Abstract
Objectives
Initial studies of individuals with coronavirus disease 2019 (COVID-19) revealed that obesity, diabetes and hypertension were associated with severe outcomes. Subsequently, some authors showed that the risk could vary according to age, gender, co-morbidities and medical history. In a nationwide retrospective cohort, we studied the association between these co-morbidities and patients' requirement for invasive mechanical ventilation (IMV) or their death.
Methods
All French adult inpatients with COVID-19 admitted during the first epidemic wave (February to September 2020) were included. When patients were diagnosed with obesity, diabetes or hypertension for the first time in 2020, these conditions were considered as incident co-morbidities, otherwise they were considered prevalent. We compared outcomes of IMV and in-hospital death according to obesity, diabetes and hypertension, taking age, gender and Charlson's co-morbidity index score (CCIS) into account.
Results
A total of 134 209 adult inpatients with COVID-19 were included, half of them had hypertension (n = 66 613, 49.6%), one in four were diabetic (n = 32 209, 24.0%), and one in four were obese (n = 32 070, 23.9%). Among this cohort, IMV was required for 13 596 inpatients, and 19 969 patients died. IMV and death were more frequent in male patients (adjusted oods ratio (aOR) 2.0, 95% CI 1.9–2.1 and aOR 1.5, 95% CI 1.4–1.5, respectively), IMV in patients with co-morbidities (aOR 2.1, 95% CI 2.0–2.2 for CCIS = 2 and aOR 3.0, 95% CI 2.8–3.1 for CCIS ≥5), and death in patients aged 80 or above (aOR 17.0, 95% CI 15.5–18.6). Adjusted on age, gender and CCIS, death was more frequent among inpatients with obesity (aOR 1.2, 95% CI 1.1–1.2) and diabetes (aOR 1.2, 95% CI 1.1–1.2). IMV was more frequently necessary for inpatients with obesity (aOR 1.9, 95% CI 1.8–2.0), diabetes (aOR 1.4, 95% CI 1.3–1.4) and hypertension (aOR 1.7, 95% CI 1.6–1.8). Comparatively, IMV was more often required for patients with the following incident co-morbidities: obesity (aOR 3.5, 95% CI 3.3–3.7), diabetes (aOR 2.0, 95% CI 1.8–2.1) and hypertension (aOR 2.5, 95% CI 2.4–2.6).
Conclusions
Among 134 209 inpatients with COVID-19, mortality was more frequent among patients with obesity and diabetes. IMV was more frequently necessary for inpatients with obesity, diabetes and hypertension. Patients for whom these were incident co-morbidities were particularly at risk. Specific medical monitoring and vaccination should be priorities for patients with these co-morbidities.
Keywords: Coronavirusdisease 2019, Diabetes, Hospital mortality, Hypertension, Mechanical ventilation, Obesity, Risk factors
Graphical abstract
Introduction
Coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization on 11 March 2020 [1] and rapidly overwhelmed healthcare resources across the world [2]. Initial descriptive data from China [3,4] showed that patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection admitted to hospital and at risk for severe disease resulting in the need for invasive mechanical ventilation (IMV) or in death, were more often those with diabetes or hypertension, and a meta-analysis confirmed these findings [5]. Identifying individuals most at risk is of paramount importance to adequately target the currently available anti-COVID-19 vaccines.
In the western world, studies of co-morbidities associated with an unfavourable outcome pointed to obesity [6] and diabetes [7] as the main risk factors [8,9]. Concomitantly, a nationwide study conducted on 87 000 American army veterans who had been screened for SARS-CoV-2 failed to demonstrate a correlation between obesity and the occurrence of severe disease, mechanical ventilation or death, after adjusting for co-morbidities [10].
In a study conducted in England by Williamson et al. investigating factors associated with 10 926 COVID-19-related deaths, diabetes was shown to be significantly associated with mortality, as were a large number of other medical conditions [11]. Similarly, a national Danish study of 11 222 patients with positive SARS-CoV-2 PCR test also revealed a significant association between hypertension and risk of death [12]. Recently, a meta-analysis pooling 3700 patients hospitalized with COVID-19 has found a high proportion of individuals with newly diagnosed diabetes, who are suspected to be more at risk of severe outcomes than patients with pre-existing diabetes [13].
In France, the French National Health Insurance Information System [14,15] records the care pathways of all patients admitted to hospital over the previous 10 years, i.e. between 2010 and 2020 for the present study. This provides an opportunity to distinguish between patients previously diagnosed with obesity, diabetes or hypertension between 2010 and 2019 and those admitted in 2020 and diagnosed with one of these conditions for the first time.
