Abstract
The coronavirus 2019 (COVID‐19) pandemic caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has led to more than 160 million infections and 3.5 million deaths globally. Men are disproportionately affected by COVID‐19, having more severe disease with higher mortality rates than women. Androgens have been implicated as the underlying cause for more severe disease, as the androgen receptor has been noted to upregulate the cell surface receptors that mediate viral cell entry and infection. Unfortunately, despite testosterone’s potential role in COVID‐19 prognosis, androgen deprivation therapy is neither protective nor a treatment for COVID‐19. Interestingly, the male reproductive organs have been found to be vulnerable in moderate to severe illness, leading to reports of erectile dysfunction and orchitis. COVID‐19 viral particles have been identified in penile and testis tissue, both in live patients who recovered from COVID‐19 and post mortem in men who succumbed to the disease. Although sexual transmission remains unlikely in recovered men, moderate to severe COVID‐19 infection can lead to germ cell and Leydig cell depletion, leading to decreased spermatogenesis and male hypogonadism. The objective of this review is to describe the impact of SARS‐CoV‐2 on male reproductive health. There are still many unanswered questions as to the specific underlying mechanisms by which COVID‐19 impacts male reproductive organs and the long‐term sequelae of SARS‐CoV‐2 on male reproductive health.
Keywords: men's health, COVID‐19, male fertility, testosterone, spermatogenesis
Introduction
Coronavirus 2019 (COVID‐19) is a novel viral respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), first reported in Wuhan China in December 2019. Since then, COVID‐19 has spread globally and has infected more than 166 million people and caused more than 3.5 million deaths around the world [1, 2]. Although there is no difference in the percentage of men and women diagnosed with COVID‐19, large‐scale, multi‐country statistical analyses have shown that men experience greater disease severity and a higher mortality rate compared with women [3, 4]. While the underlying reason for worse prognosis of COVID‐19 in men has not been fully elucidated, it does appear that male gender is a risk factor for severe infection worldwide.
Researchers have been attempting to discover the underlying mechanisms causing the disproportionate impact of COVID‐19 on men. The underlying mechanisms for viral entry of SARS‐CoV‐2 could be influenced by androgens, which are well known to have higher serum concentrations in men [5]. The potential role of androgens in COVID‐19 severity has been investigated, and medical castration has even been explored as a potential treatment [6, 7]. The male reproductive organs have also been described as being vulnerable to infection believed to be due to direct viral entry. Erectile dysfunction (ED) has been reported after COVID‐19 recovery and viral particles have even been documented in penile tissue up to 7 months after infection [8]. Epididymo‐orchitis has been reported in almost a quarter of infected men, raising concerns about the ability of SARS‐CoV‐2 to enter the testis, break down the testis−blood barrier and effect spermatogenesis, and potentially be spread through sexual contact [9, 10]. In the present review, we will briefly describe the underlying mechanisms for entry of SARS‐CoV‐2 into human cells and describe how this may lead to worse infection in men, review the role of androgens in COVID‐19 infection and implications for treatment, and discuss the impact of the virus impact on male reproductive organs in order to highlight comprehensively the current evidence regarding the impact of SARS‐CoV‐2 on male reproductive health.
Viral Mechanism of Entry
SARS‐CoV‐2 is a single‐stranded RNA virus approximately 30kb in size, primarily spread through the air by an infected person’s cough or sneeze and, less commonly, by touching surfaces containing live virus droplets [11]. Similarly to other coronaviruses, SARS‐CoV‐2 contains four main structural proteins, including envelope (E), membrane (M), nucleocapsid (N) and spike (S) [12, 13] The E protein has a crucial role in the permeability of the host cells’ membrane [11]. The M protein is the most abundant molecule on the virus surface and is the main organizer of the virion assembly [14]. The N protein forms a complex with the viral genome and enhances the efficiency of SARS‐CoV‐2 transcription and assembly in human cells [15]. Finally, the S protein, located on the surface of SARS‐CoV‐2, is responsible for viral attachment and fusion between the virus and the host cell, leading to infection [13].
The S protein includes two subunits: S1 mediates the attachment process; S2 is involved in membrane fusion. The receptor‐binding domain on S1 binds to angiotensin‐converting enzyme 2 (ACE2). ACE2 is a cell entry receptor and is found in a variety of human tissue, in particular lung cells, but also male organs including the testis, notably in the Leydig and Sertoli cells [16, 17, 18, 19, 20]. After binding to ACE2 through the receptor‐binding domain, the virus uses the host’s cellular transmembrane serine protease type II (TMPRSS2) and is proteolytically activated [21]. TMPRSS2 is expressed in many cell types, including lung, intestine, colon, salivary gland, stomach and prostate [5, 22]. Furthermore, expression of TMPRSS2 is reported in all cell clusters within the testis, such as spermatogonia and spermatids [23]. Under the influence of A disintegrin and metalloproteinase 17 (ADAM17), ACE2 can become soluble and is believed to be protective against severe disease (Figure 1); however, expression of ADAM17 is not well understood [24]. The presence of target proteins for SARS‐CoV‐2 may help explain the unique consequences of COVID‐19 infection in males.
