Abstract
COVID‐19 (coronavirus disease 2019) represents a pandemic, and several vaccines have been produced to prevent infection and/or severe sequelae associated with SARS‐CoV‐2 (severe acute respiratory syndrome coronavirus 2) infection. There have been several reports of infrequent post vaccine associated thrombotic events, in particular for adenovirus‐based vaccines. These have variously been termed VIPIT (vaccine‐induced prothrombotic immune thrombocytopenia), VITT (vaccine‐induced [immune] thrombotic thrombocytopenia), VATT (vaccine‐associated [immune] thrombotic thrombocytopenia), and TTS (thrombosis with thrombocytopenia syndrome). In this report, the laboratory test processes, as utilised to assess suspected VITT, are reviewed. In published reports to date, there are notable similarities and divergences in testing approaches, potentially leading to identification of slightly disparate patient cohorts. The key to appropriate identification/exclusion of VITT, and potential differentiation from heparin‐induced thrombocytopenia with thrombosis (HITT), is identification of potentially differential test patterns. In summary, testing typically comprises platelet counts, D‐dimer, fibrinogen, and various immunological and functional assays for platelet factor 4 (PF4) antibodies. In suspected VITT, there is a generally highly elevated level of D‐dimer, thrombocytopenia, and PF4 antibodies can be identified by ELISA‐based assays, but not by other immunological assays typically positive in HITT. In addition, in some functional platelet activation assays, standard doses of heparin have been identified to inhibit activation in suspected VITT, but they tend to augment activation in HITT. Conversely, it is also important to not over‐diagnose VITT, given that not all cases of thrombosis post vaccination will have an immune basis and not all PF4‐ELISA positive patients will be VITT.
Keywords: COVID‐19, laboratory testing, platelet factor 4 antibodies, thrombosis with thrombocytopenia syndrome, vaccine‐associated thrombotic thrombocytopenia, vaccine‐induced (immune) thrombotic thrombocytopenia
1. INTRODUCTION
COVID‐19 (coronavirus disease 2019) is a recognised global pandemic caused by infection with SARS‐CoV‐2 (severe acute respiratory syndrome coronavirus 2). This infectious disease is thought to have originated in Wuhan, China, in late 2019, and at time of writing has infected over 168 million people and caused nearly 3.5 million deaths.1 Severe COVID‐19 reflects primarily a prothrombotic disorder, with thrombosis appearing in various forms.2, 3, 4, 5, 6, 7 Indeed, COVID‐19 appears to affect all facets of hemostasis, including primary hemostasis (ie, platelets, von Willebrand factor, endothelium), secondary hemostasis and fibrinolysis.8, 9, 10, 11, 12 In addition, thromboses may arise from disturbances in immune response, creating cytokine disturbance (so‐called ‘cytokine storm’), according to immunothrombosis‐/endotheliitis‐type mechanisms.2 Perhaps unsurprisingly, then, several autoimmune events have also been associated with COVID‐19, including presence of antiphospholipid antibodies.13, 14, 15 Of greater relevance, however, are reports of platelet factor 4/heparin (PF4/H) antibodies being present in COVID‐19 patients, as recently reviewed in this journal.16 In brief, PF4/H antibodies can be observed in COVID‐19 patients, and they may occur at higher incidence than in historical non–COVID‐19 cohorts. However, the situation is complex, since not all PF4/H antibodies may lead to platelet activation, and not all identified antibodies are heparin‐dependent. Thus, such antibodies may or may not identify the condition called heparin‐induced thrombocytopenia (HIT), and not all such occurrences are associated with thrombosis (or ‘HITT’). Quite recently, a ‘HIT‐like’ syndrome has also been reported in patients who have been vaccinated against COVID‐19. The current review looks at this ‘thrombotic condition’ with a focus on laboratory testing. It is also important to recognise the potential to over‐diagnosis of this disorder, if based solely on laboratory parameters.
2. MATERIALS AND METHODS
This is a narrative review. The PubMed database (https://pubmed.ncbi.nlm.nih.gov) was searched as required for both background information as well as specific papers related to post vaccination–related thrombosis. For the latter, the author primarily used various search terms and most notably “((Vaccine induced immune thrombotic thrombocytopenia) OR (vaccine associated thrombotic thrombocytopenia)) OR (thrombosis with thrombocytopenia syndrome) OR (Vaccine Induced Prothrombotic Immune Thrombocytopenia))”. An initial search performed on 8th May, 2021, identified 2236 publications, which was reduced to 66 after including only publications published in 2021, which represents the year in which the first cases of post COVID‐19 vaccination–associated events was published in preprint form by the German group of Greinacher et al.17 The search was updated to be current of 27th May, 2021, when a total of 76 publications were identified, which included the recent associated review.16 These publications were further screened by title and abstract to remove non‐relevant publications and to identify additional relevant content.
3. RESULTS
3.1. Background information on vaccine‐associated (immune) thrombotic thrombocytopenia terminology
The condition, as reported by different groups, has been given several different names, as summarised in Table 1. In the initial report,17 the German researchers coined the condition “VIPIT”, for “vaccine‐induced prothrombotic immune thrombocytopenia”. In their subsequent publication,18 the same workers instead used the term “VITT” for “vaccine‐induced immune thrombotic thrombocytopenia”. Interestingly, a separate group of Norwegian workers, also publishing in the same issue of the journal, also used the term “VITT”,19 suggesting some “harmonisation” of terminology among the separate groups, perhaps facilitated by editorial guidance. A third series of patients was reported by a UK group,20 in the same journal, and the publication also used the termed “VITT”, perhaps for consistency. The term “VITT” also links to a separate, perhaps pathophysiologically related, condition termed “HITT”, for “Heparin‐induced thrombocytopenia with thrombosis”.
TABLE 1.
Terms used to describe post COVID‐19 vaccine associated thrombosis with thrombocytopenia
| Abbreviation | Stands for | Comments |
|---|---|---|
| VIPIT | Vaccine‐induced prothrombotic immune thrombocytopenia | Original term reported by German researchers.17 |
| VITT | Vaccine‐induced immune thrombotic thrombocytopenia or vaccine‐induced immune thrombosis with thrombocytopenia | Term used in subsequent report by the German group,18 as well as separate case series by Norwegian19 and UK20 based groups publishing in the same journal. Perhaps reflecting a variation of the abbreviation HITT, representing the condition of ‘heparin‐induced thrombocytopenia with thrombosis’. |
| VATT | Vaccine‐associated (immune) thrombotic thrombocytopenia (or thrombosis with thrombocytopenia) | A term supported by other researchers concerned with the terminology ‘induced’ where a clear pathological link to the vaccine is not clear. A broader term excluding ‘immune’ potentially captures other thrombotic events associated with thrombocytopenia and vaccine use, but where an immune relationship is unclear. |
| TTS | Thrombosis with thrombocytopenia syndrome | A term favoured by some reporting agencies which does not specifically indicate any “vaccine” association. Term not typically utilised by researchers for the condition associated with COVID‐19 vaccine use, since essentially can encompass any condition where thrombosis can be associated with thrombocytopenia, including HITT and thrombotic thrombocytopenia purpura (TTP). |
Nevertheless, a few other terms have alternatively been proposed by others. One potentially useful term is “VATT”, for “vaccine‐associated (immune) thrombotic thrombocytopenia”. This term is supported by some researchers concerned with use of the term “induced”, where a clear pathological link to the vaccine is not apparent. As a broader term excluding “immune”, the term can also potentially capture other thrombotic events associated with both thrombocytopenia and vaccine use, but where an immune relationship is unclear. As an alternative to VATT, and for similar purpose, some researchers may instead use the term “suspected VITT” for “suspected vaccine‐induced thrombotic thrombocytopenia”. Finally, the term “TTS”, for “thrombosis with thrombocytopenia syndrome” has also been used by most official reporting agencies, including the CDC (Centers for Disease Control [and Prevention]) in the US, the EMA (European Medicines Agency), and TGA (Therapeutic Goods Administration) in Australia, which does not specifically indicate any ‘vaccine’ association. However, the term TTS is unlikely to be adopted by researchers in the field for the condition associated with COVID‐19 vaccine use, since the term can essentially encompass any condition where thrombosis can be associated with thrombocytopenia, including HITT, catastrophic antiphospholipid (antibody) syndrome (CAPS) and also thrombotic thrombocytopenia purpura (TTP).
