Abstract
Introduction
Previous evidence has been conflicting regarding the effect of coronavirus disease 2019 (COVID‐19) pandemic lockdowns on obstetric intervention and preterm birth rates. The literature to date suggests potentially differential underlying mechanisms based on country economic setting. We aimed to study these outcomes in an Icelandic population where uniform lockdown measures were implemented across the country.
Material and methods
The study included all singleton births (n = 20 680) during 2016–2020 identified from the population‐based Icelandic Medical Birth Register. We defined two lockdown periods during March–May and October–December in 2020 according to government implemented nationwide lockdown. We compared monthly rates of cesarean section, induction of labor and preterm birth during lockdown with the same time periods in the 4 previous years (2016–2019) using logit binomial regression adjusted for confounders.
Results
Our results indicated a reduction in the overall cesarean section rate, which was mainly evident for elective cesarean section, both during the first (adjusted odd ratio [aOR] 0.71, 95% CI 0.51–0.99) and second (aOR 0.72, 95% CI 0.52–0.99) lockdown periods, and not for emergency cesarean section. No change during lockdown was observed in induction of labor. Our results also suggested a reduction in the overall preterm birth rate during the first lockdown (aOR 0.69, 95% CI 0.49–0.97) and in the months immediately following the lockdown (June–September) (aOR 0.67, 95% CI 0.49–0.89). The reduction during the first lockdown was mainly evident for medically indicated preterm birth (although not statistically significant) and the reduction during June–September was mainly evident for spontaneous preterm birth.
Conclusions
This study suggested a reduction in elective cesarean section during COVID‐19 lockdown, possibly reflecting changes in prioritization of non‐urgent health care during lockdown. We also found a reduction in overall preterm birth during the first lockdown and spontaneous preterm birth following the first lockdown, but further research is needed to shed light on the underlying mechanisms for these findings.
Keywords: cesarean section, COVID‐19, induction of labor, lockdown, preterm birth
Abbreviations
- aOR
adjusted odds ratio
- COVID‐19
coronavirus disease 2019
- CS
cesarean section
- ICD‐10
International Classification of Disease, 10th revision
- IOL
induction of labor
Key message.
We found a reduction in elective cesarean section during COVID‐19 lockdown, likely reflecting changes in prioritization of non‐urgent procedures. We also found a reduction in overall preterm birth during the first lockdown and in the months immediately following lockdown.
1. INTRODUCTION
Lockdown measures such as have been implemented during the coronavirus disease 2019 (COVID‐19) pandemic worldwide are unprecedented in modern history and we do not fully understand the impact of these measures on human mental and physical health. Many countries underwent major reorganization of healthcare facilities during lockdown in order to accommodate the additional workload related to COVID‐19 patients. All non‐urgent surgeries were cancelled in many places during the most stringent lockdown,1 to reduce the workload on healthcare staff, and a reduction in non‐urgent surgical admission has been found following lockdown compared with the previous year.2
The lockdown periods during the COVID‐19 pandemic and the effect on health services is likely to have also affected obstetric interventions and birth outcomes. However, previous studies have found either no change in cesarean section (CS) rates3 or a slight increase in rates,4 but research is lacking, particularly for elective and emergency CS. What has been studied to a great extent in the past year is the effect of COVID‐19 lockdowns on preterm birth rates. Previous studies have found reductions in preterm birth rates during lockdown compared with before lockdown in Denmark, Ireland, the USA, and Australia,5, 6, 7, 8 but other studies from Spain, China, and the USA have not found such associations.9, 10, 11 A recent meta‐analysis found a reduction in overall preterm birth for high‐income countries only.12 The reason for this welcome, albeit mysterious, improvement in preterm birth rates is not known. As an attempt to further address these questions, a global collaboration, The International Perinatal Outcomes in the Pandemic (iPOP) Study,13 has been established, which includes population‐based data from both low‐ to middle‐income countries and high‐income countries.