Fig. 1.
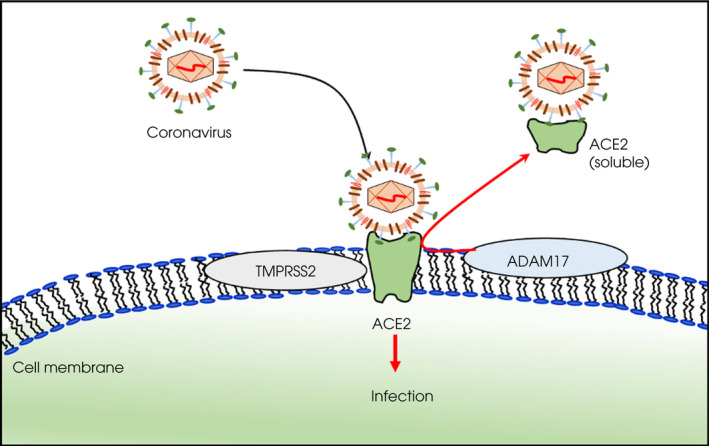
Entry of SARS‐CoV‐2 into the cell. The spike protein binds to angiotensin‐converting enzyme 2 (ACE2) and is then cleaved by transmembrane serine protease type II (TMPRSS2), leading to cell membrane fusion and viral entry. A disintegrin and metalloproteinase 17 (ADAM17) can induce a soluble form of ACE2, which may be protective against severe infection [24].
COVID‐19 Infection differences in Men and Women
While the susceptibility to SARS‐CoV‐2 infection is similar between men and women, several studies have shown that the severity of the disease and the rate of death is higher in men [3, 4]. Importantly, no difference has been reported in the number of infected patients with COVID‐19 between the men and women, suggesting that either differences in lifestyle or an underlying physiological reason could account for the increased disease severity in men [16, 17, 18, 19, 20, 25, 26]. Unhealthy lifestyles, which are known to be prominent in men, such as smoking, physical inactivity and obesity were associated with hospitalization for COVID‐19 in a large population‐based study [27]. By contrast, there is growing evidence that ACE2 and TMPRSS2 expression mediates the sex disparity during the mechanism of viral entry [5]. In women, oestrogen has been shown not only to regulate the expression of ACE2 in differentiated airway epithelial cells, but also to increase the immune response [28, 29]. Expression and activity of TMPRSS2 within alveolar type I and II epithelial cells are increased in men compared to women, which may partly explain higher severity of COVID‐19 in men [5]. TMPRSS2 is expressed in various male reproductive parts, such as the testes and the genital tract, as well as in the prostate epithelial cells, which implies its presence in the seminal fluid [22, 23, 30]. Since the effect of SARS‐CoV‐2 infection is increased by TMPRSS2 proteolysis, there is probably a positive association between TMPRSS2 expression and disease severity. Furthermore, androgen receptors (ARs) modulate the expression of TMPRSS2, and due to significantly higher expression of AR in men compared to women, this probably causes the increased risk in men for more severe COVID‐19 [4].
Androgens and SARS‐CoV‐2
The relatively high incidence of complications from severe COVID‐19 in men led investigators to study the relationship between androgens and infection early on in the pandemic. While there have been competing theories about whether testosterone improves or worsens the severity of COVID‐19, it is likely that testosterone is a key factor in infection and progression of the illness. [25, 31, 32, 33, 34]. Testosterone is a co‐regulator of expression of both ACE2 and TMPRSS2, which probably aides in the internalization of SARS‐CoV‐2. Interestingly, in contrast, both obesity and advanced age, both of which commonly result in low levels of serum testosterone, have also been associated with the development of complications from COVID‐19 [6, 33].
Testosterone’s influence on severity of COVID‐19 may be illuminated by an association between milder cases of infection and androgen deprivation therapy (ADT). ADT, the mainstay treatment for metastatic prostate cancer, lowers serum testosterone to castrate levels. Two studies have evaluated COVID‐19 in patients receiving ADT: one found no relationship [35], whereas the other found ADT to be partially protective [36]. While both of these conflicting studies included large cohorts (>4000), Klein et al. [35] evaluated an even larger number of patients on ADT and found no association between ADT and COVID‐19 severity.
