Abstract
Aim
We evaluated the prevalence of paediatric severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infections using antibody testing and characterised antibody titres by time from exposure.
Methods
This was a single‐centre, prospective, cross‐sectional cohort study. Patients under 18 years old were eligible to participate if they attended the paediatric emergency department at the tertiary Shaare Zedek Medical Center, Jerusalem, Israel, from 18 October 2020 to 12 January 2021 and required blood tests or intravenous access. SARS‐CoV‐2 seropositivity and antibody levels were tested by a dual‐assay model.
Results
The study comprised 1138 patients (56% male) with a mean age of 4.4 years (interquartile range 1.3–11.3). Anti‐SARS‐CoV‐2 antibodies were found in 10% of the patients. Seropositivity increased with age and 41% of seropositive patients had no known exposure. Children under 6 years of age had higher initial antibody levels than older children, followed by a steeper decline. The seropositivity rate did not vary during the study, despite schools re‐opening. The findings suggest that children's immunity may start falling 4 months after the initial infection.
Conclusion
Immunity started falling after just 4 months, and re‐opening schools did not affect infection rates. These findings could aid decisions about vaccinating paediatric populations and school closures.
Keywords: antibody levels, asymptomatic infection, coronavirus, immunity, school closures
Abbreviations
- AU
arbitrary units
- IgG
immunoglobulin G
- PCR
polymerase chain reaction
- PED
paediatric emergency department
Key Notes.
This prospective, cross‐sectional study found anti‐severe acute respiratory syndrome coronavirus 2 antibodies in 10% of the children sampled, and 41% of the seropositive patients had no known exposure to the virus.
Antibody levels began to fall four months after the initial infection and the seropositivity rate did not rise when schools re‐opened.
These findings are important when making decisions about vaccinating paediatric populations and ongoing school closures.
1. BACKGROUND
COVID‐19 is a highly infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and it has become a major global public health challenge. 1 The disease is usually mild in children, and mortality and morbidity are significantly lower than in adults. 2 , 3 Most children will have mild symptoms or asymptomatic infections, 4 , 5 with younger children and chronic illness being the major risk factors for a more serious disease course. 6 However, due to viral shedding, children may have significant potential to transmit the virus to other vulnerable populations, even if they have mild or no symptoms. 7 Furthermore, children may go on to develop multisystem inflammatory syndrome in children, 8 a syndrome that affects primarily school children. This syndrome is characterised by elevated inflammatory markers and can involve gastrointestinal, cardiovascular, haematopoietic and respiratory systems, as well as significant dermatological manifestations. 8
The internationally accepted gold standard for identifying SARS‐CoV‐2 infections is real‐time polymerase chain reaction (PCR) tests using nasopharyngeal swab samples. 9 Serology testing is not appropriate for identifying acute COVID‐19 and is primarily used for epidemiological purposes. 10 The median time from a positive PCR to immunoglobulin G (IgG) seroconversion in children is 18 days, 11 which is longer than the six to 14 days in adults. 12 As a result, the sensitivity of SARS‐CoV‐2 serology assays have only been 57%–70% if performed less than 14 days from the time of infection. 12 The clinical significance of anti‐SARS‐CoV‐2 antibody titre values is still being explored. Several studies have found that a correlation may exist between the severity of the primary disease, peak antibody titre values and the neutralising effect of these antibodies. 13 However, uncertainty remains regarding the long‐term serological response in children.
The purpose of this study was to evaluate the incidence of paediatric asymptomatic SARS‐CoV‐2 infections in Jerusalem, Israel, by using SARS‐CoV‐2 IgG antibody testing. In addition, when patients had a known history of SARS‐CoV‐2 infection, we sought to characterise antibody titres by the time from a positive PCR result and by the severity of the primary disease. Finally, we wanted to study the effect of closing schools on COVID‐19 infections in children and adolescents under 18 years of age.
2. METHODS
2.1. Study design and population
A prospective, cross‐sectional epidemiological survey was conducted between 18 October 2020 and 12 January 2021 in the paediatric emergency department (PED) of the Shaare Zedek Medical Center, a public, tertiary medical centre in Jerusalem, Israel. The area covered by the hospital had a high prevalence at the time of the study, with more than 4500 cumulative cases per one hundred thousand population. All patients under 18 years of age who presented to the PED during the study period were eligible to participate if they required blood tests or intravenous access for any clinical reason. Parents and, or, legal guardians gave oral consent for subjects to participate in the study. The study was approved by the hospital's Institutional Research Ethics Board (reference number 0387‐20‐SZMC). Children under the age of three months were later excluded from the analysis, as antibodies detected in their blood may have been maternal antibodies that transferred though the placenta in‐utero. Patients with blood samples that were unsuitable for laboratory testing, for technical reasons, were also excluded from the final analysis (Figure 1).
