Summary
Background
Cutaneous reactions after severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccines are poorly characterized.
Objective
To describe and classify cutaneous reactions after SARS‐CoV‐2 vaccination.
Methods
A nationwide Spanish cross‐sectional study was conducted. We included patients with cutaneous reactions within 21 days of any dose of the approved vaccines at the time of the study. After a face‐to‐face visit with a dermatologist, information on cutaneous reactions was collected via an online professional survey and clinical photographs were sent by email. Investigators searched for consensus on clinical patterns and classification.
Results
From 16 February to 15 May 2021, we collected 405 reactions after vaccination with the BNT162b2 (Pfizer‐BioNTech; 40·2%), mRNA‐1273 (Moderna; 36·3%) and AZD1222 (AstraZeneca; 23·5%) vaccines. Mean patient age was 50·7 years and 80·2% were female. Cutaneous reactions were classified as injection site (‘COVID arm’, 32·1%), urticaria (14·6%), morbilliform (8·9%), papulovesicular (6·4%), pityriasis rosea‐like (4·9%) and purpuric (4%) reactions. Varicella zoster and herpes simplex virus reactivations accounted for 13·8% of reactions. The COVID arm was almost exclusive to women (95·4%). The most reported reactions in each vaccine group were COVID arm (mRNA‐1273, Moderna, 61·9%), varicella zoster virus reactivation (BNT162b2, Pfizer‐BioNTech, 17·2%) and urticaria (AZD1222, AstraZeneca, 21·1%). Most reactions to the mRNA‐1273 (Moderna) vaccine were described in women (90·5%). Eighty reactions (21%) were classified as severe/very severe and 81% required treatment.
Conclusions
Cutaneous reactions after SARS‐CoV‐2 vaccination are heterogeneous. Most are mild‐to‐moderate and self‐limiting, although severe/very severe reactions are reported. Knowledge of these reactions during mass vaccination may help healthcare professionals and reassure patients.
Short abstract
What is already known about this topic?
In clinical trials, COVID‐19 vaccines were associated with cutaneous adverse events, especially local injection site reactions.
Previous descriptions of cutaneous reactions beyond the injection site were case reports or mostly reported by non‐dermatologists and lacked clinical images.
What does this study add?
We describe and classify a large, representative sample of patients with unexplained skin manifestations after COVID‐19 vaccination, using consensus to define associated morphological patterns.
We describe six morphological reaction patterns and herpesvirus reactivations, and their association with demographic factors and the medical record, and provide illustrations to allow for easy recognition.
Linked Comment: V. Bataille and S. Puig. Br J Dermatol 2022; 186:15.
Plain language summary available online
The search for an effective vaccine has been unrelenting since 31 December 2019, when the first cases of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) were reported in China. 1 As of 4 June 2021, COVID‐NMA, an international World Health Organization‐supported research initiative that live‐maps and reviews SARS‐CoV‐2 trials, had compiled 256 vaccine trials (https://covid‐nma.com/vaccines/mapping/).
Vaccine development can take more than 15 years. 2 SARS‐CoV‐2 vaccines have had an accelerated timeline and were approved in record time, 3 showing good safety and immunogenicity profiles in randomized controlled trials (RCTs). 4 , 5 , 6 , 7 Currently, the European Medicines Agency (EMA) has authorized four vaccines: BNT162b2 (Pfizer‐BioNTech), mRNA‐1273 (Moderna), AZD1222 (AstraZeneca) and Ad26.COV2.S (Janssen).
SARS‐CoV‐2 is associated with a wide spectrum of skin manifestations. 8 , 9 , 10 , 11 Some may appear after immunization with vaccines expressing the SARS‐CoV‐2 spike (S) protein. The Spanish Agency for Medicines and Health Products (AEMPS) pharmacovigilance report found that, as of 25 April 2021, of 14 290 507 vaccine doses administered in Spain (70% BNT162b2, 24% AZD1222 and 6% mRNA‐1273), 1468 nonspecified cutaneous adverse events (AEs; 0·01%) had been notified. 12 Cutaneous AEs reported in clinical and postauthorization trials include local injection site reactions and local or generalized reactions beyond the injection site. Local injection site reactions, both immediate or delayed (≥ 4 days after vaccination), were the most frequent manifestation. 4 , 5 , 6 , 13 , 14 , 15 , 16 Apart from anaphylactic rashes, 17 less frequent cutaneous reactions have been described in case reports and small case series: urticaria, maculopapular or morbilliform rash, pityriasis rosea‐like rash, chilblain‐like lesions, facial dermal filler reactions, reactivation of varicella zoster virus (VZV), lichen planus, erythema multiforme and nonspecific hypersensitivity eruptions. 4 , 5 , 7 , 13 , 15 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 An American registry‐based study analysed 414 cases after mRNA vaccination. 28 Most reactions were reported by nondermatologists and a small number of clinical images were shown.
Since the beginning of mass vaccination in Spain, dermatologists have treated skin rashes in vaccinees. The reactions were poorly characterized and some observers considered them more frequent than previously reported and mimicking some reactions described after SARS‐CoV‐2 infection. 8 , 9 , 10 , 11
The primary objective of our study was to characterize and classify the clinical features of cutaneous reactions after SARS‐CoV‐2 vaccination. Secondary objectives were to identify the timing of reactions, associations with other dermatological or allergic conditions, and possible relationships with diagnoses of SARS‐CoV‐2 or SARS‐CoV‐2‐associated cutaneous reactions.
Materials and methods
We conducted a nationwide, multicentre, cross‐sectional observational study. The study was endorsed by the Spanish Academy of Dermatology and all Spanish dermatologists were invited to participate.
The planned recruitment period lasted 3 months (16 February–15 May 2021). Inclusion criteria were people of any age vaccinated against SARS‐CoV‐2 with any skin manifestation within 21 days after any dose of a vaccine approved by the EMA and AEMPS. Exclusion criteria were explainable causes other than SARS‐CoV‐2 vaccination and injection site reactions lasting ≤ 3 days, as this reaction was very common in SARS‐CoV‐2 vaccine RCTs. 4 , 5 , 6 , 7
Data were collected and managed using an electronic case report form (e‐CRF) and a questionnaire administered using an online professional survey company (LimeSurvey GmbH, Hamburg, Germany). Data treatment complied with the European Commission General Data Protection Regulation and Information Security regulations. After a face‐to‐face visit, patient data were recorded and clinical pictures, if available, were sent by email. Data were encrypted, patient and investigator anonymity were assured, and no external servers were used. Case entry was restricted to dermatologists, to provide a more accurate description and classification of the morphology of the lesions. As in a previous study of SARS‐CoV‐2 skin manifestations, 8 reporting dermatologists preclassified skin rashes in a predefined cutaneous reaction pattern, with an option for a free clinical description. Only the three principal investigators had access to the clinical image dataset and independently reviewed the photographs and clinical data, and sought consensus on the cutaneous patterns. If clinical images were not available, the case was considered as missing data, unless the clinical pattern described was unequivocal. If consensus was not initially reached but histopathology was available, the case was classified according to an agreed clinicopathological correlation. If consensus was not reached and histopathology was not available or not diagnostic, the reporting dermatologist was consulted, and if clinical consensus was not reached, the case was not classified.
