Abstract
Objective
Severe acute respiratory syndrome coronavirus 2 through angiotensin‐converting enzyme 2 receptor can harm testes function. The objectives were to analyse the prevalence of low serum testosterone (LT) and impaired fertility potential (Leydig and Sertoli cells dysfunction, respectively) in coronavirus disease 2019 (COVID‐19) male survivors and to evaluate acute infection‐related associated factors. Also, we explore its association with post‐acute COVID‐19 syndrome (PCS) and quality of life (QOL).
Materials and Methods
Male adults recovered from polymerase chain reaction‐confirmed COVID‐19 were offered a structured evaluation 8–12 weeks after recovery. The main outcome measure(s) were as follows: LT, defined as total testosterone (TT) < 2 ng/ml or if TT levels 2–4 ng/ml as calculated free testosterone < 6.36 ng/dl; Sertoli cell dysfunction was defined as inhibin‐B < 89 pg/ml. Secondary outcome‐associated factors were analysed by multiple logistic regression (odds ratio; 95% confidence interval [CI]). QOL was evaluated by SF‐36 v.2.
Results
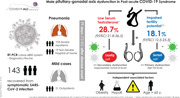
One hundred and forty‐three patients were evaluated at a median (interquartile range) of 77 days (72–83) after disease onset; 72% of them recovered from severe pneumonia. LT was detected in 41 patients (28.7%; 95% CI: 21.8–36.5). Low levels of inhibin‐B were detected in 25 patients (18.1%; 95% CI: 12.5–25.3). After multivariate adjustment, obesity and hypokalaemia were associated with LT, whereas age more than 65 was an independent predictor of Sertoli cell dysfunction. LT or Sertoli cell dysfunction was not associated with PCS. Patients with LT had a lower score in four domains of QOL.
Conclusions
Prevalence of male LT and impaired fertility potential in COVID‐19 survivors is high in the medium term. Traditional risk factors and severity markers for COVID‐19 could be predictive.
Keywords: COVID‐19, fertility, hypogonadism, low serum testosterone, quality of life, sequelae, Sertoli cell
1. INTRODUCTION
The battle against coronavirus disease 2019 (COVID‐19) seems not limited to screening and management of the acute disease. COVID‐19 also has widespread effects throughout the body with lesser known clinical manifestations, and the medium‐ and long‐term health consequences experienced by survivors of COVID‐19, if any, are currently unknown. 1 Knowledge about the impact of this virus on the endocrine system is growing. 2
Severe acute respiratory syndrome coronavirus (SARS‐CoV) was able to cause orchitis and germ cell destruction in human testes. 3 The messenger RNA for angiotensin‐converting enzyme 2 (ACE2) (genomic coordinates (GRCh38): X:15,518,196‐15,602,157), the receptor for COVID‐19, is highly expressed in human testes, primarily in spermatogonia, Leydig cells and Sertoli cells. 4 , 5 Hence, ACE2 plays vital roles in spermatogenesis—reproductive function and the regulation of steroidogenesis. Therefore, there is potential for SARS‐CoV‐2 to invade the testes via ACE2 and interfere with testosterone release and Sertoli cells function.
A pattern of elevated luteinising hormone (LH) without testosterone changes has been reported in hospitalised patients with confirmed COVID‐19. It suggests an early gonadal failure and speaks against a direct COVID‐19 effect at hypothalamus or pituitary gland. 6 This testicular defect could be caused by direct testicular damage by the virus or by an indirect inflammatory/immune response in the testicles. 7 We lack data about the pituitary–gonadal axis of male patients recovered from COVID‐19 acute infection in the medium and long term, as well as the possible effects of disease severity and the treatments used for the disease.
This study aims to analyse the medium‐term Leydig and Sertoli cells dysfunction through a hormonal evaluation in COVID‐19 survivor male patients and to evaluate the acute infection phase‐associated factors. In addition, we explore its association with post‐acute COVID‐19 syndrome (PCS) and quality of life (QOL).
2. MATERIALS AND METHODS
2.1. Patients and design
This is a cross‐sectional study of adult patients with COVID‐19, attended in the Alicante General University Hospital, Emergency Department, from 27 February to 29 April 2020. SARS‐CoV‐2 infection was confirmed by reverse transcription polymerase chain reaction (PCR) (in nasopharyngeal swab or lower respiratory tract sample) or subsequent seroconversion. Patients were classified according to the World Health Organization Clinical Progression Scale into hospitalised patients (ordinal scale ≥ 3) and ambulatory (ordinal scale < 3, which includes nonsevere pneumonia managed as hospital follow‐up at home and mild cases without pneumonia managed by primary care). 8
Surviving patients of COVID‐19 were offered an assessment by COVID‐19 medical team 8–12 weeks after ambulatory COVID‐19 recovery or discharge from hospital. A structured evaluation was performed in the same visit: clinical examination, fasting (8–10 am) blood test, Chest‐X‐ray, pulmonary function test and quality‐of‐life assessment by EuroQol Visual Analogue Scale. 9 Of 237 male patients evaluated in the emergency department, 37 (15.6%) died, and 28 were not included in the present study: 18 with severe comorbidity, 5 with follow‐up in other health areas and 5 being monitored by other physicians. Also, 3 patients refused to participate, 8 patients did not attend the face‐to‐face assessment (although a telephone interview was conducted) and 18 patients were lost to follow‐up. Finally, 143 (71.5% of the survivors) were included in the study. Patients without assessment, excluded from the analysis, did not differ in severity from the study population. No patient was on testosterone as hormone replacement therapy before admission or during follow‐up.