The Icelandic government has implemented uniform lockdown measures across the country since the beginning of the COVID‐19 pandemic. The first case in Iceland was diagnosed on February 28, 2020, and as of April 16, 2021 there were 6286 confirmed cases in the country with a 14‐day incidence of 12.5 per 100 000.14 Iceland has uniform maternity care across the country with a single tertiary hospital in the capital and equal access to high‐quality maternity care. In light of the previous evidence, this study aimed to compare CS rates, induction of labor (IOL) rates, and preterm birth rates during the lockdown periods in 2020 in Iceland with rates during the same periods in 2016–2019 using logit binomial regression. We divided CS into emergency and elective CS and preterm birth into spontaneous and medically indicated preterm birth, which has been particularly lacking in previous literature.
2. MATERIAL AND METHODS
2.1. Study setting
In Iceland, health care is publicly funded, maternity care is easily accessible, it is uniform across the country, and mostly free of charge. There is a single tertiary maternity hospital in the capital and almost all births are attended by midwives, in collaboration with obstetricians when problems arise.
The country’s lockdown procedures and assembly ban timeline during 2020 were as follows:15 The first COVID‐19 lockdown period in Iceland began on March 13, 2020 with a 100‐people assembly ban, 2‐m social distancing rule, and partial closure of schools. The assembly ban went down to 20 people on March 24, 2020 and fitness centers closed. This first lockdown was mostly lifted on May 25, 2020 with a 200‐people assembly ban, relaxation of the 2‐m social distancing rule, and the fitness centers opening. On October 5, 2020 the second lockdown began with a 20‐people assembly ban, 2‐m social distancing rule, and closure of pubs and fitness centers. The lockdown became more stringent on October 30, 2020 with partial closure of schools and masks a requirement in shops. The lockdown remained in effect until the end of the year.
2.2. Data sources and study population
We identified all births in 2016–2020 in Iceland (n = 21 287) from the Icelandic Medical Birth Register, which includes information on all infants born at or after 22 weeks of gestation. Only singleton births were included in this study (n = 20 680). Information on maternal demographic variables and delivery characteristics were obtained from the Medical Birth Register.
The lockdown periods under study reflected the two most stringent periods of lockdown in Iceland when the first and second waves of the pandemic occurred: (a) March 13 to May 25, 2020 and (b) October 5 to December 31, 2020. We did not have the exact date of birth in our data, only the month of birth, so could not calculate the birth rate for the exact dates of the lockdown periods. Lockdown 1 therefore included the months of March through May and Lockdown 2 included October through December. In the analysis, we therefore divided the year into four time periods, January–February, March–May, June–September, and October–December.
2.3. Outcome measures
We identified emergency CS with International Classification of Diseases 10th revision (ICD‐10) code O82.1 and elective CS with ICD‐10 code O82.0. IOL was identified with ICD‐10 code O83.8 and Nomesco Classification of Surgical Procedures codes MASC00, MAXC02, and MAXC09. Preterm birth was identified from gestational age at birth according to ultrasound and was categorized into severe (22+0–31+6 weeks) and moderate (32+0–36+6 weeks). Preterm birth was also categorized into spontaneous labor (including intact membranes and premature rupture of the membranes) and medically indicated (IOL or prelabor CS) according to the onset of labor. Information on stillbirth was obtained from the variable ‘born alive’ (yes/no) from the Medical Birth Register.
2.4. Covariates
Essential hypertension and pre‐existing diabetes mellitus were added as covariates in the models as they may increase the likelihood of preterm birth and birth interventions and have been increasing in recent years. They were defined with ICD‐10 codes O10.0, and O24.0 and O24.1, respectively. Hypertensive disorders in pregnancy and gestational diabetes were not added as covariates in the models, as they could be intermediates in the pathway between the lockdown measures and preterm birth and birth interventions, but the distribution is shown in Table 1. Hypertensive disorders in pregnancy were defined with ICD‐10 codes O11, O13, O14.0, O14, and O15 and gestational diabetes with ICD‐10 codes O24.4 and O24.9. Fetal growth restriction (identified with ICD‐10 code O36.5) was also not added in the models as it could be an intermediate in the pathway between the lockdown measures and medically indicated preterm birth, but the distribution is shown in Table 1. Information on parity, maternal age, country of origin, residential area, cohabitation, employment, and birthweight were obtained from variables in the Medical Birth Register.
TABLE 1.