For the purpose of “harmonisation”, this review will use the term “suspected VITT”, similar to most publications to date, but qualified with “suspected” since it is not always clear from the literature and in clinical practice if a post‐vaccination thrombotic event is truly induced by the vaccine or can be proven to have an immune basis. The term also suits the utility within the context of laboratory testing for the condition in typical clinical practice, as here the search is for “suspected VITT”, and most cases investigated for post‐vaccine thrombosis will not prove to be VITT, just as most cases investigated for HITT, will not prove to be HITT.
3.2. Literature on suspected VITT
A summarised review of the published case series and case studies identified by the Medline search is presented in Table 2. A total of 16 studies reporting cases of suspected VITT have been published to date, all being case series with case numbers ranging from 1 to 43, for a total of 81‐133 cases.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 The uncertainty in total case numbers is because there may be duplication of cases in at least four publications.17, 18, 20, 24 Most reports, in keeping with early reports, have been in women (in total, n = 69, vs 21 males).
TABLE 2.
Summary of literature on suspected vaccine‐induced thrombotic thrombocytopenia (VITT)
| Publication | Country | Vaccine | Case number | F | M | Age (range) | Daya post vaccine (range) | Case fatality | General laboratory findings | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet count (range) (109/L) | D‐dimer (range) (mg/L) | Fibrinogen (range) (g/L) | Platelet factor 4 antibodies? | Functional testing? | ||||||||||
| Greinacher et al17 | Germany | AZ | 9 | 8 | 1 | 22‐49 | 4‐16 | 4/8 (50%) died | 9‐100 | NR | NR | 4/4 pos by ELISA (in‐house assays) | 4/4 pos by platelet activation assay (HIPA) | PT & APTT NR. |
| Greinacher et al18 | Germany | AZ | 11 | 9 | 2 | 22‐49 | 5‐16 | 5/10 (50%) died | 8‐107 | 2‐142 | 0.4‐5.7 | 9/9 pos by ELISA (in‐house assays) | 9/9 pos by platelet activation assay (HIPA) | 4/6 fib <2.0; PT & APTT often abnormal. |
| Schultz et al19 | Norway | AZ | 5 | 4 | 1 | 32‐54 | 7‐10 | 3/5 (60%) died | 14‐70 | 13‐>35 | 0.8‐2.3 | 5/5 pos by ELISA (Lifecodes) | 4/5 pos by platelet activation assay (Multiplate) | 3/5 fib <2.0; PT & APTT generally normal. |
| Scully et al20 | UK | AZ | 23 | 14 | 9 | 21‐71 | 6‐24 | 7/23 (30.4%) died | 7‐113 | 5.1‐80 | 0.3‐4.5 | 22/23 pos by ELISA (Lifecodes or Stago); 9/9 neg by CLIA | 5/7 pos by flow‐cytometry based assay | 14/23 fib <2.0; 1/23 neg for PF4 ab; functional testing rarely done; PT & APTT generally normal. |
| Mehta et al21 | UK | AZ | 2 | 0 | 2 | 25,32 | 6,9 | 2/2 (100%) died | 30,19 | NR | 1.4, 1.3 | 1/1 pos (method NR) | NR | |
| Tiede et al22 | Germany | AZ | 5 | 5 | 0 | 41‐67 | 5‐11 | 0/5 (0%) died | 27‐105 | 22.4‐>35 | 0.9‐3.6 | 5/5 pos by ELISA (Hyphen & Lifecodes), but all neg by CLIA | 4/5 pos by modified HIPA | 3/5 fib <2.0; 1/5 neg by functional testing; PT & APTT NR. |
| See et al23 | USA | JJ | 12 | 12 | 0 | 18‐60 | 6‐15 | 3/12 (25%) died | 9‐127 | 1.1‐112 | 0.6‐3.3 | 11/11 pos for PF4 ab by ELISA (unspecified methods); 3/3 neg by LIA | 1/10 pos by SRA | 8/12 fib <2.0; 9/10 negative by functional SRA; PT & APTT generally normal. |
| Platton et al24 | UK | AZ | 43 (27 ‘probable’; 7 ‘possible’; 9 ‘unlikely’) | NR | NR | NR | NR | NR | NR | NR | NR | All samples assessed for PF4 ab using 6 ELISA assays; most samples also assessed using rapid assays; probable VITT cases were mostly pos by ELISA and neg by rapid assays | NR | Most ELISA assays identified PF4 ab in VITT, with some variable sensitivity and specificity; rapid assays had poor sensitivity for VITT in comparison to ELISA |
| Vayne et al25 | France | AZ | 9 | 7 | 2 | 24‐73 | 9‐18 | NR | 9‐61 | >4‐105 | 0.4‐2.0 | 7/9 pos by ELISA (Lifecodes; all neg by CLIA & STiC); 2/9 ELISA neg unlikely VITT | 5/7 pos ELISA also pos by standard SRA; 7/7 pos by PF4 boosted SRA | 5/7 VITT with fib <2.0; PAGIA & LIA if tested mostly neg |
| Althaus et al26 | Germany | AZ | 8 | 5 | 3 | 24‐53 | 6‐20 | 3/8 (37.5%) died | 8‐92 | 9‐>35 | 1.1‐2.7 | 8/8 pos by ELISA (Hyphen) | Extensively assessed | 3/5 fib <2.0 |
| Castelli et al27 | Italy | AZ | 1 | 0 | 1 | 50 | 11 | 1/1 died | 20 | >10 | 1.0 | NR | NR | |
| Muir et al28 | USA | JJ | 1 | 1 | 0 | 48 | 14 | Critically ill at time of report | 13 | 117.5 | 0.9 | Pos by ELISA; neg by ‘screen’ (methods unspecified) | NR | APTT prolonged |
| Blauenfeldt et al29 | Denmark | AZ | 1 | 1 | 0 | 60 | 7 | 1/1 died | 118 (nadir 5) | 41.8 (peak 106.2) | 3.7 (nadir 2.3) | Pos (method NR) | NR | |
| D'Agostino et al30 | Italy | AZ | 1 | 1 | 0 | 54 | 12 | 1/1 died | “low” | “elevated” | “normal” | NR | NR | PT and APTT prolonged |
| Bjørnstad‐Tuveng et al31 | Norway | AZ | 1 | 1 | 0 | 30’s | 3 | 1/1 died | 37 | 7.0 | 2.2 | 1/1 pos (method NR) | NR | |
| Xie et al32 | UK | AZ | 1 | 1 | 0 | 23 | 7 | 0/1 died | 73 | 17.5 | NR | NR | NR | Recovered |
| Total or range | 81‐133 | 69 | 21 | 18‐77 | 3‐25 | 31/79 (39.2%) | 5‐127 | 1.1‐142 | 0.3‐5.7 | |||||
Abbreviations: APTT, activated partial thromboplastin time; AZ, AstraZeneca; CLIA, chemiluminescence immunoassay; ELISA, enzyme‐linked immunosorbent assay; HIPA, heparin‐induced platelet activation; JJ, Johnston & Johnston (Janssen); LIA, latex immunoassay; NR, not reported; PAGIA, particle gel immunoassay; PF4, platelet factor 4; PT, prothrombin time; SRA, serotonin release assay.
Symptoms or hospitalisation, according to each study.
