Abstract
Objectives
To evaluate the impact of neutralizing monoclonal antibody (mAb) treatment and to determine whether the selective pressure of mAbs could facilitate the proliferation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants with spike protein mutations that might attenuate mAb effectiveness.
Patients and methods
We evaluated the impact of mAbs on the nasopharyngeal (NP) viral load and virus quasispecies of mAb-treated patients using single-molecule real-time sequencing. The mAbs used were: Bamlanivimab alone (four patients), Bamlanivimab/Etesevimab (23 patients) and Casirivimab/Imdevimab (five patients).
Results
The NP SARS-CoV-2 viral load of mAb-treated patients decreased from 8.2 log10 copies/mL before administration to 4.3 log10 copies/mL 7 days after administration. Five immunocompromised patients given Bamlanivimab/Etesevimab were found to have mAb activity-reducing spike mutations. Two patients harboured SARS-CoV-2 variants with a Q493R spike mutation 7 days after administration, as did a third patient 14 days after administration. The fourth patient harboured a variant with a Q493K spike mutation 7 days post-treatment, and the fifth patient had a variant with a E484K spike mutation on day 21. The emergence of the spike mutation was accompanied by stabilization or rebound of the NP viral load in three of five patients.
Conclusion
Two-mAb therapy can drive the selection of resistant SARS-CoV-2 variants in immunocompromised patients. Patients given mAbs should be closely monitored and measures to limit virus spread should be reinforced.
Keywords: Coronavirus disease 2019, Neutralizing monoclonal antibodies, Mutations, Quasispecies, Receptor-binding domain, Severe acute respiratory syndrome coronavirus 2, Single-molecular real-time sequencing, Spike protein
Introduction
Neutralizing monoclonal antibodies (mAbs) are promising tools for protecting at-risk patients from a severe severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. They prevent the virus attaching to human angiotensin-converting enzyme 2 receptors and entering cells by targeting the receptor-binding domain of the virus spike protein [1]. The mAbs available in France were Bamlanivimab (LY-CoV555) until March 2021, then combinations of Bamlanivimab/Etesevimab (LYCoV016) and Casirivimab/Imdevimab (REGEN-COV) [2,3]. New SARS-CoV-2 variants containing key changes in the receptor-binding domain have recently appeared that can be more transmissible, more pathogenic, and resistant to endogenous or exogenous antibodies than the original virus [4,5]. The full spectrum of key spike mutations associated with resistance to mAbs is not yet established, but mutations K417N, E484D/K/Q, Q493R/K and S494P seem to be involved in virus escape and resistance to mAbs [[6], [7], [8]]. It is not clear how frequently these mutations occur in mAb-treated patients or how they influence virus clearance.
We evaluated the impact of neutralizing mAb therapy on the nasopharyngeal viral load and the diversity of the virus genome encoding the spike protein by single-molecule real-time (SMRT) sequencing.
Materials and methods
Nasopharyngeal samples (NPs) were taken from SARS-CoV-2-infected patients not requiring oxygen who were treated with neutralizing mAbs at the Toulouse University Hospital. These patients were immunosuppressed, aged over 80 years, or had a combination of risk factors for severe coronavirus disease 2019 (COVID-19). Bamlanivimab (700 mg) was used from 27 February to 15 March 2021, and Bamlanivimab/Etesevimab (700 mg/1400 mg) or Casirivimab/Imdevimab (1200 mg/1200 mg) from 16 March to 20 May 2021. NPs were collected before treatment (Day 0), 7 days after infusion (Day 7) and then weekly until the viral load reached the 31 cycle-threshold (Ct). The controls for solid organ transplant (SOT) patients were an historical control group of untreated SARS-CoV-2-infected SOT patients.
NP SARS-CoV-2 RNA was extracted with MGI Easy Nucleic Acid Extraction kits (MGI, Shenzhen, China) and quantified using the Ct values obtained with the TaqPath COVID-19 RT-PCR assay (Thermo Fisher Scientific, Waltham, MA, USA) and digital-droplet-RT-PCR (BioRad, Hercules, CA, USA). SARS-CoV-2 RNA from NPs with N gene values of <25 Ct was amplified and sequenced (PacBio SMRT; Pacific BioSciences, Menlo Park, CA, USA) (see Supplementary material, Appendix S1). Haplotypes were aligned on the reference genome (NC_045512.2) to detect mutations associated with reduced mAb activity [6,7].