Our study, among patients admitted to hospital for COVID-19, focused on severe outcomes, i.e. death and patients with severe respiratory distress requiring IMV. The aim was to compare the frequency of occurrence of severe forms of the disease among patients with obesity, diabetes or hypertension admitted to hospital for COVID-19, with the frequency of occurrence among patients admitted to hospital for COVID-19 without these co-morbidities, taking their age, gender, number of other co-morbidities and past medical history into account.
Materials and methods
Data source and study population
All patients aged 18 years or more, admitted to hospital for COVID-19 during the first epidemic wave, between February and September 2020, were included in this national retrospective cohort study, after retrieving the data from the French National Health Insurance Information System. This is the largest medical and administrative database available for research [16]. All public and private French hospital admissions between 2010 and 2020 can be linked and include diagnoses and management details, thanks to the International Disease Classification, tenth revision (ICD-10) and the French medical classification for clinical procedures (Classification Commune des Actes Médicaux: CCAM), respectively.
Our cohort comprises all the patients admitted with an ICD-10 code for COVID-19 (U071, U0710, U0711, U0712, U0714 and U0715), which was included in the ICD-10 code in France as early as 31 January 2020 and updated on 23 October 2020 by the Technical Agency for Information on Hospital admissions, which collects all public and private hospital admission and discharge summaries [17].
Patients who had required IMV were identified by the following CCAM codes: GLLD002, GLLD004, GLLD006, GLLD007, GLLD008, GLLD012, GLLD013, GLLD015 and GLLD021.
Hospital mortality due to COVID-19 was informed by patient outcome data. The discharge summary, in all public and private French hospitals, states whether the patient is alive or dead at the end of the hospital stay. These discharge summaries are standardized, mandatory and have constituted the basis of hospital funding since 2008. This study was approved by the Health Data Hub (registration number no. F20200924115342) and declared (Déclaration CNIL no. 2211454 v 0) to the French National Data Protection Authority (Commission Nationale Informatique et Liberté).
Variables
Three patient categories were defined according to hospital admissions during the decade preceding admission for COVID-19: (a) a reference category including patients admitted for COVID-19 with no diagnosis of hypertension, diabetes or obesity during their current nor any previous hospitalization between 2010 and 2020; (b) patients admitted for COVID-19 and diagnosed during a previous hospitalization between 2010 and 2019 and still presenting with one or more of these conditions (prevalent co-morbidity); (c) patients diagnosed with hypertension, diabetes or obesity for the first time during their current admission for COVID-19 (incident co-morbidity).
Co-morbid conditions considered in this study are those defined by the Charlson co-morbidity index score (CCIS) [18]. ICD-10 codes matching each item for this index were extracted according to the algorithm developed by Quan et al. [19]. To investigate the impact of the number of co-morbidities, we compared patients without CCI conditions, i.e. CCIS = 0, and patients with at least one of them, ranked from CCIS = 1 to CCIS >12 (CCIS = 1 versus CCIS = 0, CCIS = 2 versus CCIS = 0 and so on). The variables gender, age category by 10-year increments, and CCIS stratified according to eight categories (0, 1, 2, 3, 4, 5–7, 8–12 and > 12) were used in multivariate analysis.
Statistical analysis
Categorical variables are shown as frequency rates and percentage, and continuous variables as means and standard deviation. Chi-squared test and Student's t test or analysis of variance were used. We applied a logistic regression to test whether obesity, diabetes and hypertension were associated with severe outcomes in patients admitted to hospital for COVID-19, even after adjusting for previously described predictors of death and IMV. Variables included in the final model, i.e. age, gender and CCIS, were selected on an a priori decision. The Wald method was used for the OR confidence interval calculation. The overall evaluation and predictive accuracy of the final model were calculated with likelihood ratio and C-statistic.
The main end points were death, IMV, and death following IMV. We employed univariate and multivariate models to explore the association between these end points and obesity, diabetes and hypertension. For the percentage of death following IMV, only patients undergoing IMV were considered. The specific multivariate analysis for diabetes was performed adjusting for the 15 Charlson's index items, excluding complicated and uncomplicated diabetes. All statistical analyses were performed using SAS© version 9.4 (SAS Institute, Cary, NC, USA).
Results
This study was conducted on 134 209 patients aged 18 years or above, admitted to hospital for COVID-19 in France between February and September 2020. Mean age was 63.8 (±19.8) years, sex ratio was 1.1 and median CCIS was 1.0 (interquartile range 0.0–4.0). Fig. 1 shows the proportion of patients who required mechanical ventilation, as well as in-hospital mortality by age and gender.
Fig. 1.
Mechanical ventilation, mortality and Charlson's score according to age and gender.