Maternal and pregnancy characteristics in 2016–2019 and 2020 for 20 680 singleton births in Iceland.
|
2016–2019 (n = 16 280) |
2020 (n = 4400) |
|
|---|---|---|
| Maternal age, mean ± SD | 29.9 ± 5.3 | 30.0 ± 5.1 |
| Gestational age, mean ± SD | 39.3 ± 1.8 | 39.3 ± 1.8 |
| Parity, n (%) | ||
| Primipara | 7023 (43.1) | 2110 (48.0) |
| Multipara | 9357 (56.9) | 2290 (52.1) |
| Missing | 0 | 0 |
| Country of origin, n (%) | ||
| Iceland | 13 824 (84.9) | 3648 (82.9) |
| Other | 2456 (15.1) | 752 (17.1) |
| Missing | 0 | 0 |
| Residential area, n (%) | ||
| Capital area | 10 674 (65.6) | 2893 (65.8) |
| Outside capital area | 5606 (34.4) | 1507 (34.2) |
| Missing | 0 | 0 |
| Cohabitation, n (%) | ||
| Yes | 13 331 (81.9) | 3341 (75.9) |
| No | 2018 (12.4) | 400 (9.1) |
| Missing | 931 (5.7) | 659 (15.0) |
| Employment, n (%) | ||
| Employed | 12 560 (77.2) | 3248 (73.8) |
| Student | 1645 (10.1) | 432 (9.8) |
| Disability pension | 276 (1.7) | 64 (1.5) |
| Homemaker | 449 (2.8) | 94 (2.1) |
| Unemployed | 389 (2.4) | 184 (4.2) |
| Missing | 961 (5.9) | 378 (8.9) |
| Essential hypertension, n (%) | ||
| Yes | 233 (1.4) | 44 (1.0) |
| No | 16 047 (98.6) | 4356 (99.0) |
| Missing | 0 | 0 |
| Pre‐existing diabetes mellitus, n (%) | ||
| Yes | 122 (0.8) | 30 (0.7) |
| No | 16 158 (99.2) | 4370 (99.3) |
| Missing | 0 | 0 |
| Hypertensive disorders in pregnancy, n (%) | ||
| Yes | 1139 (7.0) | 369 (8.4) |
| No | 15 141 (93.0) | 4031 (91.6) |
| Missing | 0 | 0 |
| Gestational diabetes, n (%) | ||
| Yes | 2464 (15.1) | 722 (16.4) |
| No | 13 816 (84.9) | 3678 (83.6) |
| Missing | 0 | 0 |
| Fetal growth restriction, n (%) | ||
| Yes | 277 (1.7) | 80 (1.8) |
| No | 16 003 (98.3) | 4320 (98.2) |
| Missing | 0 | 0 |
| Birthweight (g), n (%) | ||
| ≥2500 | 15 780 (96.9) | 4276 (97.2) |
| <2500 | 500 (3.1) | 124 (2.8) |
| Missing | 0 | 0 |
2.5. Statistical analyses
We calculated rates as the number of CS/IOL/preterm births per 100 births each month for 2020 and for 2016–2019 combined. We also calculated rates of stillbirth in the same way (Table S1). We used generalized linear mixed models (proc glimmix) with binomial distribution and logit link to account for clustering due to correlation between births to the same mother. To assess changes in rates during lockdown periods, we calculated odds ratios, adjusted odds ratios (aOR) and 95% CI for the risk of CS/IOL/preterm birth during lockdown in 2020 compared with the same periods in 2016–2019. We adjusted the models for parity (primipara/multipara), maternal age (continuous), country of origin (Iceland, other), residential area (capital area, outside capital area), cohabitation (yes/no), employment (employed/student/homemaker/disability pension/unemployed), essential hypertension (yes/no), and pre‐existing diabetes mellitus (yes/no). The models for CS and IOL were additionally adjusted for gestational age at birth (continuous).
All analyses were conducted using the statistical software SAS version 9.4 (SAS Institute Inc.).
2.6. Ethical approval
This study was approved on October 13, 2020 by the National Bioethics Committee in Iceland (VSNb2020080003/03.01) and performed in accordance with the Declaration of Helsinki.
3. RESULTS
We identified 20 680 singleton births during 2016–2020 in Iceland. Table 1 shows the distribution of maternal and pregnancy characteristics in 2016–2019 and 2020. In 2020, there was a slightly higher proportion of primipara, women with a foreign country of origin, women who were unemployed, hypertensive disorders in pregnancy, and gestational diabetes (Table 1). There was a slightly lower proportion of women cohabiting with a partner, women with essential hypertension, and infants with low birthweight (Table 1).
