Abstract
Episodic ataxia type 2 (EA2) is a rare autosomal dominant disorder characterized by motor incoordination, paroxysmal dystonia, vertigo, nystagmus and more recently cognitive deficits. To date over 100 mutations in the CACNA1A gene have been identified in EA2 patients leading to a loss of P/Q-type channel activity, dysfunction of cerebellar Purkinje cells and motor incoordination. To determine if the cerebellum is contributing to these cognitive deficits, we examined two different EA2 mouse models for cognition impairments where CACNA1A was removed specifically from cerebellar Purkinje or granule cells postnatally. Both mutant mouse models showed anxiolytic behavior to lighted, open areas in the open field and light/dark place preference tests but enhanced anxiousness in the novel suppressed feeding test. However, EA2 mice continued to show augmented latencies in the light/dark preference test and when the arena was divided into two dark zones in the dark/dark preference test. Moreover, increased latencies were also displayed in the novel object recognition test, indicating that EA2 mice are indecisive and anxious to explore new territories and objects and may have memory recognition deficits. Exposure to a foreign mouse led to deficiencies in attention and sniffing as well as in social and genital sniffing. These data suggest that postnatal removal of the P/Q type calcium channel from the cerebellum regulates neuronal activity involved in anxiety, memory, decision making and social interactions. Our EA2 mice will provide a model to identify the mechanisms and therapeutic agents underlying cognitive and psychiatric disorders seen in EA2 patients.
Introduction
Episodic ataxia type 2 (EA2) is an autosomal dominant inherited disorder, characterized by sporadic episodes of ataxia and dystonia, vertigo and nausea lasting for hours to days. These attacks can be induced by stress, startle, physical or mental exertion and chemicals such as caffeine and alcohol (1). EA2 is a rare disease affecting < 1 out of 100 000 individuals. To date >100 mutations in the pore-forming α1 subunit of the voltage-gated P/Q type calcium channel, Cacna1a, have been identified in EA2 patients which causes a decrease to complete loss of P/Q type calcium channel activity and ultimately cerebellar degeneration and motor incoordination (2,3). The voltage-gated P/Q calcium channel is highly expressed in the cerebellum and especially on cerebellar Purkinje cells (PC), where it plays an important role in integrating, transmitting and encoding motor and sensory information for coordinated movement. Coordinated movement is finely tuned by granule cell (GC) excitation of PCs with a counter balance of feedforward inhibition of cerebellar interneurons (4). Disruption of this balance of PC simple spike activity leads to motor incoordination (5). Further sensory inputs from climbing fibers serve as a prediction error signal to reduce PC inputs to allow for sustained fine motor learning (6).
Historically, the cerebellum is known to be important for motor coordination. However, increasing evidence shows that the cerebellum is also modulating higher cognitive functions such as problem solving, socialization and refining one’s emotions (7). The neurologist Dr Jeremy Schmahmann was one of the pioneers showing that lobules VI, Crus I and II, VIIb and X of the cerebellum are active during cognitive tasks and may be involved in psychological disorders such as attention deficit, hyperactivity disorder, autism spectrum, schizophrenia and anxiety and panic disorder (8–10). He defined the term and paradigms to diagnose patients with cerebellar cognitive affective syndrome (CCAS also known as Schmahmann’s syndrome). Patients suffering from CCAS have lesions confined to their cerebellar posterior lobes and show cognitive and emotional abnormalities unrelated to motor function. Moreover, Argyropoulos and colleagues showed that the cerebellum contributes to social cognition in a cohort of patients suffering from a diversity of cerebellar diseases including two EA2 cases. EA2 patients where degeneration is isolated to the cerebellum also reported cognitive deficiencies (10). During childhood, EA2 individuals often display developmental delays in speech and intellectual abilities in addition to severe behavioral deficits such as attention deficit hyperactivity, poor control of emotions (i.e. aggression), impatience and following rules and instructions (11–14). The majority of adult EA2 patients displayed cognitive dysfunctions in attention, memory both working, visual and figural, visuoconstructive abilities and executive functions. Additionally, some EA2 individuals showed psychiatric symptoms including attention deficit hyperactivity disorder (ADHD), anxiety and personality disorders, psychosis, autism, schizophrenia and depression (11,13–15). Case studies from cerebellar lesion and EA2 patients strengthen the hypothesis that the cerebellum may be involved in the processing of our emotions and cognitive functions.
Functional connectivity MRI studies in humans show that the cerebellum projects to higher cerebral areas via the cortico-ponto-cerebellar and cerebello-thalamo-cortical loops (16,17). Moreover, past tracing studies in mice and monkeys demonstrate that the cerebellum is either directly or indirectly connected to key circuitry involved in higher cognitive functions such as the hippocampus, prefrontal cortex (PFC), periaqueductal grey area (PAG), ventral tegmental area (VTA), basal ganglia (BG) and amygdala (18). Recent behavior studies in mice have strongly implicated the cerebellar involvement in reward (19,20), social behavior (20) and fear memories (21) via the PAG and VTA. All together this strengthens the hypothesis that the cerebellum is contributing to cognition by projecting to higher cortical areas via the thalamus (16). To date there have been no reports investigating cognitive impairments in cerebellar EA2 mutant mice.
Using our conditional Cacna1aCitrine knockout mouse models, Cacna1apurk(−/−) (postnatal removal from Purkinje cells) and Cacna1aquirk(−/−) (postnatal removal from GCs), we investigated whether EA2 mouse models displayed cognitive dysfunctions in anxiety, memory and social behavior (22,23). We compared our EA2 mice, Cacna1apurk(−/−) and Cacna1aquirk(−/−), to littermate controls Cacna1aCitrine, in a battery of behavioral tests for cognition and social skills and found that EA2 mice show dysfunction in anxiety, decision making, memory and social behavior which cannot be attributed to prenatal effects.
Results
Episodic ataxia type 2 mouse models, Cacna1apurk(−/−) and Cacna1aquirk(−/−), exhibit increased anxiety
To initially evaluate any anxiolytic deficits in the disease EA2, we utilized our conditional knock in mouse line (Cacna1aCitrine), where the P/Q-type channel can be visualized by a third generation, yellow fluorescent protein (YFP) variant, citrine, but more importantly can be deleted by crossing Cacna1aCitrine mice with mice expressing Cre recombinase under cell-type specific promoters. In previous studies we deleted the P/Q-type channel from cerebellar PCs (Cacna1apurk(−/−)) or GCs (Cacna1aquirk(−/−)) in mice by crossing Cacna1aCitrine mice with the PC specific promotor (TgPCP2-cre) or GC specific promotor (Tgma6-cre), respectively (22,23). Both promoters are active after postnatal day 6 and are fully active after 2–3 weeks of age, therefore eliminating any prenatal effects. Surprisingly, we found that the postnatal knockout of the P/Q-type channel from either cerebellar PCs or GCs exhibits the full spectrum of neurological deficits, including ataxia, dyskinesia and absence seizures as seen in mice with genomic CACNA1A ablation and loss/reduction of function mutations. Most likely due to the mosaic expression of the Cre protein, we observed a mild and severe form of Cacna1aquirk(−/−) mice where the severe showed dyskinesia and the mild Cacna1aquirk(−/−) did not. For this study, we used only the severe Cacna1aquirk(−/−) and Cacna1apurk(−/−) mice to analyze the contribution of the cerebellar cortex to anxiety compared with their littermate controls, Cacna1aCitrine. Therefore, the mice were subjected to the open field, novelty suppressed feeding and light/dark place preference behavior tests (Figs 1–3).
Figure 1 .
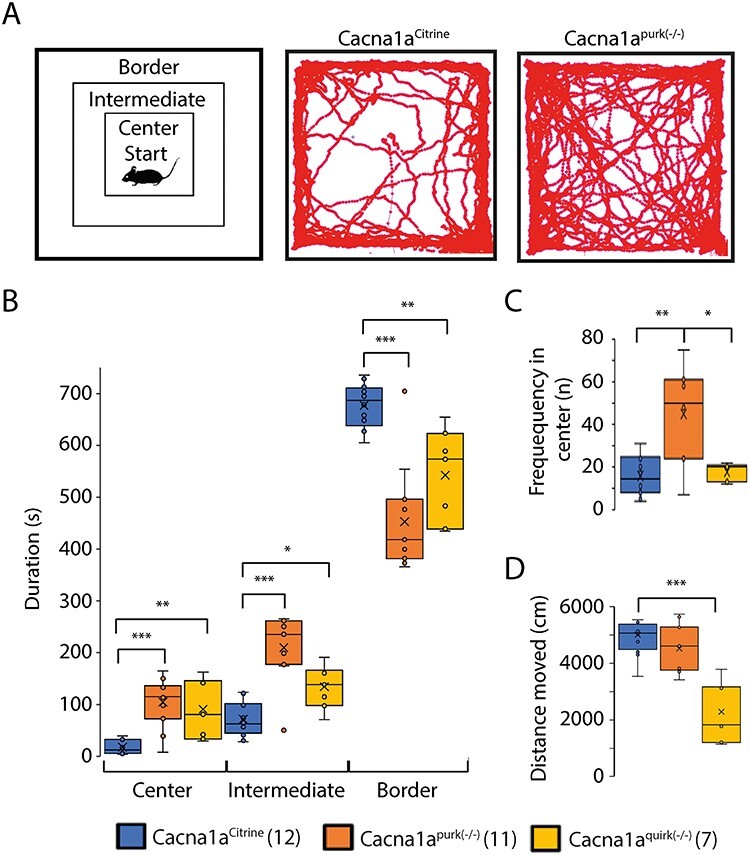
EA2 mouse models, Cacna1apurk(−/−) and Cacna1aquirk(−/−) show anxiolytic behavior in open field test. (A) Schematic of the different areas analyzed in the open field (left) which includes the border, intermediate and center regions. Mice were placed in the center at the start of the test and allowed to explore for 15 min. An example trace from one Cacna1aCitrine (middle) and Cacna1apurk(−/−) (right). (B) Average time Cacna1aCitrine (blue), Cacna1apurk(−/−) (orange) and Cacna1aquirk(−/−) (yellow) mice spent in the center, intermediate or border areas of the open field. (C) Frequency each mouse group visited the center region. (D) Total distance moved for Cacna1aCitrine (blue), Cacna1apurk(−/−) (orange) and Cacna1aquirk(−/−) (yellow) mice. Cacna1apurk(−/−) and Cacna1aquirk(−/−) mice tended to spent less time in the border and more time in the center and intermediate regions than the Cacna1aCitrine. The number of mice tested/group is indicated in parentheses in legend. Statistical significance was evaluated with one-way ANOVA (*P ≤ 0.05, **P ≤ 0.01; ***P ≤ 0.001).
Figure 3 .
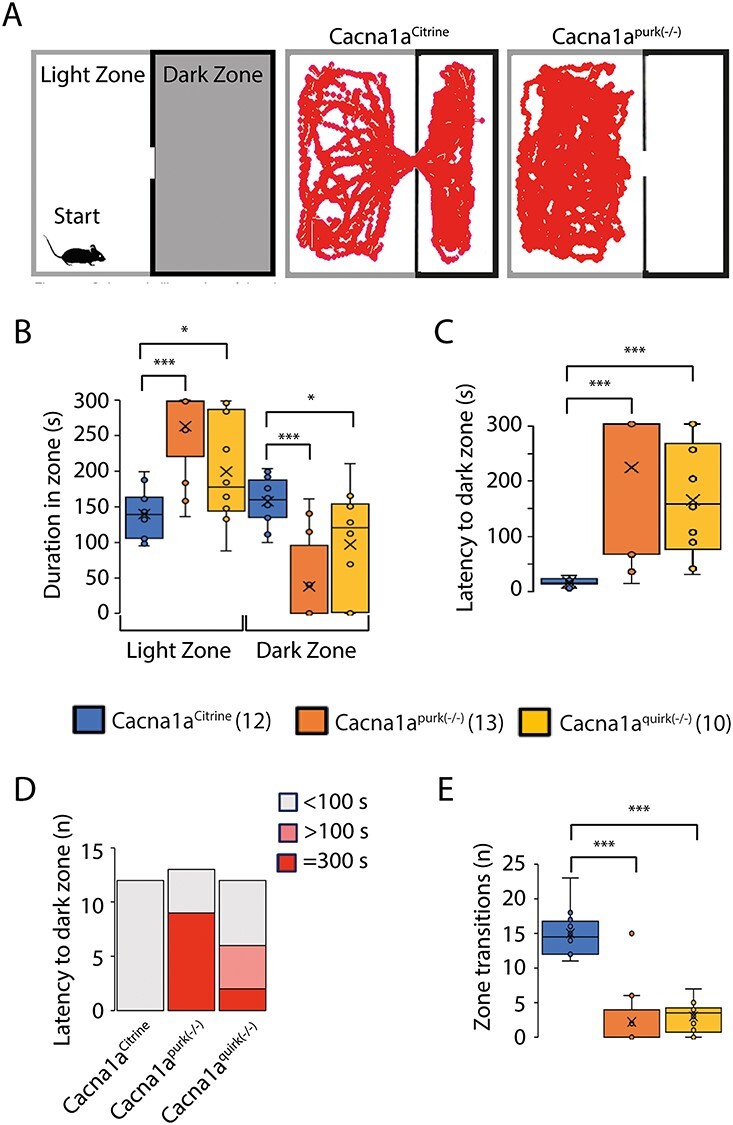
EA2 mouse models, Cacna1apurk(−/−) and Cacna1aquirk(−/−) demonstrate anxiolytic and indecisive behavior in place preference test. (A) Schematic of the place preference test (left) which was divided into a light and dark zone. Mice were started in the light zone in the far corner opposite to the dark zone entrance and given 5 min to explore both zones. Representative traces from a Cacna1aCitrine (middle) and Cacna1apurk(−/−) (right) mouse. Whisker boxplots from the duration spent in the light or dark zone (B), latency to the dark zone (C), number of mice (n) with <100 s or >100 s latency to light zone times (D) and the number of transitions (n) between the light and dark zones (E) for Cacna1aCitrine (blue), Cacna1apurk(−/−) (orange) and Cacna1aquirk(−/−) (yellow) mice are depicted. Cacna1apurk(−/−) and Cacna1aquirk(−/−) mice spent more time in the light zone due to their inability to transition to the dark zone as evident from their long latencies to dark zone and low number of transitions. In fact 9 Cacna1apurk(−/−) and 2 Cacna1aquirk(−/−) mice did not leave the light zone. Whereas the Cacna1aCitrine mice spent equal time in both arenas. The number of mice tested/group is indicated in parentheses in legend. Statistical significance was evaluated with one-way ANOVA (*P ≤ 0.05, ***P ≤ 0.001).
