Abstract
Most surgical procedures require general anesthesia, which is a reversible deep sedation state lacking all perception. The induction of this state is possible because of complex molecular and neuronal network actions of general anesthetics (GAs) and other pharmacological agents. Laboratory and clinical studies indicate that the effects of GAs may not be completely reversible upon anesthesia withdrawal. The long-term neurocognitive effects of GAs, especially when administered at the extremes of ages, are an increasingly recognized health concern and the subject of extensive laboratory and clinical research. Initial studies in rodents suggest that the adverse effects of GAs, whose actions involve enhancement of GABA type A receptor activity (GABAergic GAs), can also extend to future unexposed offspring. Importantly, experimental findings show that GABAergic GAs may induce heritable effects when administered from the early postnatal period to at least young adulthood, covering nearly all age groups that may have children after exposure to anesthesia. More studies are needed to understand when and how the clinical use of GAs in a large and growing population of patients can result in lower resilience to diseases in the even larger population of their unexposed offspring. This minireview is focused on the authors’ published results and data in the literature supporting the notion that GABAergic GAs, in particular sevoflurane, may upregulate systemic levels of stress and sex steroids and alter expressions of genes that are essential for the functioning of these steroid systems. The authors hypothesize that stress and sex steroids are involved in the mediation of sex-specific heritable effects of sevoflurane.
Keywords: general anesthetic, sevoflurane, K+-2Cl- (KCC2) Cl- exporter, Na+-K+-Cl- (NKCC1) Cl- importer, GABA type A receptor, corticosterone, estradiol, testosterone, DNA methylation, intergenerational effects
GABAergic general anesthetics, in particular sevoflurane, induce acute and long-term neuroendocrine abnormalities and epigenomic alterations in germ cells, as well as epigenomic, transcriptomic, and behavioral abnormalities in male offspring.
Introduction
Stress, endocrine-disrupting chemicals (EDCs), alcohol, and other environmental factors can lower the resilience to neuropsychiatric and other disorders in offspring of exposed parents. This notion has evolved from denial to become a data-driven phenomenon [1–16]. General anesthetics (GAs) are among the most frequently used pharmacological agents in medicine, and their use is rapidly expanding. As one example, the global number of surgeries, which would not be possible without the use of anesthesia, rose from 226.4 million in 2004 to 312.9 million in 2012 [17]. The known molecular targets for GAs include various voltage- and ligand-gated ion channels, ion transporters, proteins regulating neurogenesis, synaptogenesis, synaptic transmissions, neurotransmitter and hormonal levels and actions, genomic and epigenomic activities, immune and metabolic processes, and others [18–24]. By modulating numerous molecular targets, GAs share mechanisms of action with alcohol, stressors, and EDCs [24–33]. Although monitored macrophysiological parameters rapidly return to normal upon anesthesia withdrawal [34, 35], some of the molecular effects of GAs initiated during GA exposure may persist. As a result, significant neurocognitive abnormalities can occur later in life. The long-term neurocognitive adverse effects of GAs, especially after prolonged and/or repeated procedures, are the subject of extensive clinical and laboratory research [36–49].
Initial evidence of heritability of the adverse effects of GAs was published in the early 1980s [50, 51], long before the publication of the first studies on the neurotoxic developmental effects of GAs in the exposed animals [52, 53]. According to those early studies, offspring of mice exposed to the halogenated GAs halothane and enflurane exhibited deficiencies in learning behavioral paradigms [50, 51] (reviewed in [54]). The revolutionary interpretations of those findings, for example, GA-induced “genetic aberrations” of the germline [50, 51], were not experimentally tested at the time (likely because of limited technical tools). However, a recent human study designed to assess effects of changes in obesity after bariatric surgery on DNA methylation patterns in spermatozoa found lasting changes in multiple genes [55]. Detection of such changes as early as 1 week after the surgery suggests that GA/surgery (not just changes in obesity) could be contributing factors [55]. Sevoflurane is a commonly used halogenated GA whose polyvalent actions include enhancement of GABA type A receptor (GABAAR) signaling, similar to halothane and enflurane [21, 22, 56, 57]. We found that prolonged exposure to sevoflurane in neonatal rats or repeated exposure to sevoflurane in adult rats (F0 generation) caused changes in DNA methylation in spermatozoa and ovarian cells. It also caused epigenomic, transcriptomic, and behavioral defects in male, offspring (F1 generation) [58–60]. A recent study by Chastain-Potts and colleagues further supports the possibility of intergenerational effects of sevoflurane administered to neonatal rats [61] (reviewed in [54] and in this issue). Considering the wide and growing use of GAs [17], focused mechanistic studies that more closely model clinical settings and ultimately studies in patients of heritable effects of GAs are needed. It is critically important to elucidate when and how the use of GAs in patients can lower resilience to neurocognitive diseases in the even larger population of their offspring; to identify safer GAs and prevention strategies; and to assess whether heritable effects of GAs contribute to the rise in neurodevelopmental disorders, whose causes are often unknown [62–65].
In this minireview, we discuss our published experimental findings and data in the literature demonstrating that sevoflurane, and potentially other GABAergic GAs, act as potent stressors and EDCs. The adverse effects of sevoflurane include acute and long-term neuroendocrine abnormalities, epigenomic changes in germ cells, and epigenomic and transcriptomic changes in the brains of male offspring, as well as behavioral deficiencies. We hypothesize that stress- and EDC-like effects of sevoflurane contribute to the anesthetic’s sex-specific heritable effects.
Sevoflurane induces upregulation of stress and sex steroid production and a shift in GABAAR signaling toward excitatory
Neuroendocrine effects of sevoflurane in neonatal rats
The changes in levels of cortisol and sex steroids associated with exposure to anesthesia/surgery or to anesthesia without surgery support the acute neuroendocrine effects of GAs in patients [24, 26–33]. Consistent with the strong, stressor-like effects of GABAergic GAs, a single exposure to sevoflurane or propofol was sufficient to cause multifold increases in the secretion of corticosterone in neonatal rats [59, 66–69]. Furthermore, sevoflurane increased systemic levels of the sex steroid hormones testosterone (T) in males and 17-β-estradiol (E2) in males and females within 1 h of the GA exposure [69].
Our findings suggest that sevoflurane enhances systemic levels of E2 in neonatal rats by increasing synthesis of E2 in the brain that is independent of testis-produced T [69]. Figure 1 illustrates the hypothetical signaling pathways mediating the effects of sevoflurane; these pathways are supported by published experimental findings, which are discussed throughout this minireview. E2 is synthesized in the brain through aromatization of testis-derived T in males and via aromatization of de novo synthesized T in the brains of both sexes. Although sevoflurane increased systemic levels of T in males only, systemic levels of E2 were increased in males and females to a similar extent. These findings support the idea that sevoflurane-increased systemic levels of E2 originate in the brain. Pretreatments with the GABAAR antagonist bicuculline methiodide or E2 synthesis inhibitor formestane deterred sevoflurane-induced increases in E2 levels in both sexes, but not sevoflurane-induced increases in T levels in males [69]. The female rat ovary remains quiescent at this age, but male and female rat pups have similar serum levels of E2 during this age period [70–74]. These facts also support the possibility that de novo synthesized T in the brain is the source of sevoflurane-heightened systemic levels of E2.
Figure 1.
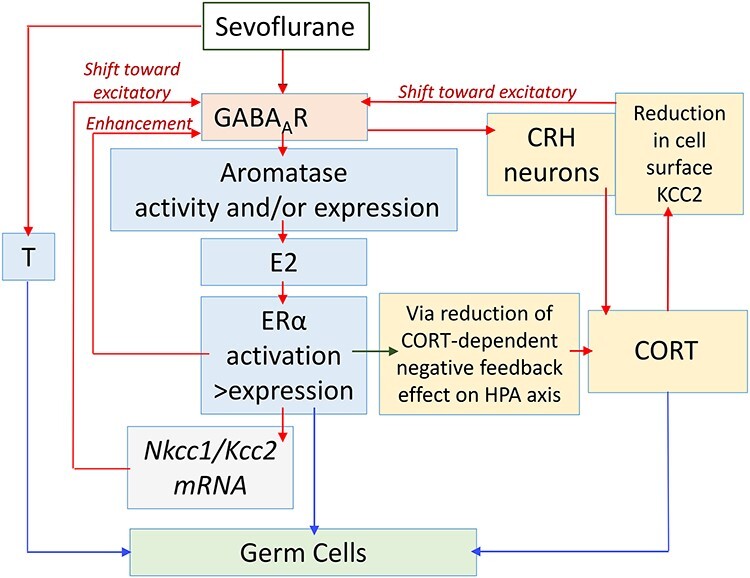
A diagram illustrating hypothetical pathways that involve the positive feedback interaction between GABAAR signaling, stress, and sex steroids in the mediation of heritable effects of sevoflurane. (See text for details.) These hypothetical pathways are proposed based on our published experimental findings and/or data in the literature that are discussed in this minireview; nevertheless, these are hypotheses that require rigorous experimental verification. The red arrows illustrate positive, stimulatory effects and the green arrow illustrates a negative, inhibitory effect. The blue arrows illustrate effects of steroids on parental germ cells. Our experimental data indicate that sevoflurane acts via GABAAR-independent mechanisms to increase systemic levels of testosterone (T) in males only [69]. Sevoflurane, by acting via GABAAR-mediated mechanisms, increases systemic levels of 17-β-estradiol (E2) in males and females [68, 69]. Our findings show that sevoflurane increases expressions of aromatase and estrogen receptor α (Erα), but not estrogen receptor β (Erβ), and systemic E2 levels. E2, by acting via ERα, increases the Na+-K+-Cl− cotransporter (Nkcc1)/K+-Cl− cotransporter (Kcc2) ratio. Therefore, E2 may shift GABAAR signaling toward excitatory and further enhances GABAAR excitatory signaling through direct interaction with GABAAR, as evident from increased sevoflurane-induced electroencephalography (EEG)-detectable seizure-like activities [68, 69]. The enhanced excitatory GABAAR signaling leads to a further increase in sevoflurane-stimulated production of E2. Sevoflurane, by enhancing excitatory GABAAR signaling in corticotrophin-releasing hormone (CRH) neurons in the paraventricular nucleus (PVN) of the hypothalamus, stimulates the hypothalamic–pituitary–adrenal (HPA) axis and corticosterone (CORT) production [59, 67–69]. The stress response reduces the KCC2 cell-surface expression in the hypothalamic neurons [59], leading to a shift in GABAAR signaling toward excitatory, and, as a result, to greater upregulation of the HPA and hypothalamic-pituitary-gonadal (HPG) axes. Sevoflurane, by increasing E2 levels and expression of Erα, reduces the CORT-dependent negative feedback on the HPA axis [101, 102], leading to greater stress response, more excitatory GABAAR signaling via reduced cell surface KCC2 expression, and greater GABAAR signaling-mediated production of E2. Based on data in the literature on epigenetic effects of stress in germ cells [138, 140–143] and on our own findings [58, 59], we hypothesize that CORT mediates effects of sevoflurane in parental germ cells, at least in part through epigenetic modifications. The heritable effects of EDCs, as well as transcriptomic activities of ERα and androgen receptors (AR) [9, 15, 136, 137, 145–147], allow us to hypothesize that E2 and T may also mediate heritable effects of sevoflurane through their actions in parental germ cells.
