and cardiovascular prognosis: practitioner-based nationwide JAMP study. Circulation. 2020;142:1810–1820. doi: 10.1161/CIRCULATIONAHA.120.049730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller Karsten, Stelzer Kathrin, Ostad M.A., Post F. Impact of exaggerated blood pressure response in normotensiveindividuals on future hypertension and prognosis: systematic reviewaccording to PRISMA guideline. Adv Med Sci. 2017;62:317–329. doi: 10.1016/j.advms.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Mariampillai J.E., Liestøl K., Kjeldsen S.E., Prestgaard E.E., Engeseth K., Bodegard J., et al. Exercise systolic blood pressure at moderate workload is linearly associated with coronary disease risk in healthy men. Hypertension. 2020;75:44–50. doi: 10.1161/HYPERTENSIONAHA.119.13528. [DOI] [PubMed] [Google Scholar]
- 12.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao C., Gao C., Cai Y., Cai Y., Zhang K., Zhang K., et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41:2058–2066. doi: 10.1093/eurheartj/ehaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albenmousa A.H., Idrees M.M. Characteristics and outcome of viral pneumonia caused by influenza and Middle East respiratory syndrome - coronavirus infections : a 4 - year experience from a tertiary care center. Ann Thorac Med. 2019;14:179–185. doi: 10.4103/atm.ATM_179_18. https://www.thoracicmedicine.org/text.asp?2019/14/3/179/261448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z., Chen Zuo, Zhang L., Wang X., Hao G., Zhang Z., et al. Status of hypertension in China results from the China hypertension survey, 2012–2015. Circulation. 2018;137:2344–2356. doi: 10.1161/CIRCULATIONAHA.117.032380. [DOI] [PubMed] [Google Scholar]
- 17.Li G., Li H., Lu J. No adequate evidence indicating hypertension as an independent risk factor for COVID - 19 severity. Clin Res Cardiol. 2021;110:146–147. doi: 10.1007/s00392-020-01653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaduganathan M., Vardeny Orly, Michel Thomas, Mcmurray J.J.V., Pfeffer MA, Ph D, et al. Renin – angiotensin – aldosterone system inhibitors in patients with covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kintscher U., Slagman A., Domenig O., Röhle R., Konietschke F., Poglitsch M., et al. Plasma angiotensin peptide profiling and ACE2-activity in COVID-19 patients treated with pharmacological blockers of the renin angiotensin system. Hypertension. 2020;76:e34–e36. doi: 10.1161/HYPERTENSIONAHA.120.15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giovanni de Simone . 2020. Position statement of the ESC council on hypertension on ACE-inhibitors and angiotensin receptor blockers.https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang [Google Scholar]
- 21.Trump S., Lukassen S., Anker M.S., Chua R.L., Liebig J., Thürmann L., et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nat Biotechnol. 2021;39:705–716. doi: 10.1038/s41587-020-00796-1. [DOI] [PubMed] [Google Scholar]
- 22.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/nejmoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., et al. Renin–angiotensin–aldosterone system inhibitors and risk of covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/nejmoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semenzato L., Botton J., Drouin J., Baricault B., Vabre C., Cuenot F., et al. Antihypertensive drugs and COVID-19 risk. Hypertension. 2021:833–842. doi: 10.1161/hypertensionaha.120.16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen J.B., Hanff T.C., William P., Sweitzer N., Rosado-Santander N.R., Medina C., et al. Continuation versus discontinuation of renin–angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9:275–284. doi: 10.1016/S2213-2600(20)30558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopes R.D., Macedo A.V.S., De Barros E Silva P.G.M., Moll-Bernardes R.J., Dos Santos T.M., Mazza L., et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA, J Am Med Assoc. 2021;325:254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benetos A., Bulpitt C.J., Petrovic M., Ungar A., Rosei E.A., Cherubini A., et al. An expert opinion from the european society of hypertension-European Union geriatric medicine society working group on the management of hypertension in very old, frail subjects. Hypertension. 2016;67:820–825. doi: 10.1161/HYPERTENSIONAHA.115.07020. [DOI] [PubMed] [Google Scholar]