To eliminate the influence of motor deficits on cognition tests and to test for anxiety, we firstly performed the open field test. Surprisingly, both EA2 mouse models, Cacna1apurk(−/−) and Cacna1aquirk(−/−), spent significantly more time in the center and intermediate regions compared with their control littermates (Cacna1aCitrine) and less time in the border region (Fig. 1B), implying that EA2 mice are not anxious (Fig. 1, Table 1). These differences do not seem to be due to their ataxic phenotype since our more severe ataxic EA2 mice, Cacna1apurk(−/−), moved the same distance as their control littermates, Cacna1aCitrine (Fig. 1D). However, we cannot exclude this possibility for the less ataxic Cacna1aquirk(−/−) mice which traveled less than controls. In contrast, when EA2 mice were starved for 24 h and underwent the novelty suppressed feeding test, where a regular food pellet is given in the center region of the open field arena (Fig. 2A), they displayed prolonged latency to feed (Fig. 2B) and reduced food consumption (Fig. 2C) compared with control littermates, implying that the EA2 mice are more anxious or have a motivation issue. However, when the food pellet was placed in the border, equidistant from the start to the center (Fig. 2D), Cacna1aquirk(−/−) mice continued to display prolonged latencies to feed (Fig. 2E; Cacna1aCitrine 108.4 ± 10.6 s, Cacna1aquirk(−/−) 222.1 ± 49.7 s) and lower consumption levels (Fig. 2F; Cacna1aCitrine 0.07 ± 0.009 g, Cacna1aquirk(−/−) 0.04 ± 0.007 g). On the other hand a familiar reward, a Froot Loop, enticed the Cacna1aquirk(−/−) mice to feed with similar latencies (Fig. 2H, Cacna1aCitrine 29.7 ± 11.9 s, Cacna1aquirk(−/−) 36.9 ± 9.2 s) and greater consumptions (Fig. 2I; Cacna1aCitrine 0.03 ± 0.006 g, Cacna1aquirk(−/−) 0.14 ± 0.01 g) as their Cacna1aCitrine littermates, suggesting that the Cacna1aquirk(−/−) mice are less motivated or need greater benefits to outweigh the risks than their controls and their motor deficits or potential fatigue do not appear to affect their abilities to perform simple behavior tasks. To investigate the anxiety in our EA2 mice further, we performed the light/dark place preference test. EA2 mice spent extensive time in the light zone compared with the dark zone in the light/dark preference test (Fig. 3B), which was reflected by the high latencies to enter the dark zone for the first time (Fig. 3C) and decreased number of transitions between the light and dark zones (Fig. 3E, Table 1). In fact, 69.2% of Cacna1apurk(−/−) and 20% of Cacna1aquirk(−/−) mice remained in the light zone the entire testing period and half of the Cacna1aquirk(−/−) mice took > 100 s to visit the dark zone, implicating an increased anxiolytic response to open lit areas (Fig. 3D).
Table 1.
Summary of cognitive analyses for EA2 mouse lines.
| Parameter | Cacna1a Citrine | Within group | Cacna1a purk(-/-) | Cacna1a quirk(-/-) | Within group | |||
| Mean ± SEM | P value | Mean ± SEM | P value | Mean ± SEM | P value | P value | ||
| Open field | ||||||||
| Duration in border (s) | 681.6 ± 11.7 | 457.1 ± 29.4 | ≤ 0.001 | 546.8 ± 31.3 | 0.008 | |||
| Duration in intermediate (s) | 75.6 ± 9.1 | 214.0 ± 18.5 | ≤ 0.001 | 138.4 ± 14.8 | 0.032 | |||
| Duration in center (s) | 21.7 ± 3.8 | 108.5 ± 13.7 | ≤ 0.001 | 94.8 ± 20.3 | 0.006 | |||
| Frequency in center (n) | 15.8 ± 2.6 | 44.9 ± 6.5 | ≤ 0.001 | 17.7 ± 1.6 | 0.62 | |||
| Distance moved (cm) | 5008 ± 214.9 | 4472 ± 238.5 | 0.12 | 3383 ± 573.8 | 0.014 | |||
| Novelty suppressed feeding | ||||||||
| Food pellet in center | ||||||||
| Latency to feed (s) | 22.0 ± 14.3 | 275.4 ± 43.9 | ≤ 0.001 | 115 ± 48.3 | 0.05 | |||
| Food consumption (g) | 0.18 ± 0.01 | 0.10 ± 0.01 | ≤ 0.001 | 0.09 ± 0.01 | ≤ 0.001 | |||
| Food pellet in border | ||||||||
| Latency to feed (s) | 108.4 ± 10.6 | 222.1 ± 49.7 | 0.05 | |||||
| Food consumption (g) | 0.07 ± 0.009 | 0.04 ± 0.007 | 0.04 | |||||
| Froot loop in border | ||||||||
| Latency to feed (s) | 29.7 ± 11.9 | 36.9 ± 9.2 | 0.67 | |||||
| Food consumption (g) | 0.03 ± 0.006 | 0.14 ± 0.01 | ≤ 0.001 | |||||
| Light/Dark place preference | ||||||||
| Duration in light zone (s) | 136.7 ± 9.6 | 263.4 ± 16.3 | ≤ 0.001 | 199.6 ± 22.1 | 0.02 | |||
| Duration in dark zone (s) | 157.7 ± 9.1 | 38.0 ± 17.3 | ≤ 0.001 | 97.0 ± 22.7 | 0.012 | |||
| Latency to dark zone (s) | 13.0 ± 2.1 | 220.8 ± 33.2 | ≤ 0.001 | 161.3 ± 30.2 | ≤ 0.001 | |||
| Latency to dark zone (n) | ||||||||
| <100 s | 12 | 4 | 3 | |||||
| >100 s | 0 | 0 | 5 | |||||
| =300 s | 0 | 9 | 2 | |||||
| Zone transitions (n) | 15.0 ± 0.9 | 2.2 ± 1.2 | ≤ 0.001 | 3.0 ± 0.68 | ≤ 0.001 | |||
| Dark/Dark place preference | ||||||||
| Duration in dark zone A (s) | 257 ± 22.5 | 354.8 ± 35.4 | 0.04 | 301.6 ± 37.9 | 0.33 | |||
| Duration in dark zone B (s) | 340.5 ± 22.6 | 243.6 ± 35.2 | 0.04 | 294.9 ± 38.1 | 0.32 | |||
| Latency to zone B (s) | 41.1 ± 12.4 | 221.0 ± 43.7 | 0.001 | 132.0 ± 38.4 | 0.032 | |||
| Latency to zone B (n) | ||||||||
| <100 s | 11 | 0 | 6 | |||||
| >100 s | 1 | 11 | 2 | |||||
| =300 s | 0 | 2 | 2 | |||||
| Zone transitions (n) | 13.33 ± 1.4 | 6.0 ± 1.3 | 0.001 | 7.0 ± 1.0 | 0.003 | |||
| Distanced moved (cm) | 2642 ± 150.8 | 2927 ± 90.9 | 0.12 | 1997 ± 164.6 | 0.012 | |||
| Novel object recognition | ||||||||
| Preference % to equal objects | 49.9 ± 2.2 | 56.6 ± 3.2 | 0.11 | 52.0 ± 8.0 | 0.77 | |||
| Preference % to novel object | 80.7 ± 3.5 | ≤ 0.001 | 66.5 ± 4.6 | 0.02 | 57.6 ± 3.6 | 0.02 | ||
| Latency to novel object (s) | 14.6 ± 3.6 | 43.6 ± 11.4 | 0.03 | 107.6 ± 31.9 | 0.013 | |||
| Frequency to novel object (n) | 119.8 ± 15.2 | 49.9 ± 8.3 | 0.002 | 44.6 ± 6.2 | ≤ 0.001 | |||
| Distance moved (cm) | 3302.9 ± 333.9 | 3127.4 ± 470.7 | 0.76 | 2229.9 ± 314.8 | 0.032 | |||
| Object relocation | ||||||||
| Day 1 | ||||||||
| Preference % | ||||||||
| Left object | 47.7 ± 5.6 | 0.61 | 53.2 ± 2.1 | 0.43 | ||||
| Right object | 52.3 ± 5.6 | 0.06 | 46.8 ± 2.1 | 0.43 | ||||
| Day 2 | ||||||||
| Preference % | ||||||||
| Left object | 44.1 ± 3.5 | 0.05 | 41.9 ± 2.4 | 0.65 | ||||
| Right object | 55.9 ± 3.5 | ≤ 0.001 | 58.1 ± 2.4 | 0.65 | ||||
| Latency to right object (s) | 52.8 ± 21.3 | 86.0 ± 27.0 | 0.38 | |||||
| Frequency to right object (n) | 30.3 ± 3.5 | 29.6 ± 3.1 | 0.89 | |||||
| Distance moved (cm) | 2795.5 ± 209.9 | 2368.1 ± 206.4 | 0.19 | |||||
| Velocity (cm/s) | 4.7 ± 0.35 | 4.0 ± 0.34 | 0.19 | |||||
| T maze | ||||||||
| Alternation (%) | 65.7 ± 8.4 | 67.5 ± 7.5 | 0.88 | |||||
| Social interaction with intruder | ||||||||
| Attention Time (s) | 12.1 ± 4.2 | 66.2 ± 6.5 | ≤ 0.001 | 38.2 ± 7.6 | 0.02 | |||
| Events (n) | 3.6 ± 1.2 | 10.0 ± 1.5 | 0.011 | 5.0 ± 1.1 | 0.47 | |||
| Sniff Time (s) | 65.3 ± 11.3 | 60.2 ± 8.3 | 0.76 | 52.6 ± 18.2 | 0.05 | |||
| Events (n) | 19.1 2.9 | 10.6 ± 0.5 | 0.05 | 7.4 ± 0.96 | 0.01 | |||
| Social sniff Time (s) | 90.4 ± 13.1 | 82.8 ± 8.1 | 0.69 | 96.6 ± 16.8 | 0.79 | |||
| Events (n) | 23.1 ± 2.3 | 18 ± 0.9 | 0.12 | 15.8 ± 1.4 | 0.04 | |||
| Genital sniff Time (s) | 23.6 ± 7.1 | 15 ± 12.5 | 0.58 | 5.2 ± 1.7 | 0.08 | |||
| Events (n) | 6.9 ± 1.8 | 2.0 ± 1.4 | 0.09 | 1.2 ± 0.4 | 0.04 | |||
| Standing on back limbs Time (s) | 15.4 ± 5.8 | 5.4 ± 4.4 | 0.27 | 0.4 ± 0.4 | 0.08 | |||
| Events (n) | 5.9 ± 2.0 | 1.0 ± 0.7 | 0.09 | 0.2 ± 0.2 | 0.06 | |||
| Walking Time (s) | 19.9 ± 5.9 | 33.8 ± 8.8 | 0.24 | 18.8 ± 3.6 | 0.9 | |||
| Events (n) | 7.1 ± 2.0 | 7.4 ± 1.3 | 0.93 | 4.4 ± 0.5 | 0.33 | |||
| In nest Time (s) | 0 | 0 | 0 | |||||
| Events (n) | 0 | 0 | 0 | |||||
| Follows Time (s) | 36.3 ± 14.9 | 29.8 ± 10.7 | 0.77 | 56 ± 17.1 | 0.45 | |||
| Events (n) | 7.4 ± 2.6 | 5.6 ± 1.7 | 0.64 | 8.2 ± 1.7 | 0.84 | |||
| Mount Time (s) | 11.1 ± 4.9 | 2.6 ± 1.5 | 0.22 | 6.0 ± 1.9 | 0.42 | |||
| Events (n) | 2.8 ± 1.2 | 1.0 ± 0.6 | 0.29 | 1.8 ± 0.6 | 0.54 | |||
| Tail rattles Time (s) | 0.3 ± 0.26 | 0 | 0.4 | 0 | 0.4 | |||
| Events (n) | 0.1 ± 0.13 | 0 | 0.4 | 0 | 0.4 | |||
| Attacks Time (s) | 17.1 ± 7.2 | 0 | 0.1 | 0 | 0.1 | |||
| Events (n) | 5.0 ± 2.1 | 0 | 0.1 | 0 | 0.1 | |||
| Bites Time (s) | 0 | 0 | 0 | |||||
| Events (n) | 0 | 0 | 0 | |||||
| 3 Chamber social interaction | ||||||||
| Non-social trial | ||||||||
| Duration (s) in | ||||||||
| Start chamber | 89.1 ± 11.4 | 144.4 ± 10.7 | 0.004 | |||||
| Empty chamber | 137.7 ± 20.9 | 144.0 ± 12.6 | 0.81 | |||||
| Non-social chamber | 285.3 ± 21.1 | ≤ 0.001 | 219.5 ± 11.4 | 0.02 | ≤ 0.001 | |||
| Non-social zone | 54.3 ± 6.1 | 32.6 ± 6.1 | 0.03 | |||||
| Frequency (n) | ||||||||
| Start chamber | 15.7 ± 1.2 | 13.3 ± 0.87 | 0.15 | |||||
| Empty chamber | 10.5 ± 1.8 | 0.05 | 12.2 ± 1.2 | 0.48 | ||||
| Non-social chamber | 71.1 ± 4.4 | ≤ 0.001 | 54.9 ± 4.6 | 0.03 | ≤ 0.001 | |||
| Non-social zone | 39.6 ± 2.9 | 30.7 ± 2.8 | 0.05 | |||||
| Distance moved with cage (cm) | 2113.2 ± 124.6 | 2033.1 ± 94.3 | 0.64 | |||||
| Velocity with cage (cm/s) | 3.8 ± 0.2 | 3.8 ± 0.2 | 0.89 | |||||
| Social trial | ||||||||
| Duration (s) in | ||||||||
| Start chamber | 57.2 ± 8.2 | 116.8 ± 16.2 | 0.006 | |||||
| Non-social chamber | 143.7 ± 20.7 | 0.005 | 144.8 ± 11.9 | 0.97 | ||||
| Social chamber | 270.0 ± 25.4 | 0.03 | 0.08; 0.012 | |||||
| Non-social zone | 19.3 ± 3.4 | 12.0 ± 1.2 | 0.08 | |||||
| Social zone | 65.5 ± 8.6 | 45.4 ± 7.3 | 0.05 | ≤ 0.001 | ||||
| Frequency (n) | ||||||||
| Start chamber | 18.7 ± 0.8 | 0.001 | 12.8 ± 1.0 | ≤ 0.001 | ≤ 0.001 | |||
| Non-social chamber | 36.6 ± 4.1 | 32.3 ± 2.7 | 0.42 | 0.004 | ||||
| Social chamber | 87.7 ± 8.4 | 55.2 ± 6.6 | 0.01 | |||||
| Non-social zone | 22.5 ± 2.6 | 19.9 ± 2.1 | 0.48 | |||||
| Social zone | 60.9 ± 6.3 | ≤ 0.001 | 41.8 ± 5.7 | 0.05 | 0.003 | |||
| Distance moved with mouse (cm) | 2547.3 ± 140.4 | 2338.0 ± 205.3 | 0.43 | |||||
| Velocity with mouse (cm/s) | 4.7 ± 0.2 | 4.7 ± 0.4 | 0.97 | |||||
| Hotplate | ||||||||
| Temperature of first paw lick (°C) | 33.9 ± 0.5 | 33.6 ± 0.7 | 0.59 | |||||
| Temperature of first jump (°C) | 38.4 ± 0.9 | 38.3 ± 1.2 | 0.89 | |||||
| Responding mice to jumps (%) | ||||||||
| 32°C | 0 | 0 | ||||||
| 33°C | 0 | 0 | ||||||
| 34°C | 22.2 | 0 | ||||||
| 35°C | 22.2 | 16.7 | ||||||
| 36°C | 22.2 | 33.3 | ||||||
| 37°C | 33.3 | 66.7 | ||||||
| 38°C | 33.3 | 100 | ||||||
| 39°C | 55.6 | 83.3 | ||||||
| 40°C | 77.8 | 100 | ||||||
| 41°C | 88.9 | 66.7 | ||||||
| 42°C | 100 | 66.7 | ||||||
| Buried Fruit Loop | ||||||||
| Latency to feed (s) | 82.5 ± 19.5 | 170.6 ± 36.3 | 0.06 | |||||
| Looming | ||||||||
| Responses/trial (%) | ||||||||
| Freeze | 32.2 ± 8.7 | 61.2 ± 4.3 | 0.02 | |||||
| Flight | 36.1 ± 4.2 | 17.9 ± 5.1 | 0.03 | |||||
| No response | 31.6 ± 5.4 | 20.9 ± 3.2 | 0.09 | |||||
| Response duration/trial (s) | ||||||||
| Freeze | 9.9 ± 1.4 | 8.1 ± 1.3 | 0.39 | |||||
| Flight | 4.5 ± 0.8 | 6.5 ± 1.2 | 0.21 | |||||
Figure 2 .
