Dear Editor,
We read the letter by Cohen et al. 1 related to leukocytoclastic vasculitis (LCV) after messenger ribonucleic acid (mRNA) COVID‐19 vaccine application with great interest. DNA‐based/RNA‐based vaccines, non‐replicating viral vector vaccines, and inactivated vaccines are now being administered to prevent COVID‐19. In January 2021, Turkey started a vaccination program in which two doses of inactivated vaccine are applied one month apart. We would like to share our clinical experience with a similar patient who developed LCV with IgA deposition after the first dose of inactivated COVID‐19 vaccine.
An otherwise healthy 33‐year‐old man was seen in our Dermatology Outpatient Clinic due to the emergence of widespread violaceous eruption which started three days after the first dose of inactivated COVID‐19 vaccine application. Three months ago, he had mildly symptomatic COVID‐19 for which he was treated with oral favipiravir for five days. He reported to have no recent history of infection, fever, cough, arthralgia, or dyspnea. Dermatologic examination revealed erythematous macules and palpable papules on the legs, forearms, and right belly (Fig. 1a‐c). At that time, two punch biopsies were taken from the right forearm for histopathological examination and direct immunofluorescence (DIF) analysis with a pre‐diagnosis of LCV. Histopathological examination showed hyperkeratosis in the epidermis, perivascular mixed inflammation, and erythrocyte extravasation in the dermis (Fig. 2a). DIF analysis revealed IgA deposition within small vessel walls (Fig. 2b). Five days later, his cutaneous lesions had turned into vivid purpuric palpable papules (Fig. 1d‐e). He did not have any known rheumatologic or dermatologic disorder; complete blood count, liver/kidney function tests, urinalysis, chest x‐ray, and stool guaiac test were all within normal limits. Rheumatologic markers were negative. He was diagnosed with IgA vasculitis without systemic involvement by clinicopathological correlation. Topical mometasone furoate was prescribed twice daily with partial resolution.
Figure 1.
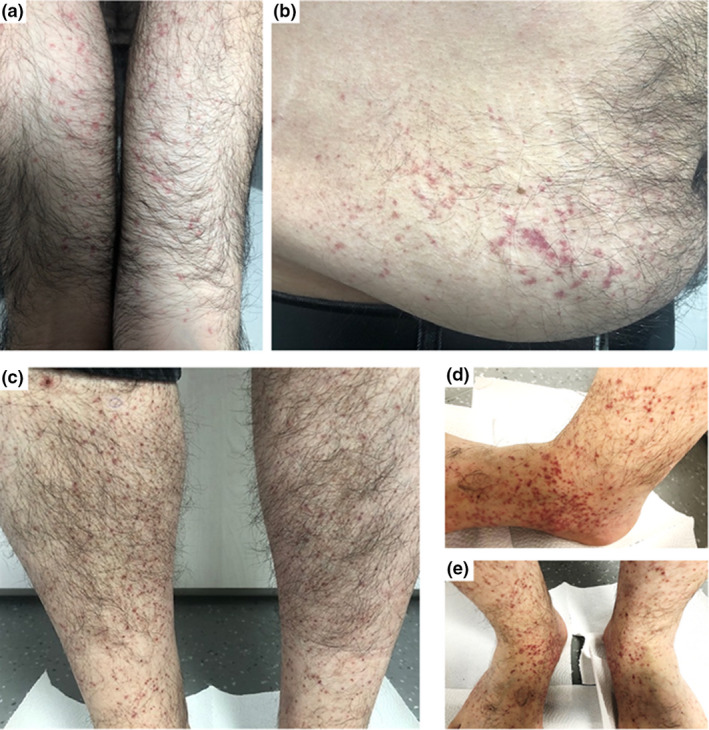
The first dermatologic examination showed purpuric‐erythematous macules prominent on the flexor forearms (a), right belly (b), and purpuric papules involving both legs (c). Five days later, palpable‐violaceous papules were more prominent on the ankles and feet (d,e)
Figure 2.
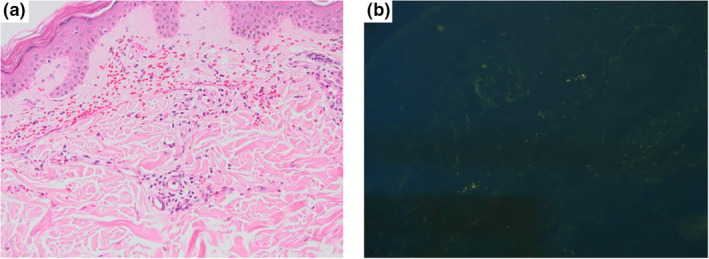
(a) Perivascular mixed inflammation and erythrocyte extravasation in the dermis, no vasculitis was present (hematoxylin and eosin, x200). (b) Direct immunofluorescence (DIF) analysis showed IgA deposition within the superficial dermal vessels (DIF, ×200)
COVID‐19 has been associated with various cutaneous manifestations. 2 Livedoid and necrotic lesions which most probably result from alterations in the coagulation cascade and increased tendency to thrombosis are believed to be associated with older age and severe disease. 2 On the other hand, skin vasculitis presenting with typical cutaneous lesions may be observed during the course of both mild and fulminant COVID‐19 disease. 2 SARS‐CoV‐2 directly invades the endothelial cells and causes hyperinflammatory response which most possibly results in immune complex deposition causing vasculitis. 1 Since our patient had COVID‐19 previously, he might have developed SARS‐CoV‐2 IgA antibodies which enhanced the development of cutaneous purpuric dermatitis with immune complex deposition after COVID‐19 vaccination. Even though similar to the case reported by Cohen et al 1 , we were not able to show fibrin deposition within small vessel walls, which is a characteristic feature of LCV, our patient’s cutaneous lesions were clinically compatible with LCV. We believe that skin biopsies might have been taken during the very early phase of LCV during which fibrin deposition and IgA deposition within vessels were not fully developed and detectable as suggested in the literature. 3 Five days after his initial presentation, our patient’s clinical picture was quite typical of LCV with palpable and violaceous papules. Different types of vasculitis induced by inactivated influenza, human papillomavirus, hepatitis A/B, and rotavirus vaccinations have been reported in the literature. 4 SARS‐CoV‐2 antigens and vaccine proteins share structural similarities, so we propose that the vaccine might have induced the same autoimmune mechanisms with the SARS‐CoV‐2 virus resulting in the activation of autoreactive B/T cells, antibody formation, and immune complex deposition within small vessels. 4 , 5
All in all, we would like to point out a possible relationship between the development of IgA vasculitis and COVID‐19 vaccination to raise awareness of cutaneous side effects of inactivated COVID‐19 vaccine.
Conflict of interest: None.
Funding source: None.
References
- 1. Cohen SR, Prussick L, Kahn JS, et al. Leukocytoclastic vasculitis flare following the COVID‐19 vaccine. Int J Dermatol. 2021. 10.1111/ijd.15623. Epub ahead of print. PMID: 33928638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020; 183: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tirumalae R. Histopathologic approach to cutaneous vasculitis. Indian J Dermatopathol Diagn Dermatol 2014; 1: 2–6. [Google Scholar]
- 4. Bonetto C, Trotta F, Felicetti P, et al. Vasculitis as an adverse event following immunization ‐ Systematic literature review. Vaccine 2016; 34: 6641–6651. [DOI] [PubMed] [Google Scholar]
- 5. Duggal T, Segal P, Shah M, et al. Antineutrophil cytoplasmic antibody vasculitis associated with influenza vaccination. Am J Nephrol 2013; 38: 174–178. [DOI] [PubMed] [Google Scholar]


