Abstract
A significant amount of epidemiological evidence has underlined that human-to-human transmission due to close contacts is considered the main pathway of transmission, however since the SARS-CoV-2 can also survive in aerosols, water, and surfaces, the development and implementation of effective decontamination strategies are urgently required. In this regard, ultraviolet germicidal irradiation (UVGI) using ultraviolet C (UVC) has been proposed to disinfect different environments and surfaces contaminated by SARS-CoV-2. Herein, we performed a systematic scoping review strictly focused on peer-reviewed studies published in English that reported experimental results of UVC-based technologies against the SARS-CoV-2 virus. Studies were retrieved from PubMed and the Web of Science database. After our criterious screening, we identified 13 eligible articles that used UVC-based systems to inactivate SARS-CoV-2. We noticed the use of different UVC wavelengths, technologies, and light doses. The initial viral titer was also heterogeneous among studies. Most studies reported virus inactivation in well plates, even though virus persistence on N95 respirators and different surfaces were also evaluated. SARS-CoV-2 inactivation reached from 90% to 100% depending on experimental conditions. We concluded that there is sufficient evidence to support the use of UVC-based technologies against SARS-CoV-2. However, appropriate implementation is required to guarantee the efficacy and safety of UVC strategies to control the COVID-19 pandemic.
Keywords: Germicidal, COVID-19, Virucidal, Photoinactivation, Physical method, Irradiation, UVGI
Graphical abstract
1. Introduction
The relentless spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the novel coronavirus disease (COVID-19), led to an unprecedented global public health crisis. SARS-CoV-2 was firstly identified in humans in December 2019 in Wuhan city (China) before it was rapidly spread worldwide [1]. The World Health Organization (WHO) declared the COVID-19 pandemic on March-11–2020 [2]. Since then, medical and scientific authorities have coordinated efforts on a scale never seen before to face the health, economic and social effects of this pandemic crisis.
The epidemiological investigations regarding the SARS-CoV-2 dynamics have demonstrated that human-to-human transmission through close contacts is the main transmission pathway [3,4]. However, it has also been confirmed that SARS-CoV-2 remains viable in aerosols, water, and surfaces, which turn the spotlight on the role of contaminated surfaces and environments for the transmission of this highly contagious pathogen [5,6]. Currently, several chemical and physical methods have been proposed for the inactivation of SARS-CoV-2 outside the human body to minimize the risks of COVID-19 transmission [7].
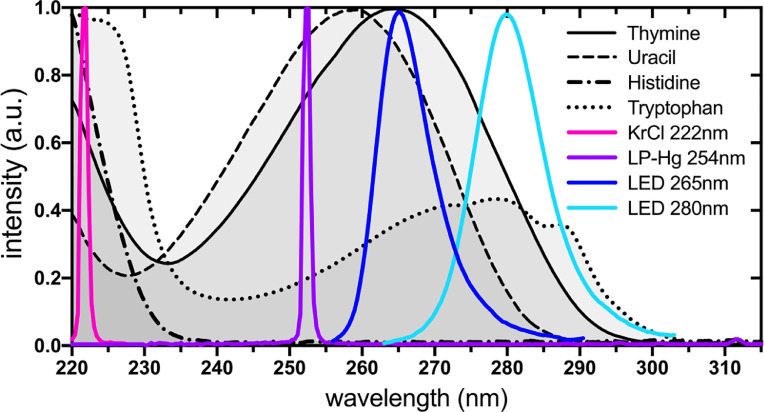
For almost a century, ultraviolet (UV) radiation has been used in hospital and industrial settings for the decontamination of surfaces, air, and water. UV spectrum can be divided into UVA (315–400 nm), UVB (280–315 nm), and UVC (100–280 nm), whereas the latter exhibits the strongest antimicrobial properties due to its absorption by nucleic acids and amino acids (Fig. 1 ). UVC absorption by RNA leads to the formation of pyrimidine dimers that block transcription while absorption by amino acids inhibits enzymatic activity and structural function of proteins. Both types of protein and RNA damage can cause viral inactivation independently or combined. Besides, UVC can cause skin erythema and cornea damage (e.g., photokeratitis and photokeratoconjuntivitis) [8] and, therefore, should not be directly exposed to humans nor other animals.
