Abstract
Thyroid hormone-clearing type 3 deiodinase is located in spermatogonia, where it may serve as a critical modulator of the thyroid hormone exposure of the male germ line and its epigenetic information, with implications for neurodevelopmental and endocrine disorders in subsequent generations.
Keywords: thyroid hormone, type 3 deiodinase, DIO3, spermatogonia, intergenerational epigenetics, neurodevelopmental disorders, endocrine function
The origins of complex human conditions (obesity, infertility, autism, and schizophrenia, to name a few) remain to be fully elucidated. Despite their relatively high heritability, genetic factors alone can only account for a relatively low percentage of clinical cases. In recent years, investigators have turned to the realm of epigenetics to identify potential nongenetic mechanisms that contribute to the missing heritability of human disease [1–3]. We have learned that male germ cells, previously thought to serve as mere vessels for the paternal genetic code, carry a wealth of epigenetic information (the “epigenome”) in the form of DNA chemical modifications, chromatin organization, noncoding RNAs and metabolites. After fecundation, this complement of nongenetic information is able to influence developmental processes in the next generation, ultimately affecting biological traits of relevance to human conditions. Moreover, germ cell epigenetic information is malleable, and can be modified by an array of environmental factors [2]. This malleability provides a mechanism by which the life context of progenitors and former ancestors affects pathological traits in the present generation, potentially explaining the unidentified heritability of disease.
Interestingly, many of the factors described so far to exert intergenerational epigenetic effects are related to cell signaling through nuclear receptors [4]. These receptors function as DNA-binding, chromatin-modifying gene transcription factors that are activated by small lipophilic compounds and hormones, including sex steroids, glucocorticoids, thyroid hormones, and fatty acid derivatives. Some chemicals in our environment (“endocrine disruptors”) mimic the structure of the endogenous molecules that normally activate these receptors [2]. As a result, excessive exposure to endocrine disruptors can alter the physiology and action of the endogenous molecules they most resemble. In this regard, many published paradigms of intergenerational epigenetic effects involve exposure to chemicals that can bind to the androgen, estrogen, or glucocorticoid receptors. Other paradigms involve models of stress or altered diet or nutritional status that may affect, respectively, signaling through the glucocorticoid receptor or other nuclear receptors activated by lipidic-like compounds.
Since thyroid hormones (TH) also signal through nuclear receptors, it is not surprising that altered thyroid hormone levels are of phenotypic consequence for descendants. In the mid-seventies, long before the word “epigenetic” was commonly used, Bakke et al. published pioneering work showing intergenerational epigenetic effects in rat models of transient neonatal thyrotoxicosis and adult hypothyroidism [5]. These conditions were modeled, respectively, by pharmacological administration of TH and by adult thyroidectomy. The offspring of those animals exhibited abnormalities in developmental maturation, growth, and endocrine organs, especially those involved the regulation of the hypothalamic–pituitary–thyroid endocrine axis [5].
To examine the intergenerational epigenetic effects of TH, we have used an efficient mouse model of developmental thyrotoxicosis based on type 3 deiodinase (DIO3) deficiency. DIO3 inactivates TH and is highly expressed in the uterine-feto-placental unit. Thus, DIO3 loss results in impaired TH clearance and subsequent thyrotoxicosis from fetal to early neonatal stages [6]. Wild type mice born to wild type mothers that were F2 generation descendants of male or female DIO3 deficient mice exhibit common patterns of altered gene expression in the neonatal brain that are associated with behavioral changes in adult life [6]. It is intriguing that the sperm methylome of DIO3-deficient males reveals an epigenetic signature of hypomethylation of CpG islands associated with the promoters of genes related to autism, cancer, schizophrenia, and addiction [6]. Support for a role of TH in the epigenetic inheritance of disease susceptibility in humans is provided by the work performed by Anselmo et al. on a unique Azorean population. Their study shows that overexposure in utero to thyroid hormone secondary to TH receptor beta haploinsufficiency leads to altered pituitary sensitivity to thyroid hormone in the individuals affected, as well as in F1 and F2 generation descendants along the paternal lineage [7]. Taken together, these observations in rodents and humans raise the possibility that abnormalities in ancestral thyroid hormone states, which can occur because of prevalent thyroid disease or exposure to chemicals that disrupt TH physiology, could contribute to the heritability of neurodevelopmental disorders and potentially other conditions.
That the Dio3 gene itself could be an integral part of the mechanisms underlying the intergenerational effects of TH is supported by the fact that it is highly expressed in the developing testis, where it largely localizes to undifferentiated spermatogonia [8]. Thus, the Dio3 in spermatogonia might be critical for the maintenance of the appropriate level of TH action in the germ line and its associated epigenetic landscape. Furthermore, Dio3 is subject to genomic imprinting, an epigenetic phenomenon that leads to preferential or exclusive allelic expression, depending on the parental origin of the allele. As such, Dio3 is preferentially expressed from the paternally inherited allele in most developing tissues, but not in the testis [9]. Because of its normal regulation by TH and by environmentally-sensitive epigenetic mechanisms related to genomic imprinting [10], Dio3 is uniquely positioned to function as a sensor of TH states and as an important modifier of the epigenetic information that the sperm will ultimately carry to the next generation.
The work of Bakke and Anselmo groups [5, 7] identifies the set point of the thyroid axis (and the subsequent TH output from the thyroid gland) as a common outcome of the intergenerational effects of TH. This suggests that there is a physiological mechanism by which the programming of TH homeostasis is continually and epigenetically adjusted across generations based on the TH levels and associated germ line exposure that occurred in ancestors (Figure 1). It is also possible that an abnormal level of TH action in previous generations produces a germ line epigenetic signature that modifies in subsequent generations the baseline and regulatory range of TH target genes, with pathophysiological consequences. The potential mediation of Dio3 in these mechanisms may have intriguing implications for evolution and environmental adaptation. Genomic imprinting is estimated to have arisen at a time of rapid mammalian speciation. Thus, variable allelic expression of Dio3 and subsequent level of germ line exposure to TH may have been a driver for the evolution and environmental adaptation of metabolic and neurological functions, which are markedly regulated by TH.