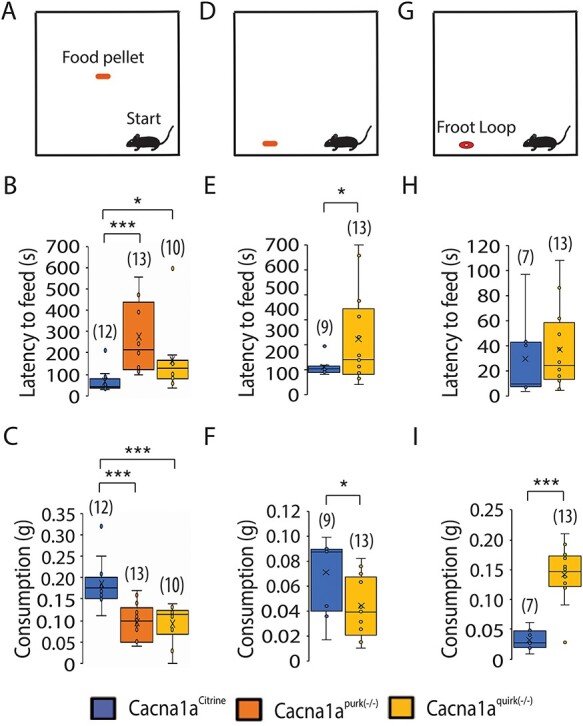
EA2 mouse models, Cacna1apurk(−/−) and Cacna1aquirk(−/−) show anxious and indecisive behavior in the novelty-suppressed feeding test. Schematic of the NSF arena, where a ~ 2 g food pellet was offered in the center (A) or border (D) or a Froot Loop was given at the border (G). Mice started in the right corner of the arena. The latency to feed (B, E, H) and food consumption (C, F, I) for Cacna1aCitrine (blue), Cacna1apurk(−/−) (orange) and Cacna1aquirk(−/−) (yellow) mice are depicted as whisker boxplots. Cacna1apurk(−/−) and Cacna1aquirk(−/−) mice demonstrated a decreased latency to feed and consumption compared with Cacna1aCitrine when the food pellet is given in the center or border of the arena (B, E). Cacna1aquirk(−/−) mice shows comparable latencies with feed (H) and had a higher food consumption (I) compared with Cacna1aCitrine mice, when a Froot Loop is given at the border. The number of mice tested/group is indicated in parentheses in legend. Statistical significance was evaluated with ANOVA (*P ≤ 0.05, **, ***P ≤ 0.001).
Episodic ataxia type 2 mouse models, Cacna1apurk(−/−) and Cacna1aquirk(−/−), show delays in making decisions
Given the conflicting results in the anxiety tests and extensive latencies to feed in the novelty suppressed feeding without a reward despite 24 h food deprivation and latency to first (to the dark zone) in the light/dark place preference tests, where mice are expected to spend more time in the protective dark zone, we wanted to examine whether the EA2 mice have deficits in making decisions. Therefore, we repeated the light/preference test but started the mice in the dark zone (data not shown). Since the mice displayed a similar exploring behavior as in the original light/dark place preference test except now remained longer in the start, dark zone, we designed a new test, the dark/dark place preference test, where we divided the arena into two dark zones (zone A and B) which are connected by an opening. The mice were started in dark zone A and given 10 min (5 min longer than in the light/dark place preference test) to explore dark zones A and B. In this scenario, we eliminated the anxiety of a well-lit fear zone and provided the mice more time to transition into zone B. Interestingly, Cacna1aquirk(−/−) mice spent equal times in both dark zones (Fig. 4B) even though their latency to first times remained high (Fig. 4C), zone transitions low (Fig. 4E) and total distance moved less (Supplementary Material, Fig. S1) compared with their control littermates (Table 1). Both EA2 mouse lines also displayed similar latency to first times in both place preference tests which indicates deficits in decision making rather than an increase in anxiolytic behavior. Cacna1apurk(−/−) mice continued to demonstrate an increased duration in the start zone A and increased latency to first times and decreased zone transitions compared with controls but less deviations compared with the light/dark place preference test. Since Cacna1apurk(−/−) mice traveled the same distance as their control littermates (Cacna1aCitrine 2642 ± 150.8 cm; Cacna1apurk(−/−) 2927 ± 90.9 cm; Supplementary Material, Fig. S1), their ataxic phenotype does not appear to hinder them from performing the test or their mobility. Considering the increased latency to first and number of EA2 mice with >100 s latency to first explore dark zone B compared with controls (Fig. 4D) but more equivalent duration times in
Figure 4 .
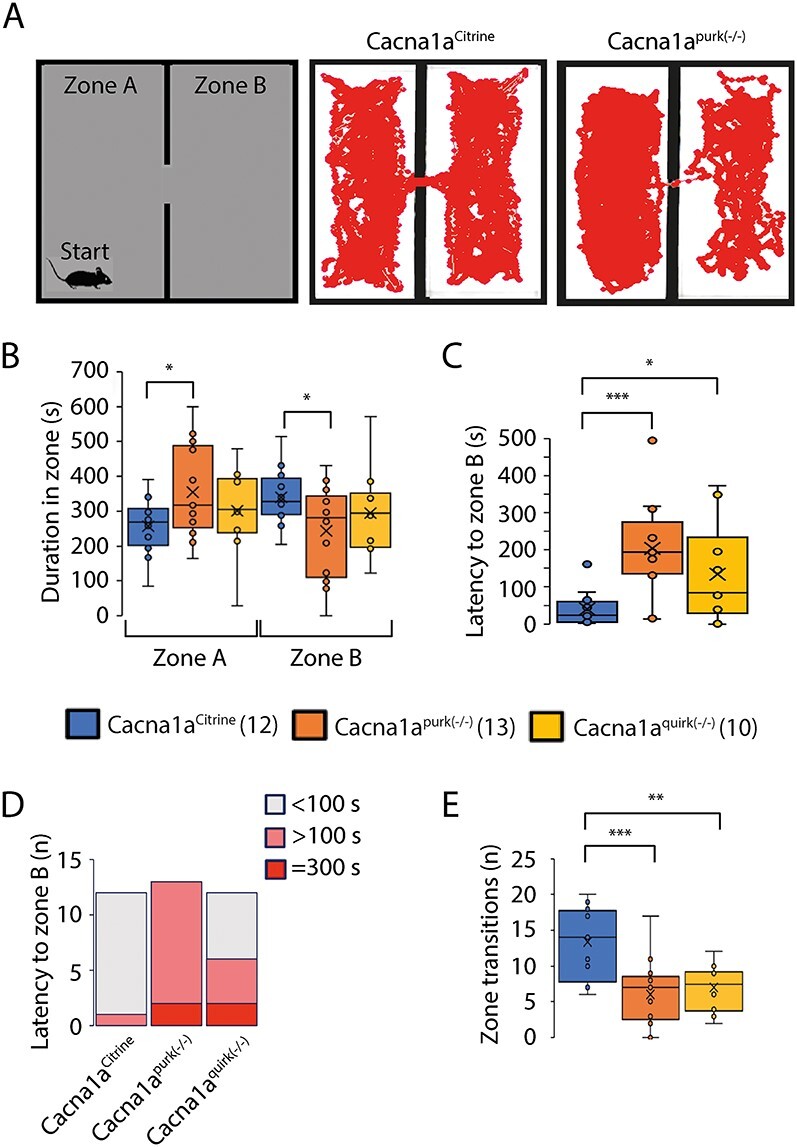
EA2 mouse models, Cacna1apurk(−/−) and Cacna1aquirk(−/−) demonstrate decision making deficits in dark/dark place preference test. (A) Schematic of the dark/dark place preference test (left) which was divided into two dark zones, A and B. Mice started in dark zone A opposite to the dark zone B entrance and given 10 min to explore both zones. Representative traces from a Cacna1aCitrine (middle) and Cacna1apurk(−/−) (right) mouse. Duration spent in the dark zone A and B (B), latency to zone B (C), number of mice (n) with <100 s (gray), >100 s (pink) or >300 s (red) latency to dark zone B times (D) and number of transitions (n) between dark zones (E) for Cacna1aCitrine (blue), Cacna1apurk(−/−) (orange) and Cacna1aquirk(−/−) (yellow) mice are depicted as whisker boxplots. Cacna1apurk(−/−) and Cacna1aquirk(−/−) mice initially spent more time in dark zone A as a reflection of the increased number of mice with >100 s latency to zone B times. Despite high latencies to zone B, Cacna1aquirk(−/−) mice spent equal time in both arenas like control Cacna1aCitrine mice. The number of mice tested/group is indicated in parentheses in legend. Statistical significance was evaluated with one-way ANOVA (*P ≤ 0.05, ***P ≤ 0.001).
both dark zones and similar latency to first times compared with the light/dark place preference test, is suggestive that EA2 mice require more time to make decisions. Although we attempted to eliminate the anxiety factor to well-lit areas in our dark/dark preference test, we cannot exclude the possibility that EA2 mice continue to experience anxiousness to new territories (i.e. dark zone B). Collectively, our results implicate that EA2 mice have deficits in decision making in addition to being more anxious to new territories.
