Abstract
Aims
Public health responses to reduce SARS‐CoV‐2 transmission have profoundly affected the epidemiology and management of other infections. We examined the impact of COVID‐19 restrictions on antibiotic dispensing in Australia.
Methods
We used national claims data to investigate antibiotic dispensing trends from November 2015 to October 2020 and whether changes reflected reductions in primary care consultations. We used interrupted time series analysis to quantify changes in monthly antibiotic dispensing and face‐to‐face and telehealth GP consultations and examined changes by recipient age, pharmacy State and prescriber specialty.
Results
Over the study period, an estimated 19 921 370 people had 125 495 137 antibiotic dispensings, 71% prescribed by GPs. Following COVID‐19 restrictions, we observed a sustained 36% (95% CI: 33–40%) reduction in antibiotic dispensings from April 2020. Antibiotics recommended for managing respiratory tract infections showed large reductions (range 51–69%), whereas those recommended for non‐respiratory infections were unchanged. Dispensings prescribed by GPs decreased from 63.5 per 1000 population for April–October 2019 to 37.0 per 1000 for April–October 2020. Total GP consultation rates remained stable, but from April 2020, 31% of consultations were telehealth.
Conclusion
In a setting with a low COVID‐19 incidence, restrictions were associated with a substantial reduction in community dispensings of antibiotics primarily used to treat respiratory infections, coincident with reported reductions in respiratory viral infections. Our findings are informative for post‐pandemic antimicrobial stewardship and highlight the potential to reduce inappropriate prescribing by GPs and specialists for respiratory viral infections.
Keywords: antibiotics, Australia, COVID‐19, epidemiology, physical distancing
What is already known about this subject
Presentations with non‐SARS‐CoV‐2 infections and in community antibiotic use fell dramatically in many countries several months into the COVID‐19 pandemic.
Australia has had a low incidence of COVID‐19, prompting the question whether similar changes in antibiotic use occurred there.
What this study adds
Community antibiotic dispensings in Australia decreased by a sustained 36% from April 2020 onwards.
Antibiotics recommended for treatment of respiratory tract infections showed large reductions (range 51–69%), but antibiotics for non‐respiratory infections were unchanged.
1. INTRODUCTION
Public health responses to reduce SARS‐CoV‐2 transmission have profoundly affected the epidemiology of non‐COVID‐19 conditions. Compared to the pre‐pandemic period, there have been marked reductions in the incidence of hospitalised and non‐hospitalised infectious diseases other than COVID‐19. 1 , 2 , 3 , 4 , 5 , 6 , 7
In regions with high rates of SARS‐CoV‐2 infection, such as Europe and the United States, there have been reductions in community antibiotic prescribing following the introduction of COVID‐19 restrictions in early 2020. 8 , 9 , 10 , 11 , 12 , 13 , 14 However, the underlying data are difficult to interpret as they reflect differing contributory and contemporaneous factors, including variation in public health restrictions and social policies, health care access, increasing use of telehealth and rates of SARS‐CoV‐2 infections.
Australia offers a unique opportunity to investigate changes in antibiotic prescribing during the pandemic; nationwide public health responses began in March 2020, with variation between States and Territories in the specific restrictions and their duration, but COVID‐19 incidence has remained low. Leveraging national population‐based claims data for medicines dispensed and primary care consultations, we evaluated the impact of COVID‐19 restrictions on community antibiotic prescribing, considering changes by specific antibiotics, prescriber specialty, recipient age and jurisdiction. We hypothesised that the marked reduction in the transmission of respiratory viruses following COVID‐19 restrictions would be reflected in a decrease in dispensing of antibiotics, especially those used to treat respiratory tract infections.
2. METHODS
2.1. Context
Australia has a universal healthcare system entitling all citizens and eligible residents to subsidised medicines through the Pharmaceutical Benefits Scheme (PBS) and subsidised medical services through the Medicare Benefits Scheme (MBS). 15
From 21 January 2020, the Australian government introduced its first response to mitigate SARS‐CoV‐2 transmission. Travel from high‐risk countries was restricted starting 30 January, which culminated in border closures and mandatory 14‐day hotel quarantine by 28 March 2020. Beginning mid‐March, the Federal, State and Territory governments issued directions restricting the size of gatherings, visits to aged care and hospitals, elective surgeries, travel to remote communities, and closed dine‐in, beauty and personal care services, and some public spaces. 16 Those who could work from home were required to do so, and schools were closed in some jurisdictions. In addition, some domestic borders were closed. Many of these restrictions were relaxed in May 2020. For most of the country (except Victoria), restrictions remained relaxed throughout the year, with the key exception of international travel.
Australia has had a low incidence of COVID‐19: at 28 February 2021, there were a cumulative 22 354 locally acquired COVID‐19 cases (88 per 100 000 population), with 87% of cases occurring in the State of Victoria (294 per 100 000) and 10% in New South Wales (27 per 100 000). 16 Cases peaked in mid‐March and late‐July, with the second outbreak occurring primarily in Victoria (July–November).
