Abstract
Background
The anti‐COVID‐19 vaccines are new, and we should be alert of potential adverse effects of them.
Aims of the Study
To report a case of a large hemorrhagic stroke 5 days after ChAdOx1 nCoV‐19 vaccination.
Methods
Clinical, imaging, and laboratory data of a patient with stroke admitted to our emergency department.
Results
A woman, 57 years old, took the first dose of the ChAdOx1 nCoV‐19 vaccine and shortly thereafter presented mild systemic symptoms and started on aspirin. On day 5, she had a sudden onset of sweating and paleness, which has followed by left hemiparesis, vomiting, and somnolence. Computed tomography showed a large right deep frontal lobe parenchymal hematoma with the inundation of the entire ventricular system. Platelets count, fibrinogen, prothrombin time, and D‐dimer were normal. Digital subtraction angiography did not show any signs of thrombosis or aneurysms in brain circulation.
Conclusions
Clinicians should be aware of cerebrovascular adverse effects of ChAdOx1 nCoV‐19, including out‐of‐context of vaccine‐induced immune thrombotic thrombocytopenia.
Keywords: case report, COVID‐19, stroke
1. INTRODUCTION
Large‐scale vaccination has emerged as a promising method to cease the COVID‐19 pandemic. Although there are concerns about side and adverse effects of vaccination, there is a consensus that the benefits of approved vaccines outweigh those risks. One of the approved vaccines, ChAdOx1 nCoV‐19 (by AstraZeneca/Oxford), is made by recombinant adenovirus technology, and adverse effects have been described, such as demyelinating disease, 1 and vaccine‐induced immune thrombotic thrombocytopenia (VITT), which may be associated with ischemic stroke, 2 cerebral sinuses, and other atypical‐sites thrombosis, 3 mainly in young women. However, to the best of our knowledge, atypical hemorrhagic stroke following ChAdOx1 nCoV‐19 vaccination, and not due to thrombocytopenia, was not reported so far.
2. CASE REPORT
A woman, 57 years old, previously healthy, without cardiovascular risk factors, and with no remarkable past medical history, took the first dose of ChAdOx1 nCoV‐19 vaccine and shortly thereafter presented fever, headache, and myalgia. On day 3, she started on aspirin 100 mg/day for those symptoms and “prevention of thrombotic complications” of the vaccine. On day 5, she had a sudden onset of sweating and paleness, which has followed by left hemiparesis, vomiting, and somnolence. She came to the emergency room with a Glasgow Coma Scale of 7 and required orotracheal intubation; blood pressure was 160 × 90 mmHg. Computed tomography showed a large right deep frontal lobe parenchymal hematoma with the inundation of the entire ventricular system (Figures 1 and 2). Platelets count, fibrinogen, prothrombin time, and D‐dimer were normal. Digital subtraction angiography did not show any signs of thrombosis or aneurysms in brain circulation. She underwent hematoma drainage and external ventricular drain, but intracranial pressure kept raising and she underwent decompressive craniectomy. We also did not find any signs of thrombosis in other sites. At the time of submitting this report (day 15), she is left hemiparetic, capable of obeying simple tasks, and on tracheostomy.
FIGURE 1.
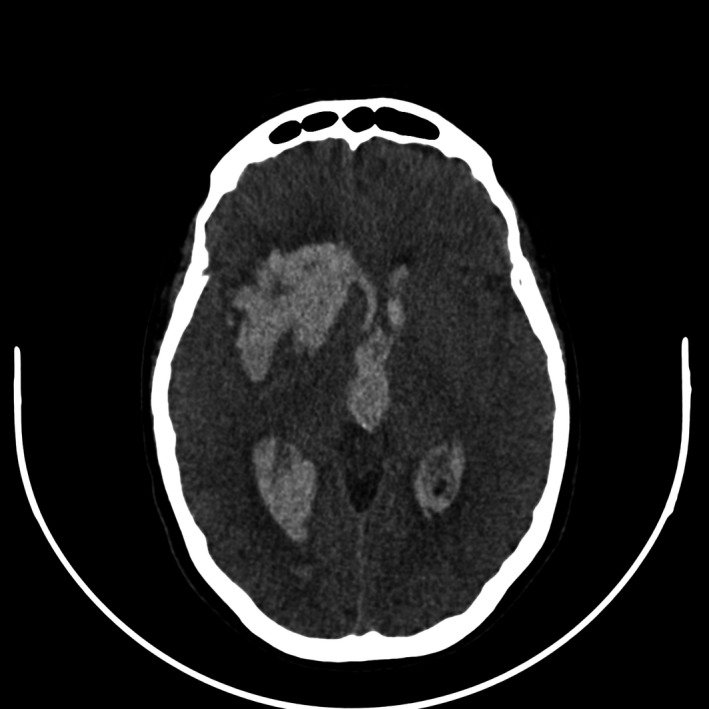
Computed tomography of brain (axial view) showing a large right deep frontal lobe parenchymal bleeding. Also note the midline shift and the black spot sign, suggesting a growing hematoma
FIGURE 2.
