Dear Editor,
Obesity is associated with adipose tissue (AT) fibrosis with aggregation or crosslinking of collagen fibers.1, 2 Lysyl oxidase (LOX) gives rise to collagen crosslinking, while upregulated LOX is reported in AT of obese humans and mice.3, 4 Our data showed higher second harmonic generation signals as well as collagen crosslinks in ob/ob AT than wild‐type (WT) AT (Figure 1A and B). In ob/ob AT, expression of LOX was highly upregulated among the LOX family, while a similar trend of LOX increment was also found in high‐fat diet‐induced obese mice (Figure 1C and S1). The ob/ob AT also exhibited higher LOX protein levels and enzymatic activity (Figure 1D and E). Increased AT stiffness was previously found in obese subjects non‐invasively.5 Our direct measurements by atomic force microscopy (AFM) showed a similar trend by yielding higher effective Young's modulus (Eeff) in ob/ob mice and obese human subcutaneous AT (Figure 1F and G). With adipose tissue derived from obese subjects before and after weight loss surgery, we observed significantly attenuated LOX expression but not that of other LOX family members (Figure 1H), accompanied by reduced stiffness (Figure 1I). While obesity‐induced physical property changes in AT may confine adipocyte functions,6 the origin and consequences of structural changes in AT as well as the augmented LOX during obesity await investigation.
FIGURE 1.

Obesity is associated with increased collagen crosslinking, LOX, and stiffening in the AT. (A) Overlaid images of second harmonic generation (SHG) and two‐photon‐excited fluorescence (TPEF) and extracted SHG signals of gonadal AT from 10‐week‐old WT and ob/ob male mice. Magnification of the blue square in the left panels is shown in the middle panels, which is then transformed into extracted SHG signals in the right panels. (B) Quantification of SHG percentage and number of crosslinks (intersections) accessed from signals in (A). (C) LOX family gene expression in gonadal AT of 10∼12 week‐old male WT and ob/ob mice. mRNA levels (n = 4∼5) are expressed relative to average expression of Lox in WT mice. (D) Immunoblot analysis of pro‐LOX and LOX and (E) LOX enzymatic activity in the gonadal AT of 10∼12 week‐old male WT and ob/ob mice. (F) Effective Young's modulus (Eeff) of 10‐week‐old male WT and ob/ob gonadal AT by AFM. (G) Eeff of the subcutaneous AT from 3 lean and obese subjects. (H) LOX family gene expression in subcutaneous AT of eight obese subjects before (Pre) and 6‐months after (Post) weight loss surgery. (I) Eeff of human subcutaneous AT obtained from three subjects before and 6‐month after weight loss surgery. *P < 0.05, **P < 0.01, and ***P < 0.001 by Student's t‐test in (B, C, and E); by paired Student's t‐test in (H); and by Wilcoxon signed‐rank test in (F, G and I). Scale bars in the left panels of (A) are 200 μm and in the middle and right panels of (A) are 50 μm. Sample numbers and measurements taken in (F, G and I) are shown in Table S1. Blue color in (G, H, and I) indicates the results from humans
Immunofluorescence staining revealed increased LOX signals in ob/ob AT, predominantly expressed in crown‐like structures and was highly associated with F4/80 signals (Figure 2A and B). To test whether obesity upregulated LOX in macrophages, we isolated peritoneal macrophages from WT and ob/ob mice and found that mRNA and protein levels and enzymatic activity of LOX were significantly augmented in ob/ob peritoneal macrophages (Figure 2C–E). Moreover, ob/ob peritoneal macrophages showed the ability to stiffen type I collagen gel, a mimicry of AT environment with its predominant extracellular matrix protein (Figure 2F). Conversely, our data demonstrated a reduction in LOX transcript levels in peripheral blood mononuclear cells (PBMCs) from human subjects 6 months after weight loss surgery, which was further reduced in PBMCs from subjects 12 months after surgery (Figure 2G). These results implied obesity was associated with increased macrophage LOX.
FIGURE 2.