2.2. Data sources
We drew on PBS dispensing claims from two national sources (see Data Availability Statement). First, we used monthly aggregate claims to analyse overall trends in antibiotic dispensing from 1 November 2015 to 30 October 2020. 17 Second, as these did not contain person‐level characteristics, we used claims for a 10% random sample of all PBS‐eligible people from 1 November 2015 to 30 October 2020 for stratified analyses. When a subsidised medicine is dispensed in Australia, the administering pharmacy or hospital provides the government with data on the prescription dispensed, identity of the patient, prescribing doctor and supplying pharmacy. The two national data sources are different deidentified extracts of these dispensing claims data, either aggregated or a 10% sample of individual records. 15
The 10% PBS sample is a standard dataset provided by Australian Government Services Australia for analysis and is selected using the last digit of each person's randomly assigned unique identifier. To protect privacy, all dispensing dates are offset by ±14 days; the direction of the offset is the same for each individual. PBS dispensing claims do not include medicines dispensed in public hospitals and private dispensings, i.e. medicines for which the consumer pays the entire cost out‐of‐pocket.
We used national MBS claims data 18 to identify general practitioner (GP) attendances during the study period. These data provide counts of services processed each month by patient age group, State/Territory of service, and MBS item category. We categorised GP consultations as face‐to‐face and telehealth services according to broad MBS item categories (Table S1 in the Supporting Information).
2.3. Medicines of interest
We included all PBS‐subsidised antibiotic medicines classified as antibacterials for systemic use (J01) using the World Health Organization (WHO) Anatomic Therapeutic Chemical (ATC) Classification and identified the ten most common individual antibiotics by dispensed volume in 2019. To explore whether changes in prescribing patterns reflected changes in epidemiology and treatment of particular types of infection, we considered the common antibiotics predominantly prescribed for upper and lower respiratory tract infections (amoxicillin, clarithromycin, roxithromycin) and those used largely for non‐respiratory infections, including skin and soft tissue infections (flucloxacillin), 19 selected gastrointestinal infections (metronidazole), and urinary tract infections (trimethoprim) and also purine‐analogues antivirals (ATC J05AB, e.g., famciclovir, valacyclovir and acyclovir). In contrast to antimicrobials commonly prescribed for acute infections, these antivirals are mainly used to treat new‐onset and recurrent herpes infections, which are less likely to be affected by social distancing and other public health measures. Changes in dispensing rates of these comparison medicines would therefore largely reflect reduced health care access rather than changes in transmission or epidemiology of herpes virus infections.
In April 2020, changes were made to the PBS subsidy of amoxicillin, amoxicillin with clavulanic acid, cefalexin and roxithromycin. This included limiting the maximum quantities dispensed and eliminating refills. However, while many antibiotics are prescribed with refills, few are actually dispensed; for example, 8% of amoxicillin dispensings in 2012–13 were refills. 20
2.4. Analysis
We calculated monthly dispensing rates per 1000 population using the Australian Bureau of Statistics quarterly population, overall, and for the top ten antibiotics and the comparison medicines listed above. We used interrupted time series (ITS) analysis to quantify changes in dispensing of antibiotics overall and of each of the top ten antibiotics, as well as of the comparison medicines. In the ITS analysis, we included variables representing a temporary increase in dispensing in March 2020 (pulse); and a level shift reduction in dispensing from 1 April to 30 October 2020. The level shift represents an immediate change that was sustained for the remainder of the study period. We included the temporary change in March due to reported increases in dispensing, likely due to stockpiling behaviour at the start of mitigation measures. 21 , 22 To control for autocorrelation and seasonality, we used seasonal autoregressive integrative moving average (ARIMA) models.
We also conducted stratified descriptive analyses by age (0–1, 2–4, 5–9, 10–17, 18–64, 65+ years), pharmacy location (by State/Territory), and prescriber (GP, dentist and other specialist). To determine the extent to which the April 2020 PBS changes had an impact on dispensing patterns, we also performed a stratified analysis for original and refill dispensings. Using the 10% PBS sample and the GP attendances data, we calculated the rate of antibiotic dispensing (GP‐prescribed original dispensings only) per 100 consultations.
Analyses were conducted using R version 4.0.4 (R Project for Statistical Computing) and the tidyverts package.
2.5. Ethics and data access
This study was approved by the New South Wales Population and Health Services Research Ethics Committee (no. 2013/11/494). Data access was granted by the Australian Services Australia External Request Evaluation Committee (no. RMS1126).
2.6. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20.
3. RESULTS
Between November 2015 and October 2020, an estimated 19 921 370 people had 125 495 137 antibiotic dispensings, of which 71% were prescribed by GPs. The most common antibiotics were cefalexin (21% of dispensings), amoxicillin (20%), amoxicillin with clavulanic acid (18%), doxycycline (9%), and roxithromycin (5%). Antibiotic dispensing was higher in the winter months (June–August), ranging from 70.7 dispensings per 1000 population in February 2019 to 100.0 dispensings per 1000 population in July 2019.