Fig. 1.
Normalized absorption spectra of major cellular UVC chromophores (thymine, uracil, histidine, and tryptophan) and UVC emission spectra of most used artificial UVC emitters (KrCl excimer lamps, low-pressure-Hg lamps, and LEDs).
Since the beginning of the COVID-19 pandemic, much has been discussed regarding the use of UVC for inactivating the SARS-CoV-2 virus. A wide range of articles has been published over the last year. The use of UVC-based technologies against the SARS-CoV-2 has been addressed mostly in perspectives and review articles, whereas some studies have demonstrated the inactivation of human coronaviruses and their viral surrogates. However, we noticed that there is limited scientific information about SARS-CoV-2 inactivation by UVC specifically, which motivated us to search for these pieces of evidence.
In this systematic scoping review, we focused our attention on the confirmation of UVC-based technologies used to strictly inactivate the SARS-CoV-2 virus. The results obtained here are discussed and future directions are addressed.
2. Methods
We followed the guidelines proposed by the PRISMA-ScR (Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews) to conduct this scoping review [9]. We searched in two databases (Pubmed and Web of Science) from Jan 2020 until April 2021. The search strategy combined the terms (UVC OR UV-C OR ultraviolet OR germicidal) and (SARS-CoV-2 OR COVID-19 OR coronavirus) to select original publications focused on UVC to fight SARS-CoV-2 and/or COVID-19. After article selection, duplicates were removed.
We screened the articles by reading titles and/or abstracts, which left out reviews, perspectives, and studies published in languages other than English. For eligibility, studies should present reliable methodology and information enough regarding the UVC system to allow calculation of light parameters by reviewers when they were not informed by authors. The articles also should contain quantitative results for SARS-CoV-2 inactivation. UV wavelengths other than UVC were excluded. SARS-CoV-2 surrogates and other coronaviruses were not included. Publications identified were read and independently evaluated by all reviewers considering the eligibility criteria.
Data were collected and inserted into an Excel spreadsheet. We extracted the authors’ names, year of publication, the purpose of the study, UVC characteristics, and outcome regarding SARS-CoV-2 inactivation. Divergences were solved after consensus by all reviewers.
3. Results and discussion
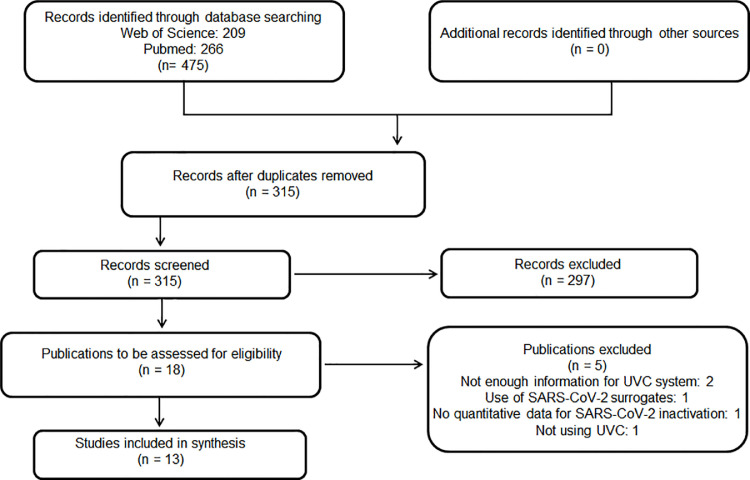
Our search retrieved 475 studies, of which 160 duplicates were removed. As a result, 315 publications were screened and 297 were excluded after reading the title and/or abstract. Eighteen records were assessed for eligibility and 13 were included in this review [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. Five studies were excluded because they did not meet our inclusion criteria, i.e., two studies did not provide enough information about the UVC system [23,24], one did not present quantitative data for SARS-CoV-2 inactivation [25], one reported data using SARS-CoV-2 surrogates (26) and one used UVA-based technology [27] (Fig. 2 ).
