Abstract
Background
Timely and accurate diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is crucial to reduce the risk of viral transmission. We investigated the diagnostic accuracy of rapid antigen detection tests (RADTs) in the diagnosis of SARS-CoV-2 infection.
Methods
A systematic literature search was performed using Pubmed, Embase, and the Cochrane Central Register. The sensitivity, specificity, diagnostic odds ratio (DOR), and a hierarchical summary receiver-operating characteristic curve (HSROC) of RADTs were pooled using meta-analysis. We used commercial and laboratory-developed reverse transcriptase-polymerase chain reaction (RT-PCR) as reference standards.
Results
We identified 24 studies comprising 14,188 patients. The overall pooled sensitivity, specificity, and DOR of RADTs for diagnosis of SARS-CoV-2 were 0.68 (95%CI, 0.59 – 0.76), 0.99 (95%CI, 0.99 – 1.00), and 426.70 (95% CI, 168.37 – 1081.65), respectively. RADTs and RT-PCR had moderate agreement with an estimated pooled Cohen's kappa statistic of 0.75 (95%CI, 0.74–0.77), and area under the HSROC of 0.98 (95%CI, 0.96 – 0.99). The pooled sensitivity of RADTs was significantly increased in subjects with viral load of Ct-value ≤25 or in those within 5 days after symptom onset than it was in subjects with lower viral loads or longer symptom duration.
Conclusions
The overall sensitivity of RADTs was inferior to that of the RT-PCR assay. The RADTs were more sensitive for samples of Ct-value ≤ 25 and might be suitable for subjects in the community within 5 days of symptom onset.
Keywords: SARS-CoV-2, COVID-19 testing, Diagnosis, Point-of-care testing, Antigens
Abbreviation list
- AUC,
area under the receiver-operating characteristic curve;
- CI,
confidence interval;
- COVID-19,
coronavirus disease 2019;
- Ct,
cycle threshold;
- DOR,
diagnostic odds ratio;
- ECDPC,
European center for Disease Prevention and Control;
- HSROC,
hierarchical summary receiver-operating characteristic curve;
- NAAT,
nucleic acid amplification test;
- NLR,
negative likelihood ratio;
- PLR,
positive likelihood ratio;
- QUADAS,
quality assessment of diagnostic accuracy studies;
- RADT,
rapid antigen detection test;
- RT-PCR,
reverse transcriptase-polymerase chain reaction;
- SARS-CoV-2,
severe acute respiratory syndrome coronavirus 2;
- WHO,
World Health Organization
1. Background
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused global health concerns since December 2019 [1, 2]. The rapid and accurate diagnosis of SARS-CoV-2 infection is very important to reduce the spread of the virus through patient management and isolation [3].
The current standard for detection of SARS-CoV-2 is viral RNA amplification testing such as reverse transcriptase-polymerase chain reaction (RT-PCR) [4]. Meanwhile, the COVID-19 pandemic has increased the demand for rapid, accurate, and convenient detection tests. The World Health Organization (WHO) and several countries have released guidelines for the use of rapid antigen detection tests (RADTs) [5], [6], [7]. These tests can be performed without a trained expert or specialized instrument and interpreted within 30 min [7]. RADTs as individual single-use tests would be useful to manage infection control during the COVID-19 pandemic [8]. Several commercially developed RADTs are available for diagnosis of SARS-CoV-2. However, data on their diagnostic performance have varied across studies [9].
2. Objectives
In this study, our objective was to evaluate the diagnostic accuracy of RADTs in the diagnosis of SARS-CoV-2 infection from respiratory tract specimens. We performed a systematic review and meta-analysis of diagnostic accuracy studies.
3. Study design
3.1. Data sources and search strategy
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses of Diagnostic Test Accuracy Studies statement [10]. We performed a comprehensive search of three electronic databases (Pubmed, Embase, and the Cochrane Central Register) through December 2020. The search terms included the following: ((("2019 nCoV" OR "2019nCoV" OR "2019 novel coronavirus" OR "COVID 19" OR "COVID19" OR "new coronavirus" OR "novel coronavirus" OR "novel corona virus" OR "SARS-CoV-2") OR (("Wuhan" AND ("coronavirus" OR "corona virus")) OR "severe acute respiratory syndrome coronavirus 2"))) AND (("rapid test*" OR "quick test*" OR "point-of-care") OR "antigen" OR ("STANDARD Q" OR "PANBIO") OR "COVID-19 testing"[MeSH terms]). Since this study was a systematic review of published articles, neither informed consent nor ethics approval was required. We also performed a manual search of the references listed in relevant review articles.
3.2. Study selection
We included studies that met the following inclusion criteria: (1) full-length reports published in peer-reviewed English language journals; (2) evaluated the performance of the RADTs for diagnosis of SARS-CoV-2 infection using respiratory samples compared to the reference standard; (3) included patients with suspected SARS-CoV-2 infection; and (4) provided sufficient data to calculate absolute numbers of true-positive, false-positive, false-negative, and true-negative results. Articles were excluded if they were review articles, case reports, commentaries, or studies reporting outcomes without raw data or peer review. The participant demographics and underlying diseases were not restricted.
The reference standard was either commercial or laboratory-developed RT-PCR. Positive results for RT-PCR were defined as a cycle threshold (Ct)-value < 40 for target genes, including the envelope gene (E) of Sarbecovirus, RNA-dependent RNA polymerase (RdRp), and nucleocapsid (N) genes of SARS-CoV-2. We allowed the following respiratory specimens: bronchoalveolar lavage, nasopharyngeal, nasal, oropharyngeal, and throat samples.
3.3. Data extraction and quality assessment
Two authors independently extracted all potentially relevant studies and reviewed each study according to the predefined eligibility criteria. After this review, the data were extracted. Any disagreements between the two authors during study selection or data extraction were resolved by discussion. A predefined form was used to extract the following data from each study: author, place of study, number of samples, the index test, comparison test, and specimen type(s). As recommended by the Cochrane Collaboration, we used the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 tool to assess the risk of bias in diagnostic test accuracy [11]. The studies were said to have a “low” risk of bias if the risk assessment was scored as "low" in the following four domains: patient selection, index test, reference standard, and flow and timing. If any domain was assessed to have a "high" risk of bias, or if two or more domains were considered as "unclear," then the study was classified at having a "high" risk of bias. If a study was assessed as being "unclear" in one of the four domains, the risk of bias was ranked as "unclear." Any discrepancies between the two authors were resolved by consensus.