Androgen deprivation therapy was also investigated as a treatment for COVID‐19 to limit the severity of symptoms [37]. Although that study was limited to a small sample size, there were significantly lower rates of hospitalization, infection and supplemental oxygen utilization in the ADT group compared to those not on ADT. There were also trends towards decreased intubation and increased overall survival. It still is not clear if medical castration could be used to treat severe COVID‐19. A sub‐analysis of the data in the study by Montopoli et al. [36] found the number needed to treat with ADT to prevent one SARS‐CoV‐2 infection was 434, and considering the adverse effects associated with ADT, O'Callaghan et al. concluded it would not be a good treatment or prevention option [38].
Interestingly, even though ADT was essentially found to be neither detrimental nor protective in COVID‐19, lower testosterone levels are also associated with severe COVID‐19. In an elegant study comparing hormonal levels among hospitalized patients with COVID‐19, patients with non‐COVID respiratory disease, and age‐matched controls, Kadihasanoglu et al. [39] found significantly lower testosterone levels in COVID‐19 patients compared to the other groups. Almost 75% of COVID‐19 patients met the criteria for testosterone deficiency, and lower testosterone levels were correlated with an increased hospitalization time. Similarly, testosterone levels were significantly decreased in severe disease compared to those with milder cases of COVID‐19 [40]. Low testosterone levels were also significantly associated with higher levels of inflammatory markers, including interleukin‐6, C‐reactive protein, interleukin‐1 receptor antagonist, hepatocyte growth factor, and interferon γ‐inducible protein 10. Testosterone can modulate immune response, so higher inflammatory markers could partly explain why severe disease has been associated with lower testosterone levels [7].
Unfortunately, as pre‐infection testosterone levels are not available in the previously mentioned studies, it remains unclear if lower testosterone levels are a risk factor for more severe disease or if severe SARS‐CoV‐2 infection leads to hypogonadism. Interestingly, in the study by Kadihasanoglu et al., serum luteinizing hormone levels were elevated, suggesting a primary hypogonadism and testicular failure, instead of a secondary cause more likely to be influenced by a severe inflammatory state [39]. Testicular compromise could be the underlying aetiology of hypogonadism in severe COVID‐19. Leydig cells, which produce testosterone, have high expressions of ACE2, although co‐expression of TMPRSS2 is unknown [41]. It is possible that direct SARS‐CoV‐2 invasion with inactivation or destruction of Leydig cells leads to testicular hypofunction in severe disease. In fact, decreased Leydig cell populations have been described in post‐mortem pathological evaluation of the testis in men who died from COVID‐19 [42]. Taken together, the association of lower testosterone levels in severe COVID‐19 appears to be attributable to the sequelae of SARS‐CoV‐2 infection, and not because men were hypogonadal before infection.
Given this information, some experts have even considered the potential use of testosterone replacement therapy (TRT) in severe infection [6]. Unfortunately, given the high rates of venous thromboembolic events (VTEs) in severe SARS‐CoV‐2 infection, concerns have been raised that TRT could accentuate VTE. Fortunately, when investigating COVID‐19 patients already on TRT compared to those who were not, Rambhatla et al. [43] did not find an increased risk of VTE. Furthermore, there was no relationship found between men taking TRT and more severe COVID‐19. Taken together, although testosterone can modulate ACE2 and TMPRSS2 expression, there is currently not enough clinical evidence to suggest a direct relationship between T levels and COVID‐19 outcomes.
Despite the well described role of androgens in SARS‐CoV‐2 cell entry, the effects of baseline testosterone levels on disease severity and any therapeutic roles remain unknown. Since the onset of the pandemic there have been conflicting data about whether castrate, low or high testosterone levels are protective or detrimental in COVID‐19, which has led to investigations into potential treatment using both ADT and TRT. Unfortunately, use of both TRT and ADT is limited by adverse effects, especially in those with severe COVID‐19. Nevertheless, larger datasets and evidence of the long‐term effects of SARS‐CoV‐2 are needed to elucidate the true relationship between androgens and COVID‐19 severity.
COVID‐19’s Impact on the Male Reproductive Organ Tract
The presence of ACE2 and TMPRSS2 throughout the male genitourinary tract make the male reproductive organs vulnerable to damage from SARS‐CoV‐2 infection. Furthermore, the influence of testosterone on these organs and the presence of the AR, which promotes the TMPRSS2 gene, would further explain viral uptake [23, 44]. One of the hallmarks of COVID‐19 infection has been the variability in symptom severity and immune response [32]. The current theory is that haematogenous viral spread, in men with more severe disease, is responsible for direct effects of the virus on the male reproductive organs [22].
Penis and Erectile Dysfunction
Endothelial dysfunction is a cornerstone of severe COVID‐19 infection and is probably the underlying pathophysiology behind the finding of ED after recovery. ACE2 and TMPRSS2 are ubiquitous in endothelial cells, the target for SARS‐CoV‐2 uptake. TMPRSS2 cleaves the S protein into S1 and S2 domains, which produces an active fusion fragment. TMPRSS2 also promotes the cleavage of ACE2, creating an increase in viral uptake via the endosomal cysteine proteases cathepsin B and L pathway. In this process, the endosome releases the virus to the cytoplasm of the cells for replication and cell damage [45, 46]. This can create a vasculopathy, which leads to microvascular damage, a hallmark of vasculogenic ED [47].