Episodic ataxia type 2 mouse models, Cacna1apurk(−/−) and Cacna1aquirk(−/−), demonstrate impairments in memory
To evaluate whether EA2 mice show cognitive deficiencies in recognition memory, Cacna1apurk(−/−), Cacna1aquirk(−/−) and control littermates were subjected to the novel object recognition test. Briefly, mice were habituated to the open field, 24 h later (testing day 1) encountered two identical objects equidistant apart and on testing day 2 one of the identical objects was replaced by a novel object. All mice displayed equal preferences to the identical objects on day 1 (Fig. 5C, left). On the next day, EA2 mice showed lower preferences for the novel object compared with controls (Fig. 5C, right). Moreover, Cacna1aCitrine controls exhibited a higher preference (Cacna1aCitrine 80.7 ± 3.5%; Cacna1apurk(−/−) 56.6 ± 3.2%; Cacna1aquirk(−/−) 44.6 ± 6.2%), shorter latency to first to the novel object (Cacna1aCitrine 14.8 ± 3.6 s; Cacna1apurk(−/−) 43.6 ± 11.4 s; Cacna1aquirk(−/−) 107.6 ± 31.9 s) and more frequent visits (Cacna1aCitrine 55.9%; Cacna1apurk(−/−) 23.4%; Cacna1aquirk(−/−) 20.8%) for the novel object compared with the EA2 mice (Table 1). EA2 mice showed the same preference to the equal object on day 1 as well as on day 2, implicating deficits in memory. These observations were also verified in the three chamber social test where a mouse is started in the middle chamber and allowed to roam a chamber containing an empty cage and the another completely empty chamber (Fig. 9A). Cacna1aquirk(−/−) mice displayed decreased durations (cage chamber: Cacna1aCitrine 285.3 ± 21.1 s; Cacna1aquirk(−/−) 219.5 ± 11.4 s; cage zone: Cacna1aCitrine 54.3 ± 6.1 s; Cacna1aquirk(−/−) 32.6 ± 6.1 s) and frequencies (cage chamber: Cacna1aCitrine 71.1 ± 4.4 s; Cacna1aquirk(−/−) 54.9 ± 4.6 s; cage zone: Cacna1aCitrine 39.6 ± 2.9 s; Cacna1aquirk(−/−) 30.7 ± 2.8 s) in the chamber and zone around the empty cage compared with control littermates (Fig. 9C, E, F; Supplementary Material, Fig. S3C). Since our EA2 mice move the same distance with similar velocities than their control littermates in the novel object recognition and three chamber social test (Fig. 5F; Supplementary Material, Fig. S3A and B), we can minimize the influence of their ataxic phenotype. However, we cannot exclude ataxia for our Cacna1Aquirk(−/−) mice in the novel object recognition test. Cacna1aquirk(−/−) mice did not show any differences in preference, latencies, visits or distance moved in another test for spatial memory and discrimination, the object relocation memory task (Fig. 6) indicating that they have no deficits in recognizing the relocated object or in physically performing the task. Together these results from the novel object recognition and object relocation memory tests indicate that EA2 mice may have impairments in their recognition memory and potentially anxiety for new objects and/or delays in deciding to approach a new object but not in their spatial memory. Mice which did not have total exploration time of ≥20 s or did not explore both objects at least once were excluded from the analysis.
Figure 5 .
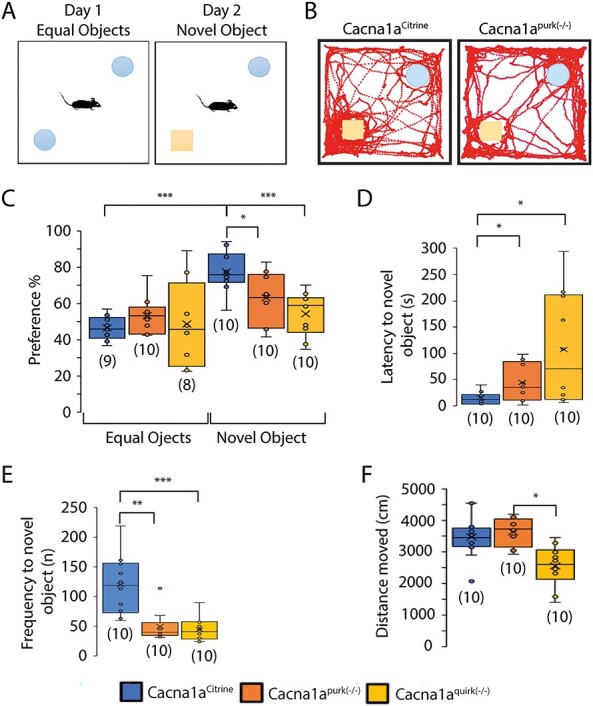
EA2 mouse models, Cacna1apurk(−/−) and Cacna1aquirk(−/−) display cognitive impairments in recognition memory. (A) Schematic of the novel object recognition test. After mice were habituated to the open field, the mice were placed in the middle of an open field between 2 equal (identical) objects on the first day and 1 familiar and 1 novel object on the second day of testing. (B) Representative traces from a Cacna1aCitrine (left) and Cacna1apurk(−/−) (right) mouse are shown on day 2. Whisker boxplots of the preference (%) for equal versus novel objects (C), latency to novel object (D), frequency (n) of visits to novel object (E) and total distance moved in the presence of the novel object (F) for Cacna1aCitrine (blue), Cacna1apurk(−/−) (orange) and Cacna1aquirk(−/−) (yellow) mice are depicted. Cacna1apurk(−/−) and Cacna1aquirk(−/−) mice demonstrated a lower preference, increased latency to first and decreased frequency of visits for the novel object compared with control Cacna1aCitrine mice. Whereas the Cacna1aCitrine mice spent more time and visits to the novel object. The number of mice tested/group is indicated in parentheses in legend. Statistical significance was evaluated with one-way ANOVA (*P ≤ 0.05, **P ≤ 0.01; ***P ≤ 0.001).
Figure 9 .
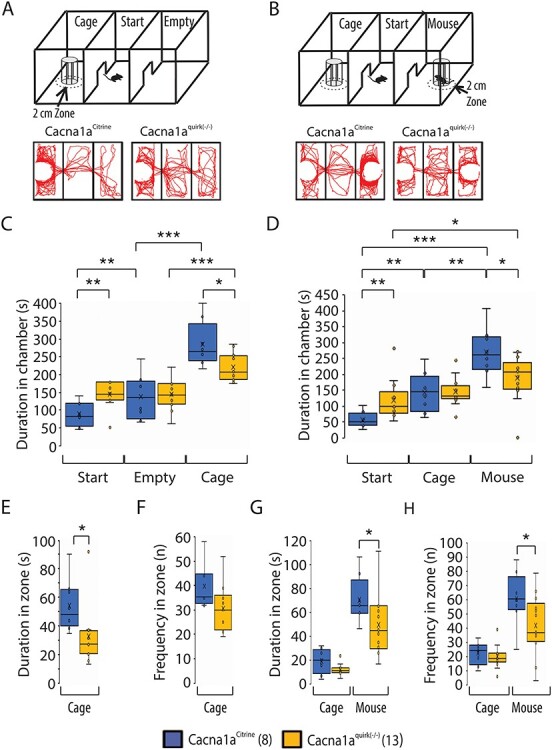
Cacna1aquirk(−/−) mice show deficits in social interaction and indifferent behavior in the three chamber social assay. (A) Schematic of the three chamber social assay with a start, non-social (cage) and empty chamber. Example traces of a Cacna1aCitrine (left) and Cacna1aquirk(−/−) (right) mouse depict the explorative behavior of the non-social chamber assay. (B) Schematic of the three chamber social assay, where the empty chamber is changed to a social (mouse) chamber. Example traces of a Cacna1aCitrine (left) and Cacna1aquirk(−/−) (right) mouse depict the explorative behavior. Cacna1aquirk(−/−) mice spent significantly more time in the start chamber compared with control Cacna1aCitrine mice for both paradigms (C, D) and showed less explorative behavior for the cage chamber than control mice (C). Additionally, Cacna1aquirk(−/−) mice spent less time in the 2 cm zone around the cage (E) and displayed less visits of the adjacent area around the cage (F) than controls, indicating a lack of curiosity and motivation. (G) Cacna1aquirk(−/−) mice spent significantly more time in the 2 cm zone around the social than the empty cage, with more visits of the social zone (H) than the cage zone. The number of mice tested/group is indicated in parentheses in legend. Statistical significance was evaluated with ANOVA (*P ≤ 0.05, **P ≤ 0.01; ***P ≤ 0.001).
Figure 6 .
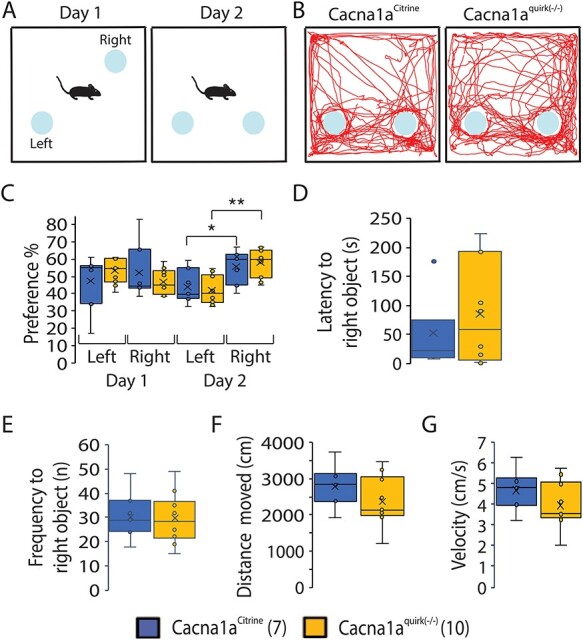
EA2 mice, Cacna1aquirk(−/−) do not display spatial memory impairments in the object relocation test. (A) Schematic of the object relocation test. The mouse was habituated to the two identical, non-social objects on the first day, while on day 2 the right object was moved. (B) Example traces of a Cacna1aCitrine (left) and Cacna1aquirk(−/−) mouse (right) after the right object was relocated. Both Cacna1aquirk(−/−) and Cacna1aCitrine mice showed equal preference for both objects on day 1, but spent significantly more time on the relocated right object on day 2 (C). (D) Cacna1aquirk(−/−) mice showed higher latency to first visit the relocated object compared with control mice, while the frequency of visits (E), the total distance moved (F) and velocity (G) were identical. The number of mice tested/group is indicated in parentheses in legend. Statistical significance was evaluated with ANOVA (*P ≤ 0.05, **P ≤ 0.01; ***P ≤ 0.001).
We also performed the T maze to test for working memory. However, EA2 mice demonstrated a high percentage of failed trials where they simply did not choose an arm and remained in the start area even after extending the test to 10 min as indicated in the Supplementary Material, Figure S2B, supporting their anxiety in decision-making or new territories. To investigate the possibility that they have anxiety to new territories, we removed the center partition (Fig. 7A) and increased the habituation trials to 3 x 10 min instead of the standard 1 x 10 min to decrease the anxiety to a new territory. Cacna1aquirk(−/−) mice now performed equally as well as the Cacna1aCitrine mice with an alternation % of 67.5 ± 7.5 compared with 67.5 ± 7.5 in controls (Fig. 7B), implicating a hesitation to exploring new territories. Together the data support that EA2 mice have cognitive impairments in not only recognition memory but also decision making and/or anxiety for new objects and territories.
Figure 7 .
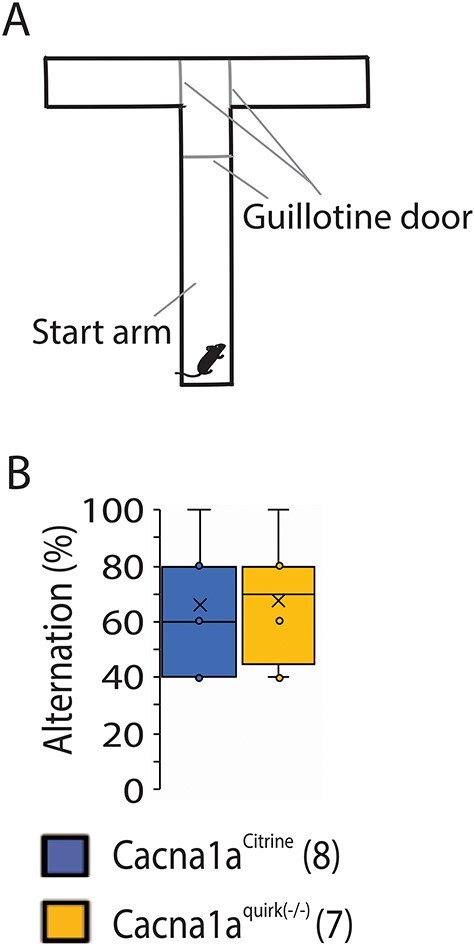
EA2 mice Cacna1aquirk(−/−) have no difficulties in decision making in the T-maze test. (A) Schematic of the T maze containing a center partition at the last third of the start arm and three guillotine doors, one at the start of the center partition and at the start of each deciding arm. (B) Cacna1aquirk(−/−) mice needed more trials to complete the T-maze test, but had no conspicuities in decision making and showed identical alternations to control Cacna1aCitrine mice. The number of mice tested/group is indicated in parentheses in legend. Statistical significance was evaluated with ANOVA.
Episodic ataxia type 2 mouse models, Cacna1apurk(−/−) and Cacna1aquirk(−/−), show social interaction deficiencies
To determine if EA2 mice have social impairments, we introduced a foreign, age and gender matched C57Bl/6 mouse for 5 min in the resident home cage and analyzed the duration and frequencies of attention and sniff events (Fig. 8). In addition, we also recorded passive behaviors such as standing up on back limbs, walking, nesting and following the intruder mouse and aggressive actions including mounting, tail rattling, attacking and biting with no obvious abnormalities in EA2 mice compared with controls (Table 1). Both EA2 mouse lines displayed a significantly enhanced duration of attention behaviors (Fig. 8A; Cacna1aCitrine 12.1 ± 4.2 s; Cacna1apurk(−/−) 66.2 ± 6.5 s; Cacna1aquirk(−/−) 38.2 ± 7.6 s) and an increase number of attention events in Cacna1apurk(−/−) mice compared with controls (Cacna1aCitrine 3.6 ± 1.2; Cacna1apurk(−/−) 10.0 ± 1.5), indicating interest in the foreign intruder. However, they demonstrated less frequent sniff events (Fig. 8B; Cacna1aCitrine 19.1 ± 2.9; Cacna1apurk(−/−) 10.6 ± 0.54; Cacna1aquirk(−/−) 7.4 ± 0.96) but comparable time spent sniffing with Cacna1aCitrine controls. Surprisingly, only Cacna1aquirk(−/−) mice exhibited less frequent social (Fig. 8C; Cacna1aCitrine 23.1 ± 2.3, Cacna1aquirk(−/−) 15.8 ± 1.4) and genital (Fig. 8D; Cacna1aCitrine 6.9 ± 1.8, Cacna1aquirk(−/−) 1.2 ± 0.44) sniff events compared Cacna1aCitrine littermates. In support of these observations, Cacna1aquirk(−/−) mice also demonstrated reduced social interactions in an independent test, the three chamber social assay where Cacna1aquirk(−/−) were first habituated to the three empty chambers, then to just an empty cage without a mouse in one of the chambers (nonsocial chamber) and finally exposed to a chamber with (social) or without a mouse (nonsocial) (Fig. 9B). Cacna1aquirk(−/−) mice spent less time and visits exploring the mouse chamber (Fig. 9D, Supplementary Material, Fig. S3F) and 2 cm zone (Fig. 9G, H) around the mouse cage compared with Cacna1aCitrine controls indicative of reduced social interest (Table 1).