It is interesting, nonetheless, that the different publications include both similarities and differences in testing approaches and case definitions. Part of this relates to publication date, given this was, and remains, an emerging situation. The original non‐peer‐reviewed German publication17 presented on 9 cases, predominantly young women, with a high fatality rate (~50%), who presented thrombotic symptoms some 4‐16 days post the AstraZeneca ChAdOx1 nCoV‐19 vaccine (AZD1222; ‘AZ vaccine’). The most striking laboratory findings reported in the manuscript was the presence of thrombocytopenia in all patients, with a platelet count ranging from 9‐100 × 109/L, and all patients that were tested (4/4) being positive for PF4 antibodies by both ELISA (enzyme‐linked immunosorbent assay) and functional platelet activation (modified ‘HIPA’) assay. Notably, D‐dimer levels and fibrinogen were not reported in this paper. The follow up paper by the German group was published in the New England Journal of Medicine (NEJM),18 and described 11 cases, presumably including the 9 cases in the preprint publication. 10/10 tested patients had thrombocytopenia (platelet count range: 8‐107 × 109/L), raised D‐dimer (range: 2‐142 mg/L), and generally low fibrinogen (4/6 tested had <2g/L). All patients tested (9/9) were positive for PF4 antibodies by ELISA and functional platelet activation by modified “HIPA” assay. Of interest, PT (prothrombin time) and APTT (activated partial thromboplastin time) were also often abnormal, something not generally reported in later case series and potentially reflecting a very ill patient cohort.
Schultz et al,19 also published in the NEJM, reported 5 cases from Norway, also with high mortality (3/5; 60% died), presenting thrombotic symptoms 7‐10 days post the AZ vaccine. Again, most striking was the marked thrombocytopenia (range 14‐70 × 109/L), raised D‐dimer (range 13‐>35 mg/L), and generally low fibrinogen (3/5 tested had <2g/L). Again, most were young (age range: 32‐54) women (4/5). All 5 had PF4 antibodies by ELISA, and 4/5 showed positive platelet activation by the multiplate method. PT and APTT were generally normal.
Scully et al,20 also published in the NEJM, reported on 23 cases from the UK, also with high mortality (7/23; 30% died), presenting thrombotic symptoms 6‐24 days post AZ vaccine. Again, most striking was the marked thrombocytopenia (range: 7‐113 × 109/L), raised D‐dimer (range 5‐80 mg/L), and generally low fibrinogen (14/23 tested had <2g/L). Diverging from the previous studies, however, the proportion of women (14/23) was not as high, and the age range (21‐71) much broader. In addition, although 22/23 had PF4 antibodies by ELISA, 1/23 did not, despite presenting clinically similarly to other patients, and only 5/7 showed positive platelet activation by a flow‐based method, with the majority not tested. PT and APTT were again generally normal.
Mehta et al21 reported on two male cases of suspected VITT post AZ vaccine, respectively after 9 and 6 days. Both were young (32 and 25 years), had thrombocytopenia (platelet counts of 30 and 19 × 109/L) and reduced fibrinogen (1.4 and 1.3 g/L). D‐dimer and other coagulation test results were not reported. PF4 antibodies was only assessed in one patient, being positive, but with unspecified method.
Tiede et al22 reported on 5 cases of suspected VITT post AZ vaccine after 5‐11 days. Cases were a little older than most published cohorts (41‐67 years); all had thrombocytopenia (platelet counts: 27‐105 × 109/L), and 3/5 had fibrinogen <2 g/L. D‐dimer was highly raised in all cases (>22 mg/L), but routine coagulation test results were not reported. PF4 antibodies were positive in all patients when using ELISA, but negative when using the chemiluminescence immunoassay (CLIA) method. A modified functional HIPA assay was positive in 4/5 cases.
See et al23 reported on 12 cases of suspected VITT post the Ad26.COV2.S COVID‐19 vaccine (Janssen/Johnson & Johnson; ‘JJ vaccine’). This report came from Emergency Use Authorization (EUA) reporting to the Vaccine Adverse Event Reporting System (VAERS). Patients’ ages ranged from 18 to younger than 60 years; all were White women, reported from 11 US states. Time from JJ vaccination to symptom onset ranged from 6 to 15 days. Platelet nadir ranged from 9 to 127 × 109/L. D‐dimer was high in all patients (1.1‐112 mg/L). All 11 patients tested for PF4 antibodies using ELISA with positive results. Interestingly, 3/3 tested for PF4 antibodies by latex immunoassay (LIA) were negative, and only 1/10 tested by functional serotonin release assay (SRA) was positive.
Platton et al24 reported an extensive study investigating PF4 antibodies in 43 patients in a UK study that may have included cases already published by Scully et al.20 They separated the 43 patients into “probable” VITT (n = 27), “possible” VITT (n = 7) and “unlikely” VITT (n = 9) and assessed six different ELISA assays as well as four different rapid assays. They were able to calculate sensitivity and specificity and compare these with previously published data for HITT. All ELISA assays had high sensitivity and “specificity” for VITT, although there was some variability, and it should be clarified here that data (especially ‘specificity’) are biased according to the study population. None of the rapid assays had any sensitivity to VITT, apart from low sensitivity using PAGIA (particle gel immunoassay).
Vayne et al25 reported a French study of 9 cases of suspected VITT in which they concluded 7/9 to be likely VITT post AZ vaccine. Findings were broadly similar to other case series, with age range 24‐73 years, all with thrombocytopenia (platelet counts 9‐61 × 109/L) and 5/7 with fibrinogen <2 g/L. D‐dimer was highly raised in all cases (>4 to 105 mg/L). PF4 antibodies were positive in all 7 patients when using ELISA, but negative when using both CLIA and lateral flow STiC (Stago) methods. Standard SRA was positive in 5/7 cases, but all 7/7 were positive using an SRA modified by addition of PF4.
Althaus et al26 reported another German study of 8 cases of VITT post AZ vaccine. Findings were again broadly similar to other case series, with age range 24‐53 years, all with thrombocytopenia (platelet counts 8‐92 × 109/L) and 3/5 with fibrinogen <2 g/L. D‐dimer was highly raised in all cases (9‐>35 mg/L). PF4 antibodies were positive in all 8 patients by ELISA, and functional testing was extensively investigated.
The remaining publications to date have been single case studies.27, 28, 29, 30, 31, 32 Castelli et al27 reported a fatal case from Italy of a 50‐year‐old male of cerebral venous sinus thrombosis (CVST) post AZ vaccine associated with thrombocytopenia and raised D‐dimer, but PF4 antibodies were not reported. Muir et al28 reported a critically ill case from the USA of a 48‐year‐old female of cerebral thrombosis post JJ vaccine associated with thrombocytopenia and raised D‐dimer; PF4 antibodies were positive by ELISA, but negative using “the screening assay” (specific methods were not reported). Blauenfeldt et al29 reported a fatal case of cerebral infarction in a 60‐year‐old female from Denmark associated with thrombocytopenia, raised D‐dimer, and positive for PF4 antibodies (method unspecified). D'Agostino et al30 reported another Italian case of post AZ vaccine thrombotic thrombocytopenia; this was apparently associated with DIC (disseminated intravascular coagulation). Laboratory test results were mostly only mentioned in general terms (“thrombocytopenia”, “elevated D‐dimer”, “normal fibrinogen”), and PF4 antibodies were not mentioned. Bjørnstad‐Tuveng et al31 reported a case of a female in her 30 seconds with a headache a week after AZ vaccine. Symptoms worsened over the next 3 days; thrombocytopenia was identified (platelet count 37 × 109/L), as was elevated D‐dimer (>7.0mg/L), and the patient died the next day. Cerebral thrombosis was identified on autopsy, as were anti‐PF4 antibodies (unspecified method). Xie et al32 reported a case of a 23‐year‐old female who recovered with pulmonary embolism (PE), thrombocytopenia and high D‐dimers. No testing was performed for PF4 antibodies.