3.1. Impact of COVID‐19 restrictions
3.1.1. Overall antibiotic dispensing
Using whole‐of‐population claims data, we observed a 17% (95% CI: 7–27%) increase in the monthly rate of antibiotic dispensing in March 2020; from April 2020 onwards, we observed a sustained 36% (95% CI: 33–40%) reduction in the monthly rate of antibiotic dispensing, adjusted for seasonality. The mean monthly antibiotic dispensing rate for April–October decreased to 56.8 per 1000 population from 91.1 per 1000 for the corresponding period in 2019 (Figure 1, Table 1, Table S2 in the Supporting Information).
FIGURE 1.
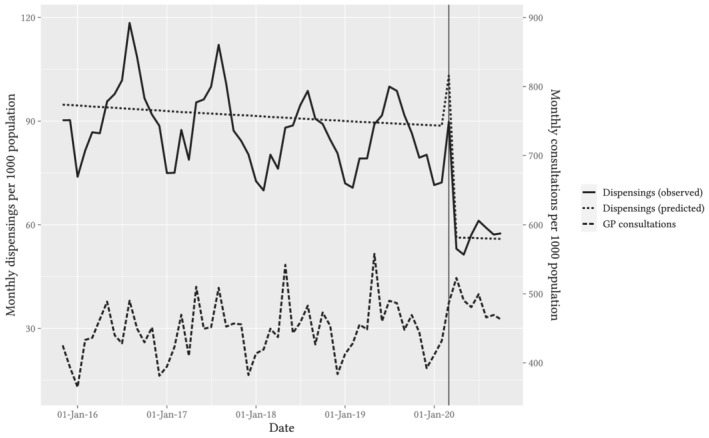
Observed and predicted trends in monthly rate of antibiotic dispensings per 1000 Australian population, superimposed with the monthly rate of reimbursements for GP consultations (combined face‐to‐face and telehealth) per 1000 Australian population. Note: Predicted line is deseasonalised. Vertical guideline marks the data for March 2020
TABLE 1.
Change in antibiotic dispensings during April–October 2020 relative to historical trend, overall and by the 10 most common antibiotics
| Antibiotic | Mean monthly dispensing per 1000 population a | Estimated level change b | ||
|---|---|---|---|---|
| 2019 | 2020 | % | 95% CI | |
| Overall | 91·1 | 56·8 | −36·4 | −39·8 to −32·7 |
| Cefalexin | 18·6 | 13·9 | −24·2 | −27·2 to −21·2 |
| Amoxicillin | 20·1 | 9·4 | −52·7 | −56·0 to −49·3 |
| Amoxicillin with clavlulanic acid | 16·7 | 8·2 | −48·0 | −52·1 to −43·6 |
| Doxycycline | 8·6 | 6·3 | −27·5 | −30·9 to −24·0 |
| Roxithromycin | 4·5 | 1·2 | −69·0 | −73·0 to −64·4 |
| Trimethoprim c | 2·9 | 2·7 | −3·1 | −6·1 to 0·1 |
| Flucloxacillin c | 2·2 | 2·5 | 2·2 | −5·7 to 10·8 |
| Clarithromycin | 2·6 | 1·1 | −51·3 | −59·5 to −41·4 |
| Metronidazole c | 2·1 | 2·0 | −4·4 | −10·0 to 1·6 |
| Phenoxymethylpenicillin | 1·9 | 1·0 | −40·9 | −46·7 to −34·4 |
Means are for the periods April–October 2019 and April–October 2020.
Estimates and 95% CIs are for the level change terms from seasonal ARIMA models.
Antibiotics listed in italics are rarely prescribed for respiratory tract infections in Australian general practice.
3.1.2. Specific antibiotics
Among the ten most common antibiotics dispensed in 2019 (Table 1), we observed the most pronounced reductions for roxithromycin 69% (95% CI: 64–73%), amoxicillin 53% (95% CI: 49–56%), and clarithromycin 51% (95% CI: 41–60%). There were no discernible reductions in flucloxacillin, metronidazole or trimethoprim dispensings (Table 1 and Figure S1 in the Supporting Information). For the comparison medicine class, purine‐analogue antivirals, we observed a transient 27% (95% CI: 20–35%) increase in dispensing in March, followed by a 3% reduction (95% CI: 0–5%) from April onwards (Table S2 and Figure S2 in the Supporting Information).
3.1.3. Stratified analyses
Using 10% sample dispensing data, the observed trend in antibiotic dispensings was similar among prescriptions written by GPs and other specialists, but antibiotic prescribing by dentists rose sharply from April through July (Figure 2). The mean monthly rate of GP‐prescribed dispensings was 63.5 per 1000 population for April–October 2019 and 37.0 per 1000 population for April–October 2020; for dentist‐prescribed dispensings it was 2.9 per 1000 population for April–October 2019, 2.3 per 1000 population in April 2020 rising to 3.2 per 1000 population in July 2020. Dispensing of prescriptions written by non‐GP medical specialists constituted about a quarter of the total volume, but followed the same pattern as GP dispensings, with an increase in March and a sustained reduction from April onwards. In the age‐stratified analysis of all dispensings, we observed similar relative reductions in antibiotic dispensing rates across all age groups (Figure 3).
