Abstract
Our understanding of the interactions between genetic and environmental factors in shaping behavioral phenotypes has expanded to include environment-induced epigenetic modifications and the intriguing possibility of their association with heritable behavioral phenotypes. The molecular basis of heritability of phenotypes arising from environment-induced epigenetic modifications is not well defined yet. However, phenomenological evidence in favor of it is accumulating rapidly. The resurgence of interest has led to focus on epigenetic modification of germ cells as a plausible mechanism of heritability. Perhaps partly because of practical reasons such as ease of access to male germ cells compared to female germ cells, attention has turned toward heritable effects of environmental influences on male founders. Public health implications of heritable effects of paternal exposures to addictive substances or to psycho-social factors may be enormous. Considering nicotine alone, over a billion people worldwide use nicotine-containing products, and the majority are men. Historically, the adverse effects of nicotine use by pregnant women received much attention by scientists and public policy experts alike. The implications of nicotine use by men for the physical and mental well-being of their children were not at the forefront of research until recently. Here, we review progress in the emerging field of heritable effects of paternal nicotine exposure and its implications for behavioral health of individuals in multiple generations.
Keywords: nicotine, germ cells, spermatozoa, behavior, epigenetics
Nicotine exposure of male experimental animals produces adverse influences not only on somatic and germ cells of the exposed males but also on the brain and behavior of multiple generations descending from the males.
Introduction
It is well established that the effects of environmental exposures are not limited to the individuals who are directly exposed but can be transmitted to their offspring. Historically, research efforts have focused on identifying environmental factors that produce adverse effects on the physical and mental development of children as a result of prenatal or early postnatal exposures. For example, the physical and mental development of children born to malnourished mothers has been the subject of intensive scientific research [1]. Fetal alcohol syndrome, a debilitating lifelong condition affecting children born to mothers who were exposed to ethanol, has also attracted significant attention from clinicians and researchers alike [2]. During the 1980s, the consequences of cocaine use by pregnant women led to the troubling notion of a “crack baby epidemic” [3]. Cigarette smoking by pregnant women and its effects on their children’s health has been the subject of extensive research [4–6]. A common theme in research on the deleterious effects of environmental factors on human development has been a focus on women, especially pregnant women. The potential consequences of environmental exposures of men for their offspring had remained largely unexplored.
All this changed around the beginning of this millennium as a result of the pioneering work by Michael Skinner and colleagues, who demonstrated that environmental exposures produce epigenetic alterations not only in the female but also in the male germline and that deleterious effects of such exposures are transmitted to the offspring [7, 8]. It was followed soon by the demonstration that paternal exposure to cocaine produced heritable effects in mice, and that the mechanisms of the heritability may involve epigenetic modification of the spermatozoal DNA [9]. Since then, the effects of exposure of males to a number of environmental factors including psycho-social factors, nicotine, alcohol, cocaine, high-fat diet, and endocrine disruptors on their progeny have been reported [10–27].
Here, we review the literature on the heritable effects of nicotine exposure of male experimental animals, with a focus on behavioral changes as well as neurochemical and molecular changes in the brain. We will discuss the role of epigenetic modification of the spermatozoa as a mechanism of the heritability.
Nicotine as an environmental toxicant
Use of nicotine-containing products and knowledge about their ill effects on human health are centuries old. Yet, nicotine use has continued to rise [Review in [28]]. Electronic nicotine delivery systems such as e-cigarettes and a false sense of safety associated with these products has led to resurgence of nicotine use and addiction [29–31]. Over a billion people worldwide, and approximately 34 million Americans aged 18 and older smoke cigarettes [29–31]. In the United States, nearly 15.6% of men and 12% of women are smokers [29–31].
A recent report suggested that nicotine use by men produces significant deficits in cognitive performance in their offspring [32]. These findings were reported at a time when studies in mouse models had offered convincing evidence that prenatal nicotine exposure produced behavioral changes not only in the prenatally exposed animals, but also in two generations descending from these animals [23]. These findings signaled the addition of nicotine to a growing list of environmental factors that produced heritable effects via the maternal or paternal lines of descent.
Experimental animal models have provided a major impetus for research on the heritable effects of environmental exposures. Animal models carry special advantages for this research because studying multiple human generations is a daunting task, notwithstanding recent progress in this arena [16, 33, 34].
Animal models of paternal nicotine exposure
The majority, if not all, of the preclinical research on paternal nicotine exposure has used rodent models (Table 1). These studies have offered valuable and unequivocal support to the idea that paternal nicotine exposure produces heritable behavioral, neurochemical, cellular, and molecular phenotypes.
Table 1.
A summary of the literature on the heritable effects of paternal exposure to cigarette smoke or nicotine in animal models.