Figures 1 and 2 show the monthly CS and IOL rates, respectively, for the year 2020, and the average for the years 2016–2019. The overall CS rate was lower in 2020 during Lockdown 1 (March–May), compared with 2016–2019 (aOR 0.81, 95% CI 0.66–0.99) (Table 2, Figure 1). It also appeared to be lower during Lockdown 2 (aOR 0.82, 95% CI 0.67–1.00). These reductions were mainly evident for elective CS, both during Lockdown 1 (aOR 0.71, 95% CI 0.51–0.99) and Lockdown 2 (aOR 0.72, 95% CI 0.52–0.99), but not for emergency CS (Table 2). We did not observe a change in the IOL rate during either lockdown in 2020 compared with the same periods in 2016–2019 (Table 2, Figure 2).
FIGURE 1.
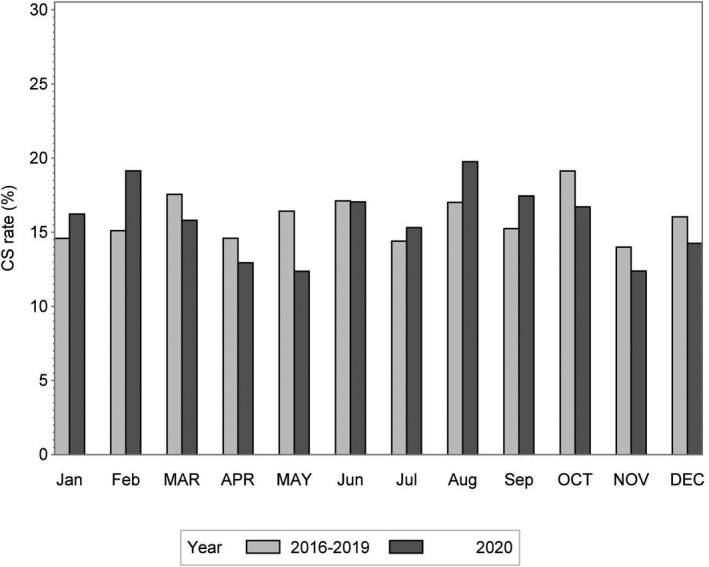
Monthly rate of cesarean section (CS) in 2020 and the average monthly rate in 2016–2019. Months during lockdown are written in uppercase letters
FIGURE 2.
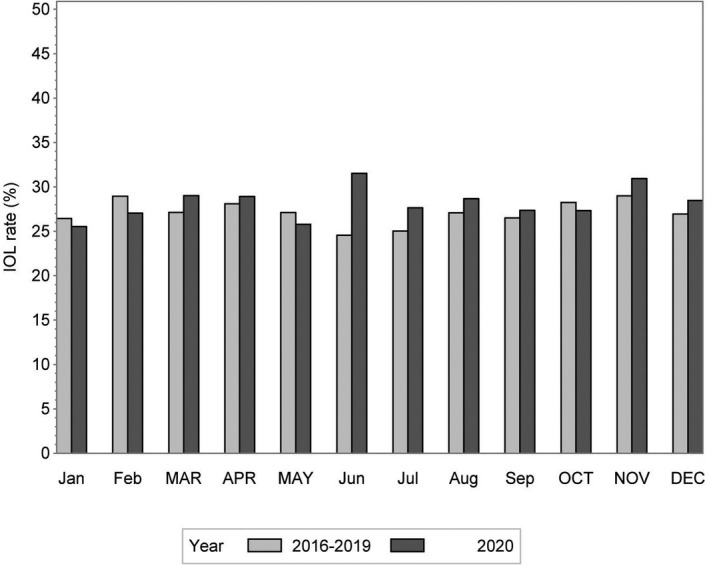
The monthly induction of labor (IOL) rate in 2020 and the average monthly rate in 2016–2019. Months during lockdown are written in uppercase letters
TABLE 2.