Fig. 2.
PRISMA flowchart for studies included in this review.
Table 1 compiles the included studies and exhibits their purpose and outcome for SARS-Cov-2 inactivation. Although the motivation has differed, all studies reported high levels of in vitro virus inactivation, ranging from 90% to 100% depending on UVC light parameters and/or material evaluated. However, remarkably, SARS-CoV-2 on contaminated wood was not efficiently inactivated.
Table 1.
Summary of the aims and results of the studies included in this review. AEC: airway epithelial cells.
| Reference | Purpose | UVC outcome for SARS-CoV-2 titer reduction |
|---|---|---|
| [10] | Identifying UVC lethal doses for SARS-CoV-2 | 90% (1-log) to 99.999% (5-log) |
| [11] | Identifying UVC lethal doses depending on SARS-CoV-2 concentration | > 99.99% (4-log) |
| [12] | Evaluating and comparing the sterilizing capability of UVC and ozone on SARS-CoV-2 adsorbed on different materials | UVC: 99.9% (3-log) on glass, plastic, and gauze; 90% (1-log) on fleece; 94.4% (>1-log) for wool; 0% for wood. UVC was better than ozone except for wood (O3: 93.3% inactivation) |
| [13] | Investigating the susceptibility of SARS-CoV-2 to combined or separated UVA and UVC | UVC was more effective: 99.999% (5-log) |
| [14] | Validating inactivation protocols from differentiated AECs cultures infected with live SARS-CoV-2 | > 99.99% (4-log) |
| [15] | Investigating UVC on SARS-CoV-2 inactivation | 99.7% (2.51-log) |
| [16] | Investigating continuous and intermittent UVC on SARS-CoV-2 inactivation | > 99.99% (> 4-log). No difference between continuous and intermittent light |
| [17] | Evaluating the antiviral efficacy of deep UV-LED on SARS-CoV-2 | 99.9% (3-log) |
| [18] | Quantifying the dose of deep UV-LED to inactivate SARS-CoV-2 | 99.9% (3-log) |
| [19] | Establishing the persistence of SARS-CoV-2 on inanimate surfaces following UVC |
99.99% (4-log) |
| [20] | Determining the effect of UVC on SARS-CoV-2 inoculated N95 respirators depending on material/model type | 99.999% (5-log) in facepieces and straps of all tested N95 respirators |
| [21] | Developing an ultra‐high power UVC irradiation source to sterilize SARS‐CoV‐2 | 100% |
| [22] | Investigating UVC on SARS-CoV-2 in wet and dried surfaces | > 99.9% (3-log) in 9 s and 4 s for wet and dried surfaces, respectively |
Regarding the UVC system, eight used wavelengths at 254 nm [[10], [11], [12], [13], [14],19,20,22], two at 222 nm [15,16], one at 280 nm [17], one at 265 nm, and 280 nm [18], and one at 275 nm [21]. We also noticed that different UVC-based technologies were used. Three studies were carried out with LED [17,18,21], while ten reported the use of lamps. From these, eight studies were performed with a mercury lamp [[10], [11], [12], [13], [14],19,20,22], and two used krypton-chloride excimer lamps [15,16] (Table 2 ). Yet, 12 studies used continuous-wave emission regimen (CW) whereas one compared CW with intermittent UV light [16]. The authors did not observe expressive differences for SARS-CoV-2 inactivation between those regimes (Table 2).
Table 2.
