Abstract
Aims
To determine if neurologic symptoms at admission can predict adverse outcomes in patients with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2).
Methods
Electronic medical records of 1053 consecutively hospitalized patients with laboratory‐confirmed infection of SARS‐CoV‐2 from one large medical center in the USA were retrospectively analyzed. Univariable and multivariable Cox regression analyses were performed with the calculation of areas under the curve (AUC) and concordance index (C‐index). Patients were stratified into subgroups based on the presence of encephalopathy and its severity using survival statistics. In sensitivity analyses, patients with mild/moderate and severe encephalopathy (defined as coma) were separately considered.
Results
Of 1053 patients (mean age 52.4 years, 48.0% men [n = 505]), 35.1% (n = 370) had neurologic manifestations at admission, including 10.3% (n = 108) with encephalopathy. Encephalopathy was an independent predictor for death (hazard ratio [HR] 2.617, 95% confidence interval [CI] 1.481–4.625) in multivariable Cox regression. The addition of encephalopathy to multivariable models comprising other predictors for adverse outcomes increased AUCs (mortality: 0.84–0.86, ventilation/ intensive care unit [ICU]: 0.76–0.78) and C‐index (mortality: 0.78 to 0.81, ventilation/ICU: 0.85–0.86). In sensitivity analyses, risk stratification survival curves for mortality and ventilation/ICU based on severe encephalopathy (n = 15) versus mild/moderate encephalopathy (n = 93) versus no encephalopathy (n = 945) at admission were discriminative (p < 0.001).
Conclusions
Encephalopathy at admission predicts later progression to death in SARS‐CoV‐2 infection, which may have important implications for risk stratification in clinical practice.
Keywords: encephalopathy, COVID‐19, SARS‐CoV‐2, neurologic symptoms
Patients with severe acute respiratory syndrome coronavirus 2 infection have a high prevalence of neurologic manifestations, including headache, encephalopathy, dizziness, taste, and smell impairment. Patients with encephalopathy at admission predict later progression to death and mechanical ventilation/ICU admission in SARS‐CoV‐2 infection, which may have important implications for risk stratification in clinical practice.
![]()
1. INTRODUCTION
The outbreak of coronavirus disease 2019 (COVID‐19), caused by novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has been a serious threat to public health. According to the World Health Organization (WHO), there have been 136,996,364 confirmed cases of COVID‐19 and 2,951,832 confirmed deaths in 216 countries, areas, and territories until April 14, 2021. Approximately 20% of patients develop a severe respiratory illness that ultimately requires mechanical ventilation, with mortality rates exceeding 50% in these severe cases.1 Due to the high risk of developing critical illness and adverse outcomes and the intensive demands placed on medical resources as the number of severe cases increases, it is critical to identify patients with COVID‐19 who may be more susceptible to advanced disease progression at an early stage, preferably at the time of hospital admission.
Recent studies have shown that severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has a myriad of neurological manifestations.2, 3, 4, 5, 6, 7, 8, 9 Several studies specifically described a high prevalence of serious neurologic manifestations in patients with severe COVID‐19, which suggests that the nervous system may become more involved as the disease progresses. 5, 9, 10, 11, 12 Despite the continuously increasing reports of the neurological symptoms of SARS‐CoV‐2, our knowledge about the possible association between early neurologic manifestations and subsequent deterioration leading to intensive care unit (ICU) admission, mechanical ventilation, and death remains unknown. Herein, we will aim to determine associations between encephalopathy and other neurologic manifestations of COVID‐19 and adverse outcomes in a large cohort of hospitalized patients. We will also aim to determine the accuracy of early neurologic manifestations in predicting subsequent adverse outcomes in COVID‐19 patients and then discuss possible routes of SARS‐CoV‐2 nervous system involvement based on the current evidence.
2. METHODS
2.1. Study design and participants
We conducted a retrospective observational cohort study using data from the hospitals in the University of Pennsylvania Healthcare System (including the Hospital of the University of Pennsylvania, Pennsylvania Hospital, Penn Presbyterian Medical Centre and Chester County Hospital) in the United States. We identified consecutive patients hospitalized with laboratory‐confirmed COVID‐19 infection between March 8, 2020 and April 23, 2020. The diagnosis of COVID‐19 was determined according to WHO interim guidance and confirmed by ribonucleic acid (RNA) detection of SARS‐CoV‐2 in our onsite clinical laboratory. A total of 1053 consecutively hospitalized patients with laboratory confirmation of SARS‐CoV‐2 were included in the analysis (Figure 1).