One hour of anesthesia with sevoflurane was also sufficient to acutely alter the expressions of several genes that encode proteins that are essential components of the E2 signaling pathway. Expressions of the aromatase gene, an enzyme that aromatases T to E2, and the estrogen receptor alpha (Erα) gene, but not estrogen receptor beta (Erβ) gene, were increased in the hypothalamus (Figure 1) [69]. ERα predominantly localizes to the brain regions involved in regulating sexual behavior such as the hypothalamus. ERβ has a broader distribution in the neurons of the hippocampus, cerebral cortex, and amygdala, as well as in the microglia and oligodendrocytes [70, 73–75]. Nevertheless, we found that the antagonist of ERα, but not ERβ, depressed sevoflurane-induced electroencephalography (EEG)-detectable seizure-like activities [69]. The EEG-detectable seizure-like patterns largely reflect the brain’s cortical activity. These findings suggest that E2/ERα is an important signaling pathway mediating effects of sevoflurane in multiple brain regions. Interestingly, the molecular mechanisms mediating the organizational effects of T in the perinatal rat brain, for example, T-regulated brain sexual differentiation, are thought to include aromatization of testis-derived T to E2 and activation primarily of ERα by E2 in the brain [71, 76–82]. Systemic levels of E2, expressions of hypothalamic aromatase and Erα, were increased by sevoflurane to a similar extent in male and female rat pups regardless of sevoflurane-induced increases in systemic levels of T in male pups only [69]. These findings suggest that sevoflurane, administered during the sensitive period in rats, may affect brain sexual differentiation.
Within 1 h of exposure, sevoflurane also increased expression of the Na+-K+-Cl− (Nkcc1) Cl− importer gene, but it reduced expression of the K+-Cl− (Kcc2) Cl− exporter gene [69]. Formed largely by NKCC1 and KCC2 activities, the neuronal transmembrane gradient of Cl− is a crucial determining factor as to whether Cl− signaling through GABAARs, the main substrate that mediates sedative effects of GABAergic anesthetics, is inhibitory or excitatory in the brain [83–90]. Intracellular concentrations of Cl−, the major charge carriers through GABAAR channels, are elevated in many neurons in the rostral regions of the neonatal brain because of the relatively high and low levels of the NKCC1 Cl− importer and KCC2 Cl− exporter, respectively [83–85, 90]. Therefore, the Cl− effluxes through GABAAR channels during this age period can be strong enough to cause membrane depolarization sufficient to activate low-threshold voltage-gated Ca++ channels and to induce depolarization-dependent relief of the Mg++-block of Ca++-permeable N-methyl-D-aspartate receptors (NMDARs) [83–85]. The GABAAR signaling-initiated Ca++ influxes through the Ca++ channels and NMDAR channels during the early stages of brain development regulate numerous developmental processes ranging from gene expression to synapse formation [84, 88–90]. Sevoflurane-caused increases in the Nkcc1/Kcc2 mRNA ratio suggest that sevoflurane may not only enhance GABAAR signaling through direct interaction with the receptor but also render GABAAR signaling even more depolarizing/excitatory by shifting the equilibrium potential for Cl− to more positive values [68, 69].
The sevoflurane-altered GABAAR signaling can further be potentiated by E2, as evident from studies demonstrating that exogenous E2 increased GABAAR-mediated currents in rat hippocampal slices and potentiated sevoflurane-caused electroencephalogram-detectable seizure-like activities in rat pups [95]. These sevoflurane-caused EEG-detectable seizure-like activities can be diminished by bicuculline methiodide, formestane, the ERα antagonist MPP (1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride), or the NKCC1 inhibitor bumetanide. The effect of those treatments supports the involvement of E2/ERα in the GABAergic excitatory effects of sevoflurane in neonatal rats (Figure 1) [95, 96].
A role of E2 in the positive modulation of the GABAergic action of sevoflurane is also evident from our findings that exogenous E2 shortened the time needed for sevoflurane to induce the loss of the righting reflex (LORR), whereas the estrogen receptor antagonist and inhibitor of synthesis of E2 lengthened it [68]. The righting reflex is predominantly mediated by neuronal circuits in the midbrain [91, 92]. Although in neonatal rats GABAAR-mediated signaling is predominantly excitatory in rostral regions, it becomes increasingly inhibitory more caudad [84–86, 90]. Therefore, exogenous E2, by enhancing excitatory and inhibitory GABAAR signaling in the neonatal rat cortex and midbrain, respectively, may increase the efficacy of sevoflurane to induce EEG-detectable epileptic seizure-like activities and LORR [95]. Also, according to data in the literature, E2-caused elevation of intracellular Ca++ in immature neurons through enhancement of the depolarizing action of GABA may explain why E2 exacerbates hippocampal cell damage in neonatal rats in the presence of a GABAAR agonist [93].
Clearly, the involvement of E2 in the mediation of sevoflurane-induced effects is more complex than just positive modulation of GABAAR activity. The alleviating effects of bicuculline and formestane on sevoflurane-increased expressions of hypothalamic aromatase, Erα, and the Nkcc1/Kcc2 mRNA ratio suggest that E2 and GABAAR signaling interact to mediate the transcriptional effects of sevoflurane (Figure 1) [69]. In further support of this interaction between E2 and GABAAR signaling, the GABAAR signaling is an important regulator of the functioning of the hypothalamic–pituitary–gonadal (HPG) axis and resulting secretion of sex steroid hormones [94–98]. Because many gonadotropin-releasing hormone (GnRH) neurons are stimulated by GABAAR signaling even under basal conditions in adulthood [94–97], the sevoflurane-initiated GABAAR/E2-mediated upregulation of excitatory GABAAR signaling in the hypothalamic neurons may lead to even greater stimulation of the HPG axis and production of sex steroids, including through gonadotrophin-releasing hormone (GRH)-regulated aromatase activity in the brain [98].
The findings that formestane and bicuculline diminished the sevoflurane-caused increase in systemic levels of corticosterone suggest that GABAAR signaling and E2 are involved in the mediation of the stress-like effects of sevoflurane in rats [68, 69]. One of the fundamental mechanisms of downregulating the stress response in the brain is the control of the corticotrophin-releasing hormone (CRH)-secreting hypothalamic paraventricular nucleus (PVN) neurons by inhibitory GABAAR signaling and positive modulation of this signaling by neuroactive steroids [99, 100]. Because of the depolarizing/excitatory GABAAR signaling in neonatal rats, which can further be shifted toward excitatory and upregulated via the E2-dependent mechanisms, sevoflurane is likely to induce an opposite, stimulating effect on hypothalamic–pituitary–adrenal (HPA) axis activity (Figure 1). Additionally, E2 may downregulate and upregulate the negative feedback effects of glucocorticoids on the HPA axis by activating and inhibiting ERα and ERβ, respectively [101, 102]. This E2-dependent upregulation of the HPA axis may be more efficacious in sevoflurane-anesthetized rat pups because the GA not only increases levels of E2 but also upregulates the expression of hypothalamic ERα, but not ERβ, receptors (Figure 1) [69].
Our findings indicate that GABAergic GA-initiated increases in the Nkcc1/Kcc2 mRNA ratio in the hypothalamus and other brain regions in neonatal rats may persist into adulthood. The adult rats, neonatally exposed to GABAergic GAs, also exhibit exacerbated HPA axis responses to stress and behavioral deficiencies [58–60, 66–69, 103–109]. Both neonatal GABAergic GA-initiated exacerbated HPA axis responses to stress and behavioral deficiencies can be deterred by pretreatments with bicuculline methiodide or formestane administered at the time of GA exposure. These findings support the important role of GABAAR signaling and sex steroids in the mediation of the long-term stress-like adverse effects of GABAergic GAs [58–60, 66–69, 103–109]. In further support of this contention, we found that NKCC1 inhibition at the time of neonatal anesthesia ameliorated most of the lasting developmental effects of GABAergic GAs in rats. The role of the Cl− transporters and, hence, excitatory GABAAR signaling, in the developmental effects of GAs, has also been confirmed by other laboratories [110–112]. The long-term developmental abnormalities similar to those induced by single early-life exposures to GABAergic GAs can be induced by prolonged and repeated maternal separations in neonatal rats [113–118]. Collectively, these findings further strengthen the idea that GABAergic GAs administered to neonatal rats induce acute and long-term neuroendocrine abnormalities similar to those induced by excessive stress and EDCs.