Risk of CS, stratified by CS type, and IOL in 2020, with lockdown periods in March–May and October–December, compared with the same periods in 2016–2019
|
2016–2019 n (%) |
2020 n (%) |
OR (95% CI) | AOR (95% CI)a | |
|---|---|---|---|---|
| Overall CS | ||||
| Jan–Feb | 363 (14.8) | 124 (17.6) | 1.22 (0.98–1.53) | 1.22 (0.96–1.54) |
| Mar–May | 643 (16.2) | 145 (13.7) | 0.82 (0.67–1.00) | 0.81 (0.66–0.99) |
| Jun–Sep | 932 (15.9) | 278 (17.4) | 1.11 (0.96–1.30) | 1.14 (0.98–1.33) |
| Oct–Dec | 663 (16.5) | 152 (14.6) | 0.87 (0.71–1.05) | 0.82 (0.67–1.00) |
| Emergency CS | ||||
| Jan–Feb | 212 (8.7) | 82 (11.6) | 1.39 (1.06–1.82) | 1.36 (1.02–1.80) |
| Mar–May | 388 (9.8) | 98 (9.3) | 0.94 (0.74–1.19) | 0.90 (0.70–1.15) |
| Jun–Sep | 572 (9.8) | 171 (10.7) | 1.11 (0.92–1.33) | 1.09 (0.90–1.32) |
| Oct–Dec | 394 (9.8) | 101 (9.7) | 0.99 (0.79–1.25) | 0.90 (0.70–1.15) |
| Elective CS | ||||
| Jan–Feb | 152 (6.2) | 42 (6.0) | 0.96 (0.67–1.36) | 0.94 (0.65–1.36) |
| Mar–May | 255 (6.5) | 47 (4.4) | 0.67 (0.49–0.93) | 0.71 (0.51–0.99) |
| Jun–Sep | 360 (6.2) | 107 (6.7) | 1.10 (0.88–1.38) | 1.19 (0.94–1.50) |
| Oct–Dec | 269 (6.7) | 51 (4.9) | 0.72 (0.53–0.98) | 0.72 (0.52–0.99) |
| IOL | ||||
| Jan–Feb | 676 (27.6) | 185 (26.2) | 0.93 (0.77–1.13) | 0.96 (0.79–1.16) |
| Mar–May | 1087 (27.4) | 295 (27.8) | 1.02 (0.88–1.19) | 1.03 (0.89–1.21) |
| Jun–Sep | 1514 (25.9) | 458 (28.7) | 1.15 (1.02–1.31) | 1.16 (1.02–1.32) |
| Oct–Dec | 1128 (28.1) | 299 (28.8) | 1.03 (0.89–1.20) | 1.08 (0.92–1.26) |
Abbreviations: aOR, adjusted odds ratio; CS, cesarean section; IOL, induction of labor; OR, odds ratio.
Adjusted for parity, maternal age, gestational age at birth, country of origin, residential area, cohabitation, employment, essential hypertension, and pre‐existing diabetes mellitus.
The overall preterm birth rate was significantly lower in 2020 during Lockdown 1 (aOR 0.69, 95% CI 0.49–0.97) and in the following months of June–September (aOR 0.67, 95% CI 0.49–0.89) compared with 2016–2019 (Figure 3, Table 3). This reduction was also evident for moderate preterm birth (32–36 weeks), but not severe preterm birth (22–31 weeks). The reduction in preterm birth rates during Lockdown 1 was mainly evident for medically indicated preterm birth (aOR 0.61, 95% CI 0.36–1.02), although not statistically significant, and the reduction during June–September was mainly evident for spontaneous preterm birth (aOR 0.52, 95% CI 0.33–0.83) (Table 3). We also assessed stillbirth in this study, but found that the numbers were too low to calculate meaningful effect measures (Table S1).
FIGURE 3.

Monthly rate of preterm birth (22–36 weeks) in 2020 and average rate in 2016–2019. Months during lockdown are written in uppercase letters
TABLE 3.