FIGURE 1.
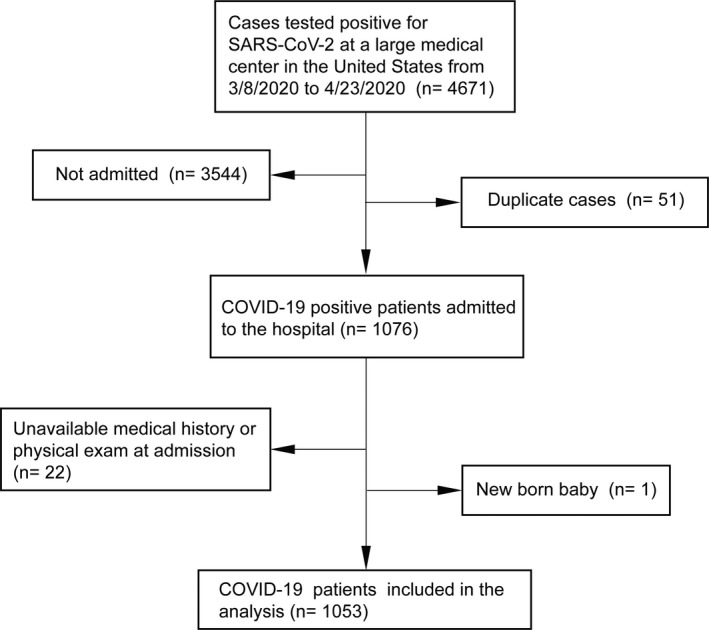
Flow chart of inclusion/exclusion
2.2. Standard protocol approvals, registrations, and patient consents
The study was approved and the requirement for informed consent was waived by our medical center's Institutional Review Board.
2.3. Data collection
Each patient's electronic medical record was reviewed, with data abstracted from physician and nursing documentation, laboratory results, and radiologic examinations, and recorded in a pre‐specified electronic collection form by two medical students in consensus under the supervision of an attending physician. Collected data included patients’ demographics (age and sex), comorbidities (hypertension, diabetes, cardiac or cerebrovascular disease, malignancy, and chronic kidney disease), typical COVID‐19 symptoms (fever, cough, dyspnea, fatigue, chill, nausea, anorexia, diarrhea, throat pain, chest pain, and abdominal pain), and neurological symptoms. Laboratory results were extracted from medical records on the day of hospital admission (before transferring into ICU). As most patients lack complete laboratory data on the day of admission, only 495 cases were included when analyzing laboratory parameters. Results of all radiologic testing (including brain CT and MRI) were also recorded.
2.4. Neurologic symptoms
We distinguished patient symptoms present at the time of hospital admission from subsequent symptoms that occurred later during hospitalization. We specifically focused on encephalopathy as our primary neurologic symptom of interest, but also included other neurologic symptoms related to the central nervous system (CNS) (dizziness/vertigo, headache, stroke, ataxia, aphasia, and seizure) and peripheral nervous system (PNS) (taste impairment, smell impairment, and vision impairment).5
Encephalopathy was defined as global disturbance in brain function, which was expressed clinically as either subsyndromal delirium, delirium or coma with possible additional features, such as seizures or extrapyramidal signs.13 Encephalopathy was categorized as severe if the patient developed coma, and mild/moderate if the patient did not develop coma.
2.5. Outcomes
For each patient, we recorded the timing of hospital admission, discharge, ICU admission, mechanical ventilation, and death, with clinical outcomes followed up to May 26, 2020. We defined mortality as our primary outcome and mechanical ventilation and ICU admission as a combined secondary outcome, and then calculated time to each event in calendar days.