Neuroendocrine effects of sevoflurane in young adult rats
Acute and chronic stress and other environmental stressors can induce a shift in GABAAR signaling from inhibitory to excitatory even in adults, at least in part by decreasing cell-surface KCC2 levels. Such changes in KCC2-GABAAR signaling are linked to a number of pathophysiological conditions [119–126]. We tested whether sevoflurane, administered to young adult Sprague Dawley rats, can also act as a stressor. We exposed male and female rats to 2.1% sevoflurane for 3 h on 3 alternating days beginning on postnatal day 56 (P56) [59]. One hour after the last exposure to sevoflurane on P60, male and female rats had multifold increases in systemic levels of corticosterone. However, only exposed males had significantly reduced KCC2 expression in the PVN neurons of the hypothalamus at that time (Figure 1) [86]. The changes in KCC2 expression in the PVN neurons of exposed females were characterized by the same trend, but they were not sufficient to achieve statistical significance [59]. This finding suggests that the threshold for similar KCC2 effects of sevoflurane in adult females is higher. Interestingly, only male rodents were tested in previous studies reporting acute stress-induced downregulation of KCC2 expression [119–125]; however, daily exposure to stress for 25 days is reported to reduce cell-surface KCC2 expression in the female mouse hippocampus as well [126]. It is important to stress that the specific functional excitatory/inhibitory outcome of GABAAR-mediated signaling depends on cellular and subcellular NKCC1/KCC2 expression patterns, which are not fully studied. Immunohistochemical and electrophysiological studies indicate that vasopressin-secreting cells in the adult rat PVN do not express detectable levels of KCC2 and their GABAergic synaptic inputs are excitatory [127, 128], as are many GnRH neurons [94–97].
Male rats that were exposed to sevoflurane for 3 h on P56, P58, and P60 developed long-term neuroendocrine and behavioral abnormalities, which were similar to those found in adult rats that were exposed to sevoflurane as neonates. More than 3 months after exposure to sevoflurane in young adulthood, male rats exhibited exacerbated HPA axis responses to stress and behavioral deficiencies. Furthermore, the serum levels of luteinizing and T hormones were significantly increased in male rats, as was their expression of the hypothalamic Gnrh gene [59]. The HPG axis in these male rats was also altered at the level of expressions of the hypothalamic aromatase, Erα, and Erβ genes. The expressions of the aromatase and Erα genes were significantly increased, whereas the expression of the Erβ gene was slightly, but significantly, decreased [59]. The latter may be an additional example of the interaction between the HPG and HPA axes in the long-term effects of sevoflurane in adult rats to induce exacerbated corticosterone responses to stress (Figure 1). As we previously discussed, data in the literature indicate that E2 could potentiate the HPA axis responses to stress by downregulating and upregulating the negative feedback effects of glucocorticoids on the HPA axis through activation and inhibition of ERα and ERβ, respectively (Figure 1) [101, 102]. The long-term changes in the HPA axis responses to stress and neurobehavioral characteristics in female rats that were exposed to sevoflurane as young adults showed similar trends as their male counterparts, but they were not sufficient to achieve significance [59]. In future studies, it will be important to test whether differences in sex hormones in young adult male and female rats at the time of anesthesia contribute to differences in acute and long-term EDC-like and stress-like effects of sevoflurane in both sexes.
Human and animal studies provide evidence that parental exposure to excessive stress and/or EDCs can impact the health of future generation(s) through nongenetic transmission, i.e., a transmission that does not include changes in DNA nucleotide sequences [1–16, 129–144]. Findings of studies that were discussed above support the idea that the GABAergic GA sevoflurane may induce acute and long-lasting changes in systemic levels of stress and sex steroids and alterations in the expressions of genes that encode proteins essential for the functioning of these steroid systems, including the Gr and Erα genes. Considering that GR, androgen receptors (ARs), and ERα also function as transcription factors [145–147], it is plausible that sevoflurane-induced changes in the functioning of the steroid systems may be involved in the mediation of nongenetic heritable effects of the GA.
Potential role of steroids in intergenerational effects of parental exposure to sevoflurane
The identification and investigation of nongenomic heritable effects of parental experiences in humans is complicated by the difficulties of assembling study cohorts from different generations spread over many years or decades and the confounding effects of social, cultural, educational, and behavioral factors, among others [1–9]. It is even more difficult to link someone’s neurodevelopmental abnormalities to the GA exposure of their parents, who have undergone a relatively short procedure(s) in the past. Moreover, the exposed parents may not exhibit symptoms that can be easily identified as those associated with GA exposure. Animal studies, which have the advantage of strictly controlled experimental conditions, strongly support multigenerational effects of stress, EDCs, and other environmental factors and implicate epigenetic alterations in the germline as a mediator of such heritable effects [10–16, 129, 133–144].
Such germline epigenetic mechanisms include changes in DNA methylation, histone modifications, and noncoding RNAs [10–16, 129, 133–144]. The relatively long-lasting stability of DNA methylation marks supports the possibility that DNA methyltransferase (DNMT)-regulated DNA methylation within a 5′-cytosine-phosphate-guanine-3′ (CpG) sequence plays a role in epigenetic inheritance [133–136]. The DNA methyltransferase family includes DNA methyltransferase 1 (DNMT1) and DNA methyltransferases 3A and 3B (DNMT3A/3B). DNMT1 is responsible for maintaining DNA methylation during the DNA replication process by reproducing the methylation patterns in newly synthesized nonmethylated strands of DNA, as DNMT1 prefers hemimethylated DNA. The activities of DNMT3A/3B, on the other hand, are modulated by internal and external factors so they belong to de novo DNMTs [148].
Findings of differentially methylated regions in multiple genes in the spermatozoa of adult patients just 1 week after bariatric surgery to treat obesity suggest that GA/surgery, not just changes in obesity, could induce DNMT-regulated changes in the methylome of human germ cells [55]. Our studies in laboratory animals, in which rats were exposed to sevoflurane only, without surgery or any other interventions, further support the possibility that GAs may induce changes in DNA methylation patterns in parental germ cells. The findings also indicate that similar DNA methylation patterns may be detected in the same gene in the brains of future offspring who were not exposed. Importantly, the offspring also exhibited altered expression of the same gene in the brain, as well as neurobehavioral deficiencies [58–60].
In these initial studies, we tested whether intergenerational neurobehavioral effects of sevoflurane were accompanied by changes in DNA methylation in the Kcc2 gene promoter in parental spermatozoa and ovarian cells (the latter were studied as a surrogate of female germ cells) and in the brains of their offspring [58–60]. We chose changes in the Kcc2 gene DNA methylation patterns as a potential epigenomic biomarker because of our previous experimental findings that sevoflurane-induced E2-dependent changes in Kcc2 gene expression may be linked to neurobehavioral effects of sevoflurane in the GA-exposed animals [58, 59, 105–107].
In one of these studies [59], male and female rats (generation F0) were exposed to 2.1% SEVO for 3 h on 3 alternating days beginning on P56 and then mated 25 days later to generate offspring (generation F1) of all four possible combinations of sevoflurane-exposed and control sires and dams. The F0 rats were the same rats whose somatic acute and long-term neuroendocrine and behavioral abnormalities induced by sevoflurane are discussed in detail in the preceding section of this minireview [59]. Consistent with stress-like effects of sevoflurane, F0 male and female rats had similar multifold increases in systemic levels of corticosterone 1 h after the last sevoflurane exposure on P60. In parallel with similar acute stress-like responses to sevoflurane, the F0 male and female rats had a similarly hypermethylated Kcc2 gene promoter in sperm and ovarian tissue, respectively, measured >2 months after GA exposure [59]. Although only exposed F0 males, not F0 females, exhibited significant long-term neurobehavioral deficiencies, both sevoflurane-exposed F0 males and females passed behavioral abnormalities to F1 males [59]. The Kcc2 gene was also hypermethylated and exhibited reduced expression in the hypothalamus and hippocampus of adult F1 male, offspring of the exposed parents [59]. The F1 male, offspring of parents exposed to the anesthetic in young adulthood exhibited neurobehavioral deficiencies during the elevated plus maze (EPM) and prepulse inhibition (PPI) of acoustic startle response tests [59].
The only detected significant somatic changes in sevoflurane-exposed F0 females were acute increases in systemic levels of corticosterone. This finding allows us to hypothesize that sevoflurane-induced upregulation of the HPA axis and corticosterone secretion at the time of anesthesia are involved in the mediation of sevoflurane-initiated epigenetic changes in the parental germlines (Figure 1). We also speculate that the threshold for parental sevoflurane exposure to induce epigenomic changes in the F0 germline and F1 epigenomic, transcriptomic, and neurobehavioral defects, is lower than that needed to induce F0 neurobehavioral defects. This idea is supported by the lack of significant long-term changes in measured neurobehavioral parameters in the exposed F0 young adult female rats [59].
In our other study, F0 male and female rats were exposed to 6 h of anesthesia with 2.1% sevoflurane on P5 [58]. On ~P90, the exposed and control F0 rats were used as breeders to produce F1 offspring of four possible combinations of exposed and control sires and dams. As we discussed in the preceding section, sevoflurane and propofol cause acute increases in systemic levels of corticosterone and E2 in rat pups of both sexes (Figure 1) [66–69]. Notably, bisulfite sequencing revealed that sevoflurane also affected the same region in the Kcc2 gene in germ cells of F0 rats neonatally exposed to sevoflurane, similar to those that were exposed to the GA in young adulthood. Adult rats neonatally exposed to sevoflurane had increased CpG site methylation in the Kcc2 promoter in sperm, with the same trend in F0 ovaries (although the latter changes were not sufficient to achieve significance) [58]. Similar to the young adult study, F1 male, offspring of parents neonatally exposed to sevoflurane had increased methylation in the same region of the Kcc2 gene in the brain.
