Abstract
The antimitogenic action of transforming growth factor β (TGF-β) in epithelial cells involves cyclin-dependent kinase (cdk) inhibitory gene responses and downregulation of c-Myc expression. Although the cdk inhibitory responses are sufficient for G1 arrest, enforced expression of c-Myc prevents G1 arrest by TGF-β. We investigated the basis of this antagonism by using Mv1Lu lung epithelial cell lines that conditionally express levels of human c-Myc. We show that c-Myc prevents induction of the cdk4 inhibitor p15Ink4b and the subsequent inhibition of G1 cdks by TGF-β. We assessed the significance of this effect by analyzing the oligomeric state of cdk4 in these cells. In proliferating cells, endogenous cdk4 is distributed among three populations: an abundant high-molecular-mass (>400-kDa) pool of latent cdk4 that serves as a source of cdk4 for cyclin D, a low-abundance pool containing active cyclin D-cdk4 complexes, and an inactive population of monomeric cdk4. Cell stimulation with TGF-β converts the latent and active cdk4 pools into inactive cdk4, an effect that is specifically mimicked by overexpression of p15 but not by other forms of G1 arrest. This process of TGF-β-induced cdk4 inactivation is completely blocked by expression of c-Myc, even though the latent and active cdk4 complexes from c-Myc-expressing cells remain sensitive to dissociation by p15 in vitro. c-Myc causes a small increase in cyclin D levels, but this effect contributes little to the loss of TGF-β responses in these cells. The evidence suggests that c-Myc interferes with TGF-β activation of the p15 G1 arrest pathway. TGF-β must therefore downregulate c-Myc in order to activate this pathway.
Transforming growth factor β (TGF-β) inhibits the proliferation of epithelial, endothelial, hematopoietic, and certain mesenchymal cell types by restricting progression through the G1 phase of the cell cycle (reviewed in references 2, 33, 44, and 49). The antimitogenic response to TGF-β is generally mediated by two classes of rapid gene responses: (i) gene responses that directly compromise the activity of G1-phase cyclin-dependent kinases (cdk4, cdk6, and cdk2), and (ii) downregulation of c-myc. The cdk-inhibitory responses can vary depending on the cell type. In some cases, TGF-β rapidly elevates the expression of p15Ink4b (henceforth referred to as p15), which is a specific inhibitor of the early G1 cyclin D-dependent cdks, cdk4 and cdk6 (15, 48). High levels of p15 can induce a redistribution of p27Kip1 from active p27-cyclin D-cdk4/6 complexes to cyclin E-cdk2, inactivating this late G1/S kinase (47). In other instances, TGF-β elevates the expression of the cdk inhibitor p21Cip1 with or without p15 induction (10, 31, 48). However, in other cases, TGF-β rapidly decreases the expression of the cdk tyrosine phosphatase Cdc25A, causing the accumulation of inhibitory tyrosine phosphorylation in cdk4 and cdk6 (20). The dependence of TGF-β gene responses on the type and developmental state of the cell is thought to be determined by the presence of specific DNA-binding cofactors that take the TGF-β signal-transducing proteins (the Smads) to the regulatory region of each target gene (reviewed in reference 32).
In contrast to this variability in cdk-inhibitory responses, a rapid and profound decline in c-myc expression is a general feature of TGF-β antiproliferative responses (2). c-Myc is short-lived, and downregulation of its mRNA by TGF-β results in a rapid loss of the protein (20, 31, 43). c-Myc is thought to act as a transcriptional activator of certain genes whose products foster G1 progression in the presence of balanced mitogenic stimuli (reviewed in references 9, 14, and 18) and lead to apoptosis under conditions of proliferative stress (12). c-Myc can also act as a transcriptional repressor (6, 18). c-Myc can favor the generation of active cdk complexes by mechanisms that remain ill-defined (1, 27, 38, 41, 60). The significance of c-Myc downregulation in TGF-β action is underscored by the observation that overexpression of exogenous c-Myc renders cells resistant to the antimitogenic effect of TGF-β (2, 57).
An important question raised by these observations is the following: if TGF-β can exert a potent cdk-inhibitory effect (e.g., through induction of p15), why is c-myc downregulation needed for a TGF-β antimitogenic response? We have investigated this question by using Mv1Lu mink lung epithelial cells that express human c-Myc under the control of a conditional promoter. The parental Mv1Lu cell line has one of the best-characterized antimitogenic responses to TGF-β. Addition of TGF-β to these cells causes a rapid increase in p15 expression, which leads to the conversion of active p27-cyclin D-cdk4 complexes into inactive p15-cdk4/6 complexes with a displacement of p27 from these kinases (47, 48). p27 then causes cdk2 inactivation by forming inactive p27-cyclin E-cdk2 complexes (22, 45, 48). This loss of G1 cdk activities and the resultant accumulation of pRb protein in the hypophosphorylated state cause an arrest of G1 progression (25). A similar increase in p15 expression is sufficient for G1 arrest in these cells (47). However, like other cell types, Mv1Lu cells rapidly downregulate c-myc in response to TGF-β (61) and, as we show here, preventing this decrease by enforced expression of exogenous c-Myc interferes with the TGF-β antiproliferative response. Investigating the mechanism by which enforced c-Myc expression silences the TGF-β antimitogenic action, we unexpectedly found that the presence of c-Myc prevents activation of the p15 pathway by TGF-β.
MATERIALS AND METHODS
Cell culture and transfection.
The human c-myc cDNA was cloned into the XbaI site of the pUHD10-3 hygromycin vector (48). The mink lung epithelial cell line Mv1Lu-tTA (48) was maintained in minimal essential medium supplemented with 10% fetal bovine serum (FBS) plus 0.5 mg of G418 per ml. Mv1Lu-tTA cells were transfected with pUHD10-3 hygromycin–c-myc by the Lipofectin procedure as specified by the manufacturer (GIBCO-BRL). c-Myc inducible clones were selected as described previously (48). Three clones, TM1, TM2, and TM3, were further subcloned by end dilution to obtain the cell lines analyzed in this study. Human cyclin D1 cDNA was subcloned into the XbaI site of pUHD10-3 hygromycin and transfected with Lipofectin, and inducible clones were selected as described above. tet-p27, tet-p15, and tet-K4 cell lines have been previously described (47, 48). All tet cell lines were selected and maintained in minimal essential medium plus 10% FBS, 0.5 mg of G418 per ml, 0.3 mg of hygromycin per ml, and 1 μg of tetracycline per ml.
HaCaT keratinocytes (a gift from N. Fusenig) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS. The simian virus 40 large T antigen-immortalized human lung epithelial cell line HPL1 (34) was a gift of T. Takahasi. Cell lines obtained from the American Type Culture Collection included SW480, K562, CA46, and ST486. The human colon adenocarcinoma cell line SW480 was maintained in DME plus 10% FBS, whereas the human leukemia cell line K562 was grown in RPMI plus 10% FBS. The Burkitt’s lymphoma cell lines CA46 and ST486 were maintained in RPMI plus 20% FBS.
Immunoprecipitation and immunoblotting assays.
The Tet-Myc cells were grown to near confluence and then split 1:3 into medium containing no tetracycline or 1 μg of tetracycline per ml. After a 20-h incubation, the cells were harvested by trypsinization or treated further with 200 pM TGF-β1 (R&D Systems, Minneapolis, Mn.). Cell pellets from tet cells were lysed by a published procedure (35). After lysates were precleared for 1 h with protein A-Sepharose and normal rabbit serum, they were immunoprecipitated with the appropriate antibodies for 3 to 16 h at 4°C. The protein complexes that bound to protein A-Sepharose were washed four times with immunoprecipitation buffer (35) and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
For Western immunoblotting analysis, the immunoprecipitates or aliquots (0.2 mg of protein) of cell lysate were separated by SDS-PAGE and transferred to polyvinylidene difluoride (Immobilon-P) membranes. The blots were probed with the appropriate primary antibody followed by a protein A-conjugated horseradish peroxidase (Sigma) or anti-mouse immunoglobulin G secondary antibody (Pierce) before being visualized by enhanced chemiluminescence (ECL or ECLplus; Amersham).
Antibodies.