Risk of preterm birth in 2020, with lockdown periods in March–May and October–December, compared with the same periods in 2016–2019, stratified by severity and type of preterm birth
| Preterm birth |
2016–2019 n preterm (%) |
2020 n preterm (%) |
OR (95% CI) | AOR (95% CI)a |
|---|---|---|---|---|
| Overall 22–36 wk | ||||
| Jan–Feb | 107 (4.4) | 28 (4.0) | 0.90 (0.59–1.39) | 0.87 (0.56–1.35) |
| Mar–May | 221 (5.6) | 43 (4.1) | 0.72 (0.51–1.00) | 0.69 (0.49–0.97) |
| Jun–Sep | 303 (5.2) | 58 (3.6) | 0.69 (0.52–0.92) | 0.67 (0.49–0.89) |
| Oct–Dec | 180 (4.5) | 58 (5.6) | 1.22 (0.90–1.66) | 1.22 (0.89–1.67) |
| Severe 22–31 wk | ||||
| Jan–Feb | 14 (0.6) | 6 (0.9) | 1.49 (0.57–3.89) | 1.35 (0.50–3.63) |
| Mar–May | 27 (0.7) | 9 (0.9) | 1.25 (0.58–2.69) | 1.15 (0.53–2.48) |
| Jun–Sep | 58 (1.0) | 11 (0.7) | 0.69 (0.36–1.32) | 0.61 (0.31–1.18) |
| Oct–Dec | 26 (0.7) | 10 (1.0) | 1.49 (0.72–3.10) | 1.45 (0.69–3.08) |
| Moderate 32–36 wk | ||||
| Jan–Feb | 93 (3.8) | 22 (3.1) | 0.81 (0.51–1.31) | 0.79 (0.49–1.29) |
| Mar–May | 194 (4.9) | 34 (3.2) | 0.64 (0.44–0.94) | 0.63 (0.43–0.92) |
| Jun–Sep | 245 (4.2) | 47 (2.9) | 0.69 (0.51–0.95) | 0.69 (0.50–0.95) |
| Oct–Dec | 159 (4.0) | 48 (4.6) | 1.17 (0.84–1.63) | 1.18 (0.84–1.66) |
| Spontaneous 22–36 wk | ||||
| Jan–Feb | 45 (2.8) | 17 (3.6) | 1.28 (0.72–2.26) | 1.18 (0.66–2.12) |
| Mar–May | 105 (4.1) | 24 (3.4) | 0.83 (0.53–1.31) | 0.75 (0.48–1.19) |
| Jun–Sep | 149 (3.8) | 22 (2.2) | 0.57 (0.36–0.89) | 0.52 (0.33–0.83) |
| Oct–Dec | 88 (3.4) | 26 (3.9) | 1.14 (0.73–1.79) | 1.19 (0.75–1.88) |
| Medically indicated 22–36 wk | ||||
| Jan–Feb | 62 (7.3) | 11 (4.8) | 0.64 (0.33–1.25) | 0.63 (0.32–1.24) |
| Mar–May | 116 (8.5) | 19 (5.5) | 0.63 (0.38–1.04) | 0.61 (0.36–1.02) |
| Jun–Sep | 154 (8.1) | 35 (6.1) | 0.74 (0.50–1.08) | 0.75 (0.50–1.11) |
| Oct–Dec | 97 (6.8) | 31 (8.6) | 1.28 (0.84–1.96) | 1.21 (0.79–1.88) |
Abbreviations: aOR, adjusted odds ratio; OR, odds ratio.
Adjusted for parity, maternal age, country of origin, residential area, cohabitation, employment, essential hypertension, and pre‐existing diabetes mellitus.
4. DISCUSSION
In this nationwide study from Iceland, we investigated CS, IOL, and preterm birth rates during two COVID‐19 lockdown periods in Iceland and compared them with rates in 2016–2019. We observed a reduction in elective CS rates during the first lockdown (March–May) and second lockdown (October–December) in 2020 compared with 2016–2019. Our results also suggested a reduction in overall preterm birth rates during the first lockdown, mainly evident for medically indicated preterm birth rates, and a reduction in spontaneous preterm birth rates during the following months (June–September).