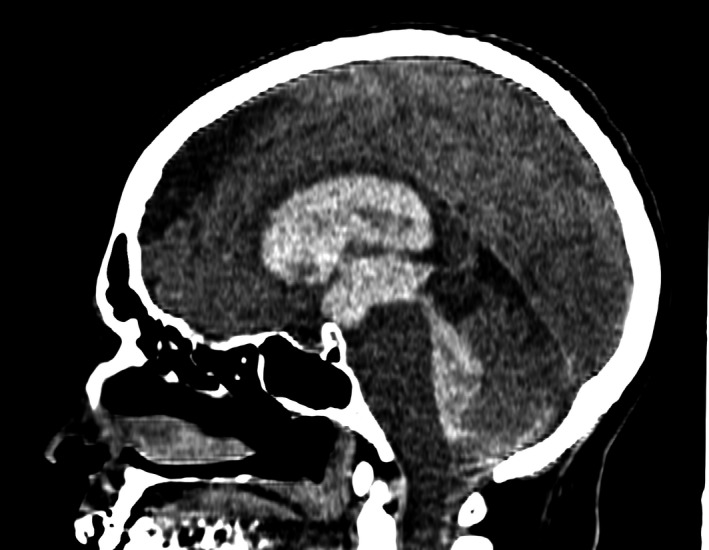
Computed tomography of brain (sagittal view) showing inundation of the entire ventricular system
3. DISCUSSION
Brain hemorrhage in the context of VITT has been described, 4 but primary hemorrhagic stroke following ChAdOx1 nCoV‐19 vaccination in a patient without thrombocytopenia, coagulation disorder, or risk factors was not. It is worth noting that this case presents an atypical site and uncommonly large volume for stroke, which raises concerns about its pathogenesis. Data 3 suggested that, besides thrombosis, there might also be a greater risk of bleeding events related to that vaccine.
Although we cannot claim causality in this report, it is important to keep surveillance on the adverse effects of vaccines.
CONFLICT OF INTEREST
The authors have no conflict of interests to declare.
AUTHOR CONTRIBUTIONS
MLM conceptualized, acquired data, and wrote the draft of the report. DPL acquired data and reviewed the report for scientific content. All the authors had access to the data and a role in writing the manuscript.
ETHICS APPROVAL
Informed consent was obtained from family of the patient prior writing this case report.
ACKNOWLEDGMENTS
Authors thank patient’s family members for consent with this publication.
de Mélo Silva ML Jr, Lopes DP. Large hemorrhagic stroke after ChAdOx1 nCoV‐19 vaccination: A case report. Acta Neurol Scand. 2021;144:717–718. 10.1111/ane.13505
The Clinical commentary refers to ANE13608 (https://onlinelibrary.wiley.com/doi/10.1111/ane.13608)
REFERENCES
- 1. Lu LU, Xiong W, Mu J, et al. The potential neurological effect of the COVID‐19 vaccines: a review. Acta Neurol Scand. 2021;144(1):3‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al‐Mayhani T, Saber S, Stubbs MJ, et al. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV‐19 vaccine‐induced immune thrombotic thrombocytopenia. J Neurol Neurosurg Psychiatry. 2021;2021‐326984. [DOI] [PubMed] [Google Scholar]
- 3. Pottegård A, Lund LC, Karlstad Ø, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford‐AstraZeneca ChAdOx1‐S in Denmark and Norway: population based cohort study. BMJ. 2021;n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384(22):2124‐2130. [DOI] [PMC free article] [PubMed] [Google Scholar]