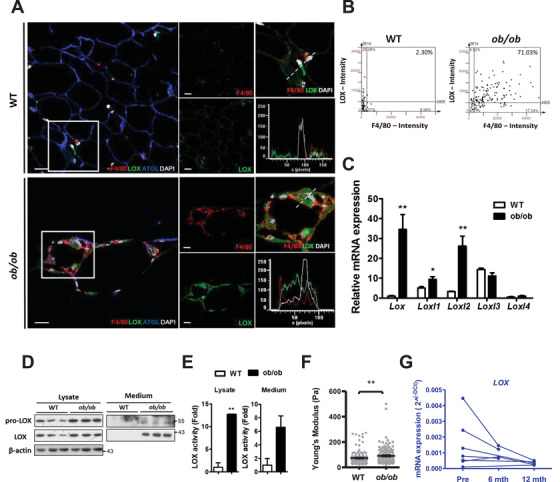
Obesity up‐regulated the crosslinking mediator, LOX, in macrophages. (A) Confocal image for co‐staining of LOX (green), F4/80 (red), ATGL (blue), and DAPI (white). The enlarged images of the squared area in the left panel are presented in the right panels, where the individual channels are shown in red (F4/80), green (LOX), and white (DAPI). The fluorescence intensity of the individual channel in the right bottom panel represents the dot line cross‐sectioning the image in the right upper panel. Scale bars are 20 μm. (B) The scattering plot from semi‐automated analysis of the fluorescent image shows an association between LOX and F4/80 in the gonadal AT of 10∼12 week‐old male mice (2.3% in control versus 71.0% in ob/ob; n = 3 in each group). (C) LOX family gene expression in the peritoneal macrophages derived from 10∼12 week‐old male mice (n = 3 in each group). mRNA levels are expressed relative to average expression of Lox in peritoneal macrophages of WT mice. (D) Immunoblot analysis of pro‐LOX and LOX; and (E) LOX enzymatic activity in the cell lysates and culture medium of peritoneal macrophages derived from 10∼12 week‐old male mice (n = 3 in each group). (F) Eeff of the collagen gel on which the peritoneal macrophages derived from WT and ob/ob male mice were cultivated. Sample numbers and measurements taken are shown in Table S1. (G) Expression of LOX in PBMCs of obese subjects before (Pre) and 6‐months (n = 4)/12‐months (n = 5) after weight loss surgery. *P < 0.05 and **P < 0.01 by Student's t‐test in (C and E) and by Wilcoxon signed‐rank test in (F). Blue color in (G) indicates the results from humans
Because LOX regulation by different cell types among distinct tissues was reported,7 we herein tested various cell types and stimuli to delineate the cause of LOX upregulation in obese AT (Figure S2A–F). Macrophage RAW 264.7 cells exhibited significant LOX induction by lipopolysaccharide (LPS; inflammation) but not by CoCl2 (hypoxia) or TGFβ (fibrosis) (Figure 3A). LPS or a combined inflammatory regimen increased pro‐LOX and LOX proteins in RAW 264.7 cells but not the other cell types (Figure 3B and S2G and H). Importantly, LPS induced LOX enzymatic activity in RAW 264.7 cells (Figure 3C) and their ability to stiffen collagen gel; however, a LOX inhibitor, β‐aminopropionitrile (BAPN) abolished this ability (Figure 3D). In mouse primary peritoneal macrophages, LPS and cytokine cocktail induced pro‐LOX and LOX proteins and their enzymatic activity as well as the ability of macrophages to stiffen collagen gel (Figure S3). Collectively, these results implied increased LOX levels in macrophages caused by inflammatory stimuli resulted in AT stiffening. Thus, we hypothesized that obesity‐associated inflammatory responses upregulate LOX and collagen crosslinking, leading to AT stiffening and dysfunction.
FIGURE 3.

Macrophage LOX elevates substratum stiffness, consequently deteriorating functions of adipocytes cultivated on it. (A) Expression of Lox in RAW 264.7 cells treated with LPS (100 ng/ml), CoCl2 (20 nM) and TGFβ (20 ng/ml) for 24 h (n = 3 each). (B) Immunoblot analysis of pro‐LOX and LOX in the cell lysate and medium of RAW 264.7 cells in response to LPS (100 ng/ml). (C) LOX enzymatic activity in the cell lysate and medium of RAW 264.7 cells treated with LPS (100 ng/ml) and BAPN (200 μM) for 24 hr (n = 3 each). (D) Eeff of decellularized collagen gel after culturing with RAW 264.7 cells with or without LPS (100 ng/mL) and BAPN (200 μM) for 24hr. At least 60 distinct points from one to two plates were indented in each group. (E) The experimental design of cultivating differentiated 3T3‐L1 cells on RAW 264.7‐processed collagen gel. Immunoblot analysis of (F) adipokine secreted in culture medium and (G) insulin‐induced Akt phosphorylation (Ser473) in cell lysate of adipocytes cultivated on RAW 264.7‐processed collagen gel, of which RAW 264.7 were treated with LPS (100 ng/mL) and BAPN (200 μM) for 24 hr. The intensities of bands, quantified densitometrically relative to the control, are shown with the sample number in parentheses. (H) Immunoblot analysis of Akt Ser473 phosphorylation after 4‐h 100 nM insulin stimulation in AT explants of male WT and ob/ob mice with overnight 200 μM BAPN treatment. (I) Immunoblot analysis of adiponectin in AT explants. (J) Eeff of AT explants from 8–12 week‐old male WT and ob/ob mice with or without 200 μM BAPN treatment for 24 h. At least 60 distinct points from one to two mice were indented in each group. (K) Eeff in the gonadal AT of 6 to 8‐week‐old male ob/ob (from 3 mice in each group) treated with 600 mg/kg/day BAPN for 2 weeks. *P < 0.05, **P < 0.01, ***P < 0.001 by one‐way ANOVA with Tukey HSD test in (A and C) and by Wilcoxon signed‐rank test in (D, J, and K). Sample numbers and measurements taken in (D, J, and K) are shown in Table S1