Male sexual dysfunction in the form of ED was explored early in the pandemic. Survey studies from China and Italy evaluated how COVID‐19 impacted sexual health and found that the prevalence of ED was higher among previously infected men [48, 49]. Aside from endothelial dysfunction, other underlying causes associated with illness recovery could impact erectile function, including subclinical hypogonadism, psychological distress, and impaired pulmonary haemodynamics [50]. While ED pathophysiology is often multi‐factorial, a recent report of SARS‐CoV‐2 within the corpora cavernosa (Figure 2) of two previously infected men strongly suggests that direct penile damage, probably from endothelial dysfunction, plays a major role in ED after severe COVID‐19 [8]. The presence of the virus within the penile tissue up to 7 months after infection illustrates how SARS‐CoV‐2 can directly damage cavernosal endothelium, leading to male sexual dysfunction. Although the majority of these studies are based on small sample sizes, it will be interesting to learn if COVID‐19 severity is indeed a risk factor for ED as longer‐term survivor data become available.
Fig. 2.
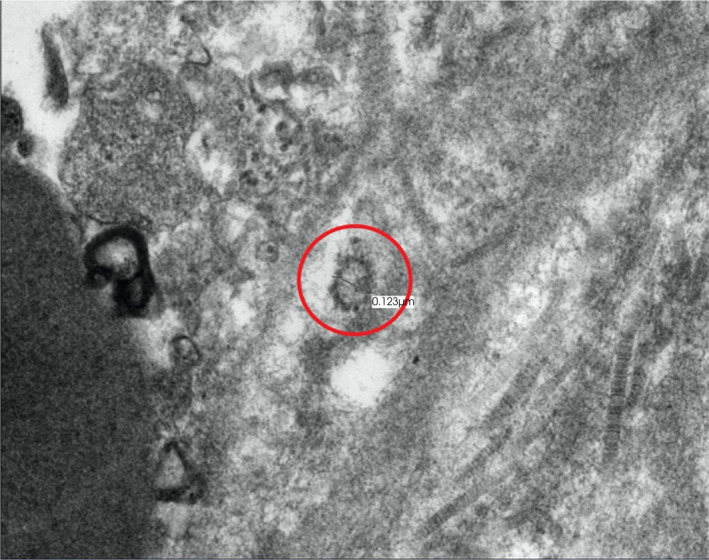
Transmission electron microscopy image of SARS‐CoV‐2 within cavernosal tissue of a previously severe COVID‐19‐infected patient undergoing penile prosthesis surgery 7 months after infection. Viral particle (red circle) exhibiting prominent spikes and nucleocapsid (electron dens material).
Prostate and Seminal Vesicles
Despite expression of both ACE2 and TMPRSS2, the prostate and seminal vesicles are the rare male reproductive organs that have not been commonly described to have been impacted in COVID‐19. This is possibly attributable to very low ACE2 expression, as shown from bioinformatic analysis from the Human Protein Atlas database [51]. Conversely, TMPRSS2 expression is noted to be at low to medium levels in the prostate. Colocalization was identified by single‐cell RNA sequencing (scRNA‐seq) in <1% of prostate epithelial cells, while 0.3% expressed ACE2 and 18.6% expressed TMPRSS2 [52]. Not surprisingly, three studies investigated whether SARS‐CoV‐2 was present in prostatic secretions, and across these studies, all 89 samples were negative for the virus [53, 54, 55].
Testis and Epididymis
Unlike the prostate, despite low co‐expression of ACE2 and TMPRSS2, the testis has been vulnerable to SARS‐CoV‐2, as there are reports of both orchitis and decreased sperm production during infection. Interestingly, scRNA‐seq data suggest that co‐expression of both ACE2 and TMPRSS2 occurs less than 0.05% of the time in testicular tissue [56, 57, 58]. ACE2 expression was found mostly in Leydig and Sertoli cells, while TMPRSS2 was concentrated in spermatogonia and spermatids [23]. Since current models suggest that both receptors are needed for SARS‐CoV‐2 entry, there may be another mechanism by which the virus infects testicular tissue.