| Direct exposure of male founders | F1 (intergenerational effects) | F2 (transgenerational effects) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure type | Length of exposure | Nicotine withdrawal prior to breeding | Behavior | Spermatozoa | Other effects | Behavior | Neurochemistry | Spermatozoa | Other effects | Behavior | Other effects | |
| Cigarette Smoke: 2× daily for 1 h; Nicotine intraperitoneal, 0.05 mg/100 g 4×/day | 5 weeks | No | Cigarette smoke increased depression like behavior and decreased locomotor activity. Nicotine exposure also increased depression like behavior but had no effect on locomotor activity. Neither exposure produced significant effect on anxiety, novel object recognition, or social behavior. | Enrichment of mRNA in the Wnt signaling pathway; increased WNT4 and DLV2 mRNA and protein expression in testis. Downregulation of Mmu-miRNA-15b with accompanying increase in methylation at CpG island shore regions. | Effects of nicotine exposure, but not cigarette smoke exposure, were examined on WNT4 and DLV2 expression in brain to compare with expression in testes. Expression was not significantly changes in the brain | Exposures to cigarette smoke and nicotine both produced significant decrease in depression like behavior, and increases in social behavior and locomotor activity. | N/A | No significant effect on Mmu-miRNA-15b expression | Nicotine exposure increased WNT4 and DVL2 protein expression in the brain. WNT4 mRNA but not DVL2 mRNA expression was increased significantly as well. Expression of Mmu-miRNA-15b, which represses WNT4 expression, was downregulated significantly in the brain. The CpG island shore regions of this miRNA showed significant hypermethylation. | No significant effect on depression like behavior, anxiety, novel object recognition, locomotor activity, and social behavior. | No significant effect on WNT and DLV4 expression | |
| Nicotine 200 μg/ml in drinking water sweetened with saccharin | 5 weeks | Yes | No significant effects on anxiety-like behavior | N/A | N/A | No significant effect on nicotine-induced suppression of locomotor activity in novel environment or on nicotine self-administration. However, paternal nicotine exposure “protected” the mice from lethal effects of high-dose nicotine, if they had been exposed previously to chronic sub-lethal doses, but not upon the first administration of the lethal dose. | N/A | N/A | Male but not female mice showed increased resistance to nicotine and cocaine toxicity and upregulation of xenobiotic metabolism. Hepatocytes showed reduced apoptosis and necrosis following cocaine exposure. | N/A | N/A | |
| Nicotine 200 μg/ml in drinking water | 12 weeks | No | No significant effect on locomotor activity, spatial working memory or object based attention | Increased global DNA methylation. Hypomethylation of DNA at dopamine D2 receptor promoter region. No significant change in DNA in methylation of dopamine D1, D3, D4, or D5 receptor promoter regions | N/A | Both male and female mice showed significant hyperactivity and reversal learning deficit. Male mice also showed significant attention deficit. No significant effects on working memory in male or female mice. | Male mice showed significant decreases in striatal tissue content of dopamine and its metabolites as well as significant increase in frontal cortical tissue content of norepinephrine. Male mice also showed significant decreases in mRNA expression for dopamine D1, D2, and D4 receptors in the striatum without significant changes in dopamine D3 and D5 receptor mRNA. Female mice showed significant increase in striatal dopamine D5 receptor mRNA without significant changes in dopamine D1, D2, D3, or D4 receptor mRNA. There was no change in striatal tissue content of dopamine or dopamine metabolites nor any significant change in frontal cortical tissue content of norepinephrine. | N/A | N/A | Male F2 mice from female F1 founders showed significant reversal learning deficit but no significant change in locomotor activity, working memory or attention. Male F2 mice from F1 male founders and female F2 mice from male or female F1 founders did not significant changes in locomotor activity, working memory, or attention. | N/A | |
| Nicotine; 12.6 mg/day/x4 via subcutaneous mini pump | 28 days | Yes | N/A | N/A | N/A | Both male and female mice showed significant decreases in nicotine self-administration and significant increases in contextual and cued fear responses. | Both male and female mice showed significant upregulation of hippocampal nicotinic acetylcholine receptor ligand binding and significant reduction in evoked hippocampal cholinergic currents. | N/A | Both male and female mice showed significant changes in DNA methylation and mRNA expression of genes related to neural development and plasticity in the hippocampus. | Contextual and cued fear responses were significantly upregulated | N/A | |
| Reference | Exposure type | Length of exposure | Nicotine withdrawal prior to breeding | Behavior | Spermatozoa | Other effects | Behavior | Neurochemistry | Spermatozoa | Other effects | Behavior | Other effects |
| Zhang et al. (2020) | Nicotine 0.05 mg/100 g x4/day intraperitoneal | 5 weeks | No | N/A | Total DNA methylation rate of dopamine transporter gene was upregulated | N/A | Male mice were significantly hyperactive | Hippocampal dopamine release and tissue content were significantly upregulated in male and female mice along with significant downregulation of dopamine transporter mRNA and protein expression. Total DNA methylation rate of dopamine transporter gene was upregulated along with activation of the dopamine D2 receptor/AKT/GSKα/β pathway. | N/A | N/A | N/A | |
| Murphy et al. (2020) | Cigarette smoke exposure 2.5 h/day for 5 days a week | 60 days | Yes | N/A | Significant up- and downregulation of DNA methylation occurred depending on the methylation status of the DNA at baseline. The baseline methylation status also impacted the rate at which the cigarette-smoke-induced changes were lost following cessation of the exposure. DNA methylation at regions that showed extreme hyper- and hypo-methylation at baseline was less likely to be affected by the smoke exposure and when changes did occur these regions were more likely to recover upon withdrawal of exposure. | N/A | N/A | N/A | There was no significant correlation between DNA methylation patterns in the spermatozoa of directly cigarette smoke exposed mice (founders) and spermatozoa of the F1 mice. | Significant changes in DNA methylation in over 28,000 regions in the prefrontal cortex in male mice and differential expression of 134 genes associated with oxidative stress. | N/A | N/A |
| Zhang et al. (2021) | Nicotine (0.01% (v/v) in saline (5 ml/kg) intraperitoneal; 4x/day | 5 weeks | No | N/A | Downregulation of mmu-miRNA-15b via hypermethylation of CpG island shore regions. | N/A | N/A | N/A | N/A | Upregulation of mRNA and protein levels of α-SMA and Col1α1 in F1 male offspring The increased hepatic fibrosis was a result of upregulation of Wnt4, Dvl2 and β-catenin and downregulation of Gsk-3β. Downregulation of mmu-miRNA-15b via hypermethylation of DNA in F1 liver (i.e. activation of Wnt pathway). | N/A | No change in Wnt4 mRNA and protein expression, mmu-miR-15b expression, or methylation state of mmu-miR-15b in liver. |
| Zeid et al. (2021) | Nicotine; 12.6 mg/day/x4 via subcutaneous mini pump | 28 days | Yes | N/A | N/A | N/A | No change in sensitivity to nicotine-induced hypo locomotion in F1 male and female offspring. | N/A | N/A | Reduced sensitivity to nicotine-induced hypothermia, and delayed nicotine clearance in F1 male mice. Reduced basal corticosterone in F1 female offspring. | N/A | N/A |
Data and conclusions from each publication are presented separately for the exposed founder, his offspring (F1) and the second generation (F2). The findings on behavior as well as neurochemistry, gene expression, and epigenetic changes in the brain and spermatozoa are presented
Figure 1.
