Summary
COVID‐19 is associated with high mortality in patients with haematological malignancies (HM) and rate of seroconversion is unknown. The ITA‐HEMA‐COV project (NCT04352556) investigated patterns of seroconversion for SARS‐CoV‐2 IgG in patients with HMs. A total of 237 patients, SARS‐CoV‐2 PCR‐positive with at least one SARS‐CoV‐2 IgG test performed during their care, entered the analysis. Among these, 62 (26·2%) had myeloid, 121 (51·1%) lymphoid and 54 (22·8%) plasma cell neoplasms. Overall, 69% of patients (164 of 237) had detectable IgG SARS‐CoV‐2 serum antibodies. Serologically negative patients (31%, 73 of 237) were evenly distributed across patients with myeloid, lymphoid and plasma cell neoplasms. In the multivariable logistic regression, chemoimmunotherapy [odds ratio (OR), 3·42; 95% confidence interval (CI), 1·04–11·21; P = 0·04] was associated with a lower rate of seroconversion. This effect did not decline after 180 days from treatment withdrawal (OR, 0·35; 95% CI: 0·11–1·13; P = 0·08). This study demonstrates a low rate of seroconversion in HM patients and indicates that treatment‐mediated immune dysfunction is the main driver. As a consequence, we expect a low rate of seroconversion after vaccination and thus we suggest testing the efficacy of seroconversion in HM patients.
Keywords: Covid‐19, SARS‐CoV‐2, leukemia, lymphoma, myeloma
Introduction
COVID‐19, the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has been declared pandemic in March 2020. According to the largest series, its mortality for patients with haematological malignancies (HM) was 37%. 1 , 2 A meta‐analysis including 3 210 HMs reported an overall 34% risk of death. 2 Among 650 patients with multiple myeloma (MM), 3 198 with chronic lymphocytic leukaemia (CLL), 4 177 with non‐Hodgkin lymphomas (NHL), 5 and 318 who received haematopoietic stem cell transplantation (HSCT) 6 case fatality was 33%, 33%, 34%, and 33%, respectively.
Several studies have shown that 95–100% of immunocompetent patients with COVID‐19 have seroconverted about three weeks after symptom onset, 7 , 8 while this information in patients with HM is scant. A low rate (67%) of anti‐SARS‐CoV‐2 IgG development has been described in 21 patients with CLL (67%); 9 a slightly better rate (88%) was found in eight patient with acute myeloid leukaemia. 10
This study was registered in the ClinicalTrials.gov Identifier, NCT04352556. We investigated patterns of seroconversion for SARS‐CoV‐2 IgG in a broad range of patients with HMs. The is a retrospective study and the cohort came from the Italian Hematology Alliance on COVID‐19 (ITA‐HEMA‐COV) project, that already provided information on mortality in HM with COVID‐19. 1 Addressing the rate of humoral immune response to SARS‐CoV‐2 in HM patients who have been exposed to SARS‐CoV‐2 is a critical step to properly inform and develop vaccination strategies and monitoring in this vulnerable patient population. In fact, there is preliminary evidence showing a low rate of seroconversion after vaccines against SARS‐CoV‐2. 11
Methods
To investigate the presence of SARS‐CoV‐2 antibodies, we analyzed serology reports within the ITA‐HEMA‐COV database, starting 23 February 2020, until 17 February 2021 (first and last observed HM patient with COVID‐19). The study was approved by the institutional review board of each centre. A total of 2 463 patients (735 myeloid, 1 214 lymphoid, 514 plasma cell neoplasms) were identified with a real‐time polymerase chain reaction (RT‐PCR) positive for SARS‐CoV‐2. We excluded 577 cases who died without performing serology and 1 649 coming from centres without test availability. Eventually, 237 patients with HM, SARS‐CoV‐2 PCR‐positive and at least one SARS‐CoV‐2 IgG test performed during their standard care, were included. For SARS‐CoV‐2 serological testing we used commercial, widely available high‐throughput immunoassays available at the centres such as the Abbott (SARS‐CoV‐2 nucleocapsid IgG, Chicago, IL, USA), the DiaSorin (SARS‐CoV‐2 Spike‐1 and Spike‐2 IgG, Saluggia, Italy), the Roche [SARS‐CoV‐2 nucleocapsid and Spike protein RBD (receptor‐binding‐domain) IgG, Basel, Switzerland] or the Siemens (Atellica, SARS‐CoV‐2 Spike protein RBD IgG, Munich, Germany) tests. Sensitivity and specificity of these tests was at least 98% in a head‐to‐head comparison study, 12 with some divergent results in seroprevalence studies on cancer patients. 13
Continuous variables are expressed as median, minimum, and maximum values. Categorical variables are presented as frequencies and percentages. Characteristics of the study population were described for patients with a positive result to the SARS‐CoV‐2 IgG test and for negative ones.
Univariate logistic regression models were applied to study the association between the absence of seroconversion and the following variables: age, sex, comorbidities, HM type, COVID‐19 disease severity, hospitalization, last therapy applied and time to COVID‐19, active therapy (time from last therapy to COVID‐19 within three months) and time from COVID‐19 to first serology evaluation. A multivariable logistic regression model, adjusted for the time from COVID‐19 to first serology evaluation, was implemented using parameters of interest. In addition, we did this latter analysis on the subgroup on active therapy. The odds ratio (OR) together with the 95% confidence interval (CI) and Wald chi‐square P‐value were calculated. All statistical analyses were done with SAS version 9.4 and R statistical software version 3.2.0 (Cary, NC, USA).
Results and discussion
A total of 237 patients were evaluated: 62 (26·2%) had myeloid neoplasms, 121 (51·1%) lymphoid neoplasms, and 54 (22·8%) had plasma cell neoplasms. As the distribution of HMs was superimposable to that of the whole series of 2 463 cases (P = 0·40), findings obtained here can be representative of a real‐life situation. Median age was 61 years (range, 19–91), male/female ratio was 1·14, with most patients having symptomatic COVID‐19 (78·5%). A detailed description of patients and HMs is available in Table I.
Table I.
Characteristics of 237 patients with haematological malignancies, exposed to SARS‐CoV‐2 infection according to IgG serology response.
| Positive serology n = 164 | Negative serology n = 73 | All patients n = 237 | |
|---|---|---|---|
| Age, years | |||
| Median [min–max] | 61 [19–91] | 61 [22–88] | 61 [19–91] |
| Sex, n (%) | |||
| Female | 75 (45·73) | 36 (49·32) | 111 (46·84) |
| Charlson comorbidity Index | |||
| Median [min–max] | 3 [0–14] | 4 [0–13] | 3 [0–14] |
| Coexisting condition, n (%) | |||
| Heart disease | 16 (9·76) | 9 (12·33) | 25 (10·55) |
| Pulmonary disease | 15 (9·15) | 6 (8·22) | 21 (8·86) |
| Vascular disease | 19 (11·59) | 4 (5·48) | 23 (9·70) |
| Connective tissue disease | 5 (3·05) | 3 (4·11) | 8 (3·38) |
| Liver disease | 3 (1·83) | 3 (4·11) | 6 (2·53) |
| Kidney disease | 5 (3·05) | 3 (4·11) | 8 (3·38) |
| Diabetes | 5 (6·85) | 13 (7·93) | 18 (7·59) |
| Non‐haematological cancer | 13 (7·93) | 7 (9·59) | 20 (8·44) |
| Type of haematological malignancies, n (%) | 47 (28·66) | 15 (20·55) | 62 (26·16) |
| Myeloid neoplasms | 14 (8·54) | 4 (5·48) | 18 (7·59) |
| Myeloproliferative neoplasm | 9 (5·49) | 0 (0·00) | 9 (3·80) |
| Myelodysplastic syndrome | 21 (12·80) | 10 (13·70) | 31 (13·08) |
| Acute myeloid leukaemia | 3 (1·83) | 1 (1·37) | 4 (1·69) |
| Acute lymphoblastic leukaemia | 4 (2·44) | 2 (2·74) | 6 (2·53) |
| Lymphomas | 71 (43·29) | 44 (60·27) | 115 (48·52) |
| Hodgkin lymphomas | 14 (8·5%) | 1 (1·4) | 15 (6·3) |
| Aggressive non‐Hodgkin lymphomas | 21 (12·80) | 24 (32·88) | 45 (18·99) |
| Indolent non‐Hodgkin lymphomas | 14 (8·54) | 15 (20·55) | 29 (12·24) |
| Chronic lymphoproliferative disorders | 21 (12·80) | 4 (5·48) | 25 (10·55) |
| Plasma cell neoplasms | 42 (25·61) | 12 (16·44) | 54 (22·78) |
| Plasma cell myeloma | 33 (20·12) | 11 (15·07) | 44 (18·57) |
| Symptomatic, n (%) | |||
| Yes | 52 (71·23) | 134 (81·71) | 186 (78·48) |
| COVID‐19 disease severity, n (%) | |||
| Mild | 78 (47·56) | 32 (43·84) | 110 (46·41) |
| Severe | 51 (31·10) | 19 (26·03) | 70 (29·54) |
| Critical | 5 (3·05) | 1 (1·37) | 6 (2·53) |
| Asymptomatic | 30 (18·29) | 21 (28·77) | 51 (21·52) |
| Last therapy type, n (%) | |||
| Chemotherapy | 47 (28·66) | 15 (20·55) | 62 (26·16) |
| Immunotherapy | 7 (4·27) | 8 (10·96) | 15 (6·33) |
| Chemoimmunotherapy | 28 (17·07) | 26 (35·62) | 54 (22·78) |
| Targeted therapy | 33 (20·12) | 13 (17·81) | 46 (19·41) |
| Autologous stem cell transplant | 10 (6·10) | 1 (1·37) | 11 (4·64) |
| Allogeneic stem cell transplant | 6 (3·66) | 3 (4·11) | 9 (3·80) |
| Active therapy, n (%) | 85 (51·83) | 48 (65·75) | 133 (56·12) |
| Hospitalized patients, n (%) | 44 (60·27) | 90 (54·88) | 134 (56·54) |
| Time from COVID‐19 to first serology, days | |||
| Median [min–max] | 34 [2–275] | 46 [7–383] | 38 [2–383] |
| Time from last therapy to COVID‐19, days | |||
| Median [min–max] | 49 [1–3 566] | 27 [1–2 628] | 42 [1–3 566] |
Most patients (83·1%, 197 of 237) received HM‐directed therapies before COVID‐19. In detail, it was chemotherapy in 62 (26·2%), chemoimmunotherapy in 54 (22·8%), immunotherapy only in 15 (6·3%), targeted therapies in 46 (19·4%), autologous and allogeneic HSCT in 11 (4·6%) and 9 (3·8%) respectively. Among these, 133 (56%) were on active therapy at the time of COVID‐19.
Overall, 69% of patients (164 of 237) tested positive for IgG SARS‐CoV‐2 antibodies and 31% (73 of 237) negative. This indicates that one‐third of patients with HM are at high risk of failing seroconversion which, contrarily, approaches 100% in the general population. 7 The median time from COVID‐19 to first SARS‐CoV‐2 IgG serology evaluation was 38 days (range, 2–383), longer than that reported in the immunocompetent population. 7 In fact, viral shedding is persistent in immunocompromised patients 14 and, as a consequence, seroconversion comes later. Testing frequencies in IgG‐positive and IgG‐negative cases is reported in Fig 1A: finding patients without seroconversion tested later (OR, 1·01; 95% CI, 1·00–1·01; P = 0·02) corroborates these results. Figure S1 reports the timing of seroconversion in seven patients who resulted negative in an early SARS‐CoV‐2 IgG evaluation and subsequently became positive. This suggests that measuring serological response too soon after SARS‐CoV‐2 infection in HM might lead to an incorrect evaluation of immune response. This probably explains the very low rate of seroconversion reported in 20 HMs (16·6%), tested after 12 days from COVID‐19. 15
Fig 1.
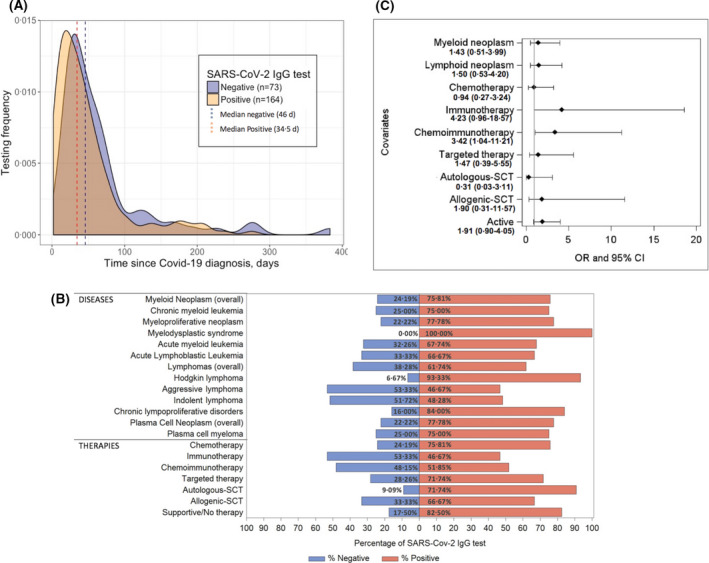
(A) Timing and antibody testing after a positive real‐time polymerase chain reaction (RT‐PCR) test. The testing frequency of patients evaluated showed that those resulting IgG‐negative have been tested later (46 days), than positive (34 days) and some patients were IgG‐positive long time after COVID‐19 diagnosis. (B) Rate of seroconversion according to haematological malignancy and treatment. Serological non‐responders were spread evenly across patients with myeloid, lymphoid and plasma cell neoplasms. Percentages of serological non‐responders and responders are reported. (C) Results of multivariable analysis for identification of factors affecting negative seroconversion for SARS‐CoV‐2 in patients with haematological malignancies. Multivariable logistic regression revealed that chemoimmunotherapy (P = 0·04) and immunotherapy (P = 0·06) were associated with a significant and borderline lower rate of seroconversion, respectively. OR values with 95% CI are reported. Plasma cell neoplasms and no therapy represent the reference for the analysis. SCT, stem cell transplantation; OR, odds ratio; CI, confidence interval.
Figure 1B shows the rate of seroconversion per HM type and specific treatments (more details in Figure S2). We found that serological non‐responders were spread evenly across patients with lymphomas, MM and myeloid neoplasms. However, the univariate logistic regression (Table SI) showed that patients with absent seroconversion more likely had lymphoid neoplasms (OR, 2·15; 95% CI, 1·03–4·50; P = 0·04), received chemoimmunotherapy (OR, 6·68, 95% CI, 2·22–20·08; P = 0·0007), immunotherapy (OR, 4·71; 95% CI, 1·07–20·79; P = 0·04), or were on active therapy at the time of COVID‐19 (OR, 1·84; 95% CI, 1·03–3·27, P = 0·04). To note, 27 out of 57 (47·4%) patients receiving immunotherapy/chemoimmunotherapy within one year from COVID‐19 resulted positive.
In the multivariable logistic regression on the whole cohort, chemoimmunotherapy (OR, 3·42; 95% CI, 1·04–11·21; P = 0.04) was associated with a significantly lower rate of seroconversion, while immunotherapy (OR, 4·23; 95% CI, 0·96–18·57; P = 0·06) was of borderline significance (Fig 1C).
When analyzing patients on active therapy, chemoimmunotherapy (OR, 8·02; 95% CI, 2·25–28·56; P = 0·001), immunotherapy (OR, 5·89; 95% CI, 1·19–29·26; P = 0·03) and targeted therapy (OR, 3·61; 95% CI, 1·07–12·18; P = 0·04) were independent predictors of lower seroconversion (Figure S3). Both analyses maintained their value even adjusting for the time from COVID‐19 to serology. In addition, the effect of chemoimmunotherapy and immunotherapy did not decline after 180 days from treatment withdrawal (OR, 0·35; 95% CI, 0·11–1·13; P = 0·08).
Conclusions
In conclusion, this study shows that COVID‐19 can elicit antibodies to SARS‐CoV‐2 in 69% of patients with HM. The mechanism for this reduced seroconversion is not fully known, but our study indicates that treatment‐mediated immune dysfunction can play an important role. Limitations of the study come from the real‐world data collection, i.e. various SARS‐CoV‐2 lgG tests used with different epitopes and different thresholds for seroconversion, and various time points for blood sampling. Then, it should be noted that the cellular response to the SARS‐CoV‐2 may also have a protective role in HM patients who have recovered from COVID‐19 but failed humoral response. We expect a lower rate of seroconversion after vaccination and thus HM patients should be tested for seroconversion and should maintain all the protective measures. Our data further highlight the importance of vaccination for caregivers and the need for effective infection treatment. HM can potentially become a reservoir for viral genetic variants.
List of ITA‐HEMA‐COV investigators
Francesco Passamonti,1,2 Alessandra Romano,3 Marco Salvini,2 Francesco Merli,4 Matteo Giovanni Della Porta,5 Riccardo Bruna,6 Elisa Coviello,7 Ilaria Romano,8 Roberto Cairoli,9 Roberto Lemoli,10 Francesca Farina,11 Adriano Venditti,12 Alessandro Busca,13 Marco Ladetto,14 Massimo Massaia,15 Antonio Pinto,16 Luca Arcaini,17 Agostino Tafuri,18 Francesco Marchesi,19 Nicola Fracchiolla,20 Monica Bocchia,21 Daniele Armiento,22 Anna Candoni,23 Mauro Krampera,24 Mario Luppi,25 Valeria Cardinali26 Sara Galimberti,27 Chiara Cattaneo,28 Elettra Ortu La Barbera,29 Roberto Mina,30 Francesco Lanza,31 Giuseppe Visani,32 Pellegrino Musto,33 Luigi Petrucci,34 Francesco Zaja,35 Enrico Derenzini,36 Monia Marchetti,37 Anna M. Scattolin,38 Alessandro Corso,39 Patrizia Tosi,40 Filippo Gherlinzoni,41 Carlo G. Passerini,42 Michele Cavo,43 Carmen Fava,44 Mauro Turrini,45 Carlo Visco,24 Paolo Antonio Grossi,1,2 Lorenza Bertù,2 Livio Pagano,46 Patrizia Zappasodi17, Michele Merli,2 Barbara Mora,1,2 Alessandro M. Vannucchi8 and Paolo Corradini.47
Affiliation of ITA‐HEMA‐COV investigators
1Dipartimento di Medicina e Chirurgia, Università dell’Insubria, Italy; 2ASST Sette Laghi, Ospedale di Circolo of Varese, Italy; 3Università degli Studi di Catania, Catania, Italy; 4Hematology, AUSL‐IRCCS, Reggio Emilia, Italy; 5Humanitas Clinical and Research Hospital – IRCCS and Department of Biomedical Sciences, Humanitas University, Milan, Italy; 6Department of Translational Medicine, University of Eastern Piedmont and Ospedale Maggiore della Carità, Novara, Italy; 7Ospedale Policlinico San Martino‐IRCCS, Genoa, Italy; 8University of Florence and AOU Careggi, Florence , Italy; 9ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy; 10University of Genoa, Genoa, Italy; 11San Raffaele, Scientific Institute, Milan, Italy; 12Ematologia, Dipartimento di Biomedicina e Prevenzione, Università Tor Vergata, Rome, Italy; 13Stem Cell Transplant Center, AOU Citta' della Salute e della Scienza of Turin, Italy; 14Dip di Medicina Traslazionale, Università del Piemonte Orientale ed AO SS Antonio e Biagio e Cesare Arrigo, Alessandria, Italy; 15Santa Croce Hospital, Cuneo, Italy; 16Istituto Nazionale Tumori IRCCS “Fondazione G. Pascale”, Naples, Italy; 17Division of Hematology, Fondazione IRCCS Policlinico San Matteo & Department of Molecular Medicine, University of Pavia, Italy; 18University Hospital Sant'Andrea, Department of Clinical and Molecular Medicine, Sapienza, Univ. of Rome, Rome, Italy; 19Hematology and Stem Cell Transplant Unit, IRCCS Regina Elena National Cancer Institute, Rome, Italy; 20Fondazione IRCCS Ca' Granda ‐ Ospedale Maggiore Policlinico, Milan, Italy; 21Azienda Ospedaliera Universitaria Senese, University of Siena, Italy; 22Unit of Hematology, Stem Cell Transplantation, University Campus Bio‐Medico, Rome, Italy; 23Division of Hematology, University Hospital of Udine ‐ASUFC, Udine, Italy; 24Department of Medicine, University of Verona, Italy; 25Department of Medical and Surgical Sciences, University of Modena and Reggio Emilia, Modena, Italy; 26University of Perugia, Italy; 27Università di Pisa, Pisa, Italy; 28ASST‐Spedali Civili, Brescia, Italy; 29Ospedale Santa Maria Goretti, Latina, Italy; 30Università di Torino, Azienda Ospedaliera Città della Salute e della Scienza, Turin, Italy; 31Santa Maria delle Croci, Ravenna, Italy; 32Azienda Ospedaliera Ospedali Riuniti Marche Nord, Pesaro, Italy; 33Department of Emergency and Organ Transplantation,"Aldo Moro" University School of Medicine and Unit of Hematology and Stem Cell Transplantation, AOU Consorziale Policlinico, Bari, Italy; 34Division of Hematology Department of Translational and Precison Medicine, Sapienza University of Rome, Italy; 35Università di Trieste, Trieste, Italy; 36Oncohematology Division, IEO European Institute of Oncology IRCCS, Milan, Italy; Department of Health Sciences, University of Milan, Italy; 37Azienda Ospedaliera SS Antonio e Biagio e Cesare Arrigo, Alessandria, Italy; 38Ospedale dell’Angelo di Mestre, Mestre, Italy; 39ASST Ovest Milanese, Legnano, Italy; 40Ospedale degli Infermi di Rimini, Rimini, Italy; 41Ospedale Ca' Foncello, Vicenza, Italy; 42Università degli Studi di Milano‐Bicocca, Italy; 43IRCCS Azienda Ospedaliero‐Universitaria, Bologna, Italy e Istituto di Ematologia “Seràgnoli”, Dipartimento di Medicina Specialistica, Diagnostica e Sperimentale, Università degli Studi, Bologna, Italy; 44University of Turin, Turin, Italy; 45Ospedale Valduce, Como, Italy; 46University Cattolica del Sacro Cuore, Rome, Italy; 47University of Milan & Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Funding information
The study has been supported by the charity AIL (Associazione italiana contro le leucemie‐linfomi e mieloma) Onlus of Varese, by grants from the Ministero della Salute, Rome, Italy (Finalizzata 2018, NET‐2018‐12365935, Personalized medicine program on myeloid neoplasms: characterization of the patient's genome for clinical decision making and systematic collection of real world data to improve quality of health care), by grants from the Ministero dell’Istruzione, dell’Università e della Ricerca, Roma, Italy (PRIN 2017, 2017WXR7ZT; Myeloid Neoplasms: an integrated clinical, molecular and therapeutic approach) and by grants from Fondazione Matarelli, Milan.
Author contributions
FP designed and performed the research, analyzed data, and wrote the paper; PC designed the research, analyzed data, and wrote the paper; MS and PAG designed the research; LB did statistical analysis; AR, FM, MGD, RB, EA, IR, RC, RL, FF, AV, AB, ML, MM, AP and LA provided and analyzed data.
Conflict of interest
The authors have no conflicts of interest to disclose.
Supporting information
Data S1. Supplemental data.
Table SI. Results of univariate logistic regression for identification of factors affecting negative seroconversion for SARS‐CoV‐2 in haematological malignancies.
Fig S1. Description of seroconversion time period in seven patients with haematological malignancies.
Fig S2. Rate of seroconversion in haematological malignancies according to detailed treatments.
Fig S3. Results of multivariable analysis for identification of factors affecting negative seroconversion for SARS‐CoV‐2 in patients with haematological malignancies on active therapy.
Acknowledgements
We thank Dr Roberta Mattarucchi and Dr Alessia Ingrassia from the Clinical Trial Center of the A.S.S.T. and Sette Laghi of Varese for managing the study protocol and procedures across many Institutional Review Boards. The list of ITA‐HEMA‐COV members is reported below: all provided and analyzed data. Open Access Funding provided by Universita degli Studi dell'Insubria within the CRUI‐CARE Agreement. [Correction added on 26 May 2022, after first online publication: CRUI funding statement has been added.]
*A list of the ITA‐HEMA‐COV Investigators is available after the Acknowledgement section.
Contributor Information
Francesco Passamonti, Email: francesco.passamonti@uninsubria.it.
the ITA-HEMA-COV Investigators*:
Enrico Derenzini, Monia Marchetti, Anna M. Scattolin, Alessandro Corso, Patrizia Tosi, Filippo Gherlinzoni, Carlo Gambacorti Passerini, Michele Cavo, Carmen Fava, Mauro Turrini, Carlo Visco, Patrizia Zappasodi, Michele Merli, Barbara Mora, and Alessandro M. Vannucchi
References
- 1. Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, et al. Clinical characteristics and risk factors associated with COVID‐19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7(10):e737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, et al. Outcomes of patients with hematologic malignancies and COVID‐19: a systematic review and meta‐analysis of 3377 patients. Blood. 2020;136(25):2881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chari A, Samur MK, Martinez‐Lopez J, Cook G, Biran N, Yong K, et al. Clinical features associated with COVID‐19 outcome in multiple myeloma: first results from the International Myeloma Society data set. Blood. 2020;136(26):3033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mato AR, Roeker LE, Lamanna N, Allan JN, Leslie L, Pagel JM, et al. Outcomes of COVID‐19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Regalado‐Artamendi I, Jiménez‐Ubieto A, Hernández‐Rivas JÁ, Navarro B, Núñez L, Alaez C, et al. Risk factors and mortality of COVID‐19 in patients with lymphoma: a multicenter study. Hemasphere. 2021;5(3):e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma A, Bhatt NS, St Martin A, Abid MB, Bloomquist J, Chemaly RF, et al. Clinical characteristics and outcomes of COVID‐19 in haematopoietic stem‐cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8(3):e185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Long Q‐X, Liu B‐Z, Deng H‐J, Wu G‐C, Deng K, Chen Y‐K, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med. 2020;26(6):845–8. [DOI] [PubMed] [Google Scholar]
- 8. Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody responses to SARS‐CoV‐2 in patients with novel coronavirus disease 2019. Clin Infect Dis. 2020;71(16):2027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roeker LE, Knorr DA, Pessin MS, Ramanathan LV, Thompson MC, Leslie LA, et al. Anti‐SARS‐CoV‐2 antibody response in patients with chronic lymphocytic leukemia. Leukemia. 2020;34(11):3047–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Nions J, Muir L, Zheng J, Rees‐Spear C, Rosa A, Roustan C, et al. SARS‐CoV‐2 antibody responses in patients with acute leukaemia. Leukemia. 2021;35(1):289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ainsworth M, Andersson M, Auckland K, Baillie JK, Barnes E, Beer S, et al. Performance characteristics of five immunoassays for SARS‐CoV‐2: a head‐to‐head benchmark comparison. Lancet Infect Dis. 2020;20(12):1390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Dam P, Huizing M, Roelant E, Hotterbeekx AN, De Winter FHR, Kumar‐Singh S, et al. Immunoglobin G/total antibody testing for SARS‐CoV‐2: a prospective cohort study of ambulatory patients and health care workers in two Belgian oncology units comparing three commercial tests. Eur J Cancer. 2021;148:328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aydillo T, Gonzalez‐Reiche AS, Aslam S, van de Guchte A, Khan Z, Obla A, et al. Shedding of viable SARS‐CoV‐2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383(26):2586–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bird PW, Badhwar V, Kennedy B, Ladani S, Tang JW. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) seroconversion in hematology‐oncology patients. J Med Virol. 2021;93(7):4585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental data.
Table SI. Results of univariate logistic regression for identification of factors affecting negative seroconversion for SARS‐CoV‐2 in haematological malignancies.
Fig S1. Description of seroconversion time period in seven patients with haematological malignancies.
Fig S2. Rate of seroconversion in haematological malignancies according to detailed treatments.
Fig S3. Results of multivariable analysis for identification of factors affecting negative seroconversion for SARS‐CoV‐2 in patients with haematological malignancies on active therapy.


