Abstract
Background and objective
Bone fragility has been linked to COVID-19 severity. The objective of this study was to evaluate whether a diagnosis of vertebral fracture (VF) increased mortality risk in COVID-19 patients and whether this effect was greater than in those without COVID-19.
Methods
We assessed VFs by computed tomography (CT) in a cohort of 501 patients consecutively admitted to the emergency department (ED) for clinical suspicion of SARS-CoV-2 infection during the first wave of pandemic emergency. Of those, 239 had a confirmed diagnosis of COVID-19.
Results
VF prevalence was similar between COVID-19 and non-COVID-19 groups (22.2 vs. 19%; p = 0.458). Death rates were similar between COVID-19 and non-COVID-19 groups at both 30 (15.8 vs. 12.2%; p = 0.234) and 120 days (21.8 vs. 17.6%; p = 0.236). The mortality risk was higher in COVID-19 patients either with one or multiple fractures compared to those without VFs, at 30 and 120 days, but statistical significance was reached only in those with multiple VFs (30-day HR 3.03, 95% CI 1.36–6.75; 120-day HR 2.91, 95% CI 1.43–5.91). In the non-COVID-19 group, the 30-day mortality risk was significantly higher in patients either with one (HR 7.46, 95% CI 3.12–17.8) or multiple fractures (HR 6.2, 95% CI 2.75–13.98) compared to those without VFs. A similar effect was observed at 120 days. After adjustment for age, sex and bone density, mortality risk remained associated with VFs in the non-COVID-19 group only.
Conclusions
VFs were not independently associated with short-term mortality in patients with COVID-19, but they strongly increased mortality risk in those without COVID-19.
Keywords: Vertebral fractures, Osteoporosis, COVID-19, Viral pneumonia, Bone metabolism
Introduction
Coronavirus disease 2019 (COVID-19) is a global outbreak of a new respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). According to the last WHO report as of 18 August 2021, there have been over 207 million confirmed cases of COVID-19 worldwide, including over four million deaths [1].
The presence of comorbidities among hospitalised COVID-19 patients [2, 3], particularly in the elderly, are linked to acute respiratory syndrome, poor clinical outcomes and increased mortality rate [4, 5]. These patients often present factors that may increase the risk of bone fragility, such as systemic inflammation, older age, glucocorticoid treatment and immobilisation. Vertebral fractures (VFs) are the most frequent fragility fracture caused by osteoporosis and commonly occur without a recognisable trauma [6]. Among women over 50 years old, the incidence of VFs increases with age following the same trend as the osteoporosis prevalence, with 25% of women older than 80 having experienced at least one VF [7]. VFs are associated with reduced pulmonary function [8–10], disability [11] and an over 60% increased risk of mortality [12, 13]. Recently, Di Filippo et al. retrospectively assessed the VFs prevalence and clinical impact in COVID-19 patients in a tertiary health care hospital in Italy. In their study, including 114 patients with available lateral chest X-rays, the authors found that 36% of COVID-19 patients had a thoracic VF [14]. Fracture severity was associated with COVID-19 mortality, implying a potential role of bone fragility in disease severity. Moreover, Kottlors et al. found that low bone mineral density (BMD) measured by computer tomography (CT) scan was a risk factor for intensive care unit (ICU) admission in a cohort of 58 COVID-19 patients, although BMD analysis did not result in a prognostic advantage over simply considering age [15]. These data suggest that VF could be even more prevalent than expected based on large cohort studies, where VF prevalence is between 8 and 25% [16]. However, it is unknown whether VF prevalence is higher compared to a background population of non-COVID-19 subjects. It is also unknown whether the effect of VF on mortality is greater in COVID-19 compared to those without COVID-19. Therefore, the aim of this study was to evaluate VF and their effect on survival in COVID-19 and non-COVID-19 patients. To this aim, we analysed a large cohort of patients consecutively admitted to the emergency department (ED) for clinical suspicion of symptomatic SARS-CoV-2 infection. We hypothesised that COVID-19 patients with VFs have lower survival compared to those without COVID-19.
Methods
Study design and population
This was a single centre retrospective cohort study including patients admitted to the ED of Trauma Center Public Hospital Bufalini, Cesena, Italy, during the first pandemic wave, who were subjected to CT scan for detection of pulmonary COVID-19 when clinical suspicion was present based on symptoms and clinical findings. The first case of COVID-19 (patient 0) at this Hospital was registered on February 26, 2020. This report includes patients admitted to the ED until April 28, 2020 for clinical suspicion of SARS-CoV-2 infection. During this time frame, CT scans were performed on a total of 501 patients [age 64.36 (18.71) years, 277 males], together with clinical evaluation and real-time reverse-transcriptase polymerase chain reaction (RT-PCR) from a nasal and/or throat swab. A diagnosis of confirmed COVID-19 was based on a SARS-CoV-2 positive RT-PCR together with signs, symptoms and radiological findings suggestive of COVID-19 pneumonia. Patients evaluated in the ED for clinical suspicion of SARS-CoV-2 infection who tested negative by RT-PCR nasopharyngeal swab served as control group (non-COVID-19). The primary exposure and outcome measures were prevalent VFs at hospital admission and death at 30 and 120 days from hospital admission, respectively. For all patients, we obtained age, gender and admission to ICU during hospital stay. In addition, the following clinical and biochemical features were collected in COVID-19 patients: leucocyte count, C-reactive protein (CRP), serum creatinine and estimated glomerular filtration rate (using the CKD-EPI equation), plasma lactic dehydrogenase, hospital length of stay. Comorbidities were identified in medical history by patients or caregivers and included: history of cardiovascular disease (any disease of the cardiovascular system including hypertension), diabetes and chronic obstructive pulmonary disease. Visceral and subcutaneous adipose tissues were assessed as previously described [3].