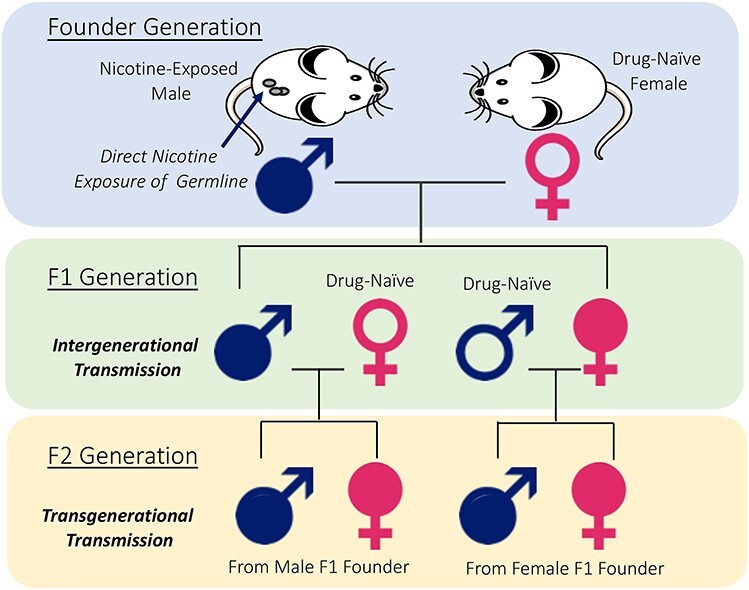
Intergenerational and transgenerational transmission. The nicotine-exposed founder male is bred with drug naïve female to produce the F1 generation. Changes in the F1 phenotype represent intergenerational transmission of nicotine’s effects from the founder to his offspring. The F1 males and females derived from the nicotine exposed founder male are bred with drug naïve females and males to produce the F2 generation. Changes in F2 phenotype represent transgenerational transmission of nicotine’s effects in the founder via F1 to the F2 generation. The intergenerational transmission is believed to occur due to epigenetic changes in the founder’s germline produced by the direct effects of nicotine. This paradigm permits analysis of the effects of nicotine on somatic and germ cells in the founder male as well as in males and females in multiple generations of descendants.
Extrapolation of findings from experimental animal models to humans requires a careful consideration of a number of factors. For example, there are significant differences in nicotine pharmacokinetics and pharmacodynamics between humans and other animals. Moreover, individual differences in nicotine metabolism among humans due to racial, ethnic, and sex differences add an additional layer of complexity for translation of findings from rodent models to humans, and vice versa [Review in [28]].
In rodent models, plasma cotinine levels are used as an index of nicotine exposure and as a comparator for human exposure levels. Cotinine is the major metabolite of nicotine, as approximately 80% of nicotine is converted into cotinine rapidly [35, 36]. In mice, plasma cotinine levels of 70–90 ng/mL are considered to be equivalent to the plasma levels in humans who smoke 20 cigarettes per day. However, the rapid rate of nicotine metabolism in rodents compared to humans warrants a careful titration of drug dosing to attain plasma levels similar to human plasma levels [37].
Other factors that impact extrapolation of data from animal studies to humans include the route of nicotine administration [37]. Rodent models employ oral, inhalation, intravenous, intraperitoneal, or subcutaneous routes of nicotine administration. Oral administration via the drinking water eliminates the stress associated with systemic nicotine administration or inhalation of the noxious cigarette smoke. The bitter taste imparted by nicotine to the drinking water can be alleviated by the addition of the artificial sweetener saccharin [38–41]. However, saccharin exposure of adult male mice produces significant behavioral alterations [42], but not if the exposure occurs via the mother during the prenatal or perinatal periods [39, 41, 43]. Another advantage of oral nicotine administration is that it is a good model for chewing tobacco use in humans.
Administration of nicotine via subcutaneously implanted osmotic minipumps is associated with stress during the surgery for pump implantation. Repeated intraperitoneal or intravenous administration of nicotine is associated with the stress of repeated, albeit, momentary, pain of injection. Neither of these two methods of systemic nicotine administration mimic human nicotine use. However, compared to oral administration, systemic administration leads to a rapid elevation of plasma nicotine levels akin to the rapid rise following cigarette smoking. Exposure via inhalation of cigarette smoke or e-cigarette aerosol may carry the maximum translational relevance, although even this exposure is associated, at least initially, with the stress of involuntary inhalation of a noxious substance.
Paternal exposure to nicotine produces behavioral changes in the offspring in the generation descending directly from the exposed individuals as well as in multiple successive generations of descendants, even though only the founder (and not the successive generations) was exposed to nicotine. These heritable effects of nicotine exposure are considered in terms of intergenerational or transgenerational effects (Figure 1).
Intergenerational effects refer to the effects observed in the offspring (first generation, F1) of the nicotine exposed males (Figure 1). The effects observed in generations descending from the first generation, for example in the second generation (F2 or grandchildren of the nicotine-exposed male), are called transgenerational effects (Figure 1).
Intergenerational transmission occurs due to the effects of direct exposure of the germline of the founder (father) to nicotine. In the case of transgenerational transmission, the effects in the second and subsequent generations cannot be attributed to the effects of direct nicotine exposure of founders’ germline because the founders (F1 generation and beyond) were not exposed to nicotine directly.
Behavioral effects of paternal nicotine exposure
The behavioral effects of paternal nicotine exposure examined in rodent models include locomotor activity, exploration in a novel environment, attention, spatial working memory, reversal learning, anxiety-like behavior, fear conditioning, learned helplessness (depression-like behavior), and drug self-administration (Table 1). Although factors such as the species and strain as well as dose, route, and duration of nicotine exposure vary among the different studies (Table 1), for the sake of brevity, the discussion below does not refer to each of these factors for each study.
Intergenerational effects
Locomotor activity is among the most common behaviors analyzed in the offspring of male rodents exposed to nicotine [25, 44–46]. The activity is measured by placing the rodent in a novel environment or in the animals’ own home cage. The novel environment (or open field) assay measures exploratory behavior as well as general locomotion, whereas the home cage activity assay is a more direct measurement of spontaneous locomotor activity. Both male and female offspring derived from nicotine exposed fathers displayed significant increases in locomotor activity (Table 1).
Another study [46] used a Figure-8 maze to analyze locomotor activity in a rat model of paternal nicotine exposure and found significant increase in locomotor activity in male offspring of nicotine-exposed male rats at adolescence and young adulthood but not at adulthood. Thus, the effects of paternal nicotine exposure showed a developmental effect, attenuating with age. The paternal nicotine exposure produced changes in habituation to a novel environment as well. For example, offspring of nicotine-exposed male rats spent a significantly longer period exploring the Figure-8 maze upon first exposure to it as well as exploring objects presented to them for the first time in a novel object recognition assay [46]. There were no significant effects on recognition memory, attention, or working memory [46].
Anxiety-like behavior was analyzed in paternally nicotine exposed rodents using the elevated plus maze or by analysis of exploration in an open field. Both assays expose the animal to anxiogenic stimuli in the form of brightly lit “unprotected” arenas. Rodents normally prefer the “comfort and safety” of darkness but do explore the brightly lit arena voluntarily. Animals with an anxiety-like phenotype tend to spend significantly reduced time under the bright light compared to controls. The elevated plus maze combines the bright light with elevation, making the task that much more anxiogenic to the animal. Assays of anxiety-like behavior not only have translational relevance for anxiety disorders but also offer significant value as an internal control, because anxiety-like behavior could interfere with performance in other behavioral assays. None of the studies showed anxiety-like behavior in male or female offspring derived from the nicotine-exposed males (Table 1).