Another relevant study was recently published by Sørvoll et al33 These workers followed on their earlier NEJM paper19 by testing 492 healthcare workers recently vaccinated with the first dose of AZ vaccine, as recruited from two hospitals in Norway. Study individuals were screened for thrombocytopenia and the presence of anti‐PF4/polyanion antibodies with a PF4/polyvinyl sulfonate (PVS) IgG ELISA immunoassay. The majority of study participants had normal platelet counts and were negative by immunoassay. Anti‐PF4/polyanion antibodies without platelet activating properties were, however, detected in 6 individuals (OD ≥0.4, range 0.58‐1.16), all with normal platelet counts. A few subjects had reduced platelet counts, but none had severe thrombocytopenia.
Another recent paper by Thiele et al34 also reported on the frequency of anti‐PF4 antibodies detectable by ELISA after COVID‐19 vaccination with either AZ or Pfizer (BNT162b2) vaccines. In total, 19 of 281 participants tested positive for anti‐PF4/polyanion antibodies post‐vaccination (all: 6.8% [95% CI, 4.4‐10.3]; BNT162b2: 5.6% [95% CI, 2.9‐10.7]; ChAdOx1 nCoV‐19:8.0% [95% CI, 4.5%‐13.7%]). ELISA optical densities were mostly low (between 0.5 and 1.0 units; reference range: <0.50), and none of the PF4/polyanion ELISA‐positive samples induced platelet activation in the presence
of PF4. They concluded that positive PF4/polyanion ELISAs can occur after SARS‐CoV‐2 vaccination with both mRNA‐ and adenoviral vector–based vaccines, but that the majority of these antibodies likely have minor (if any) clinical relevance. Accordingly, low‐titer positive PF4/polyanion EIA results should be interpreted with caution when screening asymptomatic individuals after vaccination against COVID‐19, in order to avoid false positive identification of VITT. Pathogenic platelet‐activating antibodies that cause VITT do not occur commonly following vaccination.
4. DISCUSSION
4.1. Striking similarities and some differences between published studies
Early reports identified mostly young women, but later reports have indicated both males and females across a wide age group. It appears that earlier reports, in particular with the AZ vaccine, may simply have been reporting on the predominant cohort being vaccinated at that time, being mostly (young) female healthcare workers. Thus, there may not be a gender or age restriction in regard to suspected VITT. The ages of all cases reported to date is shown in Figure 1.
FIGURE 1.
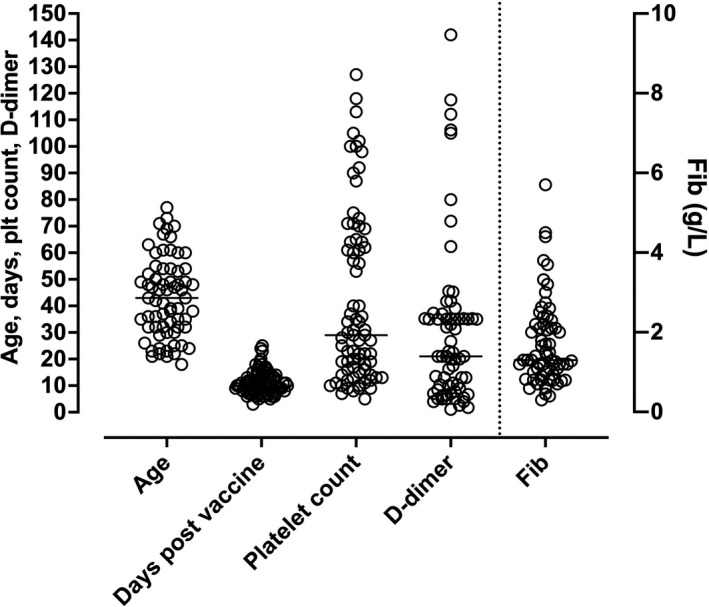
Summary of findings in the literature in regard to age (years), presenting symptoms or hospitalisation post vaccine (days), platelet count (×109/L), D‐dimer (mg/L) (all left y‐axis) or fibrinogen level (g/L; right y‐axis). Data are from the references cited in Table 2
The date of onset of symptoms or hospitalisation (depending on the study reporting) ranged from 3‐25 days post vaccination (Table 2, Figure 1). Case fatality was very high in the first few reports but seems to be falling with the newer case series descriptions, perhaps due to better understanding of the disease progression, its prompter recognition and earlier and better initiation of treatment.
Of particular relevance to this review, however, are the laboratory test findings. The most striking initial test findings are thrombocytopenia and highly elevated D‐dimer levels (Table 2, Figure 1). Overall platelet counts ranged from 5‐127 × 109/L, with worsening thrombocytopenia according to disease progression. Overall D‐dimer levels ranged from 1.1 to 142 mg/L, also with worsening according to disease progression. However, it was not always clear from the publications whether D‐dimer units were being reported in fibrinogen equivalent units (FEU) or D‐dimer units (DDU). Fibrinogen levels were often low but were normal in some cases.
PF4 antibodies were present in almost all patients when assessed by ELISA assays, and often negative (if reported) if assessed by rapid HIT assays such as LIA or CLIA. Indeed, only a single case was reported as ELISA‐negative in the UK series.20 Furthermore, if reported, ELISA OD units were often reported as high. Eight case series reported OD units for ELISA (Figures 2 and 3).18, 19, 20, 22, 23, 24, 25, 26 Greinacher et al18 appear to be reporting results of in‐house ELISA assays, using methods previously reported.35 They assessed for both PF4/H antibodies and PF4 antibodies, with similar results obtained with the two assays. Scully et al20 reported data using two different commercial ELISA assays, the Asserachrom HPIA IgG Assay (Stago Diagnostics), and the Lifecodes PF4 IgG Assay (Immucor GTI Diagnostics). Schultz et al19 also reported using the Lifecodes PF4 IgG Assay. Tiede et al22 reported using both the Lifecodes PF4 IgG Assay and the Zymutest HIT IgG assay (Hyphen BioMed); these “tested positive in all samples with very similar results”, although they reported the results of the Hyphen BioMed assay in their publication. See et al23 reported ELISA ODs in their case series but did not specify the source of the ELISA assays, potentially representing different assays from different test laboratories. Platton et al24 reported on six different ELISA assays, including both IgG and multi‐Ig class assays from Stago, Immucor, Hyphen and AEKSU (AESKULISA HiT II), as well as four rapid assays (AcuStar CLIA HIT‐IgG(PF4‐H), ACL LIA IgG‐specific HemosIL HITAb(PF4‐H), BioRad PaGIA, and Stago STic Expert lateral flow device. Finally, Vayne et al25 and Althaus et al26 assessed ELISA PF4 using Immucor Lifecodes and Hyphen methods, respectively. The potentially noteworthy fact is that the ODs reported for the Stago assay were a little lower than those of other ELISA assays in the initial case series (Figure 2), possibly linked to the assay method or assay substrate incubation times. However, in the Platton et al24 study (Figure 3), this did not appear to be the case.
FIGURE 2.
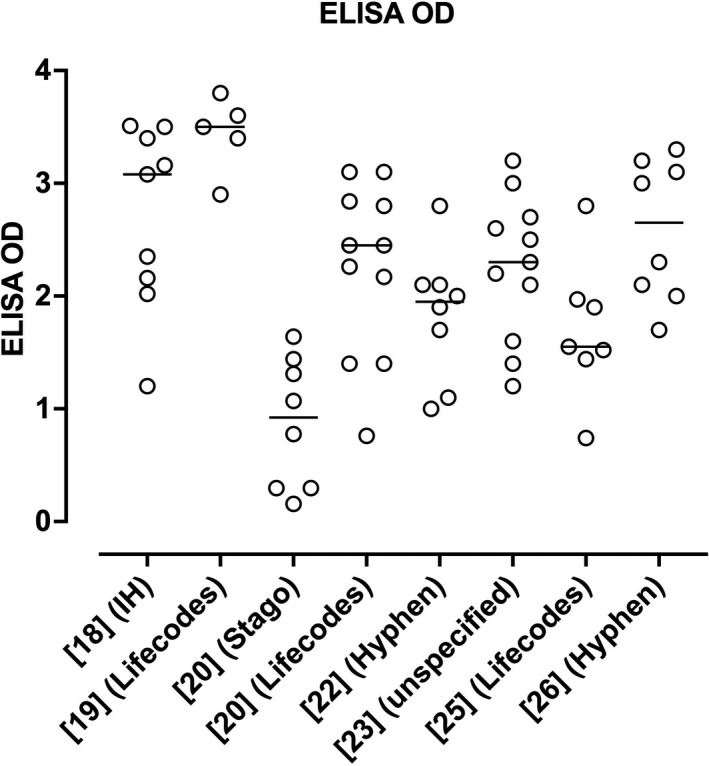
Summary of reported ELISA OD readings for PF4 antibodies according to the literature using case series cited in Table 2, excluding the study of Platton et al24 The horizontal lines indicate the median values for each group
FIGURE 3.