Vertebral fractures and bone density assessment
VFs were assessed using sagittal image reconstruction from chest CT scanner (Philips Diamond Select Brillance CT 64-slice) standard acquired covering all thorax volume from D1 to a plane transverse to L2. To define osteoporotic VFs, we used the visual semiquantitative method proposed by Genant et al. [17] by two radiologists in consensus (S.B. and M.V.). Vertebral deformities were defined as follows: (1) wedge deformity: marked reduction of the vertebral anterior height without evidence of bone discontinuity; anterior height reduced by ≥4 mm compared with posterior height; (2) endplate deformity: central height of the body reduced by ≥4 mm with respect to the posterior and anterior walls; (3) compression deformity: all three heights reduced by ≥4 mm compared with adjacent vertebrae. Spinal trabecular bone density was assessed on CT scans using Hounsfield unit (HU) quantification [18]. HUs were measured at the sagittal cross sections of the trabecular regions of L1 vertebral body, avoiding cortex value. The vertebral body was divided into three axial segments and HUs were calculated by placing a circular region of interest over an area of trabecular bone on the vertebral body. D12 or any cranial vertebral body HU value were used when L1 was subjected to VF. CT-attenuation values ≤110 HU (high specificity cut-off) were used for definition of low bone density [19, 20].
Statistical analysis
Patients’ characteristics were described using means and standard deviations or medians and interquartile ranges, as appropriate, and percentages. Student’s t test or the Wilcoxon rank-sum test were used as appropriate to compare variables between groups. When variables were not normally distributed, we used a logarithmic transformation. Categorical variables between groups were compared by the chi-square test. The risk of mortality was estimated using the Kaplan–Meier method with log‐rank test for groups comparison. All statistical tests were two-tailed. Cox regression models were used to estimate the hazard ratios (HR) of death by VFs. Statistical analyses were performed in R Statistical Software 3.3 (R Foundation for Statistical Computing, Wien, Austria).
Results
Clinical features of COVID-19 and non-COVID-19 patients
Two hundred thirty-nine patients had a confirmed diagnosis of COVID-19. Of those, 188 patients (78.7%) had CT findings suggestive of COVID-19 pneumonia. Fifty-eight (24.2%) COVID-19 patients required ICU admission (ICU-COVID-19 group). The non-COVID-19 group consisted of 262 patients (Table 1). There were no differences among COVID-19 and controls in terms of age [mean (standard deviation): 63.7 (18.1) vs. 64.9 (19.3) years, respectively; p = 0.468], gender or body mass index, although a male preponderance was observed in COVID-19 patients (58.2% vs. 52.7%, respectively, p = 0.217) (Table 1). The 30-day death rate was 15.8% (38/239) and 12.2% (32/262) in the COVID-19 and non-COVID-19 groups, respectively (p = 0.234). At the longest follow-up (120 days), number of deaths increased slightly further up to 21.8% (52/239) and 17.6% (46/262) in the two groups, respectively (p = 0.236).
Table 1.