The clinical features, comorbidity (Charlson index 10 ), complementary examinations, established therapies and evolution during the acute phase of the infection by SARS‐CoV‐2 and after recovery from the acute phase were extracted from the digital medical record. The systematic approach in patients who had recovered from COVID‐19 include the evaluation of PCS (defined as the persistence of at least one clinically relevant symptom or abnormalities in spirometry or chest radiology) 11 and the QoL by SF‐36 v.2 Health Survey. 12
2.2. Outcomes
2.2.1. Outcome variables
Primary
-
‐
Low serum testosterone was defined as total testosterone (TT) < 2 ng/ml (6.9 nmol/L) or in those with borderline TT levels 2–4 ng/ml (6.9–13.9 nmol/l) as a calculated free testosterone (CFT) < 6.36 ng/dl (<0.22 nmol/L), according to the normal range for healthy young males in our laboratory. 13 , 14 CFT was calculated by determining TT, sex hormone binding globulin (SHBG) and albumin from the equation described by Vermeulen et al. 15
-
‐
Sertoli cell dysfunction, spermatogenesis disruption, was defined as inhibin‐B < 89 pg/ml (89 ng/L), cutoff derived from 2.5 percentile inhibin‐B in a cohort of young men unbiased with regard to fertility. 16 Inhibin‐B/follicle‐stimulating hormone (FSH) ratio was calculated.
Secondary
-
‐
The pituitary–gonadal axis was evaluated determining the FSH and LH. Secondary dysfunction was defined as low or inappropriately normal gonadotropin levels, 14 LH (for hypogonadism) or FSH concentrations (for Sertoli cell dysfunction).
-
‐
Identification of risk factors for gonadal dysfunction related to the baseline characteristics of the acute episode of COVID‐19. To study the association between the presence of gonadal dysfunction and post‐COVID syndrome; post‐COVID syndrome was defined as the persistence of COVID‐19‐relevant symptoms (systemic, neurological or respiratory) or radiological or spirometric alterations.
TT (ng/ml; reference range [r.r.]: 3–10 [nmol/L; r.r.: 10.4–34.6]) (intermediate coefficients of variability [CV]: 7.9% for 1.32 nmol/L and 6.7% for 1.96 nmol/L), LH (U/L; r.r.: 2–11.2) (CV: 1.4% for 11.4 U/L and 1.1% for 63.4 U/L), FSH (U/L; r.r.: 1–8) (CV: 2.7% for 9.9 U/L and 3.1% for 48.8 U/L), SHBG (nmol/L; r.r.: 1–8) (CV: 3.5% for 3.03 nmol/L and 2.4% for 20.9 nmol/L) and prolactin (ng/ml; r.r.: 4.6–21 (µg/L; r.r.: 4.6–21]) (CV: 2.7% for 0.74 µg/L and 2.9% for 7.6 µg/L) were determined by an electrochemiluminescent immunoassay method. They were quantified in serum on a Cobas e 801 automated autoanalyser (Roche Diagnostics). Albumin was determined by an immunoturbidimetric method, in a Cobas c 702 autoanalyser (Roche Diagnostics) (CV 1.0% for 637 µmol/L and 1.2% for 1037 µmol/L). Inhibin‐B was determined by Gen II ELISA (Beckman Coulter INMUNOTECH) (intra‐assay CV: 2.9% for 82.7 ng/L and interassay CV: 6.5% for 24.36 ng/L). Hyperprolactinaemia was evaluated and iron overload syndrome was discarded.
2.3. Statistics
The prevalence of principal outcomes (95% confidence interval [CI]) was determined both in the severe pneumonia subpopulation and the global cohort. Associations between disease severity and PCS were evaluated by χ 2 test. Multiple logistic regression models were built to explore which characteristics present at COVID‐19 diagnosis could be associated with a higher prevalence of gonadal dysfunction; odds ratios (ORs) with (95% CI) were estimated. Variables were included as covariates if they showed significant associations in simple models. Some covariates could be excluded in case of being highly correlated; >20% of missing values or number of events was too small to calculate ORs. IBM SPSS Statistics v25 (Armonk, NY) was used for analyses. p < .050 indicated statistical significance. Written informed consent was obtained from all the participants, with approval by the institutional review board (EXP. 200145).
2.4. Ethical approval
Written informed consent was obtained from all the participants, with approval by the institutional review board (EXP. 200145).
3. RESULTS
One‐hundred and forty‐three patients were included, median age was 59.0 years, interquartile range (IQR) was (46.0–68.0) and 30.8% had a Charlson comorbidity index ≥ 3. One‐hundred and three (72%) required hospital admission and 98/103 had recovered from severe pneumonia. Patients were evaluated at a median (IQR) of 77 days (72–83) after disease onset. Table 1 shows the general characteristics of the study population and the main features of COVID‐19 acute phase infection and its clinical evolution (Table 2).
Table 1.
General characteristics of the global study population and patients with gonadal dysfunction