FIGURE 2.
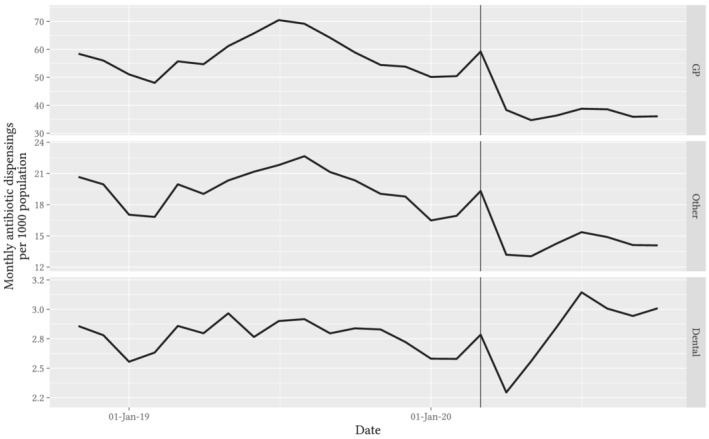
Observed trends in monthly rate of antibiotic dispensings per 1000 Australian population, stratified by prescriber specialty. Note: Vertical guideline marks the data for March 2020
FIGURE 3.
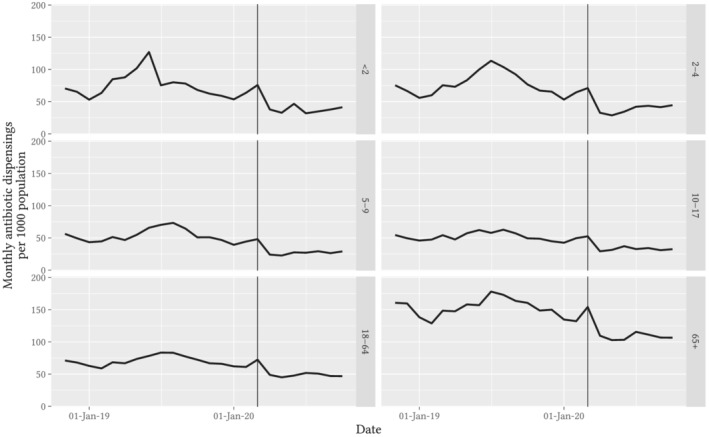
Observed trends in monthly rate of antibiotic dispensings per 1000 Australian population, stratified by age on the day of dispensing. Note: Vertical guideline marks the data for March 2020
Trends were similar across all States and Territories, with a prolonged downward slope slightly more evident for Victoria than for the other States (Figure S3 in the Supporting Information). The monthly rate of dispensings in Victoria was 52.1 per 1000 population in April 2020 and 46.5 per 1000 population in October 2020; in NSW it was 55.5 per 1000 population in April 2020 and 54.3 per 1000 population in October 2020; the mean monthly rates for April–October were 49.2 per 1000 population for Victoria and 55.0 per 1000 population for NSW.
We observed a sustained decrease in dispensing from April 2020 onwards in both original dispensings and refills (Figure S4 in the Supporting Information). The mean monthly rate of original dispensings was 71.4 per 1000 population for April–October 2019 and 46.7 per 1000 population for April–October 2020; for refill dispensings it was 16.7 per 1000 population for April–October 2019 and 7.8 per 1000 population for April–October 2020.
3.1.4. GP consultations
Prior to March 2020, GP consultation rates increased annually by a mean of 1% (Figure 1), with peaks in autumn and winter. The proportion of remote consultations (telehealth or video consultation) was less than 1%. Following COVID‐19 restrictions, overall GP consultation rates increased initially in March by 16% (95% CI: 6–27%), and then from April onwards they returned to baseline and did not differ from predicted seasonal values, changing by 2% (95% CI: −5–10%). The mean monthly proportion of telehealth consultations was 31% for April–October (Figure S5 in the Supporting Information). The rate of antibiotic dispensings per GP consultation dropped from 9.7 per 100 consultations in April 2019 to a low of 5.9 dispensings per 100 consultations in April 2020, showing a similar pattern of sustained reduction to the dispensing data (Figure 4).
FIGURE 4.