It has been estimated that 10−22% of men with acute COVID‐19 infection develop orchitis or epididymo‐orchitis, probably due to direct testicular infection [56, 59, 60, 61]. In two studies evaluating the presence of SARS‐CoV‐2 in seminal fluid, 18% of men reported scrotal discomfort [56, 59]. Similarly, when evaluating for inflammation such as tunica albuginea thickening, enhanced echogenicity of the testis or epididymis via scrotal ultrasonography, 22% of men with acute COVID‐19 were found to have acute orchitis with or without epididymitis [60]. It is important to recognize that these small studies were conducted primarily in hospitalized patients; therefore, the high rates of testicular pain and orchitis is likely more common in moderate‐to‐severe COVID‐19 compared to asymptomatic or mild disease.
Testicular inflammation from COVID‐19 could be attributable to direct viral invasion, even though SARS‐CoV‐2 receptors are infrequently expressed. In post‐mortem testicular biopsies, multiple studies using transmission electron microscopy and RT‐PCR found virus in 17% of samples, similar to the clinical rate of epididymo‐orchitis [42, 62, 63]. Although RT‐PCR was positive for SARS‐CoV‐2 in the testis, it is possible that the samples, which were predominantly composed of fibrovascular tissue, could have contained a contaminant from blood instead of testicular tissue [42]. Interestingly, Ma et al. [63] also analysed transcriptome changes within the testes with SARS‐CoV‐2, demonstrating dysfunction of the genes regulating spermatogenesis and inflammation‐related changes. Additionally, four of five samples demonstrated extensive germ cell loss, histologically similar to patients with Sertoli cell‐only syndrome, suggesting that SARS‐CoV‐2 can not only damage the testis−blood barrier, but also negatively impact male fertility.
Potential for Sexual Transmission and Effect on Male Fertility
The presence of SARS‐CoV‐2 within the testis, potential for breakdown of the testis−blood barrier, and depletion of germ cells has raised the question of whether COVID‐19 could be spread sexually and what the effects could be on male reproduction. Early in the pandemic, a study by Li et al. [64] identified SARS‐CoV‐2 viral particles in six out of 38 men who submitted a semen sample. This finding raised the question about whether the virus had the potential to be present in high enough concentrations to be transmitted through sexual contact. A recent systematic review evaluated the available data on SARS‐CoV‐2 presence in semen, using 19 articles that met the inclusion criteria. All studies investigated the presence of viral presence in the semen; however, Li et al. were the only group to detect seminal COVID‐19 [10, 64]. This is probably because the study by Li et al. was the only study that evaluated viral presence during acute infection, notably among many severely infected men. Given the low rates of SARS‐CoV‐2 within seminal fluid, andrology technicians are not likely to be at risk of contracting COVID‐19 while performing semen analysis; however, universal precautions should still be used. Similarly, although there has not been any reported evidence of strictly sexual transmission, it is unlikely, given that there is seminal presence of SARS‐CoV‐2 only during severe, acute infection and this probably resolves with resolution of the illness. Current data suggest that the risk of sexual transmission from men who have recovered from infection is negligible.
While SARS‐CoV‐2 concentrations within the testis are unlikely to be high enough for sexual transmission, the virus is able to impact male fertility through germ cell depletion [63]. An early COVID‐19 study evaluated semen characteristics in two acutely ill and 18 recovered men. Men with moderate infection had a statistically significant impairment in semen characteristics compared to those with mild infection or within the control group, even though the values were within the normal WHO ranges [59]. A prospective cross‐sectional analysis of 43 men recovered from SARS‐CoV‐2 infection found 11 men to have semen impairment; of these, eight had azoospermia and three oligospermia (sperm concentration <15million/mL) [65]. A more recent study evaluated 30 men who recovered from SARS‐CoV‐2 infection and included a follow‐up semen analysis from five men [9]. The median total sperm number in the ejaculate in these men was 12.5 million, which was significantly lower than in an age‐matched control group of healthy, non‐SARS‐CoV‐2‐infected men (Table 1). At a median of 3 months, the five men with follow‐up semen analyses had an increase in median total sperm number to 18 million. Given these findings, it appears that COVID‐19 infection can negatively impact spermatogenesis, at least temporarily. Although the long‐term effects of SARS‐CoV‐2 infection on semen quality are not yet known, after infection and normalization of semen parameters, which may take up to 3 months for spermatogenesis recovery, the sperm is probably safe enough for cryopreservation and/or use for assisted reproductive techniques such as in vitro fertilization. Since long‐term sperm quality is not yet known in men who have recovered from COVID‐19, those who wish to conceive should consider undergoing a fertility evaluation to assess sperm quality.
Table 1.