Clinical and biochemical features of the studied population
| COVID-19 (n = 239) | Non-COVID-19 (n = 262) | p value | |
|---|---|---|---|
| Age, years | 63.7 (18.1) | 64.9 (19.3) | 0.468 |
| Males, n (%) | 139 (58.2%) | 138 (52.7%) | 0.217 |
| BMI, Kg/m2 | 26.84 (5.3) | 26.33 (4.0) | 0.513 |
| CT pneumonia, n (%) | 188 (78.7%) | 83 (31.7%) | <0.0001 |
| 30-day deaths, n (%) | 38 (15.8%) | 32 (12.2%) | 0.234 |
| 120-day deaths, n (%) | 52 (21.8%) | 46 (17.6%) | 0.236 |
| Vertebral fractures, n (%) | 53 (22.2%) | 50 (19%) | 0.458 |
| Single fracture, n (%) | 30 (12.6%) | 20 (7.6%) | 0.066 |
| Multiple fractures, n (%) | 23 (9.6%) | 30 (11.5%) | 0.506 |
| Bone density, HU | 130.8 (58.6) | 119.3 (55.3) | 0.086 |
The table reports comparisons between the subjects with COVID-19 and subjects without COVID-19 (non-COVID-19 group)
Prevalence of vertebral fractures
Prevalence of VFs was similar between non-COVID-19 and COVID-19 groups [50/262 (19%) vs. 53/239 (22.2%); p = 0.458] (Table 1). A similar prevalence was found also in COVID-19 patients with CT findings of pneumonia [36/188 (19.1%)]. VFs were not associated with ICU admission (Table 2). Multiple VFs tended to be more frequent in ICU-COVID-19 than in nICU-COVID-19, but this difference was not significant (57% vs. 38% of total fracture number, respectively; p = 0.34). Consistently, spinal trabecular density was lower in fractured patients compared to those without fractures, in both COVID-19 [71.41(38.56) vs. 134.3(57.12) HU; p < 0.001; Table 2] and controls [77.03(36.99) vs. 131.4(53.64) HU; p = 0.008]. Using a high specificity cut-off (HU ≤ 110) osteoporosis was detected in 45% COVID-19 vs. 47% control subjects (p = ns). A more sensitive cut-off (HU ≤ 135) [19, 20] identified osteoporosis in 62 COVID-19 vs. 64% control subjects (p = ns). Table 2 shows differences in clinical and biochemical features of COVID-19 patients according to VFs. Patients with VFs were older than those without VFs [76.4(10.8) vs. 60.3(18); p < 0.0001] and more commonly diagnosed with cardiovascular comorbidities. Gender, BMI, TC-derived adiposity measures (visceral and subcutaneous adipose tissue), inflammatory markers (CRP, leucocyte counts) and length of in-hospital stay were similar between patients with and without VFs. Although diabetes tended to be more prevalent in the VFs group, this difference was not significant (30.2 vs. 17.3%, p = 0.065).
Table 2.
Clinical and biochemical features of COVID-19 subjects with or without vertebral fractures (VFs)
| With VF | Without VF | p value | |
|---|---|---|---|
| Age, years | 76.4 (10.8) | 60.3 (18) | <0.0001 |
| Males, n (%) | 32/53 (60.3%) | 104/188 (55.3%) | 0.702 |
| BMI, Kg/m2 | 26.9 (4.2) | 28.4 (19.2) | 0.722 |
| Diabetes, n (%) | 13/43 (30.2%) | 25/144 (17.3%) | 0.065 |
| Cardiovascular disease, n (%) | 34/41 (82.9%) | 68/133 (51.1%) | 0.0003 |
| COPD, n (%) | 7/39 (17.9%) | 15/133 (11.2%) | 0.272 |
| Hospitalisation length, daysa | 12.5 (6.6) | 10.7 (6.5) | 0.136 |
| ICU admission, n (%) | 14/53 (26.41%) | 44/188 (23.40%) | 0.650 |
| CRP, mg/lb | 77.8 (85.0) | 64.0 (176.0) | 0.641 |
| LDH, U/la | 296.5 (140.1) | 284.7 (125.9) | 0.636 |