Changes in the NP viral load of treated patients were compared using the Wilcoxon matched-pairs signed rank test. Differences between the viral loads of patients and controls were compared using the Mann–Whitney U test (GraphPad Prism 8.0; GraphPad Software, San Diego, CA, USA). These analyses were part of the national SARS-CoV-2 surveillance. French law (CSP Art.L1121-1.1) does not require institutional review board approval for anonymous retrospective studies.
Results
Patient characteristics
Thirty-two SARS-CoV-2-infected patients were treated with neutralizing mAbs at the Toulouse University Hospital (average age: 69 years; range: 23–95 years; 56% men). Seventeen of the 32 were immunocompromised, including 11 SOT patients (see Supplementary data, Table S1). Most patients (31; 97%) harboured the B.1.1.7 variant; one had the B.1, clade 20A. Four patients were given Bamlanivimab, 23 were given Bamlanivimab/Etesevimab, and five were given Casirivimab/Imdevimab.
Changes in NP viral load after mAb administration
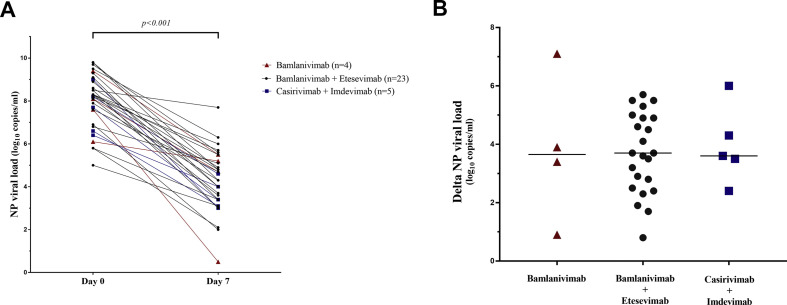
The RT-PCR N gene values became greater than 31 Ct a median of 21 days after treatment (range 7–28 days). The median SARS-CoV-2 viral load of mAb-treated patients decreased from 8.2 log10copies/mL (interquartile range (IQR), 7.1–9.0) on day 0 to 4.3 log10copies/mL (IQR, 3.4–5.2) on day 7 (p < 0.001) (Fig. 1 a). The viral loads of all three mAb groups decreased similarly: by 3.65 log10copies/mL (IQR, 1.53–6.30) for Bamlanivimab patients, 3.67 log10copies/mL (IQR, 2.40–4.90) for Bamlanivimab/Etesevimab patients and 3.60 log10copies/mL (IQR, 2.95–5.15) for Casirivimab/Imdevimab patients (Fig. 1b).
Fig. 1.
(a) Evolution of the viral load in nasopharyngeal (NP) samples from monoclonal antibody (mAb) -treated patients between days 0 and 7. The horizontal black line of the histogram indicates the median of difference (day 7 – day 0). Red: Bamlanivimab alone (n = 4), dark grey: Bamlanivimab/Imdevimab (n = 23) and blue: Casirivimab/Imdevimab (n = 5). (b) Differences in the viral load in NP samples from mAb-treated patients between days 0 and 7. The horizontal black line of the histogram indicates the median of difference (day 7 – day 0). Red: Bamlanivimab alone (n = 4), dark grey: Bamlanivimab/Etesevimab (n = 23) and blue: Casirivimab/Imdevimab (n = 5).