Comparison of semen characteristics in men with COVID‐19 infection and age‐matched controls.
| Variable | COVID (+) Cohort (n = 30) | COVID (‐) Cohort (n = 30) | P |
|---|---|---|---|
| Age, years | 40 (24.75) | 42 (9.8) | 0.8732 |
| Volume, mL | 2.1 (1.23) | 2.2 (2.15) | 0.3841 |
| pH | 7.2 (0.8) | 7.2 (0.4) | 0.2304 |
| Concentration, million/mL | 11.5 (26.8) | 21.5 (21.5) | 0.0048 |
| Total sperm number, million | 12.5 (52.1) | 59.2 (70.5) | 0.0024 |
Concentration and total sperm number were significantly lower in COVID(+) men. Values are presented as median (interquartile range). P < 0.05 was considered significant [9]
Conclusion
There are still many questions as to why the COVID‐19 pandemic has disproportionately affected men, leading to more severe disease and worse mortality rates than for women. In addition to men generally having more comorbid conditions than women, higher androgen levels have been identified as a potential mechanism for viral cell entry. The ability for the AR to upregulate ACE2 and TMPRSS2 gene could be an underlying reason for worse disease severity; however, the data are mixed on whether clinical differences exist amongst men on TRT and ADT. Furthermore, despite extremely low levels detected of both ACE2 and TMPRSS2 in reproductive organs such as the testis, there have been higher than expected rates of orchitis and spermatogenesis impairment amongst men with moderate to severe COVID‐19, suggesting that there may be another mechanism by which the virus impacts male reproductive health. As the long‐term effects of infection in males are not yet available, it is important for men who have overcome COVID‐19 with concerns about potential consequences of the disease to seek medical care. Future research is needed to elucidate the sequelae of infection, develop methods to decrease the impact of SARS‐CoV‐2 on male reproductive health, and address the long‐term consequences of severe disease.
Conflict of Interest
None declared.
Abbreviations
- ACE
angiotensin‐converting enzyme
- ADAM17
A disintegrin and metalloproteinase 17
- ADT
androgen deprivation therapy
- AR
androgen receptor
- ED
erectile dysfunction
- TMPRSS2
transmembrane serine protease type II
- scRNA‐seq
single‐cell RNA sequencing
- TRT
testosterone replacement therapy
- VTE
venous thromboembolic event
References
- 1. Weekly operational update on COVID‐19 ‐ 24 May 2021 [Internet]. [cited 2021 May 25]. Available from: https://www.who.int/publications/m/item/weekly‐operational‐update‐on‐covid‐19‐‐‐24‐may‐2021
- 2. Koutsakos M, Kedzierska K. A race to determine what drives COVID‐19 severity. Nature 2020; 583: 366–8 [DOI] [PubMed] [Google Scholar]
- 3. Peckham H, de Gruijter NM, Raine C et al. Male sex identified by global COVID‐19 meta‐analysis as a risk factor for death and ITU admission. Nat Commun 2020; 11: 6317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mukherjee S, Pahan K. Is COVID‐19 Gender‐sensitive? J Neuroimmune Pharmacol 2021; 16: 38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okwan‐Duodu D, Lim E‐C, You S, Engman DM. TMPRSS2 activity may mediate sex differences in COVID‐19 severity. Signal Transduct Target Ther. 2021; 6: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ory J, Lima TFN, Towe M et al. Understanding the complex relationship between androgens and SARS‐CoV2. Urology 2020; 144: 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Auerbach JM, Khera M. Testosterone’s Role in COVID‐19. J Sex Med 2021; 18: 843–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kresch E, Achua J, Saltzman R et al. COVID‐19 endothelial dysfunction can cause erectile dysfunction: histopathological, immunohistochemical, and ultrastructural study of the human penis. World J Men's Health 2021; 39: 466. 10.5534/wjmh.210055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Best JC, Kuchakulla M, Khodamoradi K et al. Evaluation of SARS‐CoV‐2 in human semen and effect on total sperm number: a prospective observational study. The World Journal of Men's Health 2021; 39: 489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez DC, Khodamoradi K, Pai R et al. A systematic review on the investigation of SARS‐CoV‐2 in Semen. Res Rep Urol. 2020; 12: 615–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boopathi S, Poma AB, Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J Biomol Struct Dyn 2021; 39: 3409–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sternberg A, Naujokat C. Structural features of coronavirus SARS‐CoV‐2 spike protein: Targets for vaccination. Life Sci 2020; 257: 118056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Structure, Function, and Antigenicity of the SARS‐CoV‐2 Spike Glycoprotein ‐ ScienceDirect [Internet]. [cited 2021 May 25]. Available from: https://www.sciencedirect.com/science/article/pii/S0092867420302622