Summary of the study methodology included in this review. CCID50: 50% cell culture infectious dose; MOI: multiplicity of infection; PFU: plaque-forming unit; TCID50: 50% tissue culture infectious dose.
| Reference | Viral titer | Sample holder | UVC emitter | λ(nm) | Irradiance (mW/cm2) | Dose (mJ/cm2) | Exposure time(s) |
|---|---|---|---|---|---|---|---|
| [10] | 2.8 ⋅ 106 RNA copies/mla | 24-well plates | LP-Hg | 254 | 2.2 ± 0.2 | 0.016 to 108.714 | 0.01 to 50 |
| [11] | MOI (1000, 5, 0.5) | 24-well plates | LP-Hg | 254 | 1.082 | 16.9b | 23 |
| [12] | 8.2 . 105 PFU/ml | Glass, plastic, wood, gauze, wool, fleece | LP-Hg | 254 | 1.8 | 1620 | 900 |
| [13] | 5 ⋅ 106 TCID50/ml | 24-well plates | LP-Hg | 254 | 1.94 | 1047.6 | 540 |
| [14] | 3.5 ⋅ 106 PFU/ml (MOI 0.5) | 12-well plates | LP-Hg | 254 | 0.667 d | 200 | 300 |
| [15] | 5 ⋅ 106 TCID50/ml | 90 mm Petri dishes | Kr-Cl excimer lamp | 222 | 0.1 | 3.0 | 30 |
| [16] | 5 ⋅ 106 TCID50/ml | 90 mm Petri dishesa | Kr-Cl excimer lamp | 222 | 0.05 | 15 | 300 (cw) 10 s irradiation with 380 s interval (intermittent) |
| [17] | 2 . 104 PFU/ml | 60 mm Petri dishes | LED | 280 | 3.75 | 37.5 | 10 |
| [18] | 1.2 ⋅ 104 PFU/ml | 96-well plates | LED | 265/280 | 0.092 (265 nm) 0.083 (280 nm) |
1.8 3.0 |
≈ 20 (265 nm)c ≈ 36 (280 nm)c |
| [19] | 1 ⋅ 107.5 TCID50/ml | Plastic, glass and stainless steel | LP-Hg | 254 | 0.466 | 20.06 (plastic/stainless steel) 10.25 (glass) | 36 (plastic/stainless steel) 21 (glass) |
| [20] | 8 ⋅ 107 TCID50/ml | N95 respirators | LP-Hg | 254 | 16.5 | 1500 | 70 |
| [21] | 0.1, 1, 10 and 100 CCID50/0.05ml | Not informed | LED | 275 | 94 | 94d | 1.0 |
| [22] | 7.33 ⋅ 103 PFU/ml | 60 mm Petri dishes | LP-Hg | 254 | 0.849 | 3.39 (wet virus) and 7.64 (dry) | 4.0 (wet) and 9.0 (dry) |
a: Informed by authors; b: Effective dose calculated by authors reaching the virus; c: Calculated by reviewers; d: Reported in another study.
Low-pressure mercury vapor (LP-Hg) lamps are quasi-monochromatic light sources with nearly 90% of emission at 254 nm [28]. They have been widely used because of their long-established antimicrobial properties, electrical efficiency, and low cost. Despite that, their use has raised several concerns over undesirable hazards to eyes and skin (e.g., erythema, photokeratitis, and photokeratoconjuntivitis) and environmental pollution caused by Hg content (generally less than 5 mg/lamp) [28,29].
From this perspective, other light sources have emerged as a potential alternative to LP-Hg lamps. Although LEDs emitting UVC wavelengths are expensive and have a low output power (milliwatt range) and energy efficiency (less than 5%) levels, UVC-LEDs demonstrated to be also suitable for microbial inactivation. UVC-LEDs generate narrow emission spectra that can deliver light across the UVC germicidal spectral range (i.e., 250–280 nm) [28]. Yet, UVC-LEDs might be especially useful in compact applications such as disinfection cases used for smartphones and earbuds.