| Global cohort (n = 143) | Low serum testosteronea Leydig cell dysfunction (n = 41) | Impaired fertility potentialb Sertoli cell dysfunction (n = 25) | p1 | p2 | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (median), years | 59.0 (46.0–68.0) | 65.0 (53.5–73.5) | 71.0 (57.0–77.5) | .002 | <.001 |
| Comorbidities | |||||
| Hypertension, % | 44.1 | 68.6 | 68.0 | <.001 | .007 |
| ARA2/ACEI as hypertension therapy, % | 81.5 | 85.2 | 72.2 | .523 | .269 |
| Diabetes, % | 11.9 | 26.8 | 28.0 | .001 | .010 |
| Obesity, % | 32.1 | 58.5 | 48.0 | <.001 | .079 |
| Cardiovascular disease, % | 9.6 | 15.0 | 25.0 | .202 | .008 |
| Charlson index ≥ 3, % | 30.8 | 56.1 | 56.0 | <.001 | .003 |
| Clinical presentation | |||||
| Clinical duration, daysc | 7.0 (5.0–9.0) | 7.0 (4.0–8.0) | 6.0 (2.5–10.0) | 0.222 | .329 |
| Fever, % | 81.8 | 75.6 | 72.0 | .222 | .255 |
| Dry cough, % | 66.2 | 67.5 | 48.0 | .837 | .029 |
| Dyspnoea, % | 52.5 | 55.0 | 40.0 | .706 | .191 |
| Diarrhoea, % | 23.8 | 19.5 | 16.0 | .488 | .277 |
| Confusion, % | 4.2 | 9.8 | 12.0 | .056 | .071 |
| Fatigue, % | 42.4 | 45.0 | 44.0 | .699 | .908 |
| Myalgias–arthralgias, % | 37.1 | 30.0 | 24.0 | .269 | .123 |
| Anosmia–dysgeusia, % | 15.2 | 17.5 | 12.0 | .633 | .649 |
| Initial assessment | |||||
| Oximetry at room air (%) | 96.0 (93.0–97.0) | 95.0 (92.0–97.0) | 96.0 (93.0–97.0) | .069 | .834 |
| PaO2:FiO2 | 350.0 (286.7–416.6) | 300.0 (258.0–414.5) | 319.0 (276.2–383.0) | .016 | .269 |
| Respiratory rate, breaths/min | 16.0 (14.0–18.0) | 16.0 (14.0–24.0) | 16.0 (16.0–20.0) | .385 | .135 |
| Systolic BP, mmHg | 135.0 (120.0–149.0) | 134.0 (120.0–144.0) | 134.0 (122.5–149.5) | .624 | .944 |
| Diastolic BP, mmHg | 83.0 (74.0–92.0) | 79.0 (70.0–88.0) | 77.0 (64.0–92.0) | .015 | 0.098 |
| Heart rate, beats/min | 92.0 (82.5–102.0) | 90.0 (73.0–100.0) | 89.0 (74.7–99.5) | .196 | .192 |
| BMI, kg/m2 | 27.7 (25.0–31.6) | 30.8 (27.2–34.8) | 29.9 (28.4–31.5) | <.001 | .025 |
| eGFR, ml/min/m2 | 86.0 (70.0–90.0) | 74.0 (56.1–89.5) | 80.3 (54.6–90.0) | .006 | .080 |
| eGFR < 60 ml/min/m2, % | 15.4 | 28.9 | 28.0 | .007 | 0.072 |
| Lymphocytes, per mm3 | 1130.0 (820.0–1530.0) | 1095.0 (842.5–1387.5) | 1030.0 (610.0–1450.0) | .584 | 0.260 |
| C‐reactive protein, mg/dl | 4.34 (1.53–8.49) | 5.54 (2.97–10.5) | 4.89 (2.26–8.67) | .075 | .588 |
| Procalcitonin, ng/ml | 0.08 (0.05–0.16) | 0.12 (0.06–0.19) | 0.07 (0.06–0.16) | .025 | .663 |
| Ferritin, mg/L | 854.5 (423.7–1516.0) | 811.0 (551.0–1755.0) | 780.0 (508.5–1226.0) | .825 | .609 |
| Interleukin‐6, pg/ml | 23.0 (12.5–63) | 29.0 (13.7–74.5) | 45.0 (17.2–75.5) | .398 | .263 |
| Lactate dehydrogenase, U/L | 254.5 (202.7–327.2) | 265.0 (224.0–326.7) | 228.0 (181.5–308.0) | .360 | .334 |
| d‐dimers, mg/ml | 0.52 (0.33–0.89) | 0.59 (0.36–1.03) | 0.49 (0.32–0.97) | .223 | .921 |
| Troponin T, ng/L | 8.0 (6.0–14.0) | 16.0 (8.0–22.5) | 14.5 (6.5–21.5) | <.001 | .027 |
| Brain natriuretic peptide, pg/ml | 61.0 (22.0–202.0) | 183.0 (36.5–484.5) | 176.0 (62.5–758.5) | .004 | .007 |
| Haemoglobin, g/dl | 14.8 (13.9–15.5) | 14.2 (13.5–15.2) | 14.3 (13.3–15.1) | .015 | .046 |
| Haematocrit, % | 45.1 (43.4–46.9) | 44.5 (41.8–46.8) | 44.1 (41.3–46.0) | .090 | .065 |
| Opacities > 50% of lung surface on X‐rays, % | 67.8% | 68.3 | 68.0 | .396 | .734 |
| Severe pneumonia, yes | 72.0 | 80.5 | 76.0 | .153 | .744 |
| COVID‐gram score, pointsd | 106.8 (90.0–132.9) | 127.4 (100.5–140.6) | 131.1 (98.6–145.9) | .004 | .146 |
| CURB65 ≥ 2, % | 19.0 | 38.9 | 46.7 | .036 | .007 |
| Evolution | |||||
| Hospitalisation, days | 9.0 (6.0–15.0) | 9.5 (7.25–16.0) | 9.0 (6.0–11.0) | .261 | .682 |
| Hypokalemia, % | 23.1 | 36.4 | 26.3 | .028 | .768 |
| TCZ use, % | 23.1 | 24.4 | 20.0 | .813 | .398 |
| Corticosteroids use, % | 23.8 | 19.5 | 20.0 | .448 | .567 |
| ICU, % | 11.2 | 14.6 | 0 | .395 | .076 |
| ICU, days | 11.0 (7.25–14.75) | 12.0 (6.0–27.5) | – | .704 | – |
| IMV, % | 9.8 | 12.2 | 0 | .544 | .074 |
| IMV, days | 8.5 (6.75–11.75) | 11.0 (7.5–29.0) | – | .159 | – |
| Post‐COVID syndromee | 53.8 | 61.0 | 60.0 | .870 | .503 |
| Changes in QoL, points | −3.5 (−10.0 to 0.0) | −4.0 (−15.0 to 0.0) | 0.0 (−11.25 to 0.0) | .794 | .164 |
Note: Data shown as %, median (IQR), unless specified otherwise. In bold, statistically significant differences.
Mann–Whitney's U and χ 2 tests were used for group comparisons: p1 low serum testosterone versus no low serum testosterone, p2 impaired fertility potential vs no impaired fertility potential.
Abbreviations: ARA2/ACEI, angiotensin II receptor antagonists/angiotensin‐converting enzyme inhibitors; BMI, body mass index; BP, blood pressure; COVID, coronavirus disease; CURB65, severity score for community‐acquired pneumonia 36 ; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; IMV, invasive mechanical ventilation requirement; PaO2:FiO2, ratio between partial pressure of oxygen in arterial blood (PaO2) and FiO2; QoL, quality of life; TCZ, tocilizumab.