FIGURE 1.
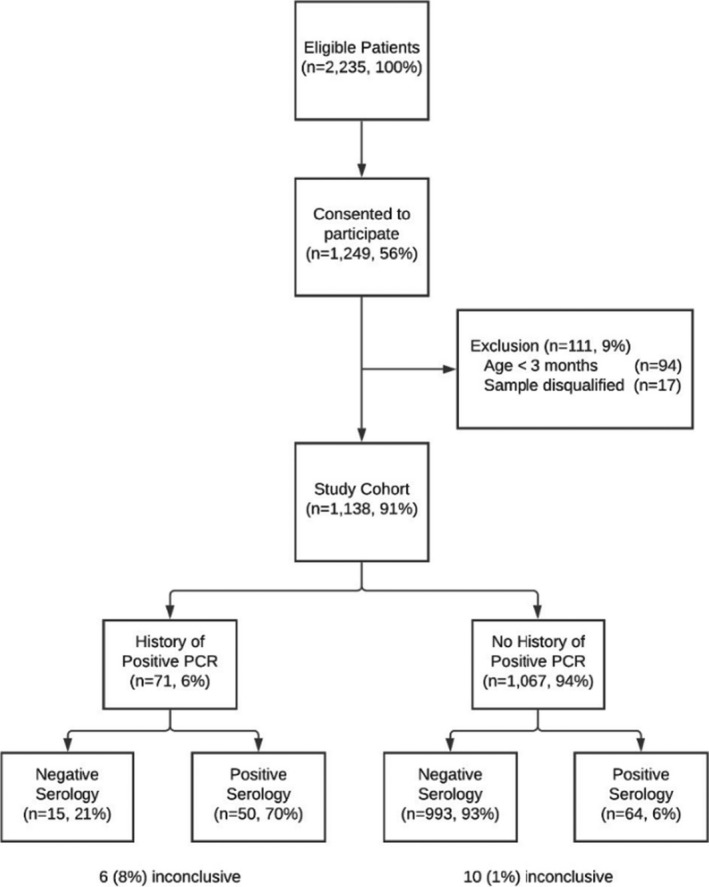
Study flow diagram. History of a positive PCR indicates a prior positive SARS‐CoV‐2 PCR on a nasopharyngeal swab test at the time of enrolment. Serology was only considered positive if antibodies were detected by both the Abbott Architect (anti‐n protein IgG antibodies) and DiaSorin Liaison (anti‐spike IgG antibodies) assays
The accompanying parent or guardian filled out a comprehensive questionnaire in Hebrew or Arabic for each patient. This covered the patient's demographic background, their past medical history and specific COVID‐19 information, such as their exposure to any individuals with the virus, previous PCR testing for SARS‐CoV‐2 and symptoms (Figure S1). All patients with positive serology results were contacted by telephone and given their results. Details from the questionnaire were confirmed during the same phone call, in particular the timing and source of the confirmed, or suspected, SARS‐CoV‐2 infection.
2.2. Laboratory testing
All the blood samples were collected in a clot‐activator test tube. Initial testing was conducted using the Abbott Architect SARS‐CoV‐2 IgG assay (Abbott Laboratories, Illinois, USA) according to the manufacturer's instructions. The presence or absence of IgG antibodies against the SARS‐CoV‐2 nucleocapsid protein was calculated by the system as an index of the chemiluminescent relative light unit in the reaction to the calibrator relative light unit. An index of 1.4 or greater was considered a positive result. All samples with positive or borderline (index 0.5–1.4) results were retested using the DiaSorin Liaison SARS‐CoV‐2 S1/S2 IgG assay (DiaSorin Spa, Piedmont, Italy), which tests antibodies against the spike protein of the virus. This assay calculates antibody concentrations, expressed as arbitrary units per millilitre (AU/mL): an AU/mL of 15 or greater is considered a positive result and 12–15 AU/mL is borderline. We used a dual‐assay model in this study, which means that a patient was only considered positive for SARS‐CoV‐2 IgG antibodies if both assay tests produced a positive result. A positive Abbott assay, with a subsequent negative or borderline DiaSorin assay, or vice‐versa, were considered inconclusive. All other results were considered negative. The manufacturers established that the tests’ sensitivity and specificity approached 100% after 14 days from infection. Accordingly, seropositivity rates were not adjusted for imperfect test performance.