Antibodies against human c-Myc (sc-40, 9E10), cyclin D1 (sc-753, sc-718), cdk6 (sc-177, C21), cyclin D2 (sc-181), or human p15 (sc-612) were purchased from Santa Cruz Biotechnology. Anti-Rb antibody (14001A from Pharmingen) recognizes the hypo- and hyperphosphorylated pRb. Anti-hsp90 antibody (NM Ab-1, anti-hsp86) was obtained from Neomarkers. The anti-p27, anti-cdk2, and anti-mouse cdk4 antibodies were described previously (47, 48). The anti-mink cdk4 antibody was raised in rabbits by using a synthetic mink cdk4 carboxyl-terminal peptide (CKRISAFRALQHSYLQKPEGNP; purchased from Chiron) by using standard methods (16).
Kinase assays.
Kinase assays were performed with cell lysates, prepared as described above for immunoprecipitations. To measure cdk2 kinase activity, 0.5 mg of lysates was immunoprecipitated and analyzed as previously described (35), using H1 histone as a substrate. Due to the inability of mink cdk4 antibodies to support Rb kinase activity, cdk4 kinase activity was measured from tet-K4 cells, which express mouse cdk4. tet-K4 cells grown in the absence of tetracycline were fractionated by Superdex 200 gel filtration chromatography and fractions were pooled as indicated. Kinase activity was analyzed as previously described (35), using the “large-pocket” region of pRb (glutathione S-transferase–Rb fusion, amino acids 379 to 928) as a substrate.
Flow cytometric cell cycle analysis.
Stained nuclei were prepared by hypotonic lysis of cells in a mixture of 0.03% Nonidet P-40, 10 mM NaCl, 1 mg of sodium citrate per ml, ethidium bromide (25 μg/ml), and RNase (10 μg/ml) at room temperature for 30 min. After addition of 80 mM citric acid, 250 mM sucrose, and 40 μg of ethidium bromide per ml, the nuclei were either immediately analyzed with a FACScan apparatus (Becton Dickinson) or stored at 4°C for later analysis.
Northern analysis.
Poly(A)+ RNA was prepared from snap-frozen cell pellets by using the Micro-FastTrack kit (Invitrogen). For Northern blot analysis, RNA samples were fractionated on 1% agarose gels containing 0.22 M formaldehyde, 1 mM EDTA, 5 mM sodium acetate, 20 mM morpholinepropanesulfonic acid (MOPS), and 0.1 μg of ethidium bromide per ml. The gels were destained by being washed in water, photographed under UV illumination to check RNA integrity, transferred onto a nylon membrane (Nytran plus; Schleicher & Schuell) by capillary blotting in 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), and fixed by UV cross-linking. Prehybridization was carried out at 42°C for 2 to 6 h in a mixture of 50% formamide, 5× SSC, 5× Denhardt’s solution, 0.1% SDS, 100 μg of salmon sperm DNA per ml, and 40 μg of tRNA per ml. Hybridization was then performed overnight at 42°C after addition of labeled DNA. The DNA probe for mink p15Ink4b was prepared and labeled as described previously (48), as was the PAI-I probe (61). To control for loading, the Northern blots were probed with the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene.
Analysis of cdk complexes.
Cell pellets were lysed as described for immunoprecipitation and cleared of cell debris by sonication and centrifugation at 14,000 × g for 10 min followed by centrifugation at 50,000 rpm (Beckman T100.2) for 30 min. Then 500 μl of the cleared lysate was injected onto a Superdex 200 column, HR 10/30 (Pharmacia Biotech Inc.), preequilibrated with column buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 1 mM EDTA), and 0.5-ml fractions (0.35 ml/min) were collected by fast protein liquid chromatography. Molecular weight markers (Sigma) were subjected to gel filtration separately and monitored by SDS-PAGE. Fractions 1 to 50 were subjected to SDS-PAGE and immunoprecipitation as described above. Recombinant p15 was prepared as previously described (48) and mixed with the cleared lysates in column buffer containing 400 mM phosphocreatine (pH 7.5), 2 mg of creatine phosphokinase per ml, and 0.3 mM ATP.
Reporter assays.
As a reporter, we used a luciferase gene under the control of the proximal promoter region (−113 to +68) of the human p15Ink4b, construct p15p113-Luc. This region was shown to respond to TGF-β in HaCaT cells (28). TM1 cells were transiently transfected with p15p113-Luc by using DEAE-dextran as previously described (4). Cells were split 24 h posttransfection into medium with or without tetracycline, plus or minus TGF-β in 10% FBS. Luciferase assays were carried out 24 h later by using the Promega luciferase assay kit and a Berthold luminometer. HaCaT cells were transfected with LipofectAMINE (Gibco-BRL), as specified by the manufacturer, with the p15p113-Luc reporter and in the presence or absence of the human c-myc expression construct, pCMV5-cMyc.
RESULTS
Normal c-Myc levels prevent TGF-β-induced G1 arrest in Mv1Lu cells.
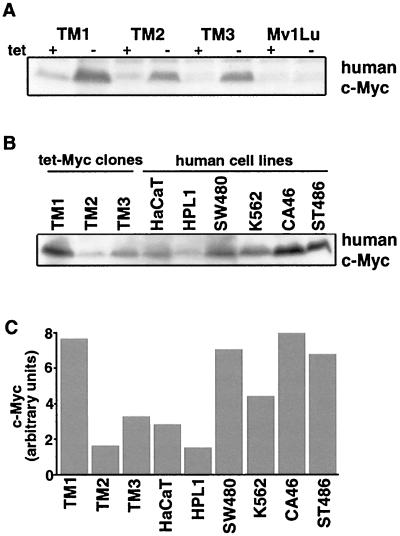
To investigate the role of c-Myc downregulation in the antimitogenic response of Mv1Lu cells to TGF-β, we generated Mv1Lu derivatives expressing a human c-myc cDNA under negative control of the tetracycline trans activator (13). Three independent cell lines (TM1, TM2, and TM3) that expressed exogenous c-Myc at different levels upon induction in tetracycline-free medium were chosen for further characterization (Fig. 1A). Of these cell lines, TM1 expressed the highest level of human c-Myc, a level that was approximately 5- and 2.5-fold higher than those in TM2 and TM3 cells, respectively, as determined by anti-human c-Myc Western immunoblotting (Fig. 1C). The levels of human c-Myc expressed in TM2 and TM3 cells were similar to the endogenous levels in two cell lines derived from normal tissue: HPL1 human lung epithelial cells (34) and HaCaT human keratinocytes (26). The level of human c-Myc expressed in TM1 cells was similar to the endogenous levels in human tumor-derived cell lines that are known to overexpress c-Myc, including SW480 colon carcinoma cells (8), K562 erythroleukemia cells (58), and the Burkitt’s lymphoma cell lines CA46 and ST486 (21) (Fig. 1B and C). Therefore, the TM cell lines allowed us to study the effects of physiologically relevant levels of c-Myc on the TGF-β response.
FIG. 1.
Generation of Mv1Lu cell lines with inducible c-Myc expression. (A) Clonal tet-Myc cell lines (TM1 to TM3) were maintained in medium containing 1 μg of tetracycline per ml and then grown in the absence of tetracycline for 18 h before being harvested and analyzed by anti-human c-Myc Western immunoblotting. Parental Mv1Lu cells were also analyzed in the presence and absence of tetracycline with the anti-human c-Myc antibody. (B) Comparison of the levels of induced human c-Myc expressed in tet-Myc clones with other human cell lines by anti-human c-Myc Western immunoblotting. The same amount of cell lysate protein was loaded in each lane. (C) The human c-Myc protein levels visualized in panel B were quantitated by densitometry of the signal and plotted as relative levels of c-Myc protein in arbitrary units.