Excimer lamps appeared as another promising option as a source of UVC light [29]. These systems generate higher-energy photons (e.g., 207 and 222 nm, often termed as far-UVC) without the need for a hot cathode electron emission to ignite the plasma discharge as used in LP-Hg lamps [29]. This characteristic can extend the number of ignitions with reduced cathode damage, reach maximum emission within a shorter time and also be able to operate within a broader environmental temperature range. Even though, when compared to LP-Hg, excimer lamps provide lower energy efficiency (e.g., ∼1% instead of ∼30%), lower lifetime (e.g., ∼3000 h instead of 9000–15,000 h), and higher costs of acquisition and implementation (i.e., cost per Watt can be up to 100 times higher). On the other hand, since far-UVC wavelengths (e.g., 200–230 nm) are intensely absorbed by amino acids, they tend to be safer to human exposure due to reduced transmission into living tissues [30,31]. Even though, being safer does not mean to be safe and, so far, there are no International Standards that establish what are the permissible levels of daily exposure to far-UVC radiation. Therefore, eyes and skin should not be exposed to far-UVC radiation without caution and appropriate personal protective equipment (PPE).
Concerning the experimental design, the initial viral titer differed hugely among studies (Table 2). Besides, ten studies were performed in vitro using 24- [10,11,13], 12- [14], or 96- [18] well plates, 60 mm- [17,22] or 90 mm- [15,16] Petri dishes, and one study did not describe the sample holder [21]. All these protocols resulted in more than a 2.5-log of viral titer inactivation.
Two studies examined SARS-CoV-2 inactivation on different surfaces [12,19]. Gidari and coworkers compared virus inactivation on plastic, glass, and stainless steel due to the ability of SARS-CoV-2 to persist differently on these materials (half-life of 4.4, 5.3, and 4.2 h in stainless steel, plastic, and glass, respectively). As a result, the authors observed a 99.99% (4-log) viral reduction for all materials. Yet, the authors reported that 36 s were necessary for plastic and stainless-steel surfaces, while 21 s was enough for viral inactivation on glass [19]. Another study has reported that 15 min of UVC exposure led to 99.9% (3-log) inactivation of viral titer on glass, plastic, and gauze, whereas 94.4% and 90.0% of viruses were inactivated on contaminated wool and fleece, respectively. Interestingly, UVC was not able to reduce the viral titer of contaminated wood probably due to its porous nature that could protect viral particles under a shadow region [12]. Of notice, in that study UVC proved to be more effective than ozone except for wood.
It is important to highlight that all included studies were set under experimental and controlled conditions. However, the viability of SARS-CoV-2 outside the host might be influenced by the nature of materials and/or other relevant factors such as temperature, light, and humidity [32]. Indeed, SARS-CoV-2, MERS-CoV, and other coronaviruses can persist on metal (stainless steel), plastic, and glass for up to nine days [33]. Thus, it is still unclear for how long SARS-CoV-2 particles remain viable under other environmental circumstances such as low temperature, high humidity, or the presence of biomass.
Although air moisture is an important factor, little is known about the susceptibility of the SARS-CoV-2 under UVC exposure on wet and dried surfaces. We only found one study comparing both conditions. Nearly 99.9% (3-log) of virus titer were inactivated within 4 s on wet plastic surfaces while more than twice as much time was required (i.e., 9 s) to reach the same levels of viral inactivation on dried surfaces [22].
Besides disinfection of air and surfaces, the lack of sufficient hospital resources and the seasonal shortage of PPE has placed UVC light systems under the spotlight as a strategy to overcome current challenges towards decontamination and reuse of filtering facepiece respirators, such as N95 masks. Although several reports are showing the potential of UVC for mask disinfection, only one eligible study reported the effects of UVC on N95 respirators contaminated with SARS-CoV-2 [20]. Even though, regulatory agencies may yet question whether this physical disinfection method alone is sufficient to allow the reuse of the same mask by different people since UVC cannot remove biological residues such as proteins, carbohydrates, and lipids. Thus, this disinfection method should only be recommended for masks that will be used by the same person.