2.6. Statistical analysis
Means and standard deviations (SD) were used to describe normally distributed baseline data and median and interquartile ranges to describe non‐normally distributed baseline data. Categorical variables were expressed as counts and percentages. Kolmogorov‐Smirnov test was performed to access the distribution of continuous variables. In this study, all laboratory parameters are subject to non‐normal distribution, and the Mann‐Whitney U test was used to assess the statistical difference between the encephalopathy group and the non‐encephalopathy group in laboratory parameters. Chi‐square test was used to compare group differences of categorical variables. Univariable Cox regression analysis based on forward likelihood ratio was performed to evaluate the association of clinical characteristics at admission with later progression to death or mechanical ventilation/ICU admission. Clinical characteristics and laboratory parameters that were significant on univariable analysis were subsequently included in multivariable Cox regression models. Due to missing part of the laboratory data of some patients, we used the following method to fill in the missing data: We first standardized the laboratory data, then filled in the missing values based on the K nearest neighbor algorithm, and finally scaled to the [0,1] interval. We then performed receiver operating characteristic (ROC) analysis with calculation of areas under the curve (AUC) to determine the accuracy of our models in predicting progression to death or mechanical ventilation/ICU admission using variables from our final multivariable Cox regression models. Concordance index (C‐index) for right‐censored data was applied to evaluate the performance of these prediction models.14 Finally, we stratified patients into subgroups based on the presence or absence of encephalopathy on admission and compared adverse outcome rates using survival statistics. In a sensitivity analysis, we separately considered patients with mild/moderate and severe encephalopathy.
All statistical analyses were performed using R version 4.0.0 software (the R Foundation). The significance threshold was set at a 2‐sided P value less than 0.05.
3. RESULTS
3.1. Baseline characteristics and clinical features
We identified a total of 1053 hospitalized patients with confirmed SARS‐CoV‐2 infection over the time period studied. Mean age was 52.4 (standard deviation [SD] 20.2) years, 48.0% (n = 505) were male, and 35.1% (n = 370) had neurologic symptoms at the time of hospital admission (CNS 31.5% [n = 332], PNS 4.8% [n = 51]). In patients with CNS symptoms on admission, the most commonly reported were headache (16.3% [n = 172]), encephalopathy (10.3% [n = 108]), and dizziness (6.3% [n = 66]). In patients with PNS symptoms, the most commonly reported were taste impairment (3.8% [n = 40]), and smell impairment (3.9% [n = 41]).
A total of 174 patients developed encephalopathy during their hospitalization, including 66 that occurred later during hospitalization. Of 108 cases that had encephalopathy on admission, 15 patients were comatose and were characterized as having severe encephalopathy, while 93 patients were non‐comatose. Encephalopathy was predominantly first recorded in documentation from emergency medicine clinicians (Table S1).
Patients with encephalopathy were significantly older and had a higher prevalence of numerous comorbidities compared to patients without encephalopathy, while symptom profiles and laboratory findings also significantly differed between groups (Table 1, Table S2 and Table S3).
TABLE 1.
Clinical characteristics of COVID‐19 patients with and without encephalopathy at admission
| Clinical and laboratory | Total (n = 1053) |
Encephalopathy (n = 108) |
No encephalopathy (n = 945) |
P value |
|---|---|---|---|---|
| Clinical characteristic, no. % | ||||
| Age, mean (SD), y | 52.4 (20.2) | 72.9 (15.3) | 50.0 (19.4) | |
| <50 | 487 (46.2) | 8 (7.4) | 479 (50.7) | <0.001 |
| ≥50 | 566 (53.8) | 100 (92.6) | 466 (49.3) | |
| Sex | ||||
| Male | 505 (48.0) | 48 (44.4) | 457 (48.4) | 0.440 |
| Female | 548 (52.0) | 60 (55.6) | 488 (51.6) | |
| Comorbidities | ||||
| Hypertension | 567 (53.8) | 87 (80.6) | 480 (50.8) | <0.001 |
| Diabetes | 349 (33.1) | 50 (46.3) | 299 (31.6) | 0.002 |
| Cardiac or cerebrovascular disease | 249 (23.6) | 59 (54.6) | 190 (20.1) | <0.001 |
| Chronic kidney disease | 164 (15.6) | 40 (37.0) | 126 (13.3) | <0.001 |