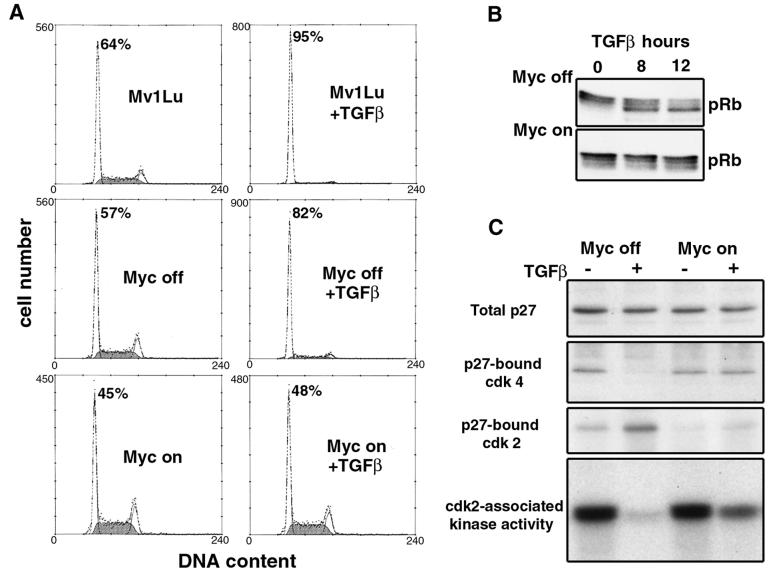
Addition of TGF-β to parental Mv1Lu cells causes an arrest of the cell cycle with extensive accumulation of cells in G1 (Fig. 2A) (25). A similar effect was observed in the TM cell lines when they were maintained in the presence of tetracycline (TM2 data in Fig. 2A; TM1 and TM3 data not shown). When cultured in tetracycline-free medium, however, the TM cell lines were fully refractory to the antiproliferative action of TGF-β, even for TM2 cells, which express the lowest level of exogenous c-Myc (Fig. 2A and data not shown). These experiments were conducted in the presence of serum-containing medium to avoid the c-Myc-induced apoptosis that can occur when c-Myc-overexpressing cells receive antiproliferative signals. No signs of apoptosis (e.g., accumulation of cells with a sub-G1 DNA content) were observed in the course of our experiments.
FIG. 2.
Analysis of cell cycle progression and G1 regulators upon treatment of tet-Myc cells with TGF-β. (A) Parental Mv1Lu cells were maintained in growth medium, and TM2 cells grown in the absence of tetracycline (Myc on) or in the presence of 1 μg of tetracycline per ml (Myc off) for 18 h before the addition of 200 pM TGF-β. Cells were harvested for flow-cytometric analysis of DNA content after 20 h in the presence or absence of TGF-β. The percentage of cells in the G1 phase at this time is indicated. (B) TM2 cells were harvested and analyzed by anti-pRB Western immunoblotting at the indicated times after TGF-β addition. (C) TM2 cells were analyzed for the presence of p27-cdk complexes following 20 h of TGF-β treatment. Immunoprecipitations were performed on cell lysates with anti-p27, and Western immunoblotting was used to determine total p27 or p27-associated cdk4 and cdk2 levels. cdk2 was also immunoprecipitated from these lysates, and the ability of these complexes to phosphorylate histone H1 in vitro was determined by kinase assays.
c-Myc prevents inhibition of G1 cdks by TGF-β.
TGF-β-induced G1 arrest in epithelial cells is accompanied by a loss of pRB phosphorylation (Fig. 2B) (25), which is a sign of failure to activate the G1 cdks that normally phosphorylate pRB (51). When TM cells were induced to express exogenous c-Myc, TGF-β failed to induce the characteristic accumulation of pRB in the hypophosphorylated state (Fig. 2B; TM1 data not shown). This result suggests that c-Myc protects cdks from inhibition by TGF-β.
p27 in proliferating Mv1Lu cells is mostly bound to cyclin D-cdk4 and cyclin D-cdk6 complexes (5, 47). p15 accumulating in response to TGF-β binds to cdk4 and cdk6, excludes p27 from these complexes, and allows it to bind to and inhibit cdk2 (47, 48). Analysis of the association of p27 with cdk4 and cdk2 provided an initial indication of the ineffectiveness of the action of TGF-β in TM cells (Fig. 2C). With exogenous c-Myc turned off (i.e., in tetracycline-containing medium), TM2 cells responded to TGF-β with the characteristic redistribution of p27 from cdk4 to cdk2 and inhibition of cdk2-associated kinase activity. However, TGF-β failed to induce these events in TM2 cells when c-Myc expression was turned on (Fig. 2C).
c-Myc increases cyclin D levels and impairs p15Ink4b gene induction by TGF-β.
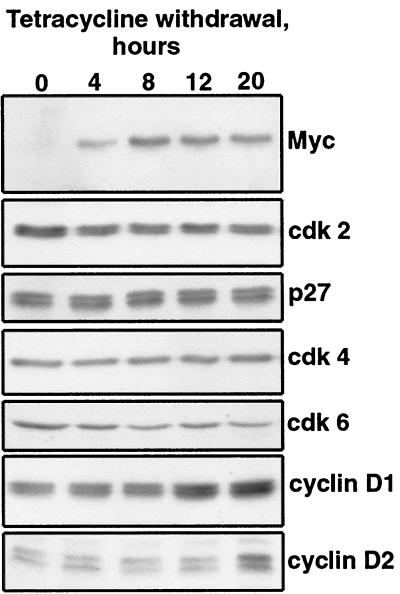
Diverse mechanisms could underlie these effects of c-Myc. One possibility is that c-Myc increases the abundance of cyclin D-cdk complexes well above the level that could be inhibited by a finite amount of TGF-β-induced p15. When TM2 cells were placed in tetracycline-free medium, the increase in the amount of exogenous c-Myc was accompanied by a two- to threefold increase in the level of cyclin D1 and, several hours later, by a similar increase in the level of cyclin D2 (Fig. 3). No detectable D3 was present in Mv1Lu cells. No changes were observed in the levels of p27, cdk2, cdk4, or cdk6. Induction of c-Myc also correlated with a twofold increase in the formation of cyclin D-cdk4 complexes, as determined by anti-cdk4 Western immunoblotting of cyclin D1 immunoprecipitates (data not shown) or by chromatographic analysis of cdk4 complexes (see Fig. 7). However, these increases were relatively small compared to the extent of inhibition of the TGF-β response. Furthermore, the increase in the levels of cyclins D1 and D2 occurred late considering that the antiproliferative effect of TGF-β was suppressed even when TGF-β was added simultaneously with the switch of TM2 cells to tetracycline-free medium (data not shown).
FIG. 3.
Effect of exogenous c-Myc expression on G1 cdk components. TM2 cells were induced to express exogenous Myc by the removal of tetracycline and harvested at the indicated times. Immunoblots were probed for exogenous human c-Myc and endogenous mink cdk2, p27, cdk4, cdk6, cyclin D1, and cyclin D2 proteins.
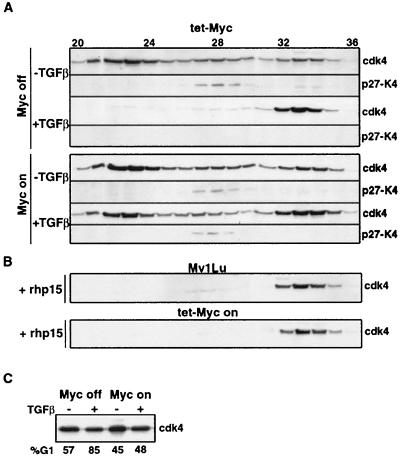
FIG. 7.
c-Myc overexpression and cdk4 complexes in Mv1Lu cells. (A) TM2 cells grown in the presence of tetracycline (Myc off) or grown for 18 h in the absence of tetracycline (Myc on) were harvested for gel filtration analysis either directly (−TGF-β) or after incubation with TGF-β for 20 h (+TGF-β). Following gel filtration of these cell lysates, fractions were subjected to anti-cdk4 Western immunoblotting either directly (cdk4) or following anti-p27 immunoprecipitation (p27-K4). (B) Lysates (3 mg of total protein) from proliferating Mv1Lu cells and TM2 cells induced to express c-Myc for 18 h in the absence of tetracycline (tet-Myc on) were incubated with 80 μg of recombinant human p15 (+rhp15) at 37°C for 30 min and then subjected to gel filtration and anti-cdk4 immunoblotting. (C) The proportion of cells in G1 phase and the level of cdk4 under each of these conditions.