Ozog et al. evaluated the potential of UVC light to reduce the viral titer of facepieces and straps of 5 types of N95 respirators (3 M 1860, 3 M 8210, 3 M 8511, 3 M 9211, and Moldex 1511) [20]. Authors showed that after 70 s, viral titer recovered was below the limits of detection on facepieces of Moldex 1511, 3 M 1860, and straps of Moldex 1511 and 3 M 8210. Even though, out of the 5 respirators, only Moldex 1511 was fully decontaminated under the experimental conditions. Because facepieces of 3 M 1860 and Moldex 1511 respirators contain hydrophobic surfaces, they are more likely to be decontaminated compared to hygroscopic ones (that easily absorb the droplets, e.g., facepiece of 3 M 8210).
Despite the ability to rapidly disinfect N95 respirators, several repeated cycles and/or very high light doses (> 120,000 mJ/cm2) may degrade certain types of polymers, influencing the elasticity and structural integrity of such materials [34]. This type of material degradation may reduce the tensile strength of filters as well as it may increase the airflow resistance reducing the mask's reusability after several UVC cycles. However, literature data are conflicting concerning the number of cycles that affect the respirator integrity since it is influenced by the respirator model and the UVC dose per cycle of disinfection. Lindsley et al. reported that all N95 respirators tested required at least 120,000 mJ/cm2 of dose to induce any variation in filter material performance or resistance [34]. Such dosage is 120 times higher than the 1000 mJ/cm2 dose that is reported for mask disinfection [35].
Concerning the UVC equipment implementation for the particular application of N95 respirator decontamination, light must be uniformly projected over the entire mask surface to promote reliable results. This limitation can be avoided if several light sources are positioned around a stationary mask being treated or the mask/light is constantly moved to allow exposure by every possible angle. Otherwise, light may not be delivered uniformly over the surface area (i.e., the apex of the respirator is more likely to receive a higher dose compared to the edges). Additionally, due to the porous characteristic of filtering facepieces, light doses required to achieve a desirable disinfection rate should be higher than those traditionally used for flat surfaces.
Last but not least, the percentage of SARS-CoV-2 inactivation occurs in a UVC dose-dependent manner according to nine studies [10,11,13,[15], [16], [17], [18], [19],22], even though light doses varied widely among studies due to different irradiances and exposure times (Table 2). Interestingly, complete SARS-CoV-2 inactivation was accomplished in 5 min or less in 11 studies [10,11,[14], [15], [16], [17], [18], [19], [20], [21], [22]]. In the other two studies, effective inactivation was observed after 9 [13] and 15 min of UVC exposure [12].
Conclusions and future directions
Considering the global health problem and the current challenges over the COVID-19 pandemic, a direct approach to prevent airborne pathogens transmission is increasingly necessary. However, an appropriate technological implementation must be of pivotal role to produce effective and safe UVC devices following recommendations by regulatory agencies. In this regard, do-it-yourself (DIY) solutions must be regarded as potential risks and liabilities for any domestic or commercial use. Noteworthy, despite the benefits and antimicrobial activities, overexposure to UVC radiation can be harmful to the human body leading to damages to skin and eye tissues, causing symptoms such as erythema, photokeratitis, and photokeratoconjuntivitis. Therefore, the use of UVC systems is limited and should only be implemented with devices that present proven efficacy and safety.
Although UVC antimicrobial properties have long been known, few reports addressing the use of UVC against SARS-CoV-2 have been published so far. For that reason, we gathered efforts to find enough evidence of UVC-based technologies to fight the COVID-19 pandemic. UVC has proven to be an effective method to promote SARS-CoV-2 inactivation, reaching a complete viral titer reduction in a few minutes or even seconds depending on the viral titer, material, and light parameters. Indeed, regardless of the different methodologies used by the authors, all studies reported effective in vitro inactivation of SARS-CoV-2. However, we could not find any support to discuss the nature of the solutions (e.g., pH, salts, etc.) or the influence of virus manipulation in these studies.