2.3. Statistical analysis
The statistical analyses were conducted using SPSS Statistics for Windows, version 25.0. (IBM Corp, New York, USA). Continuous normally distributed variables, according to the Shapiro–Wilk test, were compared using the Student's t‐test or one‐way analysis of variance and presented as means and standard deviations (SD) and 95% confidence intervals (CI). Non‐normally distributed variables were compared using the Mann–Whitney U test and presented as medians with their corresponding interquartile range (IQR). Categorical variables were compared using the chi‐square test or Fisher's exact test and presented as percentages with absolute values. Statistical tests were conducted using two‐sided tests and p values of 0.05 or less were considered statistically significant. Finally, a multivariable logistic regression analysis was performed to study the risk factors for SARS‐CoV‐2 infection. The model's dependent variable was evidence of SARS‐CoV‐2 infection, due to either a positive serology test or a history of a positive SARS‐CoV‐2 PCR test. The independent variables were: gender, age, religion, ethnicity, number of parents and children in the home, housing density and if the child's educational institution remained open during lockdowns.
3. RESULTS
There were 6264 patients treated in the PED during the 90‐day study period. Blood tests and, or, intravenous access were obtained for 2235 patients (35%) and the parent or guardian of 1249 patients (56%) provided consent to participate in the study. We excluded 111 patients (9%) and these comprised 94 infants under the age of 3 months and 17 with blood samples that were rejected by the laboratory. This means that there were 1138 patients (56% male) in the final study group (Figure 1), with a median age of 4.4 years (IQR 1.3–11.3). The cohort was similar to the general PED population during the study period (median age 4.6 [IQR 1.3–11.6] and 55.9% male, p = 0.49 and p = 0.78 respectively). Additional socio‐demographic information is presented in Table 1.
TABLE 1.
Demographics and characteristics of the patients, with a comparison of seropositive and seronegative patients
| Characteristic | All patients | Patients with positive serology results (dual assay) | Patients with negative serology results | p Value |
|---|---|---|---|---|
| n = 1138 (100%) | n = 114 (10%) | n = 1008 (88.6%) | ||
| Age median [IQR] | 4.4 [1.3–11.3] | 7.6 [2.6–14.9] | 3.8 [1.2–10.8] | <0.001 |
| Age groups n (%) | ||||
| 3–12 months | 219 (19) | 13 (11) | 201 (20) | 0.005 |
| 1–3 years | 283 (25) | 21 (18) | 259 (25) | |
| 3–6 years | 146 (12) | 12 (10) | 134 (13) | |
| 6–12 years | 222 (20) | 30 (26) | 190 (19) | |
| 12–18 years | 268 (24) | 38 (33) | 224 (22) | |
| Gender n (%) | ||||
| Male | 639 (56) | 72 (63) | 558 (55) | 0.112 |
| Female | 499 (44) | 42 (36) | 450 (44) | |
| Ethnicity/religion n (%) | ||||
| Jewish | 901 (79) | 73 (64) | 815 (81) | 0.005 |
| Ultra‐orthodox | 451 (50) | 51 (69) | 387 (47) | <0.007 |
| Traditional | 274 (30) | 9 (12) | 265 (32) | |
| Secular | 60 (6) | 2 (3) | 58 (7) | |
| Other/no answer | 116 (12) | 11 (15) | 104 (12) | |
| Muslim | 160 (14) | 24 (21) | 133 (14) | |
| Christian | 10 (1) | 1 (1) | 9 (1) | |
| Other/unknown | 67 (6) | 16 (14) | 49 (5) | |
| Family and housing | ||||
| Number of parents and children in household (SD) | 6.2 (2.6) | 7 (3.2) | 6 (2.5) | 0.004 |
| Number of rooms | 4 [3–5] | 4 [3–5] | 4 [3–5] | 0.3 |
| Size of residence (m²) | 100 [80–120] | 103 [84–120] | 100 [80–120] | 0.33 |
| Housing density | ||||
| per room | 1.3 [1–2] | 1.5 [1–2] | 1.3 [1–1.8] | 0.02 |
| per size (m2) | 17 [12–24] | 16 [1–22] | 17 [12–24] | 2.6 |
| Chronic disease n (%) | 140 (12) | 12 (10) | 126 (12) | 0.73 |
| Attended school during lockdowns n (%) | 152 (13) | 15 (13) | 134 (13) | 0.809 |
| Reason for PED referral n (%) | ||||
| Infectious disease | 730 (64) | 69 (61) | 661 (66) | 0.21 |
| Non‐infectious disease | 159 (14) | 24 (21) | 135 (13) | 0.09 |
| Trauma | 176 (15) | 16 (12) | 160 (16) | 0.58 |
Inconclusive results (n = 16, 1.4%) were not included in this comparison. Values shown with square brackets represent median and interquartile range (IQR).
Abbreviations: PED, paediatric emergency department; SD, standard deviation.