- 14. Alsaadi EA, Jones IM. Membrane binding proteins of coronaviruses. Future Virol. 2019; 14: 275–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McBride R, Van Zyl M, Fielding BC. The coronavirus nucleocapsid is a multifunctional protein. Viruses 2014; 6: 2991–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Z, Xu W, Xia S et al. RBD‐Fc‐based COVID‐19 vaccine candidate induces highly potent SARS‐CoV‐2 neutralizing antibody response. Signal Transduct Target Ther. 2020; 5: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang J, Wang W, Chen Z et al. A vaccine targeting the RBD of the S protein of SARS‐CoV‐2 induces protective immunity. Nature 2020; 586: 572–7 [DOI] [PubMed] [Google Scholar]
- 18. Dong Y, Dai T, Wei Y, Zhang L, Zheng M, Zhou F. A systematic review of SARS‐CoV‐2 vaccine candidates. Signal Transduct Target Ther. 2020; 5: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Navarra A, Albani E, Castellano S, Arruzzolo L, Levi‐Setti PE. Coronavirus disease‐19 infection: implications on male fertility and reproduction. Front Physiol [Internet]. 2020; 11: 1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amini Mahabadi J, Negaresh F. The effect of COVID‐19 disease on sperm parameters and male fertility. Sarem J Reprod Med. 2020; 5: 58–66 [Google Scholar]
- 21. Hirano T, Murakami M. COVID‐19: A new virus, but a familiar receptor and cytokine release syndrome. Immunity 2020; 52: 731–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hesari FS, Hosseinzadeh SS, Sardroud MAAM. Review of COVID‐19 and male genital tract. Andrologia 2021; 53: e13914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Z, Xu X. scRNA‐seq profiling of human testes reveals the presence of the ACE2 receptor, A Target for SARS‐CoV‐2 Infection in Spermatogonia. Leydig and Sertoli Cells Cells. 2020; 9: 920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pradhan A, Olsson P‐E. Sex differences in severity and mortality from COVID‐19: are males more vulnerable? Biol Sex Differ. 2020; 11: 53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gebhard C, Regitz‐Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID‐19 outcomes in Europe. Biol Sex Differ. 2020; 11: 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kragholm K, Andersen MP, Gerds TA et al. Association between male sex and outcomes of coronavirus disease 2019 (COVID‐19)—A Danish Nationwide, Register‐based Study. Clin Infect Dis [Internet]. 2020. 10.1093/cid/ciaa924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamer M, Kivimäki M, Gale CR, Batty GD. Lifestyle risk factors, inflammatory mechanisms, and COVID‐19 hospitalization: A community‐based cohort study of 387,109 adults in UK. Brain Behav Immun 2020; 87: 184–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Conti P, Younes A. Coronavirus COV‐19/SARS‐CoV‐2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents 2020; 34 [DOI] [PubMed] [Google Scholar]
- 29. Suba Z. Prevention and therapy of COVID‐19 via exogenous estrogen treatment for both male and female patients: Prevention and therapy of COVID‐19. J Pharm Pharm Sci 2020; 23: 75–85 [DOI] [PubMed] [Google Scholar]
- 30. Chen Y‐W, Lee M‐S, Lucht A et al. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into Semen in prostasomes, is misregulated in prostate cancer cells. Am J Pathol 2010; 176: 2986–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Q, Guan X, Wu P et al. Early transmission dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N Engl J Med 2020; 382: 1199–207. 10.1056/nejmoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jin J‐M, Bai P, He W et al. Gender Differences in Patients With COVID‐19: Focus on Severity and Mortality. Front Public Health 2020; 8: 152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou Y, Chi J, Lv W, Wang Y. Obesity and diabetes as high‐risk factors for severe coronavirus disease 2019 (Covid‐19). Diabetes Metab Res Rev 2021; 37: e3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giagulli VA, Guastamacchia E, Magrone T et al. Worse progression of COVID‐19 in men: Is testosterone a key factor? Andrology 2021; 9: 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klein EA, Li J, Milinovich A et al. Androgen deprivation therapy in men with prostate cancer does not affect risk of infection with SARS‐CoV‐2. J Urol 2021; 205: 441–3 [DOI] [PubMed] [Google Scholar]
- 36. Montopoli M, Zumerle S, Vettor R et al. Androgen‐deprivation therapies for prostate cancer and risk of infection by SARS‐CoV‐2: a population‐based study (N = 4532). Ann Oncol Off J Eur Soc Med Oncol 2020; 31: 1040–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patel VG, Zhong X, Liaw B et al. Does androgen deprivation therapy protect against severe complications from COVID‐19? Ann Oncol Off J Eur Soc Med Oncol 2020; 31: 1419–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Callaghan ME, Jay A, Kichenadasse G, Moretti KL. Androgen deprivation therapy in unlikely to be effective for treatment of COVID‐19. Ann Oncol 2020; 31: 1780–82. 10.1016/j.annonc.2020.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kadihasanoglu M, Aktas S, Yardimci E, Aral H, Kadioglu A. SARS‐CoV‐2 pneumonia affects male reproductive hormone levels: a prospective. Cohort Study. J Sex Med 2021; 18: 256–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dhindsa S, Zhang N, McPhaul MJ et al. Association of circulating sex hormones with inflammation and disease severity in patients with COVID‐19. JAMA Netw Open 2021; 4: e2111398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Douglas GC, O’Bryan MK, Hedger MP et al. The novel angiotensin‐converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology 2004; 145: 4703–11 [DOI] [PubMed] [Google Scholar]