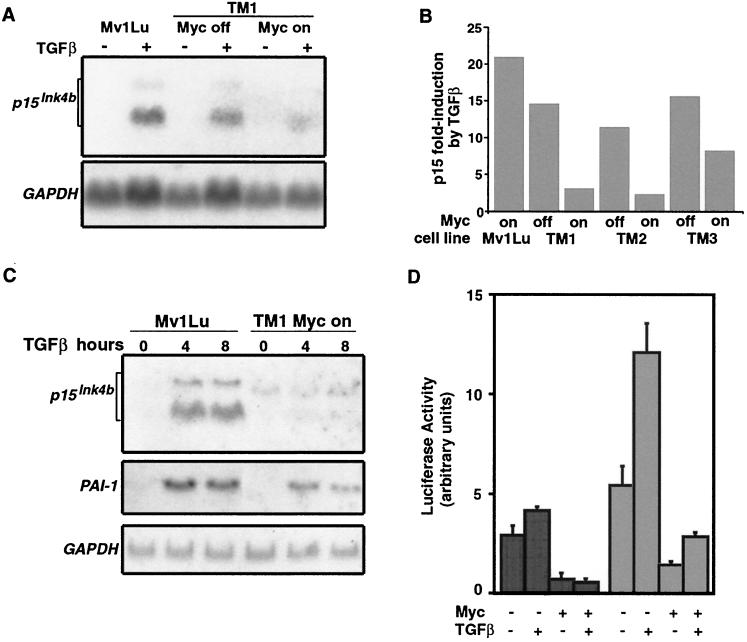
Given these results, we investigated the alternative possibility that c-Myc impairs the induction of p15 by TGF-β, thus preventing cdk inhibition. Indeed, c-Myc expression inhibited p15Ink4b mRNA induction by TGF-β (Fig. 4A to C). Another early-gene response to TGF-β, namely, induction of the plasminogen activator inhibitor 1 (PAI-1) gene, was also inhibited in the presence of c-Myc but less so than the p15 response (Fig. 4C). Thus, c-Myc preferentially inhibits the p15 response. These responses are thought to be mediated by TGF-β receptor-activated Smad2 and Smad3 (11, 19, 37, 53). The function of Smads as transcription factors requires their accumulation in the nucleus, and this process is inhibited by various pathways that antagonize TGF-β signaling (23, 24, 39, 59). However, Smad accumulation in the nucleus in response to TGF-β was not impaired by c-Myc, as determined by anti-Smad2/3 indirect immunofluorescence of TM2 cells (data not shown). These results suggest that c-Myc interferes with TGF-β induction of p15 at a level downstream of Smad nuclear accumulation.
FIG. 4.
Induction of p15Ink4b in tet-Myc clones. (A and B) Three tet-Myc clones (TM1 to TM3) were induced to express c-Myc by the absence of tetracycline for 18 h and then treated with 200 pM TGF-β. At 6 h after TGF-β addition, the cells were harvested and their RNA was analyzed by Northern blotting for p15Ink4b expression. A representative blot with the TM1 cell lines is shown (A). The same blot was probed for GAPDH expression as a loading control. Quantitation of such p15 blots for all three TM clones was carried out and is shown as the fold induction of this RNA by addition of TGF-β, normalized to GAPDH values (B). (C) p15Ink4b induction in parental Mv1Lu cells and TM1 cells after 20 h without tetracycline followed by TGF-β addition for the indicated times. The same Northern blot was probed for PAI-1 as another representative TGF-β-responsive gene and GAPDH as a loading control. (D) TM1 cells were transfected with the p15p113-Luc reporter. Transfections were carried out without or with TGFβ in the absence or presence of tetracycline for 24 h prior to analysis of luciferase activity. Results are means ± standard deviations of triplicate transfections. HaCaT cells were transfected with p15 p113-Luc reporter, without or with a human c-Myc expression vector, and analyzed as described above.
Inhibition of the p15 promoter by c-Myc.
To examine whether c-Myc inhibits the p15 promoter, we used a luciferase reporter construct, p15p113-Luc, that is driven by the proximal region of the human p15 promoter (Fig. 4D). This region of the p15 promoter exhibits TGF-β responsiveness in HaCaT cells (28). Additionally, the natural p15 transcriptional initiator site present in this reporter, CCCACTCT, fits the Inr consensus previously identified in genes that are transcriptionally repressed by c-Myc and shown to impart this type of response to c-Myc (14, 29, 42). The activity of the p15p113-Luc reporter construct was repressed upon induction of c-Myc expression by tetracycline removal in TM1 cells and by transfection of a c-Myc expression vector in HaCaT cells (Fig. 4D), suggesting that c-Myc affects p15 transcription directly.
Latent, active, and inactive cdk4 pools.
To better assess how c-Myc protects cdk4 from inhibition by TGF-β, we analyzed cdk4 from Mv1Lu and derivative clones by size fractionation chromatography on a Superdex 200 column. Using similar fractionations in different cell types, others have shown that cdk4 and cdk6 are distributed among three major populations: a high-molecular-weight pool associated with hsp90 and cdc37, an active cyclin D-associated pool, and a pool of monomeric or Ink4b-bound cdk (30, 36, 40, 56).
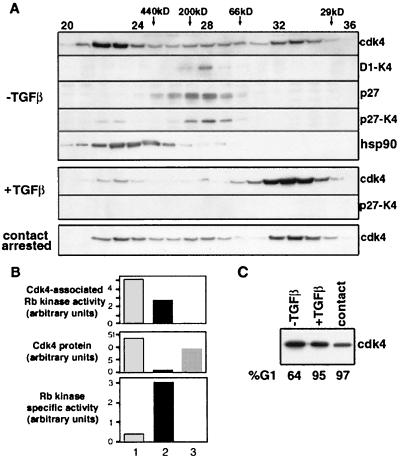
Figure 5 shows the existence of similar cdk4 pools in proliferating Mv1Lu cells. An abundant high-molecular-mass (>400-kDa) complex that coelutes with hsp90 includes as much as half of the cdk4 in these cell extracts (Fig. 5A, top). We refer to this complex as latent cdk4 because it is a source of cdk4 for cyclin D1 (see below). A second cdk4 population elutes between the 200- and 66-kDa markers and contains all the cyclin D-bound cdk4 and most of the p27-bound cdk4. Although this pool includes only approximately 10% of the total cdk4 (Fig. 5A, top), its specific activity in in vitro pRB kinase assays was approximately 10-fold higher than that of the latent pool (Fig. 5B). Thus, the middle pool, which we refer to as the active-cdk4 pool, contains active cyclin D-cdk4 complexes. Most of the p27 from these cells coeluted with this active pool (Fig. 5A), which is distinct from where excess p27 from overexpressing tet-p27 cells eluted (after the 66-kDa marker [data not shown]). The association of p27 with the active cdk4 pool is consistent with previous observations that in proliferating cells, p27 is mostly bound to cyclin-cdk complexes and p27-cyclin D-cdk4 complexes isolated from these cells are catalytically active (5, 46, 47, 54). Finally, a low-molecular-weight population, which we refer to as the inactive-cdk4 pool, contains the remainder (20 to 30%) of the cdk4 in these cells (Fig. 5A, top) and has no Rb kinase activity (data not shown).
FIG. 5.
Analysis of cdk4 complexes by gel filtration. (A) Fractions from Superdex 200 gel filtration of lysates from parental Mv1Lu cells were analyzed by immunoblotting and immunoprecipitation for the composition of endogenous cdk4 complexes. Fractions 20 to 36 are shown, with the positions of protein molecular weight markers indicated at the top. Fractions were subjected directly to anti-cdk4 immunoblotting in both proliferating cells (−TGFβ), cells treated with 200 pM TGF-β for 20 h (+TGFβ), and cells allowed to grow to confluence and maintained in this arrested state for three days in the presence of growth media (contact arrested). Western immunoblotting analysis of p27 and hsp90 was also carried out on fractions from proliferating Mv1Lu cells. Immunoprecipitations of endogenous cyclin D1 and p27 from fractions were subjected to anti-cdk4 Western immunoblotting to determine the levels of cyclin D1-bound cdk4 (D1-K4) and p27-bound cdk4 (p27-K4) respectively. (B) Fractions from Superdex 200 gel filtration of lysates from tet-K4 cells grown in the absence of tetracycline were collected. Fractions 22 to 24 (lane 1), 27 to 29 (lane 2), and 32 to 33 (lane 3) were pooled and immunoprecipitated with anti-cdk4 antibodies, followed by cdk4-associated Rb kinase assays (top) or subjected directly to anti-cdk4 immunoblotting analysis (middle). The Rb kinase specific activity for each pool was plotted as the value of Rb kinase activity over protein units (bottom). Kinase activity and protein level are plotted as arbitrary units determined by PhosphorImager quantitation and densitometry, respectively. (C) The level of cdk4 in cells under each of the conditions in panel A was determined by Western immunoblotting of the lysates loaded on the column. The percentage of cells in G1 phase under each of these conditions before lysis, as determined by flow cytometry, is indicated.