| Malignancy | 107 (10.2) | 21 (19.4) | 86 (9.1) | 0.001 |
| Typical symptoms | ||||
| Cough | 788 (74.8) | 63 (58.3) | 725 (76.7) | <0.001 |
| Fever | 776 (73.7) | 91 (84.3) | 685 (72.5) | 0.008 |
| Dyspnea | 712 (67.6) | 77 (71.3) | 635 (67.2) | 0.388 |
| Fatigue | 448 (42.5) | 37 (34.3) | 411 (43.5) | 0.066 |
| Chill | 365 (34.7) | 15 (13.9) | 350 (37.0) | <0.001 |
| Nausea | 283 (26.9) | 14 (13.0) | 269 (28.5) | 0.001 |
| Diarrheal | 271 (25.7) | 13 (12.0) | 258 (27.3) | 0.001 |
| Chest pain | 250 (23.7) | 7 (6.5) | 243 (25.7) | <0.001 |
| Throat pain | 246 (23.4) | 8 (7.4) | 238 (25.2) | <0.001 |
| Anorexia | 218 (20.7) | 15 (13.9) | 203 (21.5) | 0.065 |
| Abdominal pain | 170 (16.1) | 8 (7.4) | 162 (17.1) | 0.009 |
| Nervous system symptoms | ||||
| Headache | 172 (16.3) | 6 (5.6) | 166 (17.6) | 0.001 |
| Dizziness | 66 (6.3) | 4 (3.7) | 62 (6.6) | 0.246 |
| Seizure | 24 (2.3) | 11 (10.2) | 13 (1.4) | <0.001 |
| Acute cerebrovascular disease | 4 (0.4) | 1 (0.9) | 3 (0.3) | 0.352 |
| Ataxia | 6 (0.6) | 4 (3.7) | 2 (0.2) | 0.001 |
| Taste impairment | 40 (3.8) | 0 (NA) | 40 (4.7) | NA |
| Smell impairment | 41 (3.9) | 0 (NA) | 41 (4.7) | NA |
| Vision impairment | 2 (0.2) | 0 (NA) | 2 (0.5) | NA |
| Outcomes | ||||
| Mortality | 126 (12.0) | 58 (53.7) | 49 (5.2) | <0.001 |
| Ventilation/ICU | 221 (21.0) | 66 (61.1) | 155 (17.3) | <0.001 |
Abbreviations: SD, Standard deviation. ICU, intensive care unit.
Bold values are statistically significant.
Patients with encephalopathy at admission also had a significantly higher frequency of death, mechanical ventilation, and ICU admission in our univariable analysis, and early encephalopathy was the only significant neurologic symptom associated with mortality (mild/moderate: hazard ratio [HR] 3.005, 95% confidence interval [CI] 2.071–4.360; severe: HR 3.895, 95% CI 1.997–7.594) and ventilation/ICU (mild/moderate: HR 3.333, 95% CI 2.447–4.539) (Table S4). Of note, patients with severe encephalopathy were not considered in our ventilation/ICU analyses due to near‐perfect correlation between coma and mechanical ventilation requirements.
3.2. Multivariable Cox regression analysis and sensitivity analysis in encephalopathy
Encephalopathy was an independent predictor for mortality (HR 2.617, 95% CI 1.481–4.625) in our multivariable Cox regression models based on variables significant in univariable analysis (Table 2). Other significant risk factors for mortality included age, cardiac or cerebrovascular disease and lactate dehydrogenase, while other significant risk factors for ventilation/ICU included hypertension, malignancy, dyspnea, diastolic blood pressure, oxygen saturation, magnesium, lactate dehydrogenase, and neutrophil (Table 2 and Table S5).
TABLE 2.
Multivariable Cox regression for mortality or ventilation/ICU in clinical characteristics and laboratory parameters of COVID‐19 patients
| P | Hazard Ratio | 95% CI | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Mortality | ||||
| Age | <0.001 | 193.012 | 40.471 | 920.495 |
| Hypertension | 0.987 | 0.996 | 0.610 | 1.627 |
| Cardiac or cerebrovascular disease | 0.020 | 1.566 | 1.075 | 2.282 |
| Cough | 0.157 | 0.752 | 0.507 | 1.116 |
| Fatigue | 0.211 | 0.791 | 0.548 | 1.142 |
| Encephalopathy | <0.001 | 2.617 | 1.481 | 4.625 |
| Lactate dehydrogenase | <0.001 | 15.596 | 5.133 | 47.388 |
| Ventilation/ICU | ||||
| Age | 0.957 | 1.035 | 0.296 | 3.623 |
| Hypertension | 0.007 | 1.741 | 1.161 | 2.612 |
| Diabetes | 0.124 | 1.290 | 0.933 | 1.786 |
| Cardiac or cerebrovascular disease | 0.407 | 1.144 | 0.832 | 1.573 |
| Chronic kidney disease | 0.261 | 0.806 | 0.554 | 1.174 |
| Malignancy | 0.004 | 1.718 | 1.187 | 2.486 |
| Fever | 0.244 | 1.279 | 0.846 | 1.933 |
| Fatigue | 0.286 | 1.177 | 0.872 | 1.588 |
| Dyspnea | <0.001 | 2.843 | 1.808 | 4.471 |
| Encephalopathy | 0.255 | 1.452 | 0.764 | 2.761 |
| Diastolic blood pressure | <0.001 | 0.093 | 0.025 | 0.345 |
| Oxygen Saturation | <0.001 | 0.017 | 0.006 | 0.052 |
| Blood glucose | 0.192 | .262 | .035 | 1.964 |
| Blood urea nitrogen | 0.172 | 6.164 | 0.453 | 83.859 |
| Creatinine | 0.578 | 2.291 | 0.123 | 42.541 |
| Magnesium | 0.031 | 6.830 | 1.190 | 39.203 |
| Phosphorus | 0.188 | 7.045 | 0.384 | 129.302 |
| Lactate dehydrogenase | <0.001 | 8.883 | 3.373 | 23.397 |
| White blood cell count | 0.308 | 2.048 | 0.516 | 8.125 |
| Neutrophil | 0.015 | 4.324 | 1.325 | 14.108 |
Abbreviations: CI, confidence interval; ICU, intensive care unit.