Figure 8 .
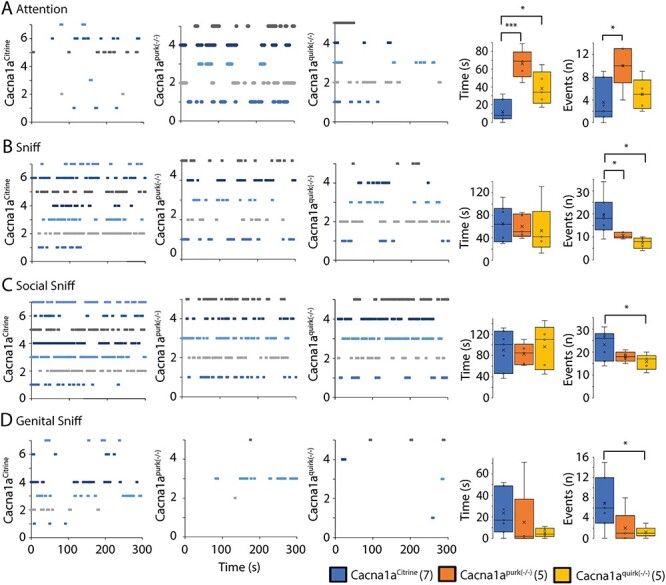
EA2 mouse models, Cacna1apurk(−/−) and Cacna1aquirk(−/−) demonstrate social interaction deficiencies. The duration and number of social events for attention (A), sniffing (B), social sniffing (C) and genital sniffing (D) were analyzed from Cacna1aCitrine (left), Cacna1apurk(−/−) (middle) and Cacna1aquirk(−/−) (right) mice after a 5 min exposure to a foreign C57/Bl6 mouse. The number and genotype of mice tested/group is indicated on y axis and plotted against the time (s) sequence of events. Each mouse is represented as a different color. Each dash represents a new event and time point of occurrence. The length of the dash represents the duration of the event. The average duration (time (s)) and number of events (n) for each mouse group is depicted as whisker boxplots. The number of mice tested/group is indicated in parentheses in legend. Statistical significance was evaluated with one-way ANOVA (*P ≤ 0.05, ***P ≤ 0.001).
Although there were no significant changes in aggressive traits such as mounting, tail rattling, attacking and biting measured, the EA2 mice tended to be more passive as evident by their reduced number of mounts, tail rattles and attacks compared with Cacna1aCitrine controls (Table 1). To further characterize their more docile phenotype directly, we implemented the tube dominance test where a control and EA2 mouse of the same gender were placed on either side of a plastic tube. The mouse which backed out of the tube first was scored as ‘loser.’ If both mice remained in the tube for the entire 10 min, then it was noted as a ‘tie.’ Cacna1apurk(−/−) mice only won 22% of their trials against controls who won 67% of their trials with very few ties (11%; Fig. 10B). On the contrary Cacna1aquirk(−/−) mice showed similar aggression levels as controls (18% wins) with 54% ties and only 29% wins (Fig. 10C). All together these results indicate that the augmented attention and sniff events in EA2 mice may have similar traits to ADHD and social abnormalities as seen in EA2 patients and that Cacna1apurk(−/−) mice may be less aggressive. Considering the more ataxic phenotype of Cacna1apurk(−/−) mice, this may be contributing to their inability to push the control mice out of the tube successfully.
Figure 10 .
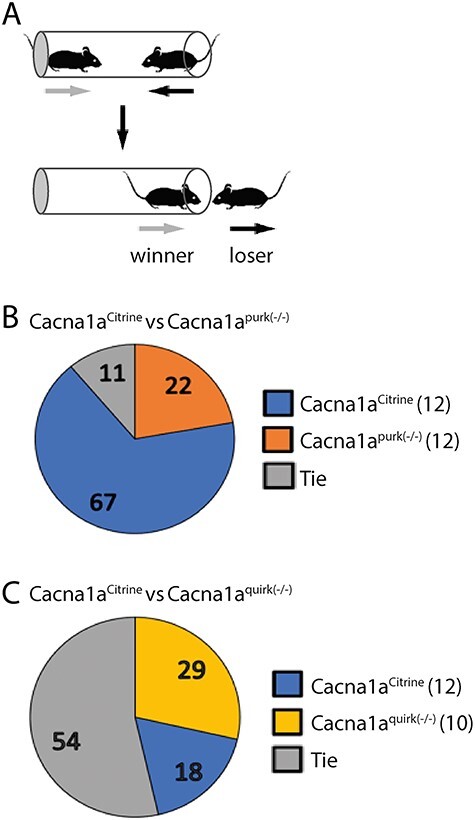
Cacna1apurk(−/−) mice are nonaggressive compared with Cacna1aquirk(−/−) and Cacna1aCitrine controls in the tube dominance test. (A) Schematic of the tube dominance test where opponents are placed on opposite ends of a tube and the winner displaces their opponent out of the tube. Cacna1aCitrine (blue) controls were paired with gender matched EA2 mice randomly for ≥ 3 trials. (B) Cacna1aCitrine versus Cacna1apurk(−/−) (orange), Cacna1apurk(−/−) mice were docile compared with controls (67% wins). (C) Cacna1aCitrine versus Cacna1aquirk(−/−) (yellow), both lines showed similar levels of aggression with a tie of 54%. The percentage of winners from each group is indicated in the pie graphs. The number of mice tested/group is indicated in parentheses.
Cacna1aquirk(−/−) demonstrate normal reward driven olfaction, heat detection and defensive responses to a visual stimulus (looming)
To examine if Cacna1aquirk(−/−) mice exhibit sensory deficits which may inhibit their cognitive abilities, we tested their sense of touch, pain, smell and sight. To test their thermoception Cacna1aquirk(−/−) mice were placed on a hotplate at 32°C with rising temperatures of 1°C/min to 42°C. The number and time to first paw lick and jump at each degree was recorded compared with controls. Cacna1aquirk(−/−) mice showed indistinguishable temperature to first responses for either paw licks (Fig. 11A; Cacna1aCitrine 33.9 ± 0.5°C, Cacna1aquirk(−/−) 33.6 ± 0.7°C) or jumps (Fig. 11A; Cacna1aCitrine 38.4 ± 0.9°C, Cacna1aquirk(−/−) 38.3 ± 1.2°C) in the hotplate test compared with controls. We did observe that the control mice display a more linear response to the hotplate, and the Cacna1aquirk(−/−) mice respond to lower temperatures than controls (Fig. 11C). Moreover, Cacna1aquirk(−/−) mice demonstrated no differences in latency to feed in the buried food assay (Fig. 12B; Cacna1aCitrine 82.5 ± 19.5 s, Cacna1aquirk(−/−) 170.6 ± 36.3 s), where a Froot Loop was hidden under 1 cm from a total of 3 cm of bedding in a mouse cage (Fig. 12A). Together the hotplate and buried food tests indicated that Cacna1aquirk(−/−) mice appear to have an intact perception of heat and smell when provided sufficient positive or negative incentive to respond. Additionally, Cacna1aquirk(−/−) mice responded to visual stimuli such as a growing black disk (looming) which represents a predator coming from above (Fig. 13A) similar to controls. The total percentage of Cacna1aquirk(−/−) mice (~79%) reacting either by freezing or by flight to the looming was comparable with controls (~68%) and the duration of freezing (Cacna1aCitrine 9.9 ± 1.4 s, Cacna1aquirk(−/−) 8.1 ± 1.3 s) and flight (Cacna1aCitrine 4.5 ± 0.8 s, Cacna1aquirk(−/−) 6.5 ± 1.2 s) responses to looming (Fig. 13B, C), indicating that Cacna1aquirk(−/−) mice respond in a timely manner in threatening situations. Overall, these results indicate that the sensory systems, i.e. olfaction, thermoception and vision and sense of innate danger are intact in Cacna1aquirk(−/−) mice.
Figure 11 .
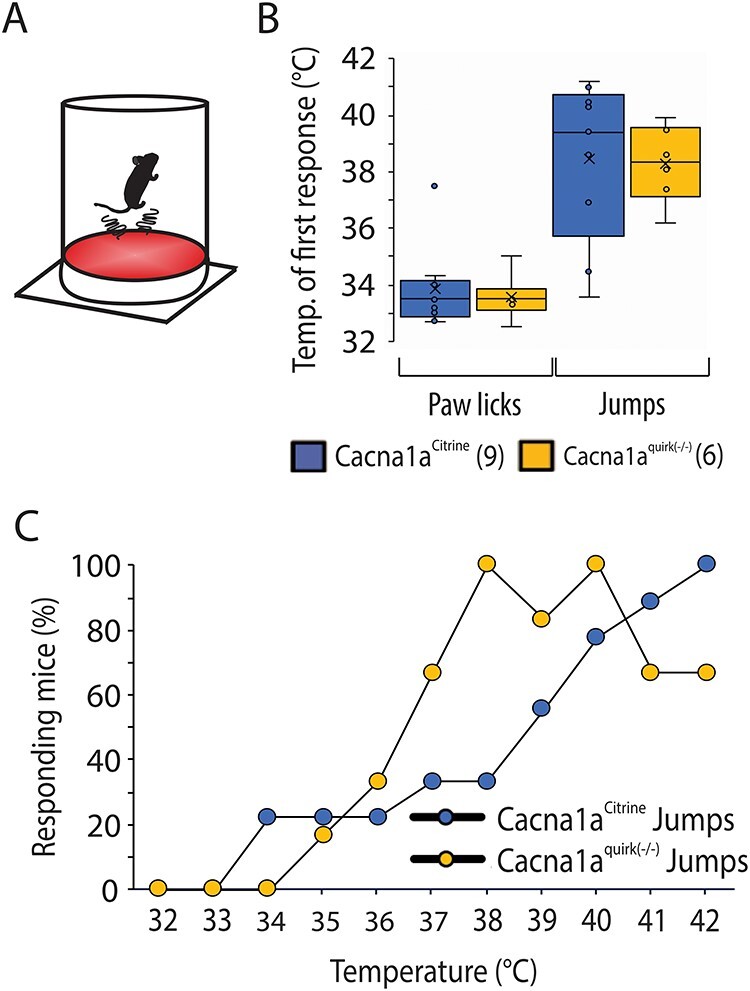
EA2 mice, Cacna1aquirk(−/−) show no sensorimotor deficits and altered nociception in the hotplate test. (A) Schematic of the test apparatus. The mouse was placed on a 32°C warm aluminum plate surrounded by a plexiglass cylinder, which is heated to 42°C with 1°C/min. (B) Average temperature for the first reaction to the increasing heat of Cacna1aCitrine (blue) and Cacna1aquirk(−/−) (yellow) mice. Licking the paws was observed at lower temperatures (~ 33°C) for both mouse lines, while first jumps were observed at temperatures above 38°C. There was no significant change in the latency to jump between the mouse lines. (C) Cacna1aquirk(−/−) (yellow) mice respond earlier to increasing temperatures than Cacna1aCitrine mice. 100% of Cacna1aquirk(−/−) mice started to jump ~ 38°C, whereas only 30% of Cacna1aCitrine were jumping as response to the heat. Control mice show a more linear response to the increasing heat compared with EA2 mice. The number of mice tested/group is indicated in parentheses in legend. Statistical significance was evaluated with ANOVA (*P ≤ 0.05, **P ≤ 0.01; ***P ≤ 0.001).
Figure 12 .
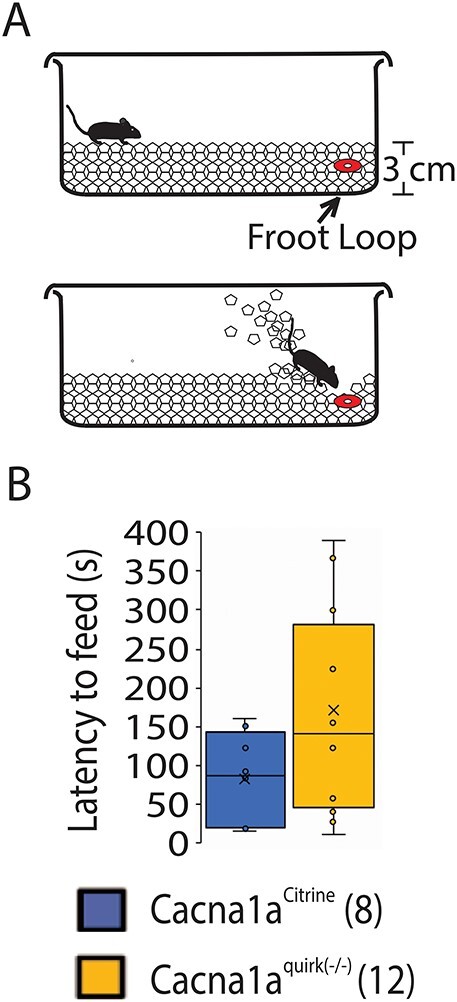
Cacna1aquirk(−/−) EA2 mice and controls do not display olfactory deficits in the buried food test. (A) Schematic of the buried food test, where a Froot Loop was buried under 1 cm from 3 cm of total regular cage bedding. Mice were starved for 24 h and given 15 min on the test day to find and bite the treat. (B) The latency to feed is not significantly altered in Cacna1aquirk(−/−) (yellow) compared with Cacna1aCitrine (blue) mice. We observed that both mouse lines detected the hidden Froot Loop, but that Cacna1aquirk(−/−) took more time to dig it out due to their motor deficits. The number of mice tested/group is indicated in parentheses in legend. Statistical significance was evaluated with ANOVA.