Overall, 114 (10%) patients had anti‐SARS‐CoV‐2 antibodies in both assays and another 16 (1.6%) had inconclusive test results. A positive serology test result was associated with older age (median 7.6 vs. 3.8 years, p < 0.001), self‐identifying as a Muslim (21% vs. 14%, p = 0.05) or an ultra‐orthodox Jew (69% vs. 50%, p < 0.001) and a larger family size (mean 7.08 ± 3.2 vs. 6.09 ± 2.5, p = 0.004) (Table 1). The rate of seropositivity increased by age, from 13/219 (5.9%) samples from infants under one year of age to 68/490 (13.8%) from adolescents aged 12 to 18 years (Figure S2). When these variables were adjusted using a logistic regression model, only four variables were significantly associated with the virus: older age, larger family size and Muslim and ultra‐orthodox Jewish religions. Other patient characteristics, such as gender, housing density and whether the child's school was open or closed during lockdowns were insignificant (Table S1).
Of the 114 seropositive patients, only 50 (44%) had a known history of a PCR‐confirmed SARS‐CoV‐2 infection. Another 17 (15%) had not been tested but strongly suspected that they had contracted the virus due to clinical symptoms or exposure to a first‐degree relative or non‐relative with COVID‐19. Thus, 47 (41%) seropositive patients had no knowledge of any prior infection or exposure. Of note, only 17/73 (23%) of the patients who suspected they had COVID‐19 were seropositive for anti‐SARS‐CoV‐2 antibodies. In addition, of the 179 (16%) patients with first‐degree relatives who tested positive for SARS‐CoV‐2, only 57 (31%) were seropositive.
3.1. Patients with a previously confirmed infection
A history of PCR‐confirmed SARS‐CoV‐2 infection was reported in 71 (6%) patients and only 50 (70%) of those had anti‐SARS‐CoV‐2 antibodies. The 26 (37%) of those who were asymptomatic at the time of their PCR‐confirmed infection were more likely to be seronegative, but this was not statistically significant (p = 0.34) (Table 2). Of note, the five patients with multisystem inflammatory syndrome in children had higher antibody levels than all the other seropositive patients (Abbot index 5.3 vs. 3.9, p = 0.053 and DiaSorin 108.9 vs. 84.8 AU/mL, p = 0.096), but this was not statistically significant.
TABLE 2.
Demographic and clinical characteristics of patients with a past PCR‐confirmed SARS‐CoV‐2 infection
| Characteristic | All patients | Positive Serology | Negative Serology | Inconclusive Serology | p Value |
|---|---|---|---|---|---|
| n = 71 (100%) | n = 50 (70%) | n = 15 (21%) | n = 6 (8%) | ||
| Age median [IQR] | 10.8 [3–15] | 10.8 [4–15] | 6.8 [2–13] | 7.2 [1–16] | 0.18 |
| Gender n (%) | |||||
| Male | 40 (56) | 28 (56) | 8 (53) | 4 (67) | 0.85 |
| Female | 31 (44) | 22 (44) | 7 (47) | 2 (33) | |
| Symptoms n (%) | 45 (63) | 33 (66) | 8 (53) | 4 (66) | 0.34 |
| Weakness | 22 (31) | 16 (32) | 5 (33) | 1 (16) | 0.83 |
| Fever | 21 (29) | 17 (34) | 3 (20) | 1 (16) | 0.2 |
| Sore throat | 13 (18) | 10 (20) | 3 (20) | 0 (0) | 0.79 |
| Smell/taste loss | 13 (18) | 10 (20) | 1 (6) | 2 (33) | 0.15 |
| Rhinorrhoea | 12 (17) | 8 (16) | 3 (20) | 1 (16) | 0.9 |
| Cough | 12 (17) | 7 (14) | 5 (33) | 0 (0) | 0.16 |
| Headache | 10 (14) | 6 (12) | 3 (20) | 1 (16) | 0.63 |
| Days since COVID‐19 infection n (%) | |||||
| 0–21 | 8 (11) | 1 (2) | 6 (40) | 1(16) | <0.001 |
| 22–59 | 24 (33) | 22 (44) | 1 (6) | 1(16) | 0.008 |
| 60–89 | 11 (15) | 10 (20) | 1 (6) | 0 (0) | 0.22 |
| 90–119 | 19 (26) | 15 (30) | 4 (26) | 0 (0) | 0.80 |
| 120+ | 9 (13) | 2 (4) | 3 (20) | 4 (67) | 0.04 |
Abbreviation: IQR, interquartile range.
3.2. Time from infection and antibody levels
The time interval between the primary infection, established by the initial positive SARS‐CoV‐2 PCR test, and the serology testing significantly affected the sensitivity of the anti‐SARS‐CoV‐2 antibody assay. There was a peak sensitivity rate of 47/54 (87%) when it was taken 22–119 days after the infection, (p < 0.001, Table 2). The same temporal effect was also noted in the levels of antibodies detected over time, with significantly lower antibody levels in samples taken more than 4 months after the infection. Samples taken within 22–119 days of the infection showed higher antibody levels than tests taken within 21 days or more than 120 days after the initial infection: Abbott index 3.2 versus 0.7 within 21 days (p = 0.001) and 3.2 versus 1.2 after 120 days (p = 0.005) and DiaSorin levels 80 versus 25 AU/mL within 21 days (p = 0.015) and 80 versus 57 AU/mL after 120 days (p = 0.1) (Figure 2A, Table S2). The antibody levels were also affected by the age of the patient. Children under the age of 6 years had higher antibody levels than older children in the first 60 days after infection, followed by a steeper decline in antibody levels (Figure 2B and C, Table S2).
FIGURE 2.
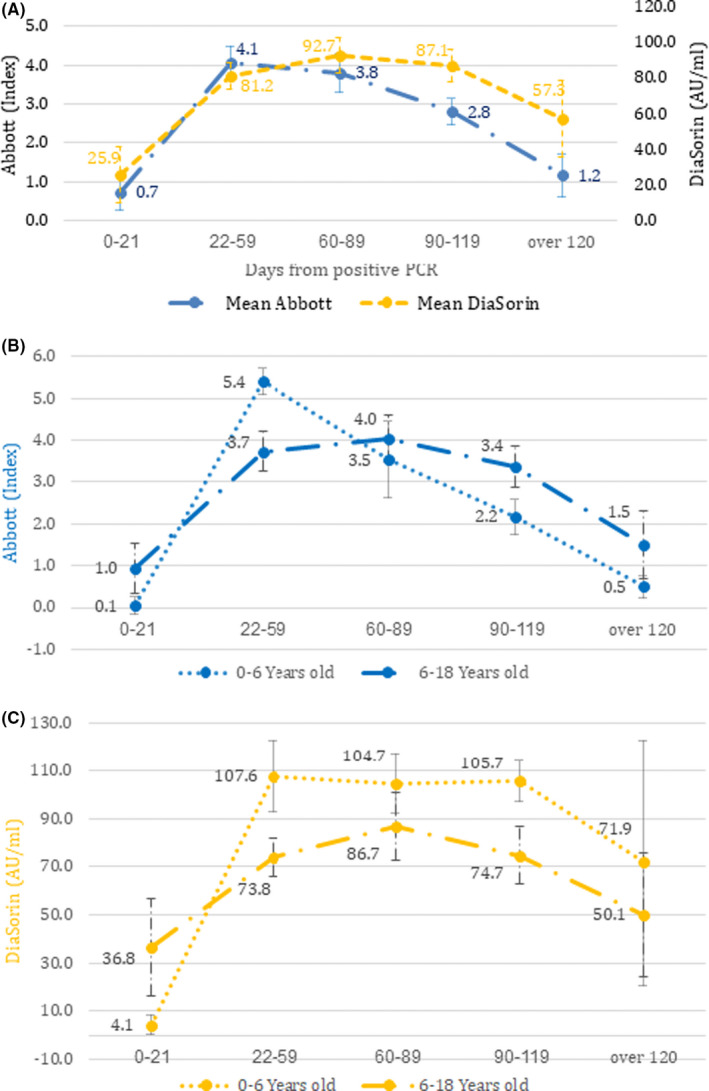
Antibody titre levels by time from infection. (A) Mean antibody levels and standard error, for both Abbott and DiaSorin assays, of all patients with a past PCR confirmed SARS‐Cov‐2 infection (n = 71). (B) Antibody levels obtained using the Abbott assay, divided by older (n = 47) or younger (n = 24) than 6 years old. (C) Antibody levels obtained using the DiaSorin assay, divided by older (n = 40) or younger than 6 years old (n = 17)
3.3. School closures and virus infections
Attending school was not associated with an increased risk of infection. The weekly rate of seropositive cases did not vary over the study period, even though the re‐opening of schools coincided with the start of the study (Figure S3). In addition, only 9/71 (12%) of the patients with a history of a SARS‐CoV‐2 infection reported that they contracted the infection at school. Finally, 152/816 (19%) respondents said they still attended school during lockdown. The rate of SARS‐CoV‐2 seropositive cases in this cohort was not different to the children who stayed at home during lock‐down: 3% versus 7.5% for day care or preschool children aged 0–6 years old (p = 0.19), 14% versus 13% for elementary school children aged 6 to 12 (p = 0.82) and 15% versus 14% for high school children aged 12 to 18 (p = 0.83). Overall, there were no differences in the seroprevalence rate between children who went to school, stayed at home during lockdown or whose parents did not answer this question (13%, 13%, and 12%, respectively, p = 0.809) (Table 1).
4. DISCUSSION
We found an overall 10% seropositivity rate among children in this large, prospective cohort of paediatric patients presenting to the PED in a region with a high prevalence of COVID‐19 cases. Seroprevalence rates varied from 7.7% for children under the age of 6 years to 16.4% in older children. Of the seropositive patients, 41% had no known past exposure to COVID‐19, indicating a significant rate of undetected, asymptomatic infection and carriage in children. 14 This was similar to the rate in a large meta‐analysis. 15 The paediatric seroprevalence rate found in this study was higher than previously reported in Israel 16 and similar to other areas worldwide that have been heavily affected by COVID‐19. 17 This was in contrast to studies from earlier in the pandemic, which demonstrated significantly lower seroprevalence in children. 18 The high seropositivity rate we observed among ultra‐orthodox Jews and Muslims contributed to the total seroprevalence rate found in this study. Previous studies have described how religious affiliation and communal ceremonies may have influenced SARS‐CoV‐2 transmission. 19 We also found that a larger family size was associated with seropositivity and this may provide another explanation for the high seroprevalence rate in this group.
Less than a third (29%) of the patients with a PCR‐confirmed virus infection did not have conclusive positive serology test results. The nucleocapsid‐based antibody test was chosen as the primary screening antibody test for this study, based on preliminary studies that found it showed superior sensitivity to spike‐based antibody kits. 20 This was in accordance with the Israeli Ministry of Health guidelines for SARS‐CoV‐2 serology testing at the time of the study. Overall, our dual‐assay model only had a sensitivity of 70% for detecting anti‐SARS‐CoV‐2 antibodies in patients with a history of COVID‐19. This poor sensitivity was pronounced in samples taken within 21 days of infection (13% sensitivity) or after 120 days of infection (22% sensitivity). Samples taken 21–119 days after infection had a dual‐assay sensitivity of 87%, which was slightly lower than the 90%–97% described in adults. 12 However, it is important to note that a lack of detectable antibodies does not necessarily correlate to a lack of neutralising ability and specific, acquired SARS‐CoV‐2 immunity may still exist. 21 , 22 Of note, no correlation was found between symptomatic and asymptomatic COVID‐19 cases and the level of anti‐SARS‐CoV‐2 antibodies. 23
We found that the temporal trend of anti‐SARS‐CoV‐2 antibodies over time in children was different to the trends in adults. Children experienced a delayed initial rise in antibody levels, followed by a rapid decline and even the disappearance of detectable antibodies 4 months after the initial infection. This was particularly pronounced in children under the age of 6 years (Figure 2). These findings are in contrast to the current evidence in adults, 24 and may point to children having a distinct, weaker long‐term immune response. 25 That latter study focused on a pseudo‐virus in vitro assay of antibodies from the serum of children who had reduced neutralising activity compared to those taken from adults. The results may indicate reduced serological protection and susceptibility to recurrent infection in the paediatric population. 25 This attenuated immune response may lead to an underestimation of the true rate of prior, undetected virus infections in children, as negative serology test results will be interpreted as a lack of past infection, and not as a patient recovering from COVID‐19 with undetectable antibodies. This may also have implications for studies exploring long COVID‐19 in children, 26 as a cohort of seronegative children may not represent a reliable control group. 27 In addition, this may have implications on the efficacy and lasting, long‐term immunogenicity of SARS‐CoV‐2 vaccines in the paediatric population.
Our study began immediately after a month‐long nationwide Israeli lockdown, between 18 September and 17 October 2020, in contrast to previous large paediatric seroprevalence studies, which were conducted during extended lockdown periods when infection rates were low. 18 Interestingly, there was a relatively stable week‐by‐week seropositivity rate despite the gradual re‐opening of schools during the study period. In addition, school closures were not uniformly or completely enforced during this lockdown. This included a government‐approved strategy to keep schools open for children with special needs and the children of essential workers. It also included children who attended schools that remained open in defiance of government‐mandated closures. 28 The 152 children who reported that they continued to attend school during the lockdown period did not have an increased risk of infection and this remained when the data were corrected for all other patient characteristics. These results were consistent with other findings that children were mostly infected by a first‐degree relative in their home (55%) and that there were relatively low rates of infection in childcare facilities and schools (12%). 29 , 30 These data may encourage policymakers to carefully reconsider widespread school closures. 31
4.1. Strengths and limitations
The strengths of this study included its large, prospective, population‐based cohort in a region with high rates of SARS‐CoV‐2 infections and a diverse paediatric population. This enabled us to identify asymptomatic carriers and to characterise the serological response over time. We also noted that previous studies found lower rates of unknown, asymptomatic infections in children. 32 Moreover, we were able to assess the role schools played in infections among children in a way that was not possible in previous studies. Other strengths of this study were the comprehensive patient questionnaire and further telephone‐based epidemiological investigations of seropositive children, as well as the use of two distinct SARS‐CoV‐2 antibody assays.
This study had several limitations. The single‐site sample collection may have led to a geo‐demographic population bias, as seroprevalence in other regions with different rates of infection may have been different. Similarly, these findings may only apply to the SARS‐CoV‐2 variants in Israel at the time of the study. Of note, the study only included Israeli residents and patients from the Palestinian Authority were not represented in this cohort. Also, voluntary enrolment in the study may have led to selection bias, as the parents of patients with a past history of exposure to SARS‐CoV‐2 may have been more interested in participating in the study. Thus, the true rate of seroprevalence may be lower than reported here. On the other hand, since the assays used in this study were not 100% sensitive and specific, the true seropositivity rate in the general population may be somewhat different to our results. It is worth noting that the Abbott assay used in this study is intended to be used for the qualitative, and not quantitative, detection of antibodies, unlike the DiaSorin assay. Despite this, we have demonstrated significant differences in the test values, with respect to the time from infection, and our results can help guide clinicians when they are interpreting the results of this assay. In addition, this study sampled the paediatric population presenting to the PED for varied reasons, and may not reflect the general, healthy paediatric population. Nevertheless, refusal to participate in the study was minimal. This was because of the high infection rates in the region before and during the study, rising public awareness that children are often asymptomatic and the fact that serological tests were not easily accessible at the time of the study. Moreover, our findings on the serological responses over time, as well as differences in seropositivity rates among different age groups, were not site‐specific and not affected by these biases. This means that they should be generalisable to broader paediatric populations. We did not collect data on the parents’ occupation, as well as the child's involvement in extra‐curricular group activities, which may have had an effect on the rate of seropositivity. These data may have revealed additional risk factors for infection.
5. CONCLUSION
This study found that 41% of children with SARS‐CoV‐2 antibodies had no known or suspected exposure to the virus. We also found that anti‐SARS‐CoV‐2 antibodies declined rapidly in young children and even disappeared 4 months after the initial infection. This may indicate a distinct immune response pattern among children, which may be important when weighing up the cost‐effectiveness and advantages of vaccinating healthy paediatric populations. In addition, we did not find that opening schools increased the infection rates among children. These findings, along with the ongoing vaccination of adults, may help policymakers to decide whether to keep schools open and restore an element of normalcy and stability in the lives of children worldwide.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
This study was performed in collaboration with the Israeli Ministry of Health. The authors would like to thank Dror Ben Moshe for his support and the hospital's clinical endocrinology laboratory staff for processing the samples.
Breuer A, Raphael A, Stern H, et al. SARS‐CoV‐2 antibodies started to decline just four months after COVID‐19 infection in a paediatric population. Acta Paediatr. 2021;110:3054–3062. 10.1111/apa.16031
Breuer and Raphael contributed equally to the work.
Barak‐Corren and Heiman contributed equally to the work.
Funding information
This study received funding from the Israeli Ministry of Health.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020;145(6):e20200702. 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 3. Ludvigsson JF. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeBiasi RL, Delaney M. Symptomatic and asymptomatic viral shedding in pediatric patients infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): under the surface. JAMA Pediatr. 2021;175(1):16‐18. [DOI] [PubMed] [Google Scholar]
- 5. Israel Ministry of Health, Public Health Services . Analysis of Covid‐19 in children. Published October 18, 2020. https://www.gov.il/BlobFolder/reports/bz‐400844120/he/files_publications_corona_bz‐400844120.pdf. Accessed June 27, 2021.
- 6. Bellino S, Punzo O, Rota MC, et al.; COVID‐19 WORKING GROUP . COVID‐19 disease severity risk factors for pediatric patients in Italy. Pediatrics. 2020;146(4):e2020009399. [DOI] [PubMed] [Google Scholar]
- 7. Yonker LM, Neilan AM, Bartsch Y, et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): clinical presentation, infectivity, and immune responses. J Pediatr. 2020;227:45‐52.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feldstein LR, Rose EB, Horwitz SM, et al. Overcoming COVID‐19 Investigators ; CDC COVID‐19 Response Team . Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Center for Diseases Control and Prevention, USA . Interim guidelines for collecting, handling, testing clinical specimens from persons for coronavirus disease 2019 (COVID‐19). Updated February 26, 2021. https://www.cdc.gov/coronavirus/2019‐ncov/lab/guidelines‐clinical‐specimens.html. Accessed June 27, 2021.