Another study [47] examined hippocampus-mediated behaviors and found that paternal nicotine exposure produced significant increases in contextual fear conditioning and cued fear responses. Since nicotine is an addictive substance, the same study [47] examined whether paternal nicotine exposure produced changes in nicotine preference. Intravenous drug self-administration is a reliable measure of the abuse potential of the drug in humans. Nicotine self-administration was reduced significantly in the offspring of male mice exposed to nicotine [47]. These findings corroborated similar findings from an earlier study [48]. The attenuation of drug self-administration is not unique to paternal nicotine exposure, as paternal cocaine, ethanol, and morphine exposure reduce preference for those drugs as well [21, 24, 27].
The offspring of male mice exposed to nicotine developed “protective” responses not only to nicotine but also to cocaine [48]. That is, male (but not female) offspring of nicotine-exposed fathers survived toxic doses of nicotine or cocaine [48]. Interestingly, the protective response occurred only following chronic exposure to sub-lethal doses of the drug (nicotine or cocaine) rather than acute exposure (i.e. exposure for the first time to lethal dose). The tolerance was associated with upregulation of hepatic xenobiotic processing genes and increased metabolic clearance of both the drugs [48]. These findings suggest a potential adaptation of metabolic pathways to fathers’ nicotine use, which may confer protection on the offspring not only to the specific drug used by the father but also to a broad category of substances. This finding has interesting potential implications for evolutionary significance of heritable effects of paternal exposures.
Reversal learning is a measure of cognitive flexibility, which is the ability to seamlessly shift between tasks by quickly modifying strategies to suit the changing demands. Poor cognitive flexibility is a hallmark of autism spectrum disorder and it is associated with ADHD as well [49–51]. Exposure of male mice to nicotine in drinking water for 12 weeks produced significant reduction in reversal learning in male and female offspring [25].
Another study examined the effects of cigarette smoke exposure and nicotine exposure on depression-like behavior using the forced swim test or sucrose preference test [45]. The forced swim test is a measure of learned helplessness, a behavior in animals that is believed to have bearing on human depression. The sucrose preference test is a measure of hedonic behavior, and the reduced sucrose preference is considered to be a reflection of anhedonia. Neither test showed significant adverse effects of paternal cigarette smoke or nicotine exposure although, interestingly, both exposures attenuated the learned helplessness phenotype, which could mean a reduction in depression-like behavior [45].
Attention, working memory, or impulsivity were not significantly affected by paternal nicotine exposure in male or female offspring [25].
In another study, the intergenerational effects of co-exposure to nicotine and saccharin were examined [42]. Such co-exposure occurs in individuals consuming some smokeless tobacco products [52]. The co-exposure produced significant locomotor hyperactivity and significant deficits in spatial working memory in the male but not female offspring.
Transgenerational effects
Transgenerational effects of paternal nicotine exposure were examined in male and female descendants in the second generation derived from male and female founders who were direct descendants of nicotine-exposed males [25]. Reversal learning deficits were found in male mice in the second generation of offspring of nicotine-exposed males only when the male mice were derived from female first-generation founders but not from a male first-generation founder. The female counterparts in the second generation did not show reversal learning deficits. Thus, the transgenerational transmission showed a sex-bias twice in the line of descent—once in each generation. Incidentally, the reversal learning deficit was observed in male and female offspring from the first generation descending from the nicotine-exposed male (intergenerational effect). However, although the first generation showed locomotor hyperactivity and attention deficit (intergenerational effect), neither behavior was observed in the second generation (transgenerational) regardless of the sex of the founder in the first generation.
In another study that used only male founders from the first generation derived from nicotine exposed fathers [47], significant increases were found in contextual and cued fear conditioning in the second generation. In this study as well, the behaviors observed in the first generation namely, locomotor hyperactivity and attenuated depression-like phenotype were not seen in the second generation. Consistent with these findings, another study showed that paternal cigarette smoke exposure (rather than direct nicotine exposure) did not produce transgenerational transmission of locomotor hyperactivity, depression-like behavior, or social interaction [45].
These findings, although from only three independent studies, suggest that behavioral changes produced by the paternal nicotine exposure show a sex-bias and attenuation with each successive generation.
Effects on neurotransmitter signaling
Nicotine binds to nicotinic acetylcholine receptors. However, a major focus of research on the effects of nicotine has been on nicotine-induced changes in the brain’s reward system, especially on dopamine neurotransmission. In the mesolimbic system, a major component of the brain’s reward circuitry, nicotine binds to nicotinic acetylcholine receptors in the ventral tegmental area and promotes the release of dopamine in the ventral striatum [53]. Dopamine release is considered to be the prime driver of nicotine addiction [54].
Intergenerational effects
We showed that nicotine exposure of male mice produces significant reductions in tissue content of dopamine and its metabolites in the striatum of the offspring. These changes were accompanied by decreased striatal D1, D2, and D4 mRNA expression [25]. Another study showed that paternal nicotine exposure increased hippocampal dopamine tissue content as well as dopamine release and produced decreases in dopamine transporter protein and mRNA expression in the offspring [44]. Yet another study examined cholinergic signaling in the hippocampus of offspring derived from nicotine-exposed male mice and found that nicotinic acetylcholine receptor binding was increased, and hippocampal evoked cholinergic currents were decreased [47].
Epigenetic changes in germ cells
Heritability of nicotine-induced phenotypes in a non-Mendelian pattern suggests epigenetic changes in germ cells as a likely mechanism of the heritability. Epigenetic modifications of DNA and histones collectively regulate gene expression in somatic cells. However, in germ cells, the majority (but not all [55, 56]) of the histones are replaced with protamines during the germ cell development [57]. Therefore, although histone modification may play a significant role in epigenetic regulation of spermatozoal DNA [56], DNA methylation and the action of non-coding RNAs such as miRNAs [57, 58] are considered to be candidate mechanisms for heritability of paternal nicotine-induced phenotypes.
Nicotine exposure of male mice produced significant DNA hypomethylation at promoter regions of the dopamine receptor genes [25]. Another study found hypermethylation of the dopamine transporter gene following nicotine exposure [44]. Cigarette smoke exposure produced changes in methylation across the entire spermatozoal DNA landscape within 3 days, but the changes that occurred at CpG-dense regions such as promoters and enhancers were highly transient and returned to baseline within 28 days of cessation of the exposure compared to methylation at individual CpGs and at CpG-poor regions [59]. Overall, both up- and down-regulation of DNA methylation occurred depending on the methylation status of the DNA at baseline, i.e. prior to cigarette smoke exposure. The baseline methylation status also impacted the rate at which the cigarette-smoke-induced changes were lost following cessation of the exposure. DNA methylation at regions that showed extreme hyper- and hypo-methylation at baseline was less likely to be affected by the smoke exposure compared to intermediate levels of methylation, and when changes did occur due to the smoke exposure these regions were more likely to recover upon cessation of exposure.