Bold values are statistically significant.
The addition of encephalopathy to a multivariable model comprising other risk factors for adverse outcomes increased the C‐index of the model from 0.78 to 0.81 for mortality and from 0.85 to 0.86 for ventilation/ICU (Table 3).
TABLE 3.
Measures of model performance in predicting progression to mortality or ventilation/ICU
| Variables | Mortality | Ventilation/ICU | ||
|---|---|---|---|---|
| C‐index | Se | C‐index | Se | |
| Encephalopathy | 0.694 | 0.029 | 0.606 | 0.017 |
| Other factorsa | 0.784 | 0.025 | 0.851 | 0.013 |
| Encephalopathy with other factors | 0.806 | 0.022 | 0.858 | 0.013 |
Abbreviations: CI, confidence interval; ICU, intensive care unit.
Other factors represent age, cardiac or cerebrovascular disease in mortality, and age, diabetes, malignancy, dyspnea in ventilation/ICU.
In addition, the AUCs were higher when encephalopathy was added to other factors to predict mortality (0.86 vs. 0.84, p < 0.001, Figure 2a) and ventilation/ICU (0.78 vs. 0.76, p < 0.001, Figure 2b).
FIGURE 2.
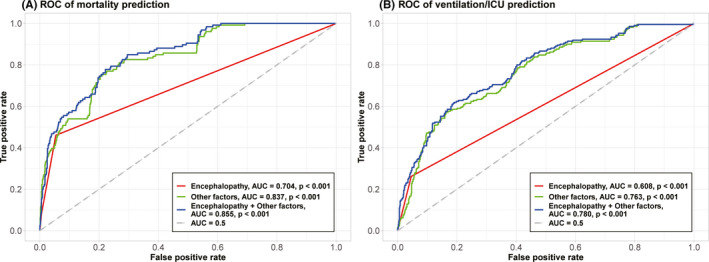
ROC‐AUCs to predict mortality or ventilation/ICU are shown in (A) and (B). In these plots, the x‐axis is false‐positive rate and y‐axis is true‐positive rate. Curves in different colors represent ROC curves based on (1) encephalopathy (2) other factors significant on multivariable Cox regression without encephalopathy (3) encephalopathy +other factors. Other factors represent age, cardiac or cerebrovascular disease, and lactate dehydrogenase in mortality, and hypertension, malignancy, dyspnea, diastolic blood pressure, oxygen saturation, magnesium, lactate dehydrogenase, and neutrophil in ventilation/ICU. ICU, intensive care unit
In a sensitivity analysis, risk stratification survival curves for mortality based on severe encephalopathy (n = 15) versus mild/moderate encephalopathy (n=93) vs. no encephalopathy (n = 945) at admission were discriminative (p < 0.001, Figure 3a). Compared to patients without encephalopathy at admission (n = 945), patients with mild/moderate encephalopathy (n = 93) had higher rates of mechanical ventilation/ICU admission (p < 0.001, Figure 3b).
FIGURE 3.

Risk stratification for mortality or ventilation/ICU based on encephalopathy for (A) mortality (B) ventilation/ICU. In these plots, the x‐axis stands for time in days, and the y‐axis is survival probability that represents the probability of not progressing to mortality or ventilation/ICU. ICU, intensive care unit
3.3. Radiologic presentations
Among 174 patients diagnosed with encephalopathy during hospitalization, 68 patients had a brain CT, of which 14 had acute abnormalities. These findings included evidence of acute ischemic stroke in four patients and intracranial hemorrhage in six patients, while four patients had other evidence of focal or global ischemia including decreased attenuation and loss of gray‐white differentiation (Figure 4A). Only four patients had a brain MRI performed in addition to their CT, with MRI confirming ischemic stroke in three patients (Figure 4B and 4C) and revealing an intracranial malignancy in one patient.