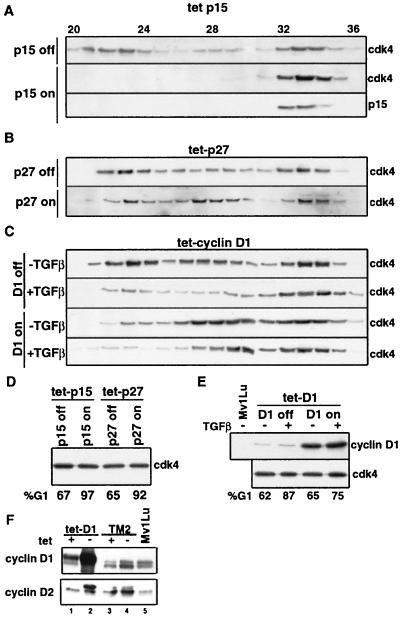
We further characterized these cdk4 populations by using tet-cyclin D1 cells, a Mv1Lu cell line expressing human cyclin D1 under the negative control of tetracycline. Induction of cyclin D1 in tet-cyclin D1 cells increased the proportion of cdk4 in the active pool with a marked loss from the latent cdk4 pool (Fig. 6C, D1-on −TGFβ panel). This effect was already somewhat apparent in the presence of tetracycline (D1-off −TGFβ panel), most probably owing to the leaky expression of exogenous cyclin D1 under these conditions (Fig. 6E). Thus, the high-molecular-weight cdk4 pool appears to serve as the main reservoir of cdk4 for cyclin D.
FIG. 6.
Effect of overexpression of cell cycle regulators on cdk4 complexes in Mv1Lu cells. (A to C) Mv1Lu cells engineered to conditionally overexpress p15 (tet-p15) (A), p27 (tet-p27) (B), or cyclin D1 (tet-D1) (C) in the absence of tetracycline were analyzed by gel filtration for cdk4 complexes. Cell lines were either maintained in the presence of tetracycline (exogenous gene off) or removed from tetracycline for 18 h (exogenous gene on) before being harvested for analysis. Exogenous p15 in tet-p15 cells (p15 on) was visualized by Western immunoblotting with an antibody specific to human p15 (A). (D and E) tet-D1 cells were removed from tetracycline for 18 h and treated with 200 pM TGF-β (+TGF-β) for 20 h before being lysed. The proportion of cells in the G1 phase and the level of cdk4 under each of these conditions are shown. The expression of the exogenous genes in tet-p15 and tet-p27 cells has been described previously (47, 48). The expression of exogenous cyclin D1 in tet-D1 cells was analyzed by Western immunoblotting with an antibody specific for human cyclin D1 and is also shown in panel E. (F) Comparison of cyclin D1 (top) and cyclin D2 (bottom) protein levels in tet-D1, TM2, and parental Mv1Lu cells in the absence and presence of tetracycline.
Latent and active cdk4 complexes as targets of TGF-β and p15.
TGF-β addition to Mv1Lu cells had a major effect on the distribution of cdk4 between these pools, causing an extensive loss of latent and active cdk4 pools and a corresponding gain in the inactive pool (Fig. 5, +TGFβ panels). This effect was not simply secondary to G1 arrest, because a similar G1 arrest by contact inhibition (G1 values in Fig. 5B) caused only a limited loss in the latent cdk4 pool (Fig. 5A, contact arrested panel; compare to top panel).
Because p15 is a central mediator of cdk inhibition by TGF-β in Mv1Lu cells, we compared these effects of TGF-β on cdk4 with those of p15 and, as a control, p27, by using Mv1Lu-derived tet-p15 and tet-p27 cell lines that conditionally express these cdk inhibitors (47, 48). Under basal conditions (in tetracycline-containing medium) the tet-p15 and tet-p27 cells yielded cdk4 profiles on Superdex similar to those of the parental Mv1Lu cells (Fig. 6A and B, p15-off and p27-off panels). Upon induction in tetracycline-free medium, both p15 and p27 caused G1 arrest (G1 values in Fig. 6D) but with an important difference: p15 caused a major shift in cdk4 distribution (Fig. 6A, p15-on), a comparable shift to that caused by TGF-β addition, whereas p27 caused only a limited loss of latent cdk4 (Fig. 6B, p27-on), comparable to that caused by contact inhibition (Fig. 5A).
The above results collectively suggest that the latent cdk4 pool, along with the active pool, is a major target of dissociation by exogenous p15 in tet-p15 cells and endogenous p15 in TGF-β-treated cells. In some experiments, the dissociation of the active cdk4 complexes in response to TGF-β or p15 was less extensive than that of the latent cdk4 pool. Crystallographic studies on Ink4-bound cdk4 and cdk6 predict that Ink4 binding may diminish the affinity of these cdks for cyclin D (7, 50), which may lead to variability in the extent of dissociation between experiments.
c-Myc prevents TGF-β inhibition of latent and active cdk4 complexes.
Our analysis of the TM cell lines suggested two potential mechanisms by which c-Myc might protect cdk4 from inhibition by TGF-β. By increasing the levels of cyclin D, c-Myc might generate a larger pool of cyclin D-cdk4 complexes with increased capacity for binding p15 and p27. In addition, by inhibiting p15 induction, c-Myc may remove a primary mechanism of cdk4 inhibition by TGF-β.
To determine the relative contribution of these two mechanisms, we analyzed the effect of c-Myc expression on the cdk4 Superdex profile. TM2 cells placed in tetracycline-containing medium (Myc-off conditions) yielded a cdk4 profile very similar to that of parental Mv1Lu cells (Fig. 7A, top panel; compare to Fig. 5 top panel; similar results not shown were obtained with TM1 cells). Under these conditions, TM2 cells responded to TGF-β with an extensive loss of latent and active cdk4 complexes and a corresponding accumulation of inactive cdk4 (Fig. 7A, Myc-off −TGFβ panel). When placed in tetracycline-free medium (Myc-on conditions), these cells showed a small increase in the active cdk4 pool (Fig. 7A, Myc-on −TGFβ panel) that may reflect the increase in cyclin D levels (Fig. 3). This increase was minimal compared to that observed upon induction of cyclin D1 in tet-cyclin D1 cells (Fig. 6C). However, c-Myc expression fully protected TM2 cells against G1 arrest by TGF-β whereas the high levels of cyclin D1 expressed in tet-cyclin D1 cells provided only a partial protection (see the corresponding G1 values in Fig. 6D and 7C and the corresponding cyclin D levels in Fig. 6F). The small increase in cyclin D induced by c-Myc in TM2 cells is therefore unlikely to play a major role in Myc-induced TGF-β resistance.
However, c-Myc totally abolished the TGF-β-induced loss of latent cdk4 and active cdk4 in these cells (Fig. 7A, Myc-on +TGFβ panels; similar results not shown were obtained with TM1 cells). Incubation of cell extracts with recombinant p15 prior to Superdex chromatography showed that p15 can dissociate the latent and active cdk4 complexes from Myc-expressing TM2 cells as effectively as it dissociates these complexes from parental Mv1Lu cells (Fig. 7B). Mock incubation prior to chromatography did not alter the cdk4 profiles, whereas addition of as little as 0.5 μg of p15 caused a shift to the inactive pool (data not shown). These results suggest that c-Myc prevents inhibition of cdk4 not by rendering cdk4 intrinsically resistant to p15 but by its ability to prevent TGF-β induction of p15.
DISCUSSION
cdk inhibitors are key mediators of antiproliferative responses to paracrine factors such as TGF-β, DNA damage response factors such as p53, and pharmacologic inhibitors of cell proliferation (52). By increasing the levels of cdk inhibitors, these various signals can directly repress the activity of cdks controlling G1 progression. For TGF-β, its action on human keratinocytes and mink lung epithelial cells involves a rapid and sustained increase of p15 levels (48), and mimicking this increase with exogenous p15 causes a full G1 arrest (47). However, TGF-β also causes a rapid downregulation of c-Myc in these cells (20). Because c-Myc controls the expression of genes involved in progression to S phase (3, 9), the decrease in c-Myc levels and the increase in p15 levels could be viewed as coincident but independent events that extinguish G1 progression in response to TGF-β.