3.4. Data synthesis and statistical analysis
For the diagnostic meta-analysis, we used the bivariate random-effects model to generate pooled estimates with 95% confidence intervals (CIs). We extracted the numbers of patients with true-positive, false-positive, false-negative, and true negative test results either directly or through a recalculation (based on the reported measures of accuracy and the prevalence and sample size of the included study).
The pooled sensitivity and specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), Cohen's kappa (κ), and area under the receiver-operating characteristic curve (AUC) were calculated by combining each study's results [12]. We also constructed hierarchical summary receiver-operating characteristic curves (HSROCs).
The current WHO emergency use listing for in vitro diagnostics detecting SARS-CoV-2 includes two RADTs: Panbio COVID-19 Ag Rapid Test Device (Abbott, Germany) and Standard Q COVID-19 Ag (SD Biosensor, South Korea), and we individually calculated the pooled results for the two tests [13]. We additionally performed subgroup analysis according to viral load (Ct-values ≤ 25 vs. > 25 and ≤ 30 vs. > 30), presence of symptoms, duration of symptoms, and age group. Publication bias was assessed using the Deeks’ funnel plot, with statistical significance being evaluated based on Deeks’ asymmetry test [14].
A P value < 0.05 was considered statistically significant. The statistical analyses were performed using Stata statistical software (Version 14.2, Stata Corp LP, College Station, TX, USA) and Review Manager (Version 5.3, Nordic Cochrane center, The Cochrane Collaboration, Copenhagen, Denmark).
4. Results
4.1. Study search and characteristics and quality of included studies
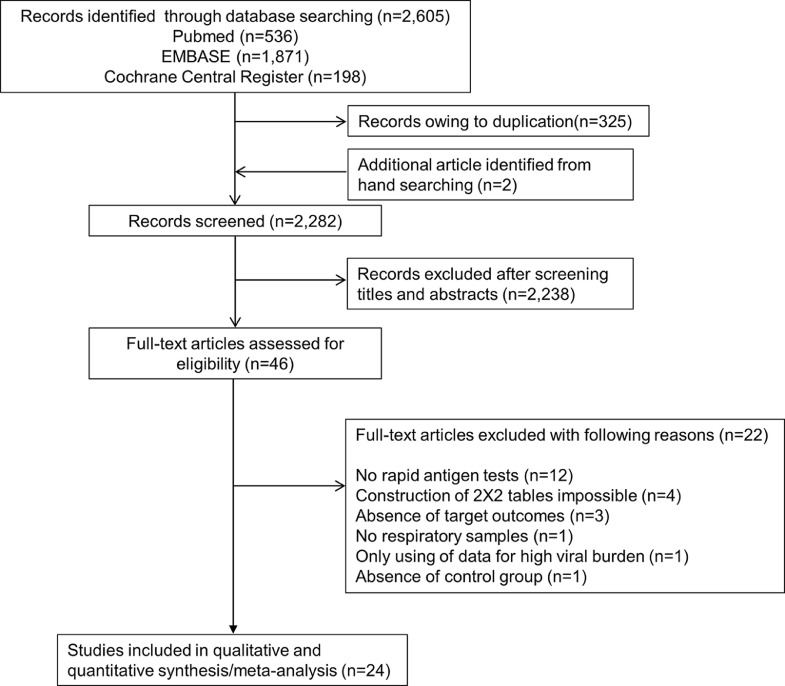
The literature search process is shown in Fig. 1 . We initially identified 536 articles from Pubmed, 1871 articles from EMBASE, 198 articles from the Cochrane library, and two additional articles from hand-searching. After removing duplicate articles, we screened 2282 potentially eligible articles. After reviewing the title and abstracts, 2238 search records were removed. The remaining 46 articles were eligible for reading the full text. Twenty-two articles were excluded for the reasons shown in Fig. 1. After qualitative and quantitative syntheses, 24 studies were included in our final analysis [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38].
Fig. 1.
Flow diagram for identification of eligible studies.
Table 1 summarizes the features of the included studies. Twenty-four studies involving 14,188 subjects met the defined inclusion criteria. The number of patients in each trial ranged from 19 to 3410. For QUADAS assessment, we only judged one study to be at low risk of bias. In contrast, the risk of bias in most studies was unclear or high because of insufficient reporting. We also had concerns about the applicability of the results across the studies included in the analysis.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Study | Country | Number of samples | Index test | Comparison test (RT-PCR) | Type of specimens | Quality assessment | |||
|---|---|---|---|---|---|---|---|---|---|
| Test name | Manufacturer | Test name | Manufacturer | Risk of bias | Concern regarding applicability | ||||
| Agulló [15] | Spain | 1311 | Panbio COVID-19 Ag rapid test device | Abbott, USA | Cobas Z 480 Real-Time PCR Analyzer | Roche, Switzerland | Nasal, saliva | High | Low |
| Albert [16] | Spain | 412 | Panbio COVID-19 Ag rapid test device | Abbott, USA | TaqPath COVID-19 Combo Kit | Thermo Fisher Scientific, USA | NP | Unclear | Low |
| Beck [17] | USA | 346 | Sofia SARS Ag assay | Quidel, USA | Hologic Aptima SARS-CoV-2 transcription-mediated amplification test | Hologic, USA | NP | High | Low |
| Cerutti [18] | Italy | 330 | Standard Q COVID-19 Ag test | SD-Biosensor, South Korea | Allplex 2019 n-CoV Assay, DiaSorin Simplexa, and Cobas 6800 | Seegene, South Korea; Diasorin, Italy; and Roche, Switzerland | NP | High | High |
