Vaccine‐induced thrombotic thrombocytopenia (VITT) is a serious side‐effect of the ChAdOx1 nCoV‐19 vaccine (Oxford/AstraZeneca BioPharmaceuticals, Cambridge, UK) occurring in approximately one in 26 000 to one in 100 000 vaccine recipients. 1 , 2 More recently similar thrombotic complications have been reported for the Ad26.COV2.S coronavirus disease 2019 (COVID‐19) vaccine (Janssen/Johnson & Johnson, New Brunswick, NJ, USA). 3
Vaccine‐induced thrombotic thrombocytopenia can result in life‐threatening thrombotic complications involving different vascular beds, including the pulmonary, cerebral and splanchnic circulations in conjunction with thrombocytopenia and markedly raised D‐dimer. 1 Although the precise mechanisms underpinning the development of VITT are unknown, the resemblance to the pathology seen in patients with heparin‐induced thrombocytopenia (HIT), has guided our understanding, research efforts and therapy of VITT. It is currently proposed that VITT is caused by pathological immunoglobulin G (IgG) antibodies directed against platelet factor 4 (PF4, potentially complexed with components of the ChAdOx1 nCoV‐19 vaccine), which can induce platelet activation via cross‐linked platelet Fc gamma receptor IIA (FcγRIIa) receptors. 4 , 5 Based on this proposed mechanism, intravenous Ig (IVIg) therapy is recommended as a cornerstone in the treatment of VITT. 6 , 7
Recently published reports suggested that components of serum/plasma from patients with VITT can activate platelets isolated from healthy donors. 8 , 9 However, to date, no direct evidence of platelet activation in patients with VITT has been reported. In the present study, we report the presence of activated circulating platelets in a patient with VITT, using whole blood flow cytometry, and confirm that IVIg treatment reduces platelet activation in VITT.
Case report
We report a 55‐year‐old female with a background history of hypertrophic cardiomyopathy, ischaemic heart disease, obstructive sleep apnoea, epilepsy, and schizophrenia, who presented to hospital with chest pain 14 days after the first dose of the ChAdOx1 nCoV‐19 vaccine. Consistent with a diagnosis of VITT, her platelet count was 45 × 109/l with a D‐dimer of 65·94 µg/ml and strongly positive HIT enzyme‐linked immunosorbent assay (ELISA, 143·9%; normal cut‐off 10·5% – Stago Asserachrom, Diagnostica Stago S.A.S., Asnières‐sur‐Seine, France) (Fig 1). A subsequent computed tomography (CT) pulmonary angiogram demonstrated bilateral pulmonary emboli and venous doppler ultrasonography revealed a proximal deep vein thrombosis involving the popliteal vein. Therapeutic anticoagulation was commenced with fondaparinux 7·5 mg subcutaneously daily and IVIg was administered at a dose of 90 g (1 g/kg). Informed consent was obtained, and blood samples were taken before and after the administration of IVIg for flow cytometry analysis of platelet activation. Over the following 5 days the platelet count steadily incremented, which was associated with a concordant fall in the D‐dimer. However, 6 days after the initial dose of IVIg the patient developed chest pain and dyspnoea, in association with a reduction in platelet count to 108 × 109/l. Subsequent imaging with a CT pulmonary angiogram revealed a reduction in thrombus burden of the left lung; however, demonstrated extension of thrombosis within the segmental and subsegmental vessels of the right lower lobe. Therefore, fondaparinux was changed to bivalirudin and a further dose of IVIg (1 g/kg) was administered. This was associated with an increment of the platelet count to 157 × 109/l. Over the following days, the platelet count remained normal and the patient at the time of reporting is stably anticoagulated with bivalirudin and being transitioned to oral anticoagulation with a vitamin K antagonist, given the known interactions of carbamazepine with direct oral anticoagulants.
Fig 1.
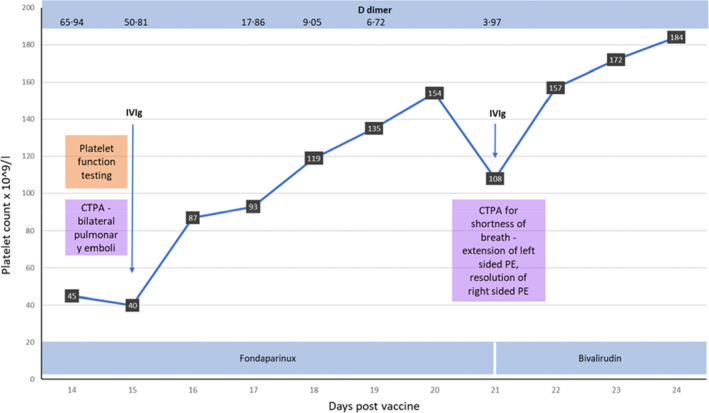
Clinical and laboratory data of the patient with vaccine‐induced thrombotic thrombocytopenia (VITT). CTPA, computed tomography pulmonary angiogram; PE, pulmonary embolism.