- 28.Sheppard J.P., Burt J., Lown M., Temple E., Lowe R., Fraser R., et al. Effect of antihypertensive medication reduction vs usual care on short-term blood pressure control in patients with hypertension aged 80 Years and older: the OPTIMISE randomized clinical trial. JAMA, J Am Med Assoc. 2020;323:2039–2051. doi: 10.1001/jama.2020.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Böhm M., Schumacher H., Teo K.K., Lonn E., Mahfoud F., Mann J.F.E., et al. Achieved diastolic blood pressure and pulse pressure at target systolic blood pressure (120-140mmHg) and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Eur Heart J. 2018;39:3105–3114. doi: 10.1093/eurheartj/ehy287. [DOI] [PubMed] [Google Scholar]
- 30.Böhm M., Schumacher H., Teo K.K., Lonn E.M., Mahfoud F., Mann J.F.E., et al. Cardiovascular outcomes and achieved blood pressure in patients with and without diabetes at high cardiovascular risk. Eur Heart J. 2019;40:2032–2043. doi: 10.1093/eurheartj/ehz149. [DOI] [PubMed] [Google Scholar]
- 31.Böhm M., Schumacher H., Teo K.K., Lonn E.M., Mahfoud F., Mann J.F.E., et al. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Lancet. 2017;389:2226–2237. doi: 10.1016/S0140-6736(17)30754-7. [DOI] [PubMed] [Google Scholar]
- 32.Böhm M., Brueckmann M., Eikelboom J.W., Ezekowitz M., Fräßdorf M., Hijazi Z., et al. Cardiovascular outcomes, bleeding risk, and achieved blood pressure in patients on long-term anticoagulation with the thrombin antagonist dabigatran or warfarin: data from the RE-LY trial. Eur Heart J. 2020;41:2848–2859. doi: 10.1093/eurheartj/ehaa247. [DOI] [PubMed] [Google Scholar]
- 33.Juraschek S.P., Miller E.R., Chang A.R., Anderson C.A.M., Hall J.E., Appel L.J. Effects of sodium reduction on energy, metabolism, weight, thirst, and urine volume: results from the DASH (dietary approaches to Stop hypertension)-sodium trial. Hypertension. 2020:723–729. doi: 10.1161/HYPERTENSIONAHA.119.13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Couch S.C., Saelens B.E., Khoury P.R., Dart K.B., Hinn K., Mitsnefes M.M., et al. Dietary approaches to Stop hypertension dietary intervention improves blood pressure and vascular health in youth with elevated blood pressure. Hypertension. 2020:241–251. doi: 10.1161/HYPERTENSIONAHA.120.16156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cosentino F., Grant P.J., Aboyans V., Bailey C.J., Ceriello A., Delgado V., et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2019;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 36.McMurray J.J.V., Solomon S.D., Inzucchi S.E., Kober L., Kosiborod M.N., Martinez F.A., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 37.Tikkanen I., Narko K., Zeller C., Green A., Salsali A., Broedl U.C., et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–428. doi: 10.2337/dc14-1096. [DOI] [PubMed] [Google Scholar]
- 38.Marso S.P., Daniels G.H., Brown-Frandsen K., Kristensen P., Mann J., Nauck M.A., et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papadopouloua E., Loutradis C., Tzatzagou G., Kotsa K., Zografou I., Minopoulou I., et al. Dapagliflozin decreases ambulatory central blood pressure and pulse wave velocity in patients with type 2 diabetes: a randomized, double-blind, placebo- controlled clinical trial. J Hypertens. 2021:749–758. doi: 10.1097/HJH.0000000000002690. [DOI] [PubMed] [Google Scholar]