| Chaimayo [19] | Thailand | 454 | Standard Q COVID-19 Ag test | SD-Biosensor, South Korea | Allplex 2019 n-CoV Assay | Seegene, South Korea | NP, Throat, endotracheal aspirates | High | Low |
| Diao [20] | China | 251 | Laboratory developed test | N/A, China | TaqMan One-Step RT-PCR Kit | Da An Gene, China | NP | Low | Low |
| Fenollar [21] | France | 341 | Panbio COVID-19 Ag rapid test device | Abbott, USA | VitaPCR SARS-Cov-2 assay | Credo Diagnostics, Singapore | NP | High | Low |
| Hirotsu [22] | Japan | 313 | Lumipulse SARS-CoV-2 Ag kit | Fujirebio, Japan | StepOnePlus Real- Time PCR System | Thermo Fisher Scientific, USA | NP | High | High |
| Krüttgen [23] | Germany | 150 | SARS-CoV-2 Rapid Antigen Test | Roche, Switzerland | Real Star SARS-CoV-2 RT PCR Kit | Altona, Germany | NP | High | High |
| Lambert-Niclot [24] | France | 138 | COVID-19 Ag Respi-strip | Coris BioConcept, Belgium | Real Star SARS-CoV-2 RT PCR Kit, Bosphore novel coronavirus (2019-nCoV) detection kit, and Cobas 6800 | Altona, Germany; Anatolia Geneworks, Turkey; and Roche, Switzerland | NP | High | High |
| Lanser [25] | Austria | 53 | Panbio COVID-19 Ag rapid test device | Abbott, USA | Cobas analyzer | Roche, Switzerland | 53 | High | Low |
| Linares [26] | Spain | 255 | Panbio COVID-19 Ag rapid test device | Abbott, USA | Allplex 2019 n-CoV Assay | Seegene, South Korea | NP | High | Low |
| Liotti [27] | Italy | 359 | Standard Q COVID-19 Ag test | SD Biosensor, South Korea | Real Star SARS-CoV-2 RT PCR Kit, Allplex 2019 n-CoV Assay, DiaSorin Simplexa, and Cobas 6800 | Altona, Germany; Seegene, South Korea; Diasorin, Italy; and Roche, Switzerland | NP | High | High |
| Mertens [28] | Belgium | 328 | COVID-19 Ag Respi-strip | Coris BioConcept, Belgium | Real Star SARS-CoV-2 RT PCR Kit, StepOnePlus Real-Time PCR System, and Panther Fusion Assay | Altona, Germany; Thermo Fisher Scientific, USA; and Hologic, USA | NP, Broncho-Alveolar Lavage | High | Low |
| Nalumansi [29] | Uganda | 262 | Standard Q COVID-19 Ag test | SD-Biosensor, South Korea | Applied Biosystems PCR platform | Thermo Fisher Scientific, USA | NP | High | Low |
| Pilarowski [30] | USA | 3302 | BinaxNOW COVID-19 Ag Card | Abbott, USA | RenegadeXP test | Renegade.bio, USA | Nasal | High | Low |
| Porte [31] | Chile | 127 | SARS-CoV-2 Ag test | Bioeasy Biotechnology, China | COVID- 19 Genesig Real-Time PCR assay | Primerdesign Ltd, UK | NP and OP | High | High |
| Pray [32] | USA | 1098 | Sofia SARS Ag assay | Quidel, USA | TaqPath COVID-19 Combo Kit | Thermo Fisher Scientific, USA | Nasal | High | Low |
| Scohy [33] | Belgium | 148 | COVID-19 Ag Respi-strip | Coris BioConcept, Belgium | COVID- 19 Genesig Real-Time PCR assay | Primerdesign Ltd, UK | NP | High | Low |
| Strömer [34] | Germany | 134 | Nadal COVID-19 Ag test | Nal von Minden GmbH, Germany | Laboratory-developed triplex RT-PCRs | N/A | NP | High | High |
| Toptan [35] | Germany | 67 | RIDA®QUICK SARS-CoV-2 Ag test | R-Biopharm, Germany | Luna Universal One-Step RT-qPCR Kit | New England Biolabs | Respiratory samples | High | High |
| Turcato [36] | Italy | 3410 | Standard Q COVID-19 Ag test | SD-Biosensor, South Korea | NA | NA | NP | High | High |
| Weitzel [37] | Chile | 348 | [A] Biocredit One Step SARS-CoV-2 Ag test, [B] StrongStep COVID-19 Ag test, [C] Huaketai SARS-CoV-2 N Protein Detection Kit, and [D] Diagnostic Kit for 2019-Novel Coronavirus Ag test | [A] RapiGen, South Korea, [B] Liming Bio-Products, China, [C] Savant Biotechnology, China, and [D] Bioeasy Biotechnology, China | NA | NA | NP, OP, Throat, and sputum | Unclear | Low |
| Young [38] | USA | 251 | Veritor System for Rapid Detection of SARS-CoV-2 | BD, USA | Lyra SARS-CoV-2 assay | Quidel, USA | Nasal | High | Low |
Ag, Antigen; COVID-19, coronavirus disease 2019; NA, not available; NP, nasopharyngeal; OP, oropharyngeal; RT-PCR, reverse-transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