Defined as total testosterone (TT) < 2 ng/ml (6.9 nmol/l) or in those with borderline TT levels 2–4 ng/ml (6.9–13.9 nmol/l) as calculated free testosterone (CFT) <6.36 ng/dl (<0.22 nmol/L), lower than the normal range for healthy young males in our laboratory. 13 , 14
Defined as inhibin‐B level < 89 pg/ml16; no inhibin‐B available in five patients. To convert into International System of Units: TT (ng/ml × 3.467) nmol/L; CFT (ng/dl × 0.03467) nmol/L; inhibin‐B (ng/L × 1).
Days of symptoms before admission.
Clinical risk score to predict the occurrence of critical illness in hospitalised patients with COVID‐19. 35
Post‐COVID syndrome, defined as the persistence of at least one clinically relevant symptom, spirometry disturbances or significant radiological alterations.
Table 2.
Hypothalamic–pituitary–gonadal axis features
| Median (IQR) | |
|---|---|
| Total testosterone, ng/ml | 4.2 (3.2–5.5) |
| CFT, ng/dl a | 5.8 (4.9–6.7) |
| SHBG | 43.0 (31.0–54.2) |
| LH, U/L | 5.1 (3.8–7.4) |
| FSH, U/L | 5.4 (3.7–10.4) |
| Inhibin‐B, pg/ml | 145.0 (103.0–188.0) |
Abbreviations: CFT, calculated free testosterone; FSH, follicle‐stimulating hormone; IQR, interquartile range; LH, luteinising hormone; SHBG, sex hormone binding globulin.
CFT was calculated in those patients with borderline total testosterone (TT) levels 2–4 ng/ml (6.9–13.9 nmol/L) by determining TT, SHBG and albumin from the equation described by Vermeulen et al. 15 To convert into the International System of Units: TT (ng/ml × 3.467) nmol/L; CFT (ng/dl × 0.03467) nmol/L; inhibin‐B (pg/ml × 1) ng/L.
Median TT was 4.2 ng/ml (3.2–5.5), and TT displayed by age quartiles was Q1 (17–45 years) 4.2 ng/ml (3.1–5.5), Q2 (46–58 years) 4.2 ng/ml (3.2–5.2), Q3 (59–67 years) 4.4 ng/ml (3.5–5.8) and Q4 (68–85 years) 3.8 ng/ml (2.8–5.1). There were no differences between age groups (p = .764).
3.1. Low serum testosterone—Leydig cell dysfunction and associated factors
Low serum testosterone was detected in 41/143 patients (28.7%; 95% CI: 21.8–36.5), 32% (95% CI: 23.8–41.5) in hospitalised patients (mainly with severe pneumonia) and 20.0% 8/40 (95% CI: 10.5–34.7) in the rest of the cohort p = .153). High LH levels were present in 9/41 patients (22%), whereas 32/41 (78%) showed low or inappropriately normal levels (p = .017), due to a predominance in the inhibition of the hypothalamic–pituitary–gonadal (HPG) axis (see Figure 1).
Figure 1.
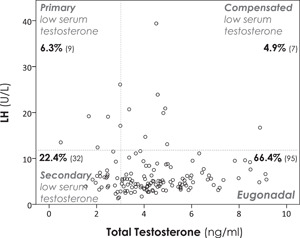
Scatter plot showing the relationship between serum total testosterone (TT) and luteinising hormone.
Low serum testosterone was defined as TT < 2 ng/ml (6.9 nmol/L) or in those with borderline TT levels 2–4 ng/ml (6.9–3.9 nmol/L) as a calculated free testosterone (CFT) < 6.36 ng/dl (<0.22 nmol/L), according to the normal range for healthy young males in our laboratory. 11 , 12 Those patients with borderline TT levels 2–4 ng/ml (6.9–13.9 nmol/L) and a CFT < 6.36 ng/dl (<0.22 nmol/L) are not represented in the scatter plot, but they have been reflected in the number and type of low serum testosterone. TT (ng/ml; reference range [r.r.]: 3–10 [nmol/L; r.r.: 10.4–34.6]); LH, luteinising hormone (U/L; r.r.: 2–11.2) (CV: 1.4% for 11.4 U/L and 1.1% for 63.4 U/L). %, percentage of patients (number of patients). To convert into the International System of Units: TT (ng/ml × 3.467) nmol/L; CFT (ng/dl × 0.03467) nmol/L; inhibin‐B (pg/ml × 1) ng/L
Eleven patients had a mild elevation of their prolactin levels (<35 ng/ml) and one a moderate elevation (<100 ng/ml); of the 32 patients with secondary low serum testosterone, only 5 had hyperprolactinaemia. After multivariate adjustment, in hospitalised patients subpopulation, obesity and hypokalaemia were independent predictors of low serum testosterone, whereas higher T‐troponin level was close to statistical significance (see Figure 2). In the global cohort model, including mild disease, only obesity (OR: 5.12 [1.77–14.76]; p = .003) was an independent predictor of low serum testosterone.
Figure 2.

Low serum testosterone and spermatogenesis disruption associated factors in patients with severe pneumonia.
Variables were included as covariates if they showed significant associations in simple models. The 95% confidence intervals (CIs) of the odds ratios have been adjusted for multiple testing. In bold, independent predictors associated with the outcomes. For the purpose of logistic regression, variables were categorised regarding their 75 percentiles within this subpopulation, to show the impact of severe extreme values in the outcomes. For the following variables, standard categorisations were followed: age ≥65 years, estimated glomerular filtration rate (eGFR) < 60 ml/min/m2, respectively. Low serum testosterone was included in the impaired fertility potential model, given its strong association at the time of assessment
3.2. Potential impaired fertility—Sertoli cell dysfunction and associated factors
Low levels of inhibin‐B were detected in 25/138 patients (18.1%; 95% CI: 12.5–25.3), 18.4% (95% CI: 12.1–27.0) and 17.1% (95% CI: 8.1–32.6) in patients with or without need for hospital admission, respectively (p = .744). High FSH levels were present in 21/25 patients (84%), whereas 4/25 (16%) showed levels in the r.r. (p = .017), translating a predominance in primary structural damage. Inhibin‐B/FSH ratio was 26.1 (10.9–50.4).
Thirteen patients presented both low serum testosterone and Sertoli cell dysfunction, the former being a risk factor of low inhibin‐B, OR: 3.49 (1.42–8.54) (p = .006).