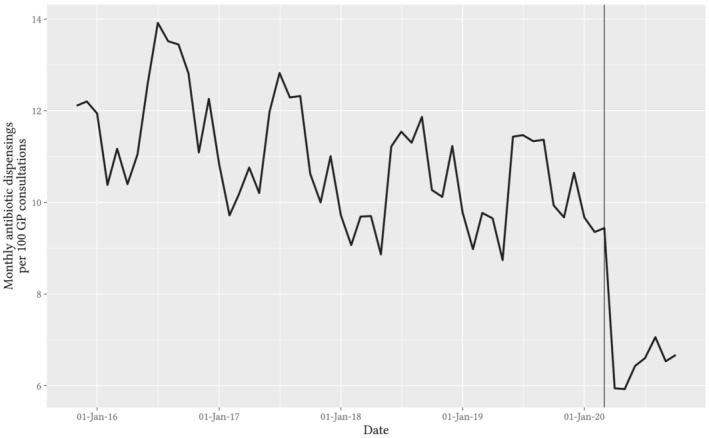
Observed trend in monthly rate of antibiotic dispensings per 100 GP consultations (combined face‐to‐face and telehealth). Note: Original prescriptions written by a GP only. Vertical guideline marks the data for March 2020
4. DISCUSSION
In this whole‐of‐population study, we observed a 36% reduction in total antibiotic dispensing in the months following the introduction of COVID‐19 pandemic restrictions. There were large reductions in GP prescribing of antibiotics primarily used for respiratory tract infections (amoxicillin, clarithromycin and roxithromycin) but no reductions in prescribing of antibiotics generally used to treat non‐respiratory infections (flucloxacillin, metronidazole and trimethoprim) 23 , 24 or ciclovirs, used for chronic viral infections. The reduction in antibiotic dispensings persisted into winter (June–August), in contrast to the seasonality of antibiotic prescribing before the pandemic. We observed similar reductions across all age groups, with no change in GP consultations over time. Dentists excepted, non‐GP prescribers showed the same pattern of changes as GPs during the pandemic months. Reductions from April onwards were similar in all Australian States and Territories, but Victoria showed lower dispensing rates after July, coinciding with more stringent Victorian measures introduced at that time.
Australia introduced several restrictions simultaneously, early in the pandemic, including travel limitations, school and childcare closures, work‐from‐home orders, and restrictions on gatherings, elective surgeries and visitors to hospital inpatients and aged care facilities. 16 Data from smartphones and other sources showed large decreases in mobility across the country in March and April 2020, 25 likely resulting in reduced person‐to‐person transmission of respiratory and gastrointestinal pathogens. Indeed, following the implementation of restrictions, dramatic falls were reported in incidence of virologically confirmed and clinical episodes of influenza, respiratory syncytial virus (RSV), and infectious gastroenteritis. 1 , 2 , 3 , 4 Importantly, to date there are no indications or reports of increases in hospitalisations arising from suboptimal community treatment of infection.
Sluggett et al. compared Australian rates of antibiotic dispensing in 2020 with those in 2019, using the same monthly aggregate claims data as our study, and observed the same pattern of increased dispensing in March 2020 followed by an overall decrease through to September 2020. They also concluded that the observations likely reflected decreased transmission of respiratory infections. 26 Other high‐income countries have recorded similar changes in community antibiotic prescribing rates during the pandemic. In the US, the community dispensing rate for the ten most common antibiotics dropped by 13% to 56% in April, but returned to pre‐pandemic levels by July 2020, except for antibiotics commonly used for respiratory infections, which showed sustained reductions. 11 In the Netherlands, there was a decrease in antibiotics for upper and lower respiratory infections, with no increase in observed complications from severe infections. 10 A UK population‐based cohort study found that the rate of GP consultations for respiratory infections fell by 68–84% from the expected rates for April to September 2020, while the proportion of respiratory infection consultations in which an antibiotic was prescribed fell by −1–18%. The rate of urinary tract infection consultations also fell, but to a lesser degree (59–72%), and the proportion of consultations where an antibiotic was prescribed did not change. 14 In contrast to Australia, the UK, US and the Netherlands have had persistently high COVID‐19 incidence rates, which may have modified clinical decision‐making about acute respiratory infections.
We found that the overall rates of GP consultations remained stable in Australia during the pandemic, albeit with a marked shift to telehealth. Remote assessment without clinical examination might have modified management decisions, but we were unable to link antibiotic prescribing patterns to GP consultation type. In England and the Netherlands, there were similar shifts from face‐to‐face to remote GP consultations 8 , 10 and concern that telehealth consultations without clinical examination might lead to diagnostic uncertainty and inappropriate antibiotic prescribing. 27
We observed an increase in antibiotic prescribing by dental practitioners following the COVID‐19 restrictions. Reduced access to dental care, particularly for surgical management of dental infections, is likely to have driven a switch to medical management. 28 Additionally, we saw initial increases in dispensings of ciclovirs following implementation of restrictions, suggesting patients were stockpiling in anticipation of shortage or stay‐at‐home orders; similar increases were also reported in Australia for medicines used to treat respiratory conditions, such as asthma or chronic obstructive pulmonary disease. 21
Australia is in the top quarter of countries for antibiotic use, compared with European countries and Canada. 29 Inappropriate antibiotic prescribing contrary to clinical guidelines is prevalent in both hospitals and the community in many countries and is a major driver of antimicrobial resistance. 30 Unnecessary antibiotics for viral respiratory infections managed in the community are a particular concern and a target for interventions; inappropriate prescribing for viral upper and lower respiratory tract infections has been estimated to account for roughly 40% of antibiotics prescribed by Australian GPs. 29 , 31 These observations are consistent with our finding of a sharp decrease in antibiotic prescription rates during the COVID‐19 pandemic, despite stable rates of GP consultations, given the reported falls in the incidence of viral respiratory tract infections cited above.