4.2. Diagnostic accuracy of RADTs in the diagnosis of SARS-CoV-2 infection
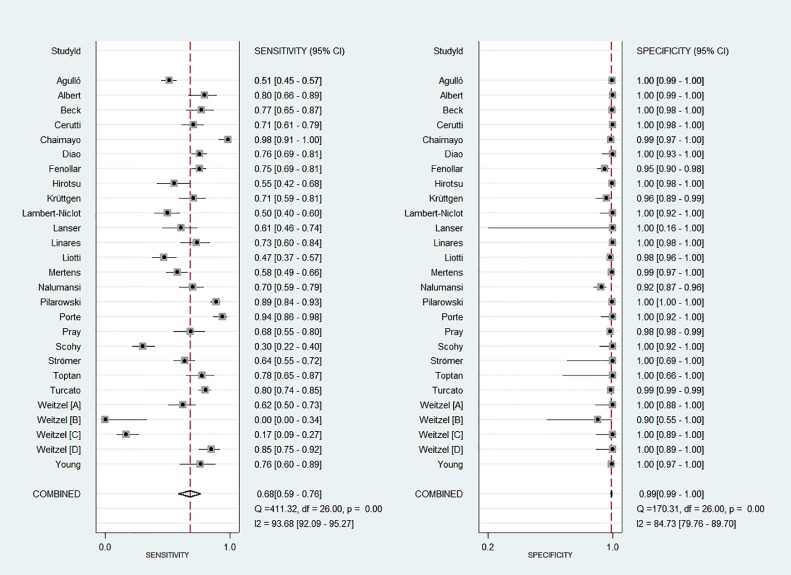
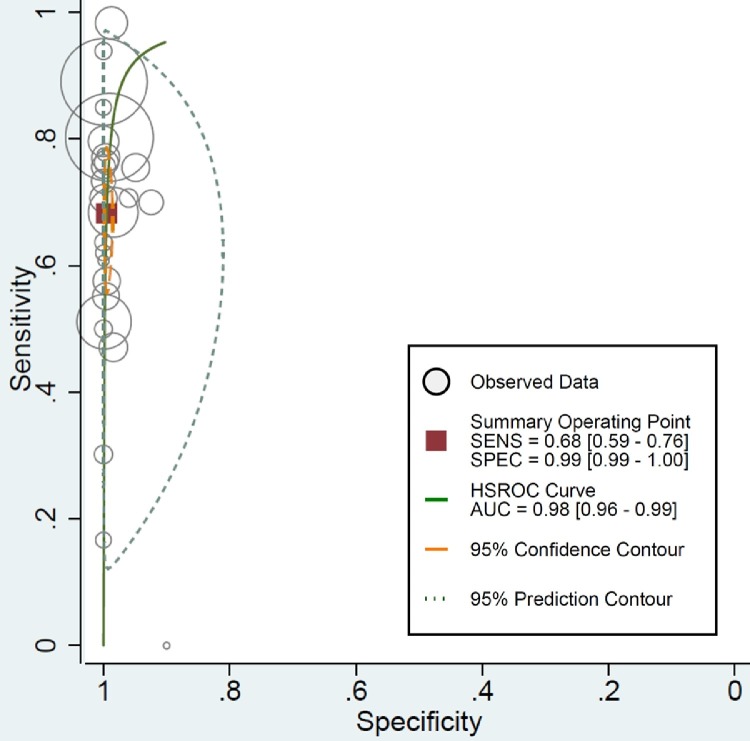
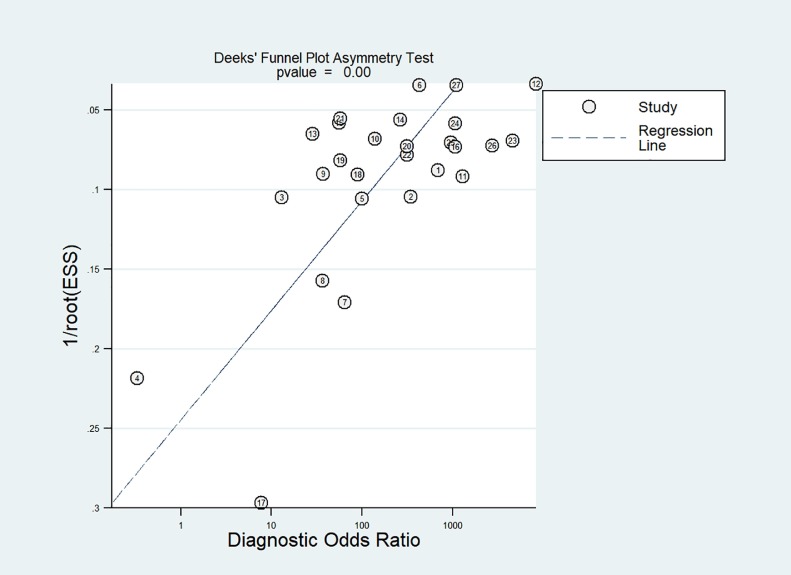
Fig. 2 shows paired forest plots of the sensitivity and specificity of RADTs in the diagnosis of SARS-CoV-2 infection. The pooled sensitivity across the studies was 0.68 (95%CI, 0.59 – 0.76). The pooled specificity was 0.99 (95%CI, 0.99 – 1.00). The pooled PLR and NLR were 136.02 (95%CI, 59.67 – 310.09) and 0.32 (95%CI, 0.24 – 0.42), respectively. The DOR for RADTs was 426.70 (95%CI, 168.37 – 1081.65). There was moderate agreement between RADTs and RT-PCR in all included studies, with an estimated pooled Cohen's kappa (κ) statistic of 0.75 (95%CI, 0.74 – 0.77). The AUC of the index tests was 0.98 (95%CI, 0.96 – 0.99, Fig. 3 ). Deeks’ funnel plot and the results of regression test for asymmetry of the included studies indicated significant publication bias (P = 0.005, Fig. 4 ).
Fig. 2.
Paired forest plots of sensitivity and specificity of rapid antigen detection tests in diagnosis of SARS-CoV-2.
Fig. 3.
Hierarchical summary receiver operating characteristic curves for rapid antigen detection tests in diagnosis of SARS-CoV-2.
Fig. 4.
Funnel plot of publication bias of the included studies.
We examined two RADTs corresponding to an emergency use listing that was recommended by the WHO for in vitro diagnostics identifying SARS-CoV-2 infection. For the Panbio COVID-19 Ag rapid test device [15, 16, 21, 25, 26], the pooled sensitivity across the studies was 0.68 (95%CI, 0.58 – 0.77), while the pooled specificity was 1.00 (95%CI, 0.97 – 1.00). The pooled PLR and NLR were 468.04 (95%CI, 22.92 – 9558.01) and 0.32 (95%CI, 0.24 – 0.43), respectively. The pooled DOR was 1464.64 (95%CI, 72.33 – 29,658.73). For the Standard Q COVID-19 Ag test [18, 19, 29, 36], the pooled sensitivity across studies was 0.83 (95%CI, 0.63 – 0.94), while the pooled specificity was 0.99 (95%CI, 0.95 – 1.00). The pooled PLR and NLR were 66.69 (95%CI, 16.99 – 261.75) and 0.17 (95%CI, 0.07 – 0.41), respectively. The pooled DOR was 397.14 (95%CI, 66.15 – 2384.50).
4.3. Subgroup analysis
Table 2 summarizes the results of subgroup analyses for the pooled sensitivity of the included studies. We first investigated the relationship between the SARS-CoV-2 viral load (as Ct values determined by viral RNA amplification tests) and the positive results of RADTs. We extracted sensitivity data associated with viral loads from 16 studies of RADTs [15, 16, 18, 21, [23], [24], [25], [26], [27], [28], [29], [30], [31], 33, 34, 37]. In 15 evaluations of the 13 studies, we extracted data according to Ct-values ≤ 25 and >25 [15, 18, 21, [23], [24], [25], [26], [27], [28], 31, 33, 34, 37]. Data extracted from the 12 studies of Ct-values ≤ 30 and >30 were evaluated [15, 16, 18, 21, [23], [24], [25], [26], 29, 30, 33, 34]. Nine studies included all data for Ct-values ≤ 25, >25, ≤30, and >30 [15, 18, 21, [23], [24], [25], [26], 33, 34].
Table 2.
Subgroup analysis for the pooled sensitivity of rapid antigen tests according to study design.
| Variable | No. of studies | No. of patients | Sensitivity (95% CI) | P value |
|---|---|---|---|---|
| Viral load | ||||
| 1. Ct ≤ 25 | 13 | 676 | 0.94 (0.84–0.98) | <0.001 |
| Ct > 25 | 13 | 821 | 0.38 (0.29–0.48) | |
| 2. Ct ≤ 30 | 12 | 873 | 0.84 (0.77–0.93) | <0.001 |
| Ct > 30 | 12 | 447 | 0.30 (0.17–0.48) | |
| Presence of symptoms | ||||
| Symptomatic | 15 | 1531 | 0.72 (0.57–0.83) | 0.220 |
| Asymptomatic | 9 | 314 | 0.52 (0.36–0.67) | |
| Duration of symptoms | ||||
| ≤5 days | 5 | 234 | 0.87 (0.82–0.91) | 0.003 |
| >5 days | 5 | 88 | 0.73 (0.62–0.82) | |
| Age groups | ||||
| Adult | 3 | 299 | 0.86 (0.82–0.90) | <0.001 |
| Child | 3 | 83 | 0.52 (0.41–0.63) |
Ag, antigen; CI, confidence interval; Ct, cycle threshold.