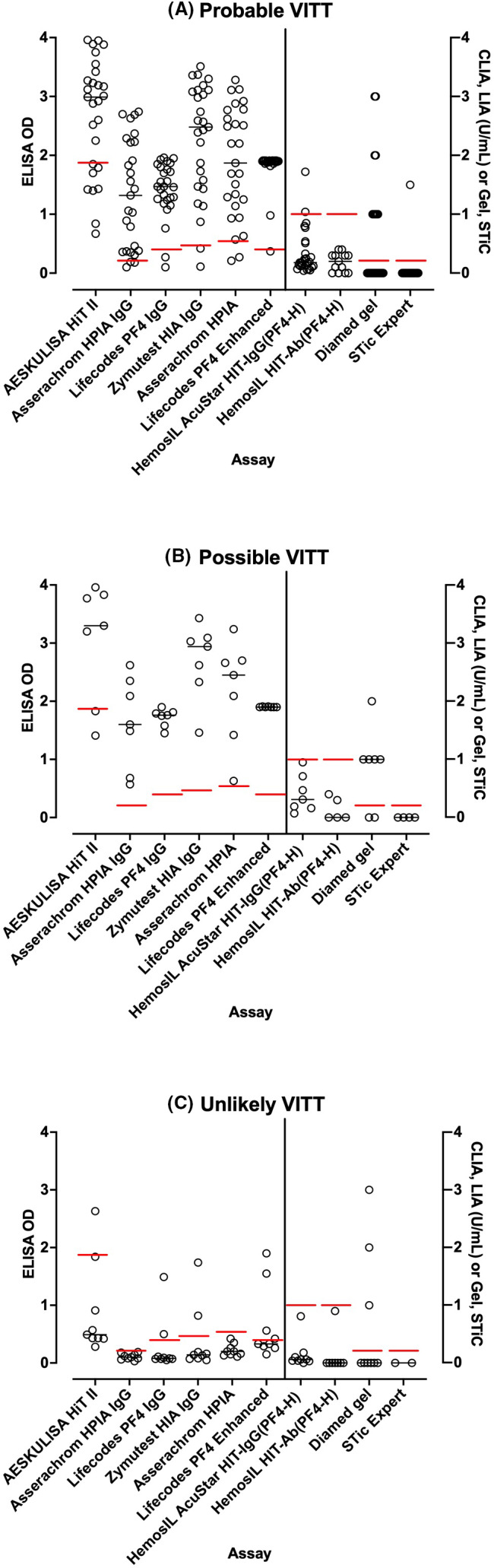
Summary of reported ELISA OD readings for PF4 antibodies and results of rapid assays, according to the study of Platton et al24 For rapid assays, CLIA and LIA results given in U/mL; for PAGIA, the numbers refer to the grade of response, and, for STiC, a value of 0 represents negative and a value of 1.5 represents positive. The horizontal lines indicate the negative/positive cut‐off values
4.2. Selection bias in the literature?
It is important to always note the potential for bias due to the type of reported study. In particular, case reports and small cases series, comprising some the current literature (Table 2), would be biased simply due to patient selection. Thus, authors are more likely to publish positive rather than negative findings. Second, researchers may actively look for anti‐PF4 antibodies in their suspected VITT patient cohorts and potentially discount VITT if ELISA results are negative. Only one case series20 has reported a single case as PF4 ELISA negative to date, although Platton et al24 noted additional cases negative with one assay but positive with others. These cases at least highlight the potential for cases of thrombosis associated with thrombocytopenia post vaccine that are potentially unrelated to PF4 antibody mediated pathophysiological mechanisms, or cases that may need testing by more than one ELISA assay before PF4 antibodies can be proven. On the other hand, not all PF4‐antibody ELISA‐positive patients will have VITT, and there is a background post vaccination rate of 1%‐10% in normal individuals.26, 33, 34 In any case, potential selection bias in the literature always needs to be considered in any evaluation of suspected VITT, or perhaps for any vaccine associated “TTS”. Thus, whether the literature is describing the same condition in all patients remains to be clarified.
4.3. Incidence of suspected VITT
It is not possible to be entirely sure of the true incidence of suspected VITT, given limited publications. Notably, a high relative proportion of cases based on the vaccinated population was reported in the Norwegian study,19 as compared to the other large case series. However, further reports are obviously pending, and published case series do not necessarily reflect all cases of suspected VITT from a particular geography. A recent Norwegian population–based cohort study by Pottegård et al36 calculated 11 excess events of venous thromboembolism per 100 000 AZ vaccinations, including 2.5 excess cerebral venous thrombosis events per 100 000 vaccinations. A recent safety monitoring report from the CDC reported 17 cases of ‘TTS’ from 7.98 million administered doses of JJ vaccine, or around 1 case in every 500 000 doses.37 The EMA estimates the incidence of “TTS” at around 1 in every 100 000 vaccinated people.38 In Australia, the latest report from the TGA identifies 24 cases of ‘TTS’ from approximately 2.1 million administered doses of the AZ vaccine, which would also estimate the incidence at around 1 in every 88 000 vaccinated people.39
4.4. Detection of PF4 antibodies in suspected VITT
As noted, PF4 antibodies in suspected VITT are consistently only detected by ELISA, with in‐house and 6 commercial methods used in the published studies. Where tested, other methods typically used for assessment of PF4/H antibodies, including LIA, CLIA, and STiC were negative, with PAGIA showing some low frequency positivity. The reasons for this presumably relate to the type of PF4 antibodies generated in suspected VITT vs those generated in HITT. Of interest, the 3 main commercial methods so far reported (Stago Asserachrom HPIA IgG Assay, Lifecodes PF4 IgG Assay, and Hyphen BioMed Zymutest HIT IgG assay) differ in terms of their methodology. The Stago assay uses wells coated with "a stoichiometric mixture of heparin‐PF4 by covalent linkage”. After addition of diluted patient plasma or serum, 1‐hour incubation, and some washing steps, goat anti‐human IgG antibodies coupled with peroxidase is added. After an additional 1‐hour incubation, and additional washing, ready for use tetramethylbenzidine (TMB) is added; after a brief (‘exactly 5 minutes’) incubation, the reaction is stopped using 1 M sulfuric acid, and color then read on a plate‐reader. In the Lifecodes assay, microwells are coated with PF4 in complex with polyvinyl sulfonate (PVS). After addition of diluted patient plasma or serum, 30‐35 minutes incubation, and washing, diluted goat anti‐human IgG antibodies coupled with alkaline phosphatase are added. After an additional 30‐35 minutes incubation, with additional washing, p‐nitrophenyl phosphate (PNPP) is added; after a 30 minutes incubation, the reaction is stopped, and color is read on a plate‐reader. Finally, the Hyphen Biomed Zymutest HIA IgG kit (‘for the detection and quantitation of heparin dependent antibodies of the IgG isotype’) has microwells coated with unfractionated heparin, “biologically available, saturated, and then stabilized”. After addition of diluted plasma or serum, supplemented with a kit provided platelet lysate, any heparin‐dependent antibody in the added plasma/serum is expected to bind any complexes formed on the immobilised heparin. After 1 hour incubation and washing steps, goat anti‐human IgG antibodies coupled with horse radish peroxidase (HRP) were added. After an additional 1hr incubation, with additional washing, TMB is added; after exactly 5‐minutes incubation, the reaction is stopped with acid, and color read on a plate‐reader.