Previous studies demonstrated that stiffened environment jeopardizes adipogenesis and mature adipocyte functions.8, 9 Based on the culture system and results established in Figure 3D, providing a bidirectional tool studying not only cell‐to‐matrix but also matrix‐to‐cell communications, we then tested whether LOX‐stiffened environment mediated by inflamed macrophages changed adipocyte behavior. RAW 264.7 cells were firstly cultivated on collagen gel with LPS and BAPN. After a decellularization procedure of washing away RAW 264.7 cells and residual chemicals, differentiated 3T3‐L1 adipocytes were then seeded on the macrophage‐processed collagen gel (Figure 3E). Adipokine secretion and insulin sensitivity diminished in adipocytes seeded on LPS‐treated macrophage‐processed gel, but BAPN co‐treatment during LPS administration restored these reductions (Figure 3F and G). These results implied that LOX, produced by inflamed macrophages, mediates substratum stiffness and subsequently affects adipocyte function.
The involvement of LOX and macrophage in adipose tissue stiffening and metabolism was dissected with LOX inhibition by BAPN and macrophage depletion by clodronate. BAPN has shown a beneficial effect on glucose metabolism.4, 10 Ex vivo BAPN treatment significantly enhanced insulin‐induced Akt phosphorylation and adiponectin expression, accompanied by decreased ob/ob AT stiffness (Figure 3H–J and S4). Consistently, in vivo BAPN treatment in ob/ob mice reduced AT stiffness (Figure 3K). In vivo clodronate treatment reduced pro‐LOX and LOX proteins, LOX enzymatic activity, and Eeff in ob/ob gonadal AT (Figure 4A–C) on top of the overall improvements in metabolism previously reported.
FIGURE 4.

ob/ob mice treated with clodronate or reconstituted with LOX‐knockdowned BMCs exhibit lower AT stiffness and better glucose metabolism. (A) Immunoblot analysis for pro‐LOX and LOX, (B) LOX enzymatic activity, (C) Eeff of gonadal AT in 10∼12 week‐old male ob/ob mice treated with vehicle (n = 3) or clodronate (n = 5). Clodronate and PBS liposome were intraperitoneally injected with the dosage of 40 mg/kg for the first injection and 10 mg/kg for subsequent injections every 3 days for 6 weeks. (D) Expression of Lox (n = 3 in scramble and n = 5 in shLOX) and (E) immunoblot analysis for pro‐LOX and LOX in the gonadal AT of 10–12 week‐old ob/ob mice reconstituted with lentivirus transduced scramble or LOX‐knockdowned (shLOX) BMCs. Recipient male and female ob/ob mice were subjected to 7 Gy of irradiation one day before transplantation, of which 106 transfected donor BMCs were delivered through tail vein injection. (F) LOX enzymatic activity, (G) Eeff, and (H) expression of genes for macrophage markers (Emr1 and Cd68) and adipocyte functional signatures in the gonadal AT of ob/ob mice reconstituted with scramble or shLOX BMCs (n = 3 in each group). (I) Plasma glucose and insulin AUC and IR index during OGTT in ob/ob mice reconstituted with scramble (n = 17) or shLOX (n = 20) BMCs 5∼8 weeks after BMTP. *P < 0.05, **P < 0.01, ***P < 0.001 by Student's t‐test in (B, D, F, H, and I) and by Wilcoxon signed‐rank test in (C) and (G). Sample numbers and measurements taken in (C) and (G) are shown in Table S1
To further assess the involvement of macrophage LOX in AT stiffening and systemic metabolism in vivo, we adopted bone marrow transplantation to reconstitute LOX‐knockdown bone marrow cells (BMCs, Figure S5A) in ob/ob mice. Eight weeks after transplantation, ob/ob mice receiving LOX‐knockdown BMCs exhibited diminished Lox transcript and pro‐LOX protein levels in gonadal AT (Figure 4D and E). Transplantation markedly suppressed LOX activity and reduced AT stiffness (Figure 4F and G). LOX knockdown in BMCs did not affect macrophage infiltration into AT, reflected by persisting macrophage markers, but improved AT functioning manifested by increased expression of adipocyte functional signature genes in ob/ob AT (Figure 4H). While LOX knockdown in BMCs had no impact on the body or AT weight (Figure S5B), a reduction in insulin area under curve and insulin resistance index during oral glucose tolerance test was discovered after transplantation (Figure 4I). These results suggested the involvement of macrophage LOX in obesity‐induced AT stiffening and metabolic malfunction.