In Ct-values ≤ 25, the pooled sensitivity of RADTs was 0.94 (95%CI, 0.84 – 0.98) compared with RT-PCR, while the pooled sensitivity declined when the Ct-value was ≤30 (0.84; 95%CI, 0.77 – 0.93). For samples with Ct-values > 25 and Ct > 30, the pooled sensitivity was largely reduced to 0.38 (95%CI, 0.29 – 0.48) and 0.30 (95%CI, 0.17 – 0.48), respectively.
The pooled sensitivity of the RADTs in patients with COVID-19 symptoms tended to be higher than in those without symptoms, although there were no significant differences (0.72; 95%CI, 0.57 – 0.83 and 0.52; 95%CI, 0.36 – 0.67, respectively; P = 0.220). There were substantial differences in pooled sensitivity between symptom duration ≤5 days and > 5 days (0.87; 95%CI, 0.82 – 0.91 and 0.73; 95%CI, 0.62 – 0.82, respectively; P = 0.003). Adults had a significant association with an increased rate of pooled sensitivity compared to children (0.86; 95%CI, 0.82 – 0.90 and 0.52; 95%CI, 0.41 – 0.63, respectively; P < 0.001).
5. Discussion
In this study, we compared the diagnostic accuracy of RADTs with that of RT-PCR for the diagnosis of SARS-CoV-2 using a systemic review and meta-analysis approach. We found that RADTs and RT-PCR had moderate agreement (estimated pooled Cohen's kappa statistic of 0.75). The RADTs had overall low sensitivity of 0.68 compared to RT-PCR. The pooled sensitivity further declined to 0.30 when the Ct-value was > 30, indicating that the RADTs have high rates of false negative results when the subjects have low viral load.
The WHO currently recommends RADTs that meet the minimum performance requirements of ≥80% sensitivity and ≥97% specificity compared with that of molecular testing, while the European center for Disease Prevention and Control suggests the use of tests with performance closer to RT-PCR, i.e., ≥90% sensitivity and ≥97% specificity [7, 39]. However, because the findings of our study showed lower overall sensitivity of RADTs than that recommended by the WHO and the ECDPC, the usefulness of RADTs as a replacement for molecular testing seemed to be limited.
We included several types of RADTs whose sensitivity varied largely across studies (from 0% to 98%). This method might have caused heterogeneity in the diagnostic availability of the overall RADTs. Recently, the WHO announced an emergency use listing for in vitro diagnostics to detect SARS-CoV-2 [13]. Two RADTs, the Panbio COVID-19 Ag rapid test device and Standard Q COVID-19 Ag test, were included in the WHO statements [13]. We found that the pooled sensitivity of the Standard Q COVID-19 Ag test was relatively higher than that of the overall RADTs. In contrast, the diagnostic performances of the Panbio COVID-19 Ag rapid test device were similar to that of overall RADTs. However, because of data limitations, we could not investigate the differences of baseline characteristics among the groups. Therefore, we could not conclude the superiority of specified commercial RADTs.
Large heterogeneities generally are reported in systematic reviews of studies on diagnostic test accuracy, and one purpose of our study was to examine the evidence of heterogeneity among studies [40]. We tried to explain the heterogeneity using subgroup analysis. There are several possible sources of this heterogeneity. First, the low overall sensitivity of RADTs might be caused by differences in the diagnostic availability of tests related to viral load. RADTs have been used to identify other viruses such as influenza virus and respiratory syncytial virus [41, 42]. However, previous studies have found that RADTs had relative low sensitivity in the diagnosis of other viruses and were influenced by factors related to viral load [41, 42]. Similarly, in samples with a Ct-value ≤ 25 or ≤30, the pooled sensitivities of the tests were 0.94 and 0.84, respectively. In contrast, the pooled sensitivities of RADTs in samples with Ct > 25 or Ct > 30 were 0.38 and 0.30, respectively.
Second, the presence of symptoms was considered a possible factor of heterogeneity. There have been conflicting data on the association between the presence of symptoms and Ct-values [43], [44], [45]. While some reports have suggested that asymptomatic patients with SARS-CoV-2 infection have a lower viral load than do symptomatic patients, others showed similar viral load between asymptomatic and symptomatic patients [43], [44], [45]. This discrepancy indicates the possibility of infectivity in asymptomatic or pre-symptomatic patients [44]. Our findings revealed that asymptomatic patients tended to show relatively low sensitivity of 0.52 in the detection of SARS-CoV-2.
Third, the duration of symptoms after onset was considered another factor in study heterogeneity. The RNA concentrations of viral load peak within 5 days of the onset of symptoms [46]. A recent systematic review also suggested that the viral load of SARS-CoV-2 from upper respiratory tract specimens peaked around the time of symptom onset or a few days after [47]. In our pooled analysis of patients with duration of symptoms within 5 days, the sensitivity of RADTs increased to 0.87. In contrast, these tests might not be useful in asymptomatic patients based on a low sensitivity of 0.52. On the basis of our findings, we suggest that RADTs will provide satisfactory sensitivity in community subjects with early onset of symptoms during the pandemic period. However, we believe that the performance of rapid molecular tests is more reasonable in specific situations that require precise test results, such as emergency pre-operative/-interventional screening, inpatient admission for other diseases, or identification of symptomatic medical workers [48].
Fourth, the pooled sensitivity of RADTs in the diagnosis of SARS-CoV-2 was lower in children than it was in adults in this study. Previous studies comparing the RNA load of SARS-CoV-2 RNA across age groups have reported inconsistent results [49]. Meanwhile, recent epidemiological data revealed that children have a lower susceptibility to the virus than do adults. Children also do not seem to be major sources of viral transmission [49, 50]. We considered the possibility that the studies included in our analysis had a high proportion of children with low viral load. This might lead to lower overall sensitivity in the present study.
Finally, the impact of training for testing can be an issue. Previous studies have reported that the diagnostic accuracy of the RADTs was lower when performed by an untrained individual or in a home setting compared with healthcare professionals [51, 52]. We could not investigate the performances of the RADTs related to this factor due to the limited data.