There are other potential commercial PF4‐based ELISA assays, possibly using alternate methods, and it is not known if all such ELISA assays will detect the PF4 antibodies so far identified in patients.
Another interesting observation has been the effect of heparin on ELISA test results. For example, Tiede et al21 reported that 2 U/ml of heparin could reduce ELISA OD values from being ≥1.0 in 5/5 suspected VITT patients to <0.3. Shultz et al18 also showed ELISA inhibition with heparin, but used a concentration of 100 U/mL, being similar to that used to inhibit HIT‐antibodies in a functional assay. Greinacher et al17 also used 100 U/mL heparin to inhibit ELISA reactivity in the PF4/H ELISA assay.
4.5. Functional testing in suspected VITT
Here there was some divergence in the publications in regard to assays used for assessing platelet activation. In Greinacher et al,17 4/9 cases were tested, and all showed platelet activation in a modified HIPA assay. Importantly, reactivity could be inhibited by ‘IVIgG’, leading to a potential therapeutic solution in people with suspected VITT. Addition of PF4 or the AZ vaccine itself seemed to augment reactivity, whereas addition of 2 U/mL of a low molecular weight heparin (LMWH) inhibited reactivity. In the later peer‐reviewed publication, Greinacher et al18 reported on a greater number of cases, showing platelet activation by their modified HIPA assay in 13/24 patients with clinically suspected VITT. Addition of PF4 increased reactivity, which was then present in more of the tested patients. Interestingly, LMWH at 0.2 U/mL inhibited the reactivity in many patients, although 100 U/mL of unfractionated heparin was more inhibitory. The authors also noted that “platelet activation was enhanced when platelets were pelleted from platelet‐rich plasma, resuspended in washing buffer, preincubated (1:2000) with ChAdOx1 nCov‐19, centrifuged, and resuspended in the final suspension buffer or when they were co‐incubated in the suspension buffer with ChAdOx1 nCov‐19 (1:50).” Furthermore, the monoclonal antibody IV.3 blocked PF4‐dependent platelet activation in all 7 samples that were tested, and IVIgG was also inhibitory.
Schultz et al19 used the multiplate to measure platelet activation. The serum of 4/5 patients showed platelet activation. Low dose heparin (0.96 U/mL) showed no consistent effect, sometimes slightly increasing, sometimes decreasing activation, and potentially just reflecting assay variability. High dose heparin (96 U/mL) completely inhibited activation in 1 case, incompletely inhibited activation in 2 cases, had no effect in one case.
Scully et al20 used a flow cytometry assay to assess platelet activation, and which was positive in 5/7 patient samples tested. According to the authors, “this effect was not increased with the addition of heparin in physiologic doses but was fully suppressed with the addition of an excess of heparin.”
Tiede et al22 used a modified HIPA to test each sample with and without heparin at two concentrations (0.2 or 100 IU/ml), or with the AZ vaccine. Interestingly, both low and high concentrations of heparin inhibited reactivity in 4/5 and 5/5 cases, respectively. Addition of AZ vaccine caused one of the non‐reactive cases to become reactive.
See et al23 assessed their cases using the SRA, and interestingly found 8/9 tested samples to be negative by SRA. However, they noted that the assay was performed in “in the presence of low (0.1 U/mL) and high (100 U/mL) concentrations of heparin (and in some specialized reference laboratories, buffer control).” Thus, given the data previously noted regarding heparin inhibition, it is possible that at least some of the 8 ‘negative’ SRA cases were not tested in the presence of buffer alone, but rather where some heparin was present. The single positive SRA case was also positive using a flow cytometry–based platelet activation assay.
More recently, the German group expanded their functional assay repertoire with a flow‐based assay. Handtke et al40 modified their functional heparin‐induced platelet activation test, a washed platelet assay, to detect vaccine‐related antibodies and attempted to differentiate these from HIT antibodies. They named the modified assay the PF4‐induced platelet activation test (PIPA). They further developed a flow cytometry–based modification they termed the PIFPA test (PF4‐induced flow cytometry–based platelet activation). Samples were pre‐tested in PF4/heparin ELISA and the functional PIPA test. The flow cytometry–based PIFPA test was performed with 16 VITT samples, all available ELISA+/PIPA‐ sera, 10 representative ELISA‐/PIPA‐ sera and 4 sera of HIT patients who had a positive ELISA and positive PIPA result. The PIFPA is based on granule release of platelets measured by CD62P‐expression. Serum from patients with VITT had a significantly higher median CD62P‐expression than vaccinated controls without thrombotic complications and without functionally relevant antibodies (ELISA+/PIPA‐(P = .0009), ELISA‐/PIPA‐ (P = .0003)). However, 3 of the 16 VITT samples remained below the cut‐off. By adding 5 μg/mL PF4, all VITT samples tested positive and were clearly distinguished from sera of vaccinated, clinically healthy, asymptomatic individuals with positive ELISA but negative PIPA test (P = .0003). All 10 sera that tested ELISA‐ and PIPA‐negative also tested negative in the PIFPA, and only 1 of the 4 tested HIT sera was slightly above the cut‐off when incubated with 20 μg/mL PF4. Low doses of unfractionated heparin (1 U/mL) decreased CD62P‐expression (P = .018), and high doses of heparin further reduced the signal (P = .0058). Inhibition of the FcγRIIA by the monoclonal antibody IV.3 inhibited platelet activation by 6 tested VITT samples (P = .0032).
Vayne et al25 identified a PF4 modified SRA could identify platelet activation in 7/7 VITT cases, compared to only 5/7 cases using the standard SRA.
Most recently, Althaus et al26 extensively investigated platelet activation using a procoagulant flow cytometry assay in addition to HIPA to demonstrate the contribution of antibody‐mediated platelet activation in the pathogenesis of VITT. PF4 antibodies in VITT patients induced significant increase in procoagulant markers (P‐selectin and phosphatidylserine externalization) compared to healthy volunteers and healthy vaccinated volunteers. The generation of procoagulant platelets was PF4‐(augmented) and heparin (inhibited)‐dependent.
In summary, the flow cytometric assays described by Scully et al20 and Handtke et al40 are similar, based on previous methodology,41, 42 and also used for detection of platelet‐activating antibodies in HITT.43 Some workers have added PF4 to their functional assay and indeed have found that, in suspected VITT, most of the patients were positive. However, reaction with PF4 is not specific for VITT. Activation of platelets is enhanced by PF4 also in HITT, as well as in described auto‐immune HIT not related to heparin or vaccine.44 Thus, the activation with PF4 in the functional assay, as it is used for HIT, is interesting, but makes the assay more complex and it does not necessarily discriminate VITT from HITT. Moreover, inhibition by high concentrations of heparin, is also a hallmark for HITT antibodies. In some reports, that functional assays are inhibited by low (therapeutic concentrations) heparin was not unequivocal. Thus, that there is a reaction in the presence of serum alone, or with added buffer, as illustrated in the first reports by Greinacher et al,17, 18 and although not present in all suspected VITT patients, is perhaps so far the best discriminator to conclude for VITT, together with a positive (high OD) ELISA and potentially a negative by rapid HIT assays.
5. CONCLUSION
VIPIT/VITT/VATT/TTS represents serious clinical events that occur in a small proportion, perhaps 1 in 100 000 or so, of people vaccinated with COVID‐19 adenovirus–based vaccines (ie, AZ, JJ). Nevertheless, it is unclear if there is a single immune‐related event occurring in all cases, given at least one case has shown clinical signs of suspected VITT without positive PF4 antibodies by ELISA.20 Although a direct vaccine mediated mechanism has not been proven, some workers have shown enhancement of platelet activation by addition of vaccine.17, 18, 22 In one recently published commentary,45 the authors noted several components of the vaccine that could plausibly be “responsible for causing the production of anti‐PF4 antibodies; it could be the adenovirus itself, the spike protein cassette or other constituents of the vaccine such as polysorbate 80.” Adenovirus has also been shown in earlier animal studies to potentially lead to platelet clearance, thrombocytopenia and platelet aggregation through a von Willebrand factor‐P selectin–mediated mechanism.46 However, the animal studies utilised a high concentration of viral particles injected directly into a vein, and thrombocytopenia occurred within hours of injection.