However, this simplified view is challenged by previous (2, 57) and present observations that the enforced expression of c-Myc can prevent TGF-β induction of cell cycle arrest. Taking these observations one step further, we found that enforced expression of c-Myc prevents inhibition of G1 cdks by TGF-β. We reasoned that if the TGF-β-mediated increase in p15 is sufficient for G1 cdk inhibition, c-Myc must prevent TGF-β from acting through p15. Here we present two lines of evidence, one based on the p15 response to TGF-β in c-Myc-expressing cells and the other based on the state of cdk4 complexes in these cells, that support this hypothesis. Our results suggest that TGF-β must downregulate c-Myc in order to activate the p15 G1 arrest pathway.
c-Myc prevents TGF-β inhibition of G1 cdks.
As an experimental system for these studies, we generated various mink lung epithelial cell lines that conditionally express human c-Myc under tetracycline control. It should be noted that the level of exogenous c-Myc in these cells is comparable to the endogenous level in lung epithelial and skin keratinocyte cell lines derived from normal human tissues. Only one of the three clones (TM1) used in this work expresses c-Myc levels that approach the high levels characteristic of certain human tumor cell lines. Thus, this experimental system allows us to investigate the behavior of exponentially proliferating cells that are forced to maintain a physiologically normal level of c-Myc throughout the course of a TGF-β response.
When these cells are forced to express c-Myc, TGF-β no longer inhibits cell cycle progression or Rb phosphorylation, correlating with an absence of inhibitory effects of TGF-β on G1 cdks. In proliferating lung epithelial cells, p27 is bound predominantly to cyclin D-cdk4 and cyclin D-cdk6 complexes that remain active in spite of carrying associated p27 (5, 54). p15 that accumulates in response to TGF-β binds to cdk4 and cdk6, displacing p27, which now binds to cdk2, inhibiting this kinase (47). Enforced expression of c-Myc, we found, prevents TGF-β from triggering this set of events.
The absence of a TGF-β-induced p27 redistribution in cells expressing c-Myc provides one indication that activation of the p15 pathway is impaired in these cells. Indeed, our results show that the enforced expression of c-Myc inhibits the TGF-β-induced increase in p15 mRNA levels. Another gene response to TGF-β, PAI-1 induction, is only partially inhibited by c-Myc. Thus, c-Myc preferentially inhibits the p15 response. Gene responses to TGF-β are thought to be mediated by Smad signaling (11, 17, 32). Various mechanisms are known that inhibit Smad signaling, but most of these prevent Smad accumulation in the nucleus (23, 24, 39, 59). Such mechanisms are unlikely to mediate the effect of c-Myc, because Smad nuclear accumulation in response to TGF-β is normal in these c-Myc-expressing Mv1Lu cells. Rather, c-Myc may act by repressing the p15 promoter. We show that c-Myc represses the activity of the p15 promoter in reporter assays, with or without the presence of TGF-β. p15 contains the consensus Inr transcriptional initiator sequence previously identified in other genes that are transcriptionally repressed by c-Myc (14, 29, 42). The mechanism of p15 promoter inhibition by c-Myc is under investigation.
Latent and active cdk4 complexes as targets of TGF-β and p15.
Analysis of the oligomeric state of cdk4 in these cells by size exclusion chromatography provides novel insights into cdk4 regulation and lends further support to the hypothesis that c-Myc specifically interferes with cdk4 inhibition by p15. cdk4 from exponentially growing mink lung epithelial cells is distributed among three populations: a high-molecular-mass (>400-kDa) pool; an intermediate pool containing cyclin D-bound, catalytically active cdk4; and an inactive pool of monomeric cdk4. A similar distribution has been observed with cdk4 and cdk6 in other cell types (30, 36, 40, 56). The active cdk4 pool represents only a small fraction of the total cdk4 in the epithelial cells studied here. This pool probably contains cyclin D-cdk4 and p27-cyclin D-cdk4 complexes, since it contains p27-bound cdk4. Our previous work has shown that in these cells, p27 is mostly bound to cyclin D-cdk4 (5, 47), forming p27-cyclin D-cdk4 complexes that remain active despite containing this inhibitor (5).
The high-molecular-weight (latent) cdk4 pool, accounting for approximately half of the cdk4 in proliferating cells, is much more abundant than the active pool. A similar high-molecular-weight pool in NIH 3T3 fibroblasts contains cdk4 bound to hsp90 and cdc37 (56). It has been proposed that cdc37 targets hsp90 to cdk4, yielding a chaperone complex that may be involved in cyclin D-cdk4 complex assembly in proliferating cells (56). Consistent with this hypothesis, we show that expression of exogenous cyclin D1 in tet-D1 cells causes a depletion of this complex and an increase in the cyclin D-bound cdk4 pool. Thus, the latent cdk4 pool acts as a source of cdk4 for cyclin D and/or a reservoir that collects excess cdk4 not bound to cyclin D.
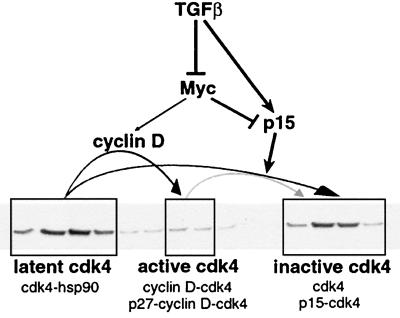
As schematically shown in Fig. 8, our evidence suggests that the latent cdk4 pool, along with the active cdk4 pool, is a major target of p15. Expression of exogenous p15 in tet-p15 cells causes the loss of both pools and a concomitant increase in inactive cdk4. A similar loss of these cdk4 populations has been observed upon p16 induction in U2-OS cells, suggesting that this may be a general Ink4 effect (36). This effect, however, is not observed when cell proliferation is arrested by other mechanisms, such as overexpression of p27 or contact inhibition. Furthermore, cell treatment with TGF-β converts the latent and active cdk4 pools into inactive cdk4, thus mimicking the characteristic effect of p15. This observation is consistent with the proposed role of p15 as a central mediator of the effects of TGF-β on G1 cdks (15, 44, 48).
FIG. 8.
Schematic representation of cdk4 complexes and their control by TGF-β, p15, and c-Myc in Mv1Lu cells. In proliferating epithelial cells, endogenous cdk4 is distributed among three populations: an abundant high-molecular-weight pool of latent cdk4, a low-abundance pool containing active cyclin D-cdk4 and p27-cyclin D-cdk4 complexes, and an inactive population of monomeric and Ink4b-bound cdk4. The latent pool serves as a source of cdk4 for cyclin D, yielding active cyclin D-cdk4 complexes. TGF-β and p15 target both the latent and active forms of cdk4, converting these to the inactive cdk4 population. TGF-β causes rapid downregulation of c-myc and upregulation of p15. cdk4 targeting by TGF-β is prevented by the enforced expression of normal levels of c-Myc, suggesting that c-Myc downregulation by TGF-β is required for activation of the p15 G1 arrest pathway.
Impairment of the p15 pathway as a major anti-TGF-β mechanism of c-Myc.
On its own, enforced expression of c-Myc has little effect on the levels and distribution of cdk4 complexes in these epithelial cells. However, c-Myc blocks the effect of TGF-β on the latent and active cdk4 pools. The state of cdk4 in c-Myc-expressing cells treated with TGF-β is almost identical to that in exponentially growing parental cells. c-Myc therefore prevents the dramatic changes in cdk4 complexes induced by TGF-β, which are changes characteristic of p15 action.
c-Myc expression in these cells causes small increases in the levels of cyclins D1 and D2 and cyclin D-bound cdk4. An increase in the levels of cyclin D-cdk could provide in principle a larger sink for a finite amount of p15 in TGF-β-treated cells. However, several observations argue against a major role of cyclin D in the TGF-β resistance imparted by c-Myc in these cells. First, the increase in cyclin D-cdk4 levels induced by c-Myc represents a minimal alteration in the size of the latent and active cdk4 pools. Second, a much larger increase in cyclin D-cdk4 levels caused by overexpression of cyclin D1 in tet-D1 cells confers only a partial resistance to TGF-β whereas c-Myc expression confers full resistance. Collectively, the evidence suggests that inhibition of p15 induction is the major mechanism by which c-Myc interferes with the TGF-β antimitogenic response in lung epithelial cells.