Table 1 shows in-hospital mortality, according to gender, age, CCIS and the association between obesity, diabetes or hypertension and deaths. Deaths were more frequent among men admitted for COVID-19 than among women, (adjusted OR (aOR) 1.5, 95% CI 1.4–1.5). In-hospital mortality was 17-fold higher, after adjusting for gender and CCIS, among patients admitted with COVID-19 aged 80 years and above, compared with those aged 18–49 years. The in-hospital mortality rate increased with the number of co-morbidities, reaching aOR 3.0 (95% CI 2.8–3.1) for patients with a CCIS between 5 and 7. Overall, after adjusting for age, gender and CCIS, in-hospital mortality was significantly higher among patients with obesity and among patients with diabetes. A statistically significant association was found between overall mortality among patients with prevalent obesity and diabetes but not among patients with incident obesity and diabetes.
Table 1.
Death according to gender, age, CCIS and association with obesity, diabetes or hypertension in 134 209 inpatients positive for SARS-CoV-2
| Patients, n | Deaths, n (%) | Univariate analysis (95% CI) | Multivariate analysis adjusted on age and CCIS (95% CI) | |
|---|---|---|---|---|
| Female | 63 872 | 8226 (12.9) | 1 (Ref.) | 1 (Ref.) |
| Male |
70 337 |
11 743 (16.7) |
1.4 (1.3–1.4) |
1.5 (1.4–1.5) |
| Patients, n |
Deaths, n (%) |
Univariate analysis (95% CI) |
Multivariate analysis adjusted on sex and CCIS (95% CI) |
|
| Age 18–49 years | 32 928 | 524 (1.6) | 1 (Ref.) | 1 (Ref.) |
| Age 50–65 years | 33 328 | 2683 (8.0) | 5.4 (4.9–6.0) | 4.1 (3.7–4.5) |
| Age 66–79 years | 32 554 | 6113 (18.8) | 14.3 (13.1–15.7) | 9.1 (8.3–10.0) |
| Age ≥80 years |
35 399 |
10 649 (30.1) |
26.6 (24.3–29.1) |
17.0 (15.5–18.6) |
| Patients, % (n) |
Deaths, % (n) |
Univariate analysis (95% CI) |
Multivariate analysis adjusted on age and sex (95% CI) |
|
| CCIS = 0 | 36.4 (48 788) | 4.9 (2412) | 1 (Ref.) | 1 (Ref.) |
| CCIS = 1 | 16.7 (22 468) | 13.3 (2985) | 2.9 (2.8–3.1) | 1.6 (1.5–1.7) |
| CCIS = 2 | 11.2 (15 088) | 18.2 (2749) | 4.3 (4.0–4.5) | 2.0 (1.8–2.1) |
| CCIS = 3 | 8.8 (11 861) | 20.8 (2462) | 5.0 (4.7–5.3) | 2.1 (1.9–2.2) |
| CCIS = 4 | 6.4 (8540) | 24.1 (2058) | 6.1 (5.7–6.5) | 2.4 (2.2–2.5) |
| CCIS from 5 to 7 | 13.9 (18 643) | 26.7 (4968) | 7.0 (6.6–7.4) | 3.0 (2.8–3.1) |
| CCIS from 8 to 12 | 5.9 (7882) | 27.5 (2167) | 7.3 (6.8–7.8) | 3.2 (3.0–3.4) |
| CCIS >12 |
0.7 (939) |
17.9 (168) |
4.2 (3.5–5.0) |
2.7 (2.2–3.2) |
| Patients, % (n) |
Deaths, % (n) |
Univariate analysis (95% CI) |
Multivariate analysis adjusted on sex, age and CCIS (95% CI) |
|
| No obesity | 76.1 (102 139) | 14.8 (15 067) | 1 (Ref.) | 1 (Ref.) |
| Obesity (total) | 23.9 (32 070) | 15.2 (4902) | 1.04 (1.01–1.08) | 1.17 (1.13–1.22) |
| Prevalent obesity | 67.9 (21 768) | 17.7 (3856) | 1.24 (1.20–1.29) | 1.27 (1.22–1.33) |
| Incident obesity | 32.1 (10 302) | 10.2 (1046) | 0.65 (0.61–0.70) | 0.94 (0.88–1.01) |
| No diabetes | 76.0 (100 200) | 13.3 (13 523) | 1 (Ref.) | 1 (Ref.) |
| Diabetes (total) | 24.0 (32 209) | 20.0 (6446) | 1.64 (1.58–1.69) | 1.16 (1.12–1.20) |
| Prevalent diabetes | 71.5 (23 040) | 22.4 (5166) | 1.89 (1.82–1.96) | 1.22 (1.17–1.27) |
| Incident diabetes | 28.5 (9169) | 14.0 (1280) | 1.06 (1.00–1.13) | 1.00 (0.94–1.07) |
| No hypertension | 50.6 (67 596) | 8.6 (5799) | 1 (Ref.) | 1 (Ref.) |
| Hypertension (total) | 49.4 (66 613) | 21.3 (14 170) | 2.88 (2.79–2.98) | 0.97 (0.94–1.01) |
| Prevalent hypertension | 73.5 (48 932) | 23.5 (11 500) | 3.27 (3.16–3.39) | 0.99 (0.96–1.04) |
| Incident hypertension | 26.5 (17 681) | 15.1 (2670) | 1.90 (1.80–1.99) | 0.92 (0.87–0.97) |
Abbreviation: CCIS, Charlson's co-morbidity index score.