FIGURE 4.
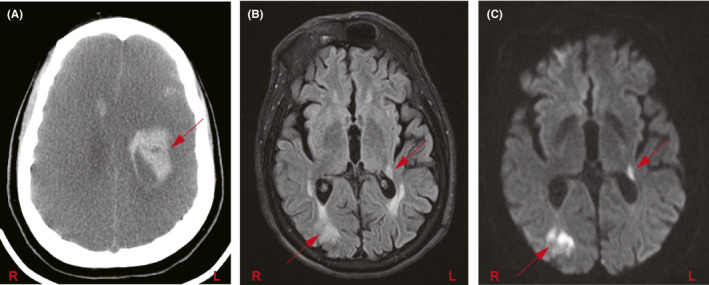
Neurological complications in COVID‐19 patients. (A) Non‐contrast CT head shows diffuse loss of gray‐white differentiation and sulcal effacement, consistent with global hypoxic ischemic encephalopathy. Additionally, there are multifocal intracranial hemorrhages, including a large hematoma in the left fronto‐parietal deep white matter (red arrow); (B) T2‐FLAIR and (C) DWI MRI brain sequences in a patient show right parieto‐occipital and left posterior thalamocapsular acute infarcts (arrows)
4. DISCUSSION
It has been widely reported in the literature that varying forms of encephalopathy are common neurological manifestations of COVID‐19, and that patients with severe COVID‐19 tend to present with more neurological symptoms. 5, 10, 11, 12, 15, 16, 17 However, these studies do not provide risk assessments based on these symptoms, which would potentially have significant clinical implications. Our study builds upon this prior work by investigating the potential utility of neurologic symptoms in predicting subsequent progression to adverse outcomes, and by building time‐to‐event models that assign risk scores to individual patients.
Our results showed that encephalopathy at admission may be an independent risk factor for later progression to death in COVID‐19 patients and that it improved the predictive accuracy of our risk factor‐based models with its addition. We also found that coma, as a marker of severe encephalopathy, provided further risk stratification and was associated with a significantly higher rate of mortality in our survival analyses. Our findings suggest that healthcare providers may consider encephalopathy and coma as key factors for the purposes of risk stratification, potentially signaling COVID‐19 patients at high risk of developing adverse outcomes. In combination with other factors, this may help inform their decisions about immediate urgency of care and longer‐term resource utilization. However, as with risk stratification in other diseases, caution must be taken that this information does not lead to a self‐fulfilling prophecy.
Other independent indicators included laboratory parameters like lactate dehydrogenase for death, and diastolic blood pressure, oxygen saturation, magnesium, lactate dehydrogenase, neutrophil for ventilation/ICU. That is understandable because patients with SARS‐CoV‐2 infection often have major organ dysfunction. Similarly, our finding that encephalopathy at admission is an independent risk factor for later progression to death may support the proposition that the CNS is directly involved in SARS‐CoV‐2 infection.
Accumulating evidence indicates that neurological complications of SARS‐CoV‐2 are highly related to immune system malfunction. 18, 19, 20, 21, 22 Overreacting of the innate immune system results in the uncontrolled release of cytokines and chemokines in patients with severe COVID‐19, leading to vascular system dysfunction and consequent brain‐blood barrier (BBB) disruption, providing a path for inflammatory mediators, immune cells, and virus particles to access the CNS. 23, 24, 25, 26, 27 It is proposed that the overproduction of inflammatory cytokines in SARS‐CoV‐2 infection may lead to inflammatory damage in the brain tissue, explaining the appearance of non‐specific complications, including headache, dizziness, taste, and smell dysfunctions. 21, 27, 28 However, as we observed, it is possible that the neurologic manifestations associated with COVID‐19 may be directly related to the infection, with encephalitis induced by hematogenous spread or neuronal retrograde transport as potential mechanisms. 15, 29, 30 These neurological manifestations have been demonstrated in other CoV infections such as SARS‐CoV and MERS‐CoV, which have provided strong evidence for CoV neuroinvasive capacity. 21, 31, 32, 33 Several reports have documented CSF samples that were positive for SARS‐CoV RNA. Also, there is evidence of monocyte and lymphocyte infiltrations in the brain, ischemic changes of neurons, and demyelinating abnormalities. 34, 35 Thus, it is proposed that neurological complications of COVID‐19 may include a rare direct infection of nerve ends. Several studies have described COVs, particularly SARS‐CoV‐2, as neurotropic viruses with neuroinvasive capabilities that directly invade the CNS through neuronal retrograde routes and result in neurological pathologies such as encephalomyelitis. 12, 36, 37, 38 Detection of SARS‐CoV‐2 and other CoVs, particularly SARS‐CoV in the cerebrospinal fluid (CSF) of infected patients, provides additional support to the potential neuroinvasive contribution of SARS‐CoV‐2. 6, 37, 39, 40, 41, 42, 43 Moreover, the endothelial cells of BBB may act as a bed for SARS‐CoV‐2 accession to CNS through angiotensin‐converting enzyme 2 (ACE2).
