Summary
Multiple myeloma (MM) patients are at excess risk for clinically significant COVID19 infection. BNT162b2 mRNA COVID19 (BNT162b2) vaccine provides effective protection against COVID19 for the general population, yet its effect in MM patients may be compromised due to disease and therapy‐related factors and was not yet evaluated. This single‐centre prospective study included MM patients tested for serological response 14–21 days post second vaccine. Vaccinated healthy volunteers served as controls. In all, 171 MM patients, median age 70 (38–94) were included; 159 active MM and 12 smouldering myeloma (SMM). Seropositive response rate (median titer) was 76% (91 U/ml) in active MM patients vs 98% (992 U/ml) in the 64 controls (P < 0·0001), and 100% (822 U/ml) in SMM patients. Multivariate analysis revealed older age (P = 0·009), exposure to ≥4 novel anti‐myeloma drugs (P = 0·02) and hypogammaglobulinaemia (P = 0·002) were associated with lower response rates. None of the novel agents significantly decreased response rate, whereas daratumumab trended towards reduced response (P = 0·08). Adverse events occurred in 53% and 55% of the MM patients and controls, respectively, all transient grade 1–2. In conclusion, BNT162b2 vaccine was safe and provided a high seropositivity rate in MM patients, independent of treatment type. Older, hypogammaglobulinaemic and heavily pretreated patients had lower response rates.
Keywords: BNT162b2, COVID19 vaccine, multiple myeloma, antibody response
Introduction
Cases of community‐acquired coronavirus disease 2019 (COVID19) are continually spreading worldwide, despite efforts of social distancing and isolation of exposed and infected persons. Patients diagnosed with multiple myeloma (MM) were found to be at high risk for significant complications, with mortality approaching 33%. 1 , 2 Moreover, recent data suggest that immunocompromised patients often experience a prolonged disease course and may serve as ‘continuous viral reservoirs’, thereby supporting the development of new viral mutations. 1 Prevention of infections, or at least reducing disease severity, is therefore warranted. BNT162b2 mRNA COVID19 vaccine has been already shown to provide 92% response rate in the general population; 3 however, the efficacy of this COVID19 vaccine in patients diagnosed with MM remained unclear. 4
The current study investigated the humoral response to COVID19 vaccine in patients with MM and evaluated predictors for the achievement of humoral response, focusing on the impact of disease status, level of immune‐paresis and treatment regimen, particularly daratumumab, hypothesizing that its plasma cell‐depleting effect might adversely affect response to COVID19 vaccine.
Patients and methods
This prospective cohort study investigated the efficacy of BNT162b2 mRNA COVID19 vaccine in patients diagnosed with MM, in a real‐world setting. The primary end‐point of the study was the seropositivity rate achieved following vaccination with BNT162b2 mRNA COVID19 vaccine, assessed by measuring the anti‐SARS‐CoV‐2S antibody titer pre and post vaccination. The study was approved by the institutional review board and is registered in ClinicalTrials.gov, number NCT04746092. All participants signed an informed consent form prior to their inclusion in the study.
Study design and patient population
The study included patients diagnosed with active or smouldering MM, 5 age >18 years, males and females, who received two consecutive vaccines and visited the Haematology division at Tel Aviv Sourasky Medical centre (TASMC) between 23 December 2020 and 17 March 2021.
Compatible with our department's policy, all MM patients [except those that underwent an autologous haematopoietic stem cell transplantation (HSCT) within the past 3 months] were advised to receive two consecutive BNT162b2 mRNA COVID19 vaccines, administered 21 days apart, as a standard of care through the national vaccination programme. Recommendations on best timing for vaccination were provided by the treating physicians and followed the international MM guidelines 6 and the department's policy. In general, patients treated with daratumumab single agent or in combination with immunomodulatory agents (IMiDs) and/or proteasome inhibitors (PI) were advised to have a 14‐days gap between their last daratumumab dose and vaccination (adjusting daratumumab schedule with possible delays). Patients treated with proteasome inhibitor (PI)/PI‐dexamethasone were advised to schedule their vaccinations 7–14 days post therapy, and patients treated with lenalidomide maintenance therapy were advised to continue therapy without interruption. There were no specific recommendations regarding the preferable timing for vaccination for treatment‐naïve patients and for those that completed therapy >3 months earlier.
In addition to the MM vaccinated cohort, described above, the study included age‐compatible healthy volunteers (mainly spouses who accompanied the myeloma patients), with no medical history of haematological malignancy or infection or known exposure to COVID19, who received two doses of vaccination 14–21 days before undergoing a serological test for COVID19, and served as controls.
Blood samples for serological test for both patients and healthy controls were drawn 14–21 days after the second vaccine. Frozen serum samples, obtained within 30 days before the first vaccine (from standard of care archive samples), were used to exclude prior exposure to COVID19 in MM participants. When unavailable, post‐vaccination samples were tested for the presence of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) nucleocapsid by the Elecsys® Anti‐SARS‐CoV‐2 assay using the cobas e 601 (Roche 5 Diagnostics). MM patients that were found to have a seropositive test prior to vaccination or positive for nucleocapsid post vaccination were excluded from further analysis.
Demographic and clinical data, focusing on histological diagnosis, fluorescence in situ hybridization (FISH) cytogenetics risk stratification [high risk including del17p, t(4:14), t(14:16), t(14:20), +1q21], disease status: active myeloma (defined as patients who had experienced at least one myeloma‐defining event) versus smouldering myeloma (SMM), as defined by International Myeloma Working Group (IMWG) criteria 5 and response to recent or current therapy (defined according to the IMWG criteria), 7 treatment regimen, lymphocyte counts and polyclonal globulin levels (calculated as total immunoglobulin minus monoclonal protein) at the time of vaccination were recorded from patient's electronic medical charts. Vaccination dates and adverse events (AEs) reported within the first seven days post each one of the two vaccines were recorded. AEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) 4·03.
Assessment of serological response
Serum samples, obtained prior to the first vaccine and 14–21 days after the second vaccine, were analyzed, using Elecsys® Anti‐SARS‐CoV‐2S immunoassay, performed on the cobas e 601 fully automated analyzer [for quantitative determination of antibodies, predominantly immunoglobulin G (IgG)] to the SARS‐CoV‐2 spike (S) protein receptor‐binding domain (RBD). 8 The assay uses a recombinant protein representing the RBD of the S antigen in a double‐antigen sandwich assay format, which favours detection of high‐affinity antibodies against SARS‐CoV‐2, and its measurement range is 0·40–250 U/ml. A concentration level <0·80 U/ml was considered a seronegative result, whereas concentration ≥0·80 U/ml was considered as positive. In patients in whom sample results exceeded the upper limit of the measuring range (reported as >250 U/ml), samples were re‐analyzed, after being diluted 1:10 or 1:100, dependent on the dilution being required (if after 1:10 dilution the sample result was >2 500 U/ml, sample was further diluted 1:100).
Statistics
Continuous variables were described as the median and range or interquartile range (IQR) of observations. Categorical data were described with contingency tables including frequency and percent. Pearson's chi‐squared test, Fisher's exact test and univariate Cox regression were used to study the crude association between studied predictors and vaccine response rate. Converting continuous variables into categorical variables was based on both frequency distributions and clinical familiarity with impact factors on response variable. A chi‐squared test for association to check the marginal relationship was performed. The Mann–Whitney U‐test was used to compare medians of concentration levels of serology antibodies (titers). Multivariate Cox regression analysis was performed using the forward method (P < 0·05 was used as criterion for entrance) in order to identify independent predictors for response rate. A two‐sided P value of < 0·05 was considered as statistically significant. Variables with trend or significant association to response rate, or those known to be of important clinical significance, were tested in the multivariate model. SPSS software (IBM SPSS Statistics for Windows, version 27, IBM corp., Armonk, NY, USA, 2017) was used for all statistical analyses.
Results
Patient characteristics
In all, 171 MM patients (study cohort) and 64 healthy volunteers (control cohort) were included. Serological tests were performed in all patients and controls. Patients with active myeloma (n = 159) had a median age of 70 (range 38–94), 57% were male, 44% had International Staging System (ISS) II–III and 26% had high‐risk cytogenetics (Table Ⅰ). Median estimated polyclonal immunoglobulin level was 6·54 (IQR 3·82–9·46) g/l. The median time from initiation of an anti‐myeloma therapy to vaccination was 32 months (range: 0–314). Twenty‐one percent (n = 34) were newly diagnosed MM patients (first therapeutic line) and 79% (n = 125) were relapse/refractory patients. Ninety‐two (147/159) percent of these patients were under active therapy at the time of vaccination. At serological testing, 98 (72%) of evaluable actively treated patients had a treatment response of very good partial response (VGPR) or better, 17 (13%) achieved less than partial response (PR). Patients with SMM (n = 12) had a median age of 72 (49–79), six females and six males. Median time from SMM diagnosis to serological testing was 2·3 (range: 0·7–7·3) years, none received anti‐myeloma therapy. The healthy control cohort (n = 64) included 27 males and 37 females; the median age was 67 (range 41–84) years. Pre‐vaccination samples, available for 82 patients, were all seronegative.
Table I.
Patients' characteristics.
| Variable | Active MM (n = 159) | SMM (n = 12) |
|---|---|---|
| Age at vaccination, years; (median, range) | 70 (38–94) | 72 (49–79) |
| Age ≥65 | 94 (59%) | 10 (83%) |
| Gender, female : male | 69 (43%): 90 (57%) | 6 (50%): 6 (50%) |
| ISS (n = 111 evaluable) | ||
| I | 61 (55%) | N/A |
| II | 26 (23%) | N/A |
| III | 24 (21%) | N/A |
| FISH cytogenetics | ||
| Standard risk | 109 (74%) | 6 (75%) |
| High risk | 38 (26%) | 2 (25%) |
| Absolute lymphocyte count, k/μl; median (IQR) | 3·13 (2·11–4) | 3·65 (3·26–4·88) |
| Estimated polyclonal Ig (g/l); median (IQR) | 6·54 (3·82–9·46) | 13·04 (6·4–16·83) |
| IVIG therapy at vaccination time | 26 (16%) | N/A |
| Time since MM treatment start, months; median (range) | 32 (0–314) | N/A |
| Actively treated at time of vaccination | 146 (92%) | N/A |
| Treatment regimen at vaccination, containing: | ||
| IMiD | 90 (57%) | N/A |
| PI | 73 (46%) | N/A |
| DARA | 72 (45%) | N/A |
| IMiD + PI | 31 (20%) | N/A |
| Lines of therapy (median, range) | 2 (1–9) | |
| 0 | 2 (1%) | N/A |
| 1 | 34 (20%) | N/A |
| 2 | 67 (42%) | N/A |
| ≥3 | 58 (37%) | N/A |
| No. anti‐myeloma drugs exposed | ||
| 0–2 | 76 (49%) | N/A |
| 3 | 40 (26%) | N/A |
| ≥4 | 40 (26%) | N/A |
| Prior HSCT | 96 (60%) | N/A |
| Time since HSCT, months; median (IQR) | 36 (20–56) | N/A |
| MM treatment response at vaccination (n = 137 evaluable) | ||
| ≥VGPR | 98 (72%) | N/A |
| ≥PR | 118 (86%) | N/A |
DARA, daratumumab; FISH, fluorescence in situ hybridization; HSCT, haematopoietic stem cell transplantation; IMiD, immunomodulatory drug; ISS, international staging system; IVIG, intravenous immunoglobulin; MM, multiple myeloma; No, number; PI, proteasome inhibitor; PR, partial response; VGPR, very good partial response.
Adverse events
All AEs reported among myeloma patients and healthy controls were grade 1–2 and transient. Fifty‐three (90/161 evaluable) percent of the myeloma patients and fifty‐five (29/53 evaluable) percent of the healthy controls experienced at least one AE related to the vaccination. The most frequent AEs in both cohorts were pain in the injection site and fatigue (Fig 1).
Fig 1.
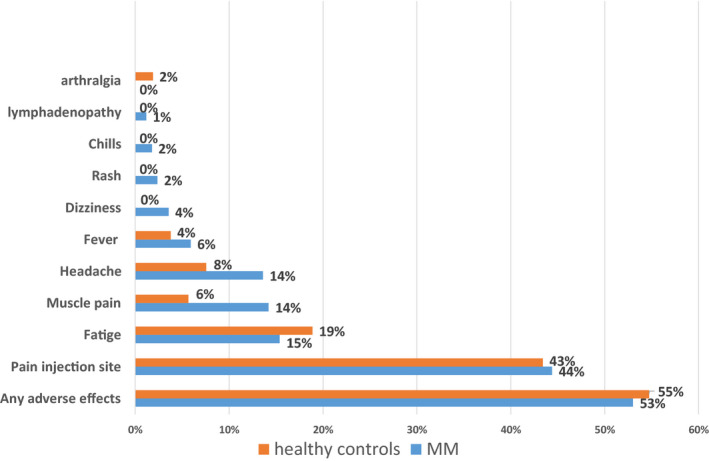
Adverse events to BNT162b2 mRNA COVID‐19 vaccine reported in patients diagnosed with multiple myeloma (MM) versus healthy controls. [Colour figure can be viewed at wileyonlinelibrary.com]
Serologic response to vaccination
All MM patients included in the study cohort either showed negative serological tests in pre‐vaccination samples (n = 82) or tested negative for nucleocapsid post vaccination (n = 89). Within a median follow‐up period of 107 (range 78–121) days from the second COVID‐19 vaccine, none of the MM patients developed COVID19 infection. Humoral response to vaccination was achieved in 133 (78%) out of 171 myeloma patients compared to 63 (98%) out of 64 subjects in the control cohort, P = 0·000132. This difference was driven by the response rates among active MM patients, 121 (76%) out of 159 compared to 63 (98%) out of 64 subjects in the control cohort, P = 0·00062. All SMM patients had a serological response to the vaccine (Table II). Median antibody titers were 91 (0–4 875) U/ml, 822 (5–2 878) U/ml and 992 (0·4–5 000) U/ml in active MM patients, SMM patients and healthy controls, respectively (Fig 2). Median antibody titer among the 121 active MM patients that responded to the vaccine was 218 (1·2–24 875) U/ml.
Table II.
Rate of serological response to BNT162b2 vaccine.
| Cohort | Antibody response n (%) | P value vs. healthy controls | |
|---|---|---|---|
| Positive | Negative | ||
| Healthy controls | 63 (98) | 1 (2) | |
| All myeloma | 133 (78) | 38 (22) | 0·000132 |
| Active myeloma* | 121 (76) | 38 (24) | 0·00062 |
| Smoldering myeloma* | 12 (100) | 0 (0) | 0·722 |
Active myeloma versus smouldering myeloma: P = 0·044.
Fig 2.
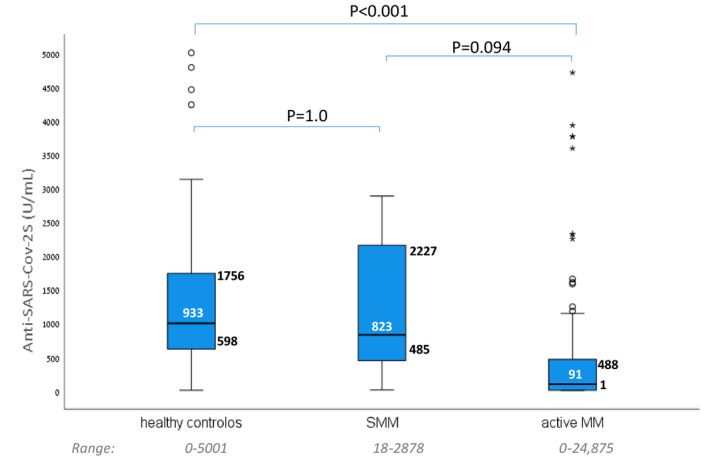
Antibody titers to BNT162b2 mRNA COVID‐19 vaccine measured in patients with active multiple myeloma (aMM) compared with patients with smouldering multiple myeloma (SMM) and healthy controls. [Colour figure can be viewed at wileyonlinelibrary.com]
Univariate analysis of the active myeloma patients, comparing vaccination responders (n = 121) versus non‐responders (n = 38) revealed that older age, high‐risk cytogenetics, lower polyclonal globulins levels, a lower lymphocyte count, advanced treatment line, greater number of novel drugs the patient was exposed up to vaccination and depth of response to anti‐myeloma therapy at vaccination time were associated with a lower response rate to the vaccination (Fig 3). Thus, vaccination response rate was 87% in patients up to 65 years vs 71% in those older than 65 (P = 0·036); 91% patients in first treatment line responded vs 60% patients in third or subsequent lines (P = 0·005); Patients with a deep myeloma response (at least VGPR) had an 81% response rate vs 69% in patients who did not achieve VGPR at the time of vaccination (P = 0·04) and response rate was 84% vs. 52% in patients exposed to 1–2 anti‐MM agents vs. ≥4 (P = 0·002). Gender, time since myeloma diagnosis, ISS, regimen combination, time on daratumumab therapy and time since autologous stem cell transplantation (ASCT) were not related to seropositive response.
Fig 3.
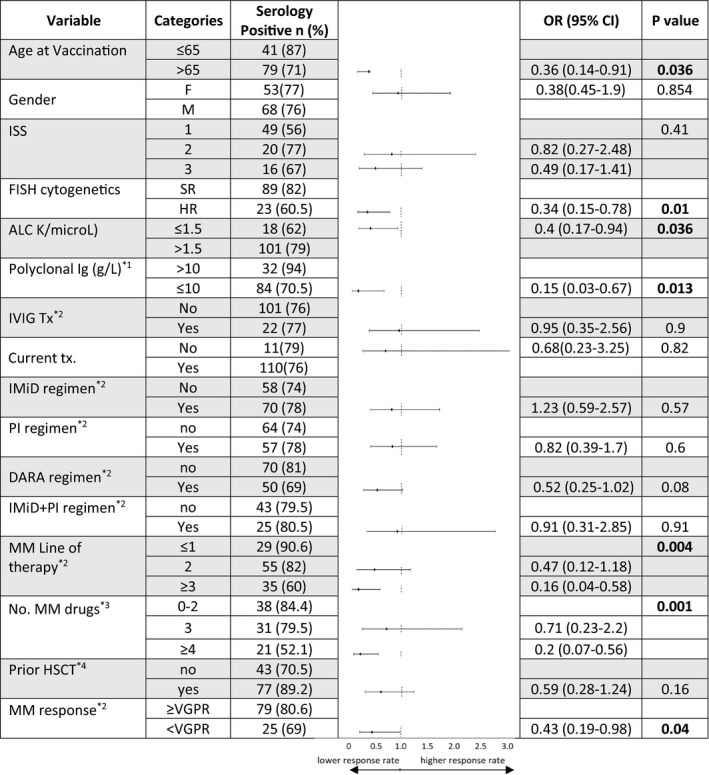
Subgroup analysis of predictors of response according to patients' characteristics. *1, polyclonal immunoglobulin estimated by total globulins minus monoclonal protein; *2, treatment at time of vaccination; *3, number of novel anti‐myeloma drugs exp;osed up to time of vaccination; *4, prior haematopoietic stem cell transplantation. ALC, absolute lymphocyte count; DARA, daratumumab; FISH, fluorescence in situ hybridization; HSCT, haematopoietic stem cell transplantation; Ig, immunoglobulin; IMiD, immunomodulatory; ISS, International Staging System; IVIG, intravenous immunoglobulin; MM,; PI, proteasome inhibitor; PR, partial response; VGPR, very good partial response.
Specifically, seropositive responses by treatment regimens were achieved in 75% (18/24) of daratumumab–lenalidomide–dexamethasone, 85% (17/20) of bortezomib–lenalidomide–dexamethasone, 63% (12/19) of daratumumab–dexamethasone, 100% (15/15) of lenalidomide maintenance, and 83% (10/12) of bortezomib maintenance patients. IMiD‐, PI‐ and IMiD + PI‐containing regimens were not associated with response rate. Daratumumab‐containing regimens trended towards a lower response rate (69% vs. 81%, P = 0·08). Titers measured among the 13 patients who were vaccinated between 3 and 12 months post‐transplant reveal numerically attenuated responses in four out of five patients vaccinated between 3·4 and eight months post ASCT; Figure S1).
Multivariate analysis confirmed older age [odds ratio (OR) 0·927, 95% confidence interval (CI) 0·876–0·981], exposure to a greater number of anti‐myeloma novel drugs (3 vs. 0–2 drugs: OR 0·333, 95% CI 0·074–1·491; ≥4 vs. 0–2 drugs: hazard ratio 0·187, 95% CI 0·046–0·767) were associated with lower humoral response, while increase in polyclonal globulin level (OR 1·3 95% CI 1·107–1·556) was associated with higher humoral response rates (Table III).
Table III.
Multivariate analysis.
| Variable | Odds ratio | 95% confidence interval | P‐value |
|---|---|---|---|
| Age | 0·927 | 0·876–0·981 | 0·009 |
| No. MM drugs exposed | |||
| No. MM drugs exposed*: 0–2 | |||
| No. MM drugs exposed: 3 | 0·333 | 0·074–1·491 | 0·150 |
| No. MM drugs exposed: ≥4 | 0·187 | 0·046–0·767 | 0·020 |
| Polyclonal Ig† | 1·313 | 1·107–1·556 | 0·002 |
Ig, immunoglobulins; MM, multiple myeloma; No, number.
Number of novel anti‐myeloma drugs patient was exposed to up to time of vaccination.
Polyclonal immunoglobulin estimated by total globulins minus monoclonal protein.
Discussion
Our study evaluated the serologic response to two doses of BNT162b2 mRNA COVID19 vaccine in 171 patients diagnosed with MM and compared their responses with those obtained in age‐compatible vaccinated healthy controls. Despite the fact that most (92%) of our patients received an anti‐myeloma therapy at the time of vaccination, response rate approached 80%. These results are highly encouraging, considering the relatively low response rates reported in MM patients that were vaccinated with anti‐influenza 9 , 10 , 11 or pneumococci vaccines 12 , 13 approaching 20%–30% only. These differences in humoral response rates may theoretically reflect variability between the characteristics of patients included these studies to our cohort, yet it is plausible this reflects a true difference in the potency of the vaccine itself. Nevertheless, response to BNT162b2 mRNA COVID19 vaccine appeared to be affected by several factors; elderly patients exhibited lower response rates, attributed to the additive effect of ageing on the immune system. 12 , 14 , 15 , 16 , 17 Immune‐compromised patients in our study cohort, as reflected by low levels of polyclonal globulins (e.g. hypogammaglobulinaemia) and lymphopenia, were found to have lesser humoral responses. Additionally, and in line with prior studies evaluating response to pneumococci and influenza vaccines, 12 , 16 response rates to BNT162b2 mRNA COVID19 vaccine were lower in those who failed to attain a deep response to anti‐myeloma therapy, most probably reflecting the greater immunodeficiency, both humoral and cellular, existing in these ‘non‐responding’ patients. 18 The highly effective anti‐MM agents employed in our patients nowadays, providing deeper and more sustainable disease control compared with those obtained earlier, in the pre‐novel agent era, might also contribute to the remarkable response rate to COVID19 vaccine, 16 compared with that obtained to influenza vaccines, investigated in the pre‐novel or early novel agent era. 9 Heavily pre‐treated patients, reflected by four or more novel anti‐myeloma drugs or third or subsequent treatment line, have also experienced lower response rates, reflecting the exhaustion of their immune system mainly attributed to their progressive/refractory disease 18 , 19 , 20 , 21 but also to the accumulated effect of multiple antimyeloma therapies being given. In line with this observation, response rate in treatment‐naïve, SMM patients was 100%. In consensus with these findings, patients with standard (vs high‐risk) disease also did better; having a relatively preserved immune function, and often being relatively less heavily pretreated.
None of the novel anti‐MM agents being administered was shown to a statistically significant impact on responsiveness to BNT162b2 mRNA COVID19 vaccine. Moreover, response rate in patients treated with lenalidomide maintenance therapy was 100%; indicating an ongoing good disease control in these patients and potentially, a contributory immunomodularory effect of lenalidomide in this setting. 16 , 22 However, daratumumab tended to be associated with reduced response rates; most probably reflecting its plasma cell‐depleting effect, affecting also normal plasma cells and associated with hypogamaglobuloumemia, 4 and also its current employment in patients with advanced, relapsed/refractory disease. Nevertheless, most daratumumab‐treated patients still responded, indicating a preserved capacity of B cells to differentiate into plasma cells, and the recovery of polyclonal IgM levels. 4 Indeed, patients treated with daratumumab were already shown to develop humoral response to pneumococcal vaccines; 4 results which support vaccines administration, including COVID19 vaccine, to all myeloma patients under active therapy, including those being currently treated with daratumumab.
As mentioned earlier, response rate in MM patients was remarkably high, almost approaching that achieved in age‐compatible healthy controls. However, antibody titer levels, often linked with the response degree and durability, 23 appeared to be lower in MM patients compared with their healthy counterparts. There are currently no sufficient data regarding the clinical significance of achieving a high‐ versus a low‐antibody titer following vaccination and the antibody titer cut‐off that predicts an efficient and durable immunity is not yet determined. However, achievement of a higher antibody titer may theoretically predict a longer immunity (as reported for some other vaccines). 10 , 11 , 23 Studies evaluating the significance and the dynamics of antibody titers achieved following vaccination and the role of booster vaccines in MM patients that lose their humeral response are warranted.
Our study has several limitations. Although most of the patients included in our cohort were actively treated, this is a real‐world study in which patients received different treatment regimens, making it difficult to define the exact impact of each treatment regimen on the response to vaccination. Additionally, our study, similar to most prior vaccination studies, focused on humoral response rate and did not assess the cellular response to vaccination. Nevertheless, the achievement of humoral response was shown to be highly important for overcoming or preventing COVID19 infection. 24 Patients who recovered from COVID19 infection attained high anti‐spike antibody levels. 24 Moreover, convalescent plasma obtained from patients that recovered from COVID19 infection was shown to be active in patients infected with COVID19. 25 , 26 Finally, optimality of the timing of vaccination post HSCT could not be evaluated in our cohort as only few patients were vaccinated within six months from HSCT; however it appears that antibody titers increase around eight months post HSCT, suggesting that patients vaccinated sooner may possibly require an additional vaccine dose.
In conclusion, considering the poor outcome of MM patients infected with COVID19, together with the high seropositivity rate to BNT162b2 mRNA COVID19 vaccine, we recommend to vaccinate all MM patients with mRNA anti‐COVID vaccine. Older, hypogammaglobulinaemic and heavily pretreated patients were at greater risk for non‐response. The best timing for vaccination in relation to treatment schedule is not fully elucidated, but it appears that the current IMWG recommendations which were applied in our practice, provide high response rates. Further research and follow‐up are required to investigate the impact of lower titers obtained, as well as the durability and extent of protection from COVID19 infections in MM patients.
Funding information
The study did not receive any external support, it was funded internally by the Hematology division at the Tel Aviv Sourasky Medical Center.
Author contributions
IA, TS, GS, MMo, AA, NL‐S, ST, CP, NB, MMi, YT, TBL, MZ, YH, EL and YCC performed the research. IA and YCC designed the research study. IA, YCC , RB and EL analyzed the data. IA, YCC , RB, TS and EL wrote the paper.
Conflicts of interest
The authors have no relevant disclosures.
Ethics approval
The study was approved and performed in accordance with the International Conference on Harmonization, the Guidelines for Good Clinical Practice, appropriate regulatory requirements and with approval of institutional review boards at the individual enrolling institutions. All patients provided written informed consent before study start.
Supporting information
Fig S1. Antibody titers to BNT162b2 mRNA COVID‐19 vaccine measured in patients who underwent an autologous haematopoietic cell transplantation (HSCT) within 3–12 months prior to vaccination.
Acknowledgements
We thank Mrs Doaa Natour, Mrs Einav Kaufman, Mrs Inna Zetserov and Mrs Maayan Jean for coordinating the study and performing the data management. We thank Ronit Benn‐Izhak for helping in performing the pre‐vaccination serological tests. We thank Gamidor Diagnostics for supplying kits for this study. We thank Dr Tomer Ziv‐Baran for his helpful statistical review.
References
- 1. Susek KH, Gran C, Ljunggren H, Alici E, Nahi H. Outcome of COVID‐19 in multiple myeloma patients in relation to treatment. Eur J Haematol. 2020;105(6):751–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chari A, Samur MK, Martinez‐Lopez J, Cook G, Biran N, Yong K, et al. Clinical features associated with COVID‐19 outcome in multiple myeloma: first results from the International Myeloma Society data set. Blood. 2020;136(26):3033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid‐19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frerichs KA, Bosman PWC, Van Velzen JF, Fraaij PLA, Koopmans MPG, Rimmelzwaan GF, et al. Effect of daratumumab on normal plasma cells, polyclonal immunoglobulin levels, and vaccination responses in extensively pre‐treated multiple myeloma patients. Haematologica. 2020;105:e302–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48. [DOI] [PubMed] [Google Scholar]
- 6. Recommendations for anti‐Covid‐19 vaccination in patients with multiple myeloma (MM) and related conditions, AL amyloidosis and other monoclonal gammopathies of clinical significance.
- 7. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–46. [DOI] [PubMed] [Google Scholar]
- 8. Elecsys® Anti‐SARS‐CoV‐2 S . Package Insert 2020‐09, V1.0; Material Numbers 09289267190 and 09289275190 ‐ Yahoo Search Results [Internet]. https://uk.search.yahoo.com/search?fr=mcafee&type=E211GB885G0&p=Elecsys®+Anti‐SARS‐CoV‐2+S.+Package+Insert+2020‐09%2C+V1.0%3B+Material+Numbers+09289267190+and+09289275190. Accessed 24 Mar 2021.
- 9. Robertson JD, Nagesh K, Jowitt SN, Dougal M, Anderson H, Mutton K, et al. Immunogenicity of vaccination against influenza, Streptococcus pneumoniae and Haemophilus influenzae type B in patients with multiple myeloma. Br J Cancer. 2000;82(7):1261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sanada Y, Yakushijin K, Nomura T, Chayahara N, Toyoda M, Minami Y, et al. A prospective study on the efficacy of two‐dose influenza vaccinations in cancer patients receiving chemotherapy. Jpn J Clin Oncol. 2016;46(5):448–52. [DOI] [PubMed] [Google Scholar]
- 11. Hahn M, Schnitzler P, Schweiger B, Kunz C, Ho AD, Goldschmidt H, et al. Efficacy of single versus boost vaccination against influenza virus in patients with multiple myeloma. Haematologica. 2015;100, e285–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hinge M, Ingels HAS, Slotved HC, Mølle I. Serologic response to a 23‐valent pneumococcal vaccine administered prior to autologous stem cell transplantation in patients with multiple myeloma. APMIS. 2012;120(11):935–40. [DOI] [PubMed] [Google Scholar]
- 13. Mustafa SS, Shah D, Bress J, Jamshed S. Response to PCV13 vaccination in patients with multiple myeloma versus healthy controls. Hum Vaccin Immunother. 2019;15(2):452–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Avivi I, Zisman‐Rozen S, Naor S, Dai I, Benhamou D, Shahaf G, et al. Depletion of B cells rejuvenates the peripheral B‐cell compartment but is insufficient to restore immune competence in aging. Aging Cell. 2019;18(4). /pmc/articles/PMC6612643/. Accessed 24 Mar 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng NY, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine‐specific antibodies. J Clin Invest. 2011;121(8):3109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Branagan AR, Duffy E, Albrecht RA, Cooper DL, Seropian S, Parker TL, et al. Clinical and serologic responses after a two‐dose series of high‐dose influenza vaccine in plasma cell disorders: a prospective, single‐arm trial. Clin Lymphoma, Myeloma Leuk. 2017;17(5):296‐304.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elias R, Hartshorn K, Rahma O, Lin N, Snyder‐Cappione JE. Aging, immune senescence, and immunotherapy: a comprehensive review. Seminars in Oncol. 2018;45:187–200. [DOI] [PubMed] [Google Scholar]
- 18. Torti L, Morelli A, Bacci F, Di Bartolomeo P. Infections and immune system impairment in multiple myeloma: increasing frequency of serious complications in the novel agents era—a retrospective real life analysis. Blood. 2017;130(Suppl 1):1875. [Google Scholar]
- 19. Blimark C, Holmberg E, Mellqvist UH, Landgren O, Bjorkholm M, Hultcrantz M, et al. Multiple myeloma and infections: a population‐based study on 9253 multiple myeloma patients. Haematologica. 2015;100(1):107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karlsson J, Andréasson B, Kondori N, Erman E, Riesbeck K, Hogevik H, et al. Comparative study of immune status to infectious agents in elderly patients with multiple myeloma, Waldenstrom’s macroglobulinemia, and monoclonal gammopathy of undetermined significance. Clin Vaccine Immunol. 2011;18(6):969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pratt G, Goodyear O, Moss P. Immunodeficiency and immunotherapy in multiple myeloma. Br J Haematol. 2007;138:563–79. [DOI] [PubMed] [Google Scholar]
- 22. Noonan K, Rudraraju L, Ferguson A, Emerling A, Pasetti MF, Huff CA, et al. Lenalidomide‐induced immunomodulation in multiple myeloma: impact on vaccines and antitumor responses. Clin Cancer Res. 2012;18(5):1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kakisaka K, Sakai A, Yoshida Y, Miyasaka A, Takahashi F, Sumazaki R, et al. Hepatitis B surface antibody titers at one and two years after hepatitis B virus vaccination in healthy young Japanese adults. Intern Med. 2019;58(16):2349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lau EHY, Tsang OTY, Hui DSC, Kwan MYW, Chan WH, Chiu SS, et al. Neutralizing antibody titres in SARS‐CoV‐2 infections. Nat Commun. 2021;12(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hueso T, Pouderoux C, Péré H, Beaumont AL, Raillon LA, Ader F, et al. Convalescent plasma therapy for B‐cell–depleted patients with protracted COVID‐19. Blood. 2020;136(20):2290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Antibody titers to BNT162b2 mRNA COVID‐19 vaccine measured in patients who underwent an autologous haematopoietic cell transplantation (HSCT) within 3–12 months prior to vaccination.