Previous studies of CS rates during the COVID‐19 pandemic lockdown have not found a reduction in CS rates,3, 4 on the contrary, one study found a slight increase in rates during lockdown compared with the year before.4 However, neither study explored CS rates according to CS type, ie whether it was an emergency or an elective CS. Our study indicated a reduction in CS rates only for elective CS. This might reflect an overall reduction in non‐urgent surgeries during lockdown, evidenced by all non‐urgent surgeries being cancelled in many places, for example England1 and Iceland, during the most stringent lockdown. A study from the UK found a reduction in non‐urgent surgical admissions following lockdown compared with the previous year.2 Explanations for reductions in non‐urgent admissions might include an increased threshold for patient admission during the COVID‐19 pandemic lockdowns as COVID‐19‐related workload was increased among all healthcare staff. For maternity care, this might be reflected in a decision to not perform elective CS in instances where this possible without increasing the risk for the mother and infant (ie CS for maternal request, for psychosocial indications, or maternal discomfort). In fact, recent research from Iceland suggests that the majority of early‐term elective CS can be postponed safely, as a medical indication was only present in 35% of elective early‐term CS.16 An overall call for reduction in non‐urgent surgeries may therefore have led to a more critical view of medical indications for elective CS, resulting in fewer elective CS during the lockdown. Interestingly, our data did not suggest that emergency CS or IOL increased in response to the reduction in elective CS. Our data therefore suggest that lowering the elective CS rate may be an effective way in reducing the overall CS rate. This supports our hypothesis that the prioritization in maternity care during COVID‐19 pandemic lockdown might be the reason for the reduction in elective CS in our study.
Results regarding preterm birth rates during COVID‐19 lockdowns are conflicting.5, 6, 7, 8, 9, 10, 11 A recent meta‐analysis of 40 studies from January 1, 2020 to January 8, 2021 found a reduction in the overall preterm birth rate for high income countries (12 studies) as an effect of COVID‐19 pandemic lockdowns.12 This reduction appeared to be driven by a reduction in spontaneous preterm birth.12 The reason for this improvement in preterm birth rates is not known but could be related to improved air quality17, 18 and/or reduced maternal non‐COVID‐19 related infections during lockdown, particularly for spontaneous preterm birth. However, the authors of the meta‐analysis conclude that it is also likely that changes in population behaviors and healthcare delivery are contributing factors.12 Few studies in the meta‐analysis included information on both stillbirth and preterm birth,12 but some have suggested that a reduction of preterm birth might come at the cost of an increase in stillbirth. Due to the small population size in Iceland and the low rate of stillbirth, we were not able to reliably detect an effect of the COVID‐19 pandemic lockdowns on the risk of stillbirth.
A reduction in medically indicated preterm birth during COVID‐19 lockdown could be due to a reduction in non‐urgent surgeries.2 Our results suggested a reduction in medically indicated preterm birth rates during the first lockdown, which could reflect the reduction in elective CS rates we also found during the same period, although the results were not statistically significant. However, we did not find a reduction in medically indicated preterm birth rates during the second lockdown, which is surprising, as the lockdown measures during these two periods were very similar. The reduction in spontaneous preterm birth rates observed during the months following the first lockdown could possibly reflect a delayed effect of improved air quality17, 18 and reduced maternal infections during the lockdowns, but further research is needed to shed light on these hypotheses.
The strength of this study is reflected in the population‐based design and the fact that we were able to include all births in Iceland during the study period. Iceland has universal health care with equal access to high‐quality maternity care. The weakness, however, is reflected in the fact that the data included few births, due to the small size of the Icelandic population. This resulted in our inability to obtain reliable results for extreme or severe preterm births. Furthermore, we did not have the exact date of birth in our data, only the month of birth, so could not calculate the birth rate for the exact dates of the lockdown periods.
5. CONCLUSION
In conclusion, we found a reduction in elective CS rates during COVID‐19 pandemic lockdown in Iceland during 2020, compared with the 4 previous years. We did not observe an increase in emergency CS or IOL in response to the reduction in elective CS. These findings likely reflect changes in prioritization of non‐urgent procedures during lockdown periods. We also found a reduction in overall preterm birth rates during the first lockdown period and in spontaneous preterm birth rates in the months immediately following the first lockdown. Further research is needed to shed light on the possible underlying mechanisms for these findings.
AUTHOR CONTRIBUTIONS
KE analyzed the data and wrote the article, EMS provided obstetric advice, and HZ oversaw the project. All authors read and approved the manuscript.
Supporting information
Table S1
ACKNOWLEDGMENT
We thank the Icelandic Directorate of Health for providing the data.