Figure 13 .
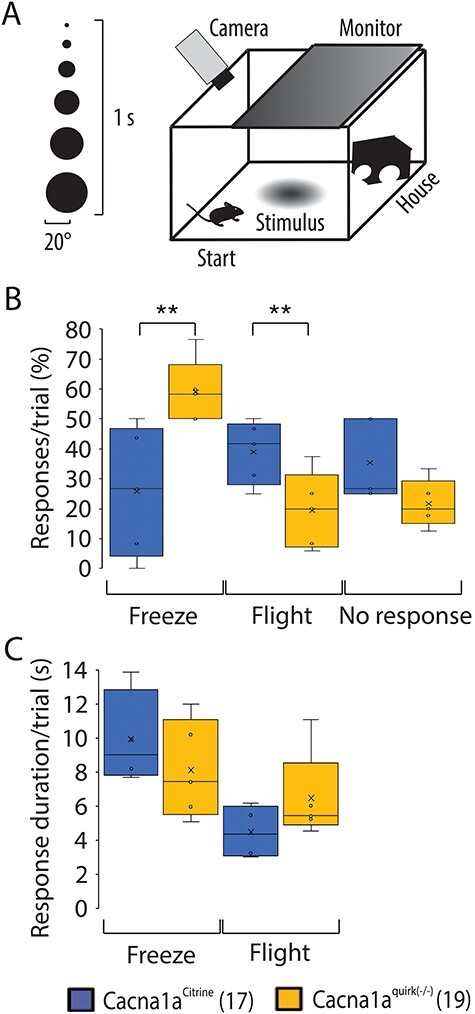
EA2 mice, Cacna1aquirk(−/−) show defensive escape behavior in the looming test. (A) Schematic of the looming test. An expanding black disk on an LCD monitor is used as looming stimulus and presented from above. (B) EA2 mice reacted with decreased impulsive flight behavior to the looming stimulus but responded with increased freezing behavior compared with control Cacna1aCitrine mice. Both Cacna1aquirk(−/−) and control mice showed the same number of unresponsive trials. (C) The response duration per trial of freezing or flight behavior in Cacna1aquirk(−/−) mice was not different from the response duration of Cacna1aCitrine mice. The number of mice tested/group is indicated in parentheses in legend. Statistical significance was evaluated with ANOVA (**P ≤ 0.01).
Discussion
In the present study, we performed the first battery of cognitive tests in two different mouse models for EA2, Cacna1apurk(−/−) and Cacna1aquirk(−/−), where we genetically removed the P/Q type calcium channel postnatally from either cerebellar Purkinje or granular cells (22,23) to eliminate prenatal defects. A previous study by Galliano et al. (24) removing the P/Q type channel from GC in the mouse cerebellum did not show motor or cognitive dysfunctions most likely due to a lower dose response effect by the CRE recombinase expression levels. Both EA2 mouse models displayed impairments in anxiety, decision making, memory and social behaviors compared with their Cacna1aCitrine littermate controls at adult ages. Our data not only support the case studies in EA2 patients that the cerebellum can contribute to cognition but also that these cognitive deficits cannot be attributed to prenatal defects since complete removal of the P/Q type calcium channel from the cerebellum occurred 2–3 weeks after birth in mice. Additionally, our results show that loss of P/Q type calcium channel postnatally from either PC or GC may contribute to anxiety, ADHD, social phobia, autism spectrum disorder (ASD), adaptation and schizophrenia disorders as seen in EA2 patients.
EA2 mice are anxious and indecisive
Using two different mouse models for EA2 where the P/Q-type calcium channel was removed specifically from cerebellar PCs (Cacna1apurk(−/−)) or GCs (Cacna1aquirk(−/−)), mice showed at first anxiolytic behavior to brightly lighted open spaces in the open field test (Fig. 1B), spending less time in the borders and more time in the intermediate and center regions, and in the place preference test (Fig. 3) where they spent more time in the light zone (start) as evident by their severely inflated latencies and few transitions (Fig. 3C and E). In fact 69.2% of the Cacna1apurk(−/−) mice did not leave the brightly lit start zone and 20% of the Cacna1aquirk(−/−) mice. This anxiolytic phenotype cannot be attributed to ataxia since the more affected Cacna1apurk(−/−) mice (22) moved the same total distances as their littermate controls and subsequent behavior test show that EA2 mice move with similar distances and velocities as their control littermates (Fig. 6, Supplementary Material, Figs S1 and S3). At first glance it would appear the EA2 mice were anxiolytic, but when the mice performed the exact same place preference test except starting in the dark zone similar results were found (data not shown) where EA2 mice spent more time in the dark zone (start) with few transitions. To examine whether the mice were anxious to new territories or to the brightly lighted open spaces, we designed a novel test which we call the dark/dark place preference test where an open field is divided into two dark zones, therefore eliminating the light zone and anxiety to brightly lighted open spaces (Fig. 4). Much to our surprise the EA2 mice spent more time in transition zone B when they were allotted twice the amount of time to explore both zones as in the light/dark preference test, suggesting difficulties in making timely decisions (Fig. 4B). In spite of this, their latencies to dark zone B remained the same as in the light/dark preference test with few transitions (Fig. 4E). An interesting observation to note is that less Cacna1apurk(−/−) mice displayed latencies >300 s (n = 2) and less Cacna1aquirk(−/−) mice had latencies >100 s (n = 2) as in the light/dark place preference test. These data indicate that the EA2 mice do have grave delays in making decisions to explore new territories due to potential fears to new situations. However, once they are given more time to overcome their delays in decision making, Cacna1aquirk(−/−) spent equal times exploring the new zone as controls with improved durations in the new zone with Cacna1apurk(−/−) mice. These observations suggest that the EA2 mice are engaged and curious when given sufficient time to overcome their anxiety and/or delays in decision making. The EA2 mice also demonstrated increased latencies in the novel suppressed feeding test with the food pellet and in the novel recognition test to the novel object supporting that they have decision making in addition to potential anxious impairments since the tests cannot completely rule out an anxiety deficit (Fig. 2B). In the T-maze test, only ~40% of the Cacna1apurk(−/−) mice completed the trials and the remaining did not choose an arm. Although 100% of the Cacna1aquirk(−/−) mice were able to complete trial 5, previous completed trials (~5–50%) were drastically diminished due to their inability to choose an arm in the T maze (Supplementary Material, Fig. S2). However, after increasing the habituation period to the T maze Cacna1aquirk(−/−) performed equally as well as control mice, supporting the idea that EA2 mice may have delayed decision making due to an anxiety to new territories. We also observed extensive periods of side to side rocking at the end of the start arm in Cacna1apurk(−/−) mice which were milder in the Cacna1aquirk(−/−) mice. This rocking behavior was reminiscence of an autistic child undergoing an episode except with forward and backwards rocking. Since a few case reports observed ASD in EA2 patients (10,11), this indecisive side to side rocking together with their nonsocial and anxious phenotype may be an indication of ASD like traits in EA2 mice. A recent study showed that the cerebellum may be regulated by the PFC to mediate autism like traits (25). Although we attempted to reduce anxiolytic responses in the dark/dark preference, novel object recognition and social tests by using dim light conditions, we cannot exclude the possibility that EA2 mice demonstrate a combination of anxiety to new territories and objects and delays in decision making.
Memory recognition impairments in EA2 mice
Both EA2 mouse lines demonstrated deficits in memory recognition in the novel object recognition test which may be compounded with anxiety for new objects and decision-making deficiencies. Mice displayed an equal preference to both identical objects like control mice though less preference, latencies and visits to the novel object on the next day indicating memory recognition impairments (Fig. 5). However, Cacna1aquirk(−/−) mice which showed decreased movement in the novel object recognition test did not demonstrate any deficiencies in their spatial memory and discrimination in a relocated familiar object (Fig. 6). To investigate their working and reference memory, EA2 mice were also subjected to the T-maze test. Control mice displayed a normal alteration rate (number of turns in each goal arm and total trial duration) of 0.73 ± 0.04 (data not shown) where EA2 mice unable to choose an arm within the extended 10 min testing time (Supplementary Material, Fig. S2). After increasing the habituation period to three trials for 10 min each, Cacna1aquirk(−/−) mice demonstrated a normal alteration rate suggesting an anxiety to new territories (Fig. 7). Together these data in EA2 mice are in agreement with EA2 patient studies that show memory deficits and anxiety disorders (11,13,14,26).
EA2 mice demonstrate deficiencies in specific social traits
Twelve different features were examined for social abnormalities in the social interaction test with same sex resident intruder (Table 1). Passive social interactions such as attention were exceptionally enhanced indicating hyperactivity and diminished ability to focus while decreases in sniffing events were observed (Fig. 8A). Only Cacna1aquirk(−/−) mice displayed less social and genital sniffing events compared with their control littermates (Fig. 8C and D) which was confirmed in the three chamber social assay (Fig. 9). No changes in standing up on back limbs, walking, time in nest or following behavior were notice in EA2 mice compared with controls (Table 1). These differences in passive social interactions may suggest ADHD and inability to focus as seen in EA2 patients (8,11–13,15). Although there were no significant differences in aggressive behaviors which include mounting, tail rattling, attacking and bites, EA2 mice appeared more passive than the Cacna1aCitrine controls which showed a tendency of more attacks and mounts (Table 1). To directly compare the aggressive behavior between the EA2 and control mouse lines, we implemented the tube dominance test where the Cacna1apurk(−/−) mice were much less aggressive with only 22.2% wins and 11.1% ties compared with 66.7% wins in controls. In contrast, Cacna1aquirk(−/−) mice showed similar aggression levels as Cacna1aCitrine mice with 54% ties and 29% wins (Fig. 10). However, we cannot exclude the possibility that the decreased aggression was due to motor incoordination.
EA2 mice do not have obvious thermoception, olfactory, visual or innate fear dysfunctions
To ensure that EA2 mouse performance was not inhibited by general sensory malfunctions, we examined the Cacna1aquirk(−/−) mice for pain and olfactory perception. Cacna1aquirk(−/−) mice showed relatively comparable reactions with heat perception in the hot plate test (Fig. 11) and finding a Froot Loop in the buried food test (Fig. 12). In addition, they displayed comparable defensive responses with a visual stimulus indicating that their visual and innate defensive systems are intact (Fig. 13). Based on these tests, it appears that the EA2 mice are physically able to perform simple tests that require unrestrained walking since in the majority of experiments they moved equal total distances with an equivalent velocity as the Cacna1aCitrine mice. Additionally, when given the proper positive (e.g. novelty suppressed feeding test with the Froot Loop, buried Froot Loop test; Figs 2 and 12) or negative (e.g. looming test, hotplate test; Fig. 13) stimulus, Cacna1aquirk(−/−) mice were able to respond in an appropriate manner as controls which seem to indicate a lack of engagement and motivation intensity without the proper enticements or habituation time like in the T maze (Fig. 7). These data also indicate an anxiety for new situations either territories or objects which influence their decision making (long delays) as was seen in the place preference tests (Figs 3 and 4). Although Cacna1aquirk(−/−) mice have no difficulties with their spatial memory as was observed in the object relocation test and T maze (Figs 6 and 7), EA2 mice may have a deficit in their recognition memory (Fig. 5). However, it is difficult to distinguish from anxiety for a new object in this test. EA2 mice also display nonsocial behaviors as was seen in the social interaction with a resident intruder and three chamber social test (Figs 8 and 9) which are in agreement with their general lack of engagement to perform certain tests without a positive or negative stimulus. More complex behavior tasks need to be implemented in the future to dissect out the different deficits contributing to cognition in EA2 mouse models.
Contribution of cerebellar PC or GC activities to cognition
We observed differences in cognitive function in both EA2 mouse lines where we removed the P/Q type calcium channel from either the PCs or the GCs which support the idea that both neurons are contributing to cognitive functions such as decision making, anxiety, social behaviors and memory. Although more detailed studies in our mouse models need to be performed to dissect the neural circuitry disrupting these cognitive functions, numerous past studies support our hypothesis that the cerebellar PC and GC activity are contributing to cognition. For example, the Wang lab showed the molecular layer interneurons in the posterior cerebellum which receive excitatory input from the GCs to inhibit PCs are important for emotional and cognitive processes (27). Moreover, they also provide evidence that PCs are involved in working memory and decision making (28) and perturbations in the GC pathway may be responsible for sensory learning defects in autistic mouse models (29). PCs activity is correlated to error predictions in an associative learning task in monkeys (30) and in reward prediction signals in mice (31). Carta et al. (20) found that the cerebellum modulates reward and social behavior via the VTA. PC activity is also important in shaping our emotions like fear (32–34). All together these findings support our results that disruptions in PC or GC activity by removing the P/Q type calcium channel which is important for neuronal activity, synaptic transmission and integration of information may lead to cognitive abnormalities in decision making, social behaviors and emotions.
Episodic ataxia type 2 is a multi-spectrum disorder
EA2 is a complex, diverse faceted neurological disease characterized by motor deficits, which include paroxysmal and stress induced ataxic episodes, nystagmus, absence epilepsy, headaches and more recently psychiatric and cognitive disorders such as anxiety, depression, attention deficit hyperactivity, autistic-like traits, personality and executive function impairments. In order to treat individual patients successfully, as many aspects of their deficiencies should be diagnosed and therapeutically managed. In this study, we aimed to investigate cognitive impairments in our mouse models for EA2 to determine whether it would be a sufficient animal model to study combined drug therapy treatments for their ataxia, absence epilepsy and cognitive abnormalities in the future. It is important to keep in mind that our mouse models for EA2 also suffer from ataxia and stress induced dystonia which can affect their cognitive performance since most of the test requires motor coordination. However, we tracked the total distanced moved in several of the tests with no changes in Cacna1apurk(−/−) mice. Cacna1aquirk(−/−) mice were not as mobile and motivated in general and moved less distance in several the tests. In our previous publications, we demonstrate in several motor coordination tests that postnatal removal of the P/Q type calcium channel have more severe effects on Cacna1apurk(−/−) compared with Cacna1aquirk(−/−) mice. Therefore, we do not think this is the reason for their poor motor performance but may be due to motivation issues. In addition, all mice experiencing a spontaneous or stress induced dystonic attack were removed from the analyses. Our EA2 mouse models as in some patients suffer from absence epilepsy (22,23,35–37) which may also contribute to their poor cognitive performance. To the best our knowledge this is the first cognitive study in EA2 mouse models identifying dysfunction in several areas such as anxiety for new situations (i.e. territories and objects), decision making, memory recognition, social interactions and aggression. Collectively, our data suggest that EA2 mice in addition to suffering from motor-related abnormalities also may display anxiety, autistic- and ADHD-like traits which include deficiencies in social, decision making and new situations. Our mouse models for EA2 would be ideal for pharmacologically dissecting out the different pathways contributing to cognitive deficits from their motor and epileptic dysfunctions which may even lead to new therapeutic treatments for EA2 patients.