Our findings demonstrate that UVC is an ally in our fight against COVID-19 pandemics and may bring some light in these times of darkness that all of us are living. Future studies should challenge the SARS-CoV-2 in aerosols and uninhabited environments. Technical analysis of the implementation site is always recommended to achieve desirable and safe results under realistic conditions.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Caetano P. Sabino is an associate at BioLambda Scientific and Commercial LTD but declares to only have a scientific interest in this study. There are no further conflicts of interest to be declared.
Acknowledgments
F. P. Sellera thanks CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for his fellowship. F. V. Cabral is grateful to CNPq (Conselho Nacional de Pesquisa e DesenvolvimentoTecnológico) for her scholarship. We thank Estevão Macedo for figure design.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China. N Engl. J. Med. 2019;382:727–733. doi: 10.1056/NEJMoa2001017. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohrabi C., Alsafi Z., O'Neill N., et al. World health organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velavan T.P., Meyer C.G. The COVID-19 epidemic. Trop. Med. Int. Health. 2020;25:278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S., Kumar V., Kapoor D., et al. Detection and disinfection of COVID-19 virus in wastewater. Environ. Chem. Lett. 2021:1–17. doi: 10.1007/s10311-021-01202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabino C.P., Ball A.R., Baptista M.S., et al. Light-based technologies for management of COVID-19 pandemic crisis. J. Photochem. Photobiol. B. 2020;212 doi: 10.1016/j.jphotobiol.2020.111999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyons A.B., Narla S., Torres A.E., et al. Skin and eye protection against ultraviolet C from ultraviolet germicidal irradiation devices during the COVID-19 pandemic. Int. J. Dermatol. 2021;60:391–393. doi: 10.1111/ijd.15255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tricco A.C., Lillie E., Zarin W., et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 10.Sabino C.P., Sellera F.P., Sales-Medina D.F., et al. UV-C (254 nm) lethal doses for SARS-CoV-2. Photodiagnosis Photodyn. Ther. 2020;32 doi: 10.1016/j.pdpdt.2020.101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biasin M., Bianco A., Pareschi G., et al. UV-C irradiation is highly effective in inactivating SARS-CoV-2 replication. Sci. Rep. 2021;11:6260. doi: 10.1038/s41598-021-85425-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Criscuolo E., Diotti R.A., Ferrarese R., et al. Fast inactivation of SARS-CoV-2 by UV-C and ozone exposure on different materials. Emerg. Microbes Infect. 2021;10:206–210. doi: 10.1080/22221751.2021.1872354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heilingloh C.S., Aufderhorst U.W., Schipper L., et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am. J. Infect. Control. 2020;48:1273–1275. doi: 10.1016/j.ajic.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrow K.A., Rich L.M., Vanderwall E.R., et al. Inactivation of material from SARS-CoV-2-infected primary airway epithelial cell cultures. Methods Protoc. 2021;4:7. doi: 10.3390/mps4010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitagawa H., Nomura T., Nazmul T., et al. Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination. Am. J. Infect. Control. 2021;49:299–301. doi: 10.1016/j.ajic.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitagawa H., Nomura T., Nazmul T., et al. Effect of intermittent irradiation and fluence-response of 222 nm ultraviolet light on SARS-CoV-2 contamination. Photodiagnosis Photodyn. Ther. 2021;33 doi: 10.1016/j.pdpdt.2021.102184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inagaki H., Saito A., Sugiyama H., Okabayashi T., Fujimoto S. Rapid inactivation of SARS-CoV-2 with deep-UV LED irradiation. Emerg. Microbes. Infect. 