- 42. Yang M, Chen S, Huang B et al. Pathological findings in the testes of COVID‐19 patients: Clinical implications. Eur Urol Focus 2020; 6: 1124–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rambhatla A, Bronkema CJ, Corsi N et al. COVID‐19 infection in men on testosterone replacement therapy. J Sex Med 2021; 18: 215–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wambier CG, Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection is likely to be androgen mediated. J Am Acad Dermatol 2020; 83: 308–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoffmann M, Kleine‐Weber H, Schroeder S et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zipeto D, da Palmeira J, Argañaraz GA, Argañaraz ER. ACE2/ADAM17/TMPRSS2 interplay may be the main risk factor for COVID‐19. Front Immunol 2020; 11: 576745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS‐CoV‐2 infection. Crit Care 2020; 24: 353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fang D, Peng J, Liao S et al. An Online questionnaire survey on the sexual life and sexual function of Chinese adult men during the coronavirus disease 2019 epidemic. Sex Med 2021; 9: 100293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sansone A, Mollaioli D, Ciocca G et al. “Mask up to keep it up”: Preliminary evidence of the association between erectile dysfunction and COVID‐19. Andrology 2021; 9: 1053–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sansone A, Mollaioli D, Ciocca G et al. Addressing male sexual and reproductive health in the wake of COVID‐19 outbreak. J Endocrinol Invest 2021; 44: 223–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tissue expression of ACE2 ‐ Summary ‐ The Human Protein Atlas [Internet]. [cited 2021 May 26]. Available from: https://www.proteinatlas.org/ENSG00000130234‐ACE2/tissue
- 52. Song H, Seddighzadeh B, Cooperberg MR, Huang FW Expression of ACE2, the SARS‐CoV‐2 receptor, and TMPRSS2 in prostate epithelial cells. Eur Urol 2020; 78: 296–8. 10.1016/j.eururo.2020.04.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Quan W, Chen J, Liu Z et al. No SARS‐CoV‐2 in expressed prostatic secretion of patients with coronavirus disease 2019: a descriptive multicentre study in China. medRxiv. 2020;2020.03.26.20044198. [Google Scholar]
- 54. Zhang S, Wang X, Zhang H et al. The absence of coronavirus in expressed prostatic secretion in COVID‐19 patients in Wuhan city. Reprod Toxicol Elmsford N 2020; 96: 90–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ruan Y, Hu B, Liu Z et al. No detection of SARS‐CoV‐2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID‐19 male patients: A perspective and urogenital evaluation. Andrology 2021; 9: 99–106 [DOI] [PubMed] [Google Scholar]
- 56. Pan F, Xiao X, Guo J et al. No evidence of severe acute respiratory syndrome‐coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril 2020; 113: 1135–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stanley KE, Thomas E, Leaver M, Wells D. Coronavirus disease‐19 and fertility: viral host entry protein expression in male and female reproductive tissues. Fertil Steril 2020; 114: 33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu X, Chen Y, Tang W et al. Single‐cell transcriptome analysis of the novel coronavirus (SARS‐CoV‐2) associated gene ACE2 expression in normal and non‐obstructive azoospermia (NOA) human male testes. Sci China Life Sci 2020; 63: 1006–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Holtmann N, Edimiris P, Andree M et al. Assessment of SARS‐CoV‐2 in human semen‐a cohort study. Fertil Steril 2020; 114: 233–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen L, Huang X, Yi Z et al. Ultrasound imaging findings of acute testicular infection in patients with coronavirus disease 2019. J Ultrasound Med 2020; 10.1002/jum.15558 [DOI] [PubMed] [Google Scholar]
- 61. Ediz C, Tavukcu HH, Akan S et al. Is there any association of COVID‐19 with testicular pain and epididymo‐orchitis? Int J Clin Pract 2021; 75: e13753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Achua JK, Chu KY, Ibrahim E et al. Histopathology and ultrastructural findings of Fatal COVID‐19 infections on testis. World J Mens Health 2021; 39: 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ma X, Guan C, Chen R et al. Pathological and molecular examinations of postmortem testis biopsies reveal SARS‐CoV‐2 infection in the testis and spermatogenesis damage in COVID‐19 patients. Cell Mol Immunol 2021; 18: 487–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open 2020; 3: e208292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gacci M, Coppi M, Baldi E et al. Semen impairment and occurrence of SARS‐CoV‐2 virus in semen after recovery from COVID‐19. Hum Reprod Oxf Engl 2021; 36: 1520–9 [DOI] [PMC free article] [PubMed] [Google Scholar]