Considering the overall low sensitivity of RADTs, future research should focus on the development of novel RADTs and the strategies for their performances. For example, lateral flow devices are a new form of testing that detect SARS-CoV-2 viral antigens by immunoassays [51]. The UK COVID-19 Lateral Flow Oversight group suggested that lateral flow devices had promising performance characteristics for mass population testing [51]. Various strategies are required to improve the sensitivity of RADTs, such as serial sampling, digital results, and enhanced training [51].
This study has some limitations. First, the studies included in our analysis had low methodological quality and significant risk of publication bias. Therefore, our results should be interpreted carefully. Second, we used various types of RADTs and RT-PCR assays. We also handled multiple kinds of repository samples without distinction. These factors might have introduced bias. Third, even though RT-PCR tests are regarded as the standard for detecting SARS-CoV-2, their sensitivity can be characterized poorly due to the lack of an international standard or difficulties in appropriate timing of testing in asymptomatic subjects for optimal sensitivity [53]. Especially, a recent pooled analysis revealed that the possibility of false negative results for RT-PCR increased in the early course of infection [54]. Depending on the low sensitivity of RT-PCR, the sensitivity of RADTs should be considered together.
6. Conclusions
We demonstrated that RADTs have good specificity in the diagnosis of SARS-CoV-2 in respiratory samples but low sensitivity (of 0.68) compared to those of RT-PCR. Therefore, when RADTs produce negative results, they should be coupled to confirmatory tests in situations that require accurate test results. The test was more sensitive in patients with viral load of Ct-value ≤ 25 and might be useful for patients within 5 days of symptoms onset (when the viral load in the upper respiratory tract is at its peak). Considering that RADTs are individual use tests that are rapid and easy to use, they can be used carefully in community patients with early onset of symptoms.
Funding
The 2021 scientific promotion program was funded by Jeju National University.
CRediT authorship contribution statement
Jonghoo Lee: Conceptualization, Data curation, Formal analysis, Writing – review & editing. Jae-Uk Song: Data curation, Formal analysis. Sung Ryul Shim: Data curation, Formal analysis.
Declaration of Competing Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Y., Ho W., Huang Y., Jin D.Y., Li S., Liu S.L., Liu X., Qiu J., Sang Y., Wang Q., Yuen K.Y., Zheng Z.M. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet. 2020;395:949–950. doi: 10.1016/S0140-6736(20)30557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rong X.M., Yang L., Chu H.D., Fan M. Effect of delay in diagnosis on transmission of COVID-19. Mathematical biosciences and engineering. Math Biosci. Eng. 2020;17:2725–2740. doi: 10.3934/mbe.2020149. [DOI] [PubMed] [Google Scholar]
- 4.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro surveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World health organization, Coronavirus Disease (COVID-19) Pandemic — Emergency Use Listing Procedure (EUL) Open for IVDs, Available from: https://extranet.who.int/pqweb/vitro-diagnostics/coronavirus-disease-covid-19-pandemic-%E2%80%94-emergency-use-listing-procedure-eul-open, 2021 (accessed 19 Aug 2021).
- 6.US Centers for disease control and prevention, Interim Guidance for Rapid Antigen Testing for SARS-CoV-2, Available from: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html, 2021 (assessed 19 Aug 2021). .
- 7.European centre for disease prevention and control, options for the use of rapid antigen tests for COVID-19 in the EU/EEA and the UK, Available from: https://www.ecdc.europa.eu/en/publications-data/options-use-rapid-antigen-tests-covid-19-eueea-and-uk, 2021 (assessed 19 Aug 2021).
- 8.Mak G.C.K., Lau S.S.Y., Wong K.K.Y., Chow N.L.S., Lau C.S., Lam E.T.K., Chan R.C.W., Tsang D.N.C. Evaluation of rapid antigen detection kit from the WHO emergency use list for detecting SARS-CoV-2. J. Clin. Virol. 2020;134 doi: 10.1016/j.jcv.2020.104712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinnes J., Deeks J.J., Adriano A., Berhane S., Davenport C., Dittrich S., Emperador D., Takwoingi Y., Cunningham J., Beese S., Dretzke J., Ferrante di Ruffano L., Harris I.M., Price M.J., Taylor-Phillips S., Hooft L., Leeflang M.M., Spijker R. Van den Bruel A, rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2020;8 doi: 10.1002/14651858.CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McInnes M.D.F., Moher D., Thombs B.D., McGrath T.A., Bossuyt P.M., The PRISMA-DTA Group. Clifford T., Cohen J.F., Deeks J.J., Gatsonis C., Hooft L., Hunt H.A., Hyde C.J., Korevaar D.A., Leeflang M.M.G., Macaskill P., Reitsma J.B., Rodin R., Rutjes A.W.S., Salameh J.P., Stevens A., Takwoingi Y., Tonelli M., Weeks L., Whiting P., Willis B.H. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319:388–396. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 11.Whiting P.F., Rutjes A.W., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B., Leeflang M.M., Sterne J.A., Bossuyt P.M. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2003;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 12.Reitsma J.B., Glas A.S., Rutjes A.W., Scholten R.J., Bossuyt P.M., Zwinderman A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005;58:982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 13.World health organization, WHO emergency use listing for in vitro diagnostics (IVDs) detecting SARS-CoV-2, Available from: https://extranet.who.int/pqweb/sites/default/files/documents/210430_EUL_SARS-CoV-2_product_list.pdf, 2021 (accessed 19 Aug 2021).