4.1. Strengths and limitations
Strengths of our study include the analysis of total population claims data and person‐level dispensing data from a random 10% of the population, allowing stratified analyses by age and practitioner type. We used robust interrupted time series methods to estimate the changes in antibiotic use at the population level, adjusting for historical trends and seasonal variation. We incorporated national data on the number and type of GP consultations, indicating that reduced health care access was unlikely to explain the reduction in antibiotic dispensings. A prior Australian study did not use interrupted time series methods, person‐level claims data or data on GP consultations. 26 Australia had a comparatively low COVID‐19 incidence and high rates of SARS‐CoV‐2 testing. In contrast to high incidence settings, GPs did not need to treat COVID‐19 patients in the community. 32
Our study limitations include a lack of information about the reason for consultation and indication for prescribing, as well as the lack of a link between individual consultation and dispensing records; therefore, we cannot determine with certainty the relative contribution of infection rates, patient presentation rates and GP management decisions to the reduction in antibiotic dispensing. We also lacked data on prescriptions that were not subsidised by the PBS; however, historical data suggest these are a small proportion of dispensed antibiotics. 29 Due to perturbation of dates in the PBS 10% sample data, we were unable to investigate differences due to State‐by‐State response measures in detail. Finally, new prescribing restrictions for selected PBS‐listed antibiotics from 1 April 2020 may have reduced rates of dispensing. However, we found that the reductions in original and refill dispensings were similar.
5. CONCLUSION
Following measures to mitigate SARS‐CoV‐2 transmission, we observed substantial reductions in community dispensings of antibiotics primarily used to treat respiratory infections, in a setting with a low COVID‐19 incidence. These reductions correspond to reported drops in respiratory viral infections and notifications; they were not accompanied by a decline in primary care consultations. The “natural experiment” arising from the public health responses to the COVID‐19 pandemic is a unique occasion to inform antimicrobial prescribing guidelines, highlighting the potential reduction in antibiotic prescribing by GPs and specialists for respiratory viral infections.
COMPETING INTERESTS
H.Z., A.L.S., M.B.G. and S.A.P. are employed by the Centre for Big Data Research in Health, UNSW Sydney, which received funding in 2020 from AbbVie Australia to conduct research, unrelated to this study. The remaining authors report no actual, potential or perceived conflict of interest regarding the submission of this manuscript.
CONTRIBUTORS
M.B.G., D.P.B., S.A.P., A.L.S. and H.Z. conceived and designed this study. M.B.G. and L.I. analysed the data. M.B.G., D.P.B., A.L.S. and H.Z. drafted the manuscript. All the authors interpreted the analyses and reviewed and approved all drafts of the manuscript.
Supporting information
TABLE S1 General practitioner attendance items: Medicare Benefits Schedule service codes (item numbers)
TABLE S2 Tabulated seasonal ARIMA model coefficients for all antibiotics and the comparison medicine classes
FIGURE S1 Observed trends in monthly rate of antibiotic dispensings per 1000 Australian population, stratified by ten most commonly prescribed antibiotics
FIGURE S2 Observed trend in monthly rate of dispensings per 1000 Australian population for comparison medicine class J05AB (ciclovirs)
FIGURE S3 Observed trends in monthly rate of antibiotic dispensings per 1000 Australian population, stratified by State or Territory of dispensing pharmacy
FIGURE S4 Observed trends in monthly rate of antibiotic dispensings per 1000 Australian population stratified by original dispensing or refill
FIGURE S5 Observed trends in general practice consultations per 1000 Australian population, stratified by mode of delivery
ACKNOWLEDGEMENTS
We thank the Australian Government Services Australia for providing the data and Melisa Litchfield for assisting with data access and ethics approval. This work was supported by the National Health and Medical Research Council (NHMRC) Centre of Research Excellence in Medicines Intelligence [grant number 1196900]. Dr Zoega is supported by a UNSW Scientia Fellowship. Dr Schaffer is supported by an NHMRC Early Career Fellowship [grant number 1158763]. Dr Burgner is supported by an NHMRC Investigator Grant [grant number 1175744]. Dr Nassar was supported by the Financial Markets Foundation for Children and NHMRC Investigator Grant [grant number APP1197940]. Research at the Murdoch Children's Research Institute is supported by the Victorian Government's Operational Infrastructure Program. The WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health. The funding bodies did not play any role in the study.
Gillies MB, Burgner DP, Ivancic L, et al. Changes in antibiotic prescribing following COVID‐19 restrictions: Lessons for post‐pandemic antibiotic stewardship. Br J Clin Pharmacol. 2022;88(3):1143-1151. 10.1111/bcp.15000
Malcolm Gillies and David Burgner are equal contributions as first authors.
Andrea Schaffer and Helga Zoega are equal contributions as last authors.
[Correction added on 21 August 2021, after first online publication: Isobel M. F. Todd's name has been corrected in this current version.]