Figure 1.
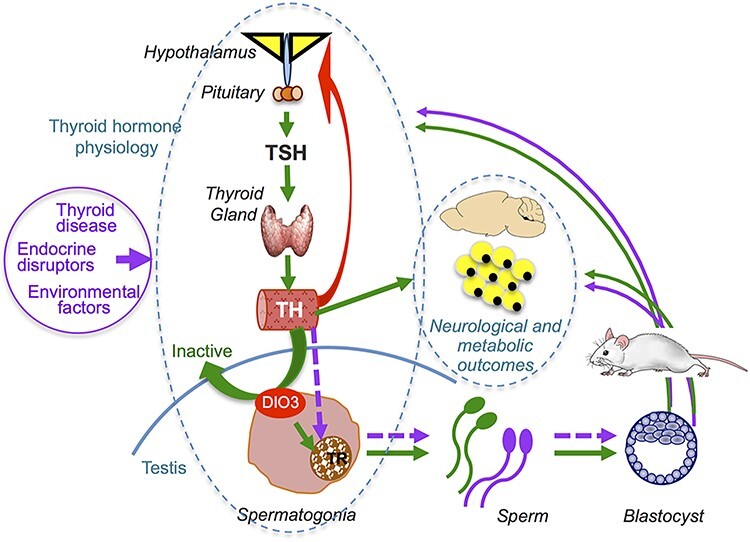
Working model for the cycle of intergenerational epigenetic effects of thyroid hormones along the male lineage. In normal physiology, TH are secreted by the thyroid gland under the control of TSH pituitary output, which is regulated by the hypothalamus (green arrows). TH will have a central negative feedback effect on the axis (red arrow) to maintain circulating TH levels in a narrow range. DIO3 modulates TH action in spermatogonia, allowing an adequate level of TH action on the thyroid receptors, resulting in normal epigenetic information in the mature sperm (green arrows). Exogenous factors (circled in purple) may alter thyroid hormone physiology at different levels, causing abnormal spermatogonial exposure to TH and changes in sperm epigenetic information, ultimately impacting phenotypic outcomes in the next generation (purple arrows). These outcomes may include the programming of thyroid hormone physiology and DIO3 expression, which will repeat the cycle. TSH, thyroid stimulating hormone; TH, thyroid hormones; TR, thyroid hormone receptors; DIO3, type 3 deiodinase.
Much is still unknown about the underlying TH-dependent cellular and molecular processes that modify the epigenetic information of the male (and female) germ line. Likewise, the principal phenotypes affected in subsequent generations, their adaptive or maladaptive nature and their parental mode of inheritance remain to be fully elucidated. Given the pleiotropic effects of TH and their essential role in regulating metabolism and brain development, it is reasonable to speculate that their intergenerational effects will also impact those critical biological processes, with important implications for the etiology of metabolic and neurodevelopmental disorders.
Acknowledgements
I am grateful to Valerie Anne Galton for critically reviewing the manuscript.
Footnotes
Grant Support: This work was supported by the National Institute of Mental Health (MH096050) and the National Institute of Diabetes, Digestive and Kidney Disease (DK095908).
Funding
National Institute of Mental Health and National Institute of Diabetes, Digestive and Kidney Diseases.
Conflict of interest
The author has nothing to disclose.
Data availability
There is no new data associated with this article.
References
- 1.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE et al. Finding the missing heritability of complex diseases. Nature 2009; 461:747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner MK. Endocrine disruptors in 2015: Epigenetic transgenerational inheritance. Nat Rev Endocrinol 2016; 12:68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohacek J, Mansuy IM. Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nat Rev Genet 2015; 16:641–652. [DOI] [PubMed] [Google Scholar]
- 4.Ozgyin L, Erdos E, Bojcsuk D, Balint BL. Nuclear receptors in transgenerational epigenetic inheritance. Prog Biophys Mol Biol 2015; 118:34–43. [DOI] [PubMed] [Google Scholar]
- 5.Bakke JL, Lawrence NL, Robinson S, Bennett J. Endocrine studies in the untreated F1 and F2 progeny of rats treated neonatally with thyroxine. Biol Neonate 1977; 31:71–83. [DOI] [PubMed] [Google Scholar]
- 6.Martinez ME, Duarte CW, Stohn JP, Karaczyn A, Wu Z, DeMambro VE, Hernandez A. Thyroid hormone influences brain gene expression programs and behaviors in later generations by altering germ line epigenetic information. Mol Psychiatry 2020; 25:939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anselmo J, Scherberg NH, Dumitrescu AM, Refetoff S. Reduced sensitivity to thyroid hormone as a transgenerational epigenetic marker transmitted along the human male line. Thyroid 2019; 29:778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez ME, Lary CW, Karaczyn AA, Griswold MD, Hernandez A. Spermatogonial type 3 deiodinase regulates thyroid hormone target genes in developing testicular somatic cells. Endocrinology 2019; 160:2929–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez ME, Charalambous M, Saferali A, Fiering S, Naumova AK, St Germain D, Ferguson-Smith AC, Hernandez A. Genomic imprinting variations in the mouse type 3 deiodinase gene between tissues and brain regions. Mol Endocrinol 2014; 28:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet 2007; 8:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no new data associated with this article.