The methylation in the Kcc2 promoter region in the hippocampus of F1 male offspring of parents that were both exposed was more profound than other groups of F1 males. Notably, F1 male offspring of parents that were both exposed was the only group of F1 males with a significant decrease in the hippocampal Kcc2 mRNA level and increase in Nkcc1/Kcc2 mRNA ratio [58]. This group of F1 males was the only F1 male group that exhibited abnormalities in spatial memory during the Morris water maze test, the behavioral paradigm during which animal behavior closely correlates with hippocampal synaptic plasticity [149].
Interestingly, the results of this initial study suggest that the effects of early-life parental exposure to sevoflurane in F1 male offspring depend on whether a sire only, a mother only, or both parents are neonatally exposed to the anesthetic. F1 males of all combinations of exposed and control parents had a hypermethylated Kcc2 promoter gene in the hypothalamic PVN, similar to the F1 male hippocampus. However, in contrast to the F1 male hippocampus, where F1 male offspring of both exposed parents had the most profound increase in Kcc2 promoter methylation, the F1 males of the exposed sires/control mother were the most affected group in terms of the hypothalamic PVN Kcc2 promoter methylation [58]. The F1 male offspring of all combinations of exposed parents had similarly reduced Kcc2 expression in the hypothalamic PVN, but the F1 progeny of exposed sires/control mothers was the only group that had an increased expression of the Nkcc1 gene in the PVN. The resulting Nkcc1/Kcc2 mRNA ratios in the hypothalamic PVN were increased in the F1 male offspring of a single exposed parent, but not in F1 males of both exposed parents. Only F1 male progeny of exposed sires/control mothers exhibited significant alterations in behavior during the EPM and PPI of acoustic startle response tests [58]. If further confirmed, these findings suggest that a reduction in the expression of the hypothalamic PVN Kcc2 gene and resulting increase in the hypothalamic PVN Nkcc1/Kcc2 mRNA ratio are not sufficient to explain deficiencies in behavior during the EPM and PPI of acoustic startle response tests in F1 male offspring of parents neonatally exposed to sevoflurane.
The neurobehavioral abnormalities during the EPM and PPI tests in adult F0 males neonatally exposed to sevoflurane and in their adult F1 male offspring were similar. However, adult F1 male offspring, in contrast to their fathers neonatally exposed to sevoflurane, responded to physical restraint with corticosterone release not different than that in F1 male offspring of control parents [58]. Findings of normal corticosterone responses to stress in F1 male offspring of the exposed parents, coupled with the effects of GABAergic anesthetics in the exposed F0 rats, suggest that GABAergic GA-induced modulation of GABAAR signaling through reduced expression of Kcc2 may be required but not sufficient to support exacerbated corticosterone responses to stress in adulthood. It will be important to test whether such differences in corticosterone responses to stress occur because of differently affected T/E2-dependent mechanisms in F1 male offspring than in their F0 parents at the time of sevoflurane exposure [69].
The possibility that sex steroids are involved in the mediation of intergenerational effects of sevoflurane is supported by the fact that the next-generation effects of early-life parental exposure to sevoflurane were sex dependent. Although male and female F0 animals were affected, only F1 males exhibited significant abnormalities at both molecular and systemic levels [58]. Similarly, parental exposure to stress may affect the offspring of one sex or both, depending on the stress paradigm and/or parental age at the time of stress exposure [16, 144, 150–154]. Despite many years of research by multiple groups, the exact mechanisms by which preconception parental stress induces germ cell epigenetic changes, how these changes translate into the reprogramming of offspring phenotypes, and why offspring of a specific sex are selectively affected remain largely unknown. Understanding such mechanisms is essential for elucidating the etiology of mental health disorders, which are often sex specific. For example, females are more likely to suffer from depression and anxiety, while neurodevelopmental disorders, including early-onset schizophrenia, autism spectrum disorder (ASD), and attention-deficit/hyperactivity disorder, are disproportionally diagnosed in males [150–155].
The findings that F1 male offspring of sevoflurane-exposed sires/control dams had a more profoundly methylated hypothalamic Kcc2 gene promoter than F1 male offspring of both exposed parents are counterintuitive. Nonetheless, findings of different heritable effects of parental experiences depending on whether both parents or a single parent was exposed are not unique to this study. For example, adult offspring of parents with paternal posttraumatic stress disorder (PTSD) showed higher DNA methylation of the exon 1F promoter of the glucocorticoid receptor (GR-1F) gene (NR3C1) in peripheral blood mononuclear cells than offspring with both maternal and paternal PTSD [8]. In future studies, it will be important to test whether stress-like effects of sevoflurane induce similar intergenerational alterations in DNA methylation in the GR gene. The sex-, brain region-, and behavioral paradigm-specific effects in F1 offspring depending on which parent was neonatally exposed to sevoflurane may provide additional guidance in investigating the mechanisms of intergenerational transmission of early-life anesthetic exposure.
Our findings also demonstrate that in addition to a downregulated Kcc2 gene in F0 and F1 males, sires neonatally exposed to sevoflurane and their F1 male offspring have a significantly upregulated expression of hypothalamic Dnmt3a and Dnmt3b [60]. The preventive effects of pretreatment with the nonselective DNMT inhibitor decitabine (5-aza-2′-deoxycytidine) in sevoflurane-exposed sires and in their unexposed male offspring suggest that DNA methylation-based mechanisms are involved in the mediation of the F0 germ cell effects and the F0 somatic (neurobehavioral) effects of neonatal exposure to sevoflurane. The findings of somatic DNMT-involved effects of sevoflurane in neonatal rats are in agreement with earlier findings of Ju and colleagues [156]. They reported that decitabine diminished a sevoflurane-induced increase in the expression of hippocampal DNMT 3A/B, methylation of the brain-derived neurotrophic factor (Bdnf) gene, and reduction in levels of methyl CpG-binding protein 2 (MeCP2), as well as behavioral abnormalities [156]. Although they did not measure effects of decitabine on sevoflurane-induced reduction in KCC2 levels, the changes in levels of MeCP2 and KCC2 may be linked. For example, in Rett syndrome, a severe form of ASD, a KCC2 downregulation linked to deficiency in MeCP2, may play a role in the pathophysiology of the disease [157–159].
Interestingly, an environmental EDC, bisphenol A, depresses Kcc2 expression in developing rodent and human cortical neurons through decitabine-sensitive mechanisms [160]. This finding is potentially relevant to sevoflurane-induced DNMT-mediated changes in Kcc2 levels, especially considering that sevoflurane can induce EDC-like effects. Notably, the decitabine-sensitive long-term neurobehavioral abnormalities in the two generations were not identical [60]. Pretreatment with decitabine prevented sevoflurane-induced exacerbated corticosterone responses to stress in the exposed F0 male rats 60]. However, such corticosterone responses to stress in the F1 male offspring of the exposed sires were not different from those in the F1 male offspring of the unexposed parents [58]. Therefore, altered DNA methylation may be needed, but not sufficient, to mediate all intergenerational effects of neonatal parental exposure to sevoflurane, at least as it relates to abnormal functioning of the HPA axis in F1 male offspring.
Our findings demonstrate that sevoflurane may induce similar intergenerational effects when administered to parents during the early postnatal period or during young adulthood. These findings are consistent with other reports of heritable effects of GAs or stress when administered to parents of different ages. For example, in an earlier study, 11-week-old male mice were exposed for 4 hr on 5 alternate days to 2% enflurane, an anesthetic agent whose polyvalent actions include enhancement of GABAAR signaling similar to sevoflurane. Eight days later, the exposed males were bred with naïve females to generate offspring. The 7-week-old offspring of exposed sires exhibited deficiencies in a Rosensweig maze behavioral paradigm [51]. On the other hand, a recent study in rats confirmed that sevoflurane can induce intergenerational effects when administered during the early postnatal period [61]. Data in the literature indicate that chronic stress administered to male mice during different age periods, specifically during the pubertal window or in adulthood, induced similar abnormalities in offspring [16].
Along with similarities between intergenerational effects of sevoflurane administered during early postnatal age and young adulthood, there were important differences between such effects. Parental exposure to sevoflurane at both ages induced similar sex-dependent effects in F1 male offspring, including changes in expressions of the hypothalamic and hippocampal Kcc2 gene, behavioral abnormalities during the EPM and PPI of the acoustic startle response tests, and a lack of exacerbated corticosterone responses to physical restraint [58, 59]. F1 males of neonatally exposed fathers/control mothers were the only F1 males that exhibited abnormalities during the EPM and PPI behavioral tests. By contrast, exposure to sevoflurane in young adulthood led to the most consistent behavioral changes during the EPM and PPI of acoustic startle response tests in F1 males of both exposed parents and to changes in hypothalamic and hippocampal Kcc2 expressions [58, 59]. Furthermore, the hypothalamic and hippocampal Kcc2 gene expressions were not affected in F1 males of control fathers/exposed mothers, but they exhibited significant deficiencies during the EPM and PPI of acoustic startle response tests [58]. These findings, along with findings of intergenerational effects of neonatal parental exposure to sevoflurane, suggest that the systemic intergenerational effects of sevoflurane are mechanistically complex phenomena that cannot be explained by changes in a single Kcc2 gene. Further studies are needed to elucidate the involvement of stress and sex steroids in the mediation of the heritable effects of sevoflurane and other GAs, as well as whether changes in DNA methylation and other epigenetic marks transmit the effects of parental exposure to GAs to offspring.