Materials and Methods
Transgenic mice
Cacna1aCitrine mice were created in our lab (22,23) and then further crossed with transgenic PC-specific CRE mice (Jackson laboratories, stock number 004146 B6.129-Tg(Pcp2-cre)2Mpin/J) (38) to create the Cacna1apurk(−/−) mice or crossed with transgenic GC-specific CRE mice (Jackson laboratories, stock number 000196-UCD; B6; D2-Tg(Gabra6-cre)B1Lfr/Mmucd) (39) to create the Cacna1quirk (−/−) mice. The genetic background of the mice was determined by PCR of genomic DNA from tail biopsy (22,23). To detect Citrine incorporation into the Cacna1 gene, PCRs were performed with primers to Cacna1a, Citrine and Cre recombinase. The following primer pairs were used to identify the mouse strains described: Cacna1a forward 5′ GGGGTCTGACTTCTGATGGA 3′, reverse 5′ AAGTTGCACACAGGGCTTCT 3′; Cacna1aCitrine forward 5′ TATATCATGGCCGACAAGCA 3′, reverse 5′ TTCGGTCTTCACAAGGAACC 3′; Tgcre forward 5′ ATTCTCCCACCACCGTCAGTACG 3′, reverse 5′ AAAATTTGCCTGCATTACCG 3′.
Adult male and female mice (9–12 mo) were used for behavior experiments. Cacna1aCitrine test group consisted of 7 males and 5 females. Cacna1apurk(−/−) test group contained 8 males and 5 females. Cacna1aquirk(−/−) test group contained 4 males and 6 females. A second group of mice consisting 7 males and 10 females for Cacna1aCitrine and 13 males and 14 females for Cacna1aquirk(−/−) at 6–12 mo of age were used for additional experiments. All mice were group housed in the behavior lab on a 12 h dark/light cycle with food and water ad libitum for the duration of the testing period. Mice were single housed for the social interaction test. Cleaning of the cages was executed at least 2 days prior to testing and not during the testing period to avoid stress and distraction. All tests were performed during their dark cycle to minimize disruption of sleep cycle and with at least 2 days in between tests to allow adequate recovery time. Mice were acclimated for at least 7 days to the dark/light cycle and behavior lab before testing. Trials were omitted with mice undergoing a dyskinetic event and repeated at a later time point if possible or excluded from the analyses. Data acquisition was performed using the Noldus video recording system and Ethovision XT 8.5 tracking software.
The present study was carried out in accordance with the European Communities Council Directive of 2010 (2010/63/EU) for care of laboratory animals and approved by a local ethics committee (Bezirksamt Arnsberg) and the animal care committee of North Rhine-Westphalia, Germany, based at the LANUV (Landesamt für Umweltschutz, Naturschutz und Verbraucherschutz, Nordrhein-Westfalen, D-45659 Recklinghausen, Germany). The study was supervised by the animal welfare commission of the Ruhr-University Bochum. All efforts were made to minimize the number of mice used for this study.
Behavior tests
Open field. The open field arena consisted of 50 x 50 cm opaque, plexiglass chamber subdivided into a center (20 x 20 cm), intermediate and border (8 cm from chamber wall) region, which was brightly illuminated with 950 lux above the arena (40). Mice were placed into the center of the open field and the following parameters were video tracked for 15 min with the Ethovision XT 8.5 software (Noldus): time spent in the center, time spent in the border, total distance traveled and border-to-center transitions. The apparatus was cleaned between subjects with 70% ethanol. Each mouse underwent 1 trial.
Novelty suppressed feeding. Mice were deprived of food for 24 h before testing with water available ad libitum (40). A familiar food pellet previously weighed (~2 g) was placed in the middle or in the border equal distance from the start to the center of a new aversive environment (arena: 50 x 50 cm), brightly illuminated with 950 lux above the arena. As an enticement, a familiar Froot Loop was placed in the border equal distance from the start to the center of a new aversive environment. Mice were placed into a plastic tube in the right corner of the arena, which was removed at the start of the test. The task ended when the mice first fed, defined as biting the food pellet with use of the forepaws. The latency to start feeding served as a measurement of anxious behavior. Subjects were recorded for 10 min with the Noldus video recording system and Ethovision XT 8.5 tracking and analysis software for further analysis. Mice were allowed to consume food an additional 5 min in the arena before returning to home cages. Food consumption was measured for potential feeding differences among test groups. Each mouse underwent 1 trial.
Light/dark place preference. The light/dark place preference test, also known as the light/dark mouse exploration test was created according to the modified specifications as Crawley and Goodwin (41). Briefly an open field arena (30 x 30 x 30 cm) was divided into two arenas (30 x 15 x 30 cm), an open, light arena and a dark closed arena consisting of a black infrared (IR) see through plexiglass box with an opening. The light arena was brightly illuminated with 950 lux above the arena. Mice were placed into one corner of the light arena and the following parameters were video tracked with an IR camera for 5 min with the Noldus video recording system and Ethovision XT 8.5 tracking and analysis software: time spent in the light and dark arena and the number of transitions between the light and dark arenas. The arenas were cleaned between subjects with 70% ethanol. Each mouse underwent 1 trial.
Dark/dark place preference. The dark/dark place preference test was created according to the modified specifications as Crawley and Goodwin (41) to examine the decisiveness of the mice. Briefly an open field arena (30 x 30 x 30 cm) was divided into two dark closed arenas (zone A and zone B) consisting of a black IR plexiglass box (30 x 15 x 30 cm) with a small opening to access both dark arenas. Tests were performed under IR light only. Mice were placed into the corner of one of the dark arenas and the following parameters were video tracked with an IR camera for 10 min with the Ethovision XT 8.5 software (Noldus): time spent in dark zone A or B and the number of transitions between the two dark zones. The arenas were cleaned between subjects with 70% ethanol. Each mouse underwent 1 trial.
Novel object recognition. The novel object recognition test was performed based on the protocol from Lueptow (42). The mice were habituated for 5 min to a 50 x 50 cm open field under dim light conditions. The next day two equal objects were placed in opposite quarters of the field. The mice were placed in the middle of the box for 10 min to explore the objects. Twenty four hours later, one of the familiar objects was replaced by a novel object. The novel object is placed alternately in one or the other quarter to avoid side preference bias. The mice were again placed in the middle of the box with 10 min to explore both objects. The mice were video tracked with an IR camera with the Noldus video recording system and Ethovision XT 8.5 tracking and analysis software for the last 2 days of the trials. The total exploration time (nose point) for both objects, latency to first for each object, duration and frequency were tracked. A preference index (p) was calculated with the equation p = (n/t) x 100 (n = time spent exploring the novel object, t = total exploration time). Mice which did not have total exploration time of ≥20 s or did not explore both of the objects at least once were excluded from the analysis. To obtain a representative sample, a group is excluded from the analysis of one phase if more than half of the mice have been excluded. Each mouse underwent 1 trial.
Object relocation test. The object relocation test also known as object location memory task was performed to examine spatial memory and cognition abilities of Cacna1aquirk(−/−) and Cacna1aCitrine mice and was created according to the protocol from Vogel-Ciermia and Wood (43). Mice were placed in the center of a 50 x 50 cm opaque, plexiglass chamber brightly illuminated with 950 lux above the arena for a 5 min habituation period. The next day, two identical objects were placed on opposite sides of the arena, before mice were placed in the middle of the field for 10 min. Twenty four hours later, one object was placed to the same side. The mice were again placed in the middle of the box with 10 min to explore. Animals were video tracked each time with an IR camera with the Ethovision XT 11.5 software (Noldus) and following parameters were analyzed: total exploration time (nose point) for both objects, latency to first, duration at each object and frequency. A preference index (p) was calculated p = (n/t) x 100. Mice which did not have total exploration time of ≥20 s or did not explore both of the objects at least once were excluded from the analysis. To obtain a representative sample, a group is excluded from the analysis of one phase if more than half of the mice have been excluded. Each mouse underwent 1 trial.
T maze. Spatial memory and cognition of the mice were investigated using the T-maze test under dim light conditions (44). Cacna1aquirk(−/−) and Cacna1aCitrine mice were habituated to the T maze for 3 x 10 min trials. Twenty-four hours later, mice were tested in 5 trials where each trial of the test consists of a sample- and a choice-phase. Mice were placed at the beginning of the start arm facing the wall. After choosing one arm, the mice were allowed 30 s to explore the chosen arm. Then the choice-phase began by placing mice again at the start arm and choose an arm. Mice were given a maximum time of 10 min to complete each phase. The chosen arm in the sample- and choice-phase and duration of arm choice were noted. Animals explored the previously unknown arm, whereas impaired animals would explore arms randomly. Five trials per animal were averaged and the alternation value was evaluated.
Social interaction with resident intruder. Transgenic mice were single housed for at least 2 days before testing (45). Transgenic mice were introduced to an age, gender and size matched C57B/6 mouse in a neutral cage for 5 min during their dark cycle. The duration and number of events for attention, sniffing, social and genital sniffing were later analyzed from video tracks (Ethovision XT 8.5 software, Noldus). Attention was defined as interest or examination of environment. Sniffing was determined by sniffing the environment but not the foreign mouse. Social sniffing was defined as sniffing the foreign mouse but not its genitals. Genital sniffing was defined as sniffing the genitals of the foreign mouse. Nesting, standing up on back limbs, walking, following, mounting, tail rattling, biting and fighting were also analyzed. Each mouse underwent 1 trial.
3 Chamber social assay. This test was performed to analyze the social skills and anxiety of Cacna1aquirk(−/−) and Cacna1aCitrine mice. We modified the protocol from Locke et al. (46). A 60 x 30 x 30 cm plexiglass arena, which was divided into three chambers of equal size (20 x 30 x 30 cm) with small openings to allow access to all chambers was brightly illuminated with 950 lux above the arena. For habituation, mice were placed in the center chamber for 10 min, freely exploring the arena. The following day an empty wire cage (10 x 15 cm) was placed in the left arena (non-social chamber) and the test was repeated. On the third day, another wire cage containing a foreign mouse was placed in the right chamber (social chamber) and the mice were tested again for 10 min. The mice were video tracked with an IR camera by the Ethovision XT 11.5 software (Noldus). The total exploration time, frequency of visits, time spent in the chambers and 2 cm zones around the wire cages were analyzed. Mice which did not have total exploration time of ≥20 s or did not explore both chambers at least once were excluded from the analysis.
Tube dominance test. Aggression was more directly tested by the tube dominance test (30 cm long and 3.0 cm diameter transparent, plexiglass tube). Mouse pairs were released into either end of the tube, the first mouse to exit was the ‘loser.’ EA2 mice were paired with Cacna1aCitrine mice of the same gender. A maximum session of 10 min was performed and ≥3 trials with randomized opponents. In the event that both mice remained in the tube for the entire 10 min or both mice exited the tube at the same time, this was considered a ‘tie.’ The plastic tube was cleaned between subjects with 70% ethanol.
Hotplate test. The hotplate test was modified according to the methods of Barrot (47). Cacna1aquirk(−/−) and Cacna1aCitrine mice were placed inside a 15 x 30 cm plexiglass cylinder, which was positioned on a metal heat plate connected to a temperature control panel. Starting at 32°C, the plate slowly heated 1°C/min to a maximum of 42°C. Animals were filmed with a conventional camcorder (Panasonic). The temperature of first jump and paw lick, total number of jumps/mouse/°C and paw licks/mouse/°C and % of mice jumping or paw licking at a given temperature were analyzed. Experiments were performed under normal light conditions. The heat plate and cylinder were disinfected with 70% ethanol. Each mouse underwent 1 trial.
Buried food test. To determine the olfactory abilities of the Cacana1aquirk(−/−) mice, overnight-fasted mice were timed how quickly they could find a Froot Loop under 1 cm of bedding from a total of 3 cm of bedding (48). Each mouse underwent 1 trial and was given a maximum of 10 min to complete the task. Average latencies to find the Froot Loop were analyzed and reported.
Looming test. Flight behavior and recognition abilities of threatening situations as a parameter for anxiety were investigated with the looming test based on the protocol from Li et al. (49). Briefly, Cacna1aquirk(−/−) and Cacna1aCitrine mice were habituated for 10 min to the 50 x 50 cm open field arena, where a black plexiglass shelter (10 x 10 cm) was placed in the upper left corner and an Acer Predator LED display (62 cm) was placed on the ceiling of the arena with only a white background. Twenty four hours later, mice were placed in the center of the arena and allowed to explore for 5 min again. Following the 5 min habituation period, the monitor presented the looming stimulus, an expanding black disk starting 2–20° in 1 s with 10 repetitions (programmed with Matlab, MathWorks®, USA). Each mouse was presented with the looming stimulus for ≤ 5 times. The stimulus was triggered manually as soon as the mouse entered the opposite quarter of the arena (maximum distance from shelter). Experiments were performed under IR conditions. Mice were recorded with an IR camera by the Ethovision XT 11.5 software (Noldus) and the following parameters were analyzed: number of responses for freezing or flight/trial and mean time freezing/stimulus/trial. Mice were given a total of 3 min to rest in the shelter. If mice did not leave the house within this time period, they were gently removed manually and placed in the center of the arena. ≤ 5 trials per animal were averaged. The arena was disinfected with 70% ethanol between subjects.
