Abstract
Recent evidence indicates that the transcription factor NF-κB is a major effector of inducible antiapoptotic mechanisms. For example, it was shown that NF-κB activation suppresses the activation of caspase 8, the apical caspase in tumor necrosis factor (TNF) receptor family signaling cascades, through the transcriptional regulation of certain TRAF and IAP proteins. However, it was unknown whether NF-κB controls other key regulatory mechanisms in apoptosis. Here we show that NF-κB activation suppresses mitochondrial release of cytochrome c through the activation of the Bcl-2 family member A1/Bfl-1. The restoration of A1 in NF-κB null cells diminished TNF-induced apoptosis by reducing the release of proapoptotic cytochrome c from mitochondria. In addition, A1 potently inhibited etoposide-induced apoptosis by inhibiting the release of cytochrome c and by blocking caspase 3 activation. Our findings demonstrate that A1 is an important antiapoptotic gene controlled by NF-κB and establish that the prosurvival function of NF-κB can be manifested at multiple levels.
Programmed cell death (or apoptosis), which is characterized by condensation of the nucleus, specific protein degradation, and DNA fragmentation, is a fundamentally important biological process that is required to maintain the integrity and homeostasis of multicellular organisms (reviewed in references 1, 2, 10, 44, and 53). Cysteine proteases (renamed caspases) related to the Caenorhabditis elegans protein Ced-3 and the mammalian homolog interleukin-1β-converting enzyme have been identified as critical components of several apoptotic pathways (reviewed in references 41, 48, and 54). Studies of knockout mice demonstrated that caspase 8 is required for tumor necrosis factor (TNF)- and Fas-mediated apoptosis but that caspase 8 knockout cells remained sensitive to DNA damage inducers such as UV irradiation and etoposide (56). In contrast, caspase 9 −/− mouse fibroblasts were resistant to stress-induced apoptosis (26, 32). Therefore, it is likely that there are at least two primary pathways to induce apoptosis, with the stress induction pathway being caspase 8 independent.
One apoptosis pathway is controlled through “death receptors” such as TNF receptor 1 (TNFR1) which transduce death signal by recruiting death domain-containing proteins which activate initiating caspases by ligand-induced oligomerization. Thus, TNF engagement of TNFR1 leads to the recruitment of TRADD (TNFR1-associated death domain protein) and RIP (receptor-interacting protein) to the receptor complex (reviewed in references 2 and 4). TRADD interacts with FADD (Fas-associated death domain protein) to initiate the death pathway and recruits several proteins such as TRAF1, TRAF2, and RIP to transduce TNF signaling pathways such as activation of NF-κB (38). FADD recruits and activates caspase 8 at the death-inducing signaling complex. The active caspase 8 is released from the complex to activate the downstream effector caspases directly or indirectly (2, 4). Recently, several groups have demonstrated that caspase 8 cleaves Bid, a Bcl-2 family member, and that the cleaved Bid translocates to mitochondria to induce the release of cytochrome c to initiate apoptosis (24, 33, 39). The second pathway involved in initiating apoptosis is activated by stress inducers such as the chemotherapeutic drug etoposide or ionizing radiation. These inducers damage mitochondria by an unknown mechanism to lead to the release of cytochrome c from mitochondria into the cytosol (14, 23, 34, 52, 63, 64). Cytochrome c along with ATP and Apaf-1, a mammalian homolog of Ced-4, recruits and processes procaspase 9 (34). The active caspase 9 activates the effector caspases such as caspase 3 to induce apoptosis (34, 36, 67).
Extensive studies have demonstrated that Bcl-2 family proteins can positively and negatively regulate apoptosis (reviewed in references 1, 10, 44, 45, and 53). Intriguingly, the Bcl-2 family possesses antiapoptotic (Bcl-2, Bcl-xL, Bcl-W, Bag-1, Mcl-1, and A1/Bfl-1) as well as proapoptotic (Bad, Bax, Bak, Bcl-xs, Bid, Bik, and Hrk) molecules (10, 44). Both the balance and interaction between Bcl-2 gene family and posttranslational modifications of Bcl-2-related proteins have been demonstrated to play an important role in regulating cell survival and death (10, 44). The ratio of anti- and proapoptotic molecules such as Bcl-2 and Bax determines the response to a death signal. Bcl-2 and Bcl-xL have been shown to form membrane pores involved in the homeostasis of cell organelles, inhibiting the mitochondrial permeability transition and cytochrome c release, functioning to block apoptosis (reviewed in references 1, 10, 44, 45, and 53). However, precluding cytochrome c release is unlikely to be the sole function of Bcl-2 and Bcl-xL since Bcl-2 and Bcl-xL have been found to block apoptotic mechanisms downstream of cytochrome c release (7, 27, 43, 46).
NF-κB, originally identified and named for its role in the regulation of immunoglobulin kappa-chain gene expression in B cells, is a dimer composed of p50 (NF-κB1), p52 (NF-κB2), c-Rel, RelB, or p65/RelA subunits. Classic NF-κB is described as the p50-p65 heterodimer which is typically found sequestered in the cytoplasm by the IκB group of inhibitory proteins. The nuclear translocation of NF-κB occurs rapidly following the induced phosphorylation and degradation of IκB (reviewed in references 3, 5, and 22). Interestingly, we and others have identified the inducible transcription factor NF-κB as playing an important role in inhibiting TNF- or chemotherapy-induced apoptosis (6, 38, 55, 57, 61). Recently, we demonstrated that activation of NF-κB by TNF blocks the induction of the caspase cascade through the positive regulation of genes encoding several inhibitors of the apoptotic pathway (58). NF-κB activation blocked caspase 8 cleavage and cytochrome c release, indicating that NF-κB suppresses the earliest signaling components of the caspase cascade. We and others found that IAP family genes c-IAP1 and c-IAP2 and TRAF family genes TRAF1 and TRAF2 are positively regulated by NF-κB with rapid kinetics following TNF addition (11, 58). Coexpression of TRAF-1, TRAF2, c-IAP1, and c-IAP2 potently blocked caspase 8 activation and TNF-mediated apoptosis (58). Another member of the IAP family, XIAP, has been shown to be activated by NF-κB in endothelial cells (16, 50). Recently, Wu et al. also identified a protein, IEX-1L, which is encoded by a gene that is transcriptionally regulated by NF-κB and which can inhibit both TNF- and Fas-induced apoptosis by an unknown mechanism (62). In this report, we show that the gene encoding A1/Bfl-1, a homologue of Bcl-2 (12, 18, 19, 28, 29, 35), is activated in response to NF-κB induction. A1 is a 175-amino-acid protein which can suppress apoptosis and which is overexpressed in certain epithelial and hematopoietic malignancies. A1 was previously shown to be activated in response to inflammatory cytokine-induced signals (29, 35). In our studies we find that A1 reduced the rate of TNF-mediated apoptosis by inhibiting the release of cytochrome c. In addition, A1 potently inhibited etoposide-induced apoptosis by strongly inhibiting the release of cytochrome c and by blocking caspase 3 activation. These studies show that NF-κB activation leads to specific gene expression which suppresses mitochondrial mechanisms associated with apoptosis through a process separate from the ability to block the activation of caspase 8. Furthermore, the data suggest that the induction of A1 by NF-κB may be important in controlling resistance to chemotherapeutic responses in tumor cells.
MATERIALS AND METHODS
Cell culture and stable transfection.
HT1080I and a control cell line (HT1080V) were cultured in Eagle minimal essential medium supplemented with 10% fetal calf serum, penicillin (100 μg/ml), streptomycin (100 μg/ml), and hygromycin (200 μg/ml) (57). For stable transfection, Flag-tagged full-length A1 (the A1 cDNA was the generous gift of A. Karsan) was subcloned into the pcDNA3 vector containing a neomycin resistance gene. HT1080I cells were transfected with pcDNA3-Flag-A1 expression plasmid or empty control vector by using Lipofectamine according to the manufacturer’s instructions. Two days after transfection, cells were selected for resistance to 600 μg of G418 (Gibco-BRL) per ml. The individual clones expressing A1 were screened after 2 weeks of selection with a monoclonal antibody against the Flag epitope.
Northern blot analysis.
Total RNA was isolated with Trizol reagent as instructed by the manufacturer (Life Technologies). Ten-microgram aliquots of RNA were fractionated on a 1.4% agarose-formaldehyde gel, transferred onto a nylon filter, and cross-linked with a UV cross-linker. Blots were hybridized overnight with random-primed 32P-labeled probe at 42°C in a mixture containing 50% formamide, 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 1× PE (50 mM Tris-HCl [pH 7.5], 0.1% sodium pyrophosphate, 1% sodium dodecyl sulfate [SDS], 0.25% polyvinylpyrrolidone, 0.25% Ficoll, 5 mM EDTA), and 150 μg of salmon sperm DNA. The probes were generated with a random-primed labeling kit (Life Technologies) in the presence of [α-32P]dCTP (NEN-Dupont). Probes were purified with a micro G-50 Sephadex column (Life Technologies). After hybridization, the blots were washed twice in 2× SSC–0.1% SDS for 10 min at room temperature and twice in 0.1× SSC–0.1% SDS for 20 min at 42°C.
Electrophoretic mobility shift assays (EMSAs).
Cells were treated with etoposide (50 μM; Sigma) for the indicated time periods, and nuclear extracts were prepared as described previously (57). Five-microgram aliquots nuclear extracts of cells were preincubated with 1 μg of poly(dI-dc) in binding buffer (10 mM Tris [pH 7.7], 50 mM NaCl, 20% glycerol, 1 mM dithiothreitol [DTT], 0.5 mM EDTA) for 10 min at room temperature. Approximately 20,000 cpm of 32P-labeled DNA probe containing the class I major histocompatibility complex NF-κB site (5′-CAGGGCTGGGGATTCCCCATCTCCACAGTTTCACTTC-3′) was then added, and binding proceeded for 15 min. The complexes were separated on a 5% polyacrylamide gel and exposed for autoradiography.
Western blot analysis.
Whole-cell extracts were prepared as described previously (57). Cytosolic extracts were prepared by the method of Yang et al. (63). Briefly, HT1080V and HT1080I cells were treated with TNF-α (20 ng/ml) or etoposide (50 μM). The detached and attached cells were collected, and cell pellets were washed twice with ice-cold phosphate-buffered saline and resuspended with 5 volumes of buffer containing 20 mM HEPES, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.1 mM phenylmethylsulfonyl fluoride, 2 μg each of aprotinin, leupeptin, and pepstatin per ml, and 250 mM sucrose. The cells were homogenized with 10 strokes of a Dounce homogenizer, and the homogenates were centrifuged twice at 14,000 rpm for 15 min at 4°C. The resulting cytosol was collected for Western blotting. The extracts were subjected to SDS–10% or 15% polyacrylamide gel electrophoresis and transferred to nitrocellulose by electroblotting. Proteins were probed with primary antibody and visualized by using an ECL kit (Amersham) according to the manufacturer’s instructions. For internal control, the blots were stripped with 62.5 mM Tris buffer (pH 8) containing 100 mM 2-mercaptoethanol and 2% SDS at 60°C for 1 h and reprobed with α-tubulin. Primary antibodies were from the following sources: monoclonal antibodies against human caspase 8 (1:1,000) and cytochrome c (1:1,000) from Pharmingen; anti-DFF45 polyclonal antibody (1:7,500) from X. Wang; monoclonal antibody against caspase 3 (1:500) from Transduction Laboratories; and monoclonal antibody against α-tubulin (1:2,000) from Sigma.
Cell death ELISA.
For cell death enzyme-linked immunosorbent assay (ELISA), 105 cells were plated onto 24-well plates the day before stimulation. Cells were treated with TNF (20 ng/ml) or etoposide (50 μM) for different times; 20 μl of supernatant was used to measure DNA fragmentation and histone release from the nucleus. The measurement was performed as specified by the manufacturer (Boehringer Mannheim).
In vitro caspase 3 and caspase 8 activity assay.
After treatment of 2 × 106 cells with TNF-α (20 ng/ml) or etoposide (50 μM) for different times, the detached and attached cells were harvested, washed with phosphate-buffered saline and lysed in 200 μl of ice-cold hypotonic buffer (20 mM Tris-HCl [pH 7.5], 1 mM EDTA, 100 μM phenylmethylsulfonyl fluoride, 2 μg each of aprotonin, pepstatin, and leupeptin per ml). The cell lysate was frozen at −80°C and was thawed quickly at 4°C. The homogenates were centrifuged, and supernatants were collected. Then 300-μg aliquots of protein extracts were incubated with DEVD-pNA (200 μM) or IETD-pNA (200 μM) substrate (Chemicon International) in reaction buffer containing 10 mM DTT for 2 to 3 h at 37°C. The samples were analyzed with a plate reader by measuring the optical density at a wavelength of 405 nm (OD405).
RESULTS
A1/Bfl-1, a Bcl-2 homologue, is induced in response to NF-κB activation.
To explore the molecular mechanisms by which NF-κB controls resistance to TNF killing, we used an HT1080 fibrosarcoma cell line (HT1080I) (57) that expresses a modified form of the NF-κB inhibitor IκBα (8). This cell line, but not a control cell line (HT1080V), strongly inhibits NF-κB nuclear function (57). Previously, we showed that inhibition of NF-κB activation rendered the HT1080I cells sensitive to TNF- and cancer therapy-induced apoptosis and that the activation of NF-κB directly or indirectly blocked the release of cytochrome c from mitochondria (57, 58). One mechanism to explain this observation was that NF-κB suppressed the activation of caspase 8 (58), which would inhibit the ability of this caspase to activate Bid which induces mitochondrial permeability transition (24, 33, 39). Additionally, we showed that Bcl-2 and Bcl-xL levels are not controlled by NF-κB in these cells (58). Thus, it was unclear whether NF-κB activation leads to a direct mechanism to suppress mitochondrial proapoptotic mechanisms.
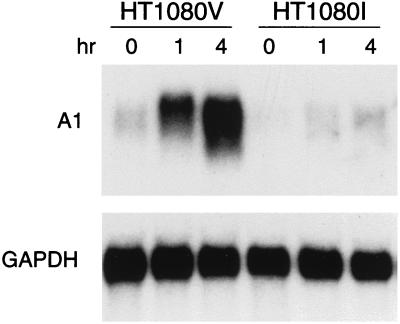
To determine if other Bcl-2 family members may be regulated by NF-κB, we analyzed whether A1/Bfl-1, a Bcl-2 homologue which is known to be induced by inflammatory cytokines, could be regulated by NF-κB. As shown in Fig. 1, A1/Bfl-1 mRNA was rapidly induced after TNF-α stimulation in HT1080V cells but not in HT1080I cells, which indicated that activation of NF-κB is required for the expression of the A1 gene. Control blotting with glyceraldehyde-3-phosphate dehydrogenase indicated equivalent loading for the different experimental conditions.
FIG. 1.
A1/Bfl-1 expression is induced by TNF through the activation of NF-κB. HT1080V and HT1080I cells were treated with TNF-α (20 ng/ml) for the indicated time. Total RNA was extracted with Trizol reagent. Northern blots were performed as described in Materials and Methods. The filter was probed with 32P-labeled human A1 cDNA probe. For the internal control, the blot was stripped and reprobed with 32P-labeled GAPDH (glyceraldehyde-3-phosphate dehydrogenase) cDNA probe.
A1/Bfl-1 partially inhibits TNF-induced apoptosis.
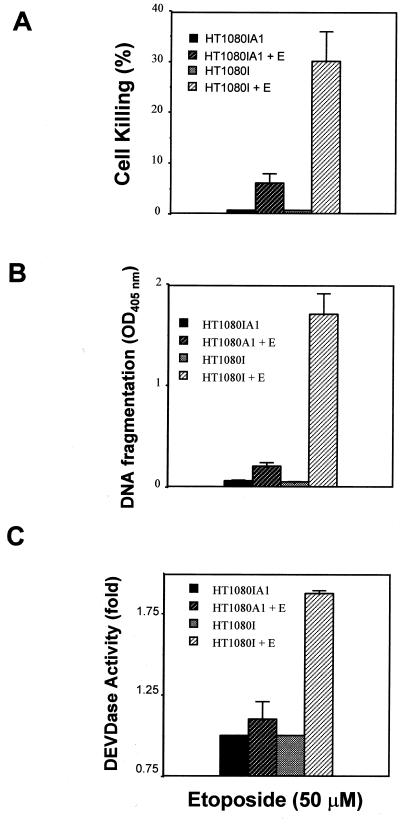
Despite the wide-ranging ability of Bcl-2 to promote cell survival, it is clear that a number of stimuli initiate an apoptotic response that is not susceptible to Bcl-2 protection. For example, studies demonstrated that TNF and Fas could bypass the Bcl-2 checkpoint to induce apoptosis in several cell types (1, 7, 24, 49, 52). In contrast to most other members of the Bcl-2 family, A1 contains only BH1 and BH2 domains and lacks the conserved hydrophobic C terminus which is required for anchoring Bcl-2 to membranes (1). Therefore, it is possible that other members of the Bcl-2 family provide protective effects in circumstances in which Bcl-2 is ineffective. To determine whether the A1 protein could protect against TNF-α-induced killing in HT1080I cells, we established A1-expressing HT1080I (HT1080IA1) cell clones (Fig. 2A). As shown in Fig. 2B, A1 partially inhibited cell death induced in HT1080I cells, with approximately 50% suppression at the 7-h time point. However, at the 14-h time point, A1-expressing cells were only weakly inhibited in the cell death response. These results were confirmed by using the cell death ELISA, which measures fragmented DNA associated with histones (Fig. 2C). As shown in Fig. 2D, A1 also partially inhibited TNF-induced DEVDase activity (a measure of caspase 3-like activity) in HT1080I cells. These data indicate that A1 expression can partially inhibit apoptosis induced by TNF when NF-κB is functionally blocked.
FIG. 2.
A1 expression in HT1080I cells partially inhibits TNF-induced apoptosis. (A) Detection of stable HT1080I transfectants expressing A1/Bfl-1. HT1080I cells were transfected with pcDNA3-Flag-A1 vector or control empty vector and selected with G418 (600 μg/ml) for 2 weeks. The clones were analyzed by monoclonal antibody against Flag epitope. The five stable clones which expressed similar levels of A1 protein were pooled (HT1080IA1). Lane 1 and 2 represent the stable HT1080I cells expressing A1; lane 3 represents the stable control clones expressing G418-resistant marker (HT1080I). (B and C) A1 partially inhibits TNF-induced apoptosis. The stable cell clones used for panel A were treated with TNF (20 ng/ml) for the indicated times. Cell viability was determined by trypan blue exclusion. The supernatants from the 14-h time point of TNF treatment were collected and measured by cell death ELISA. The results represent the mean values from three independent experiments. (D) A1 inhibits TNF-induced DEVDase activity. The stable cell clones used for panel A were treated with TNF (20 ng/ml) for 6 h. Cells were lysed in hypotonic buffer (see Materials and Methods), and 300-μg aliquots of extracts were incubated with DEVD-pNA (100 μM) substrate for 2 h at 37°C. The reaction was measured with a plate reader by determining the OD405. The results represent the mean values from three independent experiments.
A1 expression reduces TNF-α-induced cytochrome c release from mitochondria and reduces caspase 3 activation.
Biochemical and molecular studies have demonstrated that caspase 8 is at the apex of the caspase pathway (at least involving death receptor signaling) and links death domain protein signaling and caspase activation in TNF-induced apoptosis (2, 4, 48, 56). Our previous study had identified that NF-κB-regulated TRAF1 and -2 and c-IAP1 and -2 expression to cooperatively suppress caspase 8 activation (58). Therefore, we were interested in whether A1 could inhibit the processing of caspase 8 induced by TNF. As shown in Fig. 3, procaspase 8 was processed with similar kinetics after TNF treatment in both HT1080I and HT1080IA1 cells. This result indicated that A1 expression does not block processing of procaspase 8, consistent with a more downstream effect of A1 in suppressing apoptosis.
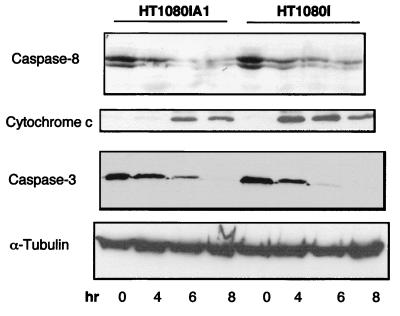
FIG. 3.
A1 partially inhibits TNF-induced cytochrome c release from mitochondria and inhibits caspase 3 processing. HT1080IA1 and HT1080I cells were treated with TNF (20 ng/ml) for the indicated times. To detect the release of cytochrome c, cytosolic proteins were extracted and separated by SDS–15% polyacrylamide gel electrophoresis. The blot was probed with monoclonal antibody to cytochrome c (1:1,000). For detecting caspase 8 and caspase 3 processing, whole-cell extracts were prepared and immunoblotted with monoclonal antibodies to caspase 8 (1:1,000) and caspase 3 (1:500). For the internal control, the blots were stripped and reprobed with antibody to α-tubulin (1:2,000).
Since Bcl-2 has been found to block the mitochondrial release of cytochrome c induced by multiple death stimuli (31, 63), we were interested to determine whether A1 would also block the release of cytochrome c. HT1080I and HT1080IA1 cells were treated with TNF for different times, and cytosolic extracts were prepared as described before. As shown in Fig. 3, the release of cytocrome c from mitochondria was partially suppressed in the A1-expressing cells but not in the control HT1080I cells. There was near-complete suppression of cytochrome c release at the 4-h time point in A1-expressing cells, but cytochrome c release did occur at the 6- and 8-h time points. Cytochrome c has been shown to function as a coactivator of caspase 9 which leads to activation of downstream caspases such as caspase 3 (34, 36, 67). Consistent with this, caspase 3 activation was processed more slowly in the HT1080IA1 cells than in the control HT1080I cells following TNF-α treatment (Fig. 3). These data indicate that A1 expression suppresses the release of cytochrome c from mitochondria following TNF-α treatment, which leads to a reduction of the apoptotic response.
A1 is induced by etoposide and strongly suppresses etoposide-induced cell death.
A variety of studies have indicated that NF-κB is antiapoptotic relative to different stimuli. For example, NF-κB activation can suppress cell death induced both by TNF-α and by chemotherapy (57). Importantly, it is known that chemotherapy induces the activation of NF-κB and that this suppresses the apoptotic potential of the chemotherapy (58). As shown in Fig. 4A, a common chemotherapeutic agent etoposide potently induced the nuclear translocation of NF-κB in HT1080V cells but not in HT1080I cells. Similar to TNF-α, etoposide strongly induced A1 mRNA expression in HT1080V cells but only weakly induced A1 mRNA in HT1080I cells as detected by Northern blotting (Fig. 4B). This experiment demonstrated that NF-κB is required for basal and elevated expression of A1 mRNA, but that NF-κB is not the only transcription factor which contributes to inducible A1 gene expression. Since A1 mRNA was induced by etoposide and since etoposide has been widely used as an inducer to stimulate the release of cytochrome c and to induce apoptosis (14, 30, 34, 47, 63), we determined whether A1 could block etoposide-induced apoptosis. As shown in Fig. 5A, A1 expression strongly inhibited etoposide-induced cell death as measured by trypan blue exclusion and inhibited apoptosis as measured by the cell death ELISA (Fig. 5B). A1 also potently inhibited DEVDase activity in HT1080I cells induced by etoposide (Fig. 5C). These data indicate that A1 strongly suppresses apoptosis induced by the chemotherapeutic compound etoposide.
FIG. 4.
A1/Bfl-1 expression is induced by etoposide through the activation of NF-κB. (A) Etoposide induces the nuclear translocation of NF-κB in HT1080V cells but not HT1080I cells. Cells were treated with etoposide (50 μM) for the indicated time. EMSAs were performed as described in Materials and Methods. (B) A1 gene expression is induced by etoposide. Cells were treated with etoposide (50 μM) for the indicated time. Northern blot analyses were performed as described for Fig. 1.
FIG. 5.
A1 inhibits etoposide-induced apoptosis. (A and B) HT1080IA1 and HT1080I cells were treated with etoposide (E; 50 μM) for 24 h. The cell viability and cell death ELISAs were performed as described for Fig. 2B and C, respectively. (C) A1 inhibits etoposide-induced DEVDase activity. Cells were treated with etoposide (50 μM) for 16 h, and DEVDase assays were performed as described for Fig. 2D. The results represent the average values from three independent experiments.
A1 effectively blocks cytochrome c release induced by etoposide.
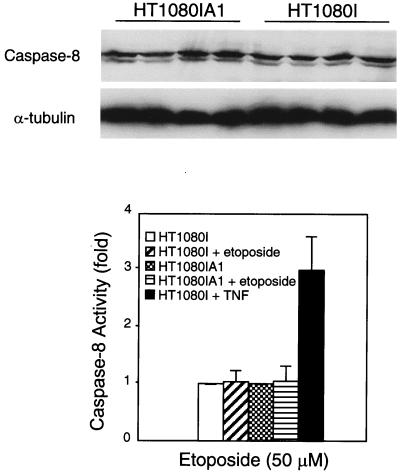
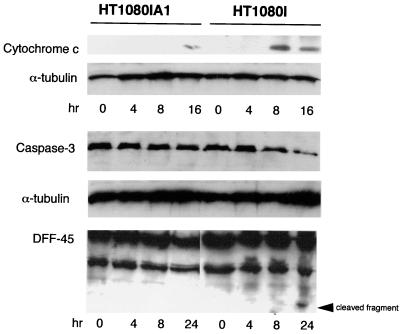
To determine at which point A1 inhibited the apoptotic response in response to etoposide, we first analyzed processing of caspase 8. Interestingly and different from TNF-α, etoposide did not activate caspase 8 either in the control HT1080I cells or in the HT1080IA1 cells, at least up to 24 h (Fig. 6). This result indicates that etoposide does not utilize caspase 8 to initiate the caspase cascade. Since cytochrome c has been identified as a coactivator with Apaf-1 to activate caspase 9 and subsequently to activate caspase 3, we are interested in whether A1 blocks etoposide-induced release of cytochrome c. As shown in Fig. 7, the release of cytochrome c was almost completely inhibited in HT1080IA1 cells but not in HT1080I cells in response to etoposide treatment. Consistent with this, the processing of caspase 3 was inhibited in HT1080IA1 but not in HT1080I cells (Fig. 7). It has been shown that ICAD/DFF45 is cleaved by caspase 3 and is the principal regulator of DNA fragmentation during apoptosis (37). As shown in Fig. 7, the cleavage of ICAD/DFF45 was inhibited in HT1080IA1 cells but not in HT1080I cells. This result is consistent with the inactivation of caspase 3 in the A1-expressing cells. Overall, these results suggest that cytochrome c is a primary initiator in etoposide-initiated apoptosis and that one mechanism whereby NF-κB inhibits chemotherapy-induced cell death is through the induction of A1, which suppresses cytochrome c release from mitochondria. The data also demonstrate that A1 is a more effective inhibitor of cytochrome c release in etoposide-treated cells than in TNF-α-treated cells.
FIG. 6.
Caspase 8 activity is not induced by etoposide. HT1080IA1 and HT1080I cells were treated with etoposide (50 μM) for 24 h. The whole-cell extracts were probed with a monoclonal antibody against caspase 8 as described for Fig. 3. The lanes are as shown in Fig. 7. For detecting caspase 8 activity, cells were treated with etoposide (50 μm) for 24 h and then lysed in hypotonic buffer as described for Fig. 2, and 300-μg aliquots of protein extracts were incubated with IETD-pNA substrate (100 μM) for 2 h at 37°C. The reaction was measured with a plate reader by determining the OD405. The results represent the average values from three independent experiments.
FIG. 7.
A1 potently inhibits etoposide-induced cytochrome c release. HT1080IA1 and HT1080I cells were treated with etoposide (50 μM) for the indicated times. Cytosolic proteins were extracted and probed with a monoclonal antibody against cytochrome c. For detecting caspase 3 and DFF45 processing, the whole-cell extracts were prepared as described for Fig. 3. For internal control, the blots were stripped and reprobed with monoclonal antibody to α-tubulin.
DISCUSSION
The transcription factor NF-κB serves as a principal mediator of resistance to a variety of apoptotic stimuli. The activation of NF-κB in response to TNF-α, ionizing radiation, oncogenic Ras expression, and chemotherapy provides a cell survival function in the face of these potential apoptotic stimuli (6, 38, 40, 55, 57, 58, 61). The multiplicity of mechanisms whereby NF-κB serves the antiapoptotic function is becoming increasingly complex. It has been shown that the activation of genes encoding TRAF and IAP proteins, IEX-1, and XIAP by NF-κB serves to block apoptosis in different cell types (11, 50, 58, 62). The mechanism of action of TRAF1 and -2 and c-IAP 1 and -2 is to block the activation of caspase 8 in response to TNF-α challenge of NF-κB null cells (58). The induction of c-IAP1 and -2 by NF-κB blocks etoposide-induced cell death at the level of caspase 3 (58), which is consistent with experiments that indicate that IAP proteins can inhibit apoptosis downstream of cytochrome c release (13, 15, 16, 20, 21, 47). It should also be noted that under some conditions NF-κB may function in a proapoptotic role (30).
Our work and that of Bradham et al. have indicated that NF-κB activation can suppress the mitochondrial permeability transition (9, 58). We had originally observed that neither Bcl-2 or Bcl-xL was activated by NF-κB (58), suggesting that the primary mechanism whereby NF-κB activation would suppress cytochrome c release associated with mitochondrial damage was through the inhibition of caspase 8 activation, which would block Bid activation (33, 39). However, in this report we report that NF-κB activation activates A1/Bfl-1, a member of the Bcl-2 family, to suppress cytochrome c release from mitochondria. Thus, NF-κB activation functions to suppress apoptosis at multiple levels (also see below).
The growing Bcl-2 family proteins include at least 15 members, which provide both positive or negative regulation of apoptosis. These family members have been indicated to play an essential role for maintenance of major organ systems, and mutation or disturbance of their expression is likely to contribute to cancer or resistance to cancer therapy (1, 10, 44, 45). The previous studies have mainly focused on the posttranslational regulation of these proteins. For example, Bcl-2 has been shown to be activated by Ser70 phosphorylation but inactivated by phosphorylation of several loop sites, perhaps by c-Jun N-terminal kinase (1). The proapoptotic protein Bad is phosphorylated by the Akt kinase, leading to sequestration by 14-3-3 proteins, precluding its inhibition of Bcl-xL (1, 59, 65). In the present study, we identified a Bcl-2 family member, A1, as a potential transcription target for the antiapoptotic action of NF-κB. Consistent with our findings, two reports published since the submission of ours demonstrated that the A1 gene is induced by NF-κB (25, 66). Both reports indicate the existence of functional NF-κB/Rel binding sites in the promoter of the A1 gene. Additionally, it was shown that A1 expression protected against TNF-α-induced cell death in IκB-expressing cells (66) and against anti-immunoglobulin M-induced cell death in c-Rel−/− B cells (25). The coexpression of A1 and c-Rel in germinal centers, in the spleen, and in inflammatory cells strongly suggests a role for NF-κB factors in protecting against apoptosis during immune and inflammatory responses (25, 66). These reports did not demonstrate how A1 functions to suppress apoptosis and did not examine the involvement of A1 expression in responses to chemotherapy.
Our study indicates that A1 functions primarily to suppress etoposide-induced cell death and can only weakly suppress TNF-α-induced cell death. This may be explained by several observations. Since caspase 8 can directly activate caspase 3 and since caspase 8 activation is required for TNF-α-induced cell death, the release of cytochrome c in response to TNF-α activation is not required for induction of cell death (51, 56). Thus, the ability of A1 to delay cytochrome c release and the subsequent caspase 3 activation likely explains the reduction of cell death induced by TNF-α at the early time. It also indicates that caspase 8 may utilize cytochrome c to amplify the caspase cascade. Furthermore, A1 is unable to block cytochrome c release at later time points, allowing full caspase 3 activation. Etoposide-induced cytochrome c release is strongly blocked by A1 expression. This may be explained by the observation that cytochrome c release occurs more slowly in response to etoposide treatment compared to TNF-α stimulation. Thus, one mechanism to explain the difference in the ability of A1 to preferentially suppress etoposide-induced cell death is that etoposide is a weaker stimulator of cytochrome c release than TNF-α and that the level of A1 expression is sufficient to block this response. We also note that A1 partially suppressed TNF-α-induced cell death even at time points where cytochrome c is fully released. Thus, we cannot rule out the possibility that A1 can function downstream of cytochrome c to block apoptosis. This would be consistent with observations that Bcl-2 and Bcl-xL can function downstream of mitochondria to suppress cell death pathways (7, 27, 43, 46). As expected, our data confirm that A1 does not block the activation of caspase 8. Although A1 does not share the conserved C-terminal transmembrane domain with other Bcl-2 family proteins, this region is still required for A1 antiapoptotic functions (1). Thus, A1 may still interact with mitochondrial membranes to suppress cytochrome c, similar to Bcl-2 and Bcl-xL.
There are a number of genes, including those encoding A20 and manganese superoxide dismutase, that have been reported to block TNF-induced apoptosis (42, 58, 62). Interestingly, these genes contain NF-κB consensus sites in their promoters and are regulated by NF-κB. However, the stable overexpression of these genes separately in vivo can only partially protect cells from TNF-induced killing (42). Our recent work identified TRAF-1, TRAF-2, c-IAP1, and c-IAP2 as TNF-induced genes that are regulated by NF-κB to control caspase 8 activation (58). This would be a primary mechanism of NF-κB inhibit apoptosis induced by TNF-α since caspase 8 serves as the apical caspase for this pathway. Here, we report that NF-κB controls the expression of a gene encoding A1, a member of the Bcl-2 family of proteins. Our evidence indicates that A1 functions downstream of caspase 8 in the apoptotic pathway and serves as an inducible factor to prevent or reduce cytochrome c release from mitochondria. Consistent with this downstream role, A1 expression was unable to strongly block TNF-α-induced cell death. Since NF-κB also plays a negative role in chemotherapy and irradiation-mediated apoptosis, the induction of A1 is likely to play a significant role in blocking cell death induced by these cancer therapies. These results and that of others indicate that complete suppression of apoptosis induced by NF-κB involves multiple functions, including inhibition of caspase 8 and of cytochrome c release from mitochondria.
ACKNOWLEDGMENTS
We gratefully acknowledge Aly Karsan for the kind gift of the A1 cDNA.
Research support was provided by NIH grant DE12823 to C.-Y.W., by NCI grant CA75080 to A.S.B. and M.W.M., by American Cancer Society grant PF9903801 to D.G., and by NIH grant AI35098 and NCI grants CA73756 and CA72771 to A.S.B.
REFERENCES
- 1.Adams J, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi A, Dixit V. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 4.Baker S, Reddy E P. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998;25:3261–3270. doi: 10.1038/sj.onc.1202568. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin A S. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 6.Beg A A, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 7.Boise L H, Thompson C B. Bcl-xL can inhibit apoptosis in cells that have undergone Fas-induced protease activation. Proc Natl Acad Sci USA. 1997;94:3759–3763. doi: 10.1073/pnas.94.8.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boothby M R, Mora A, Scherer D, Brockman J, Ballard D. Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-kappaB. J Exp Med. 1997;185:1897–1907. doi: 10.1084/jem.185.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradham C, Qian T, Streetz K, Trautwein C, Brenner D, LeMasters J. The mitochondrial permeability transition is required for tumor necrosis factor alpha-mediated apoptosis and cytochrome c release. Mol Cell Biol. 1998;18:6353–6364. doi: 10.1128/mcb.18.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao D T, Korsmeyer S J. Bcl-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 11.Chu Z L, McKinsey T, Liu L, Gentry J, Malim M, Ballard D W. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-κB control. Proc Natl Acad Sci USA. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang P, Yee E, Karsan A, Winn R, Harlan J. A1 is a constitutive and inducible Bcl-2 homologue in mature human neutrophils. Biochem Biophys Res Commun. 1998;249:361–365. doi: 10.1006/bbrc.1998.9155. [DOI] [PubMed] [Google Scholar]
- 13.Clem R J, Duckett C S. The iap genes: unique arbitrators of cell death. Trends Cell Biol. 1997;7:337–339. doi: 10.1016/S0962-8924(97)01088-X. [DOI] [PubMed] [Google Scholar]
- 14.Datta R, Banach D, Kojima H, Talanian R, Alnemri E, Wong W, Kufe D. Activation of the CPP32 protease in apoptosis induced by Ara-C and other DNA-damaging agents. Blood. 1996;86:1936–1943. [PubMed] [Google Scholar]
- 15.Deveraux Q, Roy N, Stennicke H, Van Arsdale T, Zhou Q, Srinivasula S, Alnemri E, Salvesen G, Reed J. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deveraux Q, Reed J C. IAP family proteins—suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 17.Dragovich T, Rudin C, Thompson C. Signal transduction pathways that regulate cell survival and cell death. Oncogene. 1998;25:3207–3214. doi: 10.1038/sj.onc.1202587. [DOI] [PubMed] [Google Scholar]
- 18.D’Sa-Eipper C, Subramanian T, Chinnadurai G. Bfl-1, a Bcl-2 homologue, suppresses p53-induced apoptosis and exhibits potent cooperative transforming activity. Cancer Res. 1996;56:3879–3882. [PubMed] [Google Scholar]
- 19.D’Sa-Eipper C, Chinnadurai G. Functional dissection of Bfl-1, a Bcl-3 homolog: anti-apoptosis, oncogene-cooperation and cell proliferation activities. Oncogene. 1998;16:3105–3114. doi: 10.1038/sj.onc.1201851. [DOI] [PubMed] [Google Scholar]
- 20.Duckett C S, Nava V, Gedrich R, Clem R, Van Dongen J, Gilfillan M, Shiels H, Hardwick J, Thompson C B. A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors. EMBO J. 1996;15:2685–2694. [PMC free article] [PubMed] [Google Scholar]
- 21.Duckett C S, Li F, Wang Y, Tomaselli K J, Thompson C B, Armstrong R C. Human IAP-like protein regulates programmed cell death downstream of Bcl-xL and cytochrome c. Mol Cell Biol. 1997;18:608–615. doi: 10.1128/mcb.18.1.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh S, May M, Kopp E. NF-κB and rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 23.Green D, Reed J. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 24.Gross A, Yin X, Wang K, Wei M, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer S J. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while Bcl-xL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 25.Grumont R, Rourke I J, Gerondakis S. Rel-dependent induction of A1 transcription is required to protect B cells from antigen receptor ligation-induced apoptosis. Gene Dev. 1999;13:400–411. doi: 10.1101/gad.13.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hakem R, Hakem A, Duncan G S, Henderson J T, Woo M, Soengas M S, Elisa A, Pompa J, Kagi D, Khoo W, Potter J, Yoshida R, Kaufman S A, Lowe S W, Penninger J M, Mak T W. Differential requirement for caspase-9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 27.Hu Y, Benedict M, Wu D, Inohara N, Nunez G. Bcl-xL interacts with Apaf-1 and inhibits Apaf-1 dependent caspase-9 activation. Proc Natl Acad Sci USA. 1998;95:4386–4391. doi: 10.1073/pnas.95.8.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karsan A, Yee E, Kaushansky M, Harlan J. Cloning of a human Bcl-2 homologue: inflammatory cytokines induce human A1 in cultured endothelial cells. Blood. 1996;87:3089–3096. [PubMed] [Google Scholar]
- 29.Karsan A, Yee E, Harlan J. Endothelial cell death induced by TNFα is inhibited by the Bcl-2 homologue, A1. J Biol Chem. 1996;271:27201–27204. doi: 10.1074/jbc.271.44.27201. [DOI] [PubMed] [Google Scholar]
- 30.Kasibhatla S, Genestier L, Green D R. Regulation of Fas-ligand expression during activation-induced cell death in T lymphocytes via nuclear factor κB. J Biol Chem. 1999;274:987–992. doi: 10.1074/jbc.274.2.987. [DOI] [PubMed] [Google Scholar]
- 31.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 32.Kuida K, Haydar T, Kuan C, Gu Y, Taya C, Karasuyama H, Su M, Rakic P, Flavell R. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Zhu H, Xu C-J, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 34.Li P, Nijhawan D, Budihardjo I, Srinivasula S, Ahmad M, Alnemri E, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1996;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 35.Lin E Y, Orlofsky A, Wang H, Reed J C, Prystowsky M B. A1, a Bcl-2 family member, prolongs cell survival and permits myeloid differentiation. Blood. 1996;87:983–992. [PubMed] [Google Scholar]
- 36.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 38.Liu Z-G, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector function: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 39.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 40.Mayo M W, Wang C-Y, Cogswell P C, Rogers-Graham K S, Lowe S W, Der C J, Baldwin A S. Requirement of NF-κB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 41.Nicholson D W, Thornberry N A. Caspase: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 42.Opipari A W, Hu H, Yabkowitz R, Dixit V M. The A20 zinc finger protein protects cell from tumor nectosis factor cytotoxicity. J Biol Chem. 1992;267:12424–12427. [PubMed] [Google Scholar]
- 43.Pan G, O’Rourke K, Dixit V. Caspase-9, Bcl-xL and Apaf-1 form a ternary complex. J Biol Chem. 1998;273:5841–5845. doi: 10.1074/jbc.273.10.5841. [DOI] [PubMed] [Google Scholar]
- 44.Reed J C. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 45.Reed J C. Bcl-2 family proteins. Oncogene. 1998;25:3225–3236. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- 46.Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 47.Roy N, Deveraux Q, Takashashi R, Salvesen G, Reed J. The c-IAP1 and 2 proteins are direct inhibitors of specific caspases. EMBO J. 1999;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salvesen G, Dixit V. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 49.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K, Debatin K, Krammer P, Peter M. Two CD95 (Apo-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stehlik C, de Martin R, Kumabashiri I, Schmid J, Binder B, Lipp J. NF-κB-regulated XIAP gene expression protects endothelial cells from TNFα-induced apoptosis. J Exp Med. 1998;188:211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stennicke H, Jurgensmeier J, Shin H, Devereaux Q, Wolf B, Yang X, Zhou Q, Ellerby H, Ellerby L, Bredesen D, Green D, Reed J, Froelich C, Salvesen G. Pro-caspase-3 is a major physiologic target of caspase-8. J Biol Chem. 1998;273:27084–27090. doi: 10.1074/jbc.273.42.27084. [DOI] [PubMed] [Google Scholar]
- 52.Susin S A, Zamzami N, Castedo M, Daugas E, Wang H, Geley S, Fassy F, Reed J C, Kroemer G. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson C. Apoptosis in the pathogenesis and treatment of cancer. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 54.Thornberry N, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 55.Van Antwerp D J, Martin S, Kafri T, Green D R, Verma I M. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 56.Varfolomeev E, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann J, Mett I, Rebrikov D, Brodianski V, Kemper O, Kollet O, Lapidot T, Soffer D, Sobe T, Avraham K, Goncharov T, Holtmann H, Lonai P, Wallach D. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptorsm /Fas/Apo1, and DR3 is lethal prenatally. Immunity. 1998;9:267–278. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 57.Wang C-Y, Mayo M, Baldwin A S. TNF- and cancer therapy-induced apoptosis potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 58.Wang C-Y, Mayo M W, Korneluk R C, Goeddel D V, Baldwin A S. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 59.Wang H G, Rapp U R, Reed J C. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 60.Woo M, Hakem R, Soengas M, Duncan G, Shahinian A, Kagi D, Hakem A, McCurrach M, Khoo W, Kaufman S, Senaldi G, Howard T, Lowe S, Mak T. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 1998;12:806–819. doi: 10.1101/gad.12.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu M, Lee H, Ballas R, Schauer S, Arsura M, Katz D, FitzGerald M, Rothestein T, Sherr D, Sonenshein G E. Inhibition of NF-κB/Rel induces apoptosis of murine B cells. EMBO J. 1996;15:4682–4690. [PMC free article] [PubMed] [Google Scholar]
- 62.Wu M X, Ao Z, Prasad K V S, Wu R, Schlossman S F. IEX-1L, an apoptosis inhibitor involved in NF-κB-mediated cell survival. Science. 1998;281:998–1001. doi: 10.1126/science.281.5379.998. [DOI] [PubMed] [Google Scholar]
- 63.Yang J, Liu X, Bhalla K, Kim C, Ibrado A, Cai J, Peng T-I, Jones D, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 64.Zamzami N, Susin S, Marchetti P, Hirsch T, Gomez-Monterey I, Castedo M, Kroemer G. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996;183:1523–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zha J, Hirashi H, Yang E, Jockel J, Korsmeyer S J. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-xL. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 66.Zong W, Edelstein L C, Chen C, Bash J, Gélinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-B that blocks TNF-induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zou H, Henzel W, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]