This study has several limitations. First, this was a retrospective single‐center study. Second, most of the symptoms used in our analysis were patients’ subjective descriptions as recorded via chart review, and most initial examinations were not performed by a neurologist or other clinician with neurologic expertise. It is likely that patients with encephalopathy were often too confused or stuporous to be able to report additional symptoms, which may have been underreported. Third, as many patients might be delayed for admission in the pandemic, the timing when they developed neurological symptoms could not be revealed by this study. Besides, we had limited data on advanced neurologic testing such as magnetic resonance imaging, lumbar puncture, and electromyography/nerve conduction velocity, because these tests were often purposefully avoided in COVID‐19 patients to reduce the risk of cross infection. Future prospective multi‐center studies are needed to confirm and expand on our findings, with additional testing to explore the neurologic implications of COVID‐19.
5. CONCLUSION
Encephalopathy at admission predicts later progression to death in COVID‐19 patients, which may have important implications for risk stratification in clinical practice.
IRB
Institutional Review Boards of Hospital of University of Pennsylvania was obtained for the study cohort with waiver of consent, with confirmation number (dbhbeegg) and protocol number (843448).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Supporting information
Supplementary Material
Tang L, Liu S, Xiao Y, et al. Encephalopathy at admission predicts adverse outcomes in patients with SARS‐CoV‐2 infection. CNS Neurosci Ther. 2021;27:1127–1135. 10.1111/cns.13687
Lei Tang and Shixin Liu are contributed equally to this work.
Funding information
Hunan Natural Science Foundation under Award, (Grant / Award Number: ‘2018JJ3709’) Brown University COVID‐19 seed grant, (Grant / Award Number: ‘GR399196’) Research Scholar Grant by RSNA Research & Education Foundation, National Natural Science Foundation of China grant under Award (Grant / Award ‘8181101287’,'81971696’) Amazon Web Service for the COVID‐19 Diagnostic Development Initiative.
[Correction added on 23 June 2021, after first online publication: "National Cancer Institute (NCI) of the National Institutes of Health under Award (Grant / Award Number: R03CA249554)" has been removed from the Funding Information.]
DATA AVAILABILITY STATEMENT
Data are not made publicly available due to HIPAA regulations, but anonymized data can be sent to the corresponding author by reasonable requests.