Statistics and Reproducibility
All statistical analyses were calculated with Microsoft Excel software. All statistical analyses were calculated by means of one-way ANOVA for comparison of mouse groups. Values have been considered as outliners, if there were 1.5*IQR (IQR: Interquartile range) larger than the upper quartile or smaller than the lower quartile. Outliners have been excluded from the analysis. Mice which did not meet the individual test requirements as described in the methods for each individual behavior test or had a dystonic attack were also excluded from the analyses. Data are presented as whisker boxplots or a dot plot/time sequence of events. Significance for comparisons: *P ≤ 0.05, **P ≤ 0.01; ***P ≤ 0.001. The n for every experiment is reported in the figures.
Supplementary Material
Acknowledgements
We would like to thank Dennis Piotrowski, Stefan Dobers, Winfried Junke, Margareta Möllmann, Nicole Ozdowski, Stephanie Krämer and Manuela Schmidt for their excellent technical assistance. Conflict of Interest statement. All authors declare no conflicts of interest.
Contributor Information
Pauline Bohne, Behavioral Neuroscience, Ruhr-University Bochum, D-44780 Bochum, Germany.
Damian Boden-El Mourabit, Behavioral Neuroscience, Ruhr-University Bochum, D-44780 Bochum, Germany.
Mareike Josten, Behavioral Neuroscience, Ruhr-University Bochum, D-44780 Bochum, Germany.
Melanie D Mark, Behavioral Neuroscience, Ruhr-University Bochum, D-44780 Bochum, Germany.
Funding
P.B. was supported by Project number 316803389-SFB1280. This work was supported by Deutsche Forschungsgemeinschaft (DFG) (MA 5806/2-1 and MA 5806/1-2 to M.D.M.).
References
- 1.Jen, J., Kim, G.W. and Baloh, R.W. (2004) Clinical spectrum of episodic ataxia type 2. Neurology, 62, 17–22. [DOI] [PubMed] [Google Scholar]
- 2.Nachbauer, W., Nocker, M., Karner, E., Stankovic, I., Unterberger, I., Eigentler, A., Schneider, R., Poewe, W., Delazer, M. and Boesch, S. (2014) Episodic ataxia type 2: phenotype characteristics of a novel CACNA1A mutation and review of the literature. J. Neurol., 261, 983–991. [DOI] [PubMed] [Google Scholar]
- 3.Jaudon, F., Baldassari, S., Musante, I., Thalhammer, A., Zara, F. and Cingolani, L.A. (2020) Targeting alternative splicing as a potential therapy for episodic ataxia type 2. Biomedicine, 8, 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jelitai, M., Puggioni, P., Ishikawa, T., Rinaldi, A. and Duguid, I. (2016) Dendritic excitation-inhibition balance shapes cerebellar output during motor behaviour. Nat. Commun., 7, 13722 (1-13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tara, E., Vitenzon, A., Hess, E. and Khodakhah, K. (2018) Aberrant cerebellar Purkinje cell activity as the cause of motor attacks in a mouse model of episodic ataxia type 2. Dis. Models Mech., 11, dmm034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaguchi, K., Itohara, S. and Ito, M. (2016) Reassessment of long-term depression in cerebellar Purkinje cells in mice carrying mutated GluA2 C terminus. Proc. Natl. Acad. Sci. U. S. A., 113, 10192–10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timmann, D., Drepper, J., Frings, M., Maschke, M., Richter, S., Gerwig, M. and Kolb, F.P. (2010) The human cerebellum contributes to motor, emotional and cognitive associative learning. A review. Cortex, 46, 845–857. [DOI] [PubMed] [Google Scholar]
- 8.Hoche, F., Guell, X., Vangel, M.G., Sherman, J.C. and Schmahmann, J.D. (2018) The cerebellar cognitive affective/Schmahmann syndrome scale. Brain, 141, 248–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argyropoulos, G.P.D., van Dun, K., Adamaszek, M., Leggio, M., Manto, M., Masciullo, M., Molinari, M., Stoodley, C.J., Van Overwalle, F., Ivry, R.B. and Schmahmann, J.D. (2020) The cerebellar cognitive affective/schmahmann syndrome: a task force paper. Cerebellum, 19, 102–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoche, F., Guell, X., Sherman, J.C., Vangel, M.G. and Schmahmann, J.D. (2016) Cerebellar contribution to social cognition. Cerebellum, 15, 732–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Indelicato, E., Nachbauer, W., Karner, E., Eigentler, A., Wagner, M., Unterberger, I., Poewe, W., Delazer, M. and Boesch, S. (2019) The neuropsychiatric phenotype in CACNA1A mutations: a retrospective single center study and review of the literature. Eur. J. Neurol., 26, 66–e7. [DOI] [PubMed] [Google Scholar]
- 12.Bertholon, P., Chabrier, S., Riant, F., Tournier-Lasserve, E. and Peyron, R. (2009) Episodic ataxia type 2: unusual aspects in clinical and genetic presentation. Special emphasis in childhood. J. Neurol. Neurosurg. Psychiatry, 80, 1289–1292. [DOI] [PubMed] [Google Scholar]
- 13.Nardello, R., Plicato, G., Mangano, G.D., Gennaro, E., Mangano, S., Brighina, F., Raieli, V. and Fontana, A. (2020) Two distinct phenotypes, hemiplegic migraine and episodic Ataxia type 2, caused by a novel common CACNA1A variant. BMC Neurol., 20, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guterman, E.L., Yurgionas, B. and Nelson, A.B. (2016) Pearls & Oy-sters: episodic ataxia type 2: case report and review of the literature. Neurology, 86, e239–e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laasonen, M., Salomaa, J., Cousineau, D., Leppämäki, S., Tani, P., Hokkanen, L. and Dye, M. (2012) Project DyAdd: visual attention in adult dyslexia and ADHD. Brain Cogn., 80, 311–327. [DOI] [PubMed] [Google Scholar]
- 16.Bohne, P., Schwarz, M.K., Herlitze, S. and Mark, M.D. (2019) A new projection from the deep cerebellar nuclei to the hippocampus via the ventrolateral and laterodorsal thalamus in mice. Front. Neural Circuits., 13, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apps, R. and Strata, P. (2015) Neuronal circuits for fear and anxiety - the missing link. Nat. Rev. Neurosci., 16, 642. [DOI] [PubMed] [Google Scholar]
- 18.D'Angelo, E. and Casali, S. (2013) Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front. Neural Circuits., 6, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hull, C. (2020) Prediction signals in the cerebellum: beyond supervised motor learning. elife, 9, e54073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carta, I., Chen, C.H., Schott, A.L., Dorizan, S. and Khodakhah, K. (2019) Cerebellar modulation of the reward circuitry and social behavior. Science, 363, eaav0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frontera, J.L., Baba Aissa, H., Sala, R.W., Mailhes-Hamon, C., Georgescu, I.A., Léna, C. and Popa, D. (2020) Bidirectional control of fear memories by cerebellar neurons projecting to the ventrolateral periaqueductal grey. Nat. Commun., 11, 5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mark, M.D., Maejima, T., Kuckelsberg, D., Yoo, J.W., Hyde, R.A., Shah, V., Gutierrez, D., Moreno, R.L., Kruse, W., Noebels, J.L. and Herlitze, S. (2011) Delayed postnatal loss of P/Q-type calcium channels recapitulates the absence epilepsy, dyskinesia, and ataxia phenotypes of genomic Cacna1a mutations. J. Neurosci., 31, 4311–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maejima, T., Wollenweber, P., Teusner, L.U., Noebels, J.L., Herlitze, S. and Mark, M.D. (2013) Postnatal loss of P/Q-type channels confined to rhombic-lip-derived neurons alters synaptic transmission at the parallel fiber to purkinje cell synapse and replicates genomic Cacna1a mutation phenotype of ataxia and seizures in mice. J. Neurosci., 33, 5162–5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galliano, E., Potters, J.W., Elgersma, Y., Wisden, W., Kushner, S.A., De Zeeuw, C.I. and Hoebeek, F.E. (2013) Synaptic transmission and plasticity at inputs to murine cerebellar Purkinje cells are largely dispensable for standard nonmotor tasks. J. Neurosci., 33, 12599–12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly, E., Meng, F., Fujita, H., Morgado, F., Kazemi, Y., Rice, L.C., Ren, C., Escamilla, C.O., Gibson, J.M., Sajadi, S. et al. (2020) Regulation of autism-relevant behaviors by cerebellar-prefrontal cortical circuits. Nat. Neurosci., 23, 1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brady, R.O., Jr., Gonsalvez, I., Lee, I., Öngür, D., Seidman, L.J., Schmahmann, J.D., Eack, S.M., Keshavan, M.S., Pascual-Leone, A. and Halko, M.A. (2019) Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am. J. Psychiatry, 176, 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giovannucci, A., Badura, A., Deverett, B., Najafi, F., Pereira, T.D., Gao, Z., Ozden, I., Kloth, A.D., Pnevmatikakis, E., Paninski, L. et al. (2017) Cerebellar granule cells acquire a widespread predictive feedback signal during motor learning. Nat. Neurosci., 20, 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deverett, B., Kislin, M., Tank, D.W. and Wang, S.S. (2019) Cerebellar disruption impairs working memory during evidence accumulation. Nat. Commun., 10, 3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kloth, A.D., Badura, A., Li, A., Cherskov, A., Connolly, S.G., Giovannucci, A., Bangash, M.A., Grasselli, G., Peñagarikano, O., Piochon, C. et al. (2015) Cerebellar associative sensory learning defects in five mouse autism models. elife, 4, e0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sendhilnathan, N., Semework, M., Goldberg, M.E. and Ipata, A.E. (2020) Neural correlates of reinforcement learning in mid-lateral cerebellum. Neuron, 106, 188–198.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heffley, W. and Hull, C. (2019) Classical conditioning drives learned reward prediction signals in climbing fibers across the lateral cerebellum. elife, 8, e46764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sacchetti, B., Scelfo, B., Tempia, F. and Strata, P. (2004) Long-term synaptic changes induced in the cerebellar cortex by fear conditioning. Neuron, 42, 973–982. [DOI] [PubMed] [Google Scholar]
- 33.Strata, P., Scelfo, B. and Sacchetti, B. (2011) Involvement of cerebellum in emotional behavior. Physiol. Res., 60, S39–S48. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida, M. and Kondo, H. (2012) Fear conditioning-related changes in cerebellar Purkinje cell activities in goldfish. Behav. Brain Funct., 8, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stendel, C., D'Adamo, M.C., Wiessner, M., Dusl, M., Cenciarini, M., Belia, S., Nematian-Ardestani, E., Bauer, P., Senderek, J., Klopstock, T. and Pessia, M. (2020) Association of a novel splice site mutation in P/Q-type calcium channels with childhood epilepsy and late-onset slowly progressive non-episodic cerebellar ataxia. Int. J. Mol. Sci., 21, 3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damaj, L., Lupien-Meilleur, A., Lortie, A., Riou, É., Ospina, L.H., Gagnon, L., Vanasse, C. and Rossignol, E. (2015) CACNA1A haploinsufficiency causes cognitive impairment, autism and epileptic encephalopathy with mild cerebellar symptoms. Eur. J. Hum. Genet., 23, 1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung, J., Testard, H., Tournier-Lasserve, E., Riant, F., Vallet, A.E., Berroir, S. and Broussolle, E. (2010) Phenotypic variability of episodic ataxia type 2 mutations: a family study. Eur. Neurol., 64, 114–116. [DOI] [PubMed] [Google Scholar]
- 38.Barski, J.J., Dethleffsen, K. and Meyer, M. (2000) Cre recombinase expression in cerebellar Purkinje cells. Genesis, 28, 93–98. [PubMed] [Google Scholar]
- 39.Fünfschilling, U. and Reichardt, L.F. (2002) Cre-mediated recombination in rhombic lip derivatives. Genesis, 33, 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mark, M.D., Wollenweber, P., Gesk, A., Kösters, K., Batzke, K., Janoschka, C., Maejima, T., Han, J., Deneris, E.S. and Herlitze, S. (2019) RGS2 drives male aggression in mice via the serotonergic system. Commun. Biol., 2, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crawley, J. and Goodwin, F.K. (1980) Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Behav., 13, 167–170. [DOI] [PubMed] [Google Scholar]
- 42.Lueptow, L.M. (2017) Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp., 126, 55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogel-Ciernia, A. and Wood, M.A. (2014) Examining object location and object recognition memory in mice. Curr. Protoc. Neurosci., 69, 8.31.1–8.31.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deacon, R.M. and Rawlins, J.N. (2006) T-maze alternation in the rodent. Nat. Protoc., 1, 7–12. [DOI] [PubMed] [Google Scholar]
- 45.Moretti, P., Bouwknecht, J.A., Teague, R., Paylor, R. and Zoghbi, H.Y. (2005) Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum. Mol. Genet., 14, 205–220. [DOI] [PubMed] [Google Scholar]
- 46.Locke, T.M., Fujita, H., Hunker, A., Johanson, S.S., Darvas, M., du Lac, S., Zweifel, L.S. and Carlson, E.S. (2020) Purkinje cell-specific knockout of tyrosine hydroxylase impairs cognitive behaviors. Front. Cell. Neurosci., 14, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrot, M. (2012) Tests and models of nociception and pain in rodents. Neuroscience, 211, 39–50. [DOI] [PubMed] [Google Scholar]
- 48.Yang, M. and Crawley, J.N. (2009) Simple behavioral assessment of mouse olfaction. Curr. Protoc. Neurosci., Chapter 8, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li, L., Feng, X., Zhou, Z., Zhang, H., Shi, Q., Lei, Z., Shen, P., Yang, Q., Zhao, B., Chen, S. et al. (2018) Stress accelerates defensive responses to looming in mice and involves a locus coeruleus-superior colliculus projection. Curr. Biol., 28, 859–871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