| Creatinine, mg/dlb | 1.10 (0.50) | 1.03 (0.51) | 0.497 |
| eGFR, ml/min/1.73 m2b | 66.03 (25.9) | 77.8 (26.8) | 0.017 |
| Leucocytes, x103/mclb | 7.56 (3.53) | 7.19 (4.61) | 0.650 |
| Lymphocytes, x103/mclb | 1.155 (0.757) | 1.638 (3.181) | 0.348 |
| Spinal trabecular bone density, HU | 71.41 (38.56) | 134.3 (57.12) | <0.001 |
| VAT, mm | 13.76 (6.09) | 15.02 (7.37) | 0.358 |
| SAT, mm | 18.85 (8.60) | 17.07 (8.72) | 0.285 |
| VAT/SAT | 0.906 (0.680) | 1.150 (0.928) | 0.152 |
VF were associated with older age and cardiovascular disease. Cardiovascular disease refers to any disease of the cardiovascular system, including hypertension. Data are reported for 53 subjects with VF and 188 subjects without VF, unless otherwise stated in the table
BMI body mass index, COPD chronic obstructive pulmonary disease, ICU intensive care unit, eGFR estimated glomerular filtration rate, CRP C-reactive protein, LDH lactic dehydrogenase, HU Hounsfield units, VAT visceral adipose tissue, SAT subcutaneous adipose tissue
aData available in 39 subjects with VF and 128 subjects without VF
bData available in at least 38 subjects with VF and 132 subjects without VF
Mortality risk in COVID-19 and non-COVID-19 patients according to VFs
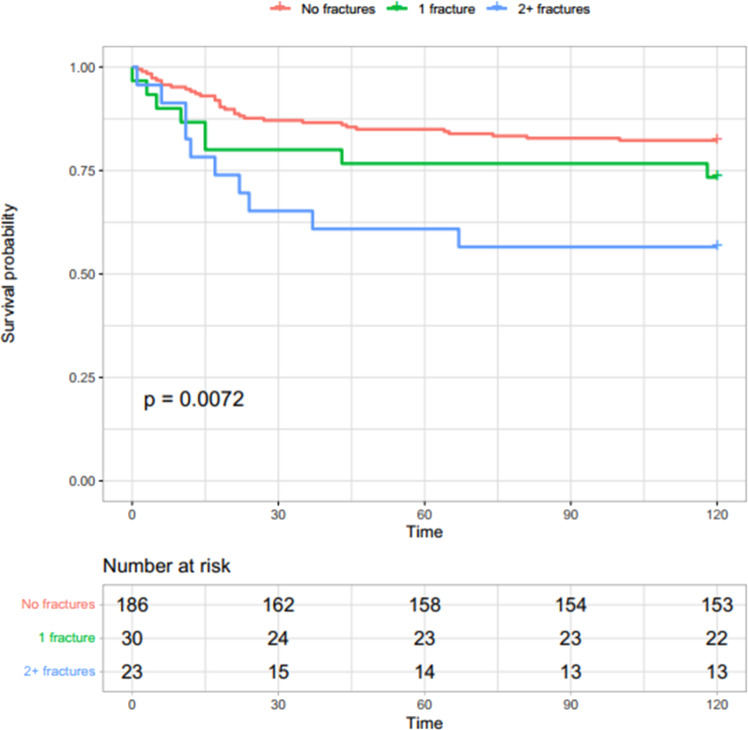
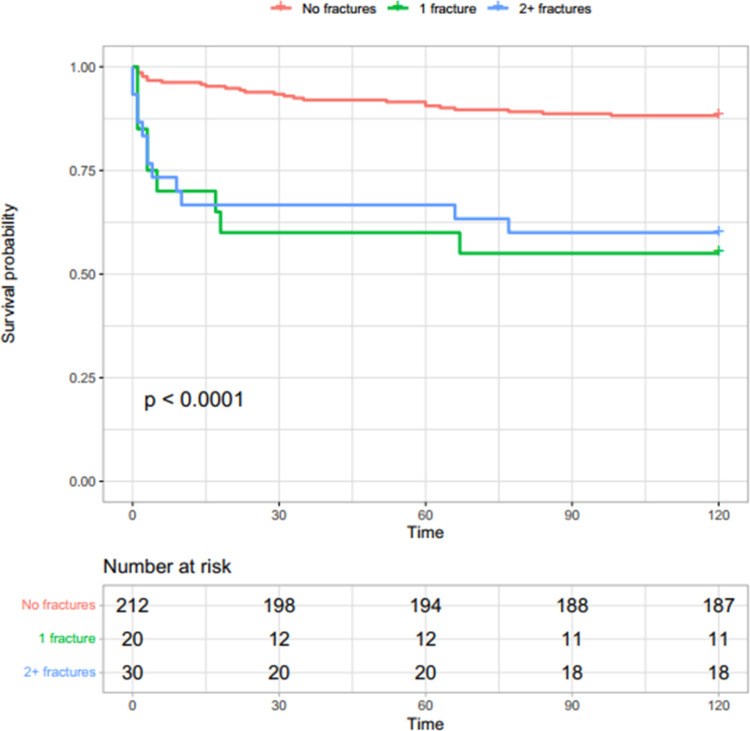
Mortality was associated with VFs in both COVID-19 and non-COVID-19 control groups. COVID-19 patients without VF had a 12.8% (95% CI: 7.9–17.5%) death probability within 30 days, which increased progressively with the number of VFs (20.7%, 95% CI 4.5–34.1%, in those with a single VF, and 34.8%, 95% CI: 12.1–51.6%, in those with multiple VFs) (Fig. 1). Non-COVID-19 patients without VF had a 6.6% (95% CI: 3.2–9.9%) death probability within 30 days from ED evaluation, which increased over five fold in fractured patients regardless of fracture number (40%, 95% CI: 14.2–58.0% in patients with a single VF; 33.3%, 95% CI: 14.1–48.2% in those with multiple VFs) (Fig. 2). Table 3 shows mortality HR in both COVID-19 and non-COVID-19 groups according to VFs. In the unadjusted models, the risk for 30 days mortality was higher in COVID-19 patients, at 30 and 120 days, but statistical significance was reached only in those with multiple VFs (30-day HR 3.03, 95% CI 1.36–6.75; and 120-day HR 2.91, 95% CI 1.43–5.91, respectively, Table 3). Risk of mortality was significantly higher in non-COVID-19 patients either with one (HR 7.46, 95% CI 3.12–17.8, p < 0.001) or multiple VFs (HR 6.2, 95% CI 2.75–13.98) compared to patients without VFs at 30 and 120 days (Table 3). After adjustment for age, sex and spinal trabecular bone density, risk of death remained associated with VFs in non-COVID-19 (30-day HR for a single VF: 3.94, 95% CI 1.57–9.87; 30-day HR for multiple VFs 3.17, 95% CI 1.30–7.74), but not in the COVID-19 group (30-day HR in patients with a single VF: 0.82, 95% CI 0.31–2.17; 30-day HR in patients with multiple VFs 1.58, 95% CI 0.45–5.57). A similar trend was shown in both groups at day 120 (Table 3). When restricting analysis to patients with COVID-19 pneumonia detected by CT, fractures were not significantly associated with mortality at either day 30 or day 120 (Table 4).