- 40.Serenelli M., Böhm M., Inzucchi S.E., Køber L., Kosiborod M.N., Martinez F.A., et al. Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA-HF) Eur Heart J. 2020;41:3402–3418. doi: 10.1093/eurheartj/ehaa496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Böhm M., Kario K., Kandzari D.E., Mahfoud F., Weber M.A., Schmieder R.E., et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–1451. doi: 10.1016/S0140-6736(20)30554-7. [DOI] [PubMed] [Google Scholar]
- 42.Kandzari D.E., Böhm M., Mahfoud F., Townsend R.R., Weber M.A., Pocock S., et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018 Jun 9;391(10137):2346–2355. doi: 10.1016/S0140-6736(18)30951-6. [DOI] [PubMed] [Google Scholar]
- 43.Böhm M., Townsend R.R., Kario K., Kandzari D., Mahfoud F., Weber M.A., et al. Rationale and design of two randomized sham-controlled trials of catheter-based renal denervation in subjects with uncontrolled hypertension in the absence (SPYRAL HTN-OFF MED Pivotal) and presence (SPYRAL HTN-ON MED Expansion) of antihypertensive medicat. Clin Res Cardiol. 2020;109:289–302. doi: 10.1007/s00392-020-01595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azizi M., Schmieder R.E., Mahfoud F., Weber M.A., Daemen J., Davies J., et al. Endovascular ultrasound renal denervation to treat hypertension ( RADIANCE-HTN SOLO ): a multicentre , international , single-blind , randomised , sham-controlled trial. Lancet. 2018;6736:1–11. doi: 10.1016/S0140-6736(18)31082-1. [DOI] [PubMed] [Google Scholar]
- 45.Azizi M., Schmieder R.E., Mahfoud F., Weber M.A., Daemen J., Lobo, et al. Six-month results of treatment-blinded medication titration for hypertension control after randomization to endovascular ultrasound renal denervation or a sham procedure in the RADIANCE-HTN SOLO trial. Circulation. 2019;139:2542–2553. doi: 10.1161/CIRCULATIONAHA.119.040451. [DOI] [PubMed] [Google Scholar]
- 46.Mahfoud F., Azizi M., Ewen S., Pathak A., Ukena C., Blankestijn P.J., et al. Proceedings from the 3rd European Clinical Consensus Conference for clinical trials in device-based hypertension therapies. Eur Heart J. 2020;41:1588–1599. doi: 10.1093/eurheartj/ehaa121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Böhm M., Mahfoud F., Townsend R.R., Kandzari D.E., Pocock S., Ukena C., et al. Ambulatory heart rate reduction after catheter-based renal denervation in hypertensive patients not receiving anti-hypertensivemedications: data from SPYRALHTN-OFFMED, a randomized, sham-controlled, proof-of-concept trial. Eur Heart J. 2019;40:743–751. doi: 10.1093/eurheartj/ehy871. [DOI] [PubMed] [Google Scholar]
- 48.Gosse P., Cremer A., Pereira H., Bobrie G., Chatellier G., Chamontin B., et al. Renal denervation twenty-four-hour blood pressure monitoring to predict and assess impact of renal denervation the DENERHTN study ( renal denervation for hypertension ) Hypertension. 2017;69:494–500. doi: 10.1161/HYPERTENSIONAHA.116.08448. [DOI] [PubMed] [Google Scholar]
- 49.Gosse P., Cremer A., Kirtane A.J., Lobo M.D., Saxena M., Daemen J., et al. Ambulatory blood pressure monitoring to predict response to renal denervation: a post hoc analysis of the RADIANCE-HTN SOLO study. Hypertension. 2021;77:529–536. doi: 10.1161/HYPERTENSIONAHA.120.16292. [DOI] [PubMed] [Google Scholar]
- 50.Mahfoud F., Townsend R.R., Kandzari D.E., Kario K., Schmieder R.E., Tsioufis K., et al. Changes in plasma renin activity after renal artery sympathetic denervation. J Am Coll Cardiol. 2021;77:2909–2919. doi: 10.1016/j.jacc.2021.04.044. [DOI] [PubMed] [Google Scholar]
- 51.Lauder L., Azizi M., Kirtane A.J., Böhm M., Mahfoud F. Device-based therapies for arterial hypertension. Nat Rev Cardiol. 2020;17:614–628. doi: 10.1038/s41569-020-0364-1. [DOI] [PubMed] [Google Scholar]