Table 2 shows the use of IMV according to gender, age, CCIS and the association between obesity, diabetes or hypertension and the requirement for IMV. Men admitted with COVID-19 were twice as likely to require IMV as women, after adjusting for age and CCIS (aOR 2.0, 95% CI 1.9–2.1). Compared with patients with no co-morbidity, i.e. a CCIS of 0, the adjusted OR for the use of IMV was 2.1 (95% CI 2.0–2.2) for those with a score of 2 adjusting for age and gender. After adjusting for age, gender and CCIS, use of IMV was significantly more frequent for patients with obesity, hypertension and diabetes. Use of IMV was three times more frequent among patients with incident obesity than among non-obese patients. Associations between obesity, diabetes or hypertension and mortality following IMV are shown in Table 3 . Overall, after adjusting for age, gender and CCIS, in-hospital mortality after IMV was significantly higher among patients with a prevalent co-morbidity: obesity, diabetes or hypertension.
Table 2.
Invasive mechanical ventilation according to gender, age, CCIS and association with obesity, diabetes or hypertension
| Patients, n | Invasive mechanical ventilation, n (%) | Univariate analysis (95% CI) | Multivariate analysis adjusted on age and CCIS (95% CI) | |
|---|---|---|---|---|
| Female | 63 872 | 3883 (6.1) | 1 (Ref.) | 1 (Ref.) |
| Male |
70 337 |
9713 (13.8) |
2.5 (2.4–2.6) |
2.0 (1.5–2.1) |
| Patients, n |
Invasive mechanical ventilation, n (%) |
Univariate analysis (95% CI) |
Multivariate analysis adjusted on sex and CCIS (95% CI) |
|
| Age ≥80 years | 35 399 | 931 (2.6) | 1 (Ref.) | 1 (Ref.) |
| Age 66 to 79 years | 32 554 | 5257 (16.2) | 7.1 (6.6–7.7) | 6.8 (6.3–7.3) |
| Age 50 to 65 years | 33 328 | 5461 (16.4) | 7.3 (6.8–7.8) | 7.8 (7.2–8.4) |
| Age 18 to 49 years |
32 928 |
1947 (5.9) |
2.3 (2.1–2.5) |
3.2 (2.9–3.4) |
| Patients, % (n) |
Invasive mechanical ventilation, % (n) |
Univariate analysis (95% CI) |
Multivariate analysis adjusted on age and sex (95% CI) |
|
| CCIS = 0 | 36.4 (48 788) | 7.2 (3495) | 1 (Ref.) | 1 (Ref.) |
| CCIS = 1 | 16.7 (22 468) | 11.6 (2596) | 1.7 (1.6–1.8) | 1.7 (1.6–1.8) |
| CCIS = 2 | 11.2 (15 088) | 13.1 (1982) | 2.0 (1.8–2.1) | 2.1 (2.0–2.2) |
| CCIS = 3 | 8.8 (11 861) | 12.6 (1496) | 1.9 (1.7–2.0) | 2.1 (2.0–2.3) |
| CCIS = 4 | 6.4 (8540) | 12.0 (1027) | 1.8 (1.6–1.9) | 2.1 (1.9–2.2) |
| CCIS from 5 to 7 | 13.9 (18 643) | 10.8 (2022) | 1.6 (1.5–1.7) | 1.6 (1.5–1.7) |
| CCIS from 8 to 12 | 5.9 (7882) | 10.7 (841) | 1.5 (1.4–1.7) | 1.3 (1.2–1.5) |
| CCIS >12 |
0.7 (939) |
14.6 (137) |
2.2 (1.8–2.6) |
1.6 (1.3–1.9) |
| Patients, % (n) |
Invasive mechanical ventilation, % (n) |
Univariate analysis (95% CI) |
Multivariate analysis adjusted on sex, age and CCIS (95% CI) |
|
| No obesity | 76.1 (102 139) | 8.0 (8152) | 1 (Ref.) | 1 (Ref.) |
| Obesity (total) | 23.9 (32 070) | 17.0 (5444) | 2.36 (2.27–2.45) | 1.88 (1.80–1.95) |
| Prevalent obesity | 67.9 (21 768) | 12.4 (2694) | 1.63 (1.56–1.71) | 1.18 (1.12–1.24) |
| Incident obesity | 32.1 (10 302) | 26.7 (2750) | 4.20 (4.00–4.41) | 3.51 (3.33–3.70) |
| No diabetes | 76.0 (100 200) | 8.7 (8902) | 1 (Ref.) | 1 (Ref.) |
| Diabetes (total) | 24.0 (32 209) | 14.6 (4694) | 1.78 (1.72–1.85) | 1.36 (1.30–1.41) |
| Prevalent diabetes | 71.5 (23 040) | 12.2 (2808) | 1.45 (1.39–1.52) | 1.06 (1.01–1.12) |
| Incident diabetes | 28.5 (9169) | 20.6 (1886) | 2.71 (2.56–2.86) | 1.95 (1.84–2.07) |
| No hypertension | 50.6 (67 596) | 7.8 (5263) | 1 (Ref.) | 1 (Ref.) |
| Hypertension (total) | 49.4 (66 613) | 12.5 (8333) | 1.69 (1.63–1.76) | 1.69 (1.62–1.77) |
| Prevalent hypertension | 73.5 (48 932) | 9.9 (4831) | 1.30 (1.25–1.35) | 1.24 (1.17–1.30) |
| Incident hypertension | 26.5 (17 681) | 19.8 (3502) | 2.93 (2.79–3.06) | 2.48 (2.36–2.61) |
Abbreviation: CCIS, Charlson's co-morbidity index score.