Conclusion
Laboratory studies have raised many compelling questions about GA-induced adverse effects and their underlying mechanisms. The experimental findings support the notion that GABAergic GAs may act like environmental stressors and EDCs in neonates and young adults. These stress-like and EDC-like effects of GABAergic GAs, in particular sevoflurane, comprise acute and long-term neuroendocrine abnormalities in exposed rodents and epigenetic changes in the parental germ cell genome, as well as epigenomic, transcriptomic, and behavioral abnormalities in offspring. Male offspring may be more vulnerable to adverse effects of parental exposure to sevoflurane than their female counterparts. Sevoflurane may induce similar intergenerational effects when administered to parents over a wide range of parental ages at the time of exposure. Additionally, the effects may occur over a wide range of time periods from when the parent is exposed to the anesthetic to when they mate to generate F1 offspring. Further laboratory and clinical studies of these translationally important heritable effects of GAs are required. A greater understanding of this phenomenon could ultimately help establish safer outcomes of GA exposure. More generally, answers to these questions may help to elucidate whether intergenerational effects of parental exposure to GAs are a contributing factor in the increasing number of neuropsychiatric disorders of unknown etiology.
Conflicts of interest: The authors have declared no conflicts of interest.
Author contributions
A.E.M., L.-S.J., and T.M. conducted literature review and analysis, drafted and critically revised the manuscript, and gave final approval.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Footnotes
✝ Grant Support: Supported in part by the National Institutes of Health (R01NS091542, R01NS091542-S, and R56HD102898 to A.E.M.), the Escher Autism Fund (A.E.M.), and the I. Heermann Anesthesia Foundation (L.-S.J.).
Contributor Information
Anatoly E Martynyuk, Department of Anesthesiology, University of Florida College of Medicine, Gainesville, FL, USA; McKnight Brain Institute, University of Florida College of Medicine, Gainesville, FL, USA.
Ling-Sha Ju, Department of Anesthesiology, University of Florida College of Medicine, Gainesville, FL, USA.
Timothy E Morey, Department of Anesthesiology, University of Florida College of Medicine, Gainesville, FL, USA.
References
- 1. Senaldi L, Smith-Raska M. Evidence for germline non-genetic inheritance of human phenotypes and diseases. Clin Epigenetics 2020; 12:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daskalakis NP, Xu C, Bader HN, Chatzinakos C, Weber P, Makotkine I, Lehrner A, Bierer LM, Binder EB, Yehuda R. Intergenerational trauma is associated with expression alterations in glucocorticoid- and immune-related genes. Neuropsychopharmacology 2021; 46:763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Letcher P, Greenwood CJ, Romaniuk H, Spry E, Macdonald JA, McAnally H, Thomson KC, Youssef G, Hutchinson D, McIntosh J, Sanson A, Ryan J et al. Adolescent and young adult mental health problems and infant offspring behavior: findings from a prospective intergenerational cohort study. J Affect Disord 2020; 272:521–528. [DOI] [PubMed] [Google Scholar]
- 4. Day GS, Cruchaga C, Wingo T, Schindler SE, Coble D, Morris JC. Association of acquired and heritable factors with intergenerational differences in age at symptomatic onset of Alzheimer disease between offspring and parents with dementia. JAMA Netw Open 2019; 2:e1913491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hendrix CL, Dilks DD, McKenna BG, Dunlop AL, Corwin EJ, Brennan PA. Maternal childhood adversity associates with frontoamygdala connectivity in neonates. Biol Psychiatry Cogn Neurosci Neuroimaging 2020; S2451-9022:30345–30341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sproul Bassett AM, Wood EK, Lindell SG, Schwandt ML, Barr CS, Suomi SJ, Higley JD. Intergenerational effects of mother's early rearing experience on offspring treatment and socioemotional development. Dev Psychobiol 2020; 62:920–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hipwell AE, Tung I, Northrup J, Keenan K. Transgenerational associations between maternal childhood stress exposure and profiles of infant emotional reactivity. Dev Psychopathol 2019; 31:887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yehuda R, Daskalakis NP, Lehrner A, Desarnaud F, Bader HN, Makotkine I, Flory JD, Bierer LM, Meaney MJ. Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. Am J Psychiatry 2014; 171:872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Curtis SW, Conneely KN, Marder ME, Terrell ML, Marcus M, Alicia K, Smith AK. Intergenerational effects of endocrine-disrupting compounds: a review of the Michigan polybrominated biphenyl registry. Epigenomics 2018; 10:845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gulyas L, Powell JR. Predicting the future: parental progeny investment in response to environmental stress cues. Front Cell Dev Biol 2019; 7:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koabel J, McNivens M, McKee P, Pautassi R, Bordner K, Nizhnikov M. The offspring of alcohol-exposed sires exhibit heightened ethanol intake and behavioral alterations in the elevated plus maze. Alcohol 2021; 92:65–72. [DOI] [PubMed] [Google Scholar]
- 12. Rompala GR, Simons A, Kihle B, Homanics GE. Paternal preconception chronic variable stress confers attenuated ethanol drinking behavior selectively to male offspring in a pre-stress environment dependent manner. Front Behav Neurosci 2018; 12:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Steenwyk G, Roszkowski M, Manuella F, Franklin TB, Mansuy IM. Transgenerational inheritance of behavioral and metabolic effects of paternal exposure to traumatic stress in early postnatal life: evidence in the 4th generation. Environ Epigenet 2018; 4:dvy023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Drobná Z, Henriksen AD, Wolstenholme JT, Montiel C, Lambeth PS, Shang S, Harris EP, Zhou C, Flaws JA, Adli M, Rissman EF. Transgenerational effects of bisphenol A on gene expression and DNA methylation of imprinted genes in brain. Endocrinology 2018; 159:132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dagher JB, Hahn-Townsend CK, Kaimal A, Mansi MA, Henriquez JE, Tran DG, Laurent CR, Bacak CJ, Buechter HE, Cambric C, Spivey J, Chuang YJ et al. Independent and combined effects of bisphenol A and diethylhexyl phthalate on gestational outcomes and offspring development in Sprague-Dawley rats. Chemosphere 2021; 263:128307. [DOI] [PubMed] [Google Scholar]
- 16. Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 2013; 33:9003–9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weiser TG, Haynes AB, Molina G, Lipsitz SR, Esquivel MM, Uribe-Leitz T, Fu R, Azad T, Chao TE, Berry WR, Gawande AA. Size and distribution of the global volume of surgery in 2012. Bull World Health Organ 2016; 94:201F–209F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wei Y, Zhang D, Liu J, Ou M, Liang P, Zuo Y, Zhou C. Effects of sevoflurane anesthesia and abdominal surgery on the systemic metabolome: a prospective observational study. BMC Anesthesiol 2021; 21:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang J, Yu P, Hua F, Hu Y, Xiao F, Liu Q, Huang D, Deng F, Wei G, Deng W, Ma J, Zhu W et al. Sevoflurane postconditioning reduces myocardial ischemia reperfusion injury-induced necroptosis by up-regulation of OGT-mediated O-GlcNAcylated RIPK3. Aging (Albany NY) 2020; 12:25452–25468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan XY, Shi G, Zhao P. Neonatal sevoflurane exposure impairs learning and memory by the hypermethylation of hippocampal synaptic genes. Mol Neurobiol 2021; 58:895–904. [DOI] [PubMed] [Google Scholar]
- 21. Nishikawa K, Harrison NL. The actions of sevoflurane and desflurane on the gamma-aminobutyric acid receptor type A: effects of TM2 mutations in the alpha and beta subunits. Anesthesiology 2003; 99:678–684. [DOI] [PubMed] [Google Scholar]
- 22. Stucke AG, Stuth EA, Tonkovic-Capin V. Effects of halothane and sevoflurane on inhibitory neurotransmission to medullary expiratory neurons in a decerebrate dog model. Anesthesiology 2002; 96:955–962. [DOI] [PubMed] [Google Scholar]
- 23. Vanlersberghe C, Camu F. Etomidate and other non-barbiturates. Handb Exp Pharmacol 2008; 182:267–282. [DOI] [PubMed] [Google Scholar]
- 24. Prete A, Yan Q, Al-Tarrah K, Akturk HK, Prokop LJ, Alahdab F, Foster MA, Lord JM, Karavitaki N, Wass JA, Murad MH, Arlt W et al. The cortisol stress response induced by surgery: a systematic review and meta-analysis. Clin Endocrinol (Oxf) 2018; 89:554–567. [DOI] [PubMed] [Google Scholar]
- 25. Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 1997; 389:385–389. [DOI] [PubMed] [Google Scholar]
- 26. Edipoglu IS, Celik F. The associations between cognitive dysfunction, stress biomarkers, and administered anesthesia type in total knee arthroplasties: prospective, randomized trial. Pain Physician 2019; 22:495–507. [PubMed] [Google Scholar]
- 27. Nakashima A, Koshiyama K, Uozumi T, Monden Y, Hamanaka Y. Effects of general anaesthesia and severity of surgical stress on serum LH and testosterone in males. Acta Endocrinol 1975; 78:258–269. [DOI] [PubMed] [Google Scholar]
- 28. Rains PC, Rampersad N, De Lima J, Murrell D, Kinchington D, Lee JW, Maguire AM, Donaghue KC. Cortisol response to general anaesthesia for medical imaging in children. Clin Endocrinol (Oxf) 2009; 71:834–839. [DOI] [PubMed] [Google Scholar]