Another study found that nicotine exposure upregulated Wnt4 signaling in the spermatozoa [45]. However, the upregulation was not associated with changes in DNA methylation at the CpG-rich Wnt4 promoter region but correlated with downregulation of mmu-miR-15b miRNA [45, 60]. These data suggest that nicotine exposure can produce changes in the expression of non-coding RNA in the spermatozoa.
The effects of cigarette smoke on germ cells are not limited to epigenetic changes, but include germline mutations as well [61–64]. For example, cigarette smoking is associated with significant increase in frequency of germline mutations in humans as well as rodents [65, 66]. This is not surprising, as cigarette smoke contains numerous chemicals with the potential for de novo mutagenesis.
Comparison between nicotine’s direct effects on the founders and heritable effects in the descendants
The research on heritable behavioral effects of paternal nicotine exposure has focused on potential conformity between the effects on the directly exposed male founder and the effects on the descendants. However, there is scant support for full alignment from behavioral or molecular studies. It is more often the case that the effects in the founder are not found in the offspring or the effects in the offspring arise de novo.
For example, although nicotine exposure did not produce significant changes in spontaneous locomotor activity, attention or reversal learning in the male mice that were directly exposed to nicotine, the offspring derived from these male mice showed significant changes in all of these behaviors [25]. In another study, nicotine exposure of male mice reduced locomotor activity and produced depression-like behavior in the exposed generation, but the offspring showed the opposite effects [45].
Other studies examined whether there was congruence between nicotine-induced epigenetic changes in the spermatozoa and gene expression in the brains of the offspring [25]. The decrease in DNA methylation at promoter regions of the dopamine receptor genes in the founder’s spermatozoa correlated with significant reductions in tissue content of dopamine and its metabolites and decreased striatal D1, D2, and D4 mRNA expression in the offspring [25]. Upregulation of Wnt4 signaling in the spermatozoa as a result of paternal nicotine exposure was also apparent in the brains of the offspring [45]. Similarly, the downregulation of mmu-miR-15b observed in the spermatozoa of the nicotine-exposed males was transmitted to their offspring.
However, the changes in spermatozoal DNA methylation patterns produced by cigarette smoke exposure were not correlated with patterns of DNA methylation in the spermatozoa of the offspring in the F1 generation or with DNA methylation in the frontal cortex of the F1 offspring [59]. Thus, whether epigenetic changes in the spermatozoa correlate with changes in gene expression in the brains of the offspring remains an open question.
A recent report examined differentially methylated regions (DMRs) in the whole blood DNA collected from adolescent and adult offspring of fathers who were cigarette smokers [67]. Six DMRs, including a DMR associated with behavioral dysfunction and drug addiction, were identified [16]. However, this study did not attempt to examine a direct correlation between changes in the methylation of spermatozoal DNA of the founders and somatic DNA of the offspring.
Role of sex in heritable phenotypes
As discussed above, nicotine’s heritable effects show a sex bias. The sex of the descendants as well as the founders influences the phenotypes (Table 1). The sex-bias occurs at the level of the founder germ cells as well as the descendant somatic cells and tissues. However, additional research focused specifically on this issue would be needed to fully understand the role of sex in heritability of behavioral or cellular phenotypes. For example, in addition to the role of genetic sex, organizational versus activational influences, imprinted genes and mitochondrial DNA [68, 69] likely influence heritability of paternal nicotine-induced phenotypes. Moreover, there are imprints/parent-of-origin effects on transcription at over 1300 loci [68, 69] and ~ 350 autosomal genes with sex-specific parent-of origin effects in the mouse brain [68, 69].
Duration of nicotine exposure and heritability of the effects
Although the duration of nicotine exposure has varied among the different studies (Table 1), the specific issue of the impact of duration of exposure on heritability of phenotypes has not been examined. One study showed that 8-weeks of nicotine exposure of mice via drinking water did not produce heritable behaviors, but a 12-week exposure did [25]. The spermatogenesis cycle in mice lasts for approximately 30 days. Therefore, the 8-weeks of exposure would have covered nearly two spermatogenesis cycles but did not produce heritable behavioral effects. In other studies, 5 weeks of cigarette smoke exposure [45] or nicotine exposure via the intraperitoneal route of administration [44] produced heritable behavioral effects. Finally, 28 days of nicotine exposure via subcutaneously implanted osmotic pumps was sufficient to produce behavioral alterations in another study [47]. Among these studies, apart from the duration of nicotine exposure, the method of nicotine administration and the specific behaviors analyzed were different as well, making firm conclusions somewhat difficult.
The consequences of a period of cessation of nicotine exposure following a period of sustained exposure for heritability of behavioral phenotypes are not fully known. A recent study [59] found that changes in mouse spermatozoal DNA methylation produced by 60 days of cigarette smoke exposure were labile, and many lasted for less than 30 days, which is the duration of one spermatogenesis cycle in mice, following cessation of cigarette smoke exposure.
The minimum duration of nicotine exposure needed to produce heritable effects may depend on the type of heritable phenotype examined (specific behavior, DNA methylation at specific regions) as well as the method of nicotine exposure (oral or cigarette smoke).
Heritable effects of co-exposures
Cigarette smoke contains thousands of chemicals in addition to nicotine. However, the effects of cigarettes on the brain and behavior are considered to be due to nicotine [70–73]. Nicotine use often accompanies the use of other neuroactive substances such as ethanol, cocaine, or cannabis. The heritable effects of co-exposures to nicotine and one or more of these substances are not yet fully understood. A recent study using a mouse model of co-exposure to nicotine and saccharin [42], an artificial sweetener contained in some forms of smokeless tobacco [52] showed that the heritable effects of the co-exposure were not merely the additive effects of each substance. In other words, the heritable effects of co-exposures to two or more substances may not be predictable based solely on knowledge of the effects of the individual substances.
Concluding remarks
Evidence from multiple lines of research demonstrates that environmental exposures are associated with heritable phenotypes [74]. Nicotine is one among many on a long list of environmental factors known to produce heritable phenotypes [10, 11, 13–18, 20, 22–27, 75–78].
An unresolved issue is how nicotine-induced epigenetic modification of the fathers’ spermatozoa translates into behavioral phenotypes in the offspring. A multitude of epigenetic reprogramming events occur involving the erasure and re-acquisition of epigenetic marks during gametogenesis, fertilization, organogenesis, and ontogenesis. Therefore, a systematic analysis of gene expression during the intervening stages (sperm-blastocyst-embryo-juvenile-adult) would be required to begin to address this issue.