Table 3.
Death after invasive mechanical ventilation according to gender, age, CCIS and association with obesity, diabetes or hypertension
| Patients with invasive mechanical ventilation, n | Death after invasive mechanical ventilation, n (%) | Univariate analysis (95% CI) | Multivariate analysis adjusted on age and CCIS (95% CI) | |
|---|---|---|---|---|
| Female | 3883 | 1116 (28.7) | 1 (Ref.) | 1 (Ref.) |
| Male |
9713 |
3015 (31.0) |
1.1 (1.0–1.2) |
1.1 (1.0–1.2) |
| Patients with invasive mechanical ventilation, n |
Death after invasive mechanical ventilation, n (%) |
Univariate analysis (95% CI) |
Multivariate analysis adjusted on sex and CCIS (95% CI) |
|
| Age 18–49 years | 1947 | 291 (15.0) | 1 (Ref.) | 1 (Ref.) |
| Age 50–65 years | 5461 | 1340 (24.5) | 1.9 (1.6–2.1) | 1.8 (1.6–2.0) |
| Age 66–79 years | 5257 | 1988 (37.8) | 3.5 (3.0–4.0) | 3.3 (2.9–3.8) |
| Age ≥80 years |
931 |
512 (55.0) |
7.0 (5.8–8.3) |
6.6 (5.5–7.9) |
| Patients with invasive mechanical ventilation, n |
Death after invasive mechanical ventilation, % (n) |
Univariate analysis (95% CI) |
Multivariate analysis adjusted on age and sex (95% CI) |
|
| CCIS = 0 | 3495 | 25.4 (887) | 1 (Ref.) | 1 (Ref.) |
| CCIS = 1 | 2596 | 28.9 (751) | 1.2 (1.1–1.3) | 1.1 (1.0–1.2) |
| CCIS = 2 | 1982 | 28.8 (570) | 1.2 (1.0–1.3) | 1.0 (0.9–1.1) |
| CCIS = 3 | 1496 | 32.0 (478) | 1.4 (1.2–1.6) | 1.1 (1.0–1.3) |
| CCIS = 4 | 1027 | 35.4 (364) | 1.6 (1.4–1.9) | 1.3 (1.1–1.5) |
| CCIS from 5 to 7 | 2022 | 37.4 (756) | 1.8 (1.6–2.0) | 1.4 (1.3–1.6) |
| CCIS from 8 to 12 | 841 | 35.4 (298) | 1.6 (1.4–1.9) | 1.3 (1.1–1.6) |
| CCIS >12 |
137 |
19.7 (27) |
0.7 (0.5–1.1) |
0.7 (0.5–1.1) |
| Patients with invasive mechanical ventilation, n |
Death after invasive mechanical ventilation, % (n) |
Univariate analysis (95% CI) |
Multivariate analysis adjusted on sex, age and CCIS (95% CI) |
|
| No obesity | 8152 | 33.1 (2696) | 1 (Ref.) | 1 (Ref.) |
| Obesity (total) | 5444 | 26.4 (1435) | 0.72 (0.67–0.78) | 0.87 (0.80–0.95) |
| Prevalent obesity | 2694 | 31.8 (856) | 0.94 (0.86–1.04) | 1.13 (1.02–1.26) |
| Incident obesity | 2750 | 21.1 (579) | 0.54 (0.49–0.60) | 0.67 (0.60–0.75) |
| No diabetes | 8902 | 28.8 (2568) | 1 (Ref.) | 1 (Ref.) |
| Diabetes (total) | 4694 | 33.3 (1563) | 1.23 (1.14–1.33) | 1.19 (1.10–1.29) |
| Prevalent diabetes | 2808 | 36.9 (1037) | 1.44 (1.32–1.58) | 1.36 (1.23–1.51) |
| Incident diabetes | 1886 | 27.9 (526) | 0.95 (0.85–1.07) | 1.00 (0.89–1.12) |
| No hypertension | 5263 | 27.0 (1423) | 1 (Ref.) | 1 (Ref.) |
| Hypertension (total) | 8333 | 32.5 (2708) | 1.30 (1.20–1.40) | 0.96 (0.88–1.04) |
| Prevalent hypertension | 4831 | 36.8 (1776) | 1.57 (1.44–1.71) | 1.12 (1.01–1.23) |
| Incident hypertension | 3502 | 26.6 (932) | 0.98 (0.89–1.08) | 0.82 (0.74–0.91) |
Abbreviation: CCIS, Charlson's co-morbidity index score.