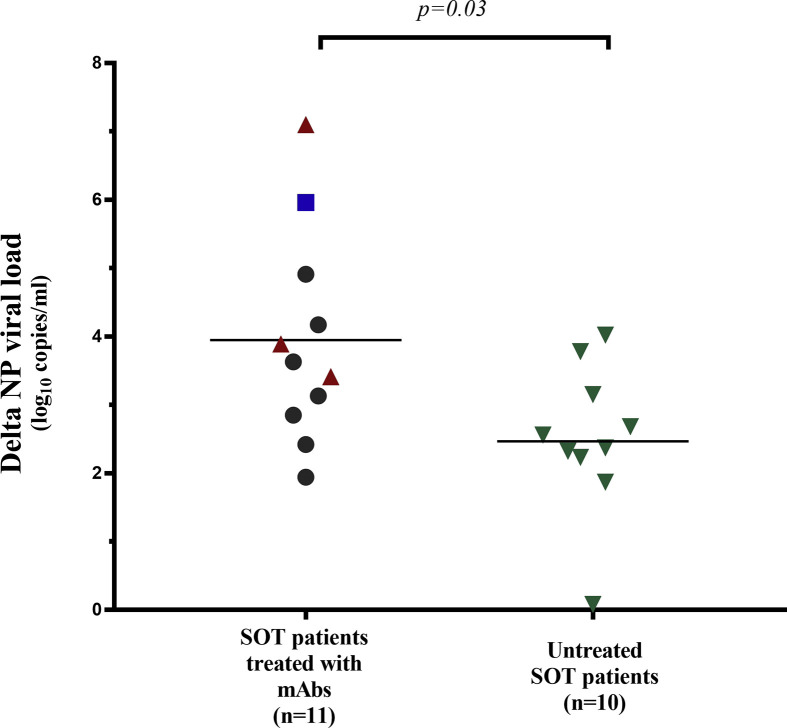
We compared the decreases in viral load of the 11 treated SOT patients and ten untreated SOT patients to determine the antiviral effect of mAb therapy in a homogeneous group. The median decreases in the NP viral load were: 3.63 log10copies/mL (IQR 2.85–4.91) for treated patients and 2.47 log10copies/mL (IQR 2.14–3.31) for controls (p = 0.03) (see Supplementary data ,Fig. S1).
SARS-CoV-2 quasispecies evolution after mAb administration
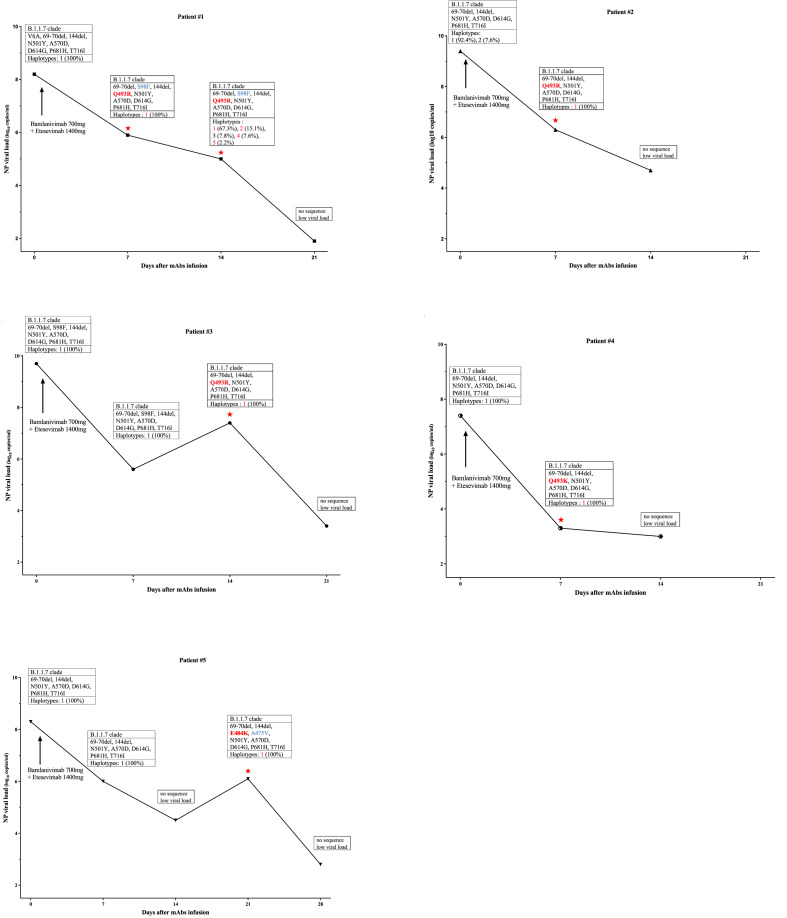
We followed up 23 (78%) of the mAb-treated patients with spike-sequencing. We detected no key mutation associated with reduced mAb activity in treated patients on day 0 (see Supplementary data, Table S2). However, several spike-haplotypes appeared over time in 2 (50%) Bamlanivimab-treated patients, 5 (31%) Bamlanivimab/Etesevimab-treated patients and one (33%) Casirivimab/Imdevimab-treated patient (see Supplementary data, Table S2). SARS-CoV-2 variants with mAb activity-reducing spike protein mutation appeared in five (31%) Bamlanivimab/Etesevimab-treated patients, all of whom were SOT patients (see Supplementary data, Appendix S2). A variant with the Q493R spike mutation was first detected in Patient#1 and Patient#2 on day 7 and in Patient#3 on day 14. The Q493K spike mutation was detected in Patient#4 on day 7 and a variant with the E484K spike mutation was detected in Patient#5 on day 21. The SARS-CoV-2 viral loads of these patients decreased over time, but the viral loads of Patient#3 and Patient#5 rebounded when the mutation was first detected; the load of Patient#1 first stabilized and then decreased (Fig. 2 ).
Fig. 2.
Changes in nasopharyngeal (NP) viral load, detection of spike protein mutations and SARS-CoV-2 genetic diversity in five Bamlanivimab/Etesevimab-treated patients. NP samples were analysed for: clade, spike protein mutations in the receptor binding domain, and percentage of haplotypes detected. Red stars indicate the appearance of key monoclonal antibody (mAb) activity-reducing mutations. Red: key mutation, blue: minor mutations.
Discussion
The impact of neutralizing anti-SARS-CoV-2 mAbs on the development of resistant variants is still unclear. Our analysis of the SARS-CoV-2 spike protein evolution in infected patients treated with mAbs indicated that key mAb activity-reducing mutations (Q493R/K, E484K) appeared in five SOT patients treated with Bamlanivimab/Etesevimab.