Fig. 1.
Survival probability in subjects with COVID-19 according to vertebral fractures. Survival probability was lower in subjects with fractures and decreased faster in subjects with multiple vertebral fractures compared to those without fractures
Fig. 2.
Survival probability in subjects without COVID-19 according to vertebral fractures. Survival probability decreased faster in subjects with vertebral fractures compared with those without fractures, regardless the number of fractures
Table 3.
Risk of death at days 30 and 120 in both COVID-19 and non-COVID-19 groups according to vertebral fractures
| Unadjusted hazard ratio | Adjusted hazard ratioa | |||
|---|---|---|---|---|
| 30-day | 120-day | 30-day | 120-day | |
| COVID-19 | ||||
| No fractures | Reference | Reference | Reference | Reference |
| Single fracture | 1.68 (0.69–4.12) | 1.64 (0.76–3.54) | 0.82 (0.31–2.17) | 0.70 (0.29–1.71) |
| Multiple fractures | 3.03 (1.36–6.75) | 2.91 (1.43–5.91) | 1.58 (0.45–5.57) | 1.13 (0.33–3.85) |
| Age | 1.10 (1.06–1.15) | 1.09 (1.06–1.12) | ||
| Sex (male) | 2.44 (1.07–5.58) | 1.48 (0.75–2.94) | ||
| Bone density (HU) | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | ||
| Non-COVID-19 | ||||
| No fractures | Reference | Reference | Reference | Reference |
| Single fracture | 7.46 (3.12–17.8) | 5.02 (2.34–10.78) | 3.94 (1.57–9.87) | 2.65 (1.19–5.94) |
| Multiple fractures | 6.2 (2.75–13.98) | 4.34 (2.18–8.64) | 3.17 (1.30–7.74) | 2.15 (1.02–4.52) |
| Age | 1.05 (1.01–1.08) | 1.06 (1.03–1.09) | ||
| Sex (male) | 0.79 (0.38–1.64) | 1.05 (0.58–1.90) | ||
| Bone density (HU) | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | ||
Data are reported as hazard ratio (95% confidence interval)
aData adjusted for age, sex and spinal trabecular bone density
Table 4.
Risk of death in subjects with COVID-19 pneumonia according to vertebral fractures
| Unadjusted hazard ratio | Adjusted hazard ratioa | |||
|---|---|---|---|---|
| 30-day | 120-day | 30-day | 120-day | |
| No fractures | Reference | Reference | Reference | Reference |
| Single fracture | 1.95 (0.66–5.73) | 1.88 (0.72–4.92) | 1.00 (0.31–3.19) | 0.89 (0.29–2.74) |
| Multiple fractures | 2.18 (0.74–6.40) | 2.57 (1.05–6.26) | 1.43 (0.31–6.61) | 1.14 (0.26–5.11) |
| Age | 1.11 (1.06–1.16) | 1.10 (1.06–1.14) | ||
| Sex (male) | 1.56 (0.63–3.91) | 1.14 (0.51–2.55) | ||
| Bone density (HU) | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | ||
Data are reported as hazard ratio (95% confidence interval)
aData are adjusted for age, sex and spinal trabecular bone density
Discussion
In this study, we found that 22% of COVID-19 patients have at least one CT-detectable VF. VFs were associated with mortality although this effect was not significant in COVID-19 patients after adjusting for age and sex. By contrast, VFs strongly increased mortality risk in non-COVID-19 patients in the short term after hospitalisation.
In agreement with our data, Di Filippo et al. did not find a significant association between VFs prevalence and mortality, although survival was lower in those with severe VFs in comparison with moderate or mild VFs [14]. Compared to Di Filippo et al., we observed a lower prevalence of fractures in COVID-19 patients (22.2%), which was similar to the non-COVID-19 control group (19%). Our finding is in line with data from the European population [16] where 18–26% of women older than 50 years, and 8–23% of men aged 50–75 years had a morphometric fracture. Differences between our data and those by De Filippo may be explained by study design, patient’s selection and different clinical conditions.
Our findings are also consistent with previous literature that identified an association between VFs and increased mortality [13, 21]. In a prospective Australian cohort study mortality rates were higher for men than women during the 18-year follow-up period and with greater risk soon after the fracture event [21]. Another prospective cohort study has shown a 1.6 relative risk for mortality in post-menopausal women followed for an average of 2.9 years [12]. Besides, a retrospective study of Swedish elderly women followed for an average of 5 years following a VF found that risk of mortality was increased immediately after the fracture event and decreased by 16% per annum [13]. However, such studies focused mainly on long-term mortality risk after a VF. To the best of our knowledge, our study is the first to assess the association between prevalent VFs and short-term mortality. In our non-COVID-19 cohort, VF determined a spike in mortality risk (up to seven times within 30 days from admission), which tended to plateau over the total observation timeframe of 6 months. We may speculate that VF may exacerbate mortality risk in presence of weakened clinical condition. Therefore, our data expand previous findings highlighting the importance of VFs as strong predictors of short-term mortality in hospitalised patients.