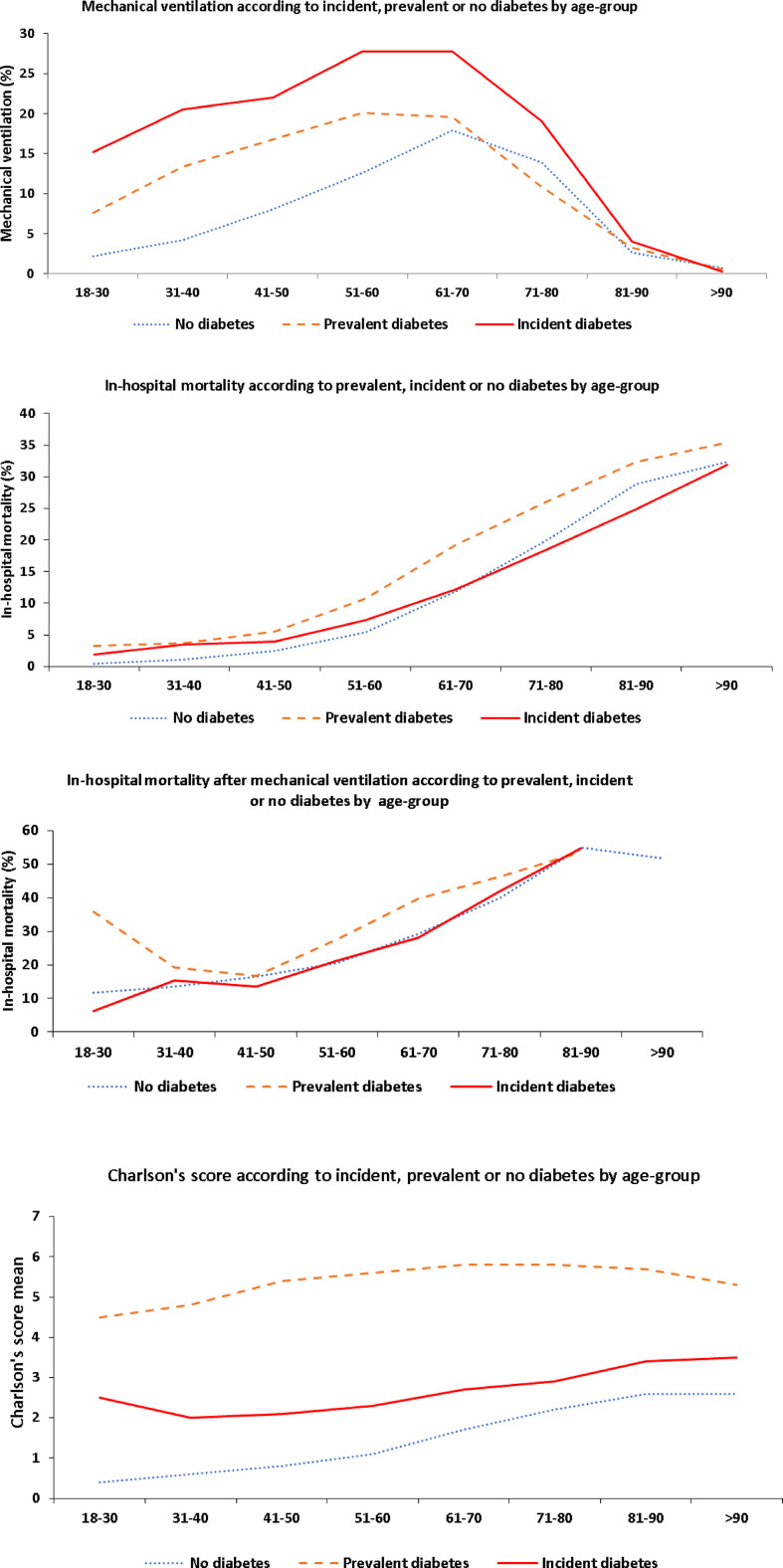
Fig. 2 shows total in-hospital mortality, use of IMV and mortality following IMV, as well as mean CCIS among non-obese patients, those with incident or with prevalent obesity, by age group. Total in-hospital mortality was higher among patients with prevalent obesity than among those with incident obesity or non-obese patients. In contrast, among patients with incident obesity, requirement for IMV was threefold that of non-obese patients, and twice that of patients with prevalent obesity. In the 51- to 60-year age group, 33.4% of patients with incident obesity (766 out of 2294) required IMV versus 18.7% of previously diagnosed obese patients (697 out of 3726) and 11.2% of non-obese patients (1577 out of 14 144). Similar results were observed among patients whose CCIS was 0, i.e. 22.3% patients with incident obesity (893 out of 3999) required IMV versus 9.1% with prevalent obesity (288 out of 3171) and 5.6% of non-obese patients (2314 out of 41 618). Fig. 3 and Fig. 4 illustrate use of IMV and in-hospital mortality among patients with prevalent and incident diabetes, and hypertension, per age group.