In hospitalised patients after adjustment for confounding factors, no baseline clinical features behave as independent predictors of low levels of inhibin‐B (see Figure 2). In the global cohort model, age > 65 years (OR: 10.04 [1.75–57.62]; p = .01) was an independent predictor of Sertoli cell dysfunction—spermatogenesis disruption.
In patients without low serum testosterone and with inhibin‐B levels in the r.r., isolated elevations of LH or FSH were found in 2/143 (1.3%) and 17/143 (11.8%).
Admission to the intensive care unit, length of hospital stay or administration of corticosteroids, tocilizumab or antiviral therapy during the acute infection phase did not show an effect on gonadal dysfunction in the medium term. Low serum testosterone or Sertoli cell dysfunction was not associated with a post‐COVID syndrome (clinically relevant symptom or abnormalities in spirometry or chest radiology). There were differences in the scores of 4 dimensions of the SF‐36 between low serum testosterone subpopulation and the remainder of the study population: general perception of health (62.0 [47.0–77.0] vs. 72.0 [60.0–87.0]; p = .012), role limitations due to physical health problems (56.3 [25.0–100.0] vs. 93.8 [62.5–100.0]; p = .016), physical functioning (65.0 [40.0–75.0] vs. 80.0 [65.0–85.0], p = .001) and body pain (60.0 [50.0–90.0] vs. 80.0 [60.0–90.0]; p = .027); without differences in social functioning (93.7 [46.8–100.0] vs. 75.0 [62.5–100.0]; p = .858], emotional well‐being (80.0 [57.5–92.5] vs. 80.0 [60.0–95.0]; p = .757], role limitations due to personal or emotional problems (100.0 [50.0–100.0] vs. 100.0 [75.0–100.0]; p = .357) and vitality (energy or fatigue) (56.3 [28.1–81.3] vs. 68.8 [50.0–81.3]; p = .091).
4. DISCUSSION
To the best of our knowledge, this is the first study that has evaluated male gonadal dysfunction in a large cohort of patients recovered from COVID‐19. The assessment shows a high prevalence of low serum testosterone or Sertoli cell dysfunction, spermatogenesis disruption, around 40%, 8–12 weeks after disease onset. Low serum testosterone is mainly functional, secondary, associated with low or inappropriately normal LH levels, whereas Sertoli cell dysfunction seems mediated by primary structural damage in the seminiferous tubules. In hospitalised patients (mainly severe pneumonia patients), obesity as comorbidity, hypokalaemia and higher T‐troponin as biomarkers of severity disease in acute infection phase were associated with Leydig cell dysfunction; neither other baseline characteristics of the patients nor the COVID‐19 disease features were associated with potential impaired fertility. In the global cohort, obesity and older age were associated with low serum testosterone and Sertoli cell dysfunction development, respectively. Low serum testosterone or Sertoli cell dysfunction were not associated with a COVID‐19 sequelae, nevertheless, patients with low serum testosterone had a poorer QoL and physical functioning. The absence of a relationship between low serum testosterone and post‐COVID syndrome makes plausible a low serum testosterone‐related worsening in some SF‐36 dimensions; however, the study design prevents us from obtaining solid conclusions in this regard.
These findings can be extremely relevant for male sexual health as the consequences of COVID‐19 pandemic can extend to sexual and reproductive health.
The prevalence of low serum testosterone detected in this post‐COVID‐19 cohort greatly exceeds that reported in the general population. In the European Male Aging Study in middle‐aged and elderly men (between the ages of 40 and 79 years, mean age: 59.7 ± 0.3), 4.1% of subjects had a TT level of less than 8.0 nmol/L (<2.3 ng/ml) and 17.0% had a TT level of less than 11 nmol/L (<3.2 ng/ml). 17 However, the community prevalence estimates of potentially functional hypogonadism in middle‐aged and older men vary from 2.1% to 12.3%. 18
Evidence of gonadal involvement in COVID‐19 is scarce and heterogeneous.
In the acute phase of the infection, Ma et al., 6 in nonsevere ill hospitalised patients (n = 81), found a higher LH and low TT:LH ratio, without changes in TT versus age‐matched healthy men; only C‐reactive protein was significantly associated with T:LH ratio after adjustment on multivariable analysis. Rastrelli et al. 19 evaluated 31 patients under noninvasive mechanical ventilation (non‐IMV) and found a lower TT and CFT (increased LH) in those who evolved unfavourably requiring IMV or deceased, compared with those who improved or maintained a stable condition. After adjustment for age and comorbidities, lower TT and CFT were significantly associated with higher serum LDH, ferritin and procalcitonin, as well as with an increased level of neutrophils and decrease in lymphocyte count. The characteristics of the study populations could explain these discrepancies.
Recently, Kadihasanoglu et al. 20 reported in a cohort of hospitalised patients that COVID‐19 (n = 89, 49.9 ± 12.5 years) was associated with decreased level of TT and increased level of LH and prolactin, compared with cases with non‐COVID‐19 respiratory tract infection (n = 30, 52.7 ± 9.6 years, and age‐matched controls admitted to urologic outpatient clinic for reproductive function evaluation (n = 143, 50 ± 7.8 years). The proportion of patients with testosterone deficiency (TT < 3 ng/ml) in Groups 1, 2 and 3 was 74.2%, 53.3% and 37.8%, respectively (p < .0001). These findings, despite being limited to patients evaluated in the acute infection phase, support a greater impairment of gonadal function in COVID‐19 infection than in other infectious processes and support a hypothalamic–pituitary origin of the dysfunction of the Leydig cells in the medium term, according to our findings.