- 29. Hsu AA, von Elten K, Chan D, Flynn T, Walker K, Barnhill J, Naun C, Pedersen AM, Ponaman M, Fredericks GJ, Crudo DF, Pinsker JE. Characterization of the cortisol stress response to sedation and anesthesia in children. J Clin Endocrinol Metab 2012; 97:E1830–E1835. [DOI] [PubMed] [Google Scholar]
- 30. Soules MR, Sutton GP, Hammond CB, Haney AF. Endocrine changes at operation under general anesthesia: reproductive hormone fluctuations in young women. Fertil Steril 1980; 33:364–371. [DOI] [PubMed] [Google Scholar]
- 31. Kaya A, Sogut E, Cayli S, Suren M, Arici S, Karaman S. Evaluation of effects of repeated sevoflurane exposure on rat testicular tissue and reproductive hormones. Inhal Toxicol 2013; 25:4. [DOI] [PubMed] [Google Scholar]
- 32. Chinn GA, Sasaki Russell JM, Yabut NA, Maharjan D, Sall JW. Androgenic modulation of the chloride transporter NKCC1 contributes to age-dependent isoflurane neurotoxicity in male rats. Anesthesiology 2020; 133:852–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sasaki Russell JM, Hagelstein M, Lee BH, Sall JW. Anesthesia-induced recognition deficit is improved in postnatally gonadectomized male rats. J Neurosurg Anesthesiol 2021; 33:273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lewis LD, Weiner VS, Mukamel EA, Donoghue JA, Eskandar EN, Madsen JR, Anderson WS, Hochberg LR, Cash SS, Brown EN, Purdon PL. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci USA 2012; 109:E3377–E3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brown EN, Pavone KJ, Naranjo M. Multimodal general anesthesia: Theory and practice. Anesth Analg 2018; 127:1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Culley DJ, Baxter M, Yukhananov R, Crosby G. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesth Analg 2003; 96:1004–1009. [DOI] [PubMed] [Google Scholar]
- 37. Crosby C, Culley DJ, Baxter MG, Yukhananov R, Crosby G. Spatial memory performance 2 weeks after general anesthesia in adult rats. Anesth Analg 2005; 101:1389–1392. [DOI] [PubMed] [Google Scholar]
- 38. Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology 2009; 110:834–848. [DOI] [PubMed] [Google Scholar]
- 39. Landin JD, Palac M, Carter JM, Dzumaga Y, Santerre-Anderson JL, Fernandez GM, Savage LM, Varlinskaya EI, Spear LP, Moore SD, Swartzwelder HS, Fleming RL et al. General anesthetic exposure in adolescent rats causes persistent maladaptations in cognitive and affective behaviors and neuroplasticity. Neuropharmacology 2019; 150:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Banerjee P, Rossi MG, Anghelescu DL, Liu W, Breazeale AM, Reddick WE, Glass JO, Phillips NS, Jacola LM, Sabin ND, Inaba H, Srivastava D et al. Association between anesthesia exposure and neurocognitive and neuroimaging outcomes in long-term survivors of childhood acute lymphoblastic leukemia. JAMA Oncol 2019; 5:1456–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jacola LM, Anghelescu DL, Hall L, Russell K, Zhang H, Wang F, Peters JB, Rossi M, Schreiber JE, Gajjar A. Anesthesia exposure during therapy predicts neurocognitive outcomes in survivors of childhood medulloblastoma. J Pediatr 2020; 223:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hu D, Flick RP, Zaccariello MJ, Colligan RC, Katusic SK, Schroeder DR, Hanson AC, Buenvenida SL, Gleich SJ, Wilder RT, Sprung J, Warner DO. Association between exposure of young children to procedures requiring general anesthesia and learning and behavioral outcomes in a population-based birth cohort. Anesthesiology 2017; 127:227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ing C, Sun M, Olfson M, DiMaggio CJ, Sun LS, Wall MM, Li G. Age at exposure to surgery and anesthesia in children and association with mental disorder diagnosis. Anesth Analg 2017; 125:1988–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Graham MR, Brownell M, Chateau DG, Dragan RD, Burchill C, Fransoo RR. Neurodevelopmental assessment in kindergarten in children exposed to general anesthesia before the age of 4 years: a retrospective matched cohort study. Anesthesiology 2016; 125:667–677. [DOI] [PubMed] [Google Scholar]
- 45. Sun LS, Li G, Miller TL, Salorio C, Byrne MW, Bellinger DC, Ing C, Park R, Radcliffe J, Hays SR, DiMaggio CJ, Cooper TJ et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA 2016; 315:2312–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Davidson AJ, Disma N, de Graaff JC, Withington DE, Dorris L, Bell G, Stargatt R, Bellinger DC, Schuster T, Arnup SJ, Hardy P, Hunt RW et al. GAS consortium. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet 2016; 387:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post-operative cognitive dysfunction. Lancet 1998; 351:857–861. [DOI] [PubMed] [Google Scholar]
- 48. Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg 2005; 100:4–10. [DOI] [PubMed] [Google Scholar]
- 49. Zurek AA, Yu J, Wang DS, Haffey SC, Bridgwater EM, Penna A, Lecker I, Lei G, Chang T, Salter EW, Orser BA. Sustained increase in γ5GABAA receptor function impairs memory after anesthesia. J Clin Invest 2014; 124:5437–5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chalon J, Tang CK, Ramanathan S, Eisner M, Katz R, Turndorf H. Exposure to halothane and enflurane affects learning function of murine progeny. Anesth Analg 1981; 60:794–797. [PubMed] [Google Scholar]
- 51. Tang CK, Chalon J, Markham JP, Ramanathan S, Turndorf H. Exposure of sires to enflurane affects learning function of murine progeny. Anesth Analg 1984; 63:729–730. [PubMed] [Google Scholar]
- 52. Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vöckler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 1999; 283:70–74. [DOI] [PubMed] [Google Scholar]
- 53. Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 2003; 23:876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Escher J, Ford D. General anesthesia, germ cells and the missing heritability of autism: an urgent need for research. Environ Epigenet 2020; 6:dvaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Donkin I, Versteyhe S, Ingerslev LR, Qian K, Mechta M, Nordkap L, Mortensen B, Appel EV, Jørgensen N, Kristiansen VB, Hansen T, Workman CT et al. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab 2016; 23:369–378. [DOI] [PubMed] [Google Scholar]
- 56. Kotani N, Akaike N. The effects of volatile anesthetics on synaptic and extrasynaptic GABA-induced neurotransmission. Brain Res Bull 2013; 93:69–79. [DOI] [PubMed] [Google Scholar]
- 57. Ogawa SK, Tanaka E, Shin MC, Kotani N, Akaike N. Volatile anesthetic effects on isolated GABA synapses and extrasynaptic receptors. Neuropharmacology 2011; 60:701–710. [DOI] [PubMed] [Google Scholar]
- 58. Ju LS, Yang JJ, Morey TE, Gravenstein N, Seubert CN, Resnick JL, Zhang JQ, Martynyuk AE. Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats. Br J Anaesth 2018; 121:406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ju LS, Yang JJ, Xu N, Li J, Morey TE, Gravenstein N, Seubert CN, Setlow B, Martynyuk AE. Intergenerational effects of sevoflurane in young adult rats. Anesthesiology 2019; 131:1092–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xu N, Lei L, Lin Y, Ju LS, Morey TE, Gravenstein N, Yang JJ, Martynyuk AE. A methyltransferase inhibitor (decitabine) alleviates intergenerational effects of paternal neonatal exposure to anesthesia with sevoflurane. Anesth Analg 2020; 131:1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chastain-Potts SE, Tesic V, Tat QL, Cabrera OH, Quillinan N, Jevtovic-Todorovic V. Sevoflurane exposure results in sex-specific transgenerational upregulation of target IEGs in the subiculum. Mol Neurobiol 2020; 57:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zablotsky B, Black L, Maenner M, Schieve LA, Danielson ML, Bitsko RH, Blumberg SJ, Kogan MD, Boyle CA. Prevalence and trends of developmental disabilities among children in the United States: 2009-2017. Pediatrics 2019; 144:e20190811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Reiner RC Jr, Olsen HE, Ikeda CT et al. GBD 2017 child and adolescent health collaborators. Diseases, injuries, and risk factors in child and adolescent health, 1990 to 2017: findings from the global burden of diseases, injuries, and risk factors 2017 study. JAMA Pediatr 2019; 29:e190337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Arora NK, Nair MKC, Gulati S, Deshmukh V, Mohapatra A, Mishra D, Patel V, Pandey RM, Das BC, Divan G, Murthy GVS, Sharma TD et al. Neurodevelopmental disorders in children aged 2-9 years: population-based burden estimates across five regions in India. PLoS Med 2018; 15:e1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Robinson Rosenberg C, White T, Durkin MS, Imm P et al. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ 2018; 67:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tan S, Xu C, Zhu W, Willis J, Seubert CN, Gravenstein N, Sumners C, Martynyuk AE. Endocrine and neurobehavioral abnormalities induced by propofol administered to neonatal rats. Anesthesiology 2014; 121:1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xu C, Tan S, Zhang J, Seubert CN, Gravenstein N, Sumners C, Vasilopoulos T, Martynyuk AE. Anesthesia with sevoflurane in neonatal rats: developmental neuroendocrine abnormalities and alleviating effects of the corticosteroid and cl(−) importer antagonists. Psychoneuroendocrinology 2015; 60:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang J, Xu C, Puentes DL, Seubert CN, Gravenstein N, Martynyuk AE. Role of steroids in hyperexcitatory adverse and anesthetic effects of sevoflurane in neonatal rats. Neuroendocrinology 2016; 103:440–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li N, Xu N, Lin Y, Lei L, Ju L-S, Morey TE, Gravenstein N, Zhang J, Martynyuk AE. Roles of testosterone and estradiol in mediation of acute neuroendocrine and electroencephalographic effects of sevoflurane during the sensitive period in rats. Front Endocrinol 2020; 11:545973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McCarthy MM. The two faces of estradiol: effects on the developing brain. Neuroscientist 2009; 15:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McCarthy MM, Nugent BM, Lenz KM. Neuroimmunology and neuroepigenetics in the establishment of sex differences in the brain. Nat Rev Neurosci 2017; 18:471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Konkle AT, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology 2011; 152:223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brock O, De Mees C, Bakker J. Hypothalamic expression of oestrogen receptor α and androgen receptor is sex-, age- and region-dependent in mice. J Neuroendocrinol 2015; 27:264–276. [DOI] [PubMed] [Google Scholar]