In terms of laboratory tests, the key initial findings in suspected VITT are thrombocytopenia, highly raised D‐dimer, potentially reduced fibrinogen, and in almost all patients the presence of PF4 antibodies detected by ELISA assay. Why other HIT‐based tests such as CLIA and LIA tend to be negative has not yet been satisfactorily explained, although some hypotheses have been raised in the medical and scientific community.47, 48, 49 Douxfils et al47 proposed a number of potential hypotheses, whilst Dotan et al48 proposed that as vaccination leads to the synthesis of specific SARS‐CoV‐2‐proteins they may trigger a production of PF4 autoantibody though molecular mimicry phenomena, while vaccination compounds lead to a rigorous bystander activation of immune cells. In a non‐peer‐review preprint, Kowarz et al49 propose that “transcription of wildtype and codon‐optimized Spike open reading frames enable alternative splice events that lead to C‐terminal truncated, soluble Spike protein variants. These soluble Spike variants may initiate severe side effects when binding to ACE2‐expressing endothelial cells in blood vessels. In analogy to the thromboembolic events caused by Spike protein encoded by the SARS‐CoV‐2 virus, [they] termed the underlying disease mechanism the “vaccine‐induced covid‐19 mimicry” syndrome (VIC19 M syndrome)”. Another interesting fact is that the effect of heparin in the assays is used to identify suspected VITT, potentially inhibiting ELISA OD and some platelet activation tests, at both low and high concentration, although this was not evident in all cases. Nevertheless, this is in contrast with HIT, where low doses of heparin would be expected to augment reactivity, and only a high dose to inhibit. These features also provide potential clues to the differential identification of HIT vs suspected VITT and should be further assessed in future studies. On the other hand, not all positive ELISA findings will necessarily identify pathogenic antibodies. It is well known in HIT studies that around 50% of HIT detected immunologically by ELISA will not cause platelet activation.50 A recent publication by the Norwegian team33 identified 6 cases positive by PF4 ELISA by serological testing post COVID‐19 vaccination in otherwise healthy Norwegian healthcare workers, none of whom had thrombocytopenia. Thiele et al34 and Althaus et al26 also identified a background of upwards of 5%‐10% PF4 ELISA antibody positivity post COVID‐19 vaccination in healthy control subjects. In this respect, investigating patients for “suspected VITT” may end up being similar to investigating patients for “suspected HITT” (ie, potential for high false positive rate if only based on positive ELISA). That the majority of ELISA positive cases, apart from the US study, also show platelet activation may also reflect some selection bias, with these patients being highly clinically suspected to have VITT. A broader investigation of cases will likely find more cases positive by ELISA but negative by functional assay, as partly suggested by more recent studies. However, in several other ways, ‘VITT’ is dissimilar to HITT, particularly the markedly elevated D‐dimer and the heparin independence being potentially critical to their differentiation.
Finally, not all thrombocytopenia post vaccination will be “VITT”. For example, there have been several cases of apparent secondary immune thrombocytopenia (ITP) after SARS‐CoV‐2 vaccination with both the Pfizer and Moderna vaccines.51
CONFLICT OF INTEREST
The author has no competing interests.
ACKNOWLEDGEMENTS
The opinions expressed in this review are those of the author and do not necessarily reflect the opinion of NSW Health Pathology.
Favaloro EJ. Laboratory testing for suspected COVID‐19 vaccine–induced (immune) thrombotic thrombocytopenia. Int J Lab Hematol. 2021;43:559–570. 10.1111/ijlh.13629
Funding information
There was no funding for this work.
DATA AVAILABILITY STATEMENT
This is a review of the existing literature; no new data were created. All data shown are condensed/summarized from the reviewed literature.
References
- 1.COVID‐19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University . COVID‐19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2021. Available at. <https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6> Accessed 27th May.
- 2.Lippi G, Sanchis‐Gomar F, Favaloro EJ, Lavie CJ, Henry BM. Coronavirus disease 2019‐associated coagulopathy. Mayo Clin Proc. 2021;96(1):203‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thachil J, Srivastava A. SARS‐2 coronavirus‐associated hemostatic lung abnormality in covid‐19: is it pulmonary thrombosis or pulmonary embolism? Semin Thromb Hemost. 2020;46(7):777‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulman S. Coronavirus disease 2019, prothrombotic factors, and venous thromboembolism. Semin Thromb Hemost. 2020;46(7):772‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Minno A, Ambrosino P, Calcaterra I, Di Minno MND. COVID‐19 and venous thromboembolism: A meta‐analysis of literature studies. Semin Thromb Hemost. 2020;46(7):763‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenner WJ, Kanji R, Mirsadraee S, et al. Thrombotic complications in 2928 patients with COVID‐19 treated in intensive care: a systematic review. J Thromb Thrombolysis. 2021;14:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uaprasert N, Moonla C, Sosothikul D, Rojnuckarin P, Chiasakul T. Systemic coagulopathy in hospitalized patients with coronavirus disease 2019: A systematic review and meta‐analysis. Clin Appl Thromb Hemost. 2021;27:107602962098762. 10.1177/1076029620987629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Favaloro EJ, Henry BM, Lippi G. Increased VWF and decreased ADAMTS13 in COVID‐19: creating a milieu for (micro) thrombosis? Semin Thromb Hemost. 2021;47(4):400‐418. 10.1055/s-0041-1727282 [DOI] [PubMed] [Google Scholar]
- 10.Levi M, Thachil J. Coronavirus disease 2019 coagulopathy: disseminated intravascular coagulation and thrombotic microangiopathy‐either, neither, or both. Semin Thromb Hemost. 2020;46(7):781‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwaan HC. Coronavirus disease 2019: The role of the fibrinolytic system from transmission to organ injury and sequelae. Semin Thromb Hemost. 2020;46(7):841‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen JB, Pasalic L, Hvas AM. Platelets in coronavirus disease 2019. Semin Thromb Hemost. 2020;46(7):823‐825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Favaloro EJ, Henry BM, Lippi G. Is lupus anticoagulant a significant feature of COVID‐19? a critical appraisal of the literature. Semin Thromb Hemost. 2021. 10.1055/s-0041-1729856 [DOI] [PubMed] [Google Scholar]
- 14.Favaloro EJ, Henry BM, Lippi G. COVID‐19 and antiphospholipid antibodies: Time for a reality check? Semin Thromb Hemost. 2021. 10.1055/s-0041-1728832 [DOI] [PubMed] [Google Scholar]
- 15.Castillo‐Martinez D, Torres Z, Amezcua‐Guerra LM, Pineda C. Are antiphospholipid antibodies just a common epiphenomenon or are they causative of immune‐mediated coagulopathy in COVID‐19? Clin Rheumatol. 2021;7:1‐5. 10.1007/s10067-021-05724-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Favaloro EJ, Henry BM, Lippi G. The complicated relationships of heparin‐induced thrombocytopenia and platelet factor 4 antibodies with COVID‐19. Int J Hematol. 2021. 10.1111/ijlh.13582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle P, Eichinger S. A prothrombotic thrombocytopenic disorder resembling heparin‐induced thrombocytopenia following coronavirus‐19 vaccination. Preprint: https://www.researchsquare.com/article/rs‐362354/v1. 10.21203/rs.3.rs-362354/v1 [DOI]