Fig. 2.
Mortality, mechanical ventilation and Charlson's score according to obesity by age group.
Fig. 3.
Mechanical ventilation, mortality and Charlson's score according to diabetes by age group.
Fig. 4.
Mechanical ventilation, Charlson's index and mortality according to hypertension by age group.
Discussion
This study of over 130 000 patients admitted to hospital with COVID-19 in France confirms that obesity, diabetes and arterial hypertension are indeed associated with severe forms of the disease. Mortality following hospital admission for COVID-19 was more frequent among patients with obesity and diabetes compared with patients who were free of these co-morbidities. Patients with obesity, diabetes and hypertension were also more at risk of requiring IMV.
The pathophysiological process underlying such risks could be explained by a state of chronic inflammation [8,20] in patients with obesity and by overexpression of angiotensin-converting enzyme (ACE) receptors [21,22] in individuals with diabetes. Such an increase in the number of ACE receptors, which allows COVID-19 to invade cells, might also be associated with hypoglycaemic medications [23], angiotensin II inhibitors [24] and statins [25], all of which enhance the expression of ACE receptors. Our results also show that the occurrence of severe forms of the disease, among patients admitted for COVID-19 in France, differs according to patient status regarding prevalent or incident obesity, diabetes or hypertension. After adjusting for age, gender and CCIS, requirement for IMV was two-fold to three-fold more frequent among patients with incident co-morbidity, whereas in-hospital mortality for these patients was comparable to those who were not obese, diabetic or hypertensive. For patients with prevalent co-morbidities, use of IMV was more frequent compared with those who had none, but was half as frequent compared with patients with incident co-morbidities. This difference in disease course can help to assess patients' prognosis [26] and the occurrence of severe forms so as to identify those patients who may require IMV and thus provide optimal management. These results should encourage the implementation of suitable preventive and treatment strategies for adults presenting with obesity, diabetes or hypertension.
It could be argued that an indication bias led to the prioritization of patients with incident co-morbidities in the context of overwhelmed emergency care facilities, in view of their perceived higher chances of improvement [27] with IMV compared with patients with prevalent co-morbidities. Moreover, the fact that, during the first epidemic wave, there was no established strategy to control the dysfunctional inflammatory response and disease progression to acute respiratory distress could also account for the use of IMV in these patients. Nevertheless, among patients whose CCIS was 0, with none of the index co-morbidities, those with newly diagnosed co-morbidities were twice as likely to need IMV compared with patients with pre-existing conditions and three times more compared with patients with none of these co-morbidities.
Mortality following IMV was not higher among patients with incident obesity and diabetes or hypertension than among patients free of these conditions, as opposed to patients in whom they were prevalent. A lack of association between obesity and in-hospital mortality has already been reported among patients with class I and II obesity by the American Heart Association COVID-19 Cardiovascular Disease Registry [28], whereas, according to that study, patients with obesity required IMV more frequently, regardless of the class.
Our study presents some limits, first COVID-19 patient identification relies on ICD-10 coding for this condition, and not directly from laboratory test results, but this disease identification method based on ICD-10 codes has already been used in France for obesity [16], namely for patients with COVID-19 [14]. Our hospital-based cohort only includes COVID-19 patient deaths that occurred in hospital, so that any death following discharge, for example, in nursing homes for the elderly, was not recorded. Lastly, the degree of severity of the co-morbidities of interest could not be assessed, which would have allowed us to adjust the results, notably for patients with diabetes and hypertension.
Conclusion
Our study confirms the link between obesity, diabetes and hypertension and the occurrence of severe forms of COVID-19. It also reveals significant differences between patients according to whether these co-morbidities are incident or prevalent, with a much more frequent use of IMV among patients in whom these are incident and a higher mortality rate among patients in whom these are prevalent.
Transparency declaration
The authors have stated that there are no conflicts of interest. Funding: The Agence Technique de l’Informatique Hospitalière provided equipment used for data collection.