- 74. Fan X, Xu H, Warner M, Gustafsson JA. ERbeta in CNS: new roles in development and function. Prog Brain Res 2010; 181:233–250. [DOI] [PubMed] [Google Scholar]
- 75. Hara Y, Waters EM, McEwen BS, Morrison JH. Estrogen effects on cognitive and synaptic health over the life course. Physiol Rev 2015; 95:785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci 2011; 14:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav 1997; 31:232–243. [DOI] [PubMed] [Google Scholar]
- 78. Ogawa S, Eng V, Taylor J, Lubahn DB, Korach K, Pfaf DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology 1998; 139:5070–5081. [DOI] [PubMed] [Google Scholar]
- 79. Khbouz B, de Bournonville C, Court L, Taziaux M, Corona R, Arnal JF, Lenfant F, Cornil CA. Role for the membrane estrogen receptor alpha in the sexual differentiation of the brain. Eur J Neurosci 2020; 52:2627–2645. [DOI] [PubMed] [Google Scholar]
- 80. Zafer D, Aycan N, Ozaydin B, Kemanli P, Ferrazzano P, Levine JE, Cengiz P. Sex differences in hippocampal memory and learning following neonatal brain injury: is there a role for estrogen receptor-α? Neuroendocrinology 2019; 109:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wu MV, Tollkuhn J. Estrogen receptor alpha is required in GABAergic, but not glutamatergic, neurons to masculinize behavior. Horm Behav 2017; 95:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McCarthy MM, Herold H, Stockman SL. Fast, furious and enduring: sensitive versus critical periods in sexual differentiation of the brain. Physiol Behav 2018; 187:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist 2012; 18:467–486. [DOI] [PubMed] [Google Scholar]
- 84. Ben-Ari Y. The GABA excitatory/inhibitory developmental sequence: a personal journey. Neuroscience 2014; 279:187–219. [DOI] [PubMed] [Google Scholar]
- 85. Khazipov R, Valeeva G, Khalilov I. Depolarizing GABA and developmental epilepsies. CNS Neurosci Ther 2015; 21:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ito S. GABA and glycine in the developing brain. J Physiol Sci 2016; 66:375–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Farrant M, Kaila K. The cellular, molecular and ionic basis of GABAA receptor signaling. Prog Brain Res 2007; 160:59–87. [DOI] [PubMed] [Google Scholar]
- 88. Dehorter N, Vinay L, Hammond C, Ben-Ari Y. Timing of developmental sequences in different brain structures: physiological and pathological implications. Eur J Neurosci 2012; 35:1846–1856. [DOI] [PubMed] [Google Scholar]
- 89. Merner N, Chandler M, Bourassa C, Liang B, Khanna A, Dion P, Rouleau G, Kahle K. Regulatory domain or CpG site variation in SLC12A5, encoding the chloride transporter KCC2, in human autism and schizophrenia. Front Cell Neurosci 2015; 9:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med 2005; 11:1205–1213. [DOI] [PubMed] [Google Scholar]
- 91. Masuda K, Yamaguchi T. Abnormal air-righting reflex in striatal rats. Jpn J Physiol 2000; 50:163–166. [DOI] [PubMed] [Google Scholar]
- 92. Musienko PE, Zelenin PV, Lyalka VF, Orlovsky GN, Deliagina TG. Postural performance in decerebrated rabbit. Behav Brain Res 2008; 190:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nuñez JL, McCarthy MM. Estradiol exacerbates hippocampal damage in a model of preterm infant brain injury. Endocrinology 2003; 144:2350–2359. [DOI] [PubMed] [Google Scholar]
- 94. Melón LC, Maguire J. GABAergic regulation of the HPA and HPG axes and the impact of stress on reproductive function. J Steroid Biochem Mol Biol 2016; 160:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bhattarai JP, Park SA, Park JB, Lee SY, Herbison AE, Ryu PD, Han SK. Tonic extrasynaptic GABA(A) receptor currents control gonadotropin-releasing hormone neuron excitability in the mouse. Endocrinology 2011; 152:1551–1561. [DOI] [PubMed] [Google Scholar]
- 96. DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type gamma-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol 2002; 16:2872–2891. [DOI] [PubMed] [Google Scholar]
- 97. Yin C, Ishii H, Tanaka N, Sakuma Y, Kato M. Activation of A-type gamma-amino butyric acid receptors excites gonadotrophin-releasing hormone neurones isolated from adult rats. J Neuroendocrinol 2008; 20:566–575. [DOI] [PubMed] [Google Scholar]
- 98. Prange-Kiel J, Jarry H, Schoen M, Kohlmann P, Lohse C, Zhou L, Rune GM. Gonadotropin-releasing hormone regulates spine density via its regulatory role in hippocampal estrogen synthesis. J Cell Biol 2008; 180:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mody I, Maguire J. The reciprocal regulation of stress hormones and GABA(A) receptors. Front Cell Neurosci 2012; 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kakizawa K, Watanabe M, Mutoh H, Okawa Y, Yamashita M, Yanagawa Y, Itoi K, Suda T, Oki Y, Fukuda A. A novel GABA-mediated corticotropin-releasing hormone secretory mechanism in the median eminence. Sci Adv 2016; 2:e1501723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience 2009; 159:883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci 2006; 26:1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Edwards DA, Shah HP, Cao W, Gravenstein N, Seubert CN, Martynyuk AE. Bumetanide alleviates epileptogenic and neurotoxic effects of sevoflurane in neonatal rat brain. Anesthesiology 2010; 112:567–575. [DOI] [PubMed] [Google Scholar]
- 104. Cao W, Pavlinec C, Gravenstein N, Seubert CN, Martynyuk AE. Roles of aldosterone and oxytocin in abnormalities caused by sevoflurane anesthesia in neonatal rats. Anesthesiology 2012; 117:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Seubert CN, Zhu W, Pavlinec C, Gravenstein N, Martynyuk AE. Developmental effects of neonatal isoflurane and sevoflurane exposure in rats. Anesthesiology 2013; 119:358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ju LS, Yang JJ, Gravenstein N, Seubert CN, Morey TE, Sumners C, Vasilopoulos T, Yang JJ, Martynyuk AE. Role of environmental stressors in determining the developmental outcome of neonatal anesthesia. Psychoneuroendocrinology 2017; 81:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yang J, Ju L, Jia M, Zhang H, Sun X, Ji M, Yang J, Martynyuk AE. Subsequent maternal separation exacerbates neurobehavioral abnormalities in rats neonatally exposed to sevoflurane anesthesia. Neurosci Lett 2017; 661:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lin Y, Lei L, Ju LS, Xu N, Morey TE, Gravenstein N, Yang J, Martynyuk AE. Neonatal exposure to sevoflurane expands the window of vulnerability to adverse effects of subsequent exposure to sevoflurane and alters hippocampal morphology via decitabine-sensitive mechanisms. Neurosci Lett 2020; 735:135240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang J, Yang B, Ju LS, Yang JJ, Allen A, Zhang JQ, Martynyuk AE. The estradiol synthesis inhibitor formestane diminishes the ability of sevoflurane to induce neurodevelopmental abnormalities in male rats. Front Syst Neurosci 2020; 14:546531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Liu G, Zhu T, Zhang A, Li F, Qian W, Qian B. Heightened stress response and cognitive impairment after repeated neonatal sevoflurane exposures might be linked to excessive GABAAR-mediated depolarization. J Anesth 2016; 30:834–841. [DOI] [PubMed] [Google Scholar]
- 111. Sasaki Russell JM, Chinn GA, Maharjan D, Eichbaum Y, Sall JW. Female rats are more vulnerable to lasting cognitive impairment after isoflurane exposure on postnatal day 4 than 7. Br J Anaesth 2019; 122:490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cabrera OH, Tesic V, Tat QL, Chastain S, Quillinan N, Jevtovic-Todorovic V. Sevoflurane-induced dysregulation of cation-chloride cotransporters NKCC1 and KCC2 in neonatal mouse brain. Mol Neurobiol 2020; 57:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Furukawa M, Tsukahara T, Tomita K, Iwai H, Sonomura T, Miyawaki S, Sato T. Neonatal maternal separation delays the GABA excitatory-to-inhibitory functional switch by inhibiting KCC2 expression. Biochem Biophys Res Commun 2017; 493:1243–1249. [DOI] [PubMed] [Google Scholar]
- 114. Patchev VK, Montkowski A, Rouskova D, Koranyi L, Holsboer F, Almeida OFA. Neonatal treatment of rats with the neuroactive steroid tetrahydrodeoxycorticosterone (THDOC) abolishes the behavioral and neuroendocrine consequences of adverse early life events. J Clin Invest 1997; 99:962–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci 2005; 25:9328–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. O'Malley D, Dinan TG, Cryan JF. Neonatal maternal separation in the rat impacts on the stress responsivity of central corticotropin-releasing factor receptors in adulthood. Psychopharmacology (Berl) 2011; 214:221–229. [DOI] [PubMed] [Google Scholar]