- 18.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov‐19 Vaccination. N Engl J Med. 2021;384(22):2092‐2101. 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV‐19 Vaccination. N Engl J Med. 2021;384(22):2124‐2130. 10.1056/NEJMoa2104882. Epub ahead of print. PMID: 33835768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scully M, Singh D, Lown R, et al. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV‐19 Vaccination. N Engl J Med. 2021;384(23):2202‐2211. 10.1056/NEJMoa2105385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta PR, Apap Mangion S, Benger M, et al. Cerebral venous sinus thrombosis and thrombocytopenia after COVID‐19 vaccination ‐ A report of two UK cases. Brain Behav Immun. 2021;1591(21):514‐517. 10.1016/j.bbi.2021.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiede A, Sachs UJ, Czwalinna A, et al. Prothrombotic immune thrombocytopenia after COVID‐19 vaccine. Blood. 2021. 10.1182/blood.2021011958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA. 2021:e217517. 10.1001/jama.2021.7517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Platton S, Bartlett A, MacCallum P, et al. Evaluation of laboratory assays for anti‐Platelet Factor 4 antibodies after ChAdOx1 nCOV‐19 vaccination. J Thromb Haemost. 2021. 10.1111/jth.15362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vayne C, Rollin J, Gruel Y, et al. PF4 Immunoassays in Vaccine‐Induced Thrombotic Thrombocytopenia. N Engl J Med. 2021. 10.1056/NEJMc2106383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Althaus K, Möller P, Uzun G, et al. Antibody‐mediated procoagulant platelets in SARS‐CoV‐2‐ vaccination associated immune thrombotic thrombocytopenia. Haematologica. 2021. 10.3324/haematol.2021.279000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castelli GP, Pognani C, Sozzi C, Franchini M, Vivona L. Cerebral venous sinus thrombosis associated with thrombocytopenia post‐vaccination for COVID‐19. Crit Care. 2021;25(1):137. 10.1186/s13054-021-03572-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N Engl J Med. 2021;384(20):1964‐1965. 10.1056/NEJMc2105869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector‐based COVID‐19 vaccine. J Thromb Haemost. 2021. 10.1111/jth.15347. Epub ahead of print. PMID: 33877737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Agostino V, Caranci F, Negro A, et al. A rare case of cerebral venous thrombosis and disseminated intravascular coagulation temporally associated to the COVID‐19 vaccine administration. J Pers Med. 2021;11(4):285. 10.3390/jpm11040285. PMID: 33917902; PMCID: PMC8068274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjørnstad‐Tuveng TH, Rudjord A, Anker P, Fatal cerebral haemorrhage after COVID‐19 vaccine. Tidsskr Nor Laegeforen. 2021:141. 10.4045/tidsskr.21.0312. PMID: 33928772. [DOI] [PubMed] [Google Scholar]
- 32.Xie C, Vincent L, Chadwick A, Peschl H. COVID‐19 vaccine induced prothrombotic immune thrombocytopenia. Eur Heart J. 2021:ehab237. 10.1093/eurheartj/ehab237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sørvoll IH, Horvei KD, Ernstsen SL, et al. An observational study to identify the prevalence of thrombocytopenia and anti‐PF4/polyanion antibodies in Norwegian health care workers after COVID‐19 vaccination. J Thromb Haemost. 2021. 10.1111/jth.15352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thiele T, Ulm L, Holtfreter S, et al. Frequency of positive anti‐PF4/polyanion antibody tests after COVID‐19 vaccination with ChAdOx1 nCoV‐19 and BNT162b2. Blood. 2021:2021012217. 10.1182/blood.2021012217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juhl D, Eichler P, Lubenow N, Strobel U, Wessel A, Greinacher A. Incidence and clinical significance of anti‐PF4/heparin antibodies of the IgG, IgM, and IgA class in 755 consecutive patient samples referred for diagnostic testing for heparin‐induced thrombocytopenia. Eur J Haematol. 2006;76:420‐426. [DOI] [PubMed] [Google Scholar]
- 36.Pottegård A, Lund LC, Karlstad Ø, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford‐AstraZeneca ChAdOx1‐S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. 10.1136/bmj.n1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shay DK, Gee J, Su JR, et al. Safety Monitoring of the Janssen (Johnson & Johnson) COVID‐19 Vaccine ‐ United States, March‐April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):680‐684. 10.15585/mmwr.mm7018e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.European Medicines Agency . European Medicines Agency News 23/04/2021. Accessed 9th May, 2021. Available at: https://www.ema.europa.eu/en/news/astrazenecas‐covid‐19‐vaccine‐benefits‐risks‐ context
- 39.Therapeutic Goods Administration . Therapeutic Goods Administration COVID‐19 vaccine weekly safety report ‐ 20‐05‐2021. Accessed 27th May, 2021. Available at: https://www.tga.gov.au/periodic/covid‐19‐vaccine‐weekly‐safety‐report‐20‐05‐ 2021
- 40.Handtke S, Wolff M, Zaninetti C, et al. A Flow cytometric assay to detect platelet activating antibodies in VITT after ChAdOx1 nCov‐19 vaccination. Blood. 2021:2021012064. 10.1182/blood.2021012064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomer A, Masalunga C, Abshire TC. Determination of heparin‐induced thrombocytopenia: a rapid flow cytometric assay for direct demonstration of antibody‐mediated platelet activation. Am J Hematol. 1999;61:53‐61. [DOI] [PubMed] [Google Scholar]
- 42.Vitale M, Tazzari P, Francesca R, et al. Comparison between different laboratory tests for detection and prevention of heparin‐induced thrombocytopenia. Cytom Commun Clin Cytom. 2001;46:290‐295. [DOI] [PubMed] [Google Scholar]
- 43.Denys B, Stove V, Philippé J, Devreese K. A clinical‐laboratory approach contributing to a rapid and reliable diagnosis of heparin‐induced thrombocytopenia. Thromb Res. 2008;123(1):137‐145. 10.1016/j.thromres.2008.04.020 [DOI] [PubMed] [Google Scholar]
- 44.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin‐induced thrombocytopenia. J Thromb Haemost. 2017;15:2099‐2114. [DOI] [PubMed] [Google Scholar]
- 45.Makris M, Pavord S, Lester W, Scully M, Hunt BJ. Vaccine‐induced Immune Thrombocytopenia and Thrombosis (VITT). RPTH. 2021;5(5). 10.1002/rth2.12529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Othman M, Labelle A, Mazzetti I, Elbatarny HS, Lillicrap D. Adenovirus‐induced thrombocytopenia: the role of von Willebrand factor and P‐selectin in mediating accelerated platelet clearance. Blood. 2007;109(7):2832‐2839. [DOI] [PubMed] [Google Scholar]
- 47.Douxfils J, Favresse J, Dogné JM, et al. Hypotheses behind the very rare cases of thrombosis with thrombocytopenia syndrome after SARS‐CoV‐2 vaccination. Thromb Res. 2021;15(203):163‐171. 10.1016/j.thromres.2021.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dotan A, Shoenfeld Y. Perspectives on vaccine induced thrombotic thrombocytopenia. J Autoimmun. 2021;18(121):102663. 10.1016/j.jaut.2021.102663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kowarz E, Krutzke L, Reis J, Bracharz S, Kochanek S, Marschalek R. Vaccine‐Induced Covid‐19 Mimicry” Syndrome: Splice reactions within the SARS‐CoV‐2 Spike open reading frame result in Spike protein variants that may cause thromboembolic events in patients immunized with vector‐based vaccines. Available at https://www.researchsquare.com/article/rs‐558954/v1. 10.21203/rs.3.rs-558954/v1 [DOI]
- 50.Favaloro EJ, McCaughan G, Mohammed S, et al. HIT or miss? A comprehensive contemporary investigation of laboratory tests for heparin induced thrombocytopenia. Pathology. 2018;50(4):426‐436. [DOI] [PubMed] [Google Scholar]
- 51.Lee EJ, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS‐CoV‐2 vaccination. Am J Hematol. 2021;96(5):534‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review of the existing literature; no new data were created. All data shown are condensed/summarized from the reviewed literature.