Post‐mortem examinations of testicular tissue from 12 COVID‐19 patients showed significantly reduced Leydig cells, as well as oedema and inflammation in the interstitium. 21 Whether SARS‐CoV‐2 virus can be found in semen is still debated. 22 , 23
The factors found associated with low serum testosterone (obesity, hypokalaemia and higher T‐troponin) seem to reflect a greater severity of the past illness. Obesity relationship was expected as it may play a key role and contribute to the apparent age‐related decline of testosterone, as observed in middle‐aged and older men in the European Male Aging Study study 24 ; moreover, obesity represents a risk factor for higher severity and worse prognosis in patients with COVID‐19 infection. 25 Hypokalaemia is prevalent in patients with COVID‐19 pneumonia, and is an independent predictor of IMV requirement and seems to be a sensitive biomarker of severe progression of COVID‐19, 26 whereas high T‐troponin levels are associated with disease severity and poor prognosis. 27
Impact of SARS‐CoV‐2 infection on male fertility is still lacking. The studies had primarily focused on the detection of the virus in the male reproductive tract. Recently, Li et al. 28 performed a thorough investigation of male reproductive health in a hospital‐based observational study that included autopsied testicular and epididymal specimens of deceased COVID‐19 male patients (n = 6) and recruited recovering COVID‐19 inpatients (n = 23) with an equal number of age‐matched controls. Li et al. 28 identified significant impacts of SARS‐CoV‐2 on the male reproductive system as demonstrated by impaired spermatogenesis (39.1% oligozoospermia); inflammatory response in testis and epididymis (interstitial oedema, red blood cell exudation, thinning of seminiferous tubules, higher number of apoptotic cells within seminiferous tubules, an increased concentration of CD3+ and CD68+ in the interstitial cells of testicular tissue and the presence of immunoglobulin G within seminiferous tubules); and altered seminal immune markers (increased seminal levels of interleukin‐6, tumour necrosis factor‐α and MCP‐1 compared with control males) signifying immune impairment by COVID‐19 illness. All of these signs would be, for the authors, due to an autoimmune orchitis. However, the ability of this novel coronavirus to induce an autoimmune orchitis is not proven to date. 29 These recent data reinforce and give plausibility to our findings in pituitary–gonadal axis of COVID‐19 recovered patients, whereas Sertoli cell dysfunction seems mediated by primary structural damage in the seminiferous tubules.
Sertoli cell function declines with age in general population. 16 The loss of the association between older age and impaired fertility in the population with severe pneumonia suggests that other COVID‐19‐related conditions have a more relevant role.
It is important to point out that the time point of evaluation of the pituitary–gonadal axis in the natural history of COVID‐19 can be essential to interpret testicular damage, since the alteration patterns can be dynamic, as in other clinical entities. The absence of association between gonadal dysfunction and post‐COVID syndrome makes it unlikely that alterations in the pituitary–gonadal axis are related to another systemic sequel.
Several viruses may cause orchitis in men. Among them, the mumps virus is well known for its testicular tropism and for inducing inflammation, decreased androgen production and degeneration of the seminiferous epithelium that can lead to sterility. 30 Orchitis develops in 5%–37% of all adult patients infected with mumps. 31 Unilateral involvement is the most common, whereas bilateral involvement occurs in 15%–30% of the patients with orchitis. 32 Bilateral orchitis leads to hypofertility with oligospermia and testicular atrophy in 13% of those patients. 33 Morphological studies of mumps‐associated orchitis showed the focused nature of the inflammation with interstitial oedema, 30 mimicking COVID‐19 features. 21 While seminiferous epithelium degenerated, Sertoli cells seemed little affected, 30 in contrast to the marked decrease in the Sertoli cells observed in COVID‐19. 21
Concerning the consequences of orchitis on testicular endocrine function, Adamopoulos et al. 34 described a severe alteration of Leydig cell function during the acute phase of the disease, with a drop in the testosterone level together with an increase in LH, a pattern of primary origin of low serum testosterone, which differ with our findings of secondary HPG axis damage in patients recovered from active infection.
4.1.
Limitations include the possible presence of undetected pre‐COVID abnormalities in the patients, the exclusion of some patients with severe comorbidity, deceased patients and the single‐centre design; due to shortage of PCR testing during the first wave of the pandemic, some mild ambulatory cases of COVID‐19 could had been clinically diagnosed and likely not enroled in this study. TT was assessed only once and not with mass spectrometry, and seminal analysis was not conducted. Genetic testing of the patients was not performed to exclude potential genetic reasons of gonadal dysfunction. Given the absence of a comprehensive evaluation of symptoms and signs of testosterone deficiency, our findings of low serum testosterone cannot be translated into the presence of hypogonadism as a clinical and biochemical state. We compared the prevalence of low serum testosterone using studies that had significantly larger cohorts (i.e., European Male Aging Study), 17 potentially with different baseline medical comorbidities; hence, whether the low serum testosterone/infertility rates are truly higher in this population is unclear. Finally, there was no control group; therefore, we cannot rule out that our results are superimposable with the sequelae of other febrile viral processes and not specific findings of SARS‐CoV‐2 infection. Long‐term follow‐up to confirm these preliminary results and the potentially reversible gonadal dysfunction is mandatory.
4.2.
This study has several strengths: (1) The size and the representativeness of the sample (wide clinical spectrum of severity and representative of comorbidity in western countries). (2) The re‐evaluation time, after recovering from the acute phase. (3) The low serum testosterone evaluation approach including CFT to avoid bias and the measurement of inhibin‐B as a sensitive marker of male fertility potential. (4) The analysis of the characteristics of acute infection associated with gonadal damage, adjusted for confounding factors.
In conclusion, this study provides the first direct evidence about the medium‐term influence of COVID‐19 on pituitary–gonadal axis and helps to bridge the existing gap in the field, suggesting that more attention to the global gonadal function among patients recovered from SARS‐CoV‐2 infection is needed. The low serum testosterone does not appear to be related with sequelae of the disease, but it is associated with a worse QoL. Independently of the limitations of these preliminary results, it should be acknowledged that the testis and pituitary–gonadal axis are a target for SARS‐CoV‐2 and the possibility for long‐lasting consequences on the endocrine function exists, even for recovered patients. We should be cautious while drawing conclusions from this medium‐term data, whether this state of low serum testosterone and impaired fertility potential is permanent or temporary is a question that remains unanswered.