Author contributions
All authors discussed the results and contributed to the final manuscript. LB, RF and CP contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript.
Acknowledgements
Brigitte Dunais, MD, formerly employed by the Nice University Public Health Department, made substantial contributions to the writing and editing of this article. The Agence Technique de l’Informatique Hospitalière provided equipment used for data collection.
Editor: L. Scudeller
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.09.010.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Internet]. Available at:
- 2.COVID-19 pandemic European centre for disease prevention and control. https://www.ecdc.europa.eu/en/covid-19-pandemic [Internet] Available at:
- 3.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.-J., Liang W.-H., Zhao Y., Liang H.-R., Chen Z.-S., Li Y.-M., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu Y., Sun J., Dai Z., Deng H., Li X., Huang Q., et al. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020 doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovani A., Byrne C.D., Zheng M.-H., Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis NMCD. 2020;30:1236–1248. doi: 10.1016/j.numecd.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korakas E., Ikonomidis I., Kousathana F., Balampanis K., Kountouri A., Raptis A., et al. Obesity and COVID-19: immune and metabolic derangement as a possible link to adverse clinical outcomes. Am J Physiol Endocrinol Metab. 2020;319:E105–E109. doi: 10.1152/ajpendo.00198.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barron E., Bakhai C., Kar P., Weaver A., Bradley D., Ismail H., et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ioannou G.N., Locke E., Green P., Berry K., O’Hare A.M., Shah J.A., et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reilev M., Kristensen K.B., Pottegård A., Lund L.C., Hallas J., Ernst M.T., et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020;49:1468–1481. doi: 10.1093/ije/dyaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sathish T., Kapoor N., Cao Y., Tapp R.J., Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Obes Metab. 2021;23:870–874. doi: 10.1111/dom.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piroth L., Cottenet J., Mariet A.-S., Bonniaud P., Blot M., Tubert-Bitter P., et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2020;9:P251–P259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Didier R., Gouysse M., Eltchaninoff H., Le Breton H., Commeau P., Cayla G., et al. Successful linkage of French large-scale national registry populations to national reimbursement data: improved data completeness and minimized loss to follow-up. Arch Cardiovasc Dis. 2020;113:534–541. doi: 10.1016/j.acvd.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Bailly L., Fabre R., Pradier C., Iannelli A. Colorectal cancer risk following bariatric surgery in a nationwide study of French individuals with obesity. JAMA Surg. 2020;155:395–402. doi: 10.1001/jamasurg.2020.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mise à jour des consignes de codage des séjours COVID-19 Publication ATIH [Internet]. Available at: https://www.atih.sante.fr/mise-jour-des-consignes-de-codage-des-sejours-covid-19; accessed 14 January 2021.
- 18.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Quan H., Sundararajan V., Halfon P., Fong A., Burnand B., Luthi J.-C., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 20.Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16:341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wysocki J., Ye M., Soler M.J., Gurley S.B., Xiao H.D., Bernstein K.E., et al. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55:2132–2139. doi: 10.2337/db06-0033. [DOI] [PubMed] [Google Scholar]
- 23.Romaní-Pérez M., Outeiriño-Iglesias V., Moya C.M., Santisteban P., González-Matías L.C., Vigo E., et al. Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of type 1 diabetes rats. Endocrinology. 2015;156:3559–3569. doi: 10.1210/en.2014-1685. [DOI] [PubMed] [Google Scholar]
- 24.Asperen R.M.W., Lutter R., Specht P.A., Moll G.N., Woensel JB van, van der Loos C.M., et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1–7) or an angiotensin II receptor antagonist. J Pathol. 2011;225:618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 25.Tikoo K., Patel G., Kumar S., Karpe P.A., Sanghavi M., Malek V., et al. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem Pharmacol. 2015;93:343–351. doi: 10.1016/j.bcp.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Bartoletti M., Giannella M., Scudeller L., Tedeschi S., Rinaldi M., Bussini L., et al. Development and validation of a prediction model for severe respiratory failure in hospitalized patients with SARS-CoV-2 infection: a multicentre cohort study (PREDI-CO study) Clin Microbiol Infect. 2020;26:1545–1553. doi: 10.1016/j.cmi.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yordanov Y., Dinh A., Bleibtreu A., Mensch A., Lescure F.-X., Debuc E., et al. Clinical characteristics and factors associated with hospital admission or death in 43,103 adult outpatients with COVID-19 managed with the Covidom telesurveillance solution: a prospective cohort study. Clin Microbiol Infect. 2021;27:1158–1166. doi: 10.1016/j.cmi.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendren N.S., de Lemos J.A., Ayers C., Das S.R., Rao A., Carter S., et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19: results from the American Heart Association COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143:135–144. doi: 10.1161/CIRCULATIONAHA.120.051936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.