Funding information UNSW Scientia Program; Financial Markets Foundation for Children, Grant/Award Number: APP1197940; National Health and Medical Research Council, Grant/Award Numbers: 1158763, 1175744, 1196900
DATA AVAILABILITY STATEMENT
The PBS and MBS claims data are publicly available at https://www.pbs.gov.au/info/statistics/dos-and-dop/dos-and-dop and http://medicarestatistics.humanservices.gov.au/statistics/mbs_item.jsp. Access to the 10% PBS sample was granted by the Services Australia External Request Evaluation Committee (approval number: RMS1128). Direct access to these data and analytical files to other individuals or authorities is not permitted without the express permission of the approving human research ethics committees and data custodians.
REFERENCES
- 1. Sutherland K, Chessman J, Zhao J, et al. Impact of COVID‐19 on healthcare activity in NSW, Australia. Public Health Res Pract. 2020;30(4):e3042030. [DOI] [PubMed] [Google Scholar]
- 2. Bruggink LD, Garcia‐Clapes A, Tran T, Druce JD, Thorley BR. Decreased incidence of enterovirus and norovirus infections during the COVID‐19 pandemic, Victoria, Australia, 2020. Commun Dis Intell. 2021;44:1‐8. 10.33321/cdi.2021.45.5 [DOI] [PubMed] [Google Scholar]
- 3. Sullivan SG, Carlson S, Cheng AC, et al. Where has all the influenza gone? The impact of COVID‐19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Eurosurveillance. 2020;25(47):2001847. 10.2807/1560-7917.ES.2020.25.47.2001847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeoh DK, Foley DA, Minney‐Smith CA, et al. The impact of COVID‐19 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis. 2021;72(12):2199‐2202. 10.1093/cid/ciaa1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Angoulvant F, Ouldali N, Yang DD, et al. COVID‐19 pandemic: Impact caused by school closure and national lockdown on pediatric visits and admissions for viral and non‐viral infections, a time series analysis. Clin Infect Dis. 2021;72(2):319‐322. 10.1093/cid/ciaa710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olsen SJ. Decreased influenza activity during the COVID‐19 pandemic—United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1305‐1309. 10.15585/mmwr.mm6937a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Todd I, Miller J, Rowe S, Burgner D, Sullivan S. Changes in infection‐related hospitalisations in children following pandemic restrictions: an interrupted time‐series analysis of total population data. Int J Epidemiol. 2021:1‐9. 10.1093/ije/dyab101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Armitage R, Nellums LB. Antibiotic prescribing in general practice during COVID‐19. Lancet Infect Dis. 2021;21(6):e144. 10.1016/S1473-3099(20)30917-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Malcolm W, Seaton RA, Haddock G, et al. Impact of the COVID‐19 pandemic on community antibiotic prescribing in Scotland. JAC‐Antimicrob Resist. 2020;2(4):dlaa105. 10.1093/jacamr/dlaa105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van de Pol AC, Boeijen JA, Venekamp RP, et al. Impact of the COVID‐19 pandemic on antibiotic prescribing for common infections in The Netherlands: a primary care‐based observational cohort study. Antibiotics. 2021;10(2):1‐10. 10.3390/antibiotics10020196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buehrle DJ, Nguyen MH, Wagener MM, Clancy CJ. Impact of the coronavirus disease 2019 pandemic on outpatient antibiotic prescriptions in the United States. Open Forum Infect Dis. 2020;7:ofaa575. 10.1093/ofid/ofaa575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blix HS, Høye S. Use of antibiotics during the COVID‐19 pandemic. Tidsskr Nor Legeforen. 2021;141(4). 10.4045/tidsskr.20.1003 [DOI] [PubMed] [Google Scholar]
- 13. Peñalva G, Benavente R, Pérez‐Moreno M, et al. Effect of the coronavirus disease 2019 pandemic on antibiotic use in primary care. Clin Microbiol Infect. 2021;27(7):1058‐1060. 10.1016/j.cmi.2021.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rezel‐Potts E, L'Esperance V, Gulliford M. Antimicrobial stewardship in the UK during the COVID‐19 pandemic: a population‐based cohort study and interrupted time‐series analysis. Br J Gen Pract. 2021;71(706):e331‐e338. 10.3399/BJGP.2020.1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mellish L, Karanges EA, Litchfield MJ, et al. The Australian Pharmaceutical Benefits Scheme data collection: a practical guide for researchers. BMC Res Notes. 2015;8(1):1‐13. 10.1186/s13104-015-1616-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. COVID‐19 National Incident Room Surveillance Team . COVID‐19 Australia: Epidemiology Report 36: Reporting period ending 28 February 2021. Commun Dis Intell. 2021;45:1‐26. 10.33321/cdi.2021.45.14 [DOI] [PubMed] [Google Scholar]
- 17. Australian Government Department of Health . PBS and RPBS Section 85 Date of Supply Data. 2021. https://www.pbs.gov.au/info/statistics/dos-and-dop/dos-and-dop. Accessed February 3, 2021.
- 18. Australian Government Services Australia . Statistics—Medicare Item Reports. http://medicarestatistics.humanservices.gov.au/statistics/mbs_item.jsp. Accessed March 1, 2021.