REFERENCES
- 1.Ahn DG, Shin HJ, Kim MH, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID‐19). J Microbiol Biotechnol. 2020;30(3):313‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad I, Rathore FA. Neurological manifestations and complications of COVID‐19: a literature review. J Clin Neurosci. 2020;77:8‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID‐19. J Neurol Neurosurg Psychiatry. 2020;91(8):889‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harapan H, Itoh N, Yufika A, et al. Coronavirus disease 2019 (COVID‐19): a literature review. J Infect Public Health. 2020;13(5):667‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurology. 2020;77(6):683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nath A. Neurologic complications of coronavirus infections. Neurology. 2020;94(19):809‐810. [DOI] [PubMed] [Google Scholar]
- 7.Paybast S, Emami A, Koosha M, Baghalha F. Novel coronavirus disease (COVID‐19) and central nervous system complications: what neurologist need to know. Acta neurologica Taiwanica. 2020;29(1):24‐31. [PubMed] [Google Scholar]
- 8.Zhou Y, Li W, Wang D, et al. Clinical time course of COVID‐19, its neurological manifestation and some thoughts on its management. Stroke Vasc Neurol. 2020;5(2):177‐179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID‐19): encephalopathy. Cureus. 2020;12(3):e7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin H, Hong C, Chen S, et al. Consensus for prevention and management of coronavirus disease 2019 (COVID‐19) for neurologists. Stroke Vasc Neurol. 2020;5(2):146‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Najjar S, Najjar A, Chong DJ, et al. Central nervous system complications associated with SARS‐CoV‐2 infection: integrative concepts of pathophysiology and case reports. J Neuroinflammation. 2020;17(1):231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID‐19 in 153 patients: a UK‐wide surveillance study. Lancet Psychiatry. 2020;7(10):875‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slooter AJC, Otte WM, Devlin JW, et al. Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Med. 2020;46(5):1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brentnall AR, Cuzick J. Use of the concordance index for predictors of censored survival data. Stat Methods Med Res. 2018;27(8):2359‐2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helms J, Kremer S, Merdji H, et al. Neurologic Features in Severe SARS‐CoV‐2 Infection. N Engl J Med. 2020;382(23):2268‐2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID‐19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID‐19 in patients presenting with influenza‐like symptoms. Int. Forum Allergy Rhinol. 2020;10(7):806‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al‐Obaidi MMJ, Bahadoran A, Wang SM, Manikam R, Raju CS, Sekaran SD. Disruption of the blood brain barrier is vital property of neurotropic viral infection of the central nervous system. Acta Virol. 2018;62(1):16‐27. [DOI] [PubMed] [Google Scholar]
- 19.Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID‐19). J Gen Intern Med. 2020;35(5):1545‐1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Wang Y, Shao C, et al. COVID‐19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451‐1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID‐19 and other coronaviruses. Brain Behav Immun. 2020;87:18‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao X. COVID‐19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID‐19 cytokine storm: the anger of inflammation. Cytokine. 2020;133:155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pilotto A, Odolini S, Masciocchi S, et al. Steroid‐responsive encephalitis in coronavirus disease 2019. Ann Neurol. 2020;88(2):423‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singhal T. A Review of coronavirus disease‐2019 (COVID‐19). Indian J Pediatr. 2020;87(4):281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID‐19. Brain Behav Immun. 2020;88:945‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Laurent S, Onur OA, et al. A systematic review of neurological symptoms and complications of COVID‐19. J Neurol. 2021;268(2):392‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gklinos P. Neurological manifestations of COVID‐19: a review of what we know so far. J Neurol. 2020;267(9):2485‐2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and Neurologic Manifestations of the Coronaviruses in the Age of Coronavirus Disease 2019: A Review. JAMA neurology. 2020;77(8):1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai LK, Hsieh ST, Chang YC. Neurological manifestations in severe acute respiratory syndrome. Acta neurologica Taiwanica. 2005;14(3):113‐119. [PubMed] [Google Scholar]
- 32.Arabi YM, Harthi A, Hussein J, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS‐CoV). Infection. 2015;43(4):495‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JE, Heo JH, Kim HO, et al. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol (Seoul, Korea). 2017;13(3):227‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaya Tutar N, Omerhoca S, Coban E, Kale N. Sars‐Cov‐2 infection related inflammatory and demyelinating disease; a brief case series. Mult Scler Relat Disord. 2021;51:102900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang ZW, Zhang LJ, Zhang SJ, et al. A clinicopathological study of three cases of severe acute respiratory syndrome (SARS). Pathology. 2003;35(6):526‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau KK, Yu WC, Chu CM, Lau ST, Sheng B, Yuen KY. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10(2):342‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS‐Coronavirus‐2. Int J Infect Dis. 2020;94:55‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yavarpour‐Bali H, Ghasemi‐Kasman M. Update on neurological manifestations of COVID‐19. Life Sci. 2020;257:118063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID‐19 virus targeting the CNS: tissue distribution, host‐virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995‐998. [DOI] [PubMed] [Google Scholar]
- 40.Steardo L, Steardo L Jr, Zorec R, Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID‐19. Acta Physiol (Oxf). 2020;229(3):e13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113(1 Pt 1):e73‐e76. [DOI] [PubMed] [Google Scholar]
- 42.Zhou L, Zhang M, Wang J, Gao J. Sars‐Cov‐2: Underestimated damage to nervous system. Travel medicine and infectious disease. 2020;36:101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS‐CoV‐2 by full‐length human ACE2. Science. 2020;367(6485):1444‐1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data are not made publicly available due to HIPAA regulations, but anonymized data can be sent to the corresponding author by reasonable requests.