The biological explanation on the difference in risk of mortality related to VFs among COVID-19 and non-COVID-19 patients still needs to be clarified. We may speculate that COVID-19 patients carry a background mortality risk higher than that simply conferred by VFs. In this context, age and gender have been consistently associated with COVID-19 severity and fatality [22–24] as they are among the main factors shaping immune response and susceptibility to infection [25, 26]. Thus, while gender and age are main drivers of COVID-19 fatality, our data do not support the role of VFs as independent cause of death in this population. In contrast, given the potential heterogeneity of the non-COVID-19 group, we were unable to account for potential confounding factors other than age and gender. Lack of clinical information on the non-COVID-19 patients does not allow us to determine a causal relationship between VFs and mortality. However, soon after the spread of the COVID-19 pandemic and social lockdown restrictions, health care systems have been re-organised with detrimental effects on the care of non-COVID-19 patients [27]. Accordingly, a 30% reduction in admissions for acute cardiovascular events [28–37] and an increased rate of deaths not related to COVID-19 during pandemic have been described [36–42]. We may speculate that by refraining from seeking medical attention, some patients reached the hospital later and in worse clinical condition during the pandemic. VFs, despite the barriers imposed by the pandemic [43], should not be neglected from healthcare providers even during hospitalisation. Increasing the risk of future fragility fractures and mortality, VFs should be carefully evaluated and treated, as indicated by recent ASBMR guidelines [44].
Our study has limitations and strengths. In the non-COVID-19 patients we were not able to obtain clinical information related to admission in ED, cause of death or possible clinical complications. Despite these limitations, both controls and COVID-19 patients were selected based on the same criterion (clinical suspicion for COVID-19), from the same geographic area and timeframe of the first COVID-19 pandemic wave. The total lumbar spine scan was not available and therefore VFs prevalence might be underestimated. We did not have information on a previous diagnosis of osteoporosis, recency of VF or anti-osteoporotic treatments, but differences between COVID-19 and the non-COVID-19 patients were unlikely. Indeed, main factors associated with osteoporosis such as age and CT-derived bone density were similar between the two groups. Furthermore, our data may not be generalised to other COVID-19 cohorts as the clinical algorithm implemented for ICU admission and hospital/ICU capacity may vary according to different clinical settings. Strengths of our approach include: the use of CT scans which have higher accuracy over plain X-rays; the systematic assessment of VFs in a large cohort of unselected COVID-19 patients consecutively admitted to the ED, which minimised selection biases; the inclusion of a large control group of non-COVID-19 patients from same geographic area and timeframe as COVID-19 patients.
In conclusion, VF did not have an independent effect on mortality in COVID-19 patients. They strongly increased mortality risk in non-COVID-19 patients. However, VF remain often neglected and associated with worse outcome. We advocate prompt recognition and treatment with vitamin D and anti-osteoporosis drugs as soon as fragility fractures are identified in both COVID-19 and non-COVID-19 patients [45].
Acknowledgements
We thank Marica Capacci, Matteo Succi and Sonia Santucci for their help in providing some of the clinical features of the patients and for the assistance in imaging acquisition. R.S. is supported by the European Foundation for the Study of Diabetes (EFSD) Mentorship programme 2018, by the Italian Ministry of Health (Grant number #GR-2018-12365982), and by the Young Investigator fellowship by the Salerno Academy of Physician (OMCeO Salerno).
Author contributions
S.B. conceived the study, collected the data, contributed to the interpretation of the data and the writing of the manuscript. N.N. conceived the study, contributed to interpretation of the data and the writing of the manuscript. M.L., V.A., M.V., L.V., E.G. contributed to data collection and interpretation of the data. C.P. analysed the data and contributed to the interpretation of the data. F.T. contributed to the writing the manuscript, data analysis and interpretation of the data. G.L. contributed to write the first draft of the manuscript and to data interpretation. M.F. contributed to manuscript writing and interpretation. R.S. wrote the first draft, contributed to data analysis and data interpretation. S.B. and R.S. are the guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data availability
The full datasets or part of it will be made available upon reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Ethical approval