COVID19‐ALC RESEARCH GROUP
The members of COVID19‐ALC research group are as follows: Esperanza Merino, Joan Gil, Vicente Boix, Ximo Portilla, Oscar Moreno‐Pérez, Mariano Andrés, Jose‐Manuel Leon‐Ramirez, Santos Asensio, Cleofé Fernandez, Alfredo Candela, Ma del Mar García, Rosario Sánchez, Diego Torrus, Sergio Reus, Pilar González, Silvia Otero, Jose M Ramos, Beatriz Valero, Alex Scholz, Antonio Amo, Héctor Pinargote, Paloma Ruiz, Raquel García‐Sevila, Ignacio Gayá, Violeta Esteban, Isabel Ribes, Julia Portilla, Cristina Herreras, Alejando Cintas, Alicia Ferradas, Ana Martí, Blanca Figueres, Marcelo Giménez, María‐Ángeles Martínez, María‐Mar García‐Mullor, María Angeles Martínez, Irene Calabuig, Marisa Peral, Ernesto Tovar, M Carmen López, Paloma Vela, Pilar Bernabeú, Ana Yuste, José Ponce, Bertomeu Massuti, Vicente Climent, Vicente Arrarte, Fernando Torres, Laura Valverde, Laura Delegido, Cristina Cambra, Miriam Sandín, Teresa Lozano, Amaya García‐Fernández, Alejandro Do Campo, Eduardo Vergara, Nicolás López, Elena Elvira, Fátima López, Fernando Dahl, Blanca Serrano, Sarai Moliner, Carmina Díaz, Dolores Castaño, Beatriz López; Antonio Picó, Joaquín Serrano, Sol Serrano, María Marín‐Barnuevo, María Díaz, Cristina Gilabert, Estela Martínez, Elena Vivó, Noelia Balibrea, Miguel Perdiguero, Carolina Mangas, Lucía Medina, Oscar Murcia, María Rodríguez, Rodrigo Jover, Javier López, Marina Morillas, Mercedes Khartabil, Cristina Gil, Carlos Salazar, Eva Vera, Helena López, Vanesa Rodríguez, Sandra Baile, Norma Guerra, Mar Blanes, Jaime Guijarro, José Carlos Pascual, Iris Gonzalez, Pedro Sanso, José Manuel Ramos, Jaime Javaloy, Clara Llopis, Olga Coronado, Esther García, Gonzalo Rodríguez, Paola Melgar, Mariano Franco, Félix Lluís, Carmen Zaragoza, Cándido Alcaraz, Ana Carrión, Celia Villodre, Emilio Ruiz de la Cuesta, Cristina Alenda, Francisca Peiró, María Planelles, Laura Greco, Sandra Silvia, Antonio Francia, Iván Verdú, Juan Sales, Ana Palacios, Hortensia Ballester, Antonio García‐Valentín, Marta Márquez, Eva Canelo, Andrea Juan, Elena Vives, Andrea Revert, Gonzalo Fuente, Ester Nofuentes, Carolina Mangas, Eva Vera, Alicia Ferradas, Helena López, Cristian Herrera, Beatriz López, Marina Morillas, Vanesa Rodríguez, Mercedes Khartabil, Mario Giménez, Ernesto Tovar, Estela Martínez, Lucia Medina, Sandra Baile, Carlos Salazar, Norma Guerra, Sarai Moliner, Mari‐Carmen López‐González, Blanca Figueres.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Writing – original draft: Oscar Moreno‐Perez and Esperanza Merino; writing – review and editing: Esperanza Merino, Oscar Moreno‐Perez, Vicente Boix, Mariano Andres, Jose‐Manuel Leon‐Ramirez, Joan Gil, Antonio Pico, Rocio Alfayate and Maria Eugenia Torregrosa; Conceptualisation: Oscar Moreno‐Perez, Antonio Pico, and Esperanza Merino; Investigation: Esperanza Merino, Oscar Moreno‐Perez, Vicente Boix, Mariano Andres, Jose‐Manuel Leon‐Ramirez, Joan Gil, Antonio Pico, Rocio Alfayate and Maria Eugenia Torregrosa; Methodology: Esperanza Merino, Oscar Moreno‐Perez and Vicente Boix; Formal Analysis: Oscar Moreno‐Perez; Project Administration: Esperanza Merino; Funding Acquisition: Esperanza Merino.
ACKNOWLEDGEMENTS
This study was supported by the Alicante Institute of Health and Biomedical Research (ISABIAL), Alicante, Spain (Grant Number 2020‐379); the information of this article has not been presented in any meeting(s).
Moreno‐Perez O, Merino E, Alfayate R, et al. Male pituitary–gonadal axis dysfunction in post‐acute COVID‐19 syndrome—prevalence and associated factors: a Mediterranean case series. Clin Endocrinol (Oxf). 2022;96:353–362. 10.1111/cen.14537
Oscar Moreno‐Perez and Esperanza Merino contributed to the manuscript equally and shares the first authorship.