- 14.Deeks J.J., Macaskill P., Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005;58:882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Agulló V., Fernández-González M., Ortiz de la Tabla V., Gonzalo-Jiménez N., García J.A., Masiá M., Gutiérrez F., Evaluation of the rapid antigen test panbio COVID-19 in saliva and nasal swabs in a population-based point-of-care study, J. Infect. (2020) S0163-4453(20)30768-4. [DOI] [PMC free article] [PubMed]
- 16.Albert E., Torres I., Bueno F., Huntley D., Molla E., Fernández-Fuentes M.Á., Martínez M., Poujois S., Forqué L., Valdivia A., Solano de la Asunción C., Ferrer J., Colomina J., Navarro D. Field evaluation of a rapid antigen test (Panbio COVID-19 Ag rapid test device) for COVID-19 diagnosis in primary healthcare centres. Clin. Microbiol. Infect. 2020;27:472. doi: 10.1016/j.cmi.2020.11.004. e7-472.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck E.T., Paar W., Fojut L., Serwe J., Jahnke R.R. Comparison of quidel sofia SARS FIA test to hologic aptima SARS-CoV-2 TMA test for diagnosis of COVID-19 in symptomatic outpatients. J. Clin. Microbiol. 2021;59:e02727. doi: 10.1128/JCM.02727-20. -20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerutti F., Burdino E., Milia M.G., Allice T., Gregori G., Bruzzone B., Ghisetti V. Urgent need of rapid tests for SARS CoV-2 antigen detection: evaluation of the SD-Biosensor antigen test for SARS-CoV-2. J. Clin. Virol. 2021;132 doi: 10.1016/j.jcv.2020.104654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaimayo C., Kaewnaphan B., Tanlieng N., Athipanyasilp N., Sirijatuphat R., Chayakulkeeree M., Angkasekwinai N., Sutthent R., Puangpunngam N., Tharmviboonsri T., Pongraweewan O., Chuthapisith S., Sirivatanauksorn Y., Kantakamalakul W., Horthongkham N. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol. J. 2020;17:177. doi: 10.1186/s12985-020-01452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diao B., Wen K., Zhang J., Chen J., Han C., Chen Y., Wang S., Deng G., Zhou H., Wu Y. Accuracy of a nucleocapsid protein antigen rapid test in the diagnosis of SARS-CoV-2 infection. Clin. Microbiol. Infect. 2020;27:289. doi: 10.1016/j.cmi.2020.09.057. e1-289.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenollar F., Bouam A., Ballouche M., Fuster L., Prudent E., Colson P., Tissot-Dupont H., Million M., Drancourt M., Raoult D., Fournier P.E. Evaluation of the panbio Covid-19 rapid antigen detection test device for the screening of patients with Covid-19. J. Clin. Microbiol. 2021;59:e02589. doi: 10.1128/JCM.02589-20. -20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirotsu Y., Maejima M., Shibusawa M., Nagakubo Y., Hosaka K., Amemiya K., Sueki H., Hayakawa M., Mochizuki H., Tsutsui T., Kakizaki Y., Miyashita Y., Yagi S., Kojima S., Omata M. Comparison of automated SARS-CoV-2 antigen test for COVID-19 infection with quantitative RT-PCR using 313 nasopharyngeal swabs, including from seven serially followed patients. International journal of infectious diseases. Int. J. Infect. Dis. 2020;99:397–402. doi: 10.1016/j.ijid.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krüttgen A., Cornelissen C.G., Dreher M., Hornef M.W., Imöhl M., Kleines M. Comparison of the SARS-CoV-2 rapid antigen test to the real star Sars-CoV-2 RT PCR kit. J. Virol. Methods. 2021;288 doi: 10.1016/j.jviromet.2020.114024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert-Niclot S., Cuffel A., Le Pape S., Vauloup-Fellous C., Morand-Joubert L., Roque-Afonso A.M., Le Goff J., Delaugerre C. Evaluation of a rapid diagnostic assay for detection of SARS-CoV-2 antigen in nasopharyngeal swabs. J. Clin. Microbiol. 2020;58:e00977. doi: 10.1128/JCM.00977-20. -20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanser L., Bellmann-Weiler R., Öttl K.W., Huber L., Griesmacher A., Theurl I., Weiss G. Evaluating the clinical utility and sensitivity of SARS-CoV-2 antigen testing in relation to RT-PCR Ct values. Infection. 2020;13:1–3. doi: 10.1007/s15010-020-01542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linares M., Pérez-Tanoira R., Carrero A., Romanyk J., Pérez-García F., Gómez-Herruz P., Arroyo T., Cuadros J. Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J. Clin. Virol. 2020;133 doi: 10.1016/j.jcv.2020.104659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liotti F.M., Menchinelli G., Lalle E., Palucci I., Marchetti S., Colavita F., La Sorda M., Sberna G., Bordi L., Sanguinetti M., Cattani P., Capobianchi M.R., Posteraro B. Performance of a novel diagnostic assay for rapid SARS-CoV-2 antigen detection in nasopharynx samples. Clin. Microbiol. Infect. 2021;27:487–488. doi: 10.1016/j.cmi.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mertens P., De Vos N., Martiny D., Jassoy C., Mirazimi A., Cuypers L., Van den Wijngaert S., Monteil V., Melin P., Stoffels K., Yin N., Mileto D., Delaunoy S., Magein H., Lagrou K., Bouzet J., Serrano G., Wautier M., Leclipteux T., Van Ranst M., Vandenberg O. Development and potential usefulness of the COVID-19 Ag respi-strip diagnostic assay in a pandemic context. Front. Med. 2020;7:225. doi: 10.3389/fmed.2020.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nalumansi A., Lutalo T., Kayiwa J., Watera C., Balinandi S., Kiconco J., Nakaseegu J., Olara D., Odwilo E., Serwanga J., Kikaire B., Ssemwanga D., Nabadda S., Ssewanyana I., Atwine D., Mwebesa H., Bosa H.K., Nsereko C., Cotten M., Downing R., Lutwama J., Kaleebu P. Field evaluation of the performance of a SARS-CoV-2 antigen rapid diagnostic test in Uganda using nasopharyngeal samples. Int. J. Infect. Dis. 2021;104:282–286. doi: 10.1016/j.ijid.2020.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilarowski G., Marquez C., Rubio L., Peng J., Martinez J., Black D., Chamie G., Jones D., Jacobo J., Tulier-Laiwa V., Rojas S., Rojas S., Cox C., Petersen M., DeRisi J., Havlir D.V., Field performance and public health response using the BinaxNOW TM Rapid SARS-CoV-2 antigen detection assay during community-based testing, Clin. Infect. Dis. (2020) DOI: 10.1093/cid/ciaa1890. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 31.Porte L., Legarraga P., Vollrath V., Aguilera X., Munita J.M., Araos R., Pizarro G., Vial P., Iruretagoyena M., Dittrich S., Weitzel T. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int. J. Infect. Dis. 2020;99:328–333. doi: 10.1016/j.ijid.2020.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pray I.W., Ford L., Cole D., Lee C., Bigouette J.P., Abedi G.R., Bushman D., Delahoy M.J., Currie D., Cherney B., Kirby M., Fajardo G., Caudill M., Langolf K., Kahrs J., Kelly P., Pitts C., Lim A., Aulik N., Tamin A., Harcourt J.L., Queen K., Zhang J., Whitaker B., Browne H., Medrzycki M., Shewmaker P., Folster J., Bankamp B., Bowen M.D., Thornburg N.J., Goffard K., Limbago B., Bateman A., Tate J.E., Gieryn D., Kirking H.L., Westergaard R., Killerby M. Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses - Wisconsin, September-October 2020. MMWR Morb. Mortal. Wkly Rep. 2021;69:1642–1647. doi: 10.15585/mmwr.mm695152a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scohy A., Anantharajah A., Bodéus M., Kabamba-Mukadi B., Verroken A., Rodriguez-Villalobos H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J. Clin. Virol. 2020;129 doi: 10.1016/j.jcv.2020.104455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strömer A., Rose R., Schäfer M., Schön F., Vollersen A., Lorentz T., Fickenscher H., Krumbholz A. Performance of a point-of-care test for the rapid detection of SARS-CoV-2 antigen. Microorganisms. 2020;9:58. doi: 10.3390/microorganisms9010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toptan T., Eckermann L., Pfeiffer A.E., Hoehl S., Ciesek S., Drosten C., Corman V.M. Evaluation of a SARS-CoV-2 rapid antigen test: potential to help reduce community spread? J. Clin. Virol. 2020;135 doi: 10.1016/j.jcv.2020.104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turcato G., Zaboli A., Pfeifer N., Ciccariello L., Sibilio S., Tezza G., Ausserhofer D. Clinical application of a rapid antigen test for the detection of SARS-CoV-2 infection in symptomatic and asymptomatic patients evaluated in the emergency department: a preliminary report. J. Infect. 2020;82:e14–e16. doi: 10.1016/j.jinf.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weitzel T., Legarraga P., Iruretagoyena M., Pizarro G., Vollrath V., Araos R., Munita J.M., Porte L. Comparative evaluation of four rapid SARS-CoV-2 antigen detection tests using universal transport medium. Travel Med. Infect. Dis. 2020;39 doi: 10.1016/j.tmaid.2020.101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young S., Taylor S.N., Cammarata C.L., Varnado K.G., Roger-Dalbert C., Montano A., Griego-Fullbright C., Burgard C., Fernandez C., Eckert K., Andrews J.C., Ren H., Allen J., Ackerman R., Cooper C.K. Clinical evaluation of BD veritor SARS-CoV-2 point-of-care test performance compared to PCR-based testing and versus the Sofia 2 SARS antigen point-of-care test. J. Clin. Microbiol. 2020;59:e02338. doi: 10.1128/JCM.02338-20. -20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization (WHO). COVID-19 target product profiles for priority diagnostics to support response to the COVID-19 pandemic v.1.0. https://www.who.int/publications/m/item/covid-19-target-product-profiles-for-priority-diagnostics-tosupport-response-to-the-covid-19-pandemic-v.0.1 (Accessed 13 Aug 2021).
- 40.Dinnes J., Deeks J., Kirby J., Roderick P. A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy. Health Technol. Assess. (Rockv) 2005;9:1–113. doi: 10.3310/hta9120. iii. [DOI] [PubMed] [Google Scholar]
- 41.Chartrand C., Tremblay N., Renaud C., Papenburg J. Diagnostic accuracy of rapid antigen detection tests for respiratory syncytial virus infection: systematic review and meta-analysis. J. Clin. Microbiol. 2015;53:3738–3749. doi: 10.1128/JCM.01816-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moesker F.M., van Kampen J.J.A., Aron G., Schutten M., van de Vijver D.A.M.C., Koopmans M.P.G., Osterhaus A.D.M.E., Fraaij P.L.A. Diagnostic performance of influenza viruses and RSV rapid antigen detection tests in children in tertiary care. J. Clin. Virol. 2016;79:12–17. doi: 10.1016/j.jcv.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou R., Li F., Chen F., Liu H., Zheng J., Lei C., Wu X. Viral dynamics in asymptomatic patients with COVID-19. Int. J. Infect. Dis. 2020;96:288–290. doi: 10.1016/j.ijid.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karahasan Yagci A., Sarinoglu R.C., Bilgin H., Ö Yanılmaz, Sayın E., Deniz G., Guncu M.M., Doyuk Z., Barıs C., Kuzan B.N., Aslan B., Korten V., Cimsit C. Relationship of the cycle threshold values of SARS-CoV-2 polymerase chain reaction and total severity score of computerized tomography in patients with COVID 19. Int. J. Infec.t Dis. 2020;101:160–166. doi: 10.1016/j.ijid.2020.09.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 47.Walsh K.A., Jordan K., Clyne B., Rohde D., Drummond L., Byrne P., Ahern S., Carty P.G., O'Brien K.K., O'Murchu E., O'Neill M., Smith S.M., Ryan M., Harrington P. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J. Infect. 2020;81:357–371. doi: 10.1016/j.jinf.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivett L., Sridhar S., Sparkes D., Routledge M., Jones N.K., Forrest S., Young J., Pereira-Dias J., Hamilton W.L., Ferris M., Torok M.E., Meredith L., Curran M.D., Fuller S., Chaudhry A., Shaw A., Samworth R.J., Bradley J.R., Dougan G., Smith K.G., Lehner P.J., Matheson N.J., Wright G., Goodfellow I.G. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. ELife. 2020;11:e58728. doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baggio S., L'Huillier A.G., Yerly S., Bellon M., Wagner N., Rohr M., Huttner A., Blanchard-Rohner G., Loevy N., Kaiser L., Jacquerioz F., Eckerle I. SARS-CoV-2 viral load in the upper respiratory tract of children and adults with early acute COVID-19. Clin. Infect. Dis. 2020;73:148–150. doi: 10.1093/cid/ciaa1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davies N.G., Klepac P., Liu Y., Prem K., Jit M., Eggo R.M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nature Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 51.Peto T. COVID-19: rapid antigen detection for SARS-CoV-2 by lateral flow assay: a national systematic evaluation of sensitivity and specificity for mass-testing. EClinicalMedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stohr J., Zwart V.F., Goderski G., Meijer A., Nagel-Imming C.R.S., Kluytmans-van den Bergh M.F.Q., Pas S.D., Oetelaar F., Hellwich M., Gan K.H., Rietveld A., Verweij J.J., Murk J.L., Bijllaardt W., Kluytmans J., Self-testing for the detection of SARS-CoV-2 infection with rapid antigen tests for people with suspected COVID-19 in the community, Clin. Microbiol. Infect. (2021) doi: 10.1016/j.cmi.2021.07.039. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 53.Zhang Z., Bi Q., Fang S., Wei L., Wang X., He J., Wu Y., Liu X., Gao W., Zhang R., Gong W., Su Q., Azman A.S., Lessler J., Zou X. Insight into the practical performance of RT-PCR testing for SARS-CoV-2 using serological data: a cohort study. Lancet Microbe. 2021;2:e79–e87. doi: 10.1016/S2666-5247(20)30200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kucirka L.M., Lauer S.A., Laeyendecker O., Boon D., Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann. Intern. Med. 2020;173:262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]