Several mechanisms have been proposed to explain the ability of exogenous c-Myc to prevent cell cycle arrest in cells overexpressing exogenous cdk inhibitors. In Rat1 fibroblasts, exogenous c-Myc can prevent a p16-induced cell cycle arrest without restoring Rb phosphorylation (1). It has been suggested that c-Myc may induce the sequestration of p27, which would increase the activity of cyclin E-cdk2, thus compensating for the inhibitory effect of p16 on cyclin D-dependent kinases (1, 41, 55, 60). However, c-Myc expression in the epithelial cells studied here does not change the endogenous levels of p27-bound cdk4 or p27-bound cdk2. c-Myc prevents the events that would inhibit cdk4 in the course of a TGF-β response and does so without altering the level or cdk association pattern of p27. While the enforced expression of c-Myc in some cell types may cause sequestration of endogenous p27 away from cdks, our evidence does not support the idea that this mechanism is operating in mink lung epithelial cells. Our results argue that c-Myc antagonizes the normal antimitogenic effect of TGF-β by preventing, not bypassing, p15 inhibition of cyclin D-dependent kinases.
Rapid downregulation of c-myc is a common TGF-β response in epithelial and endothelial cells. As summarized in Fig. 8, the present evidence suggests that c-Myc downregulation by TGF-β is required for activation of the p15 G1 arrest pathway. Although the increase in p15 mRNA levels as a result of TGF-β is relatively rapid (half maximal in 2 h [15, 48]), the fast kinetics of c-myc repression by TGF-β (43, 61), coupled with the short half-life of the protein (9), can cause an extensive decrease in c-Myc levels prior to p15 mRNA accumulation. Downregulation of c-myc by TGF-β is generally accompanied by cdk-inhibitory gene responses that vary with the cell type. Besides induction of p15, these responses may include induction of p21 (10, 31, 48) and repression of cdc25A (20). c-Myc downregulation by TGF-β might also be a condition for induction of these responses in other cell types. A better understanding of the mechanism of gene regulation by TGF-β is required to determine how c-Myc inhibits p15 induction and possibly other antiproliferative gene responses to TGF-β.
ACKNOWLEDGMENTS
We thank Mark Ewen for the mink cdk4 sequence, Akiko Hata and Celio Pouponnot for p15 reporter constructs and advice, Inga Reynisdóttir for tet-cyclin D1 cells, and Andy Koff for critical discussion.
S.W.B. is a Fellow of the Leukemia Society of America. J.S. is a Fellow of the Ministerio de Educación y Cultura of Spain. B.J.W. and J.M. are, respectively, a Fellow and an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Alevizopoulos K, Vlach J, Hennecke S, Amati B. Cyclin E and c-Myc promote cell proliferation in the presence of p16Ink4a and of hypophosphorylated retinoblastoma family proteins. EMBO J. 1997;16:5322–5333. doi: 10.1093/emboj/16.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexandrow M G, Moses H L. Transforming growth factor β and cell cycle regulation. Cancer Res. 1995;55:1452–1457. [PubMed] [Google Scholar]
- 3.Amati B, Alevizopoulos K, Vlach J. Myc and the cell cycle. Front Biosci. 1998;3:250–268. doi: 10.2741/a239. [DOI] [PubMed] [Google Scholar]
- 4.Attisano L, Cárcamo J, Ventura F, Weis F M B, Massagué J, Wrana J L. Identification of human Activin and TGF-β type I receptors that form heteromeric kinase complexes with type II receptors. Cell. 1993;75:671–680. doi: 10.1016/0092-8674(93)90488-c. [DOI] [PubMed] [Google Scholar]
- 5.Blain S W, Montalvo E, Massague J. Differential interaction of the cyclin-dependent kinase (cdk) inhibitor p27 with cyclin A-cdk2 and cyclin D2-cdk4. J Biol Chem. 1997;272:25863–25872. doi: 10.1074/jbc.272.41.25863. [DOI] [PubMed] [Google Scholar]
- 6.Bouchard C, Staller P, Eilers M. Control of cell proliferation by Myc. Trends Cell Biol. 1998;8:202–206. doi: 10.1016/s0962-8924(98)01251-3. [DOI] [PubMed] [Google Scholar]
- 7.Brotherton D H, Dhanaraj V, Wick S, Brizuela L, Domaille P J, Volyanik E, Wu W, Parisini E, Smith B O, Archer S J, Serrano M, Brenner S L, Blundell T L, Laue E D. Crystal structure of the complex of the cyclin D-dependent kinase cdk6 bound to the cell-cycle inhibitor p19Ink4D. Nature. 1998;395:244–250. doi: 10.1038/26164. [DOI] [PubMed] [Google Scholar]
- 8.Cherif D, Le Coniat M, Suarez H G, Bernheim A, Berger R. Chromosomal localization of amplified c-myc in a human colon adenocarcinoma cell line with a biotinylated probe. Cancer Genet Cytogenet. 1988;33:245–249. doi: 10.1016/0165-4608(88)90034-9. [DOI] [PubMed] [Google Scholar]
- 9.Dang C V. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datto M B, Li Y, Panus J F, Howe D J, Xiong Y, Wang X-F. Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanisms. Proc Natl Acad Sci USA. 1995;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derynck R, Gelbart W M, Harland R M, Heldin C-H, Kern S E, Massagué J, Melton D A, Mlodzik M, Padgett R M. Nomenclature: vertebrate mediators of TGFβ family signals. Cell. 1996;87:173. doi: 10.1016/s0092-8674(00)81335-5. [DOI] [PubMed] [Google Scholar]
- 12.Evan G I, Brown L, Whyte M, Harrington E. Apoptosis and the cell cycle. Curr Opin Cell Biol. 1995;7:825–834. doi: 10.1016/0955-0674(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 13.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grandori C, Eisenman R. Myc target genes. Trends Biochem Sci. 1997;22:177–181. doi: 10.1016/s0968-0004(97)01025-6. [DOI] [PubMed] [Google Scholar]
- 15.Hannon G J, Beach D. p15INK4B is a potential effector of TGF-β-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 16.Harlow E, Lane D. Antibodies: a laboratory handbook. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 17.Heldin C-H, Miyazono K, ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 18.Henriksson M, Luscher B. Proteins of the Myc network: essential regulators of cell growth and differentiation. Adv Cancer Res. 1996;68:109–182. doi: 10.1016/s0065-230x(08)60353-x. [DOI] [PubMed] [Google Scholar]
- 19.Hua X, Liu X, Ansari D O, Lodish H F. Synergistic cooperation of TFE3 and Smad proteins in TGF-β-induced transcription of the plasminogen activator inhibitor-1 gene. Genes Dev. 1998;12:3084–3095. doi: 10.1101/gad.12.19.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iavarone A, Massagué J. Repression of the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-β in cells lacking the CDK inhibitor p15. Nature. 1997;387:417–422. doi: 10.1038/387417a0. [DOI] [PubMed] [Google Scholar]
- 21.Khaira P, James C D, Leffak M. Amplification of the translocated c-myc genes in three Burkitt lymphoma cell lines. Gene. 1998;211:101–108. doi: 10.1016/s0378-1119(98)00104-8. [DOI] [PubMed] [Google Scholar]
- 22.Koff A, Ohtsuki M, Polyak K, Roberts J M, Massagué J. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-β. Science. 1993;260:536–539. doi: 10.1126/science.8475385. [DOI] [PubMed] [Google Scholar]
- 23.Kretzschmar M, Doody J, Massagué J. Opposing BMP and EGF signalling pathways converge on the TGFβ family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- 24.Kretzschmar M, Doody J, Timokhina I, Massagué J. A mechanism of repression of TGF-β/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laiho M, DeCaprio J A, Ludlow J W, Livingston D M, Massagué J. Growth inhibition by TGF-β1 linked to suppression of retinoblastoma protein phosphorylation. Cell. 1990;62:175–185. doi: 10.1016/0092-8674(90)90251-9. [DOI] [PubMed] [Google Scholar]
- 26.Lehman T A, Modali R, Boukamp P, Stanek J, Bennet W P, Welsh J A, Metcalf R A, Stampfer M R, Fusenig N, Rogan E M, Harris C C. p53 Mutations in human immortalized epithelial cell lines. Carcinogenesis. 1993;14:833–839. doi: 10.1093/carcin/14.5.833. [DOI] [PubMed] [Google Scholar]