2020;9:1744–1747. doi: 10.1080/22221751.2020.1796529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minamikawa T., Koma T., Suzuki A., et al. Quantitative evaluation of SARS-CoV-2 inactivation using a deep ultraviolet light-emitting diode. Sci. Rep. 2021;11:5070. doi: 10.1038/s41598-021-84592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gidari A., Sabbatini S., Bastianelli S., et al. SARS-CoV-2 Survival on surfaces and the effect of UV-C light. Viruses. 2021;13:408. doi: 10.3390/v13030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozog D.M., Sexton J.Z., Narla S., et al. The effect of ultraviolet C radiation against different N95 respirators inoculated with SARS-CoV-2. Int. J. Infect. Dis. 2020;100:224–229. doi: 10.1016/j.ijid.2020.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S., Luo W., Li D., et al. Sec-eliminating the SARS-CoV-2 by AlGaN based high power deep ultraviolet light source. Adv. Funct. Mater. 2020 doi: 10.1002/adfm.202008452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Storm N., McKay L.G.A., Downs S.N., et al. Rapid and complete inactivation of SARS-CoV-2 by ultraviolet-C irradiation. Sci. Rep. 2020;10:22421. doi: 10.1038/s41598-020-79600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson E.I., Prince T., Anderson E.R., et al. Methods of inactivation of SARS-CoV-2 for downstream biological assays. J. Infect. Dis. 2020;222:1462–1467. doi: 10.1093/infdis/jiaa507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmons S.E., Carrion R., Alfson K.J., et al. Deactivation of SARS-CoV-2 with pulsed-xenon ultraviolet light: implications for environmental COVID-19 control. Infect. Control Hosp. Epidemiol. 2021;42:127–130. doi: 10.1017/ice.2020.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnewall R.E., Bischoff W.E. Removal of SARS-CoV-2 bioaerosols using ultraviolet air filtration. Infect. Control Hosp. Epidemiol. 2021:1–2. doi: 10.1017/ice.2021.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A., Kasloff S.B., Leung A., et al. Decontamination of N95 masks for re-use employing 7 widely available sterilization methods. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0243965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi H.K., Cui C., Seok H., et al. Feasibility of ultraviolet light-emitting diode irradiation robot for terminal decontamination of coronavirus disease 2019 (COVID-19) patient rooms. Infect. Control Hosp. Epidemiol. 2021:1–6. doi: 10.1017/ice.2021.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sholtes K.A., Lowe K., Walters G.W., Sobsey M.D., Linden K.G., Casanova L.M. Comparison of ultraviolet light-emitting diodes and low-pressure mercury-arc lamps for disinfection of water. Environ. Technol. 2016;37:2183–2188. doi: 10.1080/09593330.2016.1144798. [DOI] [PubMed] [Google Scholar]
- 29.Hadi J., Dunowska M., Wu S., Brightwell G. Control measures for SARS-CoV-2: a review on light-based inactivation of single-stranded RNA Viruses. Pathogens. 2020;9:737. doi: 10.3390/pathogens9090737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukui T., Niikura T., Oda T., et al. Exploratory clinical trial on the safety and bactericidal effect of 222-nm ultraviolet C irradiation in healthy humans. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0235948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamano N., Kunisada M., Kaidzu S., et al. Long-term effects of 222-nm ultraviolet radiation C sterilizing lamps on mice susceptible to ultraviolet radiation. Photochem. Photobiol. 2020;96:853–862. doi: 10.1111/php.13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue X., Ball J.K., Alexander C., Alexander M.R. All surfaces are not equal in contact transmission of sars-cov-2. Matter. 2020;3:1433–1441. doi: 10.1016/j.matt.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindsley W.G., Martin S.B., Jr, Thewlis R.E., et al. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J. Occup. Environ. Hyg. 2015;12:509–517. doi: 10.1080/15459624.2015.1018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.N95 Decon. Technical report for UV-C-based N95 reuse risk management. Available at: http://jrgoicp.umin.ac.jp/ppewg/n95decon/en/2020-04-23_N95DECON_UV-C_Technical_Report_v2.0_final.pdf2020. Accessed on June 2021.