All clinical investigations have been conducted according to the principles expressed in the Declaration of Helsinki. This study was approved by the CEROM (Comitato Etico della Romagna) Ethical Committee with number Prot. 8654/2020.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Coronavirus (COVID-19) Dashboard. (WHO, 2020). https://www.covid19.who.int
- 2.Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch. Acad. Emerg. Med. 2020;8(1):e35. [PMC free article] [PubMed] [Google Scholar]
- 3.Battisti S, Pedone C, Napoli N, Russo E, Agnoletti V, Nigra SG, et al. Computed tomography highlights increased visceral adiposity associated with critical illness in COVID-19. Diabetes Care. 2020;43(10):e129–e130. doi: 10.2337/dc20-1333. [DOI] [PubMed] [Google Scholar]
- 4.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 6.Aoyagi K, Ross PD, Davis JW, Wasnich RD, Hayashi T, Takemoto T. Falls among community-dwelling elderly in Japan. J. Bone Miner. Res. 1998;13(9):1468–1474. doi: 10.1359/jbmr.1998.13.9.1468. [DOI] [PubMed] [Google Scholar]
- 7.Riggs BL, Melton LJ., 3rd Involutional osteoporosis. N. Engl J. Med. 1986;314(26):1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- 8.Schlaich C, Minne HW, Bruckner T, Wagner G, Gebest HJ, Grunze M, et al. Reduced pulmonary function in patients with spinal osteoporotic fractures. Osteoporos. Int. 1998;8(3):261–267. doi: 10.1007/s001980050063. [DOI] [PubMed] [Google Scholar]
- 9.Lombardi I, Jr., Oliveira LM, Mayer AF, Jardim JR, Natour J. Evaluation of pulmonary function and quality of life in women with osteoporosis. Osteoporos. Int. 2005;16(10):1247–1253. doi: 10.1007/s00198-005-1834-3. [DOI] [PubMed] [Google Scholar]
- 10.Munhoz da Rocha Lemos Costa T, Costa FM, Hoffman Jonasson T, Aguiar Moreira C, Boguszewski CL, Cunha Borges JL, et al. Bone mineral density and vertebral fractures and their relationship with pulmonary dysfunction in patients with chronic obstructive pulmonary disease. Osteoporos. Int. 2018;29(11):2537–2543. doi: 10.1007/s00198-018-4643-1. [DOI] [PubMed] [Google Scholar]
- 11.Francis RM, Aspray TJ, Hide G, Sutcliffe AM, Wilkinson P. Back pain in osteoporotic vertebral fractures. Osteoporos. Int. 2008;19(7):895–903. doi: 10.1007/s00198-007-0530-x. [DOI] [PubMed] [Google Scholar]
- 12.Ensrud KE, Thompson DE, Cauley JA, Nevitt MC, Kado DM, Hochberg MC, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J. Am. Geriatr. Soc. 2000;48(3):241–249. doi: 10.1111/j.1532-5415.2000.tb02641.x. [DOI] [PubMed] [Google Scholar]
- 13.Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, et al. Mortality after osteoporotic fractures. Osteoporos. Int. 2004;15(1):38–42. doi: 10.1007/s00198-003-1490-4. [DOI] [PubMed] [Google Scholar]
- 14.di Filippo L, Formenti AM, Doga M, Pedone E, Rovere-Querini P, Giustina A. Radiological thoracic vertebral fractures are highly prevalent in COVID-19 and predict disease outcomes. J. Clin. Endocrinol. Metab. 2021;106(2):e602–e614. doi: 10.1210/clinem/dgaa738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kottlors J, Große Hokamp N, Fervers P, Bremm J, Fichter F, Persigehl T, et al. Early extrapulmonary prognostic features in chest computed tomography in COVID-19 pneumonia: Bone mineral density is a relevant predictor for the clinical outcome—a multicenter feasibility study. Bone. 2021;144:115790. doi: 10.1016/j.bone.2020.115790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Prospective Osteoporosis Study Group, D. Felsenberg, A.J. Silman, M. Lunt, G. Armbrecht, A.A. Ismail et al. Incidence of vertebral fracture in Europe: results from the European Prospective Osteoporosis Study (EPOS). J. Bone Miner. Res. 17(4), 716–724 (2002). 10.1359/jbmr.2002.17.4.716 [DOI] [PubMed]
- 17.Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis The Study of Osteoporotic Fractures Research Group. J. Bone Miner. Res. 1996;11(7):984–996. doi: 10.1002/jbmr.5650110716. [DOI] [PubMed] [Google Scholar]
- 18.Buckens CF, Dijkhuis G, de Keizer B, Verhaar HJ, de Jong PA. Opportunistic screening for osteoporosis on routine computed tomography? An external validation study. Eur. Radiol. 2015;25(7):2074–2079. doi: 10.1007/s00330-014-3584-0. [DOI] [PubMed] [Google Scholar]
- 19.Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann. Intern. Med. 2013;158(April):588–595. doi: 10.7326/0003-4819-158-8-201304160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahern DP, McDonnell JM, Riffault M, Evans S, Wagner SC, Vaccaro AR, Hoey DA, Butler JS. A meta-analysis of the diagnostic accuracy of hounsfield units on computed topography relative to dual-energy X-ray absorptiometry for the diagnosis of osteoporosis in the spine surgery population. Spine J. 2021;S1529-9430(March):00119–4. doi: 10.1016/j.spinee.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301(5):513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 22.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R.H. Du, L.R. Liang, C.Q. Yang, W. Wang, T.Z. Cao, M. Li, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur. Respir. J. 55(5), (2020). 10.1183/13993003.00524-2020 [DOI] [PMC free article] [PubMed]