Whether the heritable phenotypes become permanent, and, therefore, evolutionarily meaningful is another critical question [79, 80]. Nicotine-induced behavioral phenotypes attenuate with successive generations, and almost all environment-induced phenotypes reported thus far in experimental models, appear to follow this pattern. From the perspective of evolution, transience of environment-induced phenotypes may be a distinct advantage for the survival of the species. The environmental factors that produce heritable phenotypes tend to be transient, i.e. last for only a generation or two. Examples include drugs that are abused, nutritional factors, psychosocial factors, and endocrine disruptors. Therefore, at least in theory, permanent adaptation in response to transient causal factors could be counterproductive. On the other hand, transient adaptation to tide over transient environmental adversity could become an advantage. This idea calls for transience of not only behavioral phenotypes but also the cellular and molecular mechanisms that produce the phenotype. It is here that epigenetic modification of the germ cell genome becomes an exquisitely suitable mechanism for mediating environment-induced heritable phenotypes vis-à-vis genetic mechanism (e. g. mutations), which generally produces “permanent” adaptation. Environmental insults that persist for longer than one or two generations (e. g. changes in habitat produced by changing global environment, introduction of a new species) may produce heritable phenotypes that remain robust over multiple generations, a tribute to neo-Lamarckian principles of heritability.
Research using experimental animal models has offered unequivocal data albeit at a phenomenological level that paternal nicotine exposure produces heritable phenotypes. Some of the heritable behavioral phenotypes namely hyperactivity, attention deficit, and cognitive inflexibility are consistent with behavioral changes associated with human neurodevelopmental disorders such as ADHD and autism spectrum disorder. A recent report examined differentially methylated regions (DMRs) in the whole blood DNA collected from adolescent and adult offspring of fathers who were cigarette smokers. Six DMRs, including a DMR associated with behavioral dysfunction and drug addiction, were identified [16]. Thus, the findings from preclinical models together with preliminary support from human studies help increase public awareness and influence public policy about the heritable effects of paternal nicotine exposure.
Author contributions
DMMcC and PGB reviewed the literature and wrote the manuscript.
Conflict of interest
The authors do not have any potential or actual conflict of interest with respect to the work reported in the article.
Grant Support: This work was supported by the Escher Fund for Autism, NIH (DA043848), the Florida Department of Health (James E. King Trust Grant # 20 K01), the Jim and Betty Ann Rodgers Chair Fund, and the Florida State University Council on Research and Creativity.
Contributor Information
Deirdre M McCarthy, Department of Biomedical Sciences, Florida State University College of Medicine, Tallahassee, FL, 32306, USA.
Pradeep G Bhide, Department of Biomedical Sciences, Florida State University College of Medicine, Tallahassee, FL, 32306, USA.
References
- 1.Waber DP, Bryce CP, Fitzmaurice GM, Zichlin ML, McGaughy J, Girard JM, Galler JR. Neuropsychological outcomes at midlife following moderate to severe malnutrition in infancy. Neuropsychology 2014; 28:530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams JF, Smith VC. Committee on substance a. Fetal Alcohol Spectrum Disorders Pediatrics 2015; 136:e1395–e1406. [DOI] [PubMed] [Google Scholar]
- 3.Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Dev Neurosci 2009; 31:23–35. [DOI] [PubMed] [Google Scholar]
- 4.Wickstrom R. Effects of nicotine during pregnancy: Human and experimental evidence. Curr Neuropharmacol 2007; 5:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn HG, Mcburney AK, Sandraingram, Hunter CM. Maternal cigarette smoking during pregnancy and the child's subsequent development: II. Neurological and intellectual maturation to the age of 6 1/2 years. Canadian journal of public health. Revue Canadienne de Sante Publique 1977; 68:43–50. [PubMed] [Google Scholar]
- 6.Milberger S, Biederman J, Faraone SV, Chen L, Jones J. Further evidence of an association between attention-deficit/hyperactivity disorder and cigarette smoking. Findings from a high-risk sample of siblings. Am J Addict 1997; 6:205–217. [PubMed] [Google Scholar]
- 7.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 2005; 308:1466–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nilsson EE, Sadler-Riggleman I, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of disease. Environ Epigenet 2018; 4:dvy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He F, Lidow IA, Lidow MS. Consequences of paternal cocaine exposure in mice. Neurotoxicol Teratol 2006; 28:198–209. [DOI] [PubMed] [Google Scholar]
- 10.Courtney Jones SK, Byrne PG. What role does heritability play in transgenerational phenotypic responses to captivity? Implications for managing captive populations. Zoo Biol 2017; 36:397–406. [DOI] [PubMed] [Google Scholar]
- 11.Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK. Epigenetic transgenerational inheritance of altered stress responses. Proc Natl Acad Sci U S A 2012; 109:9143–9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babenko O, Kovalchuk I, Metz GA. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev 2015; 48:70–91. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg LR, Gould TJ. Multigenerational and transgenerational effects of paternal exposure to drugs of abuse on behavioral and neural function. Eur J Neurosci 2019; 50:2453–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS One 2010; 5: e13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holloway AC, Cuu DQ, Morrison KM, Gerstein HC, Tarnopolsky MA. Transgenerational effects of fetal and neonatal exposure to nicotine. Endocrine 2007; 31:254–259. [DOI] [PubMed] [Google Scholar]