Einarsdóttir K, Swift EM, Zoega H. Changes in obstetric interventions and preterm birth during COVID‐19: A nationwide study from Iceland. Acta Obstet Gynecol Scand. 2021;100:1924–1930. 10.1111/aogs.14231
Funding information
HZ was supported by a University of New South Wales Scientia Fellowship. No other funding was obtained for this study.
REFERENCES
- 1.Iacobucci G. Covid‐19: all non‐urgent elective surgery is suspended for at least three months in England. BMJ. 2020;368:m1106. [DOI] [PubMed] [Google Scholar]
- 2.Callan R, Assaf N, Bevan K. Impact of the COVID‐19 pandemic on acute general surgical admissions in a district general hospital in the United Kingdom: a Retrospective Cohort study. Surg Res Pract. 2020;2020:2975089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M, Yin H, Jin Z, et al. Impact of Wuhan lockdown on the indications of cesarean delivery and newborn weights during the epidemic period of COVID‐19. PLoS One. 2020;15:e0237420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia K, Columb M, Bewlay A, et al. The effect of COVID‐19 on general anaesthesia rates for caesarean section. A cross‐sectional analysis of six hospitals in the north‐west of England. Anaesthesia. 2021;76:312‐319. [DOI] [PubMed] [Google Scholar]
- 5.Hedermann G, Hedley PL, Bækvad‐Hansen M, et al. Danish premature birth rates during the COVID‐19 lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106:93‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemon L, Edwards RP, Simhan HN. What is driving the decreased incidence of preterm birth during the coronavirus disease 2019 pandemic? Am J Obstet Gynecol MFM. 2021;3:m1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matheson A, McGannon CJ, Malhotra A, et al. Prematurity Rates During The Coronavirus Disease 2019 (COVID‐19) pandemic lockdown in Melbourne, Australia. Obstet Gynecol. 2021;137:405‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philip RK, Purtill H, Reidy E, et al. Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID‐19 lockdown in Ireland: a ‘natural experiment’ allowing analysis of data from the prior two decades. BMJ Glob Health. 2020;5:e003075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnaez J, Ochoa‐Sangrador C, Caserío S, et al. Lack of changes in preterm delivery and stillbirths during COVID‐19 lockdown in a European region. Eur J Pediatr. 2021;180:1997‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du M, Yang J, Han N, Liu M, Liu J. Association between the COVID‐19 pandemic and the risk for adverse pregnancy outcomes: a cohort study. BMJ Open. 2021;11:e047900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood R, Sinnott C, Goldfarb I, Clapp M, McElrath T, Little S. Preterm birth during the coronavirus disease 2019 (COVID‐19) pandemic in a large hospital system in the United States. Obstet Gynecol. 2021;137:403‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chmielewska B, Barratt I, Townsend R, et al. Effects of the COVID‐19 pandemic on maternal and perinatal outcomes: a systematic review and meta‐analysis. Lancet Glob Health. 2021;9:e759‐e772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stock SJ, Zoega H, Brockway M, et al. The international Perinatal Outcomes in the Pandemic (iPOP) study: protocol. Wellcome Open Research. 2021;6:21. 10.12688/wellcomeopenres.6507.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Icelandic Directorate of Health‐Covid‐19 information. 2020. Available from: https://www.covid.is/tolulegar‐upplysingar.
- 15.McGuinness MJ, Hsee L. Impact of the COVID‐19 national lockdown on emergency general surgery: Auckland City Hospital's experience. ANZ J Surg. 2020;90:2254‐2258. [DOI] [PubMed] [Google Scholar]
- 16.Vigdis Rikhardsdottir J, Hardardottir H, Thorkelsson T. The majority of early term elective cesarean sections can be postponed. J Maternal‐Fetal Neonatal Med. 2019;2:1‐6. [DOI] [PubMed] [Google Scholar]
- 17.Briz‐Redon A, Belenguer‐Sapina C, Serrano‐Aroca A. Changes in air pollution during COVID‐19 lockdown in Spain: a multi‐city study. J Environ Sci (China). 2021;101:16‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu CL, Wang HW, Cai WJ, He HD, Ni AN, Peng ZR. Impact of the COVID‐19 lockdown on roadside traffic‐related air pollution in Shanghai, China. Build Environ. 2021;194:107718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