- 19. Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists, Pharmaceutical Society of Australia, Royal Australian College of General Practitioners . Australian Medicines Handbook. https://amhonline.amh.net.au/auth (login required).
- 20. Australian Government Department of Health . Antibiotics: PBS/RPBS utilisation, Oct 2014 and Feb 2015. https://www.pbs.gov.au/pbs/industry/listing/participants/public-release-docs/antibiotics/antibiotics-oct-14-feb-15. Published online 29 May 2015. Accessed March 1, 2021.
- 21. Australian Institute of Health and Welfare . Impacts of COVID‐19 on Medicare Benefits Scheme and Pharmaceutical Benefits Scheme service use, Summary. Australian Institute of Health and Welfare. https://www.aihw.gov.au/reports/health-care-quality-performance/covid-impacts-on-mbs-and-pbs/contents/impact-on-pbs-service-use. Accessed February 3, 2021.
- 22. Mian M, Sreedharan S, Giles S. Increased dispensing of prescription medications in Australia early in the COVID‐19 pandemic. Med J Aust. 2021;214(9):428‐429. 10.5694/mja2.51029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Therapeutic Guidelines Ltd . eTG complete: Therapeutic Guidelines. https://tgldcdp.tg.org.au/etgAccess. Published 30 November 2020. Accessed November 30, 2020.
- 24. Dallas A, Magin P, Morgan S, et al. Antibiotic prescribing for respiratory infections: a cross‐sectional analysis of the ReCEnT study exploring the habits of early‐career doctors in primary care. Fam Pract. 2015;32(1):49‐55. 10.1093/fampra/cmu069 [DOI] [PubMed] [Google Scholar]
- 25. Beck MJ, Hensher DA. Insights into the impact of COVID‐19 on household travel and activities in Australia—the early days under restrictions. Transp Policy. 2020;96:76‐93. 10.1016/j.tranpol.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sluggett JK, Dinh YH, Wesselingh SL, Inacio MC, Caughey GE. National changes in outpatient systemic antibiotic use during the coronavirus disease 2019 pandemic in Australia. Clin Infect Dis. 2021;ciab241. 10.1093/cid/ciab241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han SM, Greenfield G, Majeed A, Hayhoe B. Impact of remote consultations on antibiotic prescribing in primary health care: systematic review. J Med Internet Res. 2020;22(11):e23482. 10.2196/23482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mian M, Teoh L, Hopcraft M. Trends in dental medication prescribing in Australia during the COVID‐19 pandemic. JDR Clin Transl Res. 2021;6(2):145‐152. 10.1177/2380084420986766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Australian Commission on Safety and Quality in Health Care (ACSQHC) . AURA 2019: Third Australian Report on Antimicrobial Use and Resistance in Human Health. ACSQHC; 2019. https://www.safetyandquality.gov.au/publications-and-resources/resource-library/aura-2019-third-australian-report-antimicrobial-use-and-resistance-human-health. Accessed November 30, 2020. [Google Scholar]
- 30. World Health Organization . Antimicrobial Resistance: Global Report on Surveillance. World Health Organization; 2014. https://apps.who.int/iris/handle/10665/112642. Accessed March 1, 2021. [Google Scholar]
- 31. McCullough AR, Pollack AJ, Plejdrup Hansen M, et al. Antibiotics for acute respiratory infections in general practice: comparison of prescribing rates with guideline recommendations. Med J Aust. 2017;207(2):65‐69. 10.5694/mja16.01042 [DOI] [PubMed] [Google Scholar]
- 32. de Lusignan S, Joy M, Sherlock J, et al. PRINCIPLE trial demonstrates scope for in‐pandemic improvement in primary care antibiotic stewardship. BJGP Open. 2021. 10.3399/BJGPO.2021.0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 General practitioner attendance items: Medicare Benefits Schedule service codes (item numbers)
TABLE S2 Tabulated seasonal ARIMA model coefficients for all antibiotics and the comparison medicine classes
FIGURE S1 Observed trends in monthly rate of antibiotic dispensings per 1000 Australian population, stratified by ten most commonly prescribed antibiotics
FIGURE S2 Observed trend in monthly rate of dispensings per 1000 Australian population for comparison medicine class J05AB (ciclovirs)
FIGURE S3 Observed trends in monthly rate of antibiotic dispensings per 1000 Australian population, stratified by State or Territory of dispensing pharmacy
FIGURE S4 Observed trends in monthly rate of antibiotic dispensings per 1000 Australian population stratified by original dispensing or refill
FIGURE S5 Observed trends in general practice consultations per 1000 Australian population, stratified by mode of delivery
Data Availability Statement
The PBS and MBS claims data are publicly available at https://www.pbs.gov.au/info/statistics/dos-and-dop/dos-and-dop and http://medicarestatistics.humanservices.gov.au/statistics/mbs_item.jsp. Access to the 10% PBS sample was granted by the Services Australia External Request Evaluation Committee (approval number: RMS1128). Direct access to these data and analytical files to other individuals or authorities is not permitted without the express permission of the approving human research ethics committees and data custodians.