DATA AVAILABILITY STATEMENT
Oscar Moreno‐Perez and Esperanza Merino have full access to the data and are the guarantor for the data. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Del Rio C, Collins LF, Malani P. Long‐term health consequences of COVID‐19. JAMA. 2020;324:1723‐1724. 10.1001/jama.2020.19719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lundholm MD, Poku C, Emanuele N, Emanuele MA, Lopez N. SARS‐CoV‐2 (COVID‐19) and the endocrine system. J Endocr Soc. 2020;4(11):bvaa144. 10.1210/jendso/bvaa144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu J, Qi L, Chi X, et al. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod. 2006;74(2):410‐416. 10.1095/biolreprod.105.044776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Z, Xu X. scRNA‐seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS‐CoV‐2 infection in spermatogonia, Leydig and sertoli Cells. Cells. 2020;9(4):920. 10.3390/cells9040920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verma S, Saksena S, Sadri‐Ardekani H. ACE2 receptor expression in testes: implications in coronavirus disease 2019 pathogenesis†. Biol Reprod. 2020;103(3):449‐451. 10.1093/biolre/ioaa080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma Y, Pei S, Shaman J, Dubrow R, Chen K. Effect of SARS‐CoV‐2 infection upon male gonadal function: a single center‐based study [published online ahead of print March 30, 2020]. medRxiv. 10.1101/2020.03.21.20037267 [DOI] [Google Scholar]
- 7. Illiano E, Trama F, Costantini E. Could COVID‐19 have an impact on male fertility? Andrologia. 2020;52(6):e13654. 10.1111/and.13654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO Working Group on the Clinical Characterisation and Management of COVID‐19 infection . A minimal common outcome measure set for COVID‐19 clinical research. Lancet Infect Dis. 2020;20(8):e192‐e197. 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Badia X, Roset M, Montserrat S, Herdman M, Segura A. The Spanish version of EuroQol: a description and its applications. European Quality of Life Scale. Med Clin. 1999;112 suppl 1:79‐85. [PubMed] [Google Scholar]
- 10. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 11. Moreno‐Pérez O, Merino E, Leon‐Ramirez J‐M, et al. Post‐acute COVID‐19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82:378 383. 10.1016/j.jinf.2021.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vilagut G, Ferrer M, Rajmil L, et al. The Spanish version of the Short Form 36 Health Survey: a decade of experience and new developments. Gac Sanit. 2005;19(2):135‐150. 10.1157/13074369 [DOI] [PubMed] [Google Scholar]
- 13. González‐Sánchez V, Moreno‐Pérez O, García de Guadiana L, et al. Reference ranges for serum and salivary testosterone in young men of Mediterranean region. Endocrinol Nutr Organo Soc Espanola Endocrinol Nutr. 2015;62(1):4‐10. 10.1016/j.endonu.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 14. Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103(5):1715‐1744. 10.1210/jc.2018-00229 [DOI] [PubMed] [Google Scholar]
- 15. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666‐3672. 10.1210/jcem.84.10.6079 [DOI] [PubMed] [Google Scholar]
- 16. Hart RJ, Doherty DA, McLachlan RI, et al. Testicular function in a birth cohort of young men. Hum Reprod Oxf Engl. 2015;30(12):2713‐2724. 10.1093/humrep/dev244 [DOI] [PubMed] [Google Scholar]
- 17. Wu FC, Tajar A, Beynon JM, et al. Identification of late‐onset hypogonadism in middle‐aged and elderly men. N Engl J Med. 2010;363(2):123‐135. 10.1056/NEJMoa0911101 [DOI] [PubMed] [Google Scholar]
- 18. Grossmann M, Matsumoto AM. A perspective on middle‐aged and older men with functional hypogonadism: focus on holistic management. J Clin Endocrinol Metab. 2017;102(3):1067‐1075. 10.1210/jc.2016-3580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rastrelli G, Di Stasi V, Inglese F, et al. Low testosterone levels predict clinical adverse outcomes in SARS‐CoV‐2 pneumonia patients. Andrology. 2020;9:88‐98. 10.1111/andr.12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kadihasanoglu M, Aktas S, Yardimci E, Aral H, Kadioglu A. SARS‐CoV‐2 pneumonia affects male reproductive hormone levels: a prospective, cohort study. J Sex Med. 2021;18(2):256‐264. 10.1016/j.jsxm.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang M, Chen S, Huang B, et al. Pathological findings in the testes of COVID‐19 patients: clinical implications. Eur Urol Focus. 2020;6(5):1124‐1129. 10.1016/j.euf.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang H‐H, Wang P‐H, Yang Y‐P, et al. A review of severe acute respiratory syndrome coronavirus 2 infection in the reproductive system. J Chin Med Assoc. 2020;83(10):895‐897. 10.1097/JCMA.0000000000000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holtmann N, Edimiris P, Andree M, et al. Assessment of SARS‐CoV‐2 in human semen‐a cohort study. Fertil Steril. 2020;114(2):233‐238. 10.1016/j.fertnstert.2020.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Corona G, Goulis DG, Huhtaniemi I, et al. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: endorsing organization: European Society of Endocrinology. Andrology. 2020;8(5):970‐987. 10.1111/andr.12770 [DOI] [PubMed] [Google Scholar]
- 25. Soeroto AY, Soetedjo NN, Purwiga A, et al. Effect of increased BMI and obesity on the outcome of COVID‐19 adult patients: a systematic review and meta‐analysis. Diabetes Metab Syndr. 2020;14(6):1897‐1904. 10.1016/j.dsx.2020.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moreno‐P O, Leon‐Ramirez J‐M, Fuertes‐Kenneally L, et al. Hypokalemia as a sensitive biomarker of disease severity and the requirement for invasive mechanical ventilation requirement in COVID‐19 pneumonia: a case series of 306 Mediterranean patients. Int J Infect Dis. 2020;100:449‐454. 10.1016/j.ijid.2020.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaze DC. Clinical utility of cardiac troponin measurement in COVID‐19 infection. Ann Clin Biochem. 2020;57(3):202‐205. 10.1177/0004563220921888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li H, Xiao X, Zhang J, et al. Impaired spermatogenesis in COVID‐19 patients. Lancet. 2020;28:100604. 10.1016/j.eclinm.2020.100604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bendayan M, Boitrelle F. Covid‐19 and impairment of spermatogenesis: What if fever was the only cause? Lancet. 2020;29:100670. 10.1016/j.eclinm.2020.100670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dejucq N, Jégou B. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol Mol Biol Rev. 2001;65(2):208‐231. 10.1128/MMBR.65.2.208-231.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Freeman R, Hambling MH. Serological studies on 40 cases of mumps virus infection. J Clin Pathol. 1980;33(1):28‐32. 10.1136/jcp.33.1.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manson AL. Mumps orchitis. Urology. 1990;36(4):355‐358. 10.1016/0090-4295(90)80248-l [DOI] [PubMed] [Google Scholar]
- 33. Casella R, Leibundgut B, Lehmann K, Gasser TC. Mumps orchitis: report of a mini‐epidemic. J Urol. 1997;158(6):2158‐2161. 10.1016/s0022-5347(01)68186-2 [DOI] [PubMed] [Google Scholar]
- 34. Adamopoulos DA, Lawrence DM, Vassilopoulos P, Contoyiannis PA, Swyer GI. Pituitary‐testicular interrelationships in mumps orchitis and other viral infections. Br Med J. 1978;1(6121):1177‐1180. 10.1136/bmj.1.6121.1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID‐19. JAMA Intern Med. 2020;180(8):1081‐1089. 10.1001/jamainternmed.2020.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377‐382. 10.1136/thorax.58.5.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Oscar Moreno‐Perez and Esperanza Merino have full access to the data and are the guarantor for the data. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