In conclusion, we identified inflamed macrophages as key players in substratum stiffening by upregulating and releasing LOX, which further stiffened the environment where the cells were cultivated, leading to dysfunctional adipocytes. Macrophage depletion or LOX inhibition attenuated obesity‐induced LOX and AT stiffening. Furthermore, ob/ob mice reconstituted with LOX‐knockdown BMCs showed decreased AT stiffness and improved metabolism. Ongoing studies have gradually untangled underlying mechanisms of obesity, our study at least supports LOX derived from inflamed macrophages exerts AT stiffening and malfunction in obesity. This research also extends our perception of well‐known chemical effects of macrophages to a newly explored mechanical impact in obesity.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We thank Dr. Wan‐Chun Chen, Dr. Hsiu‐Kuan Lin, and Dr. Ching‐Yi Liu at Department of Physiology, National Cheng Kung University for critical suggestions; Dr. Chia‐Jung Li, Dr. Chia‐Liang Yen, and Chang‐Jung Chen at Institute of Clinical Medicine; the Core Laboratories of Center for Clinical Medicine Research at National Cheng Kung University Hospital; the Laboratory Animal Center, College of Medicine, National Cheng Kung University and Taiwan Animal Consortium; and Dr. Dean Tai and Ya‐yun Ren of Histoindex Pte. Ltd. for technical supports. We thank Editage for language editing. This work was supported by grants from Ministry of Science and Technology (MOST‐107‐2320‐B‐006‐063MY3 and MOST‐107‐2320‐B‐006‐003), National Health Research Institutes (NHRI‐EX107‐10511SI), and National Cheng Kung University Top‐Notch Project.
REFERENCES
- 1.Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18(4):470‐477. 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Aron‐Wisnewsky J, Marcelin G, et al. Accumulation and changes in composition of collagens in subcutaneous adipose tissue after bariatric surgery. J Clin Endocrinol Metab. 2016;101(1):293‐304. 10.1210/jc.2015-3348. [DOI] [PubMed] [Google Scholar]
- 3.Pastel E, Price E, Sjöholm K, et al. Lysyl oxidase and adipose tissue dysfunction. Metabolism. 2018;78:118‐127. 10.1016/j.metabol.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Miana M, Galán M, Martínez‐Martínez E, et al. The lysyl oxidase inhibitor β‐aminopropionitrile reduces body weight gain and improves the metabolic profile in diet‐induced obesity in rats. Dis Model Mech. 2015;8(6):543‐551. 10.1242/dmm.020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdennour M, Reggio S, Le Naour G, et al. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: links with diabetes and BMI loss after gastric bypass. J Clin Endocrinol Metab. 2014;99(3):898‐907. 10.1210/jc.2013-3253. [DOI] [PubMed] [Google Scholar]
- 6.Pellegrinelli V, Heuvingh J, du Roure O, et al. Human adipocyte function is impacted by mechanical cues. J Pathol. 2014;233(2):183‐195. 10.1002/path.4347. [DOI] [PubMed] [Google Scholar]
- 7.Chen J‐Y, Tsai P‐J, Tai H‐C, et al. Increased aortic stiffness and attenuated lysyl oxidase activity in obesity. Arterioscler Thromb Vasc Biol. 2013;33(4):839‐846. 10.1161/ATVBAHA.112.300036. [DOI] [PubMed] [Google Scholar]
- 8.Young DA, Choi YS, Engler AJ, Christman KL. Stimulation of adipogenesis of adult adipose‐derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials. 2013;34(34):8581‐8588. 10.1016/j.biomaterials.2013.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Hosaka T, Jambaldorj B, Nakaya Y, Funaki M. Extracellular matrix with the rigidity of adipose tissue helps 3T3‐L1 adipocytes maintain insulin responsiveness. J Med Invest. 2009;56(3‐4):142‐149. 10.2152/jmi.56.142. [DOI] [PubMed] [Google Scholar]
- 10.Halberg N, Khan T, Trujillo ME, et al. Hypoxia‐inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29(16):4467‐4483. 10.1128/mcb.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