Previous in vitro studies showed that mAbs can induce the production of SARS-CoV-2 variants with mutation E484K and/or Q493R/K [[9], [10], [11]]. We have demonstrated that exposure to mAbs in vivo induces the emergence of variants harbouring these mutations in the spike receptor-binding domain of immunocompromised-patients 7–21 days after Bamlanivimab/Etesevimab treatment. The Q493R mutation has also been reported in one patient with cholangiocarcinoma [12].
A clinical study of Bamlanivimab and Bamlanivimab/Etesevimab (2800 mg/2800 mg) treatment found that new spike variants emerged in 11% of Bamlanivimab/Etesevimab-treated patients [13]. However, this study only looked for mutations E484K/Q, F490S and S494P. We found that a spike mutation in position 493 was selected in four mAb-treated patients. The high proportion of patients who developed key mutations could be a result of the lower dose of Bamlanivimab/Etesevimab (700 mg/1400 mg) used or of patient characteristics. Key mutations emerged in five of our mAb-treated SOT patients, suggesting that immunosuppression could restrict virus elimination, enhancing the risk of virus mutation under mAbs. Published clinical studies of Casirivimab/Imdevimab treatment have not looked for new treatment-induced variants [14] and we found no new key mutations in our Casirivimab/Imdevimab-treated patients.
The PacBio SMRT system has been invaluable for analysing virus diversity, as it provides all the haplotypes, enabling us to detect all variants, even minor ones. This is the first time this approach has been used to evaluate the impact of neutralizing anti-SARS-CoV-2 mAbs on a patient's virus population. We tracked the changes in viral load in addition to monitoring the emergence of new variants. Although we found that mAbs reduced the viral load of treated patients, this change did not occur in all the patients who harboured key mutations.
The limitation of this study is the small number of patients in each group, all of whom were treated at a single centre. However, we have shown that key mutations emerged in a substantial number of Bamlanivimab/Etesevimab-treated patients. Only five patients were treated with Casirivimab/Imdevimab and we detected no key mutation, which was in agreement with a recent study [15]. Although we had no control for all the patient categories, we could compare the decreases in the viral loads of mAb-treated and untreated SOT patients.