- 16.Morkve Knudsen T, Rezwan FI, Jiang Y, Karmaus W, Svanes C, Holloway JW. Transgenerational and intergenerational epigenetic inheritance in allergic diseases. J Allergy Clin Immunol 2018; 142:765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinnies KM, Harris EP, Snyder RW, Sumner SS, Rissman EF. Direct and transgenerational effects of low doses of perinatal di-(2-ethylhexyl) phthalate (DEHP) on social behaviors in mice. PLoS One 2017; 12:e0171977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rehan VK, Liu J, Sakurai R, Torday JS. Perinatal nicotine-induced transgenerational asthma. Am J Physiol Lung Cell Mol Physiol 2013; 305:L501–L507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci U S A 2015; 112:13699–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skinner MK. Environmental epigenomics and disease susceptibility. EMBO Rep 2011; 12:620–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vassoler FM, Byrnes EM, Pierce RC. The impact of exposure to addictive drugs on future generations: Physiological and behavioral effects. Neuropharmacology 2014; 76:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yohn NL, Bartolomei MS, Blendy JA. Multigenerational and transgenerational inheritance of drug exposure: The effects of alcohol, opiates, cocaine, marijuana, and nicotine. Prog Biophys Mol Biol 2015; 118:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J, Lee KP, Spencer TJ, Biederman J, Bhide PG. Transgenerational transmission of hyperactivity in a mouse model of ADHD. J Neurosci 2014; 34:2768–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci 2013; 16:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy DM, Morgan TJ Jr, Lowe SE, Williamson MJ, Spencer TJ, Biederman J, Bhide PG. Nicotine exposure of male mice produces behavioral impairment in multiple generations of descendants. PLoS Biol 2018; 16:e2006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahnke AH, Miranda RC, Homanics GE. Epigenetic mediators and consequences of excessive alcohol consumption. Alcohol 2017; 60:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rompala GR, Homanics GE. Intergenerational effects of alcohol: A review of paternal preconception ethanol exposure studies and epigenetic mechanisms in the male germline. Alcohol Clin Exp Res 2019; 43:1032–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centner AM, Bhide PG, Salazar G. Nicotine in senescence and atherosclerosis. Cell 2020; 9:1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creamer MR, Wang TW, Babb S, Cullen KA, Day H, Willis G, Jamal A, Neff L. Tobacco product use and cessation indicators among adults - United States, 2018. MMWR Morb Mortal Wkly Rep 2019; 68:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Report USSGs . The Health Consequences of Smoking —50 Years of Progress: A Report of the Surgeon General. Washington, DC: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2014. [PubMed] [Google Scholar]
- 31.Report USSGs . E-cigarette use among youth and young adults: A report of the surgeon general. In. Washington, D.C.: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2016. [Google Scholar]
- 32.Biederman J, Fitzgerald M, Spencer TJ, Bhide PG, McCarthy DM, Woodworth KY, Saunders A, Faraone SV. Is paternal smoking at conception a risk for ADHD? A controlled study in youth with and without ADHD. J Atten Disord 2020; 24:1493–1496. [DOI] [PubMed] [Google Scholar]
- 33.Golding J, Ellis G, Gregory S, Birmingham K, Iles-Caven Y, Rai D, Pembrey M. Grand-maternal smoking in pregnancy and grandchild's autistic traits and diagnosed autism. Sci Rep 2017; 7:46179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams C, Suderman M, Guggenheim JA, Ellis G, Gregory S, Iles-Caven Y, Northstone K, Golding J, Pembrey M. Grandmothers' smoking in pregnancy is associated with a reduced prevalence of early-onset myopia. Sci Rep 2019; 9:15413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benowitz NL, Porchet H, Jacob P 3rd. Nicotine dependence and tolerance in man: Pharmacokinetic and pharmacodynamic investigations. Prog Brain Res 1989; 79:279–287. [DOI] [PubMed] [Google Scholar]
- 36.Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev 2005; 57:79–115. [DOI] [PubMed] [Google Scholar]
- 37.Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007; 190:269–319. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J, Zhang X, Xu Y, Spencer TJ, Biederman J, Bhide PG. Prenatal nicotine exposure mouse model showing hyperactivity, reduced cingulate cortex volume, reduced dopamine turnover, and responsiveness to oral methylphenidate treatment. J Neurosci 2012; 32:9410–9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu J, Fan F, McCarthy DM, Zhang L, Cannon EN, Spencer TJ, Biederman J, Bhide PG. A prenatal nicotine exposure mouse model of methylphenidate responsive ADHD-associated cognitive phenotypes. Int J Dev Neurosci 2017; 58:26–34. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Spencer TJ, Biederman J, Bhide PG. Attention and working memory deficits in a perinatal nicotine exposure mouse model. PLoS One 2018; 13:e0198064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L, McCarthy DM, Eskow Jaunarajs KL, Biederman J, Spencer TJ, Bhide PG. Frontal cortical monoamine release, attention, and working memory in a perinatal nicotine exposure mouse model following kappa opioid receptor antagonism. Cereb Cortex 2021; 31:483–496. [DOI] [PubMed] [Google Scholar]
- 42.McCarthy DM, Lowe SE, Morgan TJ, Cannon EN, Biederman J, Spencer TJ, Bhide PG. Transgenerational transmission of behavioral phenotypes produced by exposure of male mice to saccharin and nicotine. Sci Rep 2020; 10:11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin MM, McCarthy DM, Schatschneider C, Trupiano MX, Jones SK, Kalluri A, Bhide PG. Effects of developmental nicotine exposure on frontal cortical GABA-to-non-GABA neuron ratio and novelty-seeking behavior. Cereb Cortex 2020; 30:1830–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang M, Zhang D, Dai J, Cao Y, Xu W, He G, Wang Z, Wang L, Li R, Qiao Z. Paternal nicotine exposure induces hyperactivity in next-generation via down-regulating the expression of DAT. Toxicology 2020; 431:152367. [DOI] [PubMed] [Google Scholar]
- 45.Dai J, Wang Z, Xu W, Zhang M, Zhu Z, Zhao X, Zhang D, Nie D, Wang L, Qiao Z. Paternal nicotine exposure defines different behavior in subsequent generation via hyper-methylation of mmu-miR-15b. Sci Rep 2017; 7:7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hawkey AB, White H, Pippen E, Greengrove E, Rezvani AH, Murphy SK, Levin ED. Paternal nicotine exposure in rats produces long-lasting neurobehavioral effects in the offspring. Neurotoxicol Teratol 2019; 74:106808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldberg LR, Zeid D, Kutlu MG, Cole RD, Lallai V, Sebastian A, Albert I, Fowler CD, Parikh V, Gould TJ. Paternal nicotine enhances fear memory, reduces nicotine administration, and alters hippocampal genetic and neural function in offspring. Addict Biol 2019; 26: e12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vallaster MP, Kukreja S, Bing XY, Ngolab J, Zhao-Shea R, Gardner PD, Tapper AR, Rando OJ. Paternal nicotine exposure alters hepatic xenobiotic metabolism in offspring. Elife 2017; 6: e24771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dajani DR, Uddin LQ. Demystifying cognitive flexibility: Implications for clinical and developmental neuroscience. Trends Neurosci 2015; 38:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A. The neural basis of reversal learning: An updated perspective. Neuroscience 2016; 345: 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCarthy DM, Bell GA, Cannon EN, Mueller KA, Huizenga MN, Sadri-Vakili G, Fadool DA, Bhide PG. Reversal learning deficits associated with increased frontal cortical brain-derived neurotrophic factor tyrosine kinase B Signaling in a prenatal cocaine exposure mouse model. Dev Neurosci 2016; 38:354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miao S, Beach ES, Sommer TJ, Zimmerman JB, Jordt SE. High-intensity sweeteners in alternative tobacco products. Nicotine Tob Res 2016; 18:2169–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: Underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol 2009; 78:756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baumeister RF. Addiction, cigarette smoking, and voluntary control of action: Do cigarette smokers lose their free will? Addict Behav Rep 2017; 5:67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller D, Brinkworth M, Iles D. Paternal DNA packaging in spermatozoa: More than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction 2010; 139:287–301. [DOI] [PubMed] [Google Scholar]
- 56.Brunner AM, Nanni P, Mansuy IM. Epigenetic marking of sperm by post-translational modification of histones and protamines. Epigenetics Chromatin 2014; 7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carrell DT. Epigenetics of the male gamete. Fertil Steril 2012; 97:267–274. [DOI] [PubMed] [Google Scholar]
- 58.Hamatani T. Human spermatozoal RNAs. Fertil Steril 2012; 97:275–281. [DOI] [PubMed] [Google Scholar]
- 59.Murphy PJ, Guo J, Jenkins TG, James ER, Hoidal JR, Huecksteadt T, Broberg DS, Hotaling JM, Alonso DF, Carrell DT, Cairns BR, Aston KI. NRF2 loss recapitulates heritable impacts of paternal cigarette smoke exposure. PLoS Genet 2020; 16:e1008756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang D, Dai J, Zhang M, Xie Y, Cao Y, He G, Xu W, Wang L, Qiao Z, Qiao Z. Paternal nicotine exposure promotes hepatic fibrosis in offspring. Toxicol Lett 2021; 343:44–55. [DOI] [PubMed] [Google Scholar]
- 61.Beal MA, Meier MJ, Williams A, Rowan-Carroll A, Gagne R, Lindsay SJ, Fitzgerald T, Hurles ME, Marchetti F, Yauk CL. Paternal exposure to benzo(a)pyrene induces genome-wide mutations in mouse offspring. Commun Biol 2019; 2:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Demarini DM. Declaring the existence of human germ-cell mutagens. Environ Mol Mutagen 2012; 53:166–172. [DOI] [PubMed] [Google Scholar]
- 63.Bline AP, Dearfield KL, DeMarini DM, Marchetti F, Yauk CL, Escher J, Workshop P. Heritable hazards of smoking: Applying the "clean sheet" framework to further science and policy. Environ Mol Mutagen 2020; 61:910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beal MA, Yauk CL, Marchetti F. From sperm to offspring: Assessing the heritable genetic consequences of paternal smoking and potential public health impacts. Mutat Res 2017; 773:26–50. [DOI] [PubMed] [Google Scholar]
- 65.Marchetti F, Rowan-Carroll A, Williams A, Polyzos A, Berndt-Weis ML, Yauk CL. Sidestream tobacco smoke is a male germ cell mutagen. Proc Natl Acad Sci U S A 2011; 108:12811–12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yauk CL, Berndt ML, Williams A, Rowan-Carroll A, Douglas GR, Stampfli MR. Mainstream tobacco smoke causes paternal germ-line DNA mutation. Cancer Res 2007; 67:5103–5106. [DOI] [PubMed] [Google Scholar]
- 67.Morkve Knudsen GT, Rezwan FI, Johannessen A, Skulstad SM, Bertelsen RJ, Real FG, Krauss-Etschmann S, Patil V, Jarvis D, Arshad SH, Holloway JW, Svanes C. Epigenome-wide association of father's smoking with offspring DNA methylation: A hypothesis-generating study. Environ Epigenet 2019; 5:dvz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gregg C, Zhang J, Butler JE, Haig D, Dulac C. Sex-specific parent-of-origin allelic expression in the mouse brain. Science 2010; 329:682–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gregg C, Zhang J, Weissbourd B, Luo S, Schroth GP, Haig D, Dulac C. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science 2010; 329:643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abreu-Villaca Y, Seidler FJ, Tate CA, Slotkin TA. Nicotine is a neurotoxin in the adolescent brain: Critical periods, patterns of exposure, regional selectivity, and dose thresholds for macromolecular alterations. Brain Res 2003; 979:114–128. [DOI] [PubMed] [Google Scholar]
- 71.Hall BJ, Cauley M, Burke DA, Kiany A, Slotkin TA, Levin ED. Cognitive and Behavioral impairments evoked by low-level exposure to tobacco smoke components: Comparison with nicotine alone. Toxicol Sci 2016; 151:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slotkin TA, Cho H, Whitmore WL. Effects of prenatal nicotine exposure on neuronal development: Selective actions on central and peripheral catecholaminergic pathways. Brain Res Bull 1987; 18:601–611. [DOI] [PubMed] [Google Scholar]
- 73.Navarro HA, Seidler FJ, Schwartz RD, Baker FE, Dobbins SS, Slotkin TA. Prenatal exposure to nicotine impairs nervous system development at a dose which does not affect viability or growth. Brain Res Bull 1989; 23:187–192. [DOI] [PubMed] [Google Scholar]
- 74.Bohacek J, Mansuy IM. Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nat Rev Genet 2015; 16:641–52. [DOI] [PubMed] [Google Scholar]
- 75.Gapp K, Soldado-Magraner S, Alvarez-Sanchez M, Bohacek J, Vernaz G, Shu H, Franklin TB, Wolfer D, Mansuy IM. Early life stress in fathers improves behavioural flexibility in their offspring. Nat Commun 2014; 5:5466. [DOI] [PubMed] [Google Scholar]
- 76.Rodgers AB, Bale TL. Germ cell origins of posttraumatic stress disorder risk: The transgenerational impact of parental stress experience. Biol Psychiatry 2015; 78:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vassoler FM, Sadri-Vakili G. Mechanisms of transgenerational inheritance of addictive-like behaviors. Neuroscience 2013; 264:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holloway ZR, Hawkey AB, Pippin E, White H, Wells C, Kenou B, Rezvani AH, Murphy SK, Levin ED. Paternal factors in neurodevelopmental toxicology: THC exposure of male rats causes long-lasting neurobehavioral effects in their offspring. Neurotoxicology 2020; 78:57–63. [DOI] [PubMed] [Google Scholar]
- 79.Bell AM, Hellmann JK. An integrative framework for understanding the mechanisms and multigenerational consequences of transgenerational plasticity. Annu Rev Ecol Evol Syst 2019; 50:97–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Donelan SC, Hellmann JK, Bell AM, Luttbeg B, Orrock JL, Sheriff MJ, Sih A. Transgenerational plasticity in human-altered environments. Trends Ecol Evol 2020; 35:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]