Results
Prior to IVIg administration a significant percentage of platelets were positive for markers of platelet activation. Indeed, 13% of platelets expressed activated glycoprotein IIb/IIIa (GPIIb/IIIa, CD41/CD61, αIIbβ3) with 19·1% demonstrating P‐selectin [CD62P, granule membrane protein‐140 (GMP‐140)] surface expression (Fig 2). In keeping with the significant degree of platelet activation there was a significant increase in platelet–monocyte aggregates, with 26·5% of monocytes displaying evidence of bound platelets. Strikingly, repeat testing after IVIg administration demonstrated that levels of PAC‐1 binding, P‐selectin expression, and platelet–monocyte aggregates had returned to a level comparable to a healthy donor. These data suggest that a significant percentage of circulating platelets in patients with VITT are highly activated. The finding of elevated levels of circulating platelet–monocyte aggregates is indicative of platelet activation; however, also suggests that monocyte activation is a feature of VITT. Overall, these findings are consistent with the pro‐thrombotic presentation of our patient with VITT.
Fig 2.
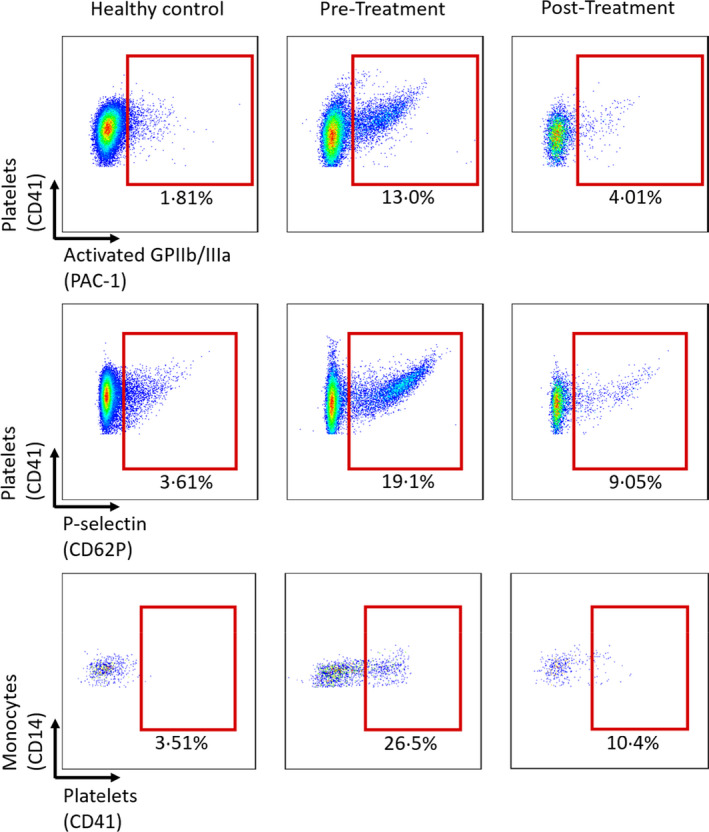
A significant proportion of circulating platelets of a vaccine‐induced thrombotic thrombocytopenia (VITT) patients display glycoprotein IIb/IIIa (GPIIb/IIIa) activation [procaspase‐activating compound 1 (PAC‐1) binding], P‐selectin expression and platelet–monocyte aggregate formation, which is revered after treatment with intravenous immunoglobulin (IVIg). Flow cytometry of PAC‐1 binding (upper row), P‐selectin expression (middle row), and platelet–monocyte aggregate formation (lower row) in a patient with VITT. The comparisons show flow cytometry event plots of a healthy control (left column), before IVIg treatment (middle column) and after IVIg treatment (right column).
Discussion
To our knowledge, the present case provides the first direct evidence of a patient with VITT presenting with activated platelets in the circulation and the first direct evidence of the ability of IVIg to abrogate this enhanced platelet activation state in VITT. Recent reports have suggested that plasma/serum of patients with VITT have the propensity to activate platelets from healthy donors. 10 Our present report is the first to show that a large number of activated platelets are present in the circulating blood of a patient with VITT. The flow cytometric data demonstrating platelet activation is very robust: (i) Binding of PAC‐1 identifies platelets with the activated GPIIb/IIIa receptor. The conformational change of this integrin from a low‐ to a high‐affinity state for its ligand is reflecting the final common pathway of platelet activation. (ii) P‐selectin expression on the platelet surface represents platelet degranulation, as P‐selectin is ‘stored’ in α‐granules, which are fused with the membrane upon platelet stimulation. (iii) Platelet–monocyte aggregate formation is a sensitive marker of platelet activation and has previously been found to be elevated in various thrombotic disorders such as acute coronary syndromes. Moreover, GPIIb/IIIa activation and P‐selectin expression have previously been used to demonstrate platelet activation associated with HIT and GPIIb/IIIa inhibitor‐induced thrombocytopenia. 10 , 11 The reported data are also unique in respect to the high percentage of platelets circulating in an activated state, further highlighting the acuteness and severity of VITT.
The finding of increased numbers of platelet–monocyte aggregates is important as they are not only a sensitive marker of platelet activation but are also likely reflective of enhanced monocyte activation as a feature of VITT. Activated monocytes have been implicated in the pathogenesis of HIT, given they express the FcγRIIa receptor and therefore are also liable to activation by pathogenic antibodies. 12 Whilst platelet–monocyte aggregates have previously been associated with venous thromboembolic disease, HIT, and severe COVID‐19 infection, 13 the number of platelet–monocyte aggregates detected, and the finding that this number is reduced after IVIg administration is a novel and important finding. This also strongly suggests that these changes are the result of pathological VITT antibodies. 14 Similar trends were seen in our present patient with platelet–neutrophil aggregates, which is of particular relevance as neutrophil extracellular trap formation has been proposed to be involved in VITT. 15 Although not elucidated in the present report, previous reports from both patients with VITT and HIT suggest the beneficial effects of IVIg in modulating platelet activation are likely due to the ability to competitively inhibit pathological antibody binding to platelet FcγRIIa receptors. 2 , 7 Notably, the clinical course of our present patient experiencing thrombus extension 6 days after IVIg likely suggests that high levels of Ig are required to competitively inhibit platelet FcγRIIa receptor binding. This raises the interesting possibility of whether changes in platelet activation markers can be utilised to assess the response to IVIg treatment and/or potentially help predict treatment relapse. Collectively, these findings support the role of platelet activation as a central mechanism of VITT and confirm the beneficial role of IVIg in preventing platelet activation.
Funding information
The study was funded by the National Health and Medical Research Council of Australia.
Supporting information
Supplementary Methods.
Acknowledgements
James D. McFadyen designed research, analysed data and wrote the paper. Prerna Sharma performed research and analysed data. Mitchell J. Moon performed research and analysed data. Jonathon Noonan analysed data. Elizabeth Goodall analysed data. Huyen A. Tran analysed data and wrote the paper. Karlheinz Peter designed research, analysed data and wrote the paper.
References
- 1. Cines DB, Bussel JB. SARS‐CoV‐2 vaccine‐induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384:2254–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund‐Johansen F, Ahlen MT, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384(22):2124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384:2202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med. 2021;384(22):2092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bourguignon A, Arnold DM, Warkentin TE, Smith JW, Pannu T, Shrum JM, et al. Adjunct immune globulin for vaccine‐induced thrombotic thrombocytopenia. N Engl J Med. 2021. 10.1056/NEJMoa2107051 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uzun G, Althaus K, Singh A, Möller P, Ziemann U, Mengel A, et al. The use of intravenous immunoglobulin in the treatment of vaccine‐induced immune thrombotic thrombocytopenia. Blood. 2021. 10.1182/blood.2021012479 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Handtke S, Wolff M, Zaninetti C, Wesche J, Schönborn L, Aurich K, et al. A flow cytometric assay to detect platelet‐activating antibodies in VITT after ChAdOx1 nCov‐19 vaccination. Blood. 2021;137:3656–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Althaus K, Möller P, Uzun G, Singh A, Beck A, Bettag M, et al. Antibody‐mediated procoagulant platelets in SARS‐CoV‐2‐ vaccination associated immune thrombotic thrombocytopenia. Haematologica. 2021. 10.3324/haematol.2021.279000 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peter K, Straub A, Kohler B, Volkmann M, Schwarz M, Kübler W, et al. Platelet activation as a potential mechanism of GP IIb/IIIa inhibitor‐induced thrombocytopenia. Am J Cardiol. 1999;84:519–24. [DOI] [PubMed] [Google Scholar]
- 11. Newman PM, Chong BH. Heparin‐induced thrombocytopenia: new evidence for the dynamic binding of purified anti‐PF4‐heparin antibodies to platelets and the resultant platelet activation. Blood. 2000;96:182–7. [PubMed] [Google Scholar]
- 12. Rauova L, Hirsch JD, Greene TK, Zhai LI, Hayes VM, Kowalska MA, et al. Monocyte‐bound PF4 in the pathogenesis of heparin‐induced thrombocytopenia. Blood. 2010;116:5021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McFadyen JD, Stevens H, Peter K. The emerging threat of (micro)thrombosis in COVID‐19 and its therapeutic implications. Circ Res. 2020;127(4):571–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Warkentin TE, Climans TH, Morin PA. Intravenous immune globulin to prevent heparin‐induced thrombocytopenia. N Engl J Med. 2018;378:1845–8. [DOI] [PubMed] [Google Scholar]
- 15. Greinacher A, Selleng K, Welsche J. Towards understanding ChAdOx1 nCov‐19 vaccine‐induced immune thrombotic thrombocytopenia (VITT). Research Square. 2021. 10.21203/rs.3.rs-440461/v1 (Preprint available). [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods.