Our findings highlight the need for close virological monitoring of mAb-treated patients to limit the spread of SARS-CoV-2 variants.
Author contributions
CV and JI designed the study. GMB, PD, NK, ADB, AD, ZS and LT provided medical care to the participants and collected NPs; CV and NR collected biological data; NJ processed the data, CV analysed the results, prepared the figures and performed statistical analyses; CV and JI drafted the initial version of the paper. All the authors revised the manuscript and approved the final version.
Transparency declaration
The authors declare no conflict of interest. Funding was received from the Toulouse Institute for Infectious and Inflammatory Diseases (Infinity) INSERM UMR1291—CNRS UMR5051—Université Toulouse III and ANRS-MIE (Emergen, Quasicov study).
Acknowledgement
Dr Owen Parkes edited the English text.
Editor: L. Kaiser
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.09.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Table S1. Clinical characteristics of patients treated with monoclonal antibodies
Table S2. Changes in the SARS-CoV-2 quasispecies in monoclonal antibody-treated patients
Appendix S1. SARS-CoV-2 RNA sequencing
Appendix S2. Alignment of SARS-CoV-2 spike sequences from five patients
Fig. S1.
Differences in the viral loads (days 0 to 7) in NPs from untreated control solid organ transplant patients and from monoclonal antibody-treated solid organ transplant patients.
References
- 1.Tuccori M., Ferraro S., Convertino I., Cappello E., Valdiserra G., Blandizzi C., et al. Anti-SARS-CoV-2 neutralizing monoclonal antibodies: clinical pipeline. MAbs. 2020;12:1854149. doi: 10.1080/19420862.2020.1854149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Food Drug and Administration . US FDA; Washington, D.C.: 2021. Fact Sheet for Health Care Providers Emergency Use Authorization (Eua) of Bamlanivimab and Etesevimab; p. 34. [Google Scholar]
- 3.US Food Drug and Administration . US FDA; Washington, D.C.: 2021. Fact Sheet for Health Care Providers Emergency Use Authorization (Eua) of REGEN-COV (Casirivimab and Imdevimab) [Google Scholar]
- 4.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021:1–16. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 6.Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021 doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 7.Wang P., Casner R.G., Nair M.S., Wang M., Yu J., Cerutti G., et al. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe. 2021;29:747–751. doi: 10.1016/j.chom.2021.04.007. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starr T.N., Greaney A.J., Dingens A.S., Bloom J.D. Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016. Cell Rep Med. 2021;2:100255. doi: 10.1016/j.xcrm.2021.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baum A., Fulton B.O., Wloga E., Copin R., Pascal K.E., Russo V., et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369:1014–1018. doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C., et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. ELife. 2020;9 doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q., Nie J., Wu J., Zhang L., Ding R., Wang H., et al. SARS-CoV-2 501Y.V2 variants lack higher infectivity but do have immune escape. Cell. 2021;184:2362–2371. doi: 10.1016/j.cell.2021.02.042. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Focosi D., Novazzi F., Genoni A., Dentali F., Gasperina D.D., Baj A., et al. Emergence of SARS-COV-2 spike protein escape mutation Q493R after treatment for COVID-19. Emerg Infect Dis. 2021;27 doi: 10.3201/eid2710.211538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottlieb R.L., Nirula A., Chen P., Boscia J., Heller B., Morris J., et al. Effect of Bamlanivimab as monotherapy or in combination with Etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Copin R., Baum A., Wloga E., Pascal K.E., Giordano S., Fulton B.O., et al. The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies. Cell. 2021;184:3949–3961. doi: 10.1016/j.cell.2021.06.002. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical characteristics of patients treated with monoclonal antibodies
Table S2. Changes in the SARS-CoV-2 quasispecies in monoclonal antibody-treated patients
Appendix S1. SARS-CoV-2 RNA sequencing
Appendix S2. Alignment of SARS-CoV-2 spike sequences from five patients