Variables collected through the e‐CRF included patient characteristics (geographical area, age, sex, history of allergy, atopic dermatitis, urticaria and/or cutaneous reactions to other vaccines before SARS‐CoV‐2 diagnosis, previous SARS‐CoV‐2‐associated cutaneous manifestations and new drugs prescribed in the 5 weeks before the reaction). Vaccine reaction data included type of vaccine, dose at the time of the cutaneous reaction and days between doses. Cutaneous reaction data included day of onset, duration, injection site involvement (local or generalized beyond the injection site), location, clinical pattern of the reaction (predefined or free description), cutaneous and systemic symptoms, treatment, photographs and histopathological findings, if available.
The severity of reactions was classified as grade 1 or mild (local macular or papular erythematous rash without associated systemic symptoms); grade 2 or moderate (the same as grade 1 plus systemic symptoms); grade 3 or severe (generalized erythematous macular or papular or vesicular rash); and grade 4 or very severe (generalized erythrodermic or exfoliative or ulcerative or bullous rash).
The study was authorized by the ethics committees of the three principal investigation centres and the regional drug regulatory agency for postauthorization of observational studies (Generalitat de Catalunya, registry number: 9015‐363592/2021). All patients gave written informed consent to participate and explicit consent to publish images.
The sample size could not be determined a priori because of the uncertain number of reported reactions and participating dermatologists. We planned for 3 months of recruitment to include the AstraZeneca vaccine (approved in Spain after the RNA‐based vaccines) and to cover populations other than healthcare workers and older people. The analysis included description of the data and distribution tests (χ2‐test for qualitative variables and anova for quantitative variables). Patients with missing data for a specific mandatory parameter were excluded. A P‐value < 0·05 was considered to be statistically significant in univariate analyses. The analysis was done with SPSS (version 22·0; IBM, Armonk, NY, USA).
Results
We collected 419 cases of cutaneous reactions from 31 public hospitals and private clinics. Fourteen cases not meeting the inclusion criteria and/or with missing data were excluded. The final sample included 405 reactions in 391 patients after BNT162b2 [n = 163 (40·2%)], mRNA‐1273 [n = 147 (36·3%)] and AZD1222 [n = 95 (23·5%)] vaccination. Owing to delayed authorization, only one reaction after Janssen vaccination was reported, which was excluded from the final analysis. A flowchart of patient inclusion is shown in Figure 1. Skin biopsies were performed in 50 cases (12·3%).
Figure 1.
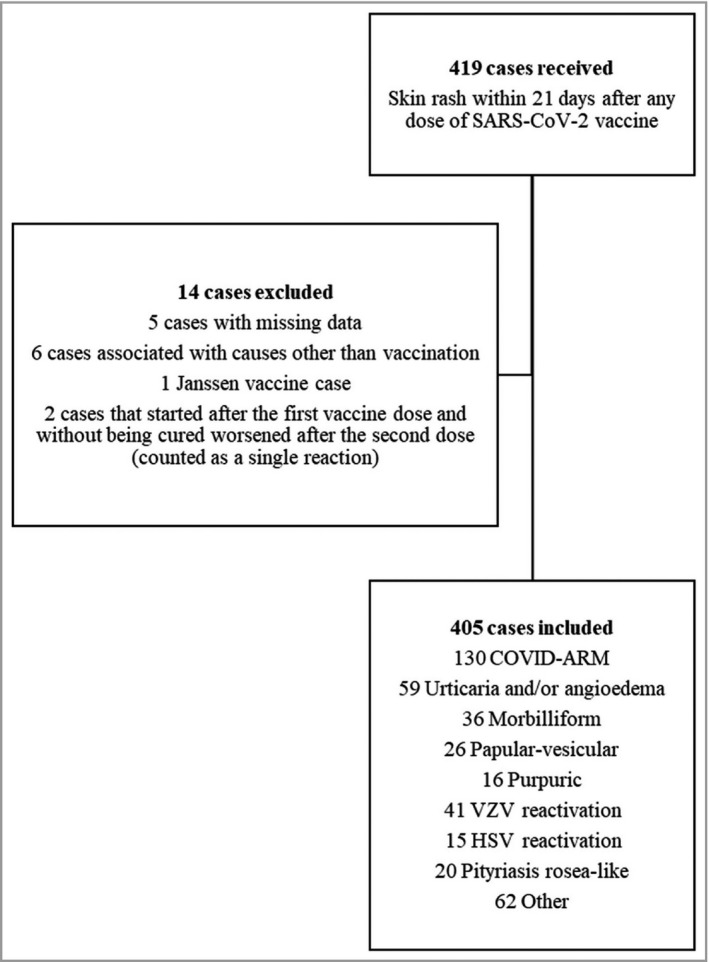
Study flowchart of the inclusion and exclusion of reported reactions. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; HSV, herpes simplex virus; VZV, varicella zoster virus.
Baseline patient characteristics are shown in Table 1. All patients were white, with a mean (SD) age of 50·7 (17·6) years and 80·2% were female. Regarding the mRNA vaccines, 165 reactions (53·2%) appeared after the first dose and 145 (46·8%) after the second. We could not evaluate the AZD1222 vaccine as second doses were not administered during the study period. Fourteen patients with first‐dose reactions [n = 14/165 (8·5%)] after mRNA vaccines developed a second‐dose reaction, of whom seven had the same reaction and seven had different reactions.
Table 1.
Baseline patient characteristics
| No. of patients | 391 |
| No. of reactions | 405 |
| No. of patients with reported reactions after both doses | 14 |
| Mean (SD) age (years) | 50·7 (17·6) |
| Range | 20–95 |
| Sex | |
| Female | 325 (80·2) |
| Male | 80 (19·8) |
| Medical history | |
| Atopic dermatitis | 28 (6·9) |
| Allergic asthma | 24 (5·9) |
| Allergic rhinitis | 42 (10·4) |
| Urticaria | 26 (6·4) |
| History of allergy to drugs or excipients | |
| Yes | 47 (11·6) |
| No | 358 (88·4) |
| Any antibiotic | 23 (5·7) |
| ASA and/or NSAIDs | 16 (4·0) |
| Iodine | 4 (1·0) |
| History of cutaneous reactions to other vaccines | 9 (2·2) |
| Previous diagnosis of SARS‐CoV‐2 infectiona | |
| Yes | 45 (11·1) |
| No | 360 (88·9) |
| Clinical suspicion only | 2 (4·4) |
| PCR+ | 33 (73·3) |
| Antibody+ | 11 (24·4) |
| Rapid antigen test+ | 3 (6·7) |
| Cutaneous manifestations after SARS‐CoV‐2 infection | |
| Yes | 7/45 |
| Maculopapular rash | 3/7 |
| Urticaria | 2/7 |
| Morbilliform rash | 1/7 |
| Pseudovesicular rash | 1/7 |
Data are n (%) unless otherwise indicated. ASA, acetylsalicylic acid; NSAID, nonsteroidal anti‐inflammatory drugs; PCR, polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2. aSome patients were diagnosed by one or more methods.
Reactions were located at the injection site in 131 cases (32·3%) and beyond the injection site in 274 (67·7%) (138 local and 136 generalized). The mean (SD) time to onset was 5·1 (4·4) days after vaccination and the mean (SD) duration was 12·2 (13·1) days.
Clinical images were available for 293 reactions (72·3%). Six major clinical morphological reaction patterns were described in 287 reactions (70·9%). Other miscellaneous cutaneous reactions were reported after vaccination. Photographic examples and the main features of each pattern are shown in Figure 2 and Table 2, and Appendix S1 (Photographic atlas, see Supporting Information). The six major patterns described were (in order of frequency): (i) local injection site reactions [commonly known as ‘COVID arm’; n = 130 (32·1%)] – erythematous patches or swollen plaque at the injection site, of which 53·8% were delayed (≥ 4 days after vaccination); (ii) urticaria and/or angioedema [n = 59 (14·6%)] – hives mostly distributed on the trunk, or generalized, and usually appearing > 24 h postvaccination (93·2%); (iii) morbilliform [n = 36 (8·9%)] – an erythematous, maculopapular rash reminiscent of measles, mostly generalized affecting the trunk and limbs; (iv) papulovesicular or pseudovesicular [n = 26 (6·4%)] – small papules/vesicles with surrounding erythema, without herpetiform arrangement; (v) pityriasis rosea‐like [n = 20 (4·9%)] – erythematous, scaly oval‐shaped plaques in a ‘Christmas tree’ distribution on the trunk; and (vi) purpuric rashes [n = 16 (4·0%)] – mostly located in the limbs. According to the histopathology, four reactions were consistent with small‐vessel vasculitis.
Figure 2.
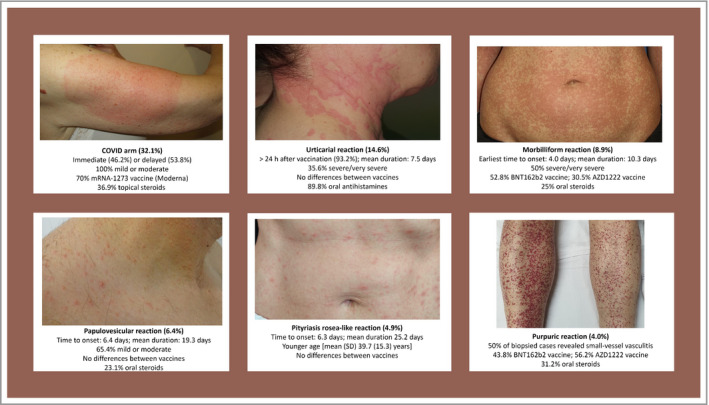
Summary of the main features of the six reaction patterns.
Table 2.
Characteristics of patients with cutaneous reactions (n = 405) after severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccination
| Characteristics | COVID arm | HSV reactivation | VZV reactivation | Papular vesicular | Pityriasis rosea‐like | Morbilliform | Urticaria and/or angioedema | Purpuric | P‐value |
|---|---|---|---|---|---|---|---|---|---|
| No. of cases | 130 (32·1) | 15 (3·7) | 41 (10·1) | 26 (6·4) | 20 (4·9) | 36 (8·9) | 59 (14·6) | 16 (4·0) | – |
| Mean (SD) age (years) | 48·8 (15·7) | 44·0 (14·6) | 60·6 (17·4) | 43·5 (15·4) | 39·7 (15·3) | 50·4 (20·8) | 47·9 (15·5) | 55·9 (20·5) | < 0·001 |
| Sex | < 0·001 | ||||||||
| Female | 124 (95·4) | 12 (80·0) | 25 (61·0) | 22 (84·6) | 15 (75·0) | 27 (75·0) | 46 (78·0) | 11 (68·8) | |
| Male | 6 (4·6) | 3 (20·0) | 16 (39·0) | 4 (15·4) | 5 (25·0) | 9 (25·0) | 13 (22·0) | 5 (31·2) | |
| Medical history | 0·0 | ||||||||
| Atopic dermatitis | 12 (9·2) | 0 (0·0) | 1 (2·4) | 1 (3·8) | 2 (10·0) | 4 (11·1) | 6 (10·2) | 1 (6·3) | 0·714 |
| Allergic asthma | 6 (4·6) | 2 (13·3) | 1 (2·4) | 4 (15·4) | 0 (0·0) | 5 (13·9) | 1 (1·7) | 0 (0·0) | 0·030 |
| Allergic rhinitis | 13 (10·0) | 2 (13·3) | 2 (4·9) | 2 (7·7) | 5 (25·0) | 8 (22·2) | 5 (8·5) | 1 (6·3) | 0·147 |
| Urticaria | 6 (4·6) | 0 (0·0) | 2 (4·9) | 1 (3·8) | 2 (10·0) | 2 (5·6) | 11 (18·6) | 0 (0·0) | 0·053 |
| History of allergy to drugs or excipients | 19 (14·6) | 0 (0·0) | 2 (4·9) | 1 (3·8) | 0 (0·0) | 8 (22·2) | 5 (8·5) | 4 (25·0) | 0·023 |
| History of cutaneous reactions to other vaccines | 5 (3·8) | 0 (0·0) | 0 (0·0) | 1 (3·8) | 0 (0·0) | 0 (0·0) | 1 (1·7) | 0 (0·0) | 0·835 |
| Vaccine | < 0·001 | ||||||||
| BNT162b2 (Pfizer) | 23 (17·7) | 5 (33·3) | 28 (68·3) | 11 (42·3) | 11 (55·0) | 19 (52·8) | 24 (40·7) | 7 (43·8) | |
| mRNA‐1273 (Moderna) | 91 (70·0) | 4 (26·7) | 6 (14·6) | 7 (26·9) | 5 (25·0) | 6 (16·7) | 15 (25·4) | 0 (0·0) | |
| AZD1222 (AstraZeneca) | 16 (12·3) | 6 (40·0) | 7 (17·1) | 8 (30·8) | 4 (20·0) | 11 (30·5) | 20 (33·9) | 9 (56·2) | |
| Vaccination dose at the time of cutaneous reaction | 0·969 | ||||||||
| First | 85 (65·4) | 9 (60·0) | 26 (63·4) | 18 (69·2) | 12 (60·0) | 25 (69·4) | 35 (59·3) | 11 (68·8) | |
| Second | 45 (34·6) | 6 (40·0) | 15 (36·6) | 8 (30·8) | 8 (40·0) | 11 (30·6) | 24 (40·7) | 5 (31·2) | |
| Mean (SD) time to onset after vaccination (days) | 4·9 (3·7) | 4·6 (4·0) | 6·9 (6·4) | 6·4 (5·2) | 6·3 (3·6) | 4·0 (3·9) | 4·9 (3·4) | 7·6 (5·4) | 0·002 |
| Mean (SD) duration of the reaction (days)a | 7·4 (4·1) | 9·3 (5·9) | 12·1 (6·8) | 19·3 (17·2) | 25·2 (14·5) | 10·3 (12·0) | 7·5 (10·0) | 15·7 (11·9) | < 0·001 |
| Photograph availability | 83 (63·8) | 10 (66·7) | 30 (73·2) | 26 (100) | 19 (95) | 29 (80·5) | 35 (59·3) | 15 (93·8) | < 0·001 |
| Associated skin symptoms | |||||||||
| Yes | 118 (90·8) | 14 (93·3) | 38 (92·7) | 24 (92·3) | 11 (55·0) | 30 (83·3) | 54 (91·5) | 9 (56·2) | < 0·001 |
| No | 12 (9·2) | 1 (6·7) | 3 (7·3) | 2 (7·7) | 9 (45·0) | 5 (13·9) | 5 (8·5) | 7 (43·8) | |
| Itch | 74 (56·9) | 3 (20·0) | 14 (34·1) | 23 (88·5) | 10 (50·0) | 28 (77·8) | 49 (83·1) | 5 (31·2) | < 0·001 |
| Pain | 62 (47·7) | 4 (26·7) | 34 (82·9) | 2 (7·7) | 0 (0·0) | 0 (0·0) | 6 (10·2) | 4 (25·0) | < 0·001 |
| Stinging | 25 (19·2) | 8 (53·3) | 14 (34·1) | 2 (7·7) | 0 (0·0) | 6 (16·7) | 8 (13·6) | 0 (0·0) | < 0·001 |
| Burning | 19 (14·6) | 1 (6·7) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | < 0·001 |
| Dysaesthesia | 0 (0·0) | 0 (0·0) | 1 (2·4) | 0 (0·0) | 0 (0·0) | 1 (2·8) | 1 (1·7) | 0 (0·0) | 0·354 |
| Painful lymph node | 8 (6·2) | 1 (6·7) | 4 (9·8) | 0 (0·0) | 0 (0·0) | 1 (2·8) | 0 (0·0) | 0 (0·0) | 0·175 |
| Systemic symptoms | |||||||||
| Yes | 84 (64·6) | 8 (53·3) | 20 (48·8) | 11 (42·3) | 8 (40·0) | 16 (44·4) | 29 (49·2) | 5 (31·2) | 0·046 |
| No | 46 (35·4) | 7 (46·7) | 21 (51·2) | 15 (57·7) | 12 (60·0) | 20 (55·6) | 30 (50·8) | 11 (68·8) | |
| Cough | 1 (0·8) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 3 (5·1) | 0 (0·0) | 0·377 |
| Dyspnoea | 1 (0·8) | 0 (0·0) | 0 (0·0) | 1 (3·8) | 0 (0·0) | 1 (2·8) | 2 (3·4) | 0 (0·0) | 0·520 |
| Low fever (37·1–38 °C) | 31 (23·8) | 1 (6·7) | 10 (24·4) | 5 (19·2) | 4 (20·0) | 5 (13·9) | 8 (13·6) | 1 (6·3) | 0·416 |
| Fever (> 38 °C) | 28 (21·5) | 4 (26·7) | 1 (2·4) | 1 (3·8) | 0 (0·0) | 5 (13·9) | 6 (10·2) | 0 (0·0) | 0·002 |
| Myalgia | 37 (28·5) | 4 (26·7) | 2 (4·9) | 4 (15·4) | 3 (15·0) | 8 (22·2) | 10 (16·9) | 2 (12·5) | 0·060 |
| Asthaenia | 38 (29·2) | 5 (33·3) | 8 (19·5) | 5 (19·2) | 1 (5·0) | 11 (30·6) | 15 (25·4) | 5 (31·2) | 0·342 |
| Headache | 29 (22·3) | 3 (20·0) | 6 (14·6) | 3 (11·5) | 3 (15·0) | 8 (22·2) | 13 (22·0) | 3 (18·8) | 0·891 |
| Nausea/vomiting/diarrhoea | 17 (13·1) | 1 (6·7) | 0 (0·0) | 1 (3·8) | 1 (5·0) | 4 (11·1) | 6 (10·2) | 1 (6·3) | 0·236 |
| Anosmia/ageusia | 0 (0·0) | 0 (0·0) | 0 (0·0) | 1 (3·8) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0·224 |
| Severity of cutaneous reaction | < 0·001 | ||||||||
| Mild (grade 1) | 66 (50·8) | 9 (60·0) | 13 (31·7) | 10 (38·5) | 5 (25·0) | 10 (27·8) | 21 (35·6) | 9 (56·3) | |
| Moderate (grade 2) | 64 (49·2) | 6 (40·0) | 23 (56·1) | 7 (26·9) | 2 (10·0) | 8 (22·2) | 17 (28·8) | 4 (25·0) | |
| Severe (grade 3) | 0 (0·0) | 0 (0·0) | 5 (12·2) | 9 (34·6) | 13 (65·0) | 17 (47·2) | 20 (33·9) | 2 (12·5) | |
| Very severe (grade 4) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 1 (2·8) | 1 (1·7) | 1 (6·3) | |
| Medical sick leave | < 0·001 | ||||||||
| Yes | 10 (7·7) | 1 (6·7) | 15 (36·6) | 3 (11·5) | 0 (0·0) | 8 (22·2) | 10 (16·9) | 5 (31·2) | |
| No | 120 (92·3) | 14 (93·3) | 26 (63·4) | 23 (88·5) | 20 (100) | 28 (77·8) | 49 (83·1) | 11 (68·8) | |
| Treatment of cutaneous reactions | |||||||||
| Yes | 93 (71·5) | 12 (80·0) | 40 (97·6) | 22 (84·6) | 13 (65·0) | 30 (83·3) | 57 (96·6) | 8 (50·0) | < 0·001 |
| No | 37 (28·5) | 3 (20·0) | 1 (2·4) | 4 (15·4) | 7 (35·0) | 6 (16·7) | 2 (3·4) | 8 (50·0) | |
| Topical corticosteroids | 48 (36·9) | 1 (6·7) | 1 (2·4) | 12 (46·2) | 9 (45·0) | 12 (33·3) | 16 (27·1) | 4 (25·0) | < 0·001 |
| Systemic corticosteroids | 3 (2·3) | 0 (0·0) | 0 (0·0) | 6 (23·1) | 1 (5·0) | 9 (25·0) | 15 (25·4) | 5 (31·2) | < 0·001 |
| Topical antibiotics | 5 (3·8) | 1 (6·7) | 7 (17·1) | 5 (19·2) | 1 (5·0) | 1 (2·8) | 3 (5·1) | 0 (0·0) | 0·024 |
| Oral antibiotics | 4 (3·1) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 1 (5·0) | 1 (2·8) | 0 (0·0) | 0 (0·0) | 0·601 |
| Paracetamol | 42 (32·3) | 0 (0·0) | 6 (14·6) | 2 (7·7) | 1 (5·0) | 3 (8·3) | 3 (5·1) | 1 (6·3) | < 0·001 |
| NSAIDs | 12 (9·2) | 0 (0·0) | 7 (17·1) | 1 (3·8) | 0 (0·0) | 1 (2·8) | 1 (1·7) | 0 (0·0) | 0·057 |
| Oral antihistamines | 33 (25·4) | 0 (0·0) | 3 (7·3) | 17 (65·4) | 7 (35·0) | 22 (61·1) | 53 (89·8) | 2 (12·5) | < 0·001 |
| Adrenaline | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 1 (1·7) | 0 (0·0) | 0·621 |
| Systemic antiviral | 0 (0·0) | 10 (66·7) | 38 (92·7) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | < 0·001 |
| New drugs (last 5 weeks) before the onset of cutaneous reaction | 9 (6·9) | 0 (0·0) | 4 (9·8) | 3 (11·5) | 1 (5·0) | 3 (8·3) | 4 (6·8) | 4 (25·0) | 0·370 |
| Prior diagnosis of SARS‐CoV‐2 infection | 19 (14·6) | 2 (13·3) | 3 (7·3) | 1 (3·8) | 2 (10·0) | 5 (13·9) | 6 (10·2) | 1 (6·3) | 0·808 |
Data are presented as n (%), unless otherwise stated. P‐values are from χ2‐tests for qualitative variables and anova for quantitative variables. HSV, herpes simplex virus; NSAID, nonsteroidal anti‐inflammatory drug; VZV, varicella zoster virus. aMissing data for 12 patients; the percentages were calculated using the available data.
Cutaneous findings not included in this classification were grouped as: (i) flare/reactivation of latent pre‐existing cutaneous infection or condition [VZV, n = 41 (10·1%); herpes simplex virus (HSV), n = 15 (3·7%); psoriasis (n = 6); lichen planus (n = 3)]; (ii) new‐onset condition [n = 31 (7·6%)], listed in Table 3; and (iii) nonclassifiable [n = 22 (5·4%)].
Table 3.
Characteristics of patients with cutaneous reactions (n = 405) according to vaccine
| Characteristics | BNT162b2 (Pfizer‐BioNTech) |
mRNA‐1273 (Moderna) |
AZD1222 (AstraZeneca) |
P‐value |
|---|---|---|---|---|
| No. of cases | 163 (40·2) | 147 (36·3) | 95 (23·5) | |
| Mean (SD) age (years) | 55·3 (20·7) | 46·1 (13·8) | 50·0 (15·2) | < 0·001 |
| Sex | ||||
| Female | 114 (69·9) | 133 (90·5) | 78 (82·1) | < 0·001 |
| Male | 49 (30·1) | 14 (9·5) | 17 (17·9) | |
| Medical history | ||||
| Atopic dermatitis | 9 (5·5) | 9 (6·1) | 10 (10·5) | 0·278 |
| Allergic asthma | 11 (6·7) | 10 (6·8) | 3 (3·2) | 0·426 |
| Allergic rhinitis | 19 (11·6) | 14 (9·5) | 9 (9·5) | 0·784 |
| Urticaria | 9 (5·5) | 11 (7·5) | 6 (6·3) | 0·780 |
| History of allergy to drugs or excipients | ||||
| Yes | 20 (12·2) | 18 (12·2) | 9 (9·5) | 0·760 |
| No | 143 (87·7) | 129 (87·8) | 86 (90·5) | |
| History of cutaneous reactions to other vaccines | ||||
| Yes | 5 (3·1) | 4 (2·7) | 0 (0·0) | 0·261 |
| No | 158 (96·9) | 143 (97·3) | 95 (100) | |
| Cutaneous reaction | ||||
| COVID arm | 23 (14·1) | 91 (61·9) | 16 (16·8) | < 0·001 |
| HSV reactivation | 5 (3·1) | 4 (2·7) | 6 (6·3) | 0·301 |
| VZV reactivation | 28 (17·2) | 6 (4·1) | 7 (7·4) | < 0·001 |
| Papulovesicular | 11 (6·7) | 7 (4·8) | 8 (8·4) | 0·371 |
| Pityriasis rosea‐like | 11 (6·7) | 5 (3·4) | 4 (4·2) | 0·419 |
| Morbilliform | 19 (11·7) | 6 (4·1) | 11 (11·6) | 0·037 |
| Urticaria and/or angioedema | 24 (14·7) | 15 (10·2) | 20 (21·1) | 0·065 |
| Purpuric | 7 (4·3) | 0 (0·0) | 9 (9·5) | 0·001 |
| Othera | 35 (21·5) | 13 (8·8) | 14 (14·7) | 0·008 |
| Vaccination dose at the time of cutaneous reaction | ||||
| First | 82 (50·3) | 83 (56·5) | 95 (100) | < 0·001 |
| Second | 81 (49·7) | 64 (43·5) | 0 (0·0) | |
| Systemic symptoms | ||||
| No | 104 (63·8) | 54 (36·7) | 40 (42·1) | |
| Cough | 2 (1·2) | 3 (2·0) | 0 (0·0) | 0·374 |
| Dyspnoea | 3 (1·8) | 4 (2·7) | 0 (0·0) | 0·309 |
| Low fever (37·1–38 °C) | 21 (12·9) | 33 (22·4) | 16 (16·8) | 0·084 |
| Fever (> 38°C) | 6 (3·7) | 30 (20·4) | 16 (16·8) | < 0·001 |
| Myalgia | 20 (12·3) | 37 (25·2) | 22 (23·2) | 0·010 |
| Asthaenia | 27 (16·6) | 44 (29·9) | 32 (33·7) | 0·003 |
| Headache | 17 (10·4) | 34 (23·1) | 25 (26·3) | 0·002 |
| Nausea/vomiting/diarrhoea | 8 (4·9) | 18 (12·2) | 10 (10·5) | 0·062 |
| Anosmia/ageusia | 0 (0·0) | 1 (0·7) | 0 (0·0) | 0·598 |
| Severity of cutaneous reaction | ||||
| Mild (grade 1) | 66 (40·5) | 64 (43·5) | 36 (37·9) | 0·002 |
| Moderate (grade 2) | 52 (31·9) | 68 (46·3) | 34 (35·8) | |
| Severe (grade 3) | 41 (25·2) | 15 (10·2) | 24 (25·3) | |
| Very severe (grade 4) | 4 (2·4) | 0 (0·0) | 1 (1·0) | |
| Medical sick leave | ||||
| Yes | 30 (18·4) | 10 (6·8) | 18 (18·9) | 0·005 |
| No | 133 (81·6) | 137 (93·2) | 77 (81·1) | |
Data are presented as n (%) unless stated otherwise. P‐values are derived from χ2‐tests for qualitative variables and anova for quantitative variables. HSV, herpes simplex virus; VZV, varicella zoster virus. a‘Other’ includes: (i) flare/reactivation of latent pre‐existing cutaneous infection or condition [VZV, n = 41 (10·1%); HSV, n = 15 (3·7%); psoriasis, n = 6; lichen planus, n = 3]; (ii) new‐onset condition (psoriasis, n = 3; eczema, n = 7; chilblain‐like/pernio, n = 3; acute generalized exanthematous pustulosis, n = 2; Raynaud syndrome, n = 2; bullous pemphigoid, n = 2; erythema multiforme, n = 2; generalized morphoea, n = 1; cutaneous B lymphoma, n = 1; livedo reticularis, n = 1; symmetrical drug‐related intertriginous and flexural exanthema‐like eruption, n = 1; erythema nodosum, n = 1; reaction to facial dermal fillers, n = 1; scrotal tongue, n = 1; xantonichia (n = 1), staphylococcal skin infection, n = 1; ankle oedema secondary to deep vein thrombosis, n = 1); and (iii) nonclassifiable.
The most frequently reported reactions were injection site reactions in women [n = 124/325 (38·1%)] and VZV reactivation in men [n = 16/80 (20%)]. Systemic symptoms associated with the skin rash were present in 207 patients (51·1%), particularly in those with the COVID arm pattern (64·6%), with low fever/fever being the most frequent symptom in this group (45·3%). The earliest pattern that appeared was the morbilliform pattern (mean 4 days), the last was VZV reactivation (mean 6·9 days) and the longest lasting was pityriasis rosea‐like (mean 25·2 days).
Thirty‐one patients (7·7%) were taking new drugs at the time of the cutaneous reaction, of which acetaminophen was the most frequent [n = 9/31 (29%)].
Forty‐five patients (11·1%) had been diagnosed with mild or asymptomatic SARS‐CoV‐2 infection. Seven (15·5%) had cutaneous reactions after both infection and vaccination. Cutaneous reactions after vaccination and their severity in this group are shown in Table S1 (see Supporting Information). There were no significant differences in the severity of cutaneous reactions between this group and patients with no prior SARS‐CoV‐2 infection (22·1% vs. 21% of severe/very severe reactions).
Dermatological findings and systemic symptoms according to type of vaccine are shown in Table 3. There were more reactions in men who received the BNT162b2 [n = 49 (30·1%)] vaccine than with the mRNA‐1273 [n = 14 (9·5%)] and AZD1222 [n = 17 (17·9%)] vaccines. Nearly all patients with a reaction to the Moderna vaccine were women (90·5%). The most frequently reported patterns in each vaccine group were VZV infection (BNT162b2, 17·2%), COVID arm (mRNA‐1273, 61·9%) and urticaria (AZD1222, 21·1%).
In total, 166 reactions (41·0%) were classified as grade 1 (mild), 154 (38·0%) as grade 2 (moderate), 80 (19·8%) as grade 3 (severe) and five (1·2%) as grade 4 (very severe). Very severe reactions included one case each of morbilliform rash progressing to erythroderma, bullous pemphigoid, acute generalized exanthematous pustulosis, vasculitis and urticaria. Fifty‐eight patients (14·3%) took sick leave, mostly due to herpes zoster [n = 15/58 (25·9%)] and urticaria [n = 10/58 (17·2%)]. Severe/very severe cases were reported more frequently with the BNT162b2 (25·2% and 2·4%, respectively) and AZD1222 (25·3% and 1·0%, respectively) vaccines. No patient died. Treatment was required in 328 cases (81%) and is detailed in Table 2.
Discussion
We described dermatological reactions after vaccination with three SARS‐CoV‐2 vaccines (two mRNA and one adenovirus‐vectored vaccines) and classified them into six well‐defined morphological reactions patterns and new‐onset or reactivation of dermatosis.
Initial reports mostly described local injection site reactions and, subsequently, other miscellaneous skin reactions after mRNA vaccination. 4 , 5 , 7 , 13 , 15 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 In 2021, McMahon et al. published a large registry‐based study (mostly with the mRNA‐1273 vaccine) in healthcare workers and older people, describing not only injection site reactions, but also urticarial and morbilliform rashes. 28
Unlike the study of McMahon et al., 28 our data entry, description and assignment of clinical patterns were made by dermatologists and were mostly supported by photographs. Case collection throughout Spain and the 3‐month recruitment period permitted a more representative sample beyond healthcare workers and older people.
Reactions were more frequent in women (80·2%), which may reflect a real difference or reporting bias, although women are known to have greater reactogenicity to vaccines, 29 and 60% of vaccinated people in Spain were women at the time of the study. 12 Therefore, women’s immune systems may be more reactive to SARS‐CoV‐2 proteins, which would result in lower susceptibility to the disease and greater reactogenicity to vaccines.
Few people had previous atopic dermatitis (6·9%) or urticaria (6·4%). In the general population, the prevalence of atopic dermatitis is around 10%, 30 , 31 and the lifetime prevalence of acute urticaria is approximately 20%, 32 so it cannot be concluded that previous atopy or acute urticaria predisposes to SARS‐CoV‐2 vaccine cutaneous reactions. However, 18·6% of patients with acute urticarial reactions to vaccines in our study had a history of urticaria. Case–control studies are needed to clarify this association. Only 7·7% of people were receiving new drugs (mainly acetaminophen) at the time of the reaction, and this factor was therefore unlikely to be related to cutaneous reactions.
There was a previous diagnosis of SARS‐CoV‐2 in 11·1% of cases, similar to the seroprevalence in Spain at the time of writing (9·9%). 33 The severity of cutaneous reactions in this group did not differ from the rest of the sample. Thus, prior SARS‐CoV‐2 infection does not seem to predispose to cutaneous reactions or more severe reactions, after vaccination.
The COVID arm, the most reported pattern was described after vaccination with all three vaccines, particularly mRNA‐1273 (70·0%), and almost exclusively in women (95·4%). This pattern had the closest association with systemic symptoms (64·6%).
Two‐thirds of reported reactions were beyond the injection site. Each morphological pattern seems to correspond to a different spectrum of delayed hypersensitivity reaction, with most of the few skin biopsies that were performed showing nonspecific changes consistent with this reaction. In contrast to previous series, 28 some reactions were scarce (chilblain‐like/pernio) or unrepresented (erythromelalgia), while other reactions were more frequently reported (pityriasis rosea‐like, VZV reactivations and papulovesicular rashes). The morbilliform and purpuric patterns were reported mostly after BNT162b2 and AZD1222 vaccination, and were associated with more severe reactions. VZV reactivation was more frequent after BNT162b2 vaccination and in men.
UK spontaneous AE reports are the main information source on AZD1222 vaccine cutaneous reactions, 34 which, in our series, were mainly acute urticaria (21·1%), injection site reactions (16·8%) and morbilliform rash (11·6%). Owing to the precautionary suspension of the vaccine in the initial target population, we could not study the second dose.
We found a large number of herpes reactivations (VZV and HSV, 13·8%). For VZV, the number [n = 41 (10·1%)], severity (36·6% took sick leave) and the percentage in healthy people aged < 50 years (29·2%) were particularly striking. 35 , 36 There were fewer HSV than VZV reactivations, probably because patients with HSV do not usually seek medical care. We also found pityriasis rosea‐like eruptions, which might have been due to human herpesvirus 6 and 7 reactivation. These herpetic reactivations were also described after SARS‐CoV‐2 infection and other vaccinations. 8 , 9 , 37 , 38 Taken together, these data strengthen a causal link between herpesvirus reactivation and the SARS‐CoV‐2 vaccine. A plausible mechanism is that a strong specific immune response against SARS‐CoV‐2 or the S protein from vaccines may distract the cell‐mediated control of another, latent virus.
New‐onset or worsening of inflammatory conditions were also reported, including psoriasis, lichen planus and bullous pemphigoid. These conditions were previously described after SARS‐CoV‐2 and other vaccinations. 20 , 28 , 39 , 40 , 41 , 42 , 43 As previously stated, 43 vaccines may exacerbate skin manifestations in patients with immune‐mediated skin diseases, but further investigation is necessary.
The patterns found in this and previous studies are heterogeneous and similar to those described in association with SARS‐CoV‐2 infection. 8 , 9 , 28 One case repeated the same papulovesicular rash after SARS‐CoV‐2 infection and vaccination. Therefore, the host immune response to the infection, and not direct viral damage, may cause these skin manifestations. However, a delayed hypersensitivity reaction against vaccine excipients cannot be ruled out.
Although most reactions were classified as mild/moderate, 21% were considered severe/very severe. This degree of severity was not reported in the study by McMahon et al. 28 This percentage is most likely over‐represented (reporting bias) but should not be ignored, as some reactions may be life threatening.
The study has some limitations. Firstly, the design did not permit causal associations or the measurement of risks or incidence. We could not compare the incidence or severity of cutaneous reactions by vaccine type, as vaccine distribution depended on availability during the study period. Secondly, the data collection period was short, which might limit study of the comprehensive data and evolution, especially after the second doses and AZD1222 vaccination. Thirdly, only 12·3% of cases were biopsied and histopathology might have prevented misclassification. Fourthly, there was a possible reporting bias towards previously reported or more serious reactions. Fifthly, SARS‐CoV‐2 infection after vaccination cannot be excluded as a plausible cause of cutaneous reactions. Finally, the lack of ethnic diversity in our sample does not permit generalization of the results.
In conclusion, we have described and classified cutaneous reactions reported after SARS‐CoV‐2 vaccination in a large Spanish case series. Most reactions were mild/moderate and self‐limiting, but some were severe/very severe and required treatment. Better knowledge of these reactions may aid physicians during mass vaccination and reassure patients seeking advice.
Author Contribution
Alba Català: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Methodology (lead); Supervision (lead); Writing‐original draft (supporting). Carlos Muñoz‐Santos: Conceptualization (lead); Formal analysis (supporting); Methodology (lead); Supervision (lead); Writing‐original draft (lead). Cristina Galvan Casas: Conceptualization (supporting); Supervision (lead); Writing‐original draft (supporting). Monica Roncero Riesco: Data curation (supporting); Validation (supporting). David Revilla Nebreda: Data curation (supporting); Validation (supporting). Amador Solà‐Truyols: Data curation (supporting); Validation (supporting). Priscila Giavedoni: Data curation (supporting); Validation (supporting). Mar Llamas‐Velasco: Data curation (supporting); Validation (supporting). Carlos González‐Cruz: Data curation (supporting); Validation (supporting). Xavier Cubiró: Data curation (supporting); Validation (supporting). Ricardo Ruiz‐Villaverde: Data curation (supporting); Validation (supporting). Sara Gómez: Data curation (supporting); Validation (supporting). Maria del Pino Gil Mateo: Data curation (supporting); Validation (supporting). David Pesqué: Data curation (supporting); Validation (supporting). Orianna Yilsy Marcantonio Santa Cruz: Data curation (supporting); Validation (supporting). Diego Fernandez‐Nieto: Data curation (supporting); Validation (supporting). Jorge Romani: Data curation (supporting); Validation (supporting). Nicolás Iglesias Pena: Data curation (supporting); Validation (supporting). Lucia Carnero‐González: Data curation (supporting); Validation (supporting). Jesus Tercedor: Data curation (supporting); Validation (supporting). Gregorio Carretero: Data curation (supporting); Validation (supporting). Teresa Masat‐Ticó: Data curation (supporting); Validation (supporting). Pedro Rodriguez‐Jiménez: Data curation (supporting); Validation (supporting). Ana Maria Giménez‐Arnau: Data curation (supporting); Validation (supporting). Marta Utrera‐Busquets: Data curation (supporting); Validation (supporting). Elena Vargas Laguna: Data curation (supporting); Validation (supporting). Ana G Angulo Menéndez: Data curation (supporting); Validation (supporting). Eliana San Juan Lasser: Data curation (supporting); Validation (supporting). Maribel Iglesias‐Sancho: Data curation (supporting); Validation (supporting). Laura Alonso Naranjo: Data curation (supporting); Validation (supporting). Ingrid Hiltun: Data curation (supporting); Validation (supporting). Eugenia Cutillas Marco: Data curation (supporting); Validation (supporting). Isabel Polimon Olabarrieta: Data curation (supporting); Validation (supporting). Silvia Marinero Escobedo: Data curation (supporting); Validation (supporting). Xavier García‐Navarro: Data curation (supporting); Validation (supporting). María José Calderón Gutierrez: Data curation (supporting); Validation (supporting). Gloria Baeza‐Hernández: Data curation (supporting); Validation (supporting). Lola Bou Camps: Data curation (supporting); Validation (supporting). TOMAS TOLEDO‐PASTRANA: Data curation (supporting); Validation (supporting). Antonio Guilabert: Data curation (supporting); Writing‐original draft (supporting); Writing‐review & editing (supporting).
Supporting information
Powerpoint S1 Journal Club Slide Set.
Appendix S1 Photographic atlas: Cutaneous reactions after severe acute respiratory syndrome coronavirus 2 vaccination.
Table S1 Cutaneous reactions and their severity after severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccines in people who had previous SARS‐CoV‐2 infection.
Video S1 Author video.
Acknowledgments
We thank the following Spanish dermatologists for their generous contributions: I. García‐Doval, J. Sola‐Ortigosa, S. Mateo Suárez, M. Elosua González, D. Ruiz Sanchez, B. Roque Quintana, M. Sidro Sarto, C.F. Vásquez Chinchay, J.F. Millán Calletano, A. Rodríguez‐Villa Lario, E. Amores Martin, A. Suarez Valle, J. Fernández Vazquez, J. Riera Monroig, L. Armillas‐Lliteras, M. Corral Forteza, J. Arandes Marcocci, J. Jimenez Cauhe, G. Selda Enriquez, L. Haya Martinez, A. Frías Sanchez, A. Pérez Pérez, M. Salleras Redonnet, A. Tuneu Valls, I. Fuertes De la Vega, M. Alegre Fernández, E. Baselga Torres, I. Escandel Gonzalez, A. Fernández Orland, A. Lopez Valle, V. García‐Patos Briones, M.C. Juarez Dobjanschi, G. Aparicio Español, C. Ferrándiz Pulido, A. Castany Pich, V. Cabezas Calderón and C. Plà Ferrer. We also thank primary care physicians from the Granollers‐Valles Oriental area, Spain (J. Alonso, M. Vilageliu, A. Estefanell, M. Manzano, M Martín‐Gil), Dr M. Ribell (Internal Medicine Department, Hospital General de Granollers), and the Pharmacovigilance and Occupational Health departments and technical committee of the Hospital Clinic of Barcelona, Maria Angeles Diaz Sotero (Hospital de Móstoles) and Joana Guerrero (Hospital General de Granollers), Spain. This study formed part of the PhD degree of Alba Català, Department of Medicine, Dermatology, Universidad Autónoma de Barcelona, Spain.
Funding sources None.
Conflicts of interest The authors declare they have no conflicts of interest.
Data availability statement The data that support the findings of this study are available from the corresponding author upon request. The data are not publicly available for privacy/ethical reasons.
A.C, C. M.‐S. and C.G.‐C. contributed equally as first authors.
Plain language summary available online
References
- 1. Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA 2020; 323:709–10. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Draft landscape of COVID‐19 candidate vaccines – 20 March 2020. Available at: https://www.who.int/blueprint/priority‐diseases/key‐action/novel‐coronavirus‐landscape‐ncov.pdf?ua=1 (last accessed 20 July 2021).
- 3. Krammer F. SARS‐CoV‐2 vaccines in development. Nature 2020; 586:516–27. [DOI] [PubMed] [Google Scholar]
- 4. Baden LR, El Sahly HM, Essink B et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polack FP, Thomas SJ, Kitchin N et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramasamy MN, Minassian AM, Ewer KJ et al. Safety and immunogenicity of ChAdOx1 nCoV‐19 vaccine administered in a prime‐boost regimen in young and old adults (COV002): a single‐blind, randomised, controlled, phase 2/3 trial. Lancet 2021; 396:1979–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sadoff J, Le Gars M, Shukarev G et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid‐19 vaccine. N Engl J Med 2021; 384:1824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Galván Casas C, Català A, Carretero Hernández G et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020; 183:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tan SW, Tam YC, Oh CC. Skin manifestations of COVID‐19: a worldwide review. JAAD Int 2021; 2:119–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marzano AV, Genovese G, Moltrasio C et al. The clinical spectrum of COVID‐19‐associated cutaneous manifestations: an Italian multicenter study of 200 adult patients. J Am Acad Dermatol 2021; 84:1356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Genovese G, Moltrasio C, Bert E et al. Skin manifestations associated with COVID‐19 current knowledge and future perspectives. Dermatology 2021; 237:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gobierno de España, Ministerio de Sanidad . 5° Informe de Farmacovigilancia sobre Vacunas COVID‐19. Available at: https://www.aemps.gob.es/informa/boletines‐aemps/boletin‐fv/2021‐boletin‐fv/5o‐informe‐de‐farmacovigilancia‐sobre‐vacunas‐covid‐19/ (last accessed 20 July 2021).
- 13. Fernandez‐Nieto D, Hammerle J, Fernandez‐Escribano M et al. Skin manifestations of the BNT162b2 mRNA COVID‐19 vaccine in healthcare workers. ‘COVID‐arm’: a clinical and histological characterization. J Eur Acad Dermatol Venereol 2021; 35:e425–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blumenthal KG, Freeman EE, Saff RR et al. Delayed large local reactions to mRNA‐1273 vaccine against SARS‐CoV‐2. N Engl J Med 2021; 384:1273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wei N, Fishman M, Wattenberg D et al. ‘COVID arm’: a reaction to the Moderna vaccine. JAAD Case Rep 2021; 10:92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnston MS, Galan A, Watsky KL, Little AJ. Delayed localized hypersensitivity reactions to the Moderna COVID‐19 vaccine: a case series. JAMA Dermatol 2021; 157:716–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID‐19 vaccines in the US – December 14, 2020–January 18, 2021. JAMA 2021; 325:1101–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Munavalli GG, Guthridge R, Knutsen‐Larson S et al. COVID‐19/SARS‐CoV‐2 virus spike protein‐related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment. Arch Dermatol Res 2021. 10.1007/s00403-021-02190-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Munavalli GG, Knutsen‐Larson S, Lupo MP, Geronemus RG. Oral angiotensin‐converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID‐19 vaccination‐a model for inhibition of angiotensin II‐induced cutaneous inflammation. JAAD Case Rep 2021; 10:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hiltun I, Sarriugarte J, Martínez‐de‐Espronceda I et al. Lichen planus arising after COVID‐19 vaccination. J Eur Acad Dermatol Venereol 2021; 35:e414–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burlando M, Russo R, Cozzani E, Parodi A. COVID‐19 “second wave” and vaccines: the dermatologists' perspective. Int J Dermatol 2021; 60:889–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gambichler T, Scholl L, Ocker L, Stranzenbach R. Prompt onset of erythema multiforme following the first BNT162b2 SARS‐CoV‐2 vaccination. J Eur Acad Dermatol Venereol 2021; 35:e415–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pileri A, Guglielmo A, Raone B, Patrizi A. Chilblain lesions after COVID‐19 mRNA vaccine. Br J Dermatol 2021; 185:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piccolo V, Bassi A, Argenziano G et al. BNT162b2 mRNA Covid‐19 Vaccine‐induced chilblain‐like lesions reinforces the hypothesis of their relationship with SARS‐CoV‐2. J Eur Acad Dermatol Venereol 2021; 35:e493–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akdaş E, İlter N, Öğüt B, Erdem Ö. Pityriasis rosea following CoronaVac COVID‐19 vaccination: a case report. J Eur Acad Dermatol Venereol 2021; 35:e491–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cyrenne B, Al‐Mohammedi F, DeKoven J, Alhusayen R. Pityriasis rosea‐like eruptions following vaccination with BNT162b2 mRNA COVID‐19 Vaccine. J Eur Acad Dermatol Venereol 2021; 35:e546–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Busto‐Leis JM, Servera‐Negre G, Mayor‐Ibarguren A et al. Pityriasis rosea, COVID‐19 and vaccination: new keys to understand an old acquaintance. J Eur Acad Dermatol Venereol 2021; 35:e489–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McMahon DE, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol 2021; 85:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis 2010; 10:338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mortz CG, Andersen KE, Dellgren C et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy 2015; 70:836–45. [DOI] [PubMed] [Google Scholar]
- 31. McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol 2019; 123:173–8. [DOI] [PubMed] [Google Scholar]
- 32. Zuberbier T, Aberer W, Asero R et al. The EAACI/GA²LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018; 73:1393–414. [DOI] [PubMed] [Google Scholar]
- 33. Pollán M, Pérez‐Gómez B, Pastor‐Barriuso R et al; ENE‐COVID Study Group . Prevalence of SARS‐CoV‐2 in Spain (ENE‐COVID): a nationwide, population‐based seroepidemiological study. Lancet 2020; 396:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. HM Government . COVID‐19 AstraZeneca vaccine analysis. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/983475/COVID‐19_vaccine_AstraZeneca_analysis_print.pdf (last accessed 20 July 2021).
- 35. Johnson RW, Alvarez‐Pasquin MJ, Bijl M et al. Herpes zoster epidemiology, management, and disease and economic burden in Europe: a multidisciplinary perspective. Ther Adv Vaccines 2015; 3:109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodríguez‐Jiménez P, Chicharro P, Cabrera LM et al. Varicella‐zoster virus reactivation after SARS‐CoV‐2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep 2021; 12:58–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mintz L. Recurrent herpes simplex infection at a smallpox vaccination site. JAMA 1982; 247:2704–5. [PubMed] [Google Scholar]
- 38. Drago F, Ciccarese G, Javor S, Parodi A. Vaccine‐induced pityriasis rosea and pityriasis rosea‐like eruptions: a review of the literature. J Eur Acad Dermatol Venereol 2016; 30:544–5. [DOI] [PubMed] [Google Scholar]
- 39. Baykal C, Okan G, Sarica R. Childhood bullous pemphigoid developed after the first vaccination. J Am Acad Dermatol 2001; 44(Suppl. 2):348–50. [DOI] [PubMed] [Google Scholar]
- 40. Downs AM, Lear JT, Bower CP, Kennedy CT. Does influenza vaccination induce bullous pemphigoid? a report of four cases. Br J Dermatol 1998; 138:363. [DOI] [PubMed] [Google Scholar]
- 41. Rosengard HC, Wheat CM, Tilson MP, Cuda JD. Lichen planus following tetanus‐diphtheria‐acellular pertussis vaccination: a case report and review of the literature. SAGE Open Med Case Rep 2018; 6:20503131X17750335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Munguía‐Calzada P, Drake‐Monfort M, Armesto S et al. Psoriasis flare after influenza vaccination in Covid‐19 era: a report of four cases from a single center. Dermatol Ther 2021; 34:e14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ferretti F, Cannatelli R, Benucci M. How to manage COVID‐19 vaccination in immune‐mediated inflammatory diseases: an expert opinion by the IMIDs Study Group. Front Immunol 2021; 12:656362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Powerpoint S1 Journal Club Slide Set.
Appendix S1 Photographic atlas: Cutaneous reactions after severe acute respiratory syndrome coronavirus 2 vaccination.
Table S1 Cutaneous reactions and their severity after severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccines in people who had previous SARS‐CoV‐2 infection.
Video S1 Author video.