- 27.Leone G, DeGregori J, Sears R, Jokoi L, Nevins J R. Myc and Ras collaborate in inducing accumulation of active cyclinE/cdk2 and E2F. Nature. 1997;387:422–426. doi: 10.1038/387422a0. [DOI] [PubMed] [Google Scholar]
- 28.Li J-M, Nichols M A, Chandrasekharan S, Xiong Y, Wang X-F. Transforming growth factor ß activates the promoter of cyclin-dependent kinase inhibitor p15Ink4B through an Sp1 consensus site. J Biol Chem. 1995;270:26750–26753. doi: 10.1074/jbc.270.45.26750. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Nerlov C, Prendergast G, MacGregor D, Ziff E B. c-Myc represses transcription in vivo by a novel mechanism dependent on the initiator element and Myc box II. EMBO J. 1994;13:4070–4079. doi: 10.1002/j.1460-2075.1994.tb06724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahony D, Parry D A, Lees E. Active cdk6 complexes are predominantly nuclear and represent only a minority of the cdk6 in T cells. Oncogene. 1998;16:603–611. doi: 10.1038/sj.onc.1201570. [DOI] [PubMed] [Google Scholar]
- 31.Malliri A, Yeudall W A, Nikolic M, Crouch D H, Parkinson E K, Ozanne B. Sensitivity to transforming growth factor beta-1 induced growth arrest is common in human squamous cell carcinoma cell lines: c-Myc downregulation and p21Waf1 induction are important early events. Cell Growth Differ. 1996;7:1291–1304. [PubMed] [Google Scholar]
- 32.Massagué J. TGFβ signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 33.Massagué J. The transforming growth factor-ß1 family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 34.Masuda A, Kondo M, Saito T, Yatabe Y, Kobayashi T, Okamoto M, Suyama M, Takahashi T, Takahashi T. Establishment of human peripheral lung epithelial cell lines (HPL1) retaining differentiated characteristics and responsiveness to epidermal growth factor, hepatocyte growth factor, and transforming growth factor beta1. Cancer Res. 1997;57:4898–4904. [PubMed] [Google Scholar]
- 35.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J-Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McConnell B B, Gregory F J, Stott F J, Hara E, Peters G. Induced expression of p16Ink4a inhibits both cdk4- and cdk2-associated kinase activity by reassortment of cyclin-cdk inhibitor complexes. Mol Cell Biol. 1999;19:1981–1989. doi: 10.1128/mcb.19.3.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moustakas A, Kardassis D. Regulation of the human p21/WAF1/Cip1 promoter in hepatic cells by functional interactions between Sp1 and Smad family members. Proc Natl Acad Sci USA. 1998;95:6733–6738. doi: 10.1073/pnas.95.12.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller D, Bouchard C, Rudolph B, Steiner P, Stuckmann I, Saffrich R, Ansorge W, Huttner W, Eilers M. Cdk2-dependent phosphorylation of p27 facilitates its Myc-induced release from cyclin E/cdk2 complexes. Oncogene. 1997;15:2561–2576. doi: 10.1038/sj.onc.1201440. [DOI] [PubMed] [Google Scholar]
- 39.Nakao A, Afrakhte M, Morén A, Nakayama T, Christian J L, Heuchel R, Itoh S, Kawabata M, Heldin N E, Heldin C H, ten Dijke P. Identification of Smad7, a TGFß-inducible antagonist of TGF-ß signaling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 40.Parry D, Mahony D, Wills K, Lees E. Cyclin D-cdk subunit arrangement is dependent on the availability of competing Ink4 and p21 class inhibitors. Mol Cell Biol. 1999;19:1775–1783. doi: 10.1128/mcb.19.3.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez-Roger I, Solomon D L C, Sewing A, Land H. Myc activation of cyclin E/cdk2 kinase involves induction of cyclin E gene transcription and inhibition of p27Kip1 binding to newly formed complexes. Oncogene. 1997;14:2373–2381. doi: 10.1038/sj.onc.1201197. [DOI] [PubMed] [Google Scholar]
- 42.Philipp A, Schneider A, Vasrik I, Finke K, Xiong Y, Beach D, Alitalo K, Eilers M. Repression of cyclin D1: a novel function of MYC. Mol Cell Biol. 1994;14:4032–4043. doi: 10.1128/mcb.14.6.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pietenpol J A, Stein R W, Moran E, Yacuik P, Schlegel R, Lyons R M, Pittelkow R M, Münger K, Howley P M, Moses H L. TGF-β1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming protein with pRB binding domeins. Cell. 1990;61:777–785. doi: 10.1016/0092-8674(90)90188-k. [DOI] [PubMed] [Google Scholar]
- 44.Polyak K. Negative regulation of cell growth by TGF-β. Biochim Biophys Acta. 1996;1242:185–199. doi: 10.1016/0304-419x(95)00009-5. [DOI] [PubMed] [Google Scholar]
- 45.Polyak K, Kato J-Y, Solomon M J, Sherr C J, Massagué J, Roberts J M, Koff A. p27Kip1, a cyclin-cdk inhibitor, links transforming growth factor-ß and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 46.Polyak K, Lee M-H, Erdjument-Bromage H, Koff A, Tempst P, Roberts J M, Massagué J. Cloning of p27Kip1 a cyclin-cdk inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 47.Reynisdóttir I, Massagué J. The subcellular locations of p15Ink4b and p27Kip1 coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 1997;11:492–503. doi: 10.1101/gad.11.4.492. [DOI] [PubMed] [Google Scholar]
- 48.Reynisdóttir I, Polyak K, Iavarone A, Massagué J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-β. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 49.Roberts A B, Sporn M B. The transforming growth factor-betas. Heidelberg, Germany: Springer-Verlag KG; 1990. [Google Scholar]
- 50.Russo A A, Tong L, Lee J-O, Jeffrey P D, Pavletich N. Structural basis for inhibition of the cyclin-dependent kinase cdk6 by the tumour suppressor p16Ink4a. Nature. 1998;395:237–243. doi: 10.1038/26155. [DOI] [PubMed] [Google Scholar]
- 51.Sherr C J. The ins and outs of Rb: coupling gene expression to the cell cycle clock. Trends Cell Biol. 1994;4:15–18. doi: 10.1016/0962-8924(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 52.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 53.Song C-Z, Siok T E, Gelehrter T D. Smad4/DPC4 and Smad3 mediate transforming growth factor-β (TGF-β) signaling through direct binding to a novel TGF-β-responsive element in human plasminogen activator inhibitor-1 protein. J Biol Chem. 1998;273:29287–29290. doi: 10.1074/jbc.273.45.29287. [DOI] [PubMed] [Google Scholar]
- 54.Soos T J, Kiyokawa H, Yan J S, Rubin M S, Giordano A, DeBlasio A, Bottega S, Wong B, Mendelsohn J, Koff A. Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ. 1996;7:135–146. [PubMed] [Google Scholar]
- 55.Steiner P, Philipp A, Lukas J, Godden-Kent D, Pagano M, Mittnacht S, Bartek J, Eilers M. Identification of a myc-dependent step during formation of active G1 cyclin-cdk complexes. EMBO J. 1995;14:4814–4826. doi: 10.1002/j.1460-2075.1995.tb00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stepanova L, Leng X, Parker S G, Harper J W. Mammalian p50cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes cdk4. Genes Dev. 1996;10:1491–1502. doi: 10.1101/gad.10.12.1491. [DOI] [PubMed] [Google Scholar]
- 57.Sun P, Dong P, Dai K, Hannon G J, Beach D. p53-independent role of MDM-2 in TGF-β resistance. Science. 1998;282:2270–2272. doi: 10.1126/science.282.5397.2270. [DOI] [PubMed] [Google Scholar]
- 58.Tonini G P, Radzioch D, Gronberg A, Clayton M, Blasi E, Benetton G, Varesio L. Erythroid differentiation and modulation of c-myc expression induced by antineoplastic drugs in the human leukemic cell line K562. Cancer Res. 1987;47:4544–4547. [PubMed] [Google Scholar]
- 59.Ulloa L, Doody J, Massagué J. Inhibition of transforming growth factor β/SMAD signalling by the interferon-γ/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- 60.Vlach J, Hennecke S, Alevizopoulos K, Conti D, Amati B. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J. 1996;15:6595–6604. [PMC free article] [PubMed] [Google Scholar]
- 61.Zentella A, Weis F M B, Ralph D A, Laiho M, Massagué J. Early gene responses to transforming growth factor β in cells lacking growth suppressive RB function. Mol Cell Biol. 1991;11:4952–4958. doi: 10.1128/mcb.11.10.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]