- 10. Younes N, Al‐Sadeq DW, Al‐Jighefee H, et al. Challenges in laboratory diagnosis of the novel coronavirus SARS‐CoV‐2. Viruses. 2020;12(6):582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bahar B, Jacquot C, Mo YD, DeBiasi RL, Campos J, Delaney M. Kinetics of viral clearance and antibody production across age groups in children with severe acute respiratory syndrome coronavirus 2 infection. J Pediatr. 2020;227:31‐37.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naaber P, Hunt K, Pesukova J, et al. Evaluation of SARS‐CoV‐2 IgG antibody response in PCR positive patients: comparison of nine tests in relation to clinical data. PLoS One. 2020;15(10):e0237548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anderson EM, Diorio C, Goodwin EC, et al. Severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2) antibody responses in children with multisystem inflammatory syndrome in children (MIS‐C) and mild and severe coronavirus disease 2019 (COVID‐19). J Pediatric Infect Dis Soc. 2021;10(5):669‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buonsenso D, Valentini P, De Rose C, et al. Seroprevalence of anti‐SARS‐CoV‐2 IgG antibodies in children with household exposure to adults with COVID‐19: preliminary findings. Pediatr Pulmonol. 2021;56(6):1374‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oran DP, Topol EJ. The proportion of SARS‐CoV‐2 infections that are asymptomatic: a systematic review. Ann Intern Med. 2021;174:655‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Israel Ministry of Health, Division of Epidemiology . The national sero‐epidemiological survey of Covid‐19. Published October 2020. https://www.gov.il/BlobFolder/reports/de‐covid19‐28062020‐17092020/he/files_publications_corona_DE‐covid19.pdf. Accessed June 27, 2021.
- 17. Cohen R, Jung C, Ouldali N, et al. Assessment of SARS‐CoV‐2 infection by reverse transcription‐PCR and serology in the Paris area: a cross‐sectional study. BMJ Paediatr Open. 2020;4(1):e000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tönshoff B, Müller B, Elling R, et al. Prevalence of SARS‐CoV‐2 infection in children and their parents in Southwest Germany. JAMA Pediatr. 2021;175(6):586‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buonsenso D, Malorni W, Sisti GL, Moscato U. COVID‐19 and religion: risks and opportunities. Preprints 2020. 2020;120173. 10.20944/preprints202012.0173.v1 [DOI] [Google Scholar]
- 20. Burbelo PD, Riedo FX, Morishima C, et al. Sensitivity in detection of antibodies to nucleocapsid and spike proteins of severe acute respiratory syndrome coronavirus 2 in patients with coronavirus disease 2019. J Infect Dis. 2020;222(2):206‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Criscuolo E, Diotti RA, Strollo M, et al. Weak correlation between antibody titers and neutralizing activity in sera from SARS‐CoV‐2 infected subjects. J Med Virol. 2021;93:2160‐2167. 10.1002/jmv.26605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McAndrews KM, Dowlatshahi DP, Dai J, et al. Heterogeneous antibodies against SARS‐CoV‐2 spike receptor binding domain and nucleocapsid with implications for COVID‐19 immunity. JCI Insight. 2020;5(18):e142386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Isoldi S, Mallardo S, Marcellino A, et al. The comprehensive clinic, laboratory, and instrumental evaluation of children with COVID‐19: a 6‐months prospective study. J Med Virol. 2021;93(5):3122‐3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS‐CoV‐2 infection persist for months. Science. 2020;370(6521):1227‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weisberg SP, Connors TJ, Zhu Y, et al. Distinct antibody responses to SARS‐CoV‐2 in children and adults across the COVID‐19 clinical spectrum. Nat Immunol. 2021;22(1):25‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buonsenso D, Munblit D, De Rose C, et al. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110(7):2208‐2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Radtke T, Ulyte A, Puhan MA & Kriemler S. Long‐term symptoms after SARS‐CoV‐2 infection in school children: population‐based cohort with 6‐months follow‐up. medRxiv 2021.05.16.21257255. 10.1101/2021.05.16.21257255 [DOI] [Google Scholar]
- 28. Barak Corren N & Perry‐Hazan L. Bidirectional legal socialization and the boundaries of law: the case of enclave communities’ compliance with COVID‐19 regulations. J Soc Issues. 2021; 77(2):631‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Soriano‐Arandes A, Gatell A, Serrano P, et al. Household SARS‐CoV‐2 transmission and children: a network prospective study [published online ahead of print, 2021 Mar 12]. Clin Infect Dis. 2021;ciab228. 10.1093/cid/ciab228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lachassinne E, de Pontual L, Caseris M, et al. SARS‐CoV‐2 transmission among children and staff in daycare centres during a nationwide lockdown in France: a cross‐sectional, multicentre, seroprevalence study. Lancet Child Adolesc Health. 2021;5(4):256‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buonsenso D, Roland D, De Rose C, et al. Schools closures during the COVID‐19 pandemic: a catastrophic global situation. Pediatr Infect Dis J. 2021;40(4):e146‐e150. [DOI] [PubMed] [Google Scholar]
- 32. Kellner J. Alberta childhood COVID‐19 cohort (AB3C) aim 3: longitudinal sero‐epidemiology study first interim report. Published January 31, 2021. https://prism.ucalgary.ca/bitstream/handle/1880/113084/AB3C%20Aim%203%201st%20Interim%20Report‐final.pdf?sequence=5&isAllowed=y. Accessed June 27, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