- 117. Veerawatananan B, Surakul P, Chutabhakdikul N. Maternal restraint stress delays maturation of cation-chloride cotransporters and GABAA receptor subunits in the hippocampus of rat pups at puberty. Neurobiol Stress 2015; 3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gao Y, Zhou JJ, Zhu Y, Kosten T, Li DP. Chronic unpredictable mild stress induces loss of GABA inhibition in corticotrophin-releasing hormone-expressing neurons through NKCC1 upregulation. Neuroendocrinology 2017; 104:194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ostroumov A, Thomas AM, Kimmey BA, Karsch JS, Doyon WM, Dani JA. Stress increases ethanol self-administration via a shift toward excitatory GABA signaling in the ventral tegmental area. Neuron 2016; 92:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Thomas AM, Ostroumov A, Kimmey BA, Taormina MB, Holden WM, Kim K, Brown-Mangum T, Dani JA. Adolescent nicotine exposure alters GABAA receptor signaling in the ventral tegmental area and increases adult ethanol self-administration. Cell Rep 2018; 23:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Fukuda A, Watanabe M. Pathogenic potential of human SLC12A5 variants causing KCC2 dysfunction. Brain Res 2019; 1710:1–7. [DOI] [PubMed] [Google Scholar]
- 122. Genç F, Kara M, Ünal Y, Uygur Küçükseymen E, Biçer Gömceli Y, Kaynar T, Tosun K, Kutlu G. Methylation of cation-chloride cotransporters NKCC1 and KCC2 in patients with juvenile myoclonic epilepsy. Neurol Sci 2019; 40:1007–1013. [DOI] [PubMed] [Google Scholar]
- 123. Han P, Welsh CT, Smith MT, Schmidt RE, Carroll SL. Complex patterns of GABAergic neuronal deficiency and type 2 potassium-chloride cotransporter immaturity in human focal cortical dysplasia. Neuropathol Exp Neurol 2019; 78:365–372. [DOI] [PubMed] [Google Scholar]
- 124. Luscher B, Fuchs T. GABAergic control of depression-related brain states. Adv Pharmacol 2015; 73:97–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Miller S, Maguire J. Deficits in KCC2 and activation of the HPA axis lead to depressionlike behavior following social defeat. Hormonal Studies 2014; 2:2. http://www.hoajonline.com/journals/pdf/2052-8000-2-2.pdf. [Google Scholar]
- 126. Tsukahara T, Masuhara M, Iwai H, Sonomura T, Sato T. The effect of repeated stress on KCC2 and NKCC1 immunoreactivity in the hippocampus of female mice. Data Brief 2016; 6:521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Haam J, Popescu IR, Morton LA, Halmos KC, Teruyama R, Ueta Y, Tasker JG. GABA is excitatory in adult vasopressinergic neuroendocrine cells. J Neurosci 2012; 32:572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kanaka C, Ohno K, Okabe A, Kuriyama K, Itoh T, Fukuda A, Sato K. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience 2001; 104:933–946. [DOI] [PubMed] [Google Scholar]
- 129. Yohn NL, Bartolomei MS, Blendy JA. Multigenerational and transgenerational inheritance of drug exposure: the effects of alcohol, opiates, cocaine, marijuana, and nicotine. Prog Biophys Mol Biol 2015; 118:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Fant B, Wimmer ME, Swinford-Jackson SE, Maurer J, Van Nest D, Pierce RC. Preconception maternal cocaine self-administration increases the reinforcing efficacy of cocaine in male offspring. Psychopharmacology (Berl) 2019; 236:3429–3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kaati G, Olov Bygren L, Pembry M, Sjostrom M. Transgenerational response to nutrition, early life circumstances and longevity. Eur J Hum Genet 2007; 15:784–790. [DOI] [PubMed] [Google Scholar]
- 132. Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S, Rabinowitz J, Shulman C, Malaspina D, Lubin G, Knobler HY, Davidson M et al. Advancing paternal age and autism. Arch Gen Psychiatry 2006; 63:1026–1032. [DOI] [PubMed] [Google Scholar]
- 133. Stenz L, Schechter DS, Serpa SR, Paoloni-Giacobino A. Intergenerational transmission of DNA methylation signatures associated with early life stress. Curr Genomics 2018; 19:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mychasiuk R, Harker A, Ilnytskyy S, Gibb R. Paternal stress prior to conception alters DNA methylation and behavior of developing rat offspring. Neuroscience 2013; 241:100–105. [DOI] [PubMed] [Google Scholar]
- 135. Niknazar S, Nahavandi A, Peyvandi AA, Peyvandi H, Roozbahany NA, Abbaszadeh HA. Hippocampal NR3C1 DNA methylation can mediate part of preconception paternal stress effects in rat offspring. Behav Brain Res 2017; 324:71–76. [DOI] [PubMed] [Google Scholar]
- 136. Liu Y, Wang L, Zhu L, Ran B, Wang Z. Bisphenol A disturbs transcription of steroidogenic genes in ovary of rare minnow Gobiocypris rarus via the abnormal DNA and histone methylation. Chemosphere 2020; 240:124935. [DOI] [PubMed] [Google Scholar]
- 137. Cacciola G, Chioccarelli T, Altucci L, Ledent C, Mason JI, Fasano S, Pierantoni R, Cobellis G. Low 17beta-estradiol levels in CNR1 knock-out mice affect spermatid chromatin remodeling by interfering with chromatin reorganization. Biol Reprod 2013; 88:152. [DOI] [PubMed] [Google Scholar]
- 138. Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A, Vizi S, Mansuy IM. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry 2010; 68:408–415. [DOI] [PubMed] [Google Scholar]
- 139. Baxter FA, Drake AJ. Non-genetic inheritance via the male germline in mammals. Philos Trans R Soc Lond B Biol Sci 2019; 374:20180118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Faraji J, Karimi M, Soltanpour N, Rouhzadeh Z, Roudaki S, Hosseini SA, Jafari SY, Abdollahi AA, Soltanpour N, Moeeini R, Metz GAS. Intergenerational sex-specific transmission of maternal social experience. Sci Rep 2018; 8:10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Beeler E, Nobile ZL, Homanics GE. Paternal preconception every-other-day ethanol drinking alters behavior and ethanol consumption in offspring. Brain Sci 2019; 9:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci 2011; 31:11748–11755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci USA 2015; 112:13699–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Rompala GR, Finegersh A, Slater M, Homanics GE. Paternal preconception alcohol exposure imparts intergenerational alcohol-related behaviors to male offspring on a pure C57BL/6J background. Alcohol 2017; 60:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Gui B, Gui F, Takai T, Feng C, Bai X, Fazli L, Dong X, Liu S, Zhang X, Zhang W, Kibel AS, Jia L. Selective targeting of PARP-2 inhibits androgen receptor signaling and prostate cancer growth through disruption of FOXA1 function. Proc Natl Acad Sci USA 2019; 116:14573–14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet 2011; 43:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kulik M, Bothe M, Kibar G, Fuchs A, Schöne S, Prekovic S, Mayayo-Peralta I, Chung HR, Zwart W, Helsen C, Claessens F, Meijsing SH. Androgen and glucocorticoid receptor direct distinct transcriptional programs by receptor-specific and shared DNA binding sites. Nucleic Acids Res 2021; 49:3856–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology 2013; 38:23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Vorhees CV, Williams MT. Value of water mazes for assessing spatial and egocentric learning and memory in rodent basic research and regulatory studies. Neurotoxicol Teratol 2014; 45:75–90. [DOI] [PubMed] [Google Scholar]
- 150. Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE, Nagaraja HN, Cooley WC, Gaelic SE, Bauman ML. Timing of prenatal stressors and autism. J Autism Dev Disord 2005; 35:471–478. [DOI] [PubMed] [Google Scholar]
- 151. Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress 2011; 14:348–356. [DOI] [PubMed] [Google Scholar]
- 152. May T, Adesina I, McGillivray J, Rinehart NJ. Sex differences in neurodevelopmental disorders. Curr Opin Neurol 2019; 32:622–626. [DOI] [PubMed] [Google Scholar]
- 153. Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry 2010; 22:417–428. [DOI] [PubMed] [Google Scholar]
- 154. Ferri SL, Abel T, Brodkin ES. Sex differences in autism spectrum disorder: a review. Curr Psychiatry Rep 2018; 20:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Romano E, Cosentino L, Laviola G, De Filippis B. Genes and sex hormones interaction in neurodevelopmental disorders. Neurosci Biobehav Rev 2016; 67:9–24. [DOI] [PubMed] [Google Scholar]
- 156. Ju LS, Jia M, Sun J, Sun XR, Zhang H, Ji MH, Yang JJ, Wang ZY. Hypermethylation of hippocampal synaptic plasticity-related genes is involved in neonatal sevoflurane exposure-induced cognitive impairments in rats. Neurotox Res 2016; 29:243–255. [DOI] [PubMed] [Google Scholar]
- 157. Tang X, Kim J, Zhou L, Wengert E, Zhang L, Wu Z, Carromeu C, Muotri AR, Marchetto MC, Gage FH, Chen G. KCC2 rescues functional deficits in human neurons derived from patients with Rett syndrome. Proc Natl Acad Sci USA 2016; 113:751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Banerjee A, Rikhye RV, Breton-Provencher V, Tang X, Li C, Li K, Runyan CA, Fu Z, Jaenisch R, Sur M. Jointly reduced inhibition and excitation underlies circuit-wide changes in cortical processing in Rett syndrome. Proc Natl Acad Sci USA 2016; 113:E7287–E7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Tang X, Drotar J, Li K, Clairmont CD, Brumm AS, Sullins AJ, Wu H, Liu XS, Wang J, Gray NS, Sur M, Jaenisch R. Pharmacological enhancement of KCC2 gene expression exerts therapeutic effects on human Rett syndrome neurons and Mecp2 mutant mice. Sci Transl Med 2019; 11:eaau0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Yeo M, Berglund K, Hanna M, Guo JU, Kittur J, Torres MD, Abramowitz J, Busciglio J, Gao Y, Birnbaumer L, Liedtke WB. Bisphenol a delays the perinatal chloride shift in cortical neurons by epigenetic effects on the Kcc2 promoter. Proc Natl Acad Sci USA 2013; 110:4315–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