- 24.Qian J, Zhao L, Ye RZ, Li XJ, Liu YL. Age-dependent gender differences in COVID-19 in Mainland China: comparative study. Clin. Infect. Dis. 2020;71(9):2488–2494. doi: 10.1093/cid/ciaa683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bajaj V, Gadi N, Spihlman AP, Wu SC, Choi CH, Moulton VR. Aging, immunity, and COVID-19: how age influences the host immune response to Coronavirus infections? Front. Physiol. 2020;11:571416. doi: 10.3389/fphys.2020.571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gadi N, Wu SC, Spihlman AP, Moulton VR. What’s sex got to do with COVID-19? Gender-based differences in the host immune response to Coronaviruses. Front. Immunol. 2020;11:2147. doi: 10.3389/fimmu.2020.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Center for Disease Control and Prevention. Framework for Healthcare Systems Providing Non-COVID-19 Clinical Care During the COVID-19 Pandemic. (2020). https://www.cdc.gov/coronavirus/2019-ncov/hcp/framework-non-COVID-care.html
- 28.Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur. Heart J. 2020;41(19):1852–1853. doi: 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Filippo O, D’Ascenzo F, Angelini F, Bocchino PP, Conrotto F, Saglietto A, et al. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in Northern Italy. N. Engl J. Med. 2020;383(1):88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holt A, Gislason GH, Schou M, Zareini B, Biering-Sorensen T, Phelps M, et al. New-onset atrial fibrillation: incidence, characteristics, and related events following a national COVID-19 lockdown of 5.6 million people. Eur. Heart J. 2020;41(32):3072–3079. doi: 10.1093/eurheartj/ehaa494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mesnier J, Cottin Y, Coste P, Ferrari E, Schiele F, Lemesle G, et al. Hospital admissions for acute myocardial infarction before and after lockdown according to regional prevalence of COVID-19 and patient profile in France: a registry study. Lancet Public Health. 2020;5(10):e536–e542. doi: 10.1016/S2468-2667(20)30188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Rosa S, Spaccarotella C, Basso C, Calabro MP, Curcio A, Filardi PP, et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur. Heart J. 2020;41(22):2083–2088. doi: 10.1093/eurheartj/ehaa409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer FA, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;75(22):2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solomon MD, McNulty EJ, Rana JS, Leong TK, Lee C, Sung SH, et al. The Covid-19 pandemic and the incidence of acute myocardial infarction. N. Engl J. Med. 2020;383(7):691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 35.Pandey AS, Daou BJ, Tsai JP, Zaidi SF, Salahuddin H, Gemmete JJ, et al. Letter: COVID-19 pandemic—the Bystander effect on stroke care in Michigan. Neurosurgery. 2020;87(3):E397–E399. doi: 10.1093/neuros/nyaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhatt AS, Moscone A, McElrath EE, Varshney AS, Claggett BL, Bhatt DL, et al. Fewer Hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;76(3):280–288. doi: 10.1016/j.jacc.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bromage DI, Cannata A, Rind IA, Gregorio C, Piper S, Shah AM, et al. The impact of COVID-19 on heart failure hospitalization and management: report from a Heart Failure Unit in London during the peak of the pandemic. Eur. J. Heart Fail. 2020;22(6):978–984. doi: 10.1002/ejhf.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.J.D. Figueroa, P.M. Brennan, et al. Distinguishing between direct and indirect consequences of covid-19. BMJ. 369, m2377 (2020). 10.1136/bmj.m2377 [DOI] [PubMed]
- 39.Office for National Statistics. Deaths involving COVID-19, England and Wales: deaths occurring in April 2020. (2020) https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsinvolvingcovid19englandandwales/deathsoccurringinapril2020
- 40.European Stroke Organisation. Likely increase in the risk of death or disability from stroke during the COVID-19 pandemic. (2020). https://eso-stroke.org/eso/likely-increase-in-the-risk-of-death-or-disability-from-stroke-during-the-covid-19-pandemic/
- 41.Rossen LM, Branum AM, Ahmad FB, Sutton P, Anderson RN. Excess deaths associated with COVID-19, by age and race and ethnicity—United States, January 26-October 3, 2020. Morb. Mortal. Wkly Rep. 2020;69(42):1522–1527. doi: 10.15585/mmwr.mm6942e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bilinski A, Emanuel EJ. COVID-19 and excess all-cause mortality in the US and 18 comparison countries. JAMA. 2020;324(20):2100–2102. doi: 10.1001/jama.2020.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Napoli N, Elderkin AL, Kiel DP, Khosla S. Managing fragility fractures during the COVID-19 pandemic. Nat. Rev. Endocrinol. 2020;16(9):467–468. doi: 10.1038/s41574-020-0379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu EW, Tsourdi E, Clarke BL, Bauer DC, Drake MT. Osteoporosis management in the era of COVID-19. J. Bone Miner. Res. 2020;35(6):1009–1013. doi: 10.1002/jbmr.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tramontana F, Napoli N, El-Hajj Fuleihan G, Strollo R. The D-side of COVID-19: musculoskeletal benefits of vitamin D and beyond. Endocrine. 2020;69(2):237–240. doi: 10.1007/s12020-020-02407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The full datasets or part of it will be made available